Abstract
Background
One strategy for reducing spread of COVID-19 is to contain the infection with broad screening, isolating infected individuals, and tracing contacts. This strategy requires widely available, reliable SARS-CoV-2 testing. To increase testing, rapid antigen detection tests (RADTs) were developed for self-sampling, self-testing, and self-interpretation. This study examined diagnostic performance, user acceptability, and safety of nasal self-RADTs compared with polymerase chain reaction (PCR) testing.
Methods
Self-RADT kits were distributed at a public COVID-19 test center in Aarhus, Denmark or delivered to participants. Participants reported test results and test preferences. During enrollment, participants reported occurrence and duration of symptoms consistent with COVID-19. Sensitivity and specificity of self-RADT, relative to oropharyngeal PCR testing, were calculated.
Results
Among 827 participants, 102 showed positive PCR test results. Sensitivities of the self-RADTs were 65.7% (95% confidence interval [CI]: 49.2–79.2; DNA Diagnostic) and 62.1% (95% CI: 50.1–72.9; Hangzhou), and specificities were 100% (95% CI: 99.0–100; DNA Diagnostic) and 100% (95% CI: 98.9–100; Hangzhou). The sensitivities of both self-RADTs appeared higher in symptomatic participants than in asymptomatic participants. Two of every 3 participants preferred self-RADT over PCR test.
Conclusion
Self-performed RADTs were reliable, user-acceptable, and safe among laypeople as a supplement to professionally collected oropharyngeal PCR testing.
Keywords: SARS-CoV-2, Antigen test, Rapid test, COVID-19, Diagnostics, Self-testing
Abbreviations: RADT, Rapid antigen detection test; RT-PCR, Reverse transcriptase polymerase chain reaction
Introduction
Rapid antigen detection tests (RADTs) that can be used to self-test for SARS-CoV-2 infections are available in several countries. However, few studies have examined the self-test application of RADTs. Self-tests require the individual to collect a specimen, conduct a RADT protocol, and interpret the result without assistance (Foundation for Innovative New Diagnostics, 2021).
Currently, the gold-standard testing method for SARS-CoV-2 is a nasopharyngeal swab or a combined nasopharyngeal/oropharyngeal swab, followed by a reverse transcriptase polymerase chain reaction (RT-PCR) analysis. The standard SARS-CoV-2 test method in Denmark is a swab from the oropharynx, performed by a trained health care worker, and an RT-PCR analysis (Statens Serum Institut, 2021). The disadvantages of this approach are the high cost of the RT-PCR analysis, the long response time, the need for personnel to operate the COVID-19 test centers, and the risk of virus transmission to health care workers and other citizens at the test centers.
RADTs have a short response time and require little equipment and reagents for analysis. In contrast, RADTs have lower sensitivities and specificities than the RT-PCR analysis. Nevertheless, the sensitivity of RADTs appears to correlate with the viral load (Corman et al, 2021; Krüger et al, 2020; Osmanodja et al, 2021). The viral load is estimated to be highest 2 to 3 days after symptom onset and is probably the timepoint when most infectious (Ejima et al., 2021).
Nasopharyngeal swabs and self-swabs from the anterior nasal region have comparable sensitivities when performed by professionals, particularly when the viral load is high (Hanson et al, 2020; Kojima et al, 2020; Lindner et al, 2021a, 2021b; McCulloch et al, 2020; Tsang et al, 2021; Tu et al, 2020). From a public health perspective, self-tests can offer advantages when used to complement professionally administered PCR tests or RADTs. Self-tests can improve accessibility to testing, support early detection of infectious cases, and reduce further community transmission. Therefore, self-testing could enhance disease control with prompt identification and isolation of infectious individuals (European Centre for Disease Prevention and Control, 2021).
The current study aimed to evaluate the sensitivity, specificity, user acceptability, and safety of 2 different nasal RADTs, when performed at home by participants, compared with an oropharyngeal swab performed by a trained health care worker and analyzed with RT-PCR.
Methods
Study design and participants
This study was a manufacturer-independent prospective study. We evaluated the diagnostic accuracy, user acceptability, and safety of 2 RADTs when performed unsupervised by participants at home. For comparison, the participants underwent an oropharyngeal swab, performed by a health care worker at the ambulatory public COVID-19 test center of Aarhus University Hospital, Denmark, and the sample was analyzed with RT-PCR. In addition, some participants who were recruited onsite were tested with a nasopharyngeal RADT performed by a health care worker.
Participants were eligible for inclusion when they were ≥18 years old, had made an appointment for PCR testing at the public test center, were able to conduct the RADT within 72 hours after their PCR test at the test center, and were able to understand written and spoken Danish. Participants were not eligible for inclusion if they had had a nosebleed within 24 hours before the RADT performance, any nose operation within 4 weeks before the execution of the RADT, or a previous infection with SARS-CoV-2. Previously infected individuals were not included because the PCR analysis can detect the virus weeks after an infection (Mallett et al, 2020).
