Key Points
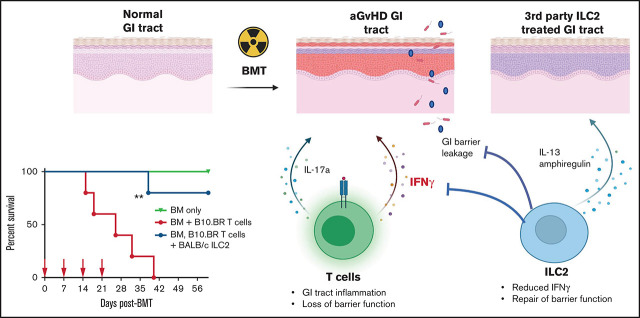
Weekly infusions of third-party ILC2s prevent, and to a lesser extent, treat GVHD via production of IL-13 and amphiregulin.
ILC2-derived IL-13 targets both host cells and the donor hematopoietic cells.
Visual Abstract
Abstract
Acute graft-versus-host disease (aGVHD), mediated by the recognition of host major histocompatibility complex/peptide polymorphisms by donor T cells, remains a significant complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT). aGVHD most commonly involves the gastrointestinal tract, liver, and skin; symptomatic aGVHD is treated with corticosteroids. Steroid-nonresponsive aGVHD is a significant problem for patients undergoing allo-HSCT, with <15% of these patients alive 1 year after diagnosis. Previously, we found that the infusion of donor innate lymphoid type 2 (ILC2) cells could prevent and treat aGVHD of the lower gastrointestinal tract with no effect on the graft-versus-leukemia response. This approach for clinical translation is cumbersome, as it would require the generation of donor-derived ILC2 cells for each recipient. Thus, the ability to use third-party ILC2 cells would provide an “off-the-shelf” reagent that could be used to treat and/or prevent aGVHD. Here, we show that third-party ILC2 cells enhance the survival of allo-HSCT recipients. Treatment required at least 4 weekly infusions of ILC2 cells. Mechanistically, we show that ILC2 cell function was completely lost if the cells could not express both interleukin-13 (IL-13) and amphiregulin. Finally, we show that the activity of IL-13 has a greater dependence on the expression of the IL-13R on host rather than donor bone marrow cells. The ability to generate third-party ILC2 cells offers a new avenue for the prevention of aGVHD.
Introduction
Acute graft-versus-host disease (aGVHD) is a significant cause of morbidity and mortality in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) for the treatment of high-risk leukemia and lymphoid malignancies.1,2 The incidence of aGVHD ranges from 10% to 80% depending on a number of variables, including the degree of HLA mismatch, age, and nature of GVHD prophylaxis.3 Although steroids are the first-line treatment of aGVHD, 30% to 50% of patients with aGVHD become steroid refractory4 or cannot be weaned from corticosteroid therapy.
More than 50 years ago, it was discovered that donor-derived T cells are critical to the pathogenesis of aGVHD; however, the mechanism(s) involved in tissue pathology during aGVHD are still not completely understood.5 In murine models of GVHD, CD4+ T cells induce inflammation by either producing effector proteins such as tumor necrosis factor or by producing pro-inflammatory cytokines such as interferon-γ (IFN-γ) and/or interleukin-12 (IL-2), which mediate allo-specific CD8+ T-cell proliferation.6 More recent research has shown that allo-reactive T helper 1 (Th1) and Th17 CD4+ T cells and their cytokines IFN-γ and IL-17A, respectively, are linked to acute lethal GVHD of the gastrointestinal (GI) tract.7 Almost 20 years ago, Krenger et al showed that shifting the donor T-cell phenotype from Th1 to Th2 (IFN-γ and IL-4 producing, respectively) could be beneficial during allo-HSCT. In addition, use of Th2/Tc2 cells in mixed lymphocyte reactions leads to reduced inflammatory cytokine production.8 In a murine model of allo-HSCT, injection of in vitro polarized Th2 and Tc2 cells at the time of transplantation attenuated aGVHD and reduced the production of tumor necrosis factor.
Over the past 2 decades, the critical role that immunosuppressive populations of immune cells have on the pathogenesis of aGVHD has become increasingly clear. Previous studies, including work from our group, have shown that the coinfusion of CD4+ FoxP3-expressing regulatory T cells (Tregs) suppresses aGVHD. These studies led to clinical trials of Treg infusions in humans; however, the infusion of donor Tregs is complicated by their lack of activity when given post–allo-HSCT.6 Our group has shown that bone marrow (BM) myeloid-derived suppressor cells (MDSCs) activated with IL-13 inhibited GVHD lethality in an arginase-1–dependent manner.9 However, similar to Tregs, MDSCs are not effective in treating aGVHD if administered after allo-HSCT unless inflammasome proteins that convert MDSCs to conventional APCs are inhibited.10 Thus, the development of a cellular therapy that could effectively treat aGVHD would be a significant advance.
More recently, a role for innate lymphoid cells (ILCs) in the biology of aGVHD has been shown. ILCs have been classified into ILC1, ILC2, and ILC3 subsets and, unlike T cells, lack the ability to rearrange germ line antigen receptors. ILC1 and natural killer cells generate cytokines and express tbx21 similar to conventional CD8+ T cells. ILC3 cells have been categorized into subsets based on their expression of natural killer–like receptors and generally secrete cytokines similar to Th17/22 cells. ILC2 cells express cytokines such as Th2 cells under the control of the transcription factors GATA3, cMAF, and RORα.11,12
Several groups, including ours, have described a role for ILCs in mitigating the pathogenesis of aGVHD. In humans, there is an increased risk of aGVHD in patients with reduced circulating CD69+ ILC2 and ILC3 cells.13 In murine models, ILC3 cells have been shown to enhance GI tract repair after radiation through production of IL-22.14 We recently showed that infusion of donor-matched ILC2 cells increased survival, lowered clinical scores, and reduced activation of donor Th1 and Th17 donor T cells.15 ILC2s also improved intestinal barrier function through production of amphiregulin (AREG) and increased MDSC numbers in the GI tract through production of IL-13. However, it was unclear if ILC2 cells were affecting T cells through direct major histocompatibility complex (MHC) interactions, which would require matching of MHC between donor ILC2 cells and the recipient.
The generation of donor ILC2s is cumbersome, requiring either a prolonged period of time to collect and expand the cells or the generation of products before the onset of GVHD, with a significant number of these products never being used clinically. Thus, the ability to use third-party ILCs that could be given “off the shelf” would be a significant benefit. Here we show that complete mismatched third-party ILC2s can suppress aGVHD and enhance survival. Although third-party ILC2 cells do not persist in GVHD target tissues, multiple infusions increase survival, with up to 80% survival when given prophylactically and ∼40% survival when given therapeutically. These studies provide the foundation for the development of third-party ILC reagents to be used in early-phase clinical trials to treat patients with steroid-nonresponsive lower GI tract aGVHD.
