Abstract
Pinned and mobile ferroelastic domain walls are detected in response to mechanical stress in a Mn3+ complex with two-step thermal switching between the spin triplet and spin quintet forms. Single-crystal X-ray diffraction and resonant ultrasound spectroscopy on [MnIII(3,5-diCl-sal2(323))]BPh4 reveal three distinct symmetry-breaking phase transitions in the polar space group series Cc → Pc → P1 → P1(1/2). The transition mechanisms involve coupling between structural and spin state order parameters, and the three transitions are Landau tricritical, first order, and first order, respectively. The two first-order phase transitions also show changes in magnetic properties and spin state ordering in the Jahn–Teller-active Mn3+ complex. On the basis of the change in symmetry from that of the parent structure, Cc, the triclinic phases are also ferroelastic, which has been confirmed by resonant ultrasound spectroscopy. Measurements of magnetoelectric coupling revealed significant changes in electric polarization at both the Pc → P1 and P1 → P1(1/2) transitions, with opposite signs. All these phases are polar, while P1 is also chiral. Remanent electric polarization was detected when applying a pulsed magnetic field of 60 T in the P1→ P1(1/2) region of bistability at 90 K. Thus, we showcase here a rare example of multifunctionality in a spin crossover material where the strain and polarization tensors and structural and spin state order parameters are strongly coupled.
Introduction
Domain wall engineering1,2 in ferroic materials (ferromagnets, ferroelectrics, ferroelastics) constitutes one of the most promising areas for new applications in nanoelectronics3 and nanomagnetism.4 It is now well-established that the properties which emerge within domain walls are distinct from the properties within the domains themselves, and thus domain walls are recognized as functional 2D objects in their own right, rather than mere barriers between ordered functional regions. Examples of novel properties include conductivity5−7 or superconductivity8 in the domain walls of insulating ferroelectrics, ferromagnetic ordering of ferroelectric antiferromagnets,3 and unexpected photovoltaic effects concentrated in the domain walls of ferroelectrics where the voltage is higher than the band gap of the parent material.9−11 The mobility of domain walls allows them to be moved by electric or magnetic fields or by stress with velocities dependent on the wall dimensions and scale of the driving field. For example, the concept of using current driven data storage in magnetic racetrack memory devices via the supersonic motion of magnetic domain walls, which tend to be relatively broad (tens of nanometers), is well advanced.12,13 On the other hand, the potential for dynamic circuitry in ferroelectrics, where domain walls have lower velocities and are typically more narrow (widths of the order of one unit cell), is only starting to be realized.14−16 Memory devices based on the wall properties of ferroelectrics therefore have the potential for higher density storage that will outperform the bulk material, as the higher number of narrow domain walls means they constitute around 1% of the material by total volume.17,18
Phase transitions within a domain wall, as opposed to those in the bulk material, may also confer new functionality. This is typified in some nonpolar ferroelastic perovskite oxides such as SrTiO3, where thermal-induced polarity appears at cryogenic temperatures, indicative of a two-dimensional phase transition within the ferroelastic twin walls that are atomistically thin.19−22 The emergence of new order parameters during a move through a phase transition can thus lead to “domains with domains and walls within walls”,19 and materials with such multicomponent order parameters should offer both novel functionality and associated switching opportunities.
An investigation of the dimensions and mobility achievable in domain walls of many different types of materials is required to determine the switching speed and power efficiency, which in turn will inform the design process for potential device applications. In this respect it is of interest to look beyond oxide physics and investigate the ferroic and multiferroic potential of molecule-based systems, where the greater scope for changes in the degrees of freedom of both translational and point group symmetries may yield multiple order parameters over a phase transition or phase transitions. In particular, the large atomic displacements and resulting volume change associated with spin state switching in spin crossover (SCO) transition-metal complexes23−28 should ensure a change in strain gradient, especially for those systems with sharp transitions. Thermal SCO is often accompanied by structural phase transitions29−47 and associated spin state ordering, a phenomenon which can be classified as ferroic and which may be periodic27,48,49 or aperiodic.50
As highlighted in recent reviews, a strong cooperative effect occurs when SCO gives rise to a sequence of phase transitions and hysteretic SCO behavior.27,48,49 The diversity of possible ordering schemes for HS and LS cations, for example, has been simulated for a 60 × 60 lattice by Cruddas and Powell.51 A formalism from Landau theory provides a convenient phenomenological means of analyzing this behavior in terms of elastic coupling between the order parameter associated with SCO and symmetry-breaking order parameters due to ordering of HS and LS atoms.52,53 In the limiting case of SCO occurring without any change in crystallographic symmetry, the evolution of a single order parameter monitoring the global evolution of the spin state is sufficient to characterize the evolution of properties such as magnetic susceptibility, unit cell volume, and elastic moduli. This parameter has the symmetry of the identity representation of the space group and is therefore a non-symmetry-breaking order parameter qspin, as it preserves spatial symmetry.54,55 Symmetry-breaking order parameters are required to define the ordered structures and will have, in the space group of the parent (HS) high-symmetry structure, the symmetry of the irreducible representation which is related to the space group of the ordered structure (HS+LS). More generally, the non-symmetry-breaking spin state order parameter can couple with the order parameter for any structural instability, leading to many possibilities for the influence of SCO in multiferroic materials.
If there is coupling between the spin state and structural order parameters, there must also be coupling of their gradients through domain walls. The emergence of multicomponent order parameters can thus be expected to give rise to a diversity of domain wall properties and behaviors in spin state ordered crystals at low temperatures. We have recently reported the use of resonant ultrasound spectroscopy (RUS) to detect stress-induced ferroelastic domain wall motion in a Mn3+ SCO complex where ferroelastic and spin state order parameters are coupled.30 The mobile domain walls are distinct from the expected mobile phase boundary that develops at the high spin/low spin (HS/LS) boundary in isostructural SCO systems, where there is no change in structural order parameter and which can be followed effectively by optical microscopy.56−66 We now employ RUS to follow the ferroelastic changes that accompany the two-step thermal SCO in a Mn3+ complex which reveals both mobile and pinned domain walls at the phase transitions.
SCO in Mn3+ systems may be particularly useful in ferroelastic applications, as the Jahn–Teller effect in the spin quintet form results in a strong and thermally switchable distortion that may enhance the spin–lattice coupling. If this results in a phase transition that leads to broken inversion symmetry, a resulting magnetoelectric (ME) coupling may be observed.67−74 The expectation must be that complexes which exhibit spin–lattice coupling will result in the development of SCO transitions that give rise to strong multiferroic coupling behavior because of the possibility of common strain providing a coupling mechanism among magnetic, ferroelectric, and ferroelastic properties. This has been extensively studied in metal oxides but has rarely been reported in SCO materials.67−74 One reported example is Mn3+ in the SCO complex Mn(taa) (H3taa = tris(1-(2-azolyl)-2-azabuten-4-yl)amine), which undergoes a magnetic-field-induced SCO between S = 1 and S = 2 states above 35 T. A C3 → C1 Jahn–Teller effect induces electric dipoles in the S = 2 state that order to create electric polarization.
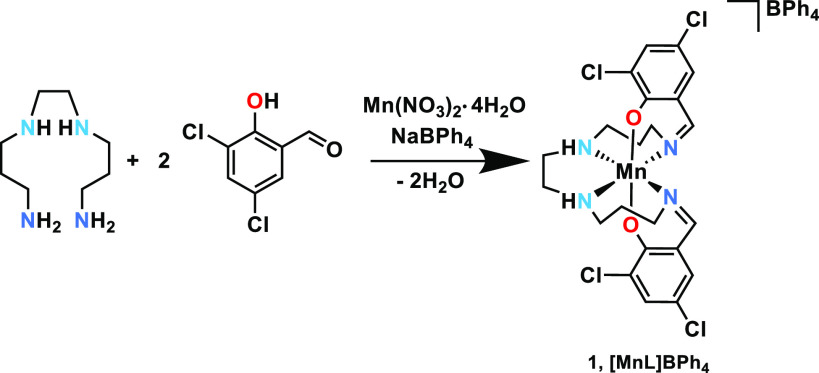
We now report hysteretic two-step SCO behavior in complex 1, [Mn(3,5-diCl-sal2(323))]BPh4 (Scheme 1), which is accompanied by structural phase transitions between polar space groups with different electric polarizations, and we also demonstrate the existence of both mobile and pinned domain walls at low temperature. We have investigated four structural phases that occur in 1 at different temperatures via single-crystal X-ray diffraction and RUS. On cooling from room temperature, the full transition sequence in terms of space groups is Cc → Pc → P1 → P1(1/2). The four distinct crystallographic phases are consistent with spin state ordering stemming from the different HS and LS populations in the sequence Cc[HS] →Pc[HS:HS]→ P1 [LS:LS:HS:HS] → P1 [LS:LS] . Elastic anomalies due to coupling of strain with the structural and spin state order parameters have been analyzed by RUS, while coupling between the spin state order parameter and electric polarization was determined from pulsed and DC magnetic field magnetization and electric polarization data. Complex 1 displays a strong magnetoelectric effect in single-crystal form.
Scheme 1. Synthesis of Complex 1, [Mn(3,5-diCl-sal2(323))]BPh4.
Results and Discussion
Two-Step Thermal Spin Transition
The temperature-dependent magnetic susceptibility (χMT) between 4 and 350 K revealed a two-step thermal SCO in complex 1. As shown in Figure 1, a χMT value of 2.60 cm3 K mol–1 was observed at 350 K, which is close to the value of 3.0 cm3 K mol–1 expected for S = 2 from the spin-only formula (with g = 2).29,30,75−79 The χMT value increases slightly to 2.80 cm3 K mol–1 on cooling to 140 K (intermediate phase 2, INT2) and then abruptly drops to 2.39 cm3 K mol–1 around 135 K, with the transition temperature T1/2,INT1↓= 139 K (intermediate phase 1, INT1). Upon further cooling, the χMT value decreases gradually to 2.07 cm3 K mol–1, indicating that the system is converging toward a 1:1 HS:LS ratio where the moment would be 1.91 cm3 K mol–1. On further cooling χMT decreases abruptly to 1.03 cm3 K mol–1, with the transition temperature T1/2,LT↓= 83 K (low-temperature (LT) phase), indicating a full conversion to the S = 1 (LS) state. Below 25 K, the χMT value falls to 0.28 cm3 K mol–1, which is attributed to zero-field splitting of the 3T1 ground state. The two-step transition is fully reversible over multiple thermal cycles in single-crystal or polycrystalline samples with hysteresis at both steps. Values of T1/2,LT↑= 86 K and T1/2,INT1↑= 140 K are recorded on warming with hysteresis widths of 3 K, centered at 84.5 K, and 1 K, centered at 139.5 K, respectively. At 350 K, the initial χMT value of 2.60 cm3 K mol–1 is recovered.
Figure 1.
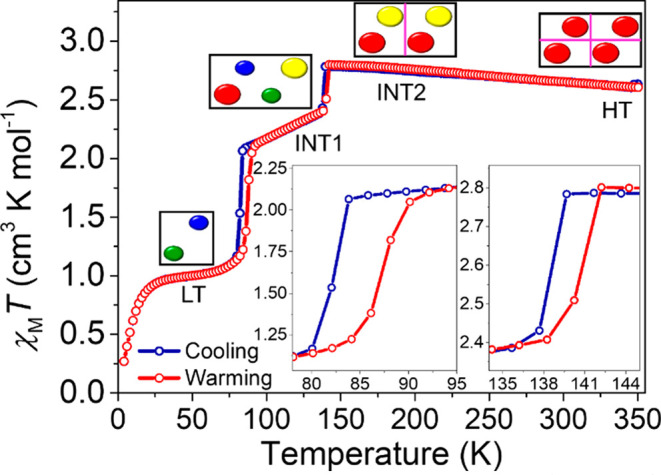
χMT versus T for complex 1 measured in cooling (blue curve) and warming (red curve) modes between 4 and 350 K with an applied magnetic field, μ0H, of 0.1 T. The insets show the 3 K wide hysteretic transition taken from the midpoints of the transition from 83 to 86 K and the 1 K hysteretic transition from 139 to 140 K. Four crystallographically distinct phases were detected by single-crystal X-ray diffraction (vide infra), and each unit cell is represented by the black box with the unit cell contents depicted with colored ellipses. The large red and yellow ellipses represent Mn3+ sites in the S = 2 HS state, and the small blue and green ellipses represent Mn3+ sites in the S = 1 LS state. Pink lines represent a simplified view of symmetry elements present in the unit cell.
We use the non-symmetry-breaking
order parameter 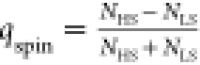 , which is convenient for monitoring
the
stepwise spin state conversion.52,53,80 In the HT and INT2 phases molecules are mainly HS and qspin ≃ 1. In the INT1 phases, qspin ≃ 0 due to the 1:1 HS:LS ratio. In the LT
phase qspin ≃ −1, as the
molecules are mainly LS. As was recently explained,52 stepwise SCO may be due to antiferroelastic coupling between
inequivalent spin-active sites or to symmetry breaking, where spin-active
sites are spatially ordered on the step. To understand the process
coming into play, it is therefore mandatory to perform a detailed
structural analysis, as discussed hereafter.
, which is convenient for monitoring
the
stepwise spin state conversion.52,53,80 In the HT and INT2 phases molecules are mainly HS and qspin ≃ 1. In the INT1 phases, qspin ≃ 0 due to the 1:1 HS:LS ratio. In the LT
phase qspin ≃ −1, as the
molecules are mainly LS. As was recently explained,52 stepwise SCO may be due to antiferroelastic coupling between
inequivalent spin-active sites or to symmetry breaking, where spin-active
sites are spatially ordered on the step. To understand the process
coming into play, it is therefore mandatory to perform a detailed
structural analysis, as discussed hereafter.
