Abstract
Background
Although severe acute malnutrition is a major public issue among HIV infected children, there is no prior evidence in Ethiopia. Hence, this study aims to assess the time to develop severe acute malnutrition and its predictors among children living with human immunodeficiency virus in Ethiopia, 2012.
Methods
An institution based retrospective cohort study was conducted in South Gondar hospitals among 363 HIV infected children from February 10, 2014, to January 7, 2021. Epi-data version 3.1 was used to enter data, which was then exported to STATA version 14 for analysis. Besides, WHO (World Health Organization) Anthro Plus software was used to assess the nutritional status of the children. A standardized data extraction tool was used to collect the data. The Kaplan Meier survival curve was used to estimate the median survival time. The Cox-proportional hazard model assumption was checked via the Schoenfeld residual ph test and a stph plot. Bivariable and multivariable Cox proportional hazard models were employed at 95% confidence intervals (CI). A variable having a p-value < 0.05 was considered a statistically significant predictor of severe acute malnutrition.
Results
A total of 363 children living with HIV, 97 (26.72%) developed severe acute malnutrition during the follow-up period. The overall incidence rate was 5.4 (95% CI: 4.7–5.9) person per year with a total of 21, 492 months or 1791 years of observation. Moreover, the median survival time was 126 months. Treatment failure [AHR =3.4 (95% CI: 2.05–5.75)], CD4 count below threshold [AHR =2.5 (95% CI: 1.64–3.95)], and WHO stage III & IV [AHR =2.9 (95% CI: 1.74–4.73)] were all significant predictors of severe acute malnutrition.
Conclusion
The time to develop severe acute malnutrition was found to be very low. Treatment failure, CD4 count below threshold, and WHO stage III were all significant predictors of severe acute malnutrition. Hence, emphasizing those predictor variables is essential for preventing and controlling the occurrence of severe acute malnutrition among HIV infected children.
Keywords: Time to develop severe acute malnutrition, Children living HIV, Ethiopia
Background
In 2019, 1.7 million children worldwide are infected with the human immunodeficiency virus (HIV) [1]; in the same year, 44,229 children in Ethiopia were infected with HIV, and 2055 died as a result of AIDS (Acquired immunodeficiency syndrome) [2].
HIV infection and malnutrition are often closely interlinked and act synergistically [3, 4]. Malnutrition increases viral replication and accelerates the progression of HIV disease [5, 6]. Likewise, the effect of HIV on nutrition includes nutrient malabsorption and complex metabolic alterations [3, 7]. As a result, a vicious cycle is formed [8, 9].
The incidence of stunting is declining too slowly, while wasting still has a great impact on many young children worldwide [10]. In 2017, 13.8 million children were wasted, of whom 4 million were severely wasted [11] and most of all were in sub-Saharan countries [12]. Each year, over one million children die and develop severe acute malnutrition (SAM), and it is more common in HIV-positive children [8, 13, 14]. Moreover, SAM among HIV-infected children is one of the leading causes of morbidity and mortality in the world, and the problems are worsening more in sub-Saharan countries [13, 15, 16], and Ethiopia is one of those countries to share this burden [13, 17].
Children are more vulnerable to severe acute malnutrition than adults with HIV, hence rapid viral replication and a higher rate of CD4 cell destruction because of immunity that has not developed well [15]. The risk of death in HIV-infected children is three times higher than in non-HIV-infected children [7, 18]. Since HIV increases the risk of loss of appetite, worsening illness, and deteriorating nutritional status [19, 20]. As a result of HIV prevalence and resource constraints, caring for severely acute malnourished children in Sub-Saharan Africa, such as Ethiopia, is difficult [6, 12].
SAM among HIV-infected children remains widespread and a serious public health problem in Ethiopia since it is interlinked with different contributing factors [13, 21, 22].
Although SAM is a leading cause of hospitalization and mortality in Ethiopia among HIV-infected children, there is no prior evidence on when to develop SAM and its predictors among HIV-infected children. Hence, this study aims to assess the time to develop severe acute malnutrition and its predictors among HIV-infected children at South Gondar hospital, Ethiopia. Additionally, the finding hopes that it will provide important information to be understood by all stakeholders, reducing the burden.
Methods
Study area, design and study participants
A retrospective follow-up study was conducted from February 10, 2014, to January 7, 2021, at South Gondar hospitals. The South Gondar Zone is one of the zonal states of the Amhara region. Based on the 2007 census conducted by the Ethiopian Central Statistical Agency, this zone has a total population of 2,051,738, of whom 1,041,061 are males, while 1,010,677 are females. South Gondar hospitals provide different services for the South Gondar Zone population, such as services in the MCH unit, genecology and obstetrics unit, laboratory unit, minor surgery, inpatient unit, and outpatient unit, major surgery, ophthalmic unit, pharmacy unit, and ART. Besides, the South Gondar public hospitals have 612 health professionals. During the study period, there were 1027 children (age 15 years) on ART, according to the South Gondar administrator report.
All HIV infected children from February 10, 2014, to January 7, 2021, at South Gondar hospitals were a study population. All children with HIV after the beginning of test and treat strategies until the end of the study period were eligible for the study. Whereas, children with unrecorded nutritional status were excluded from the study.