Materials and methods
Mice
C57BL/6J (termed B6), BALB/cJ, B10.BR, and C57BL/6J × DBA/2 F1 (termed B6D2) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The generation of enhanced green fluorescent protein–expressing C57BL/6 mice has been described previously.16 C57BL/6 IL-13Ra1−/− mice have been described elsewhere.17 Donor and recipient mice were age-matched male and female mice between 8 and 16 weeks old. All experiments were performed in accordance with protocols approved by the University of North Carolina Institutional Animal and Care Use Committee.
Isolation and expansion of murine ILC2 cells
Ten- to 12-week-old B10.BR and BALB/c mice were given 0.4 µg of recombinant mouse IL-17E/IL-25 (R&D Systems, Minneapolis, MN) for 4 days. On day 5, cells were isolated from mesenteric lymph nodes and peritoneum by peritoneal lavage using RPMI with 10% fetal bovine serum (complete media). ILC2s were isolated by negative selection with a MACs column using biotinylated antibodies [anti-CD8α (53-6.7), anti-CD4 (RM 4.4), anti-CD3ε (145-2C11), anti-γδTCR (UC7-13DS), anti-TER119 (TER-119), anti-B220 (RA3-6B2), anti-CD11b (M1/70), and anti-NK1.1 (PK136) (all; eBioscience, San Diego, CA); anti-CD11c (N418), anti-CD19 (MB19-1), anti-Ly6G (1A8), and anti-CD49b (DX5) (all; BioLegend, San Diego, CA); and Streptavidin Microbeads (130-048-101; Miltenyi Biotec, Cambridge, MA)]. Cells were cultured for 6 days in complete media with recombinant IL-7 and recombinant IL-33 (10 ng/mL) (PeproTech, Rocky Hill, NJ), changing the media every 2 days. ILC2 activation was evaluated by using flow cytometry on day 6 by intracellular cytokine staining of lineage-specific markers, ST2, KLRG1, and expression of IL-13. For steroid response assays, dexamethasone (10 nM) (D4902; MilliporeSigma, Burlington, MA) was added to ILC2 cultures on day 4 and evaluated 48 hours later.
Transplantation models
Total T cells were isolated by using a Cedarlane T-cell recovery column kit (Cedarlane Laboratories, Burlington, NC), followed by antibody depletion using phycoerythrin-conjugated anti-mouse B220 (RA3-B62) and anti-mouse CD25 (3C7) antibodies (eBioscience) and magnetic bead selection using anti-phycoerythrin beads (130-0480801; Miltenyi Biotec). T cell–depleted (TCD) BM was prepared as described previously.18 The day before transplantation, recipient B6D2 and B6 mice were given 950 cGy of total body irradiation. For the B6 into B6D2 or B10.BR into B6 transplants, recipients were intravenously injected with either 4 × 106 T cells and 3 × 106 TCD BM cells, or 1 × 106 total T cells and 4 × 106 TCD BM cells, respectively. For ILC2 treatment groups, B6D2 or B6 recipients also received 4 × 106 or 1 × 106 CD90+ ILC2s, respectively. Recipients were monitored twice a week and scored for clinical GVHD symptoms (designated as “Clinical Score”) by using a semi-quantitative scoring system as previously described19,20; animals were coded for these evaluations. For all experiments, a control group received TCD BM alone without additional T cells, which controlled for the presence of T cells in the marrow inoculum and potential infectious complications during aplasia.
Cell isolation from GVHD target organs
Lung and colon were excised and weighed. Lamina propria (LP) lymphocytes were isolated by using the Miltenyi Biotec LP dissociation kit (130-097-410) as per the manufacturer’s instructions. Lymphocytes from the lungs were isolated by using the Miltenyi Biotec Lung Dissociation kit (130-095-927). Digested tissues were treated with ACK lysis buffer to remove red blood cells and were passed through 100-µm pore size cell strainers. Leukocytes were collected at the interface of a 40%/80% Percoll (MilliporeSigma) gradient in RPMI 1640/5% newborn calf serum. The pelleted cells were washed in phosphate-buffered saline/2% fetal bovine serum.
Flow cytometry analysis
ILC2s were evaluated by flow cytometry for linage markers (88-7772), CD127 (A7R34), H-2kk (Af3-12.1), T1/ST2 (RMST2-2), CD25 (PC61.5), and KLRG1 (2F1). For steroid survival assays, cultures were stained with Fixable Aqua LIVE/DEAD dye (Invitrogen, Carlsbad, CA). T cells were evaluated by surface staining of H-2Kd (34-1-2S), CD4 (GK1.5), and CD8 (53-6.7), and intracellular staining for IFN-γ (XMG1.2), IL-17 (ebio17B7), and IL-4 (11B11). Sample acquisition was performed by using a BD FACS CANTO II or BD LSR Fortessa (BD Biosciences, East Rutherford, NJ).
Fluorescein isothiocyanate–dextran permeability assay
Intestinal permeability was assessed by luminal enteral administration of fluorescein isothiocyanate (FITC)-dextran 4000 (MilliporeSigma), a non-metabolizable macromolecule that is used as a permeability probe. Mice were fasted for 4 hours before gavage with FITC-dextran (40 mg/100 g of body weight). After 4 hours, whole blood was collected by cardiac puncture, and FITC-dextran measurements were performed in triplicate by fluorimeter using a Synergy 2 microplate reader (BioTek, Winooski, VT). Dilutions of FITC-dextran in phosphate-buffered saline were used as a standard curve, and absorption of 50 µL of serum or standard was measured at 488 nm.
Statistical evaluations
Sample sizes were chosen for the effect size needed based on our previous experience of the number of samples required to show a significant difference in GVHD scoring between control and treated groups. Investigators were blinded to the clinical score and the pathological evaluations. For the scoring evaluation experiments, the inclusion of 9 to 12 recipients provided a power of 90% to detect a difference of 14 days in the median GVHD score of >5 with an α error of <0.05 between control and treated groups. Survival differences were evaluated by using a Mantel-Cox log-rank test. Survival curves were generated by using the Kaplan-Meier method. Differences in GVHD clinical and pathology scores were determined by using two-way analysis of variance, with Bonferroni correction for repeated measures of multiple comparisons. Unless otherwise noted in the figure legends, all other continuous variables were compared by using the two-tailed Student t test with Welch’s correction. A P value <0.05 was considered statistically significant.