The thermal dependence of the derivative of the temperature-dependent molar magnetic susceptibility, dχMT/dT, the molar magnetic susceptibility, χM, and the inverse molar magnetic susceptibility, χM–1, also confirm this stepwise behavior, as shown in Figures S1 and S2. Thus, we have identified two first-order transitions: one is centered at 84.5 K with a thermal hysteresis window of 3 K, and the second is centered at 139.5 K with a thermal hysteresis window of 1 K. The thermal hysteresis windows are of width comparable to those previously observed in other Mn3+ SCO complexes.29,30,76,81 However, this is the first example of two-step hysteretic SCO behavior in a Mn3+ SCO complex. Additionally, we note that the hysteresis loops have an asymmetric shape, which is also characteristic of coupling between symmetry breaking and SCO.53
In three of the four phases (HT, INT2 and INT1), four Mn3+ sites are present in the unit cell, while in the LT phase, two Mn3+ sites are present. In the INT1 phase, two Mn3+ are in the HS S = 2 and two are in the LS S = 1 state. In the LT phase, the two Mn3+ sites are in the LS S = 1 state (vide infra). The contents of the unit cell of each phase and the respective spin states of each independent Mn3+ site are represented by colored ellipses in Figure 1. The larger red and yellow ellipses represent non-symmetry-related HS S = 2 Mn3+ sites. The smaller blue and green spheres represent LS S = 1 Mn3+ sites.
Thermochromism
Complex 1 undergoes a weak reversible color change from translucent dark ruby red to dark red when it is warmed from 40 K (LT phase, LS state) to 80 K (border of LT/INT1 phase) (Figure 2). Phase coexistence between the LT and the INT1 phases may be present, influencing the overall electronic configuration and hence the color of the crystal. The weak color change was observed when the single crystal was mounted on a cactus needle used for the single-crystal X-ray diffraction experiment. It was only observed when the crystal was at temperatures far from the lower hysteresis window and when the crystal was mounted directly into a cold helium gas stream. With liquid nitrogen, the lowest temperature that could be reliably achieved was 82 K, and no apparent visible color change was observed. It is very rare to observe color changes in Schiff base Mn3+ SCO complexes, and a recent study on a similar Mn3+ complex described a much more pronounced color change of translucent red to dark red between the fully S = 1 and S = 2 states.82
Figure 2.
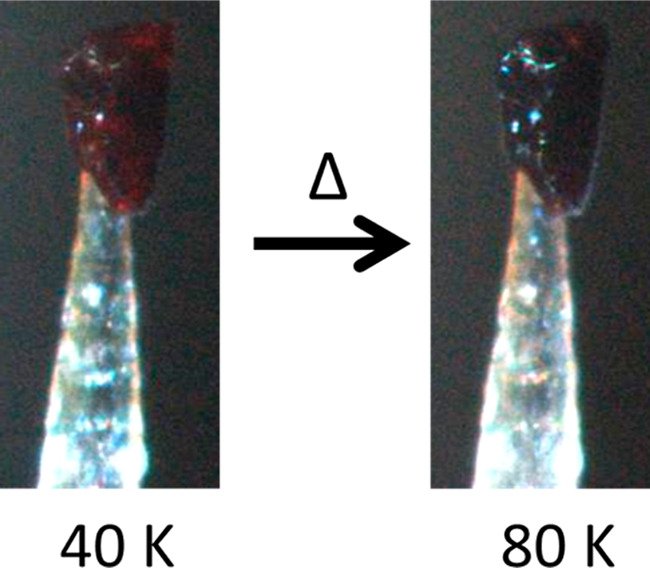
Color change of a single crystal of complex 1 mounted on a cactus needle used for the single-crystal X-ray diffraction experiment at 40 K (left, translucent ruby red) and 80 K (right, dark red) in a helium gas stream.
Structural Studies
Single-crystal X-ray diffraction data were collected at various temperatures within each of the four regions outlined on the χMT versus T plot in Figure 1. The results of full refinement of the structure in the stability field of the HT phase at 250 K are given in Table S3. The structure was refined in the noncentrosymmetric polar monoclinic space group Cc, and the asymmetric unit comprised one independent mononuclear [MnIIIL1]+ complex cation and one disordered tetraphenylborate BPh4– counteranion (Figure S3). The coordination sphere around the Mn3+ ions in the HT phase is analogous to that of the previously reported complexes. The geometry can best be described in terms of a compressed distorted octahedron formed by the trans anionic phenolate oxygen donors and pairs of cis-amine and cis-imine nitrogen atoms in the equatorial plane. The Mn–Nimine, Mn–Namine, and Mn–Ophen bond lengths measured at 250 K are summarized in Table S4. As we only observed an elongation of the bond lengths in the Mn–N equatorial plane and Mn–Ophen showed no sign of elongation, it is fair to assume that one electron occupies the dx2–y2 antibonding orbital. The bond lengths observed are in the range expected for HS S = 2 Mn–N bonds,75−77,79,83 and the spin state is in line with the observed χMT value measured at 250 K. Figure S14 shows variable-temperature single-crystal X-ray diffraction data for the unit cell parameters measured from 250 to 83 K in cooling mode and 83 to 200 K in warming mode in 3 K intervals. There are obvious but continuous changes in the trend of unit cell parameters at ∼204 K, associated with a symmetry breaking from Cc to Pc, characterized by the appearance of hkl (and h0l reflections) with h + k odd below 204 K (Figure S15). The Cc phase above 204 K is a high-spin high-symmetry phase (HS) with four symmetry-equivalent HS molecules in the unit cell (Figure 3).
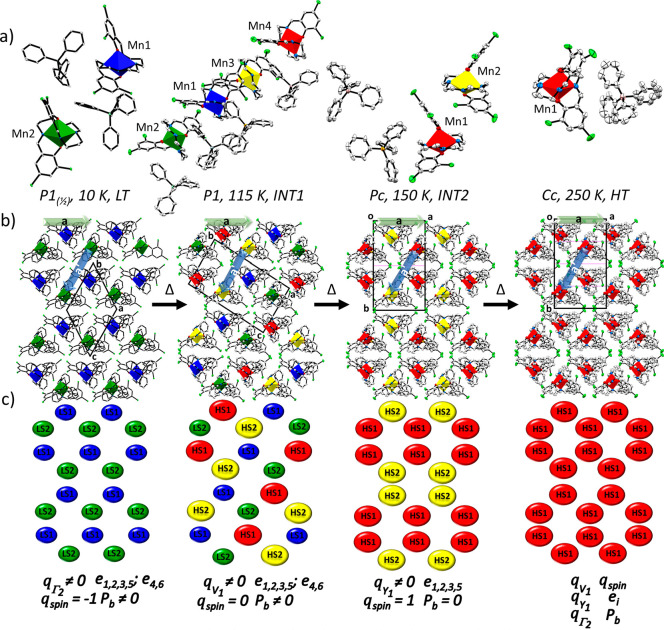
Figure 3.
Perspective view of the LT P1(1/2) (10 K), INT1 P1 (115 K), INT2 Pc (150 K), and HT Cc (250 K) structures of complex 1 and emergent order parameters (q) and polarization (P). (a) View of the asymmetric unit with distorted [MnN4O2]+ units shown as polyhedra with the following color coding: HS1 (red), HS2 (yellow), LS1 (blue), and LS2 (green). (b) The same structures shown in a layered crystal packing arrangement and also the relationships between the conventional unit cells of each structure. Atoms are shown at 50% atomic probability distributions for ellipsoids. Hydrogen atoms are omitted for clarity. (c) Simplified representation of the four phases in terms of the Mn3+ atoms alone, with the same color coding as in (a). The symmetry-breaking events between the Cc phases and each of the other phases are represented with their respective thermodynamic order parameters, q. The Pc, P1 and P1(1/2) structures are all related by a group–subgroup relationship to the same parent Cc cell. For Cc → Pc, the phase transition is purely structural in character, qY1, and no change in spin state order parameter, qspin, is observed. By symmetry, the global polarization in the Cc and Pc space groups lies in the (a,c) plane, with the net polarization vector along b, Pb, equal to zero. The Cc → P1 transition involves coupling between the structural order parameter, qV1, and the spin state order parameter, qspin. The SCO event involves changes in spin state of two of the four Mn3+ cations in the asymmetric unit. The Cc → P1(1/2) phase transition involves a structural order parameter, qΓ1, and coupling to a change in the spin state order parameter, qspin. This is different from the spin state changes observed in the Cc → P1 phase transition, since all of the Mn3+ complex cations in the asymmetric unit undergo the HS state → LS state conversion. In each case, ei represents components of the spontaneous strain tensor that are not zero (see section S3 in the Supporting Information).
We performed a full data collection at 150 K, i.e. in the stability field of the INT2 phase, on the same single crystal as that measured at 250 K. The structure was refined in the noncentrosymmetric polar monoclinic space group Pc. Due to the reduction in symmetry, the asymmetric unit comprises two independent mononuclear [MnIIIL]+ complex cations and two tetraphenylborate, BPh4–, counteranions (Figure 3a and Figure S4). A slight increase in bond lengths was observed, especially for Mn2–Nimine and Mn2–Namine, in comparison with the equivalent bond lengths observed at 250 K (Table S4). Both Mn3+ complex cations are in the HS S = 2 state. This may account for the slight increase in χMT at 150 K in comparison with 350 K, with a weak change in slope around 205 K (Figure 1). This Pc phase corresponds to a HS low-symmetry phase (INT2). There are four HS molecules within the unit cell, splitting into two symmetry-equivalent HS1 and two symmetry-equivalent HS2 sites due to the c glide plane.
Lattice parameter variations show a steep decrease in a, increases in b and c, and a steep increase in the β angle below the transition at ∼140 K (Figure S14). The diffraction patterns show h0l reflections with l odd appearing in the h0l plane, which is characteristic of the loss of the c glide plane below 140 K (Figure S15) and, therefore, of a symmetry reduction toward a P1 phase. This INT1 phase has different symmetry from those of the higher temperature phases INT2 and HT. A full data set was also collected at 115 K (INT1 phase) on the same crystal. The structure was refined in the polar and chiral triclinic space group P1. Due to the loss of the c glide plane, the unit cell comprises four independent mononuclear [MnIIIL]+ complex cations and four BPh4– counteranions (Figure 3a and Figure S5). Two of the four Mn3+ complex cations are in the LS S = 1 state, and two are in the HS S = 2 state (Table S4). The donor atom bond lengths clearly show that, at 115 K, a significant decrease occurred in two of the four sets of Mn–Nimine and Mn–Namine bond lengths, while the average Mn–Ophen bond length was more or less unchanged. This correlates well with the intermediate value of χMT that is observed below 140 K. The P1 (INT1) structure has four independent molecular sites: two mainly HS and two mainly LS. This phenomenon results from the coupling terms between the SCO and the symmetry change.41,52,80
A full data set was collected at the lowest temperature we could reliably access with liquid nitrogen, 82 K (LT phase), using the same crystal as for the 115 K measurement. An analysis revealed the disappearance of some Bragg peaks at lower temperature in comparison to the structure measured at 115 K. The structure was refined in the triclinic polar and chiral space group P1 but with a unit cell that is only half the volume of the P1 structure measured at 115 K. The difference is due to a halving of the c parameter, and we denote the LT phase as P1(1/2). The unit cell comprises two independent mononuclear [MnIIIL]+ complex cations and two BPh4– counteranions (Figures 3a and Figures S6 and S7). Both Mn3+ complex cations are in the LS S = 1 state (Table S4). A significant overall decrease in the Mn–Nimine and Mn–Namine bond lengths was observed in comparison to the average for the HT, INT2, and INT1 phases. The χMT value of around 1.03 cm3 K mol–1 below 83 K confirms this observation. Data collection was conducted on a single crystal of complex 1 at 10 K using helium for cooling to confirm the LT phase observed at 82 K (Table S3). An investigation of diffraction patterns from both the LT P1(1/2) and INT1 P1 phases revealed the presence of twinning, which develops as a consequence of the ferroelastic transition from monoclinic to triclinic. Therefore, during this transition ferroelastic twin walls, i.e. regions between crystallographic twins accompanied by domain formation, develop.
Bond length variations for Mn–N donors between HS S = 2 and LS S = 1 states are ∼0.13 Å, which is typical for Schiff base N4O22– Mn3+ SCO complexes.29,75−78 SCO in these types of Mn3+ Schiff base complexes is manifested as a change in trigonal distortion angle, Φ, and octahedral distortion parameter, Σ. Both parameters are used to estimate the magnitude of the deformation of the coordination geometry with respect to a perfect octahedron with Σ, Φ = 0. The larger the values for Σ and Φ, the weaker the ligand field, which will stabilize the HS S = 2 state for the Mn3+ ion. The parameters that characterize the extent of the structural changes induced by the SCO behavior are summarized in Table 1. The spin state of the [MnIIIL]+ complex cation in each case is also indicated. Only weak hydrogen-bonding interactions between the Mn3+ complex cation(s) and the BPh4– counteranion(s) were found in the HT (250 K), INT2 (150 K), INT1 (115 K), and LT (10 K) phases (Figures S8–S12). A full Hirshfeld surface analysis, mapped over dnorm, of complex 1 shows that the three main contributions to the intermolecular interactions are H···H, H···Cl, and H···C, with a decrease in H···Cl and an increase in H···H interactions in the LT phase in comparison to the INT and HT phases (Figure S13). The slight changes in intermolecular interactions may generate a change in lattice pressure that seems to directly affect the spin state and may explain the hysteretic behavior of complex 1.
Table 1. Calculated Distortion Parameters and List of Spin States for Each Asymmetric [MnL]+ Cation in Complex 1 at 10, 82, 115, 150, and 250 K.
| temperature |
||||||
|---|---|---|---|---|---|---|
| param | Mn site | 10 K | 82 K | 115 K | 150 K | 250 K |
| space group | P1(1/2) | P1(1/2) | P1 | Pc | Cc | |
| Z′a | 2 | 2 | 4 | 2 | 1 | |
| ΣMn (deg)b | Mn1 | 36.76 | 37.76 | 43.92 | 69.71 | 63.95 |
| Mn2 | 39.66 | 39.29 | 53.35 | 63.28 | ||
| Mn3 | 67 | |||||
| Mn4 | 74.6 | |||||
| Φ (deg)c | Mn1 | 120.76 | 126.42 | 143.23 | 259.87 | 240.53 |
| Mn2 | 120.76 | 122.12 | 180.54 | 237.27 | ||
| Mn3 | 253.83 | |||||
| Mn4 | 271.92 | |||||
| ζ (Å)d | Mn1 | 0.395 | 0.422 | 0.481 | 0.783 | 0.774 |
| Mn2 | 0.408 | 0.413 | 0.532 | 0.769 | ||
| Mn3 | 0.77 | |||||
| Mn4 | 0.787 | |||||
| spin state | Mn1 | S = 1 | S = 1 | S = 1 | S = 2 | S = 2 |
| Mn2 | S = 1 | S = 1 | S = 1 | S = 2 | ||
| Mn3 | S = 2 | |||||
| Mn4 | S = 2 | |||||
Z′ is the number of independent sites in the asymmetric unit.