Sample size determination and sampling procedure
The sample size was calculated by using the Cox proportional hazards model via Stata 14.0 software using the two-population proportion formula by considering the following assumptions. n = (Zα/2 + Zβ)2 * (p1(1-p1) + p2(1-p2)) / (p1-p2)2, where Zα/2 is the critical value, which is 1.96, Zβ is the critical value with a power of 80%, and the critical value is 0.84. The p1 and p2 are the expected sample proportions of the two groups. Based on the above two-population proportion formula, the survival probability in males as an exposure group (P1 = 0.323) and the survival probability in females as a non-exposure group was (P2 = 0.470) from a previous study [9]. The final sample size n = (1.96 + 0.84)2 * (0.323 (1–0.323) + 0.470 (1–0.470)) / (0.323–0.470)2 after adding 10% incompleteness data was 363. There are a total of 05 hospitals that provide Paediatric ART in the South Gondar Zone. Of these, one compressive specialized hospital and two primary hospitals were selected randomly. Namely, Debre Tabor compressive specialized hospital, Nefas Mewucha primary hospital, and Mekan Eysus primary hospital.
From a total of 1027 study participants, 719 were in the selected hospitals from February 10, 2014, and January 7, 2021. Then, eligible participants from those selected hospitals were identified at paediatric ART clinics. The investigator assigned the registration numbers from February 10, 2014, and January 7, 2021, in chronological order. Of these, the investigator drew 363 samples by lottery method that fulfilled the inclusion criteria after reviewing the medical charts and ART registration logbook after proportionally allocating them to each hospital (Fig. 1).
Fig. 1.
Schematic presentation of study participants’ recruitment and allocation process at south Gondar hospitals, Northwest, Ethiopia, 2021 (n-363)
Operational definitions
Time to SAM (outcome variable)
The time from admission to the occurrence of the event SAM during the follow-up period.
SAM is defined by the WHO as a weight-for-height z-score of less than − 3, or a mid-upper arm circumference of less than 11.5 cm in children aged 6 months to 5 years [8, 15].
Censored: if the child had lost follow-up or transferred out to another service before developing SAM, or if the child was free from SAM until the end of the follow-up time.
Adherence to ART was classified based on the percentage of drug dosage calculated from the total monthly doses of ART drugs. (Good > 95%, fair 85–94%, and poor < 85%) [23].
Anemia was defined as having a hemoglobin level ≤ 10 mg/dl [24].
CD4 count: CD4 levels below the threshold level were classified based on the child’s age (i.e. infants CD4 1500/mm3, 12–35 months 750/mm3, 36–59 months 350/mm3, and 5 years 200/mm3) [24].
Data collection procedures and quality control
Data were collected from children’s follow up by using a standard checklist that was adopted from the ART follow-up form. The data extraction also contained socio-demographic and clinical related characteristics of the children or caregivers. A pre-test was done on 10% of the sample size in Mekane Eyesus primary hospital, which is 52 km away from Debre Tabor Town. Tanning was given for four data collectors and two supervisors. Indeed, the data collectors had been working in ART clinics and knew the format. The completeness of the retrieved data was checked by the supervisor after the randomly selected medical records on a daily basis.
Data processing and analysis
For this study, Epi-data version 3.1 Epi-data version 3.1 was used to enter data, which was then exported to STATA version 14 for analysis. Moreover, WHO Anthro Plus software was used to assess the nutritional status of the children. The median, tables, and figures were used to describe and visualize the data. The incidence rate of SAM was calculated using the total number of people per year (PPY) as the denominator in the follow-up period. The total time that participants contributed to the study was thus incorporated into the analysis. The Kaplan-Meier plot estimates the median SAM free survival time.
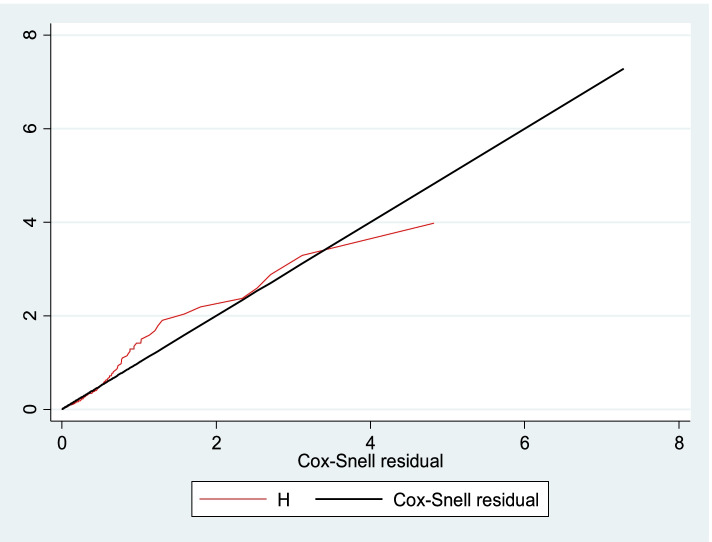
The necessary Cox-proportional hazard model assumption was checked using graphical diagnostics based on the scaled Schoenfeld residuals (log-log survival plot) (Fig. 2) and statistical tests (global test: 39.05). After the Cox-proportional hazard model was checked, both bivariate and multivariable Cox proportional hazard regression analyses at 95% CI were fitted. Then, the variables having a p-value < 0.25 in bivariate analysis were computed into the multivariable cox-proportional hazards model. Finally, a variable having a P-value < 0.05 in the multivariable cox-proportional hazards model was used to declare a significant association with SAM.
Fig. 2.