Results
Third-party ILC2s reduce aGVHD and increase recipient survival
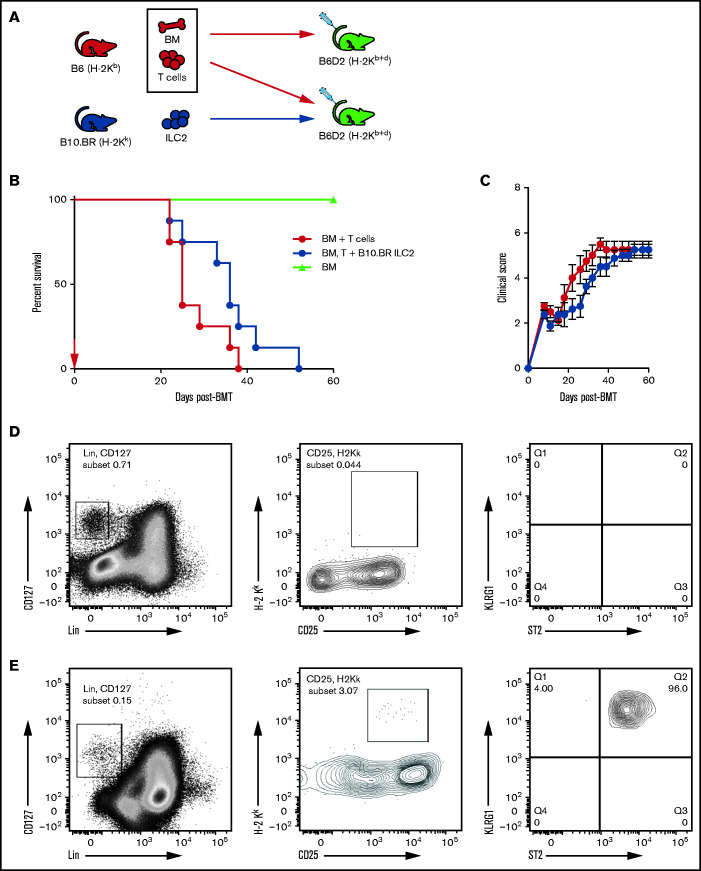
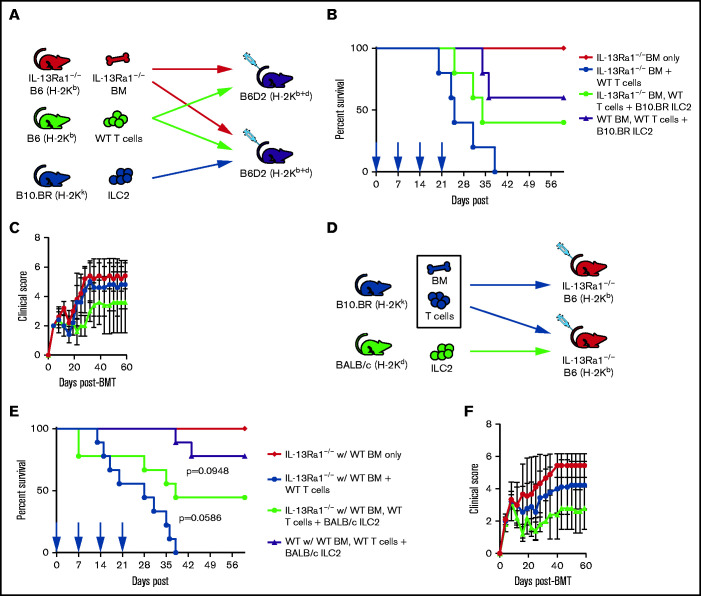
We have shown previously that a single infusion of donor-matched ILC2s significantly improved survival in a murine model of aGVHD without affecting the antitumor graft-versus-leukemia response.15 Here, we investigated the ability of third-party MHC-mismatched ILC2s to suppress aGVHD in a B6 into B6D2 haploidentical transplant model. Murine B10.BR ILC2 cells were expanded in vitro as previously described.15,21 Clinical grading of aGVHD as a surrogate for overall survival was performed in a murine model of allo-HSCT with coinfused ILC2s (Figure 1A). Cotransplantation of a single dose of third-party ILC2s significantly improved median survival of recipients (Figure 1B). However, there was no significant reduction in the clinical scores in mice given third-party ILC2s (Figure 1C). Considering this limited activity, we evaluated the persistence of third-party ILC2s after coinfusion. We previously showed that donor-matched ILC2s can be found in the GI tract and lung 21 days after transplant.15 However, third-party ILC2s were not detected in the lower GI tract as early as day 7 post–allo-HSCT (Figure 1D), and only small numbers were found in the lung at that time (Figure 1E). These findings show that a single infusion of third-party ILC2s can improve overall survival; however, they do not persist in GVHD target organs, indicating that additional treatments may be needed to significantly improve overall survival of recipients after transplant.
Figure 1.
Third-party ILC2s improve survival but do not persist. (A) Diagram for B6 into B6D2 transplantation. Each B6D2 recipient received B6 BM and B6 splenic T cells, with one group of B6D2 recipients also being given B10.BR ILC2s. Lethally irradiated B6D2 mice received TCD BM (BM only), BM plus total splenic T cells (BM + T cells), or BM plus T cells with activated B10.BR LC2s (BM, T cells + B10.BR ILC2). (B) Kaplan-Meier plot of survival following allo-HSCT; 1 representative of 2 experiments shown (n = 5 each experiment), log-rank (Mantel-Cox) test. (C) Clinical score posttransplantation, analyzed by using a two-way analysis of variance, with Bonferroni correction for repeated measures of multiple comparisons. Flow cytometry analysis of donor B10.BR (H-2Kk) ILC2 7 days’ posttransplant in the small intestine (D) and lung (E). BMT, BM transplantation.