ΣMn is the sum of the deviation from 90° of the 12 cis angles of the MnN4O2 octahedron.
Φ is the sum of the deviation from 60° of the 24 trigonal angles of the projection of the MnN4O2 octahedron onto the trigonal faces.
ζ is the distance distortion parameter, which is the sum of the deviation from individual M–X bond distances with respect to the mean metal–ligand bond distance.84
Different representations of the striped (spin state) order of the Mn3+ complex cations in each of the four phases are shown as packing diagrams viewed along the c axis for the HT Cc phase, with the other phases oriented accordingly, in Figure 3b and as a graphical representation of the Mn3+ SCO centers in Figure 3c.
Group–Subgroup Analysis, Order Parameters, Tensors, and Formal Strain Analysis
It is well understood that changes in the elastic constants of single crystals at phase transitions depend primarily on the form and strength of coupling between the driving order parameters and strain.85 In addition to the well-known coupling of a symmetry-breaking order, the non-symmetry-breaking order parameter qspin also contributes to the volume strain. This is well illustrated by the large bond contractions and consequently lattice strains exhibited by many materials undergoing SCO without a symmetry change.52−54 In a recent paper by Shatruk et al., structural phase transitions coupled to SCO have been reviewed.48 Three classifications were made where various degrees of coupling between the SCO behavior and the structural phase transition were defined. In our case, the structural phase transition sequence is most likely due to changes in Jahn–Teller distortions on adjacent Mn3+ cationic molecules, which in turn induce a strain. Collet et al. also discussed the role of the elastic coupling between SCO and symmetry-breaking order parameter.52,53 Biquadratic coupling stabilizes the intermediate HS–LS ordered phase, while linear-quadratic terms may stabilize an average HS fraction different from 1/2 on the step. Because of the strong coupling between the SCO and structural phase transition, the two SCO steps occur in an abrupt manner in complex 1.
The transitions can be analyzed from the perspective of Landau theory, for which the relevant order parameters and coupling with strain are described in the Supporting Information. Phase transitions of a crystal in which there is a group–subgroup relationship for the change in symmetry display coupling between a thermodynamic order parameter, qstruct, and strain components, ei,86 (in Voigt notation).
The parent structure for the sequence of phase transitions in complex 1 is Cc and, as summarized in Figure 4, each of Pc (INT2), P1 (INT1) and P1(1/2) (LT) are subgroups of Cc. The Cc (HT) → Pc (INT2) transition is allowed by symmetry to be second order in character, but a group–subgroup relationship does not exist between Pc (INT2) and P1 (INT1) or between P1 (INT1) and P1(1/2) (LT) so that the Pc (INT2) → P1 (INT1) and P1 (INT1) → P1(1/2) (LT) transitions are necessarily first order (reconstructive). For the Cc → Pc transition we specify the thermodynamic order parameter as qY1, the amplitude of which measures deviations from C-face centering due to a structural instability involving displacements of molecules HS1, HS2, and counteranions. Lowest order strain coupling terms have the form λieiqY12 for i = 1, 2, 3, 5 and λiei2qY12 for i = 4, 6, where λi are coupling coefficients (see Section S3 in the Supporting Information). Coupling of the form λieiqY12 gives ei ∝ qY12 in simplest form.86 This transition does not give rise to a symmetry-breaking shear strain or to electric/magnetic polarization and is therefore classified as coelastic and nonferroic.
Figure 4.
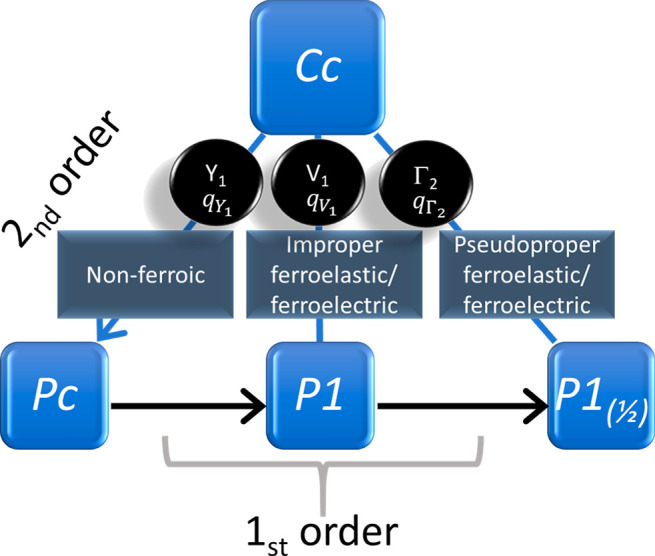
Schematic representation of group–subgroup relationships between the four phases of [MnIII(3,5-diCl-sal2(323))]BPh4 (1). Pc, P1, and P1(1/2) are each subgroups of Cc (blue lines) but do not have a group–subgroup relationship between them. Arrows on solid lines indicate the sequence of transitions with decreasing temperature. The Cc and Pc structures are related by the order parameter qY1, which belongs to the irrep Y1, and the transition is allowed by symmetry to be second order. The Cc and P1 structures are related by the order parameter qV1, which belongs to the irrep V1. Cc and P1(1/2) structures are related by the order parameter qΓ2, which belongs to the irrep Γ2. In the absence of group–subgroup relationships between the three lower temperature structures, transitions between them must necessarily be first order in character.
For the Cc → P1 transition we specify the thermodynamic order parameter as qV1, and the lowest order terms for coupling with strains are λieiqV12, i = 1–6. This would be an improper ferroelastic, improper ferroelectric transition, since the symmetry-breaking shear strains, e4 and e6, and polarization, P, each couple with qV12 rather than with qV1. For the Cc → P1(1/2) transition we specify the thermodynamic order parameter as qΓ2, and the lowest order strain coupling terms are λieiqΓ22, i = 1, 2, 3, 5 and λieiqΓ2, i = 4, 6. This transition would be ferroelectric and pseudoproper ferroelastic, where the term pseudoproper refers to the fact that the symmetry of the symmetry-breaking strains is the same as the symmetry of the structural order parameter that drives the transition.
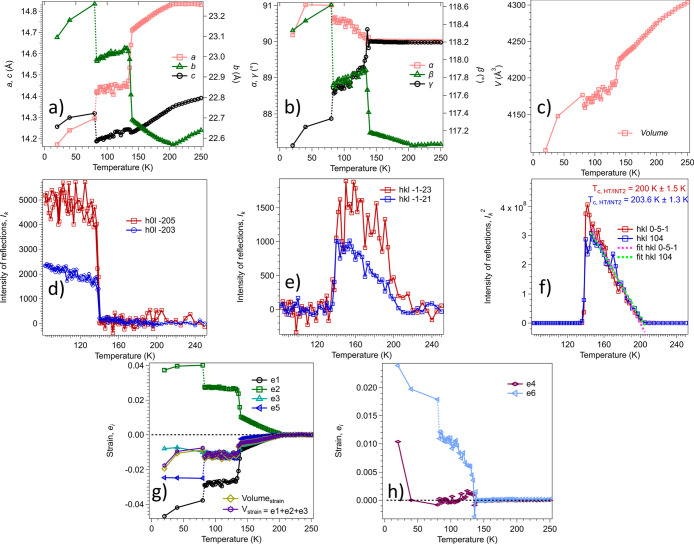
Lattice parameters determined by single crystal X-ray diffraction show a small increase in a and decrease in b and c with decreasing temperature. At ∼204 K there is a change in slope for all the parameters, indicating a change in structure (Figure 5a–c). The change in the Bragg peaks hkl associated with the appearance of superlattice reflections on cooling is shown in Figure 5d,e. In particular the development of superlattice reflections below ∼204 K (Tc,HT/INT2) associated with the Cc (HT) → Pc (INT2) transition, corresponding to Bragg peaks hkl with h + k odd, is shown in Figure 5e,f. The square of the intensities of these scales linearly with temperature, and hence, the thermodynamic character of the transition is best described as Landau tricritical (Ik2 ∝ qY14 ∝ (Tc – T)) with Tc,HT/INT2 = 202 ± 3 K. For this analysis the best reflections from the hkl family were taken into account.
Figure 5.
Changes in unit cell parameters (a) a, b, and c, (b) α, β, and γ, and (c) volume, from single-crystal measurements of complex 1 between 250 and 20 K. (d) Intensity of superstructure reflections appearing at the first-order Pc (INT2) → P1 (INT1) transition. The Bragg peaks were indexed in the P1(1/2) space group setting. Intensities of superstructure reflections through the Cc (HT) → Pc (INT2) transition, corresponding to Bragg peaks (hkl) with h + k odd shown for (e) the weaker hkl = −1–23 and −1–21 reflections and (f) the stronger hkl = 0–5–1 and 104 reflections, which appears in diffraction patterns from the Pc phase. The Bragg peaks were indexed in the Cc space group setting. The data show Ik2 ∝ (Tc – T), within experimental uncertainty, and Tc = 202 ± 3 K. (g) Temperature dependence of strain components for the Pc and P1 structures, as defined with respect to the parent Cc structure. All of the strains vary continuously through the Cc (HT) → Pc (INT2) transition at ∼204 K, discontinuously through the Pc (INT2) → P1 (INT1) transition at ∼135 K, and discontinuously through the P1 (INT1) → P1(1/2) (LT) transition at ∼80 K.
A continuous decrease in a and c and an increase in b were observed down to ∼140 K, where a steep decrease in a and increases in b and c were observed. The appearance of superstructure reflections below 140 K is evident from Figure S15. The lower limit for the temperature that can be reached with the nitrogen cryostat in this experiment is 82 K. A helium cryostat was used for measuring the unit cell parameters at 80, 40, and 20 K on a different single crystal, hence the dashed lines in the plot below 82 K. Measurements from the first single crystal between 240 and 83 K cannot necessarily be related with high precision to the unit cell of the second single crystal measured at 80, 40, and 20 K, but the discontinuities are consistent with first-order transitions at ∼140 and ∼80 K (Figure 5a,b).
A change in spin state by itself does not necessarily result in any change in symmetry; thus, the simplest spin order parameter, qspin, transforms as the identity representation. As it is the high-spin, high-symmetry phase that is taken as a reference, the convenient definition of qspin given above corresponds to qspin = 2γ – 1, where γ is the fraction of the high-spin state, varying between 0 and 1, which we use to specify the value of this order parameter.52,53,80 If the spin state transition is linked to a symmetry-breaking phase transition with order parameter qstruct and coupling with strain ei, the overall free energy change can be expressed in terms of a Landau type expansion in generalized form as
| 1 |
Here, the last term describes the Hooke’s law elastic energy; Ciko represents the elastic constants of the reference structural state (Cc in this case) and is not considered any further. Three different coupling terms are specified to draw attention to the fact that the two order parameters couple separately with strain as well as with each other.
Writing the full potential
is complex in the present case, as there
are various competing false ground states associated with SCO and/or
different structural instabilities. The generalized expression nevertheless
explains the overall pattern of behavior. On this basis, qstruct may correspond to qY1, qV1, or qΓ2, coupling between SCO, and
symmetry breaking and Gcoupling3(qstruct, qspin),
may include several terms, as explained in the framework of coupled
SCO and symmetry breaking.52 The linear-quadratic
coupling term qspinqY12 in
the case of the tricritical HT → INT2 phase transition affects
the SCO only weakly, as a slight change in slope in the thermal spin
state conversion near 205 K (Figure 1), driven by the evolution of qY1. On the other hand, the term qspinqΓ2 is responsible for the
discontinuous evolution of the high-spin fraction from INT1 to LT
because qΓ2 emerges discontinuously
at the reconstructive transition. Biquadratic coupling terms such
as 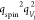 play an important role in stepwise transitions
driven by symmetry breaking, as they stabilize half-conversion around qspin = 0.52,80 The symmetry-breaking
order parameter qV1 is therefore
associated with the appearance of spin-state concentration waves41,50 that form in the INT1 phase. This corresponds to a long-range ordering
of molecules in HS and LS states over molecular sites HS1 and LS1,
which are symmetry-equivalent at higher temperature. The symmetry-breaking
order parameter qV1 scales
as the difference of HS population on these sites: qV1 ∝ |qspin, HS1 – qspin, LS1|. On this step
the linear-quadratic coupling term qspinqV12 slightly shifts the equilibrium qspin from 0. Various types of spin-state concentration
waves, forming various patterns, were theoretically found by Cruddas
et al. in the framework of a microscopic model based on elastic interactions.51 However, in addition to the HS–LS pattern
appearing in INT1 phase, the ferroelastic distortion between monoclinic
and triclinic INT1 phases plays a key role, especially for the magnetoelectric
coupling discussed below.
play an important role in stepwise transitions
driven by symmetry breaking, as they stabilize half-conversion around qspin = 0.52,80 The symmetry-breaking
order parameter qV1 is therefore
associated with the appearance of spin-state concentration waves41,50 that form in the INT1 phase. This corresponds to a long-range ordering
of molecules in HS and LS states over molecular sites HS1 and LS1,
which are symmetry-equivalent at higher temperature. The symmetry-breaking
order parameter qV1 scales
as the difference of HS population on these sites: qV1 ∝ |qspin, HS1 – qspin, LS1|. On this step
the linear-quadratic coupling term qspinqV12 slightly shifts the equilibrium qspin from 0. Various types of spin-state concentration
waves, forming various patterns, were theoretically found by Cruddas
et al. in the framework of a microscopic model based on elastic interactions.51 However, in addition to the HS–LS pattern
appearing in INT1 phase, the ferroelastic distortion between monoclinic
and triclinic INT1 phases plays a key role, especially for the magnetoelectric
coupling discussed below.
Spontaneous strain variations through the full sequence of transitions, as calculated from changes in lattice parameters using equations given in the Supporting Information, are shown in Figure 5g,h. Linear extrapolations were used to determine values of the reference parameters, and a simple test of the efficacy of this is provided by comparison of values for Vs obtained directly using eq S11 with values obtained using Vs = e1 + e2 + e3. As shown in Figure 5g, the two variations of Vs are the same within reasonable experimental uncertainty. The overall pattern of strain variations is consistent with a continuous transition at ∼204 K and discontinuous transitions at ∼135 and ∼80 K.