Cox-Snell residual Nelson -Alen cumulative hazard graph among children living with HV at south Gondar hospitals, Northwest, Ethiopia, 2021 (n-363)
Results
Socio-demographic characteristics of children living with HIV
Overall, 363 children living with HIV (Debre Tabor compressive specialized hospital: 234, Nefas Mucha primary hospital: 52, and Mekan Eyesus primary hospital: 77) were included in the study. Of the 363 children living with HIV, 190 (52.34%) and 131 (36.09%) were male and aged between 4 and 10, respectively. The majority of the 290 (79.89%) children living with HIV were in urban residences. Besides, a large proportion of 238 (67.23%), 249 (68.6%), and 281 (77.41%) of the child caregivers were married, both alive, and living with HIV, respectively. Almost half of the children (47.9%) were aware of their HIV status. Likewise, approximately 203 (55.92%) of the children’s caregivers were housewives (Table 1).
Table 1.
Socio-demographic characteristics of children living with HIV at South Gondar hospitals, Ethiopia, 2021(n = 363)
| Exposure variable | Responses | Frequency | Percent |
|---|---|---|---|
| Age of the child (years) | ≤3 | 95 | 26.17 |
| > 4–10 | 131 | 36.09 | |
| > 10 | 137 | 37.74 | |
| Sex | Male | 190 | 52.34 |
| Female | 173 | 47.66 | |
| Residence | Rural | 73 | 20.11 |
| Urban | 290 | 79.89 | |
| Caregiver’s Marital status | Married | 238 | 67.23 |
| Widowed | 106 | 29.94 | |
| Divorced | 10 | 2.82 | |
| Caregiver’s status | Both alive | 249 | 68.6 |
| Either or Bothe died | 114 | 31.4 | |
| Caregiver’s occupational status | Housewife | 203 | 55.92 |
| Governmental employee | 89 | 24.52 | |
| Merchant | 38 | 10.47 | |
| Non-governmental employee | 33 | 9.09 | |
| HIV status of the Caregiver’s | Positive | 281 | 77.41 |
| Negative | 38 | 10.47 | |
| Not Known | 44 | 12.12 | |
| Disclosure status | Yes | 174 | 47.9 |
| No | 189 | 52.1 |
Clinical characteristics of children living with HIV
Of the total of children living with HIV, 49 (13.50%), 61 (16.80%), 81 (22.31%), 40 (11.02%), and 39 (10.74%) had HGB (Hemoglobin) levels of 10 mg/dl, CD4 counts or % below the threshold, WHO stages III & IV, TB (Tuberculosis), and treatment failure in the follow-up period. Additionally, 202 (63.325) children living with HIV had OI (opportunistic infection) in the study period. ART was started on nearly half of the 188 children (51.8%) who were alive after 49 months. Of the total 363 children living with HIV, 300 (82.64%) were taking CPT (Cotrimoxazole prophylactic therapy), while 156 (42.98%) were taking IPT (Isoniazid prophylactic therapy). Besides, 327 (90.08%) and 280 (77.13%) of children living with HIV were initial non-PI (protease inhibitor) based and had a good level of adherence to ART (Table 2).
Table 2.
Clinical and immunological status characteristics of children living with HIV South Gondar hospitals, Ethiopia, 2021(n = 363)
| HGB level | < 10 mg/dl | 49 | 13.50 |
| > = 10 mg/dl | 314 | 86.50 | |
| CD4 counts or % | Below threshold | 61 | 16.80 |
| Above threshold | 302 | 83.20 | |
| WHO stages | Stage I&II | 282 | 77.69 |
| Stage III&IV | 81 | 22.31 | |
| Hx of OIs other than TB (n = 319) | Yes | 117 | 36.68 |
| No | 202 | 63.32 | |
| Regimen at baseline | PI-based | 36 | 9.92 |
| Non-PI based | 327 | 90.08 | |
| Treatment failure | Yes | 39 | 10.74 |
| No | 324 | 89.26 | |
|
Cotrimoxazole preventive therapy (CPT) |
Yes | 300 | 82.64 |
| No | 63 | 17.36 | |
| Isoniazid preventive therapy (IPT) | Yes | 156 | 42.98 |
| No | 207 | 57.02 | |
| ART adherence | Good | 280 | 77.13 |
| Poor/Fair | 83 | 22.87 | |
| TB status | Yes | 40 | 11.02 |
| No | 323 | 88.98 | |
| Duration On ART | < 49 months | 188 | 51.8 |
| ≥49 months | 175 | 48.2 |
The time to exposure for severe acute malnutrition in HIV-infected children
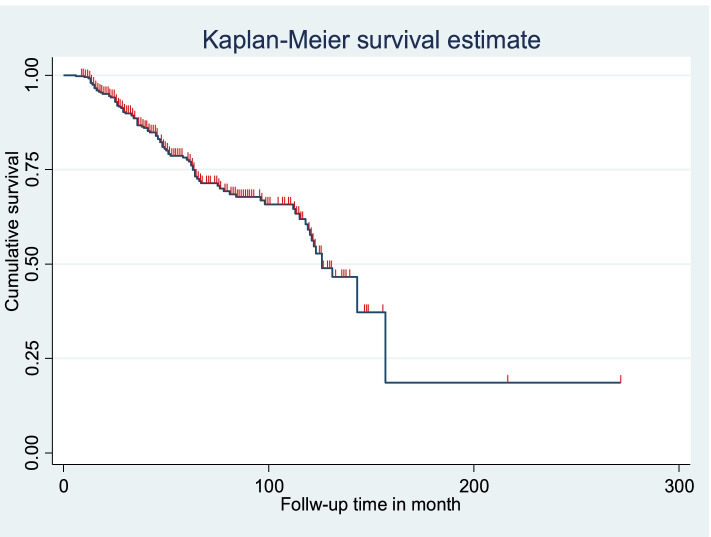
From a total of 363 children living with HIV, 97 (26.72%) developed SAM during the follow-up period. The incidence rate of SAM among children living with HIV was 5.4 (95%CI: 4.7–5.9) PPY. The study participants were followed for a total of 21, 492 months, or 1791 at different points of time. The median survival time was 126 months (Fig. 3).