Multiple injections of third-party ILC2s reduce aGVHD and increase survival
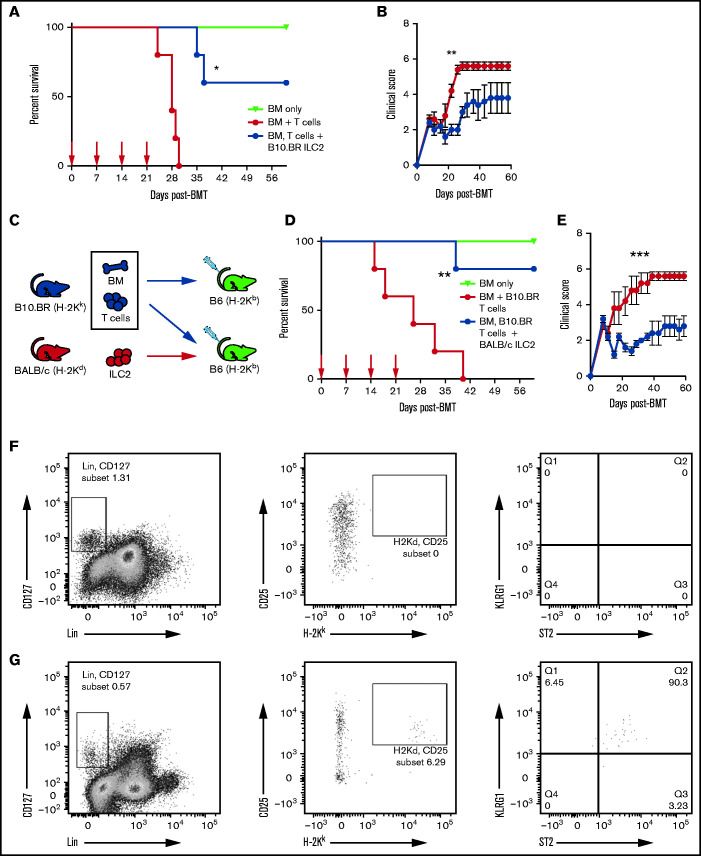
Given these findings, we investigated the ability of multiple infusions of third-party ILC2s to suppress aGVHD. Lethally irradiated recipients received expanded B10.BR ILC2s at the time of transplant with additional infusions of ILC2 cells at days 7, 14, and 21 (equal dose as day 0). Recipients were monitored for clinical score and survival. Consecutive weekly infusions significantly increased overall survival in both male (Figure 2A) and female (supplemental Figure 1A) recipients post–allo-HSCT, with 60% of the recipients surviving >60 days. In addition, multiple infusions significantly decreased clinical GVHD scores of recipients, with significant improvement found after day 20 in male recipients (Figure 2A) and day 25 in female recipients (supplemental Figure 1B).
Figure 2.
Multiple infusions of third-party ILC2s reduce aGVHD incidence. Lethally irradiated B6D2 mice received B6 TCD BM (BM only), B6 BM plus total splenic B6 T cells (BM + T cells), or BM plus T cells with activated ILC2s (BM, T cells + B10.BR ILC2). (A) Kaplan-Meier plot of male recipient survival following allo-HSCT; red arrows indicate time points for B10.BR ILC2 injections, 1 representative of 2 experiments shown (n = 5 each experiment), log-rank (Mantel-Cox) test. (B) Clinical score of recipients from panel A, analyzed by using a two-way analysis of variance, with Bonferroni correction for repeated measures of multiple comparisons. (C) Diagram for B10.BR into B6 transplantation. Each B6 recipient received WT B10.BR BM and B10.BR splenic T cells, with one group of B6 recipients also being given BALB/c ILC2s. Lethally irradiated B6 mice received TCD BM (BM only), BM plus total splenic T cells (BM + B10.BR T cells), or BM plus T cells with activated B10.BR ILC2s (BM, B10.BR T cells + BALB/c ILC2s). (D) Kaplan-Meier plot of survival after allo-HSCT; white arrows indicate time points for BALB/c ILC2 injections for those receiving infusions beginning at the time of transplant; 1 representative of 2 experiments shown (n = 5 each experiment), log-rank (Mantel-Cox) test. (E) Clinical score posttransplantation, analyzed by using a two-way analysis of variance, with Bonferroni correction for repeated measures of multiple comparisons. *P < .05, **P < .01, ***P < .001. Flow cytometry analysis of donor B10.BR (H-2Kk) ILC2 12 days’ posttransplant following ILC2 infusions on day 0 and day 7 in the small intestine (F) and lung (G).
We next established a model in which B6 mice could be used as transplant recipients, using completely MHC-mismatched B6 hosts (H-2Kb), B10.BR BM and T-cell donors (H-2Kk), and BALB/c ILC2 donors (H-2Kd) (Figure 2C). In a survival study, irradiated WT B6 mice received B10.BR BM and splenic T cells (BM + B10.BR T cells) with one group receiving expanded BALB/c ILC2s (BM, B10.BR T cells + BALB/c ILC2) (Figure 2D). In this complete MHC-mismatched study, survival was significantly improved, with 80% of recipients of ILC2s surviving (Figure 2D). Infusion of third-party ILC2s also significantly improved overall clinical scores (Figure 2E). Thus, multiple infusions of third-party ILC2s were able to significantly decrease the severity of aGVHD, presumably, by overcoming the limited persistence of these cells in vivo. In addition, these studies show that third-party ILC2 suppression of GVHD does not require MHC matching of donor T cells and ILC cells.
The increase in overall survival of B6 recipients of BALB/c ILC2s led us to hypothesize that BALB/c ILC2s may persist longer in GVHD target organs. To evaluate this hypothesis, we assessed for the presence of third-party ILC2 cells in the lung and GI tract on day 12 posttransplant. However, similar to data using B10.BR ILC2 shown in Figure 1D and E, we could not detect BALB/c ILC2 cells in the small intestine (Figure 2F) and only found small numbers in the lung (Figure 2G) on day 12 post–allo-HSCT after 2 infusions of BALB/c ILC2s.
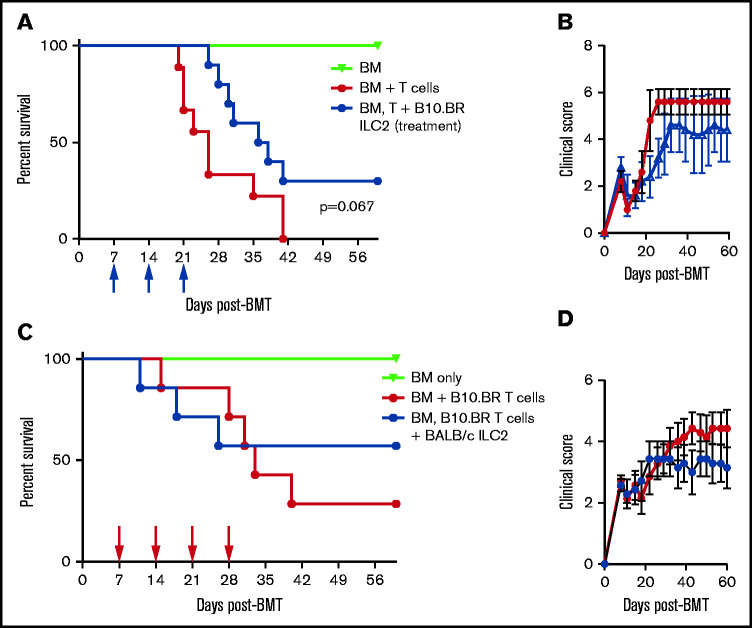
Treatment of aGVHD with multiple infusions of third-party ILC2s
Although prevention of aGVHD with ILC2 cells can be clinically beneficial, it would be more advantageous if third-party ILC2s could be used to treat active aGVHD. To evaluate their therapeutic benefit, B10.BR ILC2s were injected 7 days after transplant, a time when donor T cells are activated in GVHD target organs, with additional injections at days 14 and 21. Infusion of ILC2s as a treatment increased overall survival, with 40% of recipients surviving up to 60 days compared with 0% of control mice, with reduced clinical scores from day 20 to day 30 compared with untreated recipients (Figure 3A-B). However, this did not meet the prespecified definition of statistical significance (log-rank test, P = .067 ). Infusion of ILC2 cells was more effective when started on day 0 compared with treatment started on day 7 (Figure 2A and 3A). A similar effect was observed in B6 recipients of BALB/c ILC2 given therapeutically, with >50% of recipients surviving up to 60 days (Figure 3C-D), which also did not reach statistical significance. These findings show that multiple infusions of third-party ILC2 cells can improve the clinical score in mice after allo-HSCT. Survival was also improved, although it did not reach our predefined definition for statistical significance.
