Abstract
Background
Lipid accumulation product (LAP) and cardiometabolic index (CMI) are two novel obesity-related indexes associated with enhancing metabolic disease (MD) risk. Current evidences suggest that the differences in sex hormones and regional fat distribution in both sexes are directly correlated with MD and nonalcoholic fatty liver disease (NAFLD) risk. Hence, NAFLD incidences reflect sex differences. Herein, we examined the accuracy of LAP and CMI in diagnosing NAFLD in both sexes.
Methods
Overall, 14,407 subjects, who underwent health check-up in the northeastern China, were enrolled in this study, and their corresponding LAP and CMI were calculated. Abdominal ultrasonography was employed for NAFLD diagnosis. Multivariate analyses were analyzed potential correlations between LAP and/or CMI and NAFLD. Odds ratios (ORs) and 95% confidence intervals (CIs) were evaluated. Receiver operating characteristic curve analyses was executed for the exploration of the diagnostic accuracies. Areas under the curves (AUCs) with 95%CIs were calculated.
Results
NAFLD prevalence increased with elevated quartiles of LAP and CMI in both sexes. In multivariate logistic regression analyses, LAP and CM expressed as continuous variables or quartiles, significantly correlated with NAFLD. The ORs for the top versus bottom quartile of LAP and CMI for NAFLD were 13.183 (95%CI = 8.512–20.417) and 8.662 (95%CI = 6.371–11.778) in women and 7.544 (95%CI = 5.748–9.902) and 5.400 (95%CI = 4.297–6.786) in men. LAP and CMI exhibited larger AUCs, compared to other obesity-related indexes in terms of discriminating NAFLD. The AUCs of LAP and CMI were 0.860 (95%CI = 0.852–0.867) and 0.833 (95%CI = 0.825–0.842) in women and 0.816 (95%CI = 0.806–0.825) and 0.779 (95%CI = 0.769–0.789) in men.
Conclusions
LAP and CMI are convenient indexes for the screening and quantification of NAFLD within a Chinese adult population. Their associations with NAFLD are substantially greater in women than men.
Keywords: Nonalcoholic fatty liver disease, Lipid accumulation product, Cardiometabolic index, Sex
Background
With the recent advancements in social economy and subsequent alterations in dietary structure and living habits, nonalcoholic fatty liver disease (NAFLD) prevalence has gradually become a principal community health challenge throughout the world [1]. NAFLD represents an extensive range of liver diseases, for instance, nonalcoholic steatosis, nonalcoholic steatohepatitis, liver cirrhosis and hepatocellular carcinoma [2]. It is clearly associated with obesity, type 2 diabetes mellitus, dyslipidemia, metabolic syndrome (MS) and cardiovascular disease [3]. Thus, early NAFLD identification and diagnosis in a simple and effective manner is essential to prevent and delay the advancement of NAFLD and occurrence of its related complications.
Lipid accumulation product (LAP), which is independently calculated for males and females, is an alternative index for excess lipid accumulation [4]. Several studies revealed that LAP is a strong anthropometric indicator to predict diabetes, MS, insulin resistance and NAFLD [5–8]. Current evidence suggests that the differences in sex hormones and regional fat distribution in both sexes are directly correlated with regulating metabolic disorder (MD) and NAFLD. Hence, NAFLD prevalence reflects sex differences [9]. However, very few studies examined how sex differences affected the relationship between LAP and NAFLD.
Recently, cardiometabolic index (CMI) was proposed as a simple and reliable surrogate indicator for diabetes recognition [10]. A community-based study involving 11,478 participants from rural Northeastern China estimated diabetes prevalence using CMI, and supported CMI as an economic, stable and dose-dependent index for screening and discriminating diabetes among a general Chinese population [11]. Several studies also revealed its value in identifying the deterioration of metabolic profile and cardiovascular diseases, including hypertension, hyperuricemia, arterial stiffness, ischemic stroke, and left ventricular geometry abnormality [12–16]. Considering that MS is closely associated with NAFLD, there may be an association between CMI and NAFLD. Additionally, CMI and NAFLD prevalence often vary by sex, so there may potentially be a sex -specific difference in CMI action.