Fig. 3.
Kaplan-Meier of survival curve among children living with HV at south Gondar hospitals, Northwest, Ethiopia, 2021 (n-363)
Predictors of severe acute malnutrition among children living with HIV
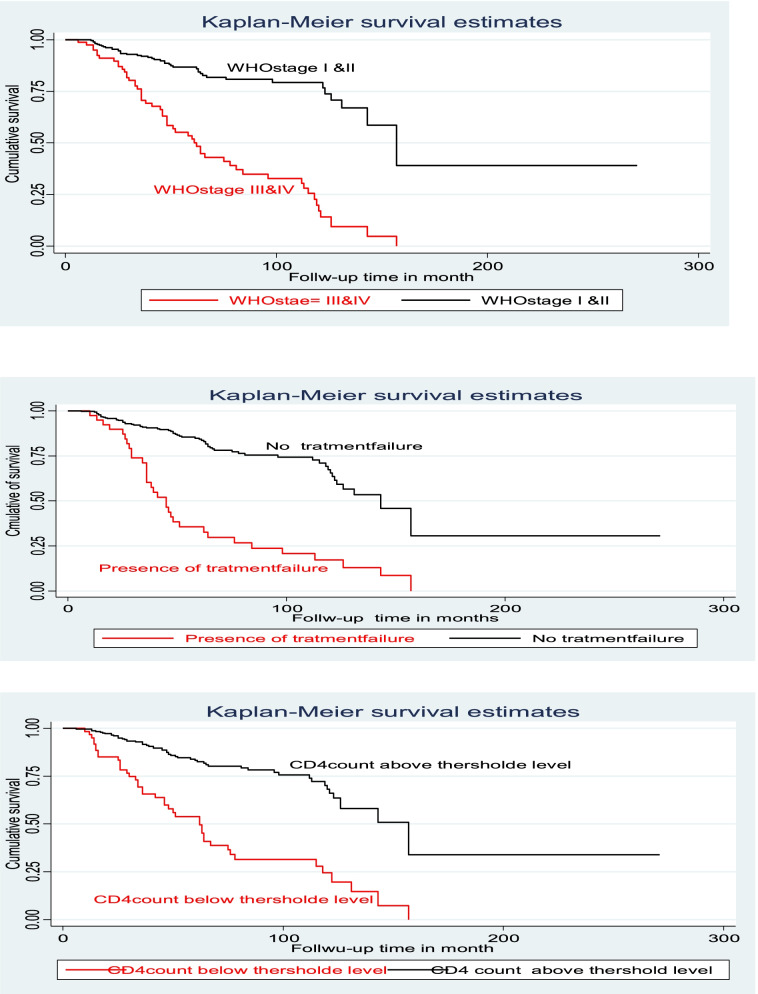
Bi-variable and multivariate cox proportional hazard models were computed to determine the relationship between each independent factor and the outcome variable. In bi-variable analysis, the variable had a P-value less than or equal to 0.25 and was entered into for multivariate cox proportional hazard after checking global and detail tests for the model fitness and for all predictor variables, respectively. In the multivariate cox proportional hazard model, treatment failure, WHO stages III & IV, and CD4 counts below threshold level were significant predictors for the occurrence of SAM among children living with HIV. The risk of SAM among children with treatment failure was 3.4 times higher than that of children without treatment failure [AHR: 3.4 (95% CI: 2.05, 5.75)]. The risk of SAM among children with CD4 counts below the threshold level was 2.5 times higher than that of children with CD4 counts above the threshold [AHR: 2.5 (95% CI: 1.64, 3.95)]. Moreover, the risk of SAM among children with WHO stage III & IV is 2.9 times higher than in children with WHO stage III & IV [AHR: 2.9 (95% CI: 1.74, 4.73)] In the follow-up period (Table 3 &Fig. 4).
Table 3.