Antigen tests
The primary tests evaluated in this study were 2 nasal RADTs: the COVID-19 Antigen Detection Kit (DNA Diagnostic A/S, Risskov, Denmark) and the SARS-CoV-2 Antigen Rapid Test (Hangzhou Immuno Biotech Co Ltd, Hangzhou, China). In addition, we evaluated a nasopharyngeal RADT, known as the COVID-19 Antigen Rapid Test Device (Abbott Rapid Diagnostic Jena GmbH, Jena, Germany). The participants swabbed themselves in each nostril for 5 seconds. Hereafter, the swab was mixed with buffer. Buffer with specimen was added to a test plate. For the test to be conclusive, a line should appear after 10 minutes in the control area of the test plate. If a line appeared in the test area, the test was positive, and no line indicated a negative result. Weak lines in the test area were also considered positive results. Both tests were validated before this study and were CE-marked.
Study procedures
Study participants were recruited either onsite at the COVID-19 test center of Aarhus University Hospital, Denmark (onsite participants), or by telephone (offsite participants). Onsite participants had come to the test center for SARS-CoV-2 testing and expressed a willingness to try a self-test at home. These participants signed a written informed consent form at the center and were given an RADT kit to be performed at home. Offsite participants were recruited after they contacted the project group after receiving a positive result on a PCR test performed at the test center. These participants received a RADT kit delivered to their address with a written informed consent form, which they signed and returned digitally to the project group. Participants were enrolled during 4 periods. Onsite participants were enrolled from January 21, 2021, to January 25, 2021, for both the DNA Diagnostic RADT and the Abbott RADT, and from March 25, 2021, to April 4, 2021, for the Hangzhou RADT. Offsite participants were enrolled from February 16, 2021, to March 10, 2021, for the DNA Diagnostic RADT, and from March 10, 2021, to March 31, 2021, for the Hangzhou RADT. All test kits contained a written, illustrated instruction pamphlet and a link to an online instruction video for self-sampling and self-testing. All instructions were translated into Danish from the manufacturers’ instruction pamphlets. The RADT results were self-interpreted by participants, and the interpretation was confirmed by the project team, based on photographs of the test plates that were sent by e-mail from the participants to the project inbox. Study participants were provided with a telephone number and a secure e-mail address for returning study-relevant material and for technical support. In case of a positive or inconclusive self-RADT result, participants were advised to call in for further instructions.
Standard reference RT-PCR
Samples for RT-PCR testing were obtained with oropharyngeal swabs. All PCR analyses for detecting SARS-CoV-2 RNA were performed by International Organization for Standardization standard accredited laboratories at the Department of Clinical Microbiology, Aarhus University Hospital or at the national reference laboratory at the Statens Serum Institute. Internationally approved PCR platforms were used. The result from the RT-PCR analysis was self-reported by each participant; however, consent was given for the project group to obtain the result from the laboratory, when necessary. At the time of participant enrollment, all citizens could make appointments for free PCR tests, and the response time was less than 48 hours. The Danish government recommended that citizens should get a PCR test if they had been in close contact with a person infected with SARS-CoV-2, if they had experienced symptoms consistent with COVID-19, and if they were about to undergo hospitalization or medical procedures. In Denmark, RADTs were recommended for routine testing for all individuals in populations with a particularly high incidence of SARS-CoV-2 and for individuals who received a notification from the COVID-19 app “SmitteStop” (Sundhedsstyrelsen, 2021).
Additional data collection
During enrollment, participants were asked to describe the reason for making an appointment for PCR testing, their symptoms, and the symptom duration. In addition, participants were asked about the number of COVID-19 vaccine injections they had received and whether they had a health professional background. When reporting their self-RADT results, participants were asked which test they preferred and why. Besides, 355 participants were asked whether performing the RADT caused nose bleeding.
Statistical analysis
Data were collected and managed with Research Electronic Data Capture (REDCap) tools. Statistical analyses were performed in Stata/MP 17.0. Sensitivity and specificity, with 95% confidence intervals (95% CIs), were calculated for self-RADTs with the Wilson test. Those results were compared with the sensitivity and specificity of the reference standard PCR test. Inconclusive RADT or PCR test results were not included in the statistical analysis of sensitivity and specificity. Descriptive statistics were used to evaluate participant characteristics, user acceptability, and safety.
Ethics
The regional Scientific Ethics Committee of the Central Denmark Region concluded that this quality assurance study did not require scientific ethical approval (reference number 1-10-72-1-20). The Danish Medicines Agency concluded that the study did not require approval from them. Data collected from the participants of this study were treated according to the General Data Protection Regulation. All participants received oral and written information about the study, and all participants consented to participate.