Two separate generic strain coupling terms, Gcoupling1 and Gcoupling2, have been included in eq 1 to emphasize the fact that the total variation of spontaneous strain through the full sequence of transitions includes separate contributions from coupling with each of the two order parameters, qstruct and qspin. Without additional information on the evolution of each of the different order parameters, it is not possible to separate out their different coupling contributions to the total strain, but some general points are clear. First, qspin = 0 in the stability field of the Pc (INT2) structure and the individual strains vary continuously up to ∼±1% by coupling only with the structural order parameter qY1. Second, an initial expectation would be that increases in qspin contribute predominantly to reductions in volume: i.e., to negative volume strains. The data for Vs in Figure 5g indeed show abrupt increases in magnitude from ∼−0.5% in the stability field of the Pc (HT) structure to ∼−1% and ∼−2% in the stability fields of the P1 (INT1) and P1(1/2) (LT) structures, respectively, which both have qspin ≠ 0. More or less constant values of Vs through the full temperature intervals of the two triclinic structures imply that the proportion of sites at which Mn is in the high-spin state and the proportion of sites at which Mn is the low-spin state remain approximately constant in each of them. Finally, the dominant ferroelastic shear strain below ∼140 K is e6, which increases up to 1% in the stability field of the P1 (INT1) structure and up to ∼2% in the stability field of the P1(1/2) (LT) structure; e4 remains close to zero at the lowest measurement temperature (Figure 5h).
The three coupling terms in eq 1 are also important in relation to the structure and properties of ferroelastic domain walls in the P1 (INT1) and P1(1/2) (LT) structures, since these represent narrow regions across which there are steep gradients in strain. There is a total strain contrast of |2e6| from one domain to the next across each wall and, in principle, qV1 or qΓ2 as qstruct and e6 as the symmetry-breaking strain will be zero at its center. Since qstruct and qspin are coupled, there will also be gradients in the degree of spin ordering within the walls, depending on the strength of the term Gcoupling3 for each of qV1 and qΓ2. It follows that any magnetic, ferroelectric, or magnetoelectric properties of the material which depend on the ordered arrangement of atoms with high and low spins will be locally different within the domain walls in comparison with the properties of bulk crystals. It follows also that the density within the domain walls might be as much as 2% lower than that within individual domains if qspin is suppressed to zero, providing the possibility of highly localized preferred sites for impurity atoms and defects or of pathways for enhanced diffusion of dopant atoms, for example. In this context it is worth noting that the strain contrast across ferroelastic domain walls in the P1(1/2) (LT) structure will be double that across domain walls in the P1 (INT1) structure and hence that contrasts in other properties will also be enhanced.
Ferroelastic Behavior
The RUS technique has previously been used to follow variations in elastic properties of materials that exhibit spin crossover behavior with and without symmetry-breaking phase transitions.30,54,55,87 Values of elastic moduli scale with f2, where f represents the frequency of an individual peak in spectra which record predominantly shear mode acoustic resonances of samples with dimensions in the vicinity of ∼1 mm. Acoustic loss is expressed in terms of the inverse mechanical quality factor, Q–1, which depends on the widths at half-height of the resonance peaks.
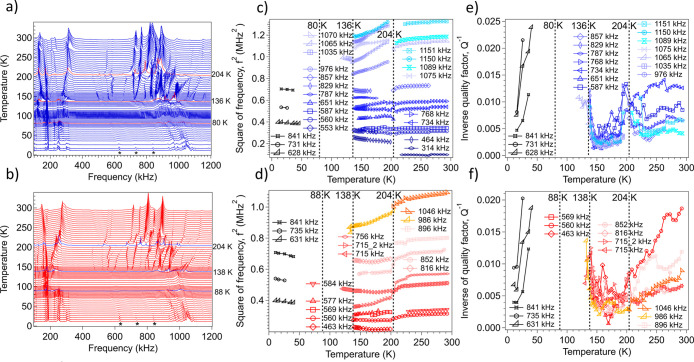
Two stacks of spectra collected during cooling and warming of a single crystal of complex 1 are shown in Figure 6a,b, respectively. Each spectrum has been offset up the y axis in proportion to the temperature at which it was collected in order to reveal the overall pattern of elastic softening/stiffening through the full transition sequence. Variations in f2 and Q–1 from peak fitting are shown in Figure 6c,e (cooling) and Figure 6d,f (heating). Individual resonance peaks display a small shift to lower frequencies (elastic softening) during cooling from 295 to ∼204 K, where the trend of softening generally continues. Almost all the resonance peaks disappeared abruptly at ∼136 K and then reappeared gradually below ∼80 K. When the temperature was increased back to room temperature from 7 K (Figure 6b), the same essential features were observed. Resonance peaks returned to the same positions as in the cooling sequence, confirming that the crystal survived all the transitions without cracking or undergoing other irreversible changes.
Figure 6.
(a, b) RUS spectra as a function of frequency for a single crystal of complex 1, stacked up the y axis in proportion to the temperature at which they were collected. The y axis is amplitude (volts) but has been relabeled as temperature to reveal the overall pattern of stiffening and softening. Spectra were collected during (a) cooling and (b) heating between ∼292 and ∼10 K. Highlighted spectra in both stacks indicate the expected positions of HT → INT2, INT2 → INT1 and INT1 → LT transitions, with thermal hysteresis between the cooling and warming data for the two lower transition points. Stars mark the three peaks that most clearly show the reappearance of resonances with relatively low attenuation at the lowest temperatures. (c, d) f2 and (e, f) Q–1 data from fitting of selected resonances with an asymmetric Lorentzian function. Vertical broken lines mark the continuous structural phase transition at ∼204 K and discontinuous transitions at ∼136/138 and ∼80/88 K. Data shown in black are for the three resonance peaks marked with stars in (a) and (b). Listed in the captions are approximate frequencies for each resonance peak used in the fitting procedure to obtain values of f2 and Q–1.
Changes in elastic constants of single crystals at phase transitions depend primarily on the form and strength of coupling between the driving order parameter with strain.85 The Cc → Pc transition is tricritical, and coupling of the form λieiqY12 would be expected to give rise to discontinuous softening as the crystal is cooled through the transition temperature.82 Instead of a discontinuity at Tc (∼200 K), however, individual resonances show more or less continuous decreases in frequency that is typical of situations where the order parameter does not relax on the time scale of the applied stress. Relaxation times of longer than ∼10–6 s (i.e. for measurements at ∼1 MHz) must derive from slow dynamics of structural changes involved in the transition mechanism. Changes in the elastic constants then vary as λiqY12 due to the next higher order coupling terms of the form λiei2qY12. The transition is accompanied by a slight peak in Q–1 values (Figure 6e), as would be typical of acoustic loss due to critical slowing of some aspect of order parameter fluctuations as Tc is approached from above and below. A quick return to low values below the transition point occurred because ferroelastic twins do not develop at a coelastic transition.
The Pc → P1 transition at ∼140 K was marked by a sudden disappearance of most of the resonance peaks (Figure 6c,d). A few may still be weakly present in the spectra, but if so, they are notably broadened in comparison with resonance peaks from the monoclinic structures. High attenuation of this type is typical of the contribution of ferroelastic domain walls appearing below a ferroelastic transition point and remaining mobile under the influence of dynamic shear stress on a time scale of ∼10–6 s.88 Resonances from the sample became detectable again below ∼88 K, initially through their interaction with weak peaks from the sample holder. This interval of strongest attenuation was ∼80–138 K during heating and corresponds to the stability field of the P1 (INT1) structure, with some small hysteresis of the first-order transition points. Peaks in the primary spectra became definitely identifiable as individual resonances of the sample below ∼40 K, indicating that the domain wall mobility was reduced by pinning/freezing effects only at the lowest temperatures.
The equivalent disappearance of acoustic resonance peaks due to the mobility of ferroelastic domain walls in LaAlO3 under the low-stress, high-frequency conditions of an RUS experiment has been described elsewhere as “superattenuation”.89 On this basis, it appears that the P1 (INT1) structure is superattenuating while the P1(1/2) (LT) is not. Key differences between the two structures are the proportions of Mn3+ in the LS state and the magnitude of the spontaneous shear strain, providing a first indication that the dynamic properties of ferroelastic domain walls in SCO structures can depend on the degree of spin ordering: i.e., on the value (in general terms) of qspin.
The mobility of ferroelastic domain walls depends on the strength of their interaction with strain fields around point defects. In the case of oxide perovskites such as LaAlO3, the most significant point defects are oxygen vacancies.90,91 A wider generalization is that thin walls will interact with point defects more strongly in comparison to thick walls92 so that domain wall pinning or freezing temperatures observed for different materials can be considered, in part at least, as a pointer to wall thickness. Again with LaAlO3 as an example, the thickness of ferroelastic domain walls has been measured by X-ray diffraction to be ∼20 Å at low temperatures93 and they become pinned below ∼400 K.90,91 No equivalent measurements have yet been made on SCO materials, but the much lower freezing temperature implied by high acoustic loss down to at least ∼35 K reported here implies a relatively weaker interaction with defects and, therefore perhaps, thicker domain walls.
Magnetoelectric Coupling
We have established that the SCO behavior in complex 1 can be induced by temperature. Now we investigate magnetic-field-induced SCO in 1. The electric polarization, ΔP ,and magnetization, M, as a function of field up to 14 T are shown in Figure 7. The data in Figure 7 were taken in a superconducting magnet with slow (100 Oe/s) sweep rates. M(μ0H) in Figure 7a shows jumps on an increase in the field for temperatures between 88 and 90.2 K. This suggests a magnetic-field-induced SCO to a state with a higher spin value. However, a comparison of the LT data to the predicted Brillouin functions of the S = 1 state shows a higher magnetization value than for pure S = 1. After the H-induced transition, M(μ0H) follows a Brillouin function consistent with the mixed S = 1,2 state. A possible explanation for this discrepancy could be different amounts of strain induced by the attachment of the sample to the measurement probe.
Figure 7.
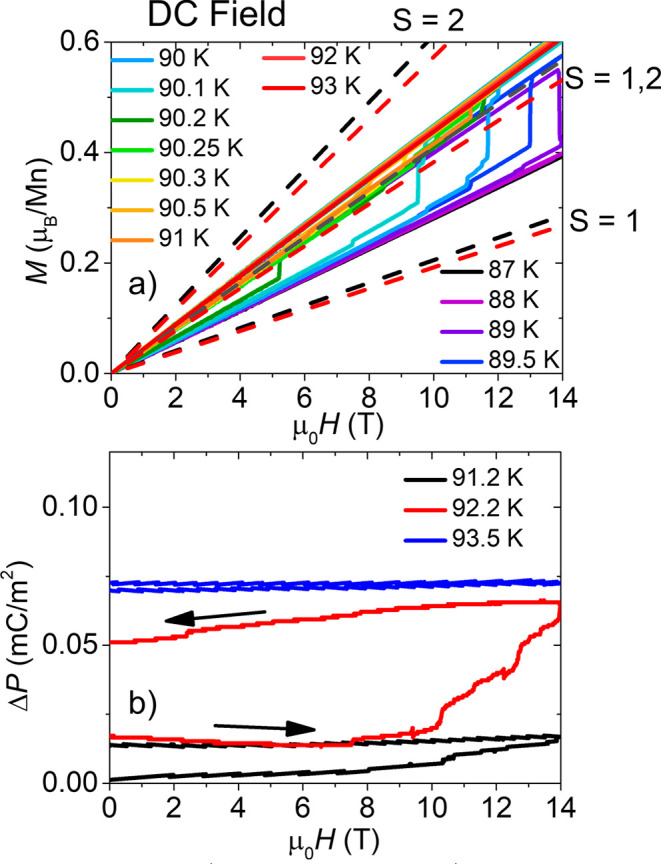
Change in (a) magnetization, M, and (b) electric polarization, ΔP, vs magnetic field, μ0H. Data were taken in magnetic fields up to 14 T in a superconducting magnet at sweep rates of 100 Oe/s for the different temperatures indicated in the legend. Arrows indicate increasing or decreasing field. Before each scan the temperature was cooled from above 100 K to the final measurement temperature. The dashed lines in (a) are calculated Brillouin functions for S = 1, S = 2, and a mixed 1:1 ratio of S = 1 and S = 2 spin states with no orbital contributions. Upper and lower lines are the lower and upper temperature limits, respectively (87 and 93 K). Data were collected using millimeter-sized single crystals of complex 1 mounted with glue to a sapphire probe with the magnetic field and electric field along the long axis. Two different crystals were measured in (a) and (b), subject to different thermal strain conditions which may have caused the difference in transition temperatures.
In Figure 7b we show that the electric polarization ΔP also undergoes a field-induced jump with a magnetic field as low as 4 T. All of the different structural states of this material are polar and can be expected to have different electric polarizations, as was also observed in the Br analogue of this compound.68 A key point is that, due to the ferroelastic transition, the electric polarization, which is confined in the (a,c) plane in the monoclinic HT and INT2 phases, gets a component along the b axis in the INT1 and LT triclinic phases. We expect a change in electric polarization at every structural phase transition, and the temperature-dependent electric polarization is shown in Figure S17 in the Supporting Information. Figure 7 demonstrates that relatively low magnetic fields can be used to induce SCO, resulting in jumps in both magnetization and electric polarization. It is also worth noting that the magnetization and the electric polarization are hysteretic—once the spin state switches with magnetic field, it remains switched as the magnetic field is removed, provided the temperature is kept within the region of bistability of the SCO. Similar behavior was also seen in the Br analogue of this compound.68 This hysteresis is to be expected, given the coupling between the spin state and the symmetry-breaking order parameters.52,53 We note that in Figure 7a,b the temperatures at which the field-induced transitions can be observed are slightly different—this may be because different crystals were measured with different strains arising from differential thermal contraction of the sample holders. The jumps within the SCO transition in the electric polarization were also observed in the Br sample and are consistent with domain reorientations occurring during the phase transition. They are evident only in the electric polarization and not in the magnetization.