Herein, a cross-sectional investigation was conducted to explore the clinical role of LAP and CMI stratified by sex-specific quartiles in the prevalence of NAFLD, and to present a theoretical foundation for the screening of NAFLD among a Chinese adult population.
Methods
Study population
A cross-sectional epidemiological investigation was performed in subjects (age ≥ 18 years) who underwent their physical examinations at the First Affiliated Hospital of the China Medical University between January 2019 and December 2019. The following subjects were excluded from analysis: (1) subjects who had long-term excessive drinking habit (alcohol intake exceeded 20 g per day for male or 10 g per day for female) [17]; (2) subjects who had viral hepatitis, drug-linked liver injury, autoimmune liver disease and other specific illnesses (i.e. Reye’s syndrome, acute fatty liver of pregnancy, Wilson’s disease) that can lead to fatty liver; (3) subjects who had consumed hepatoprotective drugs; (4) subjects with severe liver and kidney dysfunction; (5) missing data. The study was approved by the Ethics Committee of China Medical University (approval number: 2019–77). The informed consent requirement was exempted owing to the retrospective nature of this research.
Data collection
Demographic characteristics and general information of the subjects were collected by self-administered questionnaire regarding age, sex, medical history, family history, medication history and alcohol consumption. Standard weight and height were assessed while subjects had on light clothing and no shoes. Waist circumference (WC) was determined, employing a soft tape, at the midpoint of the distance between the lower edge of the costal ridge and the upper border of the iliac crest. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice following a 5 min rest, using an electronic sphygmomanometer. The average of the two values was documented as the final blood pressure. Upon overnight fasting, the samples of venous blood were accumulated and measured for biochemical markers, including serum triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), fasting blood glucose (FPG), serum uric acid (UA), serum creatinine (Scr), blood urea nitrogen (BUN), white blood cell count (WBC), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and γ-glutamyltransferase (γ-GGT) using a Cobas 8000 automatic biochemical analyzer.
Definitions
Following the Asia-Pacific Working Party criteria, the NAFLD diagnosis was made according to the results of abdominal ultrasonography scans, supporting the presence of fatty liver disease. This excluded cases of excessive alcohol consumption, hepatitis virus, hepatotoxic medicines and autoimmune liver diseases, based on the results of self-administered questionnaire [17]. Fatty liver was evaluated according to the presence or absence of hepatic steatosis, based on echo patterns, namely, hepatic versus nephritic diffuse hyper-echogenicity, limited visualization of intrahepatic structures, and ultrasound beam attenuation without semi-quantitative indices. All ultrasonographic investigations were conducted by a trained and experienced professional radiologist using a 3.5-MHz ultrasonic probe (Acuson X300, Siemens, Germany).
The body mass index (BMI) was evaluated as weight in kilograms divided by the square of height in meters. Waist-to-height (WHtR) was defined as WC divided by height in centimeters. LAP was ascertained employing the formula given below [4]: LAP = TG (mmol/L) × [WC (cm)-58] for women and LAP = TG (mmol/L) × [WC (cm)-65] for men. CMI was assessed with the following equation9: CMI = TG (mmol/L)/HDL-C (mmol/L) × WHtR.
Current smoking was described as regular cigarette smoking for over 6 months at the time of physical examination [18]. Regular exercising was described as 30 min of moderate-intensity activity for over three times a week [19].