Results
Participants
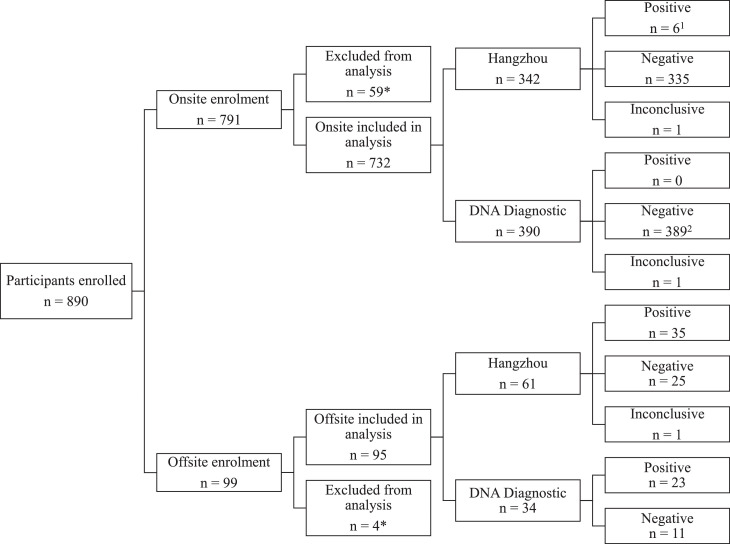
The participant inclusion process is shown in Figure 1 . Four participants were excluded from the study because they failed to return a signed consent form, and 59 participants were excluded because the test results were not reported, were inadequately reported, or they performed the self-RADT more than 72 hours after their PCR test. A total of 827 participants were included in the data analysis of self-RADTs; of these, 102 (12.3%) showed positive results on the PCR test.
Figure 1.
Study flow diagram and self-RADT results. Onsite enrollment: participants enrolled at the Aarhus University Hospital test center. Offsite enrollment: participants enrolled after a positive PCR test, and self-RADT was delivered to their home. *Participants failed to return a signed consent form, did not perform and/or report test results, or performed the self-RADT later than 72 hours after their PCR test. Positive, negative, and inconclusive: self-RADT results.1 Six participants were included onsite during the offsite inclusion period and subsequently showed a positive PCR test result. However, in the data analysis, they were included in the offsite group that used the Hangzhou RADT. They are referred to as offsite-enrolled participants in the remainder of the article.2 One participant was enrolled onsite at the test center at the time of offsite inclusion and was due to a positive PCR test analyzed with the offsite-enrolled DNA Diagnostic participants. This participant is referred to as an offsite-enrolled participant in the remainder of the article. PCR, polymerase chain reaction; RADT, rapid antigen detection test.
The clinical and demographic participant characteristics are shown in Table 1 for the overall cohort and for the 2 self-RADT groups. The mean age of the participants was 42 years, ranging from 18 to 81 years, and 50.5% were female. Of the 827 participants, 119 (14.6%) had a health professional background. Most participants had undergone PCR testing when routine tests were required before entering work or educational institution (40.9%) or when a test was taken as a precautionary measure (22.8%).
Table 1.
Demographic and clinical characteristics of participants who performed a self-RADT at home
| Overall | Hangzhou | DNA Diagnostic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR negative | PCR positive | PCR negative | PCR positive | ||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | ||
| AgeN = 827 | |||||||||||
| 18–39 years | 407 | (49.2) | 176 | (52.4) | 32 | (47.8) | 184 | (47.3) | 15 | (42.9) | |
| 40–64 years | 359 | (43.4) | 133 | (39.6) | 30 | (44.8) | 179 | (46.0) | 17 | (48.6) | |
| >65 years | 61 | (7.4) | 27 | (8.0) | 5 | (7.5) | 26 | (6.7) | 3 | (8.6) | |
| SexN = 827 | |||||||||||
| Female | 418 | (50.5) | 166 | (49.4) | 35 | (52.2) | 197 | (50.6) | 20 | (57.1) | |
| Male | 409 | (49.5) | 170 | (51.6) | 32 | (47.8) | 192 | (49.4) | 15 | (42.9) | |
| Health professional backgroundN = 814 | 119 | (14.6) | 33 | (9.9) | 6 | (9.0) | 73 | (19.4) | 7 | (20.0) | |
| Vaccines receivedN = 824 | |||||||||||
| None | 786 | (95.4) | 314 | (93.7) | 63 | (94.0) | 376 | (97.2) | 33 | (94.3) | |
| 1 | 31 | (3.8) | 16 | (4.8) | 2 | (3.0) | 11 | (2.8) | 2 | (5.7) | |
| 2 | 7 | (0.9) | 5 | (1.5) | 2 | (3.0) | 0 | 0 | |||
| Symptoms on PCR test dayN = 825 | 140 | (17.0) | 22 | (6.6) | 43 | (64.2) | 54 | (14.0) | 21 | (60.0) | |
| Symptom duration on PCR test dayN = 113 | |||||||||||
| 0–2 days | 83 | (73.5) | 8 | (57.0) | 35 | (81.4) | 25 | (71.4) | 15 | (71.4) | |