We go on to investigate the magnetic-field-induced spin crossover over a broader magnetic field range in pulsed fields. Figure 8 shows ΔP(μ0H) up to 60 T in millisecond pulsed fields for (Figure 8a) rising and (Figure 8b) falling fields. In these larger fields we can observe the magnetic-field-induced SCO over a wider range of temperatures from 60 to 90 K, which extends outside the bistable temperature region of the LT → INT1 SCO. Though there is hysteresis in the electric polarization between up and down sweeps of the magnetic field, it does switch back upon lowering the field outside the thermal region of bistability. However, in Figure 8c,d ΔP(μ0H) is shown at T = 87.5 and 90 K, respectively, which is within the thermal region of bistability and, thus, the switching appears to be permanent. In Figure 8d, the two magnetization pulses (black and red) are applied consecutively (with a 45 min waiting period for the magnet to cool off in between). The second pulse (red) does not show the transition, from which we conclude that the sample is still in the high-field state and has not relaxed back, consistent with being in the thermal range of bistability of the phase transition. We note that the measured quantity is the change in electric polarization ΔP not the absolute P. We conclude that the data during the second pulse should have an offset (shown as a red dotted line) to indicate that it remains in the HS state. In Figure 8c we also show ΔP(μ0H) at a higher temperature of 140 K, where we expect a transition from the INT2 to a field-induced state. We also see a change in electric polarization at this temperature, with a sign opposite to that of the LT → INT1 transition. Figure S18 in the Supporting Information shows the electric polarization vs temperature and magnetic field in a color contour plot for rising and falling fields.
Figure 8.
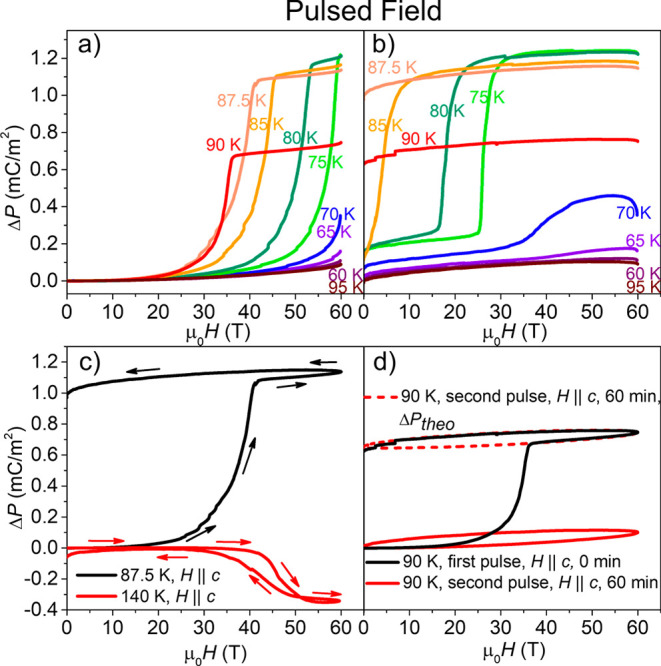
Change in electric polarization, ΔP, induced by a 60 T pulsed magnetic field, μ0H, applied to a single crystal of complex 1 for different temperatures as indicated. ΔP versus μ0H measured during (a) rising fields and (b) falling fields for temperatures between 60 and 90 K. Random errors are smaller than the line width. Systematic errors due to mechanical vibrations caused the oscillations observed in the pulsed field magnetization data. The maximum change in electric polarization induced by the 60 T pulsed magnetic field, 1.23 mC m–2, was observed at 75 K. (c) Change in sign of ΔP measured inside the two regions of bistability, the P1 → Pc (red plot) and P1(1/2) → P1 (black plot) transitions. The changes in ΔP observed in the regions of bistability for the P1 → P1(1/2) and Pc → P1 transitions were 1.14 and −0.35 mC m–2, respectively. (d) ΔP versus μ0H applied at 90 K, followed by a waiting period at 90 K for 45 min before applying a second pulse. ΔP reached a maximum of ∼ 0.75 mC m–2 during the first pulse. In the second pulse the signal showed no transition (red full line). We thus conclude that the sample remained in the HS state between pulses, and hence that the data represented by the red line should contain an additional offset (this magnetometer measures only the change in magnetization with field, not the absolute magnetization). To illustrate this, the dashed red line shows the red data with the addition of a presumed offset.
The change in electric polarization as a function of temperature and in pulsed fields is on the order of mC m–2. On the other hand, in 14 T DC fields it is only 5% of that value. This may be due to only a partial transition occurring in DC fields. The origin of this magnetoelectric coupling results from the couplings between the non-symmetry-breaking spin state order parameter qspin and the symmetry-breaking order parameter qstruct. Applying a magnetic field shifts the equilibrium HS fraction (qspin), while the couplings result in a shift of the equilibrium structure (qstruct) and, therefore, in a change of electric polarization.
Comparison with [MnIII(3,5-diBr-sal2(323))]BPh4
Substitution of the ligand substituents provides an additional means of manipulating the properties of SCO materials. The dibromo equivalent of complex 1 has the same sequence of Cc (HT) → Pc (INT2) → P1 (INT1) transitions and similar properties but does not appear to have a stability field for the P1(1/2) (LT) structure. It also does not display acoustic superattenuation in the stability field of the P1 (INT1) structure.30 The dominant effects of substituting a chloro for a bromo group are likely to be consequences simply of the change in covalent radius. The unit cell volume of the Cc structure at 250 K is ∼1.3% smaller in the case of the dichloro compound relative to the dibromo compound. Denser packing represented by this reduction in volume associated with reducing the size of the halo substituent presumably then hinders the reduction in volume achievable by increasing the proportion of the Mn3+ ions in the LS state of the P1(1/2) (LT) structure.
A more subtle difference is that all the acoustic resonances of the dibromo compound show elastic stiffening with falling temperature (Figure 4 of ref (30)), while those of the dichloro compound show elastic softening (Figure 6). The normal expectation is that the shear elastic moduli of crystalline materials will increase as the temperature falls due to increasing density arising from the effects of normal thermal expansion. Elastic softening with falling temperature is typically an indication of proximity to some approaching structural instability. If this were related to an impending Jahn–Teller transition, for example, the softening with falling temperature would be expected to occur in only one of the elastic moduli, typical of any pseudoproper ferroelastic transition.85 However, the dominant trend for resonances of the dichloro crystal is softening with falling temperature, implying that most of the shear elastic moduli soften. This is more indicative of the incipient instability being related to dynamic effects in open structures such as the high-temperature polymorph of quartz ahead of the α → β transition.85,94 Softening with falling temperature under these circumstances is attributed to fluctuations that increase in amplitude as the transition point is approached.
The most overt difference between the two compounds, however, relates to the mobility under dynamic stress of ferroelastic domain walls. Acoustic resonances were easily identifiable in RUS spectra from the P1 phase of the dibromo compound.30 Increases in Q–1 up to ∼0.01 immediately below the Pc → P1 transition point (∼85 K) were interpreted as providing evidence of some mobility of the domain walls. However, this is significantly less attenuation than is seen in spectra from the dichloro compound, where a transition to the P1 structure causes the disappearance of resonances. P1(1/2) yields values of Q–1 < 0.01 only below ∼35 K. Values of Q–1 at 10 K were found to be ∼0.001 for the dibromo compound and ∼0.005 for the dichloro compound. It is clear that the as yet undefined pinning mechanisms are sensitive to volume changes. As a speculation, it is proposed that this is a reflection of domain wall thickness—thicker in the dichloro compound than in the dibromo compound.
Conclusions
In summary, we have reported the detection of both mobile and pinned domain walls in response to mechanical stress in two spin-state ordered thermal regimes of the Mn3+ Schiff base complex [MnIII(3,5-diCl-sal2(323))]BPh4 with a two-step hysteretic SCO. From single-crystal X-ray diffraction measurements and resonant ultrasound spectroscopy it was found that four phases exist across the 350 to 4 K temperature range. A symmetry-breaking Landau tricritical phase transition occurs from the HS Cc to the HS Pc structure and two first-order phase transitions, Pc → P1 toward an ordered HS-LS state and P1 → P1(1/2) toward the LS phase. The triclinic phases are ferroelastic. Strong magnetoelectric coupling was found in the regions of bistability between the P1 → Pc and P1(1/2) → P1 phases, respectively, and a reversal in sign of the electric polarization was observed between the two regions of bistability. Moreover, a memory effect for the P1(1/2) → P1 transition is achieved.
These different characteristics must be correlated with the strong cooperativity (of elastic origin) observed in this compound that manifests itself in an extremely abrupt and robust spin transition. Strain coupling of spin and structural order parameters provides a likely mechanism for yielding magnetoelectric coupling in a two-step SCO complex undergoing multiple structural phase transitions and, thus, may provide a better understanding of the magnetostructural coupling that is observed in a large number of spin transition materials. Magnetic-field-induced transitions can also be observed on starting from the INT1 and LT phases.
In contrast to hard materials, the relatively soft lattices of molecular magnets have energy scales comparable to the energy scales of magnetic and electric orders, leading to a rich interplay and coupling of structural, magnetic, and electric degrees of freedom. In particular, spin state switching at spin crossovers can drive structural phase transitions in response to moderate temperatures and magnetic fields with energy scales at or below room temperature. In such systems large atomic displacements are commonly produced, creating exceptionally strong magnetoelectric coupling to the lattice and significant strain gradients and, as such, they hold great promise for the next generation of multiferroics.
Experimental Methods
Synthesis
[MnIII(3,5-diCl-sal)2323]BPh4 (complex 1) was synthesized by addition of a solution of N,N-bis(aminopropyl)ethylenediamine (0.25 mmol, 0.0436 g) in 1/1 CH3CN/EtOH (15 mL) to a solution of 3,5-dichlorosalicylaldehyde (0.5 mmol, 0.0955 g) also in 1/1 CH3CN/EtOH (15 mL), forming a yellow solution. This solution was stirred for 15 min before a mixture of manganese(II) nitrate tetrahydrate (0.25 mmol, 0.0628 g) and sodium tetraphenylborate (0.25 mmol, 0.0856 g) in 1/1 CH3CN/EtOH (10 mL) was added. The resulting solution was stirred, yielding a dark brown solution of the Mn3+ complex resulting from air oxidation of the Mn2+ starting salt. Dark red block crystals of suitable quality for single-crystal X-ray diffraction were formed within 1 day upon slow evaporation of the solvent (yield: 124.0 mg, 55.6%). Large single crystals (milligram sized) were obtained using a 0.1 mmol scale of reagents, a 20 mL vial, and 1/1 DMF/CH3CN as solvent, with the crystals allowed to form over several weeks. Anal. Calcd (found) for C46H44BN4O2MnCl4: C, 61.91 (61.73); H, 4.97 (4.90); N, 6.28 (6.26). IR (FT-ATR diamond anvil): ν/cm–1 3234(w), 3052(w), 1650(m), 1625(s), 1578(m), 1529(w), 1478(w), 1454(m), 1427(s), 1376(s), 1285(s), 1189(m), 1174(m), 1073(m), 985(m), 863(m), 769(s), 734(s), 708(s), 610(s), 524(m), 510(m). All chemicals were purchased from Sigma-Aldrich. All other reagents were purchased from standard sources and were used as received.
Magnetic Measurements
The magnetic properties of a polycrystalline sample of complex 1 were measured using a Quantum Design MPMS XL SQUID instrument. The molar magnetic susceptibility multiplied by the temperature (χMT) as a function of temperature of a polycrystalline sample of complex 1 was used to follow the temperature-dependent magnetic susceptibility in cooling and warming sequences in 2 K steps between 4 and 350 K with an applied magnetic field of 0.1 T. Diamagnetic corrections were calculated using Pascal’s constants and applied to all data. The calculated χMT vs T data are given in Tables S1 and S2 for cooling and warming cycles, respectively.
Single-Crystal X-ray Diffraction
Single-crystal X-ray diffraction (SCXRD) data of complex 1 were collected at 250, 150, 115, and 82 K on a first single crystal and at 10 K on a second single crystal using an Oxford Diffraction Xcalibur3 X-ray diffractometer (Oxford Instruments, Oxford, United Kingdom) fitted with an enhanced source, using Mo Kα radiation (λ = 0.71073 Å). The diffractometer is fitted with a Sapphire3 detector. The selected order of unit cell parameters allows for a direct comparison between different phases.
Changes in unit cell volume, cell parameters, and Bragg peak intensities as a function of temperature were investigated on a new single crystal of complex 1 during cooling from 250 to 83 K and warming from 83 to 200 K (2–3 K steps with 200 K/h cooling/heating rate) by means of an Agilent Technologies SuperNova Single Source X-ray diffractometer with a microsource, using Cu Kα (λ = 1.54184 Å) radiation, fitted with an EosS2 detector.
Single crystals were mounted on cactus needles with Parabar 10312 oil.
Nitrogen flow 800Plus and 700Plus series cryostats from Oxford Cryosystems were used respectively for the variable-temperature measurements and for the full data collections. A helium flow Oxford Diffraction Helijet Cryostream was used for the helium experiments performed below 80 K. Multiple crystals were tested at 80, 40, 20, and 10 K.
The CrysAlisPRO95 software package from Rigaku Oxford Diffraction was used for all data collections and data processing (indexing, integration, and reduction). Full data sets were collected on assuming that the Friedel pairs are not equivalent. This allowed for sufficient data coverage for noncentrosymmetric crystallographic systems. Data scaling and absorption corrections were applied during the final data processing. All structures were solved by dual direct methods with ShelXT96,97 and refined by full-matrix least squares on F2 using ShelXL97,98 in OLEX2GUI.99 The structures are all polar and for P1 are also chiral. In all cases, the absolute structure was determined unambiguously from the diffraction data, as confirmed by Flack parameters (Table S3).
All non-hydrogen atoms were refined anisotropically; H atoms were constrained by geometry. When applicable, ISOR restraints were applied for occupational disorder of BPh4– anions. For data collected at 10, 82, and 115 K, twin refinement was included due to the formation of ferroelastic twin domains associated with changes in symmetry from monoclinic to triclinic; only two main domains were considered in the final twin refinements.
Structural refinement details and parameters are summarized in Table S3. Bond length and bond angle details are provided in Table S4 with a comparison of Mn–donor bond lengths in the four different HT, INT2, INT1, and LT structural phases. Perspective views of the asymmetric unit of structures measured at 250, 150, 115, 82, and 10 K are provided in Figures S3–S7, shown with 50% atomic probability distributions for ellipsoids.
Precession images in the (h0l) plane were calculated for 250, 150, 115, and 82 K data sets of complex 1 and are shown in Figure S15.