Statistical analyses
All analyses were separated by sex. The Kolmogorov-Smirnov assessment was employed to investigate the normal distribution of continuous variables. The normally distributed outcomes were given as mean ± standard deviation (SD), and the intergroup comparisons were fulfilled with the Student’s t test. Non-normally distributed data were given as median with interquartile range, and the intergroup differences were carried out assessment via the Mann-Whitney U test. Categorical outcomes are presented as counts and percentages, and the intergroup differences were assessed via chi-squared test. The LAP and CMI quartiles were divided into four groups: quartile 1 (Q1) (≤P25), quartile 2 (Q2) (P25, P50), quartile 3 (Q3) (P50, P75), and quartile 4 (Q4) (>P75). The independent association of LAP and CMI was explored as continuous variables or quartiles with NAFLD occurrence. Upon adjusting for possible confounding variables, a multivariable model was utilized to evaluate the influence of LAP and CMI on NAFLD prevalence. The odds ratios (ORs) and 95% confidence intervals (CIs) were presented to predict the effect. The sex -specific estimation of the OR for 1 SD increment in LAP and CMI was obtained to ascertain NAFLD risk. A receiver operator characteristic (ROC) curve assessment was conducted for ascertaining the ability of indicators to predict NAFLD diagnosis, and to confirm the optimal cut-off values. SPSS version 23.0 (IBM, Corp., N.Y., USA) and Stata Software version 16.0 (Stata, Corp., N.Y., USA) were employed for all statistical assessments. Two-tailed P values <0.05 was set as statistically significant.
Results
Baseline characteristics
Overall, 14,407 eligible subjects (7630 females and 6777 males) were evaluated, with the median age (interquartile range) of 47 (35, 57) years. There were 2030 female participants and 4263 male participants diagnosed with NAFLD, according to the entry criteria, with a prevalence of 26.61 and 62.90%, respectively. The baseline features of the subjects, in terms of their NAFLD status, were separately described in Table 1 for each sex. Overall, NAFLD patients were advanced in age, compared to non-NAFLD subjects, and NAFLD patients exercised less regularly than non-NAFLD participants. Furthermore, regardless of sex, NAFLD patients exhibited significantly elevated BMI, WC, WHtR, LAP, CMI, WBC, SBP, DBP, TG, TC, LDL-C, FPG, ALT, AST, GGT, and UA and reduced HDL-C, relative to non-NAFLD participants. In terms of females, NAFLD patients had a significantly higher BUN than non-NAFLD participants. In terms of males, NAFLD patients had a significantly higher proportion of current smoking than non-NAFLD participants.
Table 1.
Baseline characteristics of subjects stratified by gender
| Variables | Women (n = 7630) | χ2/Z | P value a | Men (n = 6777) | χ2/Z | P value a | ||
|---|---|---|---|---|---|---|---|---|
| non-NAFLD (n = 5600) | NAFLD (n = 2030) | non-NAFLD (n = 2514) | NAFLD (n = 4263) | |||||
| Age (years) | 41 (32,53) | 55 (45,63) | − 28.372 | <0.001 | 47 (35,60) | 48 (37,57) | −2.539 | 0.011 |
| Current smoking (%) | 350 (6.25) | 112 (6.40) | 0.060 | 0.807 | 1055 (41.96) | 2260 (53.01) | 77.264 | <0.001 |