Figure 3.
Efficacy of third-party ILC2 cells when given therapeutically. (A) Kaplan-Meier plot of B6D2 recipient survival following allo-HSCT, blue arrows indicate time points for B10.BR ILC2 injections for those receiving infusions beginning 7 days posttransplant (Treatment), representative of 2 experiments shown (n = 5 each experiment). Log-rank (Mantel-Cox) test. (B) Clinical score of recipients from panel A, analyzed by 2-way analysis of variance with Bonferroni correction for repeated measures of multiple comparisons P < .05 difference clinical score on days 22-26. (C) Kaplan-Meier plot of B6 recipient survival following allo-HSCT with B10.BR BM and T cells, red arrows indicate time points for BALB/c ILC2 injections for those receiving infusions beginning 7-days posttransplant (Treatment), one representative of 2 experiments shown (n = 7 each experiment). Log-rank (Mantel-Cox) test. (D) Clinical score of recipients from panel C, analyzed by 2-way analysis of variance with Bonferroni correction for repeated measures of multiple comparisons.
ILC2 infusions lower GI tract T-cell load and reduce Th1 induction
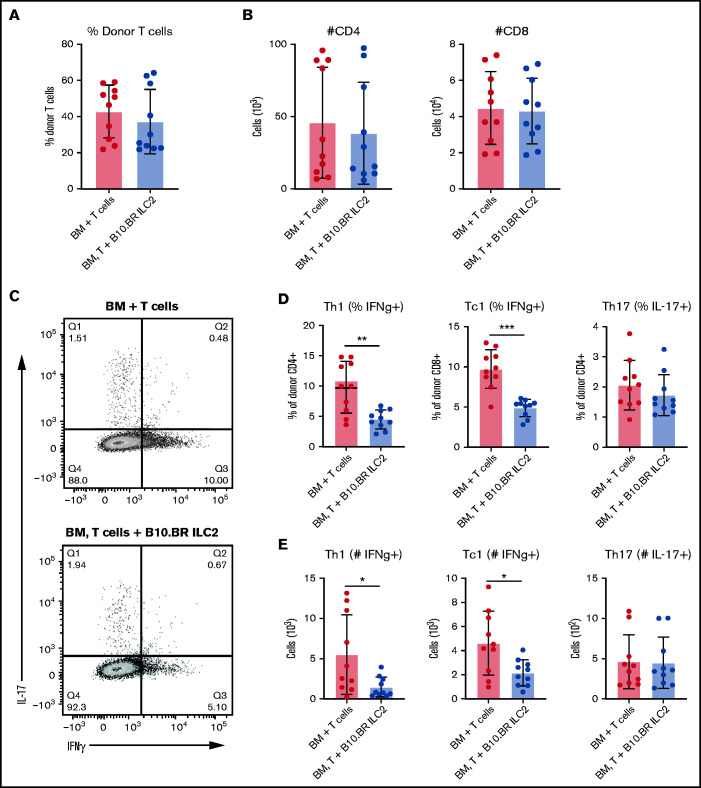
Next, we investigated the ability of third-party ILC2s to suppress donor T-cell activation in aGVHD target organs. B6 donor T-cell quantitation and activation status in the LP of the small bowel in recipient B6D2 mice was evaluated by using flow cytometry 12 days after transplant. Recipients of third-party ILC2s showed no significant difference in the number of total donor lymphocytes (Figure 4A). Interestingly, we also did not see a significant difference in the total number of donor CD4+ or CD8+ T cells (Figure 4B). However, there was a significant difference in the number and frequency of CD4+ and CD8+ T cells that expressed IFN-γ using intracellular cytokine staining (Figure 4C-E). Unlike our previous data using donor ILC2 cell infusions, no difference in IL-17–expressing donor T cells was found (Figure 4D). These data indicate that infusion of third-party ILC2s reduces the number of pro-inflammatory donor Th1 and Tc1 T cells in the LP of the small bowel.
Figure 4.
Cotransplantation of ILC2s reduces pro-inflammatory donor T-cell numbers in the GI tract. Donor T cells were evaluated in the GI tract 12 days’ posttransplant by using green fluorescent protein–positive splenic T cells alone (BM + T cells) or green fluorescent protein–positive T cells and B10.BR ILC2s (BM, T cells + B10.BR ILC2s). (A) Frequency of total LP lymphocytes. (B) Total number of donor CD4+ and CD8+ T cells in the colonic lamina propria lymphocytes. (C) Flow cytometry plots of intracellular cytokine expression from colon. Percentage (D) and total number (E) of IFN-γ producing donor CD4+ and CD8+ T cells and the number of donor CD4+ T cells producing IL-17A in the LP. These data represent 2 independent experiments (n = 5), Student’s t test with Welch’s correction; *P < .05, **P < .01, ***P < .001.
Suppressive effect of ILC2s in GVHD requires IL-13 receptor expression in donor BM as well as the host
We have previously shown that activated ILC2s express high levels of IL-13, and expression of IL-13 is a requirement for ILC2 suppression of aGVHD; we found that coinfusion of WT ILC2, but not IL-13–deficient ILC2s, significantly increased donor-derived MDSCs in GVHD target organs.15 It is unclear whether IL-13 from coinfused ILC2s directly affect the BM myeloid progenitors or alter the host microenvironment to skew myeloid progenitors toward MDSC development. To investigate the effect of IL-13 on donor BM myeloid progenitors, IL-13 receptor–deficient B6 mice (IL-13Ra1−/−) were used as BM donors in the model described in Figure 1A. Irradiated B6D2 mice received B6 IL-13Ra1−/− or WT BM and WT B6 splenic T cells (IL-13Ra1−/− BM + WT T cells), with one group receiving third-party B10.BR ILC2 (IL-13Ra1−/− BM, WT T cells + B10.BR ILC2) (Figure 5A). Infusion of third-party ILC2 cells improved survival, with 40% of recipients surviving through day 60 post–allo-HSCT (Figure 5B), which was not significantly different from the outcome of mice given wild-type (WT) BM and ILC2 cells (Figure 2A and 5C), indicating that donor expression of the IL-13 receptor is not critical to the function of ILC2 cells.