CCDC-2100797 (250 K), CCDC-2100798 (150 K), CCDC-2100799 (115 K), CCDC-2100800 (82 K), and CCDC-2100801 (10 K) contain the crystal data collection and refinement parameter details for this paper, which can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.; fax: (+44) 1223-336-033; or deposit@ccdc.ca.ac.uk).
Resonant Ultrasound Spectroscopy
The resonant ultrasound spectroscopy (RUS) technique is commonly used to investigate phase transitions88 and has been used recently to follow changes in elastic and anelastic properties at both symmetry-breaking and non-symmetry-breaking transitions in SCO materials.30,54 A single crystal of complex 1 with an irregular shape and dimensions of around 1 mm in all directions was mounted between two piezoelectric transducers.100 In the Cambridge system, the piezoelectric transducers sit in a custom built RUS head, which is inserted into a helium flow cryostat.101 The sample chamber is filled with a few millibars of helium for reliable temperature control of the crystal. The system is set up to detect acoustic resonances of small crystals or ceramic samples in the frequency range 0.1–2 MHz. For data processing, the raw spectra were transferred to the software package Igor Pro (WaveMetrics). Fits to individual peaks using an asymmetric Lorentzian function gave the values of peak frequency, f, and the peak widths at half height, Δf. f2 scales with the combination of elastic constants that determines each resonance mode.100 The inverse mechanical quality factor, Q–1, was taken as Q–1= (Δf/f) and is a measure of acoustic loss. Individual resonances are dominated by shear motions with only relative contributions from breathing motions.
Magnetoelectric Coupling
The electric polarization of complex 1 was measured in a 14 T Physical Properties Measurement System (PPMS) by Quantum Design with a custom probe. Silver paint was used to create capacitor plates on parallel faces of the crystal along the c axis and connected to Gore coaxial cables. Thermalization was provided by 4He gas and a sapphire plate on which the sample and a Cernox thermometer were mounted together with GE 7031 varnish and silver paint. Electric polarization was measured by a Keithley 6517A electrometer in charge mode, and the capacitance was measured at 1 kHz and 15 V with an Andeen-Hagerling 2500A capacitance bridge.
Electric polarization was also measured in millisecond capacitor-driven resistive magnets up to 65 T at the National High Magnetic Pulsed Field Facility at Los Alamos National Laboratory.102−104 Two single crystals were mounted on a custom-designed electric polarization probe so that the magnetic field was aligned both parallel and perpendicular to the c axis (the macroscopic long axis) of the single crystals at room temperature. The sapphire sample holder was equipped with a Cernox thermometer, and a minimum of GE 7031 varnish was employed to mount the sample. The faces of the single crystals were coated with silver paint to form capacitor plates. The sample was cooled in 4He gas between 4 and 150 K. The electric polarization in pulsed magnetic fields was measured by recording the change in surface charge using a Stanford Research 570 current to voltage converter when a pulsed magnetic field was applied up to 65 T.105,106 The pulsed field magnetization was determined by an inductive technique in compensated coils107 with sample-in sample-out background subtraction. The sample was mounted in the interior of a plastic capsule using Apiezon vacuum grease. Where not otherwise marked, measurements were conducted on one single crystal for magnetic and electric fields along the c axis. T was controlled using 4He gas or liquid via a Cernox thermometer mounted near the magnetization sample or on sapphire together with the polarization sample. Throughout, standard errors are smaller than line widths and point sizes when not shown.
Acknowledgments
We thank Science Foundation Ireland (SFI) for support via a Frontiers for the Future Project Award (19/FFP/6909 to G.G.M.). This research was also supported by the Irish Research Council GOIPG/2016/73 fellowship (V.B.J.). Travel grants for research visits to LANL, Cambridge, and Rennes for V.B.J. were funded by Augustinus Fonden (grant no. 18-0338), Oticon Fonden (grant no. 17-3813), Reinholdt W. Jorck og Hustrus Fond (grant no. 18-JI-0573), P. A. Fiskers Fond, A. P. Møller og Hustru Chastine Mc-Kinney Møllers Fond til almene Formaal, and Christian og Ottilia Brorsons Rejselegat for yngre videnskabsmænd og-kvinder. RUS facilities in Cambridge were funded by grants to M.A.C. from the Natural Environment Research Council of Great Britain (grant nos. NE/B505738/1 and NE/F17081/1) and from the Engineering and Physical Sciences Research Council (grant no. EP/I036079/1). The NHMFL facility at LANL is funded by the U.S. National Science Foundation through Cooperative Grant No. DMR-1157490, the State of Florida, and the U.S. Department of Energy. Scientific work at LANL was supported by the Laboratory-Directed Research and Development program (LDRD) followed by the Center for Molecular Magnetic Quantum Materials (M2QM), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, under Award DE SC0019330.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c08214.
Details of the magnetometry measurements, single-crystal X-ray diffraction data, resonant ultrasound spectroscopy methodology, and calculations (PDF)
Accession Codes
CCDC 2100797–2100801 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Present Address
∇ Fachbereich Chemie, Technische Universität Kaiserslautern, Erwin-Schrödinger-Strasse, 67663 Kaiserslautern, Germany
Author Present Address
○ National High Magnetic Field Laboratory, Tallahassee, Florida 32310, USA.
Author Present Address
● Idaho National Laboratory, Idaho Falls, Idaho 83402, USA.
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Notes
A report on the magnetic and dielectric properties of [MnIII(3,5-diCl-sal2(323))]BPh4 together with crystallographic data for three of the four structural phases has been published during the revision process for this article, see ref (108).
Supplementary Material
References
- Salje E. K. H. Multiferroic Domain Boundaries as Active Memory Devices: Trajectories Towards Domain Boundary Engineering. ChemPhysChem 2010, 11 (5), 940–950. 10.1002/cphc.200900943. [DOI] [PubMed] [Google Scholar]
- Nataf G. F.; Guennou M.; Gregg J. M.; Meier D.; Hlinka J.; Salje E. K. H.; Kreisel J. Domain-wall engineering and topological defects in ferroelectric and ferroelastic materials. Nature Reviews Physics 2020, 2 (11), 634–648. 10.1038/s42254-020-0235-z. [DOI] [Google Scholar]
- Catalan G.; Seidel J.; Ramesh R.; Scott J. F. Domain wall nanoelectronics. Rev. Mod. Phys. 2012, 84 (1), 119–156. 10.1103/RevModPhys.84.119. [DOI] [Google Scholar]
- Daraktchiev M.; Catalan G.; Scott J. F. Landau theory of domain wall magnetoelectricity. Phys. Rev. B: Condens. Matter Mater. Phys. 2010, 81 (22), 224118. 10.1103/PhysRevB.81.224118. [DOI] [Google Scholar]
- Seidel J.; Martin L. W.; He Q.; Zhan Q.; Chu Y. H.; Rother A.; Hawkridge M. E.; Maksymovych P.; Yu P.; Gajek M.; Balke N.; Kalinin S. V.; Gemming S.; Wang F.; Catalan G.; Scott J. F.; Spaldin N. A.; Orenstein J.; Ramesh R. Conduction at domain walls in oxide multiferroics. Nat. Mater. 2009, 8 (3), 229–234. 10.1038/nmat2373. [DOI] [PubMed] [Google Scholar]
- Guyonnet J.; Gaponenko I.; Gariglio S.; Paruch P. Conduction at Domain Walls in Insulating Pb(Zr0.2Ti0.8)O3 Thin Films. Adv. Mater. 2011, 23 (45), 5377–5382. 10.1002/adma.201102254. [DOI] [PubMed] [Google Scholar]
- Schröder M.; Haußmann A.; Thiessen A.; Soergel E.; Woike T.; Eng L. M. Conducting Domain Walls in Lithium Niobate Single Crystals. Adv. Funct. Mater. 2012, 22 (18), 3936–3944. 10.1002/adfm.201201174. [DOI] [Google Scholar]
- Aird A.; Salje E. K. H. Sheet superconductivity in twin walls: experimental evidence of WO3-x. J. Phys.: Condens. Matter 1998, 10 (22), L377–L380. 10.1088/0953-8984/10/22/003. [DOI] [Google Scholar]
- Yang S. Y.; Seidel J.; Byrnes S. J.; Shafer P.; Yang C. H.; Rossell M. D.; Yu P.; Chu Y. H.; Scott J. F.; Ager J. W.; Martin L. W.; Ramesh R. Above-bandgap voltages from ferroelectric photovoltaic devices. Nat. Nanotechnol. 2010, 5 (2), 143–147. 10.1038/nnano.2009.451. [DOI] [PubMed] [Google Scholar]
- Grinberg I.; West D. V.; Torres M.; Gou G.; Stein D. M.; Wu L.; Chen G.; Gallo E. M.; Akbashev A. R.; Davies P. K.; Spanier J. E.; Rappe A. M. Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials. Nature 2013, 503 (7477), 509–512. 10.1038/nature12622. [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y.; Li P.-F.; Liao W.-Q.; Shi P.-P.; You Y.-M.; Xiong R.-G. Multiaxial Molecular Ferroelectric Thin Films Bring Light to Practical Applications. J. Am. Chem. Soc. 2018, 140 (26), 8051–8059. 10.1021/jacs.8b04600. [DOI] [PubMed] [Google Scholar]
- Parkin S. S. P.; Hayashi M.; Thomas L. Magnetic Domain-Wall Racetrack Memory. Science 2008, 320 (5873), 190–194. 10.1126/science.1145799. [DOI] [PubMed] [Google Scholar]
- Al Bahri M.; Borie B.; Jin T. L.; Sbiaa R.; Kläui M.; Piramanayagam S. N. Staggered Magnetic Nanowire Devices for Effective Domain-Wall Pinning in Racetrack Memory. Phys. Rev. Appl. 2019, 11 (2), 024023 10.1103/PhysRevApplied.11.024023. [DOI] [Google Scholar]
- Whyte J. R.; Gregg J. M. A diode for ferroelectric domain-wall motion. Nat. Commun. 2015, 6 (1), 7361. 10.1038/ncomms8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaab J.; Skjærvø S. H.; Krohns S.; Dai X.; Holtz M. E.; Cano A.; Lilienblum M.; Yan Z.; Bourret E.; Muller D. A.; Fiebig M.; Selbach S. M.; Meier D. Electrical half-wave rectification at ferroelectric domain walls. Nat. Nanotechnol. 2018, 13 (11), 1028–1034. 10.1038/s41565-018-0253-5. [DOI] [PubMed] [Google Scholar]
- Seidel J. Nanoelectronics based on topological structures. Nat. Mater. 2019, 18 (3), 188–190. 10.1038/s41563-019-0301-z. [DOI] [PubMed] [Google Scholar]
- Harrison R. J.; Redfern S. A. T.; Buckley A.; Salje E. K. H. Application of real-time, stroboscopic x-ray diffraction with dynamical mechanical analysis to characterize the motion of ferroelastic domain walls. J. Appl. Phys. 2004, 95 (4), 1706–1717. 10.1063/1.1639949. [DOI] [Google Scholar]
- Schilling A.; Adams T. B.; Bowman R. M.; Gregg J. M.; Catalan G.; Scott J. F. Scaling of domain periodicity with thickness measured in BaTiO3 single crystal lamellae and comparison with other ferroics. Phys. Rev. B: Condens. Matter Mater. Phys. 2006, 74, 024115 10.1103/PhysRevB.74.024115. [DOI] [Google Scholar]
- Salje E. K. H.; Aktas O.; Carpenter M. A.; Laguta V. V.; Scott J. F. Domains within Domains and Walls within Walls: Evidence for Polar Domains in Cryogenic SrTiO3. Phys. Rev. Lett. 2013, 111 (24), 247603. 10.1103/PhysRevLett.111.247603. [DOI] [PubMed] [Google Scholar]
- Pesquera D.; Carpenter M. A.; Salje E. K. H. Glasslike Dynamics of Polar Domain Walls in Cryogenic SrTiO3. Phys. Rev. Lett. 2018, 121 (23), 235701. 10.1103/PhysRevLett.121.235701. [DOI] [PubMed] [Google Scholar]
- Scott J. F.; Salje E. K. H.; Carpenter M. A. Domain Wall Damping and Elastic Softening in SrTiO3 Evidence for Polar Twin Walls. Phys. Rev. Lett. 2012, 109 (18), 187601. 10.1103/PhysRevLett.109.187601. [DOI] [PubMed] [Google Scholar]
- Frenkel Y.; Haham N.; Shperber Y.; Bell C.; Xie Y.; Chen Z.; Hikita Y.; Hwang H. Y.; Salje E. K. H.; Kalisky B. Imaging and tuning polarity at SrTiO3 domain walls. Nat. Mater. 2017, 16 (12), 1203–1208. 10.1038/nmat4966. [DOI] [PubMed] [Google Scholar]