Bivariable and multivariable Cox-regression of predictor variable among children living with HIV at South Gondar hospitals, Ethiopia, 2021(n = 363)
| Exposure variable | SAM NO Yes |
CHR (95% CI) | AHR (95% CI) | P-Value | ||
|---|---|---|---|---|---|---|
| Age of the child (years) | ≤3 | 71 | 24 | 1.0 (0.63–1.78) | – | |
| > 4–10 | 96 | 35 | 1.0 (0.64–1.60) | |||
| > 10 | 99 | 38 | Ref | |||
| Sex | Male | 144 | 46 | 0.7 (0.47–1.05) | 0.9 (0.56–1.32) | 0.487 |
| Female | 122 | 51 | Ref | Ref | ||
| Residence | Rural | 51 | 22 | 1.2 (0.79–1.78) | – | |
| Urban | 215 | 75 | Ref | |||
| Caregiver’s Marital status | Married | 175 | 63 | 1.2 (0.37–3.78) | – | |
| Widowed | 79 | 27 | 1.1 (0.34–3.76) | – | ||
| Divorced | 7 | 3 | Ref | |||
| Caregiver’s status | Both alive | 186 | 63 | Ref | ||
| Either or Bothe died | 80 | 34 | 1.1 (0.71–1.65) | – | ||
| Caregiver’s occupational status | Housewife | 145 | 58 | 0.8 (0.35–1.70) | – | |
| Governmental employee | 66 | 23 | 0.7 (0.30–1.67) | – | ||
| Merchant | 29 | 9 | 0.8 (0.28–2.07) | – | ||
| Non-governmental employee | 26 | 7 | Ref | |||
| HIV status of the Caregiver’s | Positive | 218 | 63 | 0.5 (0.29–0.83) | 0.6 (0.32–1.01) | 0.053 |
| Negative | 23 | 15 | 0.9 (0.46–1.84) | 0.4 (0.18–1.01) | 0.054 | |
| Not Known | 25 | 19 | Ref | Ref | ||
| Disclosure status | Yes | 135 | 54 | Ref | Ref | |
| No | 131 | 43 | 1.3 (0.86–1.92) | 1.6 (0.98–2.47) | 0.057 | |
| Regimen at baseline | PI-based | 12 | 24 | 3.0 (1.90–4.82) | 0.9 (0.50–1.61) | 0.715 |
| Non-PI based | 254 | 73 | Ref | Ref | ||
| Treatment failure | Yes | 6 | 33 | 4.3 (2.81–6.57) | 3.4 (2.05–5.75) | 0.000 |
| No | 260 | 64 | Ref | Ref | ||
| Cotrimoxazole preventive therapy | Yes | 24 | 39 | Ref | Ref | |
| No | 242 | 58 | 3.5 (2.30–5.19) | 1.6 (0.96–2.65) | 0.070 | |
| CD4 counts or % | Below threshold | 19 | 42 | 3.9 (2.62–5.85) | 2.5 (1.64–3.95) | 0.000 |
| Above threshold | 247 | 55 | Ref | Ref | ||
| Isoniazid preventive therapy (IPT) | Yes | 125 | 31 | Ref | Ref | |
| No | 141 | 66 | 1.3 (0.85–2.02) | 0.8 (0.49–1.26) | 0.327 | |
| ART adherence | Good | 203 | 77 | Ref | ||
| Poor/Fair | 63 | 20 | 0.8 (0.49–1.32) | |||
| TB status | Yes | 12 | 28 | 3.7 (2.37–5.75) | 1.2 (0.68–2.27) | 0.471 |
| No | 254 | 69 | Ref | Ref | ||
| Hx of OIs other than TB (n = 319) | Yes | 85 | 32 | 1.1 (0.68–1.64) | – | |
| No | 149 | 53 | Ref | |||
| Hemoglobin level | < 10 mg/dl | 19 | 30 | 2.4 (1.55–3.71) | 1.6 (0.75–1.95) | 0.433 |
| > = 10 mg/dl | 247 | 67 | Ref | Ref | ||
| WHO stages | Stage I&II | 239 | 43 | Ref | Ref | |
| Stage III&IV | 27 | 54 | 4.8 (3.18–7.11) | 2.9 (1.74–4.73) | 0.000 | |
| Duration On ART | < 49 months | 131 | 57 | 0.9 (0.6–1.4) | – | |
| ≥49 months | 135 | 40 | Ref | |||
*Significant at < 0.05; ** Significant at < 0.01; CHR Crude hazard ratio, AHR Adjusted hazard ratio, Ref Reference category, CI Confidence interval
Fig. 4.
Kaplan-Meier of survival curve of among children living with HV by treatment failure, CD4 count, and WHO stage at south Gondar hospitals, Northwest, Ethiopia, 2021 (n-363)
Discussion
In this study, the average time to exposure for severe acute malnutrition at South Gondar hospitals was found to be 126 months. Besides, the incidence of severe acute malnutrition was found to be 5.4 (95% CI: 4.7–5.9) PPY with a proportion of 26.7%.
This finding is comparable to 24.65% in East Africa [8], 24.5% in sub-Saharan Africa [25], and 26% in Central and West Africa [12]. However, this finding is higher than the studies conducted in Asia 13.5% [26], 12.9% in Tanzania [27], 13.6% in Nigeria [28], 10.6% in Cameron [29], and 7.1% in Zimbabwe [30]. On the other hand, the finding was lower than the study conducted in India, it was 38.09% [31]. These differences could be explained by the level of quality care, which includes the reporting mechanism, the cut-off point for SAM diagnosis, screening, and monitoring ability of the healthcare providers. Moreover, the differences in socio-demographic characteristics, healthcare systems, study population, and study design were also considered for the variation.
The risk of SAM among children with treatment failure was 3.4 times higher than that of children without treatment failure [AHR: 3.4 (95% CI: 2.05, 5.75)]. This study’s findings were consistent with those conducted in Asia and Burkina Faso [26, 32]. This can be explained by the fact that children with treatment failure are at risk for viral load increment and the CD4 cell count continues to drop, which leads to the development of an opportunistic infection like SAM. Besides, antiretroviral therapy can prevent HIV from progressing, especially when a person starts taking it early. However, if the patients have treatment failure, HIV progressively kills the cells like CD4 T cells that help protect the body from infection, leading to complications [33–35].
The risk of SAM among children with CD4 counts below the threshold level was 2.5 times higher than that of children with CD4 counts above the threshold [AHR: 2.5 (95% CI: 1.64, 3.95)].