| 3–7 days | 24 | (21.2) | 4 | (28.7) | 7 | (16.3) | 7 | (20.0) | 6 | (28.6) | |
| >8 days | 6 | (5.3) | 2 | (14.3) | 1 | (2.3) | 3 | (8.6) | 0 | ||
| Specific symptoms on PCR test dayN = 139 | |||||||||||
| Cough | 53 | (38.1) | 2 | (9.1) | 26 | (60.5) | 14 | (26.4) | 11 | (52.4) | |
| Fever | 45 | (32.4) | 4 | (18.2) | 21 | (48.8) | 8 | (15.1) | 12 | (57.1) | |
| Unusual fatigue | 42 | (30.2) | 2 | (9.1) | 24 | (55.8) | 9 | (17.0) | 7 | (33.3) | |
| Headache | 53 | (38.1) | 6 | (27.3) | 22 | (51.2) | 9 | (17.0) | 16 | (76.2) | |
| Sore throat | 66 | (47.5) | 10 | (45.5) | 18 | (41.9) | 28 | (52.8) | 10 | (47.6) | |
| Muscle pain | 37 | (26.6) | 1 | (4.5) | 20 | (46.5) | 5 | (9.4) | 11 | (52.4) | |
| Diarrhea or stomach pain | 16 | (11.5) | 0 | 7 | (16.3) | 5 | (9.4) | 4 | (19.0) | ||
| Decreased or missing sense of taste or smell | 7 | (0.05) | 0 | 4 | (9.3) | 0 | 3 | (14.3) | |||
| Breathing problems | 7 | (0.05) | 1 | (4.5) | 3 | (7.0) | 3 | (5.7) | 0 | ||
| Runny or stuffy nose | 44 | (31.7) | 9 | (40.9) | 11 | (25.6) | 15 | (28.3) | 9 | (42.9) | |
| Symptoms on self-RADT dayN = 102 | 75 | (73.5) | 50 | (74.6) | 25 | (71.4) | |||||
| Duration of symptoms on self-RADT dayN = 75 | |||||||||||
| 0–2 days | 35 | (46.7) | 25 | (50.0) | 10 | (40.0) | |||||
| 3–7 days | 37 | (49.3) | 23 | (46.0) | 14 | (56.0) | |||||
| >8 days | 3 | (0.0) | 2 | (4.0) | 1 | (4.0) | |||||
| Specific symptoms on self-RADT dayN = 75 | |||||||||||
| Cough | 35 | (46.7) | 25 | (50.0) | 10 | (40.0) | |||||
| Fever | 31 | (41.3) | 22 | (44.0) | 10 | (40.0) | |||||
| Unusual fatigue | 26 | (34.7) | 20 | (40.0) | 8 | (32.0) | |||||
| Headache | 35 | (46.7) | 24 | (48.0) | 12 | (48.0) | |||||
| Sore throat | 29 | (38.7) | 20 | (40.0) | 10 | (40.0) | |||||
| Muscle pain | 36 | (48.0) | 27 | (54.0) | 13 | (52.0) | |||||
| Diarrhea or stomach pain | 7 | (9.3) | 4 | (8.0) | 3 | (15.8) | |||||
| Decreased or missing sense of taste or smell | 12 | (16.0) | 8 | (16.0) | 4 | (16.0) | |||||
| Breathing problems | 6 | (8.0) | 6 | (12.0) | 0 | ||||||
| Runny or stuffy nose | 16 | (21.3) | 16 | (32.0) | 10 | (40.0) | |||||
| Time between PCR test and self-RADTN = 827 | |||||||||||
| 0–12 hours | 611 | (73.9) | 241 | (71.7) | 2 | (3.0) | 368 | (94.6) | 0 | ||
| 12–24 hours | 63 | (7.6) | 47 | (14.0) | 4 | (6.0) | 9 | (2.3) | 3 | (8.6) | |
| 24–48 hours | 106 | (12.8) | 29 | (8.6) | 49 | (73.1) | 8 | (2.1) | 20 | (57.1) | |
| >48 hours | 47 | (5.7) | 19 | (5.7) | 12 | (17.9) | 4 | (1.0) | 12 | (34.3) | |
| Reason for PCR testingN = 826 | |||||||||||
| Positive RADT result at another test center | 47 | (5.7) | 33 | (9.8) | 10 | (14.9) | 1 | (0.3) | 3 | (8.6) | |
| Displaying symptoms | 77 | (9.3) | 9 | (2.7) | 14 | (20.9) | 45 | (11.6) | 9 | (25.7) | |
| Close contact with infected person | 107 | (13.0) | 10 | (3.0) | 44 | (65.7) | 36 | (9.3) | 17 | (48.6) | |
| Message from COVID-19 app | 6 | (0.7) | 1 | (0.3) | 0 | 5 | (1.3) | 0 | |||
| Routine test before entering work or educational institution | 338 | (40.9) | 142 | (42.3) | 5 | (7.5) | 182 | (46.9) | 9 | (25.7) | |
| Before appointment at hospital, doctor, dentist, and others | 12 | (1.5) | 4 | (1.2) | 2 | (3.0) | 6 | (1.5) | 0 | ||
| Before visiting a vulnerable person | 77 | (9.3) | 33 | (9.8) | 0 | 44 | (11.3) | 0 | |||
| As a precaution | 188 | (22.8) | 107 | (31.8) | 4 | (6.0) | 74 | (19.1) | 3 | (8.6) | |
| Before traveling | 8 | (1.0) | 4 | (1.2) | 0 | 4 | (1.0) | 0 | |||
| Other causes | 25 | (3.0) | 19 | (5.7) | 1 | (1.5) | 5 | (1.3) | 0 | ||
PCR negative: a negative PCR test result; PCR positive: a positive PCR test result.