Figure 5.
ILC2s suppress aGVHD, in part, through IL-13Ra1 signaling. (A) Diagram for B6 into B6D2 transplantation. Each B6D2 recipient received IL-13Ra1−/− B6 BM and B6 splenic T cells from WT donors, with one group of B6D2 recipients also being given B10.BR ILC2s. Lethally irradiated B6D2 mice received TCD BM (IL-13Ra1−/− BM only), BM plus total splenic T cells (IL-13Ra1−/− BM + WT T cells), or BM plus T cells with activated B10.BR LC2s (IL-13Ra1−/− BM, WT T cells + B10.BR ILC2). (B) Kaplan-Meier plot of survival after allo-HSCT; blue arrows indicate time points for B10.BR ILC2 injections for those receiving infusions beginning at the time of transplant. One representative of 2 experiments shown (n = 5 each experiment), log-rank (Mantel-Cox) test. (C) Clinical score posttransplantation, analyzed by using two-way analysis of variance, with Bonferroni correction for repeated measures of multiple comparisons. (D) Diagram for B10.BR into IL-13Ra1−/− B6 transplantation. Each IL-13Ra1−/− B6 recipient received WT B10.BR BM and B10.BR splenic T cells, with one group of IL-13Ra1−/− B6 recipients also being given BALB/c ILC2s. Lethally irradiated IL-13Ra1−/− B6 mice received TCD BM (BM only), BM plus total splenic T cells (BM + B10.BR T cells), or BM plus T cells with activated BALB/c ILC2s (BM, B10.BR T cells + BALB/c ILC2s). (E) Kaplan-Meier plot of survival after allo-HSCT; red arrows indicate time points for BALB/c ILC2 injections for those receiving infusions beginning at the time of transplant. Representative of 2 experiments shown (n = 5 each experiment), log-rank (Mantel-Cox) test. (F) Clinical score posttransplantation, analyzed by using a two-way analysis of variance, with Bonferroni correction for repeated measures of multiple comparisons.
To investigate a role for the IL-13 axis in recipient tissue, we used B6 IL-13Ra1−/− mice as recipients in the aGVHD model described in Figure 2C. For these experiments, IL-13Ra1−/− recipients were given B10.BR BM and T cells, with one group receiving expanded BALB/c ILC2 cells (Figure 5D). There was a nonsignificant decrease in the efficacy of third-party ILC2 cells, resulting in decreased survival, with only 50% of IL-13Ra1−/− animals surviving beyond day 50 post–allo-HSCT (Figure 5E) compared with 80% when third-party ILC2 cells were given to WT recipients (P = .09) (Figure 2A). There was a nonsignificant difference in GVHD scores comparing WT with IL-13Rα1–deficient recipients (Figure 2A and 5F). These findings indicate that the function of third-party ILC2 cells to improve GVHD scores and overall survival is partially dependent on the ability of host cells to signal via the IL-13 receptor and less dependent on the expression of the IL-13 receptor by donor BM cells.
ILC2 cells mechanism of suppression is dependent on expression of IL-13 and AREG
ILC2 cells generate the EGF family member AREG, which enhances tissue repair.22 To further evaluate the mechanism behind ILC2 cell suppression of GVHD, we produced an IL-13/AREG double-deficient strain (IL-13/AREG DKO) and used donor ILC2 cells from these mice in a haploidentical transplant model (Figure 6A). We have previously shown that the loss of either IL-13 or AREG led to decreased survival, with ∼25% of the mice receiving IL-13 or AREG-deficient ILC2 cells surviving.15 However, IL-13/AREG DKO ILC2 cells completely lost the ability to suppress GVHD, with recipients showing no improvement in overall survival and no difference in clinical scores (Figure 6B-C), with all mice dying of GVHD by day 50. These findings indicate that the ability of ILC2 cells to prevent aGVHD is completely lost if the ILC2 cells cannot express IL-13 and AREG.
Figure 6.
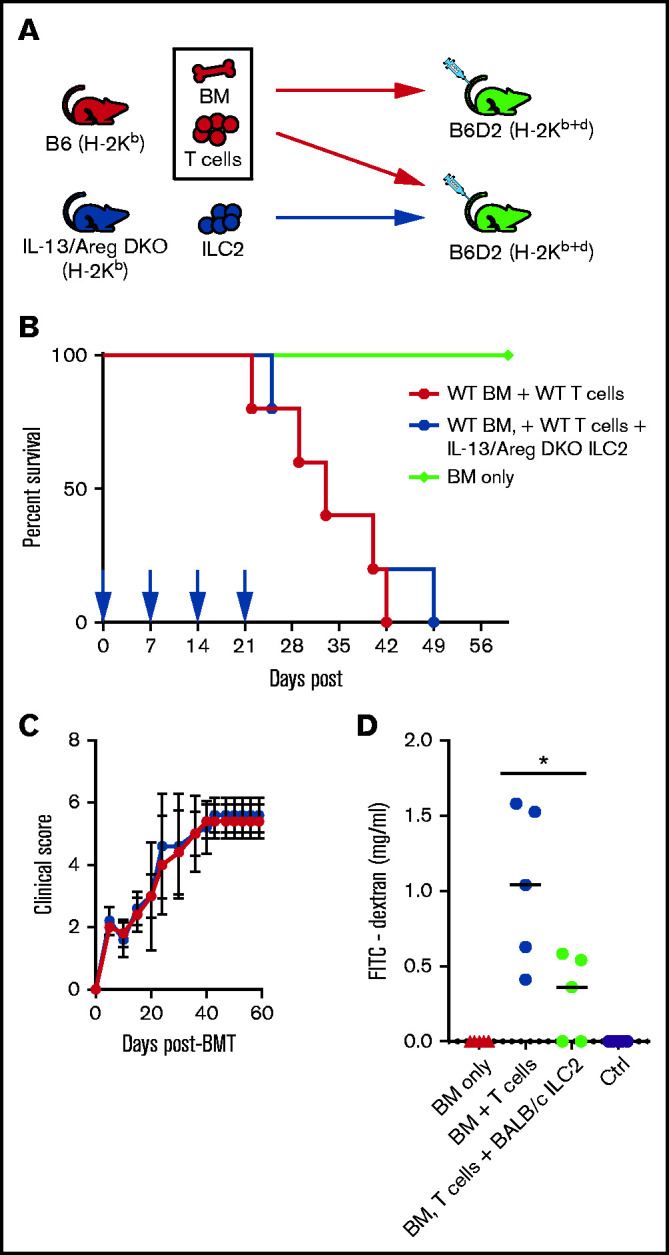
ILC2 mechanism of suppression of GVHD is dependent upon ILC2 expression of IL-13 and AREG. (A) Diagram for B6 into B6D2 transplantation. Each B6D2 recipient received B6 BM and B6 splenic T cells with one group of B6D2 recipients also being given IL-13/Areg DKO ILC2s. Lethally irradiated B6D2 mice received TCD BM (BM only), BM plus total splenic T cells (BM + T cells), or BM plus T cells with activated B10.BR ILC2s (BM, T cells + IL-13/Areg DKO ILC2s). (B) Kaplan-Meier plot of survival following allo-HSCT, 1 representative of 2 experiments shown (n = 5 each experiment), log-rank (Mantel-Cox) test. (C) Clinical score posttransplantation, analyzed by using a two-way analysis of variance, with Bonferroni correction for repeated measures of multiple comparisons. (D) Quantification of FITC-dextran in the serum of BMT recipients described in panel C 28 days after transplant. One representative of 2 independent experiments shown; mean ± standard error of the mean (n = 5 per group). Statistical analysis by Student t test, *P < .05.