| Regular exercising (%) | 2948 (52.64) | 849 (41.82) | 69.775 | <0.001 | 1236 (49.16) | 1361 (31.93) | 198.831 | <0.001 |
| BMI (Kg/m2) | 22.39 (20.67,24.28) | 26.15 (24.29,28.35) | −44.559 | <0.001 | 23.97 (22.19,25.74) | 26.90 (25.11,29.00) | −39.727 | <0.001 |
| WC (cm) | 71 (67,77) | 81 (76,86) | −42.645 | <0.001 | 80 (76,85.25) | 88 (83,93) | −37.476 | <0.001 |
| WHtR | 0.44 (0.42,0.48) | 0.51 (0.48,0.55) | − 43.521 | <0.001 | 0.47 (0.44,0.50) | 0.51 (0.48,0.54) | −36.3 | <0.001 |
| LAP (cm.mol/L) | 11.00 (6.00,19.56) | 35.41 (22.46,54.73) | −48.078 | <0.001 | 16.08 (9.00,25.76) | 39.60 (24.13,63.50) | −43.466 | <0.001 |
| CMI | 0.24 (0.15,0.39) | 0.61 (0.39,0.95) | −44.588 | <0.001 | 0.38 (0.25,0.60) | 0.81 (0.51,1.31) | −38.499 | <0.001 |
| WBC (109/L) | 5.81 (4.91,6.83) | 6.34 (5.41,7.40) | −13.730 | <0.001 | 6.10 (5.20,7.17) | 6.69 (5.77,7.72) | −14.980 | <0.001 |
| SBP (mmHg) | 120 (110,132) | 136 (122,151) | −28.521 | <0.001 | 128 (117,142) | 134 (123,147) | −12.480 | <0.001 |
| DBP (mmHg) | 71 (64,78) | 77 (70,86) | −21.709 | <0.001 | 76 (69,84) | 81 (74,90) | −16.262 | <0.001 |
| TG (mmol/L) | 0.83 (0.60,1.18) | 1.52 (1.07,2.11) | −40.024 | <0.001 | 1.03 (0.75,1.47) | 1.73 (1.20,2.53) | −34.564 | <0.001 |
| TC (mmol/L) | 4.69 (4.15,5.34) | 5.21 (4.60,5.88) | −19.221 | <0.001 | 4.61 (4.09,5.21) | 4.87 (4.33,5.48) | −11.856 | <0.001 |
| HDL-C (mmol/L) | 1.54 (1.33,1.78) | 1.28 (1.12,1.49) | −28.916 | <0.001 | 1.27 (1.09,1.48) | 1.09 (0.94,1.26) | −25.385 | <0.001 |
| LDL-C (mmol/L) | 2.80 (2.32,3.37) | 3.36 (2.83,3.96) | −24.385 | <0.001 | 2.92 (2.46,3.44) | 3.18 (2.68,3.70) | −12.513 | <0.001 |
| FPG (mmol/L) | 4.97 (4.71,5.26) | 5.37 (5.03,5.90) | −30.224 | <0.001 | 5.16 (4.85,5.51) | 5.39 (5.03,5.97) | −17.626 | <0.001 |
| ALT (U/L) | 13 (10,17) | 19 (14,26) | −33.115 | <0.001 | 17 (13,23) | 25 (18,36) | −29.760 | <0.001 |
| AST (U/L) | 17 (15,20) | 20 (17,24) | −19.249 | <0.001 | 19 (16,22) | 21 (18,25) | −14.857 | <0.001 |
| GGT (U/L) | 14 (11,18) | 21 (16,29) | −35.491 | <0.001 | 21 (16,30) | 33 (24,49) | −30.714 | <0.001 |
| BUN (mmol/L) | 4.50 (3.76,5.33) | 4.82 (4.09,5.71) | −10.698 | <0.001 | 5.23 (4.46,6.13) | 5.21 (4.47,6.08) | − 1.083 | 0.279 |
| Scr (μmol/L) | 53 (48,58) | 53 (48,59) | −1.531 | 0.126 | 73 (67,80) | 73 (66,80) | −1.549 | 0.121 |
| UA (μmol/L) | 253 (221,289) | 297 (258,343) | −28.558 | <0.001 | 343 (299,390) | 383 (334,438) | −20.998 | <0.001 |
Data were expressed as median (interquartile range) and numbers (percentage) as appropriate.
Abbreviations: NAFLD nonalcoholic fatty liver disease, BMI body mass index, WC waist circumference, WHtR waist-to-height, LAP lipid accumulation product, CMI cardiometabolic index, WBC white blood cell count, SBP systolic blood pressure, DBP diastolic blood pressure, TG triglyceride, TC total cholesterol, HDL-C high density lipoprotein cholesterol, LDL-C low density lipoprotein cholesterol, FPG fasting blood glucose, ALT alanine aminotransferase, AST aspartate aminotransferase, GGT γ-glutamyltransferase, BUN blood urea nitrogen, Scr serum creatinine, UA uric acid
a. Comparisons of continuous variables between groups were tested by Mann-Whitney U test due to skewed distribution and categorical variables between groups were tested by chi-squared test
Relationship of LAP and CMI with risk of NAFLD