- Bousseksou A.; Molnár G.; Salmon L.; Nicolazzi W. Molecular spin crossover phenomenon: recent achievements and prospects. Chem. Soc. Rev. 2011, 40 (6), 3313–3335. 10.1039/c1cs15042a. [DOI] [PubMed] [Google Scholar]
- Lefter C.; Davesne V.; Salmon L.; Molnár G.; Demont P.; Rotaru A.; Bousseksou A. Charge Transport and Electrical Properties of Spin Crossover Materials: Towards Nanoelectronic and Spintronic Devices. Magnetochemistry 2016, 2 (1), 18. 10.3390/magnetochemistry2010018. [DOI] [Google Scholar]
- Gütlich P.; Garcia Y.; Goodwin H. A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29 (6), 419–427. 10.1039/b003504l. [DOI] [Google Scholar]
- Gütlich P.; Gaspar A. B.; Garcia Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. 10.3762/bjoc.9.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet E.; Guionneau P. Structural analysis of spin-crossover materials: From molecules to materials. C. R. Chim. 2018, 21 (12), 1133–1151. 10.1016/j.crci.2018.02.003. [DOI] [Google Scholar]
- Halcrow M. A.Spin-Crossover Materials: Properties and Applications; Wiley: 2013. [Google Scholar]
- Fitzpatrick A. J.; Trzop E.; Müller-Bunz H.; Dîrtu M. M.; Garcia Y.; Collet E.; Morgan G. G. Electronic vs. structural ordering in a manganese(III) spin crossover complex. Chem. Commun. 2015, 51, 17540–3. 10.1039/C5CC05129K. [DOI] [PubMed] [Google Scholar]
- Jakobsen V. B.; Trzop E.; Gavin L. C.; Dobbelaar E.; Chikara S.; Ding X.; Esien K.; Müller-Bunz H.; Felton S.; Zapf V. S.; Collet E.; Carpenter M. A.; Morgan G. G. Stress-Induced Domain Wall Motion in a Ferroelastic Mn3+ Spin Crossover Complex. Angew. Chem., Int. Ed. 2020, 59 (32), 13305–13312. 10.1002/anie.202003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M.; Shakespeare S.; Shepherd H. J.; Harding C. J.; Létard J.-F.; Desplanches C.; Goeta A. E.; Howard J. A. K.; Powell A. K.; Mereacre V.; Garcia Y.; Naik A. D.; Müller-Bunz H.; Morgan G. G. A Symmetry-Breaking Spin-State Transition in Iron(III). Angew. Chem., Int. Ed. 2011, 50, 896–900. 10.1002/anie.201005545. [DOI] [PubMed] [Google Scholar]
- Vieira B. J. C.; Coutinho J. T.; Santos I. C.; Pereira L. C. J.; Waerenborgh J. C.; da Gama V. [Fe(nsal2trien)]SCN, a new two-step iron(III) spin crossover compound, with symmetry breaking spin-state transition and an intermediate ordered state. Inorg. Chem. 2013, 52, 3845–50. 10.1021/ic302533b. [DOI] [PubMed] [Google Scholar]
- Phonsri W.; Harding D. J.; Harding P.; Murray K. S.; Moubaraki B.; Gass I. A.; Cashion J. D.; Jameson G. N. L.; Adams H. Stepped spin crossover in Fe(III) halogen substituted quinolylsalicylaldimine complexes. Dalton Trans. 2014, 43, 17509–17518. 10.1039/C4DT01701C. [DOI] [PubMed] [Google Scholar]
- Bréfuel N.; Watanabe H.; Toupet L.; Come J.; Matsumoto N.; Collet E.; Tanaka K.; Tuchagues J.-P. Concerted Spin Crossover and Symmetry Breaking Yield Three Thermally and One Light-Induced Crystallographic Phases of a Molecular Material. Angew. Chem. 2009, 121, 9468–9471. 10.1002/ange.200904190. [DOI] [PubMed] [Google Scholar]
- Bréfuel N.; Collet E.; Watanabe H.; Kojima M.; Matsumoto N.; Toupet L.; Tanaka K.; Tuchagues J.-P. Nanoscale Self-Hosting of Molecular Spin-States in the Intermediate Phase of a Spin-Crossover Material. Chem. Eur. J. 2010, 16 (47), 14060–14068. 10.1002/chem.201001264. [DOI] [PubMed] [Google Scholar]
- Garcia Y.; Kahn O.; Rabardel L.; Chansou B.; Salmon L.; Tuchagues J. P. Two-Step Spin Conversion for the Three-Dimensional Compound Tris(4,4‘-bis-1,2,4-triazole)iron(II) Diperchlorate. Inorg. Chem. 1999, 38 (21), 4663–4670. 10.1021/ic990511q. [DOI] [PubMed] [Google Scholar]
- Harding D. J.; Phonsri W.; Harding P.; Murray K. S.; Moubaraki B.; Jameson G. N. L. Abrupt two-step and symmetry breaking spin crossover in an iron(III) complex: an exceptionally wide [LS–HS] plateau. Dalton Trans. 2015, 44, 15079–15082. 10.1039/C4DT03184A. [DOI] [PubMed] [Google Scholar]
- Zhu Y.-Y.; Liu C.-W.; Yin J.; Meng Z.-S.; Yang Q.; Wang J.; Liu T.; Gao S. Structural phase transition in a multi-induced mononuclear FeII spin-crossover complex. Dalton Trans. 2015, 44 (48), 20906–20912. 10.1039/C5DT03216D. [DOI] [PubMed] [Google Scholar]
- Murnaghan K. D.; Carbonera C.; Toupet L.; Griffin M.; Dîrtu M. M.; Desplanches C.; Garcia Y.; Collet E.; Létard J.-F.; Morgan G. G. Spin-state ordering on one sub-lattice of a mononuclear iron(III) spin crossover complex exhibiting LIESST and TIESST. Chem. Eur. J. 2014, 20, 5613–8. 10.1002/chem.201400286. [DOI] [PubMed] [Google Scholar]
- Matouzenko G. S.; Letard J.-F.; Lecocq S.; Bousseksou A.; Capes L.; Salmon L.; Perrin M.; Kahn O.; Collet A. Two-Step Spin Crossover in a Mononuclear Compound [Fe(DPEA)(bim)](ClO4)2·0.5 H2O[DPEA = (2-Aminoethyl)bis(2-pyridylmethyl)amine, bim = 2,2-Bisimidazole] – Crystal Structure, Magnetic Properties, Mössbauer Spectroscopy, and Photomagnetic Effects. Eur. J. Inorg. Chem. 2001, 2001 (11), 2935–2945. . [DOI] [Google Scholar]
- Watanabe H.; Tanaka K.; Bréfuel N.; Cailleau H.; Létard J.-F.; Ravy S.; Fertey P.; Nishino M.; Miyashita S.; Collet E. Ordering phenomena of high-spin/low-spin states in stepwise spin-crossover materials described by the ANNNI model. Phys. Rev. B: Condens. Matter Mater. Phys. 2016, 93 (1), 014419 10.1103/PhysRevB.93.014419. [DOI] [Google Scholar]
- Yamada M.; Hagiwara H.; Torigoe H.; Matsumoto N.; Kojima M.; Dahan F.; Tuchagues J.-P.; Re N.; Iijima S. A Variety of Spin-Crossover Behaviors Depending on the Counter Anion: Two-Dimensional Complexes Constructed by NH···Cl– Hydrogen Bonds, [FeIIH3LMe]Cl·X (X = PF6–, AsF6–, SbF6–, CF3SO3–; H3LMe = Tris[2-{[(2-methylimidazol-4-yl)methylidene]amino}ethyl]amine). Chem. Eur. J. 2006, 12 (17), 4536–4549. 10.1002/chem.200500972. [DOI] [PubMed] [Google Scholar]
- Sato T.; Nishi K.; Iijima S.; Kojima M.; Matsumoto N. One-Step and Two-Step Spin-Crossover Iron(II) Complexes of ((2-Methylimidazol-4-yl)methylidene)histamine. Inorg. Chem. 2009, 48 (15), 7211–7229. 10.1021/ic9006197. [DOI] [PubMed] [Google Scholar]
- Boinnard D.; Bousseksou A.; Dworkin A.; Savariault J. M.; Varret F.; Tuchagues J. P. Two-step spin conversion of [FeII(5-NO2-sal-N(1,4,7,10))]: 292, 153, and 103 K x-ray crystal and molecular structure, infrared, magnetic, Mössbauer, calorimetric, and theoretical studies. Inorg. Chem. 1994, 33 (2), 271–281. 10.1021/ic00080a015. [DOI] [Google Scholar]
- Money V. A.; Carbonera C.; Elhaïk J.; Halcrow M. A.; Howard J. A. K.; Létard J.-F. Interplay Between Kinetically Slow Thermal Spin-Crossover and Metastable High-Spin State Relaxation in an Iron(II) Complex with Similar T1/2 and T(LIESST). Chem. Eur. J. 2007, 13 (19), 5503–5514. 10.1002/chem.200601312. [DOI] [PubMed] [Google Scholar]
- Luan J.; Zhou J.; Liu Z.; Zhu B.; Wang H.; Bao X.; Liu W.; Tong M.-L.; Peng G.; Peng H.; Salmon L.; Bousseksou A. Polymorphism-Dependent Spin-Crossover: Hysteretic Two-Step Spin Transition with an Ordered [HS–HS–LS] Intermediate Phase. Inorg. Chem. 2015, 54 (11), 5145–5147. 10.1021/acs.inorgchem.5b00629. [DOI] [PubMed] [Google Scholar]
- Bonnet S.; Siegler M. A.; Costa J. S.; Molnar G.; Bousseksou A.; Spek A. L.; Gamez P.; Reedijk J. A two-step spin crossover mononuclear iron(II) complex with a [HS-LS-LS] intermediate phase. Chem. Commun. 2008, 5619–5621. 10.1039/b811746b. [DOI] [PubMed] [Google Scholar]
- Shatruk M.; Phan H.; Chrisostomo B. A.; Suleimenova A. Symmetry-breaking structural phase transitions in spin crossover complexes. Coord. Chem. Rev. 2015, 289–290, 62–73. 10.1016/j.ccr.2014.09.018. [DOI] [Google Scholar]
- Ortega-Villar N.; Muñoz M. C.; Real J. A. Symmetry Breaking in Iron(II) Spin-Crossover Molecular Crystals. Magnetochemistry 2016, 2 (1), 16. 10.3390/magnetochemistry2010016. [DOI] [Google Scholar]
- Collet E.; Watanabe H.; Bréfuel N.; Palatinus L.; Roudaut L.; Toupet L.; Tanaka K.; Tuchagues J. P.; Fertey P.; Ravy S.; Toudic B.; Cailleau H. Aperiodic Spin State Ordering of Bistable Molecules and Its Photoinduced Erasing. Phys. Rev. Lett. 2012, 109 (25), 257206. 10.1103/PhysRevLett.109.257206. [DOI] [PubMed] [Google Scholar]
- Cruddas J.; Powell B. J. Structure–property relationships and the mechanisms of multistep transitions in spin crossover materials and frameworks. Inorg. Chem. Front. 2020, 7 (22), 4424–4437. 10.1039/D0QI00799D. [DOI] [Google Scholar]
- Azzolina G.; Bertoni R.; Collet E. General Landau theory of non-symmetry-breaking and symmetry-breaking spin transition materials. J. Appl. Phys. 2021, 129 (8), 085106 10.1063/5.0041453. [DOI] [Google Scholar]
- Collet E.; Azzolina G. Coupling and decoupling of spin crossover and ferroelastic distortion: Unsymmetric hysteresis loop, phase diagram, and sequence of phases. Phys. Rev. Materials 2021, 5 (4), 044401 10.1103/PhysRevMaterials.5.044401. [DOI] [Google Scholar]
- Mason H. E.; Li W.; Carpenter M. A.; Hamilton M. L.; Howard J. A. K.; Sparkes H. A. Structural and spectroscopic characterisation of the spin crossover in [Fe(abpt)2(NCS)2 ] polymorph A. New J. Chem. 2016, 40, 2466–2478. 10.1039/C5NJ02359A. [DOI] [Google Scholar]
- Zhang Z.; Koppensteiner J.; Schranz W.; Carpenter M. A. Variations in elastic and anelastic properties of Co3O4 due to magnetic and spin-state transitions. Am. Mineral. 2012, 97 (2–3), 399–406. 10.2138/am.2012.3901. [DOI] [Google Scholar]
- Sy M.; Traiche R.; Fourati H.; Singh Y.; Varret F.; Boukheddaden K. Spatiotemporal Investigations on Light-Driven High-Spin–Low-Spin Interface Dynamics in the Thermal Hysteresis Region of a Spin-Crossover Single Crystal. J. Phys. Chem. C 2018, 122 (36), 20952–20962. 10.1021/acs.jpcc.8b05704. [DOI] [Google Scholar]
- Fourati H.; Milin E.; Slimani A.; Chastanet G.; Abid Y.; Triki S.; Boukheddaden K. Interplay between a crystal’s shape and spatiotemporal dynamics in a spin transition material. Phys. Chem. Chem. Phys. 2018, 20 (15), 10142–10154. 10.1039/C8CP00868J. [DOI] [PubMed] [Google Scholar]
- Slimani A.; Varret F.; Boukheddaden K.; Chong C.; Mishra H.; Haasnoot J.; Pillet S. Visualization and quantitative analysis of spatiotemporal behavior in a first-order thermal spin transition: A stress-driven multiscale process. Phys. Rev. B: Condens. Matter Mater. Phys. 2011, 84 (9), 094442 10.1103/PhysRevB.84.094442. [DOI] [Google Scholar]
- Chong C.; Slimani A.; Varret F.; Boukheddaden K.; Collet E.; Ameline J.-C.; Bronisz R.; Hauser A. The kinetics features of a thermal spin transition characterized by optical microscopy on the example of [Fe(bbtr)3](ClO4)2 single crystals: Size effect and mechanical instability. Chem. Phys. Lett. 2011, 504 (1), 29–33. 10.1016/j.cplett.2011.01.041. [DOI] [Google Scholar]
- Slimani A.; Varret F.; Boukheddaden K.; Garrot D.; Oubouchou H.; Kaizaki S. Velocity of the High-Spin Low-Spin Interface Inside the Thermal Hysteresis Loop of a Spin-Crossover Crystal, via Photothermal Control of the Interface Motion. Phys. Rev. Lett. 2013, 110 (8), 087208 10.1103/PhysRevLett.110.087208. [DOI] [PubMed] [Google Scholar]
- Sy M.; Varret F.; Boukheddaden K.; Bouchez G.; Marrot J.; Kawata S.; Kaizaki S. Structure-Driven Orientation of the High-Spin–Low-Spin Interface in a Spin-Crossover Single Crystal. Angew. Chem., Int. Ed. 2014, 53 (29), 7539–7542. 10.1002/anie.201403971. [DOI] [PubMed] [Google Scholar]
- Varret F.; Slimani A.; Boukheddaden K.; Chong C.; Mishra H.; Collet E.; Haasnoot J.; Pillet S. The propagation of the thermal spin transition of [Fe(btr)2(NCS)2]·H2O single crystals, observed by optical microscopy. New J. Chem. 2011, 35 (10), 2333–2340. 10.1039/c1nj20332k. [DOI] [Google Scholar]