Previously, we showed that the infusion donor ILC2 cells improved intestinal integrity, which was dependent on the expression of AREG by ILC2 cells.15 In the model described in Figure 2C, we evaluated the effects of third-party ILC2 cells on intestinal permeability on day 28 posttransplant. There was significant improvement in intestinal permeability on day 28 (Figure 6D) in recipients of third-party BALB/c ILC2 cells compared with mice receiving allogeneic T cells without third-party ILC2 cells. Thus, third-party ILC2 cells, despite their limited presence in the GI tract, can improve GI tract barrier function after allo-HSCT.
Expanded ILC2 cells are resistant to glucocorticoid treatment
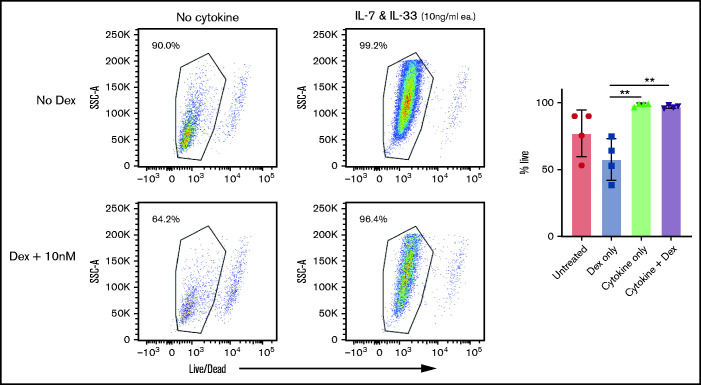
Systemic corticosteroid is the mainstay of first-line treatment in grade II to IV aGVHD.3 For ILC2 cell therapy to be clinically effective, ILC2 cells must endure in the setting of systemic exposure to corticosteroids. To investigate the impact of steroids on ILC2 persistence, ILC2 cells were treated with dexamethasone in vitro and evaluated for survival, 48 hours after treatment. As expected, ILC2 cells cultured in the absence of cytokines had diminished survival, which was nonsignificantly increased in the presence of dexamethasone. However, in the presence of pro-survival cytokines, dexamethasone had no effect on the survival of ILC2 cells (Figure 7). These findings indicate that expanded ILC2s, if they occur in the presence of the pro-survival cytokines IL-7 and IL-33 (which would be found in the GI tract), are resistant to death induced by corticosteroids. Thus, the administration of corticosteroids should not abrogate the survival of ILC2 cells when used clinically.
Figure 7.
Expanded ILC2s are resistant to glucocorticoid treatment. Flow cytometry analysis of survival of in vitro expanded ILC2s when treated with dexamethasone in the presence or absence of cytokines for 48 hours. Analyzed by using a two-way analysis of variance, with Bonferroni correction for repeated measures of multiple comparisons, **P < .01.
Discussion
aGVHD remains a significant complication after allo-HSCT. Critical to improving the outcome of patients who develop aGVHD is the ability to treat patients who do not respond to systemic corticosteroids. Previously, approaches to treat this patient group relied on methods to inhibit the persistence or function of donor T cells. Recently, our group and others have suggested that another approach to inhibiting aGVHD is to reconstitute the normal immunosuppressive mechanisms present in specific tissues. ILC2 cells play an important role in the response to parasitic infection in the GI tract. They are also critical for GI tract tissue repair and inhibit the pro-inflammatory immune response. Here, we have shown that third-party ILC2 cells significantly improve the survival of mice after allo-HSCT when given at the time of transplantation. ILC2 cells when used therapeutically were effective but less robust compared with the administration of ILC2 cells to prevent GVHD. This activity was dependent on multiple infusions of ILC2 cells as MHC-mismatched ILC2 cells did not persist in vivo. ILC2 cell function was dependent on the expression of IL-13 and AREG. Although we found no significant differences in the outcome of mice in which either BM cells or the host expressed IL-13 receptor, the loss of the IL-13 receptor on host cells had a greater impact. Finally, ILC2 cell survival in vitro was not affected by exposure to corticosteroids. These findings should greatly support the development of ILC2 cells as a clinical therapy.
We had previously shown that donor ILC2 cells could prevent aGVHD. Although these findings were exciting, translating the use of donor ILC2 cells therapeutically would be challenging. We have reported that human ILC2 cells can be isolated and expanded by three-four logs.23 However, as the expansion of human ILC2 cells takes ∼2 weeks ex vivo (H.S., Yan Xing, D.W.B., J.S.S., and B.R.B. November 2017), the use of donor ILC2 cells would require the collection and expansion of donor cells before allo-HSCT. Because 20% to 30% of patients who develop grade II to IV aGVHD are either nonresponsive or unable to wean from corticosteroids, expanding donor ILC2 cells would be complicated, inefficient, and costly. Here, for the first time, we have shown that third-party ILC2 cells, when given weekly, are as active as donor ILC2 cells in enhancing survival of recipients given on the day of transplant. In addition, ILC2 cells improved GVHD scores in mice when used therapeutically. Thus, although ILC2 cells have been shown to function as antigen-presenting cells for CD4+ T cells, this activity is not required for ILC2 cells to prevent/treat aGVHD. Given these findings, a bank of third-party ILC2 cells could be generated and used therapeutically in patients with lower GI tract aGVHD not responding to therapy.