Abbreviations: PCR, polymerase chain reaction; RADT, rapid antigen detection test.
In the Aarhus municipality, PCR tests were performed for 11% to 16% of the population per week, at the time of execution of this study. The percentage of positive test results was low, ranging from 0.1% to 0.6%, which translated to an incidence of 12 to 63 per 100,000 inhabitants (Aarhus Kommune, 2021).
Comparison between self-RADTs and RT-PCR
In this study, a COVID-19 diagnosis was solely based on the result of a single positive PCR test result. The sensitivities for the DNA Diagnostic RADT and the Hangzhou RADT were similar, 65.7% (95% CI: 49.2–79.2) and 62.1% (95% CI: 50.1–72.9), respectively (Table 2 ).
Table 2.
Self-RADT performance
| Overall | TP | FN | FP | TN | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|---|---|---|
| Hangzhou | 401 | 41 | 25 | 0 | 335 | 62.1 (50.1–72.9) | 100 (98.9–100) |
| DNA Diagnostic | 423 | 23 | 12 | 0 | 388 | 65.7 (49.2–79.2) | 100 (99.0–100) |
| Abbott | 388 | 0 | 0 | 1 | 387 | Not estimable | 100 (95.6–100) |
Six RADTs (n = 1 DNA Diagnostic, n = 2 Hangzhou, and n = 3 Abbott) were not included in the sensitivity and specificity calculations. RADT results that differed from the PCR test results were considered FP or FN; concurrences between the 2 tests were considered TP or TN.
Abbreviations: CI, confidence interval; FN, false negative; FP, false positive; RADT, rapid antigen detection test; TN, true negative; TP, true positive.
No participants enrolled onsite that underwent the professionally administrated Abbott RADT had a positive PCR test result; hence, the sensitivity could not be estimated. Only 1 presumable false positive test was detected in this study, which resulted in a high specificity for all 3 types of RADTs examined. Six tests had no control line and were considered inconclusive.
When participants were stratified into symptomatic and asymptomatic groups, the sensitivities tended to be higher in the symptomatic group than in the asymptomatic group (Table 3 ). For the symptomatic group, the test sensitivities were 76.0% (95% CI: 56.6–88.5) for the DNA Diagnostic RADT, and 66.7% (95% CI: 57.3–83.3) for the Hangzhou RADT, and for the asymptomatic group, the test sensitivities were 40.0% (95% CI: 16.8–68.7) for the DNA Diagnostic RADT and 43.8% (95% CI: 24.5–61.2) for the Hangzhou RADT.
Table 3.
Sensitivities of self-RADTs in participants with positive PCR test results that were symptomatic or asymptomatic at the time of self-testing
| Hangzhousensitivity (95% CI) | DNA Diagnosticsensitivity (95% CI) | |
|---|---|---|
| Overall | 62.1 (50.1–72.9) | 65.7 (49.2–79.2) |
| Symptomatic | 66.7 (57.3–83.3) | 76.0 (56.6–88.5) |
| Asymptomatic | 43.8 (24.5–61.2) | 40.0 (16.8–68.7) |
Abbreviation: CI, confidence interval; PCR, polymerase chain reaction; RADT, rapid antigen detection test.
User acceptability and safety
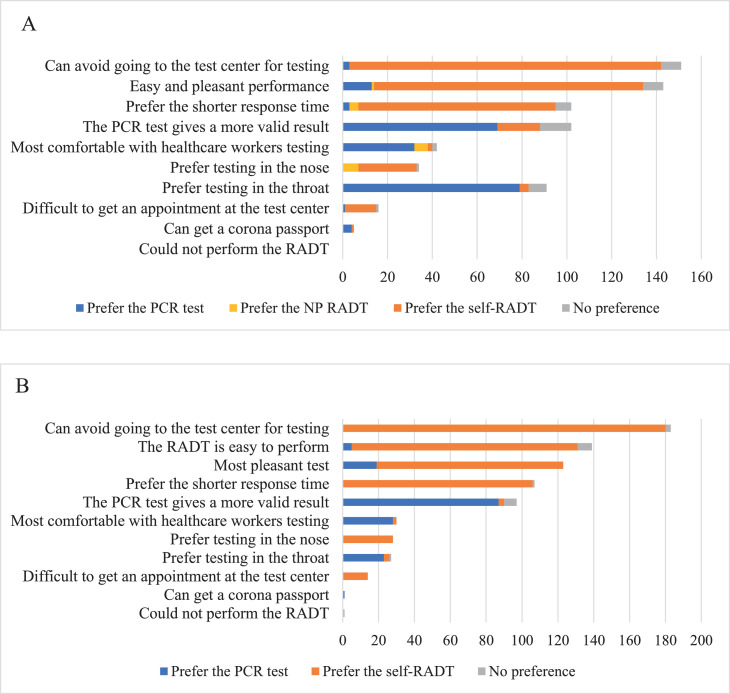
Of the 388 participants who underwent the PCR test, the nasopharyngeal RADT, and the self-RADT, 222 (57.2%) preferred the self-RADT; 128 (33.0%) preferred the PCR test; 9 (2.3%) preferred the nasopharyngeal RADT, and 29 (7.5%) had no test preference. Of the 439 participants who underwent the PCR test and the self-RADT, 280 (63.8%) preferred the self-RADT; 124 (28.2%) preferred the PCR test, and 35 (8.0%) had no test preference.