This might be because the patient who has a low CD4 count can be exposed to chronic diarrhea, tuberculosis, opportunistic infections, or anemia, which results in a significant imbalance between nutritional demand and individual intake, usually both quantitative (number of kilocalories/day) and qualitative (vitamins and minerals, etc.) deficiencies [36, 37].
Moreover, the risk of SAM among children with WHO stage III & IV is 2.9 times higher than in children with WHO stage III & IV [AHR: 2.9 (95% CI: 1.74, 4.73)] This study’s findings were consistent with the studies conducted in Asia and Burkina Faso [38, 39]. This is usually when children are presented with advanced WHO stages, where the immune system is badly damaged, which exposes them to different kinds of complications, including cancer, pneumonia, treatment failure, diarrhea, and anemia. Hence, if the children are affected severely by advanced opportunistic infections, they are unprotected against chronic and acute malnutrition [40, 41].
This finding of this study has an implication for the global rise of severe acute malnutrition, predominantly in resource-limited settings. It adds to an understanding of the links between SAM and the contributing factors that cause its occurrence in children living with HIV. These findings provide an insight into effective ways of improving the quality-of-life of children with HIV in Ethiopia, a country known for its high prevalence of severe acute malnutrition, especially among children living with HIV. Additionally, these findings may help policymakers establish context-specific strategies to reduce severe acute malnutrition, particularly in children with HIV [8, 13, 42].
This study can try to show the burden of SAM plus attempt to establish the cause-effect relationship between SAM and its predictor variable. However, the study’s inherent limitations of retrospective study design were encountered.
Conclusion
The time to develop severe acute malnutrition was found to be very low. Treatment failure, CD4 count below threshold, and WHO stage III were all significant predictors of severe acute malnutrition. Hence, emphasizing those predictor variables is essential for preventing and controlling the occurrence of severe acute malnutrition among HIV infected children.
Acknowledgments
Firstly, we would like to express our deepest gratitude to Debre Tabor University, secondly, the ART focal person of each South Gondar hospital, and lastly, our heartfelt thanks also go to all individuals who participated in the study and data collectors.
Abbreviations
- AHR
Adjusted hazard ratio
- AIDS
Acquired immunodeficiency disease
- ART
Antiretroviral therapy
- CD4
Cluster of differentiation 4
- CI
Confidence interval
- CPT
Cotrimoxazole prophylactic therapy
- HGB
Hemoglobin
- HIV
Human immunodeficiency virus
- IPT
Isoniazid prophylactic therapy
- TB
Tuberculosis
- OI
Opportunistic infection
- PI
protease inhibitor
- PYO
Person per year observation
- WHO
World health organization
Authors’ contributions
ESC and MM contributed to the conception, study design, data acquisition, data interpretation, and writing of the original article. GL, SBZ, MM, DAS, EDY, and AM contributed to visualization, supervision, and writing (review and editing) critically revising the article. MBA, MA, FTA, GY, DGF, and SA contributed to performing the statistical analysis, data curation, and critically revising the article. All authors read and approved the final manuscript.
Funding
The authors have also declared that there is no financial support for the research.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author.
Declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the Institutional Review Board (IRB) of Debre Tabor University. Informed consent was obtained from all subjects or parents on behalf of children after explaining the objective of the study to each of the study participants. All the experiment protocols for involving humans were in accordance with the guidelines of the national/international/institutional or Declaration of Helsinki in the manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ermias Sisay Chanie, Email: ermisis1888@gmail.com.
Getasew Legas, Email: getasewlegas@gmail.com.
Shimeles Biru Zewude, Email: nathanshimeles@gmail.com.
Maru Mekie, Email: maru.mekie1@gmail.com.
Dagne Addisu Sewyew, Email: addisudagne7@gmail.com.
Enyew Dagnew Yehuala, Email: enydagnew@gmail.com.
Abenezer Melkie, Email: mamushmelkie@gmail.com.
Minale Bezie Ambie, Email: minaleemu1@gmail.com.
Mengesha Assefa, Email: menge61621@gmail.com.
Fitalew Tadele Admasu, Email: fitalewtadele@gmail.com.
Getachew Yideg Yitbarek, Email: getachewyideg@gmail.com.
Sintayehu Asnakew, Email: sintie579@gmail.com.
Mekuant Mersha, Email: mequanent645@gmail.com.
Dejen Getaneh Feleke, Email: dejengetaneh38@gmail.com.
References
- 1.‘2019-UNAIDS-data_en.pdf’. Accessed: Oct. 20, 2020. *Online+. Available: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf.
- 2.Abdella S, et al. HIV Related Estimates and Projections in Ethiopia for the Year. 2019. [Google Scholar]
- 3.Duggal S, Chugh TD, Duggal AK. HIV and malnutrition: effects on the immune system. Clin Dev Immunol. 2012;2012:784740. doi: 10.1155/2012/784740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sashindran VK, Thakur R. Malnutrition in HIV/AIDS: Aetiopathogenesis. Nutr HIVAIDS Implic Treat Prev Cure. 2020; [cited 2021 Jan 25]; Available from: https://www.intechopen.com/books/nutrition-and-hiv-aids-implication-for-treatment-prevention-and-cure/malnutrition-in-hiv-aids-aetiopathogenesis.