Several groups, including our own, have shown the efficacy of infusing donor Tregs or MDSCs in recipient mice to abrogate aGVHD.6,9 Despite this promise, translation of these findings clinically has been challenging. Homogenous populations of Tregs are difficult to generate ex vivo. Inducible Tregs, which are generated by polarizing CD4+ T cells to express FoxP3, are not a fixed lineage, and there are conflicting data regarding their clinical efficacy in treating aGVHD.24 Our group has shown that MDSCs are not stable and, in the presence of inflammasome activation, generate immune-stimulatory myeloid cells. We have shown previously that ILC2 cells function upstream of MDSCs through production of IL-13.15 Thus, the infusion of ILC2 cells may be a more clinically tractable process to enhance in vivo the number and function of MDSCs post–allo-HSCT. One complication regarding the use of Tregs to treat aGVHD is their limited persistence in the presence of corticosteroids, which are the first-line therapy for patients with aGVHD. Interestingly, here we have shown that steroids do not affect the survival of ILC2 cells in the presence of trophic cytokines. These data support future studies in which patients with steroid-refractory lower GI tract aGVHD receive ILC2 cells while maintaining or tapering their dose of steroids.
In conclusion, we found that third-party ILC2 cells, if given weekly, can prevent, and to a lesser extent, decrease clinical aGVHD scores and improve survival. This process required the generation of IL-13 and AREG by the ILC2 cells. Our work provides a strong rationale for the generation of ILC2 donor banks and clinical studies to evaluate the activity of third-party ILC2 cells to treat lower GI tract aGVHD.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank A. Munitz for providing the IL-13Ra1−/− mice.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (RO1 HL139730, J.S.S.; R01 HL155098, J.S.S.; R01 HL56067, B.R.B.; and R01 HL11879, B.R.B.), National Institute of Allergy and Infectious Diseases (R37 AI34495, B.R.B.), and National Cancer Institute (P01 CA065493, H.S. and B.R.B.). It was also supported by a TRP Grant from the Leukemia and Lymphoma Society (J.S.S. and B.R.B.).
Authorship
Contribution: J.S.S. conceived of the project and directed the research; D.W.B. designed all murine experiments and evaluated the data; D.W.B. and J.S.S. wrote the manuscript; O.K., S.J.L., and H.B assisted with experiments; and H.S., B.R.B., and J.M.C. edited the manuscript and assisted in experimental protocols and discussion of the manuscript.
Conflict-of-interest disclosure: B.R.B. receives remuneration as an advisor to Magenta Therapeutics and BlueRock Therapeutics; research funding from BlueRock Therapeutics, Rheos Medicines, Equilibre Biopharmaceuticals, and Carisma Therapeutics, Inc.; and is a cofounder of Tmunity Therapeutics and an inventor on a pending patent application for use of ILC2s in GVHD. J.S.S. is supported by grants from Merck Sharpe & Dohme, Carisma Therapeutics, and GlaxoSmithKline. He has filed for intellectual property for the use of ILCs to treat and/or prevent aGVHD. D.W.B., H.S., and J.M.C. have filed for intellectual property for the use of ILCs to treat and/or prevent aGVHD. The remaining authors declare no competing financial interests.
Correspondence: Jonathan S. Serody, Room 5012, Marsico Hall, University of North Carolina School of Medicine, Chapel Hill, NC 7599-7295; e-mail: jonathan_serody@med.unc.edu.
References
- 1.Horwitz ME. Reduced intensity versus myeloablative allogeneic stem cell transplantation for the treatment of acute myeloid leukemia, myelodysplastic syndrome and acute lymphoid leukemia. Curr Opin Oncol. 2011;23(2):197-202. [DOI] [PubMed] [Google Scholar]
- 2.Kersey JH. The role of allogeneic-cell transplantation in leukemia. N Engl J Med. 2010;363(22):2158-2159. [DOI] [PubMed] [Google Scholar]
- 3.Garnett C, Apperley JF, Pavlů J.. Treatment and management of graft-versus-host disease: improving response and survival. Ther Adv Hematol. 2013;4(6):366-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatti RA, Kersey JH, Yunis EJ, Good RA.. Graft-versus-host disease. Prog Clin Pathol. 1973;5:1-18. [PubMed] [Google Scholar]
- 6.Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS.. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117(12):3268-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serody JS, Hill GR.. The IL-17 differentiation pathway and its role in transplant outcome. Biol Blood Marrow Transplant. 2012;18(suppl 1):S56-S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krenger W, Snyder KM, Byon JC, Falzarano G, Ferrara JL.. Polarized type 2 alloreactive CD4+ and CD8+ donor T cells fail to induce experimental acute graft-versus-host disease. J Immunol. 1995;155(2):585-593. [PubMed] [Google Scholar]
- 9.Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116(25):5738-5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koehn BH, Saha A, McDonald-Hyman C, et al. Danger-associated extracellular ATP counters MDSC therapeutic efficacy in acute GVHD. Blood. 2019;134(19):1670-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker JA, Barlow JL, McKenzie AN.. Innate lymphoid cells – how did we miss them? Nat Rev Immunol. 2013;13(2):75-87. [DOI] [PubMed] [Google Scholar]
- 12.Klose CS, Artis D.. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765-774. [DOI] [PubMed] [Google Scholar]
- 13.Munneke JM, Björklund AT, Mjösberg JM, et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood. 2014;124(5):812-821. [DOI] [PubMed] [Google Scholar]
- 14.Hanash AM, Dudakov JA, Hua G, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37(2):339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruce DW, Stefanski HE, Vincent BG, et al. Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease. J Clin Invest. 2017;127(5):1813-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panoskaltsis-Mortari A, Price A, Hermanson JR, et al. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103(9):3590-3598. [DOI] [PubMed] [Google Scholar]
- 17.Ramalingam TR, Pesce JT, Sheikh F, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9(1):25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coghill JM, Carlson MJ, Panoskaltsis-Mortari A, et al. Separation of graft-versus-host disease from graft-versus-leukemia responses by targeting CC-chemokine receptor 7 on donor T cells. Blood. 2010;115(23):4914-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulton LM, Carlson MJ, Coghill JM, et al. Attenuation of acute graft-versus-host disease in the absence of the transcription factor RORγt. J Immunol. 2012;189(4):1765-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Den Brink MR, Moore E, Horndasch KJ, et al. Fas-deficient lpr mice are more susceptible to graft-versus-host disease. J Immunol. 2000; 164(1):469-480. [DOI] [PubMed] [Google Scholar]
- 21.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaiss DMW, Gause WC, Osborne LC, Artis D.. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42(2):216-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing Y, Stefanski H, Bruce DW, et al. A novel process for the expansion of functional human ILC2 cells for GVHD prevention. Blood. 2017; 130(suppl 1):4432. [Google Scholar]
- 24.Romano M, Tung SL, Smyth LA, Lombardi G.. Treg therapy in transplantation: a general overview. Transpl Int. 2017;30(8):745-753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.