The main reason that the self-RADT was preferred was that it obviated a trip to the test center for testing (Figure 2 ). Furthermore, participants thought that the self-RADT was the most pleasant test, favored the shorter response time, and found self-RADT easy to perform.
Figure 2.
SARS-CoV-2 test preferences and rationales. A) Participants were first tested with a standard PCR test, then a professional nasopharyngeal (NP) RADT, and later, they performed a self-RADT at home. B) Participants were tested with a standard PCR test, and later, they performed a self-RADT at home. No preference indicates none preferred or more than 1 preferred test. PCR, polymerase chain reaction; RADT, rapid antigen detection test.
Participants who preferred the PCR test argued that the PCR test provided the most valid result and that throat sampling was more comfortable than nose sampling. Some participants mentioned that they felt more comfortable with a health care worker performing the test.
Among the 355 participants interviewed about safety issues, 12 (3.4%) reported nose bleeding. One participant had to interrupt the self-test because of nose bleeding, but no medical help was required. No other safety problems were reported.
Discussion
Findings
This study found that 2 anterior nasal self-RADTs had sensitivities of 65.7 % and 62.1%, and specificities of 100%, compared with PCR test results, among all participants, regardless of symptoms. Among individuals with symptoms, the sensitivities appeared higher than among individuals without symptoms for both self-RADTs, compared with the reference PCR test. Nevertheless, 2 of every 3 participants preferred the self-performed RADT to the PCR test and the professionally administered nasopharyngeal RADT.
Study strengths and weaknesses
The main study strength was that the participants were highly representative of the intended target populations for RADTs. Therefore, the study results reflected what could be achieved in a routine setting at an educational institution or at work, without preselecting a specific population, such as symptomatic individuals. Another strength of this study was the large number of participants enrolled and in particular, the large number of participants with positive PCR test results.
One of the major weaknesses of the study was the inaccurate nature of self-reporting. The sensitivities may have increased with a professional read-out of the lateral flow RADT device. Furthermore, the participants were not observed performing the self-sampling procedure. A study examining self-sampling made by Gertler et al associated sampling procedure mistakes with false negative results (Gertler et al., 2021). To which extent the participants followed the instructions is unknown, and inaccurate performance of the self-sampling may have caused false negative results in this study. In contrast, our study aimed to assess the effectiveness of self-tests, which entailed a real-life situation, including self-assessments of test results. Another limitation of this study was the time interval (1–3 days) between the PCR test and the self-RADT for participants enrolled offsite (Table 1). Time delays may have reduced the sensitivity of the antigen tests because the viral load might have decreased since the timepoint of PCR testing. The viral load is estimated to be highest 2 to 3 days after symptom onset; however, viral antigens can be detected with PCR testing several weeks after the initial infection (Ejima et al, 2021; Mallett et al, 2020). At the time of the PCR testing, 50 participants had had symptom onset within 2 days, and at the time of the self-RADT performance, 35 participants had had symptom onset within 2 days (Table 1).
Comparisons to other studies
Our findings support the findings from previous studies, which showed no significant difference in diagnostic performance between samples collected by health care workers and those collected by participants. The sensitivities of self-RADTs observed in other studies ranged from 49 to 96%, and the specificities ranged from 82 to 100% (Callahan et al, 2021; Tsang et al, 2021). The lowest sensitivity (49%) was observed among individuals with low viral loads. In the same study, the sensitivity was 80% for individuals with high viral loads (Callahan et al., 2021). In the current study, viral load measurements were not available, but that could have been valuable information for evaluations and comparisons with the self-RADT results.
The World Health Organization (WHO) performance criterion for RADTs is a minimum sensitivity performance of >80% (World Health Organization, 2020). Other studies on self-RADTs that observed sensitivities above that level primarily included participants with COVID-19 symptoms or participants who had been in close contact with patients with COVID-19 (Hanson et al, 2020; Klein et al, 2021; Kojima et al, 2020; McCulloch et al, 2020; Osmanodja et al, 2021; Tu et al, 2020). A meta-study of RADTs that differentiated between participants with and without symptoms found RADT sensitivities of 72.0% in symptomatic patients, and 58.1% in asymptomatic patients (Dinnes J et al, 2021). Those findings were comparable to the results obtained in the current study, with estimated sensitivities for asymptomatic participants of 43.8% and 40.0% for the Hangzhou RADT and the DNA Diagnostic RADT, respectively. However, it is debatable if these sensitivities are acceptable for the target population for whom RADTs are recommended.