- 5.Patel D, Bland R, Coovadia H, Rollins N, Coutsoudis A, Newell M-L. Breastfeeding, HIV status, and weights in south African children: a comparison of HIV-exposed and unexposed children. AIDS Lond Engl. 2010;24(3):437–445. doi: 10.1097/QAD.0b013e3283345f91. [DOI] [PubMed] [Google Scholar]
- 6.Jesson J, Koumakpaï S, Diagne NR, Amorissani-Folquet M, Kouéta F, Aka A, et al. Effect of age at antiretroviral therapy initiation on catch-up growth within the first 24 months among HIV-infected children in the IeDEA west African Pediatric cohort. Pediatr Infect Dis J. 2015;34(7):e159–e168. doi: 10.1097/INF.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taye B, Shiferaw S, Enquselassie F. The impact of malnutrition in the survival of HIV infected children after initiation of antiretroviral treatment (ART) Ethiop Med J. 2010;48(1):1–10. [PubMed] [Google Scholar]
- 8.Abate BB, Aragie TG, Tesfaw G. Magnitude of underweight, wasting and stunting among HIV positive children in East Africa: a systematic review and meta-analysis. PLoS One. 2020;15(9):e0238403. doi: 10.1371/journal.pone.0238403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sewell Y, Hailu G, Sintayehu M, Moges NA, Alebel A. Magnitude of malnutrition and associated factors among HIV infected children attending HIV-care in three public hospitals in east and west Gojjam zones, Amhara, northwest, Ethiopia, 2017: a cross-sectional study. BMC Res Notes. 2018;11(1):788. doi: 10.1186/s13104-018-3882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malnutrition in Children [Internet]. UNICEF DATA. [cited 2021 Feb 14]. Available from: https://data.unicef.org/topic/nutrition/malnutrition/
- 11.Onyango AW, Jean-Baptiste J, Samburu B, Mahlangu TLM. Regional overview on the double burden of malnutrition and examples of program and policy responses: African region. Ann Nutr Metab. 2019;75(2):127–130. doi: 10.1159/000503671. [DOI] [PubMed] [Google Scholar]
- 12.Jesson J, Masson D, Adonon A, Tran C, Habarugira C, Zio R, et al. Prevalence of malnutrition among HIV-infected children in central and west-African HIV-care programs supported by the growing up Programme in 2011: a cross-sectional study. BMC Infect Dis. 2015;15(1):216. doi: 10.1186/s12879-015-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bimerew M, Teshome GS, Abebe F, Demis A, Baye G, Adimasu M. Determinants of Severe Acute Malnutrition Among HIV-positive Children Receiving HAART in Public Health Institutions of North Wollo Zone, Northeastern Ethiopia: Unmatched Case– Control Study. Vol. 11, Pediatric Health, Medicine and Therapeutics: Dove Press; 2020. p. 313–21. [cited 2021 Jan 26] [DOI] [PMC free article] [PubMed]
- 14.Bunn J, Kerouac M, Thindwa M. Features associated with underlying HIV infection in severe acute childhood malnutrition: a cross-sectional study. 2013 Nov 5 [cited 2021 Jan 26]; Available from: https://tspace.library.utoronto.ca/handle/1807/54951 [DOI] [PMC free article] [PubMed]
- 15.Musoke PM, Fergusson P. Severe malnutrition and metabolic complications of HIV-infected children in the antiretroviral era: clinical care and management in resource-limited settings1234. Am J Clin Nutr. 2011;94(6):1716S–1720S. doi: 10.3945/ajcn.111.018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerouac M.. Improving the treatment of severe acute malnutrition in childhood: a randomized controlled trial of synbiotic-enhanced therapeutic food with long term follow-up of post-treatment mortality and morbidity [internet] [Doctoral]. Doctoral thesis, UCL (University College London). UCL (University College London); 2011 [cited 2021 Jan 25]. Available from: https://discovery.ucl.ac.uk/id/eprint/1306755/
- 17.Gebremichael DY, Hadush KT, Kebede EM, Zegeye RT. Food Insecurity, Nutritional Status, and Factors Associated with Malnutrition among People Living with HIV/AIDS Attending Antiretroviral Therapy at Public Health Facilities in West Shewa Zone, Central Ethiopia. BioMed Res Int. 2018;2018:1–9. doi: 10.1155/2018/1913534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takarinda KC, Mutasa-Apollo T, Madzima B, Nkomo B, Chigumira A, Banda M, et al. Malnutrition status and associated factors among HIV-positive patients enrolled in ART clinics in Zimbabwe. BMC Nutr. 2017;3(1):15. doi: 10.1186/s40795-017-0132-8. [DOI] [Google Scholar]
- 19.Koethe JR, Heimburger DC. Nutritional aspects of HIV-associated wasting in sub-Saharan Africa. Am J Clin Nutr. 2010;91(4):1138S–1142S. doi: 10.3945/ajcn.2010.28608D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46(10):1582–1588. doi: 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- 21.Girum T, Muktar E, Worku A. Comparative analysis of the survival status and treatment outcome of under-five children admitted with severe acute malnutrition among hospital-based and health center-based stabilization centers, South Ethiopia. Open Public Health J. 2018; May 31 [cited 2021 Jan 26];11(1).