- Boukheddaden K.; Loutete-Dangui E. D.; Codjovi E.; Castro M.; Rodriguéz-Velamazán J. A.; Ohkoshi S.; Tokoro H.; Koubaa M.; Abid Y.; Varret F. Experimental access to elastic and thermodynamic properties of RbMnFe(CN)6. J. Appl. Phys. 2011, 109 (1), 013520 10.1063/1.3528239. [DOI] [Google Scholar]
- Paez-Espejo M.; Sy M.; Varret F.; Boukheddaden K. Quantitative macroscopic treatment of the spatiotemporal properties of spin crossover solids based on a reaction diffusion equation. Phys. Rev. B: Condens. Matter Mater. Phys. 2014, 89 (2), 024306 10.1103/PhysRevB.89.024306. [DOI] [Google Scholar]
- Slimani A.; Boukheddaden K.; Varret F.; Oubouchou H.; Nishino M.; Miyashita S. Microscopic spin-distortion model for switchable molecular solids: Spatiotemporal study of the deformation field and local stress at the thermal spin transition. Phys. Rev. B: Condens. Matter Mater. Phys. 2013, 87 (1), 014111 10.1103/PhysRevB.87.014111. [DOI] [Google Scholar]
- Traiche R.; Sy M.; Oubouchou H.; Bouchez G.; Varret F.; Boukheddaden K. Spatiotemporal Observation and Modeling of Remarkable Temperature Scan Rate Effects on the Thermal Hysteresis in a Spin-Crossover Single Crystal. J. Phys. Chem. C 2017, 121 (21), 11700–11708. 10.1021/acs.jpcc.7b02816. [DOI] [Google Scholar]
- Chikara S.; Gu J.; Zhang X. G.; Cheng H.-P.; Smythe N.; Singleton J.; Scott B.; Krenkel E.; Eckert J.; Zapf V. S. Magnetoelectric behavior via a spin state transition. Nat. Commun. 2019, 10 (1), 4043. 10.1038/s41467-019-11967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen V. B.; Chikara S.; Yu J.-X.; Dobbelaar E.; Kelly C. T.; Ding X.; Weickert F.; Trzop E.; Collet E.; Cheng H.-P.; Morgan G. G.; Zapf V. S. Giant Magnetoelectric Coupling and Magnetic-Field-Induced Permanent Switching in a Spin Crossover Mn(III) Complex. Inorg. Chem. 2021, 60 (9), 6167–6175. 10.1021/acs.inorgchem.0c02789. [DOI] [PubMed] [Google Scholar]
- Olguín J. Unusual metal centres/coordination spheres in spin crossover compounds. Coord. Chem. Rev. 2020, 407, 213148. 10.1016/j.ccr.2019.213148. [DOI] [Google Scholar]
- Kühne I. A.; Barker A.; Zhang F.; Stamenov P.; O’Doherty O.; Müller-Bunz H.; Stein M.; Rodriguez B. J.; Morgan G. G. Modulation of Jahn–Teller distortion and electromechanical response in a Mn3+ spin crossover complex. J. Phys.: Condens. Matter 2020, 32 (40), 404002. 10.1088/1361-648X/ab82d1. [DOI] [PubMed] [Google Scholar]
- Kühne I. A.; Gavin L. C.; Harris M.; Gildea B.; Müller-Bunz H.; Stein M.; Morgan G. G. Mn(III) complexes with nitro-substituted ligands—Spin states with a twist. J. Appl. Phys. 2021, 129 (21), 213903. 10.1063/5.0050276. [DOI] [Google Scholar]
- Sirirak J.; Harding D. J.; Harding P.; Murray K. S.; Moubaraki B.; Liu L.; Telfer S. G. Spin Crossover in cis Manganese(III) Quinolylsalicylaldiminates. Eur. J. Inorg. Chem. 2015, 2015 (15), 2534–2542. 10.1002/ejic.201500196. [DOI] [Google Scholar]
- Shongwe M. S.; Al-Barhi K. S.; Mikuriya M.; Adams H.; Morris M. J.; Bill E.; Molloy K. C. Tuning a Single Ligand System to Stabilize Multiple Spin States of Manganese: A First Example of a Hydrazone-Based Manganese(III) Spin-Crossover Complex. Chem. -Eur. J. 2014, 20 (31), 9693–9701. 10.1002/chem.201402634. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Liang S.; Di X.; Zhang J. A manganese(III) complex that exhibits spin crossover behavior. Inorg. Chem. Commun. 2008, 11 (7), 783–786. 10.1016/j.inoche.2008.03.030. [DOI] [Google Scholar]
- Morgan G. G.; Murnaghan K. D.; Müller-Bunz H.; McKee V.; Harding C. J. A Manganese(III) Complex That Exhibits Spin Crossover Triggered by Geometric Tuning. Angew. Chem., Int. Ed. 2006, 45, 7192–7195. 10.1002/anie.200601823. [DOI] [PubMed] [Google Scholar]
- Martinho P. N.; Gildea B.; Harris M. M.; Lemma T.; Naik A. D.; Müller-Bunz H.; Keyes T. E.; Garcia Y.; Morgan G. G. Cooperative spin transition in a mononuclear manganese(III) complex. Angew. Chem., Int. Ed. 2012, 51, 12597–601. 10.1002/anie.201205573. [DOI] [PubMed] [Google Scholar]
- Pandurangan K.; Gildea B.; Murray C.; Harding C. J.; Müller-Bunz H.; Morgan G. G. Lattice effects on the spin-crossover profile of a mononuclear manganese(III) cation. Chem. Eur. J. 2012, 18, 2021–2029. 10.1002/chem.201102820. [DOI] [PubMed] [Google Scholar]
- Gildea B.; Harris M. M.; Gavin L. C.; Murray C. A.; Ortin Y.; Müller-Bunz H.; Harding C. J.; Lan Y.; Powell A. K.; Morgan G. G. Substituent effects on spin state in a series of mononuclear manganese(III) complexes with hexadentate Schiff-Base ligands. Inorg. Chem. 2014, 53, 6022–33. 10.1021/ic5003012. [DOI] [PubMed] [Google Scholar]
- Wang S.; Ferbinteanu M.; Marinescu C.; Dobrinescu A.; Ling Q.-D.; Huang W. Case Study on a Rare Effect: The Experimental and Theoretical Analysis of a Manganese(III) Spin-Crossover System. Inorg. Chem. 2010, 49, 9839–9851. 10.1021/ic100364v. [DOI] [PubMed] [Google Scholar]
- Chernyshov D.; Bürgi H.-B.; Hostettler M.; Törnroos K. W. Landau theory for spin transition and ordering phenomena in Fe(II) compounds. Phys. Rev. B: Condens. Matter Mater. Phys. 2004, 70 (9), 094116 10.1103/PhysRevB.70.094116. [DOI] [Google Scholar]
- Sim P. G.; Sinn E. First manganese(III) spin crossover, first d4 crossover. Comment on cytochrome oxidase. J. Am. Chem. Soc. 1981, 103 (1), 241–243. 10.1021/ja00391a067. [DOI] [Google Scholar]
- Villaman D.; McMonagle C. J.; Probert M. R.; Peña O.; Moreno Y.; Fuentealba M. Structural studies of a manganese(iii) complex with spin-crossover and thermochromic properties. CrystEngComm 2020, 22 (18), 3221–3233. 10.1039/C9CE01962F. [DOI] [Google Scholar]
- Barker A.; Kelly C. T.; Kühne I. A.; Hill S.; Krzystek J.; Wix P.; Esien K.; Felton S.; Müller-Bunz H.; Morgan G. G. Spin state solvomorphism in a series of rare S = 1 manganese(III) complexes. Dalton Trans. 2019, 48 (41), 15560–15566. 10.1039/C9DT02476J. [DOI] [PubMed] [Google Scholar]
- Jaffe B.; Cook W. R.; Jaffe H.. Piezoelectric Ceramics; Academic Press: 1971. [Google Scholar]
- Carpenter M. A.; Salje E. K. H. Elastic anomalies in minerals due to structural phase transitions. Eur. J. Mineral. 1998, 10 (4), 693–812. 10.1127/ejm/10/4/0693. [DOI] [Google Scholar]
- Carpenter M. A.; Salje E. K. H.; Graeme-Barber A. Spontaneous strain as a determinant of thermodynamic properties for phase transitions in minerals. Eur. J. Mineral. 1998, 10 (4), 621–691. 10.1127/ejm/10/4/0621. [DOI] [Google Scholar]
- Zhang Z.; Koppensteiner J.; Schranz W.; Prabhakaran D.; Carpenter M. A. Strain coupling mechanisms and elastic relaxation associated with spin state transitions in LaCoO3. J. Phys.: Condens. Matter 2011, 23 (14), 145401. 10.1088/0953-8984/23/14/145401. [DOI] [PubMed] [Google Scholar]
- Carpenter M. A. Static and dynamic strain coupling behaviour of ferroic and multiferroic perovskites from resonant ultrasound spectroscopy. J. Phys.: Condens. Matter 2015, 27, 263201. 10.1088/0953-8984/27/26/263201. [DOI] [PubMed] [Google Scholar]
- Carpenter M. A.; Sinogeikin S. V.; Bass J. D.; Lakshtanov D. L.; Jacobsen S. D. Elastic relaxations associated with the Pm3m-R3c transition in LaAlO3: I. Single crystal elastic moduli at room temperature. J. Phys.: Condens. Matter 2010, 22 (3), 035403 10.1088/0953-8984/22/3/035403. [DOI] [PubMed] [Google Scholar]
- Harrison R. J.; Redfern S. A. T. The influence of transformation twins on the seismic-frequency elastic and anelastic properties of perovskite: dynamical mechanical analysis of single crystal LaAlO3. Phys. Earth Planet. Inter. 2002, 134 (3), 253–272. 10.1016/S0031-9201(02)00190-5. [DOI] [Google Scholar]
- Harrison R. J.; Redfern S. A. T.; Salje E. K. H. Dynamical excitation and anelastic relaxation of ferroelastic domain walls in LaAlO3. Phys. Rev. B: Condens. Matter Mater. Phys. 2004, 69 (14), 144101. 10.1103/PhysRevB.69.144101. [DOI] [Google Scholar]
- Lee W. T.; Salje E. K. H.; Goncalves-Ferreira L.; Daraktchiev M.; Bismayer U. Intrinsic activation energy for twin-wall motion in the ferroelastic perovskite CaTiO3. Phys. Rev. B: Condens. Matter Mater. Phys. 2006, 73 (21), 214110. 10.1103/PhysRevB.73.214110. [DOI] [Google Scholar]
- Chrosch J.; Salje E. K. H. Temperature dependence of the domain wall width in LaAlO3. J. Appl. Phys. 1999, 85 (2), 722–727. 10.1063/1.369152. [DOI] [Google Scholar]
- Carpenter M. A.; Salje E. K. H.; Graeme-Barber A.; Wruck B.; Dove M. T.; Knight K. S. Calibration of excess thermodynamic properties and elastic constant variations associated with the alpha to beta phase transition in quartz. Am. Mineral. 1998, 83 (1–2), 2–22. 10.2138/am-1998-1-201. [DOI] [Google Scholar]
- CrysAlisPro v1.171.36.28.; Agilent Technologies XRD Products: 2013.
- Sheldrick G. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64 (1), 112–122. 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71 (1), 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71 (1), 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolomanov O. V.; Bourhis L. J.; Gildea R. J.; Howard J. A. K.; Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. 10.1107/S0021889808042726. [DOI] [Google Scholar]
- Migliori A.; Sarrao J. L.. Resonant Ultrasound Spectroscopy: Applications to Physics, Materials Measurements, and Nondestructive Evaluation, 1st ed.; Wiley-VCH: 1997; p 202. [Google Scholar]
- McKnight R. E. A.; Carpenter M. A.; Darling T. W.; Buckley A.; Taylor P. A. Acoustic dissipation associated with phase transitions in lawsonite, CaAl2Si2O7(OH)2·H2O. Am. Mineral. 2007, 92 (10), 1665–1672. 10.2138/am.2007.2568. [DOI] [Google Scholar]
- Yoo K.; Koteswararao B.; Kang J.; Shahee A.; Nam W.; Balakirev F. F.; Zapf V. S.; Harrison N.; Guda A.; Ter-Oganessian N.; Kim K. H. Magnetic field-induced ferroelectricity in S = 1/2 kagome staircase compound PbCu3TeO7. npj Quantum Materials 2018, 3 (1), 45. 10.1038/s41535-018-0117-0. [DOI] [Google Scholar]
- Chikara S.; Singleton J.; Bowlan J.; Yarotski D. A.; Lee N.; Choi H. Y.; Choi Y. J.; Zapf V. S. Electric polarization observed in single crystals of multiferroic Lu2MnCoO6. Phys. Rev. B: Condens. Matter Mater. Phys. 2016, 93 (18), 180405. 10.1103/PhysRevB.93.180405. [DOI] [Google Scholar]
- Kim J. W.; Artyukhin S.; Mun E. D.; Jaime M.; Harrison N.; Hansen A.; Yang J. J.; Oh Y. S.; Vanderbilt D.; Zapf V. S.; Cheong S. W. Successive Magnetic-Field-Induced Transitions and Colossal Magnetoelectric Effect in Ni3TeO6. Phys. Rev. Lett. 2015, 115 (13), 137201. 10.1103/PhysRevLett.115.137201. [DOI] [PubMed] [Google Scholar]
- Zapf V. S.; Kenzelmann M.; Wolff-Fabris F.; Balakirev F.; Chen Y. Magnetically induced electric polarization in an organometallic magnet. Phys. Rev. B: Condens. Matter Mater. Phys. 2010, 82 (6), 060402 10.1103/PhysRevB.82.060402. [DOI] [Google Scholar]
- Zapf V. S.; Sengupta P.; Batista C. D.; Nasreen F.; Wolff-Fabris F.; Paduan-Filho A. Magnetoelectric effects in an organometallic quantum magnet. Phys. Rev. B: Condens. Matter Mater. Phys. 2011, 83 (14), 140405. 10.1103/PhysRevB.83.140405. [DOI] [Google Scholar]
- Detwiler J. A.; Schmiedeshoff G. M.; Harrison N.; Lacerda A. H.; Cooley J. C.; Smith J. L. Magnetization of UBe13 to 60 T. Phys. Rev. B: Condens. Matter Mater. Phys. 2000, 61 (1), 402–404. 10.1103/PhysRevB.61.402. [DOI] [Google Scholar]
- Tiunova A. V.; Kazakova A. V.; Korchagin D. V.; Shilov G. V.; Zorina L. V.; Simonov S. V.; Zakharov K. V.; Vasiliev A. N.; Yagubskii E. B. Abrupt Spin-State Switching in Mn(III) Complexes with BPh4 Anion: Effect of Halide Substituents on Crystal Structure and Magnetic Properties. Chem. Eur. J. 2021, 27, 17609–17619. 10.1002/chem.202102666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.