In a study similar to the current study, Lindner and colleagues found a sensitivity of 82.5%, which was comparable to the sensitivities calculated in this study (Lindner et al, 2021b).
In this study, the self-RADT results were compared with results from RT-PCR analyses of oropharyngeal swab samples. However, in most other studies, RADTs were compared with RT-PCR analyses of nasopharyngeal or combined oropharyngeal/nasopharyngeal swab samples, which are more sensitive tests (Callahan et al, 2021; Klein et al, 2021; Kojima et al, 2020; Lindner et al, 2021a, 2021b; McCulloch et al, 2020; Osmanodja et al, 2021; Tsang et al, 2021; Tu et al, 2020). Thus, we might have overestimated the sensitivity of RADTs, because our reference test had a lower sensitivity than the reference tests used in other studies.
Interpretation of the study
In this study, we evaluated 2 types of self-RADTs for analyzing anterior nasal swab samples. Among all symptomatic and asymptomatic participants, the self-RADT sensitivities were 65.7% and 62.1%, and their specificities were 100% for both, compared with PCR testing.
In Denmark, several RADTs are currently available and approved for use as a self-RADT, under supervision, at schools and other educational institutions (Lægemiddelstyrelsen, 2021). The WHO has recommended that RADTs should meet the minimum performance of >80% sensitivity and 97% to 100% specificity (World Health Organization, 2020). However, recent studies have argued that the testing frequency may be more important than test sensitivity for detecting SARS-CoV-2 (Larremore et al, 2021; Paltiel et al, 2021). A modeling study suggested that rapid testing and contact tracing are important factors in stopping virus transmission (Kretzschmar et al, 2020). Compared with the PCR test, a self-RADT would significantly reduce the time delay between the test performance and an available result. Additionally, self-RADTs are cheap and easy to up- and downscale to meet the actual testing needs. These observations support the relevance of implementing self-RADTs as a supplement to professionally administered RADTs and PCR tests. Nevertheless, sufficient information should be provided to minimize the sense of false security among the individuals tested falsely negative with the RADTs.
Unanswered questions
Currently, no SARS-CoV-2 vaccine has been approved for small children. Thus, we may need to continue testing children for SARS-CoV-2. More studies are warranted that focus on self-tests or parental-administered tests for children.
Further work is required to obtain more precise estimates of the sensitivities and specificities of the RADTs examined in this study. Future studies should investigate populations with higher COVID-19 incidences than the population studied here. In addition, user acceptability of self-RADTs should be surveyed in the populations for which they are intended. More studies on self-RADT implementation are urgently needed.
Conclusion
This study has contributed new knowledge to our understanding of user feasibility and acceptability of self-RADTs among laypeople. The 2 RADTs evaluated tended to have higher sensitivities among symptomatic participants than among asymptomatic participants. Two thirds of our participants preferred the self-RADT over the PCR test or a professionally administered nasopharyngeal RADT. In conclusion, this study showed that self-RADTs for analyzing nasal swab samples was a reliable, user-acceptable, safe complementary test to PCR analyses of professionally collected oropharyngeal swab samples.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
We thank all members of the staff at the test center of the Aarhus University Hospital for their help in testing and recruiting participants throughout this study.
Author contributions
L.Ø. and S.J. conceived the project. S.J., I.M., and L.Ø. planned the study. I.M., A.U., and U.R. collected the data. I.M., S.J., and A.U. analyzed the data. I.M. and S.J. drafted the manuscript. I.M. and A. U. designed the figures. I.M., S.J., and L.Ø. interpreted the results. All authors were involved in critically revising the manuscript, and all approved the final version.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- Aarhus Kommune. Available from: https://ledelsesinformation.aarhuskommune.dk/aarhus-i-tal/default.aspx?doc=vfs://Global/AARHUS-I-TAL/CORONA_I_TAL-Sundhedsindsats-Tests.xview (Accessed 9 June 2021).
- Callahan C, Lee RA, Lee GR, Zulauf K, Kirby JE, Arnaout R. Nasal Swab Performance by Collection Timing, Procedure, and Method of Transport for Patients with SARS-CoV-2. Journal of Clinical Microbiology. 2021;59 doi: 10.1128/JCM.00569-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Haage VC, Bleicker T, Schmidt ML, Mühlemann B, Zuchowski M, et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. The Lancet Microbe. 2021:e311–e319. doi: 10.1016/S2666-5247(21)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. John Wiley & Sons, Ltd; 2021. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima K, Kim KS, Ludema C, Bento AI, Iwanami S, Fujita Y, et al. Estimation of the incubation period of COVID-19 using viral load data. Epidemics. 2021;35 doi: 10.1016/j.epidem.2021.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control European Centre for Disease Prevention and Control Considerations on the use of self-tests for COVID-19 in the EU/EEA. 2021 n.d.:21 Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Considerations-for-the-use-of-self-tests-for-COVID-19-in-the-EU-EEA.pdf. (Accessed 25 May 2021).