- 22.Tekleab AM, Tadesse BT, Grief AZ, Shimelis D, Gebre M. Anthropometric improvement among HIV infected pre-school children following initiation of first line anti-retroviral therapy: implications for follow up. PLoS One. 2016;11(12):e0167565. doi: 10.1371/journal.pone.0167565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okechukwu AA, Okechukwu OI. A clinical correlate of tuberculosis in HIV co-infected children at the University of Abuja Teaching Hospital, Gwagwalada, Nigeria. Niger J Clin Pract. 2011;14(2):206–211. doi: 10.4103/1119-3077.84018. [DOI] [PubMed] [Google Scholar]
- 24.Atalell KA, Birhan Tebeje N, Ekubagewargies DT. Survival and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. A retrospective follow-up study. PLoS ONE. 2018;13(5) May 22 [cited 2020 Jun 2]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5963769/. [DOI] [PMC free article] [PubMed]
- 25.Under-nutrition and associated factors among human immunodeficiency virus-infected children in sub-Saharan Africa: A systematic review and meta-analysis. 2020 Aug 27 [cited 2021 Jan 26]; Available from: https://www.researchsquare.com/article/rs-64695/v1
- 26.Boettiger DC, Aurpibul L, Hudaya DM, Fong SM, Lumbiganon P, Saphonn V, et al. Antiretroviral therapy in severely malnourished, HIV-infected children in Asia. Pediatr Infect Dis J. 2016;35(5):e144–e151. doi: 10.1097/INF.0000000000001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mwiru RS, Spiegelman D, Duggan C, Seage GR, Semu H, Chalamilla G, et al. Nutritional status and other baseline predictors of mortality among HIV–infected children initiating antiretroviral therapy in Tanzania. J Int Assoc Provid AIDS Care. 2015;14(2):172–179. doi: 10.1177/2325957413500852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekpo UF, Omotayo AM, Dipeolu MA. Prevalence of malnutrition among settled pastoral Fulani children in Southwest Nigeria. BMC Res Notes. 2008;1(1):7. doi: 10.1186/1756-0500-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penda CI, Moukoko ECE, Nolla NP, Evindi NOA, Ndombo PK. Malnutrition among HIV infected children under 5 years of age at the Laquintinie hospital Douala, Cameroon. Pan Afr Med J. 2018;30:91. doi: 10.11604/pamj.2018.30.91.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trehan I, O’Hare BA, Phiri A, Heikens GT. Challenges in the Management of HIV-Infected Malnourished Children in Sub-Saharan Africa [Internet]. Vol. 2012, AIDS Research and Treatment. Hindawi; 2012. p. e790786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogia P, Ambey R, Sahu S. Assessment of nutritional status and the variables affecting the nutritional status of human immunodeficiency virus-positive children in antiretroviral therapy center of J. A. Group of hospitals, Gajra Raja medic. Int J Contemp Pediatr. 2016;3(4):1227–1231. doi: 10.18203/2349-3291.ijcp20163515. [DOI] [Google Scholar]
- 32.Poda GG, Hsu C-Y, Chao JC-J. Malnutrition is associated with HIV infection in children less than 5 years in Bobo-Dioulasso City, Burkina Faso. Medicine (Baltimore). 2017;96(21) May 26 [cited 2021 Jan 26]. [DOI] [PMC free article] [PubMed]
- 33.Endalamaw A, Mekonnen M, Geremew D, Yehualashet FA, Tessera H, Habtewold TD. HIV/AIDS treatment failure and associated factors in Ethiopia: a meta-analysis. BMC Public Health. 2020;20(1):82. doi: 10.1186/s12889-020-8160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nega J, Taye S, Million Y, Rodrigo C, Eshetie S. Antiretroviral treatment failure and associated factors among HIV patients on first-line antiretroviral treatment in Sekota, Northeast Ethiopia. AIDS Res Ther. 2020;17(1):39. doi: 10.1186/s12981-020-00294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.HIV symptoms timeline: Stages, preventing progression, and outlook [Internet]. 2020 [cited 2021 Feb 14]. Available from: https://www.medicalnewstoday.com/articles/316056
- 36.Zanoni BC, Phungula T, Zanoni HM, France H, Cook EF, Feeney ME. Predictors of poor CD4 and weight recovery in HIV-infected children initiating ART in South Africa. PLoS One. 2012;7(3):e33611. doi: 10.1371/journal.pone.0033611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Severe acute malnutrition - Clinical guidelines [Internet]. [cited 2021 Feb 14]. Available from: https://medicalguidelines.msf.org/viewport/CG/english/severe-acute-malnutrition-16689141.html
- 38.Daka DW, Ergiba MS. Prevalence of malnutrition and associated factors among adult patients on antiretroviral therapy follow-up care in Jimma medical center, Southwest Ethiopia. PLoS One. 2020;15(3):e0229883. doi: 10.1371/journal.pone.0229883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moolasart V, Chottanapund S, Ausavapipit J, Ampornareekul S. Prevalence and risk factors of malnutrition among HIV-infected children aged 2-18 years: a cross-sectional study. Pediatr Infect Dis Open Access. 2017;2(1) Mar 4 [cited 2021 Jan 26].
- 40.Stages of HIV infection | Terrence Higgins Trust [Internet]. [cited 2021 Feb 14]. Available from: https://www.tht.org.uk/hiv-and-sexual-health/about-hiv/stages-hiv-infection
- 41.Pediatric HIV Infection: Practice Essentials, Background, Pathophysiology. 2020 Dec 6 [cited 2021 Feb 14]; Available from: https://emedicine.medscape.com/article/965086-overview
- 42.Mekonnen A. Assessment of magnitude and factors affecting the nutritional status of HIV infected under-five children at five public hospitals in Addis Ababa and its programmatic implication. 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author.