- Foundation for Innovative New Diagnostics Foundation for Innovative New Diagnostics SARS-CoV-2 diagnostic pipeline, 2021. FIND Available from: https://www.finddx.org/covid-19/pipeline/(Accessed 15 June 2021).
- Gertler M, Krause E, van Loon W, Krug N, Kausch F, Rohardt C, et al. Self-collected oral, nasal and saliva samples yield sensitivity comparable to professionally collected oro-nasopharyngeal swabs in SARS-CoV-2 diagnosis among symptomatic outpatients. International Journal of Infectious Diseases. 2021;110:261–266. doi: 10.1016/j.ijid.2021.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KE, Barker AP, Hillyard DR, Gilmore N, Barrett JW, Orlandi RR, et al. Self-Collected Anterior Nasal and Saliva Specimens versus Health Care Worker-Collected Nasopharyngeal Swabs for the Molecular Detection of SARS-CoV-2. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01824-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JAF, Krüger LJ, Tobian F, Gaeddert M, Lainati F, Schnitzler P, et al. Head-to-head performance comparison of self-collected nasal versus professional-collected nasopharyngeal swab for a WHO-listed SARS-CoV-2 antigen-detecting rapid diagnostic test. Med Microbiol Immunol. 2021 doi: 10.1007/s00430-021-00710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Turner F, Slepnev V, Bacelar A, Deming L, Kodeboyina S, et al. Self-Collected Oral Fluid and Nasal Swab Specimens Demonstrate Comparable Sensitivity to Clinician-Collected Nasopharyngeal Swab Specimens for the Detection of SARS-CoV-2. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar ME, Rozhnova G, Bootsma MCJ, van Boven M, van de Wijgert JHHM, Bonten MJM. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. The Lancet Public Health. 2020;5:e452–e459. doi: 10.1016/S2468-2667(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger LJ, Gaeddert M, Köppel L, Brümmer LE, Gottschalk C, Miranda IB, et al. Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. Infectious Diseases (except HIV/AIDS) 2020 [Google Scholar]
- Lægemiddelstyrelsen. COVID-19 antigen test til selvtest. Lægemiddelstyrelsen. Available from: https://laegemiddelstyrelsen.dk/da/udstyr/covid-19-antigentest-og-ivd/covid-19-antigen-test-til-selvtest/ (Accessed 25 May 2021).
- Larremore DB, Wilder B, Lester E, Shehata S, Burke JM, Hay JA, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7:eabd5393. doi: 10.1126/sciadv.abd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner AK, Nikolai O, Kausch F, Wintel M, Hommes F, Gertler M, et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur Respir J. 2021;57 doi: 10.1183/13993003.03961-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner AK, Nikolai O, Rohardt C, Kausch F, Wintel M, Gertler M, et al. Diagnostic accuracy and feasibility of patient self-testing with a SARS-CoV-2 antigen-detecting rapid test. Journal of Clinical Virology. 2021;141 doi: 10.1016/j.jcv.2021.104874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallett S, Allen AJ, Graziadio S, Taylor SA, Sakai NS, Green K, et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020;18:346. doi: 10.1186/s12916-020-01810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch DJ, Kim AE, Wilcox NC, Logue JK, Greninger AL, Englund JA, et al. Comparison of Unsupervised Home Self-collected Midnasal Swabs With Clinician-Collected Nasopharyngeal Swabs for Detection of SARS-CoV-2 Infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmanodja B, Budde K, Zickler D, Naik MG, Hofmann J, Gertler M, et al. Accuracy of a Novel SARS-CoV-2 Antigen-Detecting Rapid Diagnostic Test from Standardized Self-Collected Anterior Nasal Swabs. JCM. 2021;10:2099. doi: 10.3390/jcm10102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltiel AD, Zheng A, Sax PE. Clinical and Economic Impact of Widespread Rapid Testing to Decrease SARS-CoV-2 Transmission. Infectious Diseases (except HIV/AIDS) 2021 doi: 10.7326/M21-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statens Serum Institut. 2021. PCR-test Available from: https://covid19.ssi.dk/diagnostik/pcr-test (Accessed 25 April 2021).
- Sundhedsstyrelsen. 2021. Hvornår du skal testes. Available from: https://www.sst.dk/da/corona/hvis-du-har-symptomer_-er-syg-eller-smittet/hvornaar-du-skal-testes (Accessed 22 June 2021).
- Tsang NNY, So HC, Ng KY, Cowling BJ, Leung GM, Ip DKM. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2021;21(9):1233–1245. doi: 10.1016/S1473-3099(21)00146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y-P, Jennings R, Hart B, Cangelosi GA, Wood RC, Wehber K, et al. Swabs Collected by Patients or Health Care Workers for SARS-CoV-2 Testing. N Engl J Med. 2020;383:494–496. doi: 10.1056/NEJMc2016321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2020. WHO-2019-nCoV-Antigen_Detection-2020.1-eng.pdf. Available from: https://apps.who.int/iris/bitstream/handle/10665/334253/WHO-2019-nCoV-Antigen_Detection-2020.1-eng.pdf?isAllowed=y&sequence=1. (Accessed 22 June 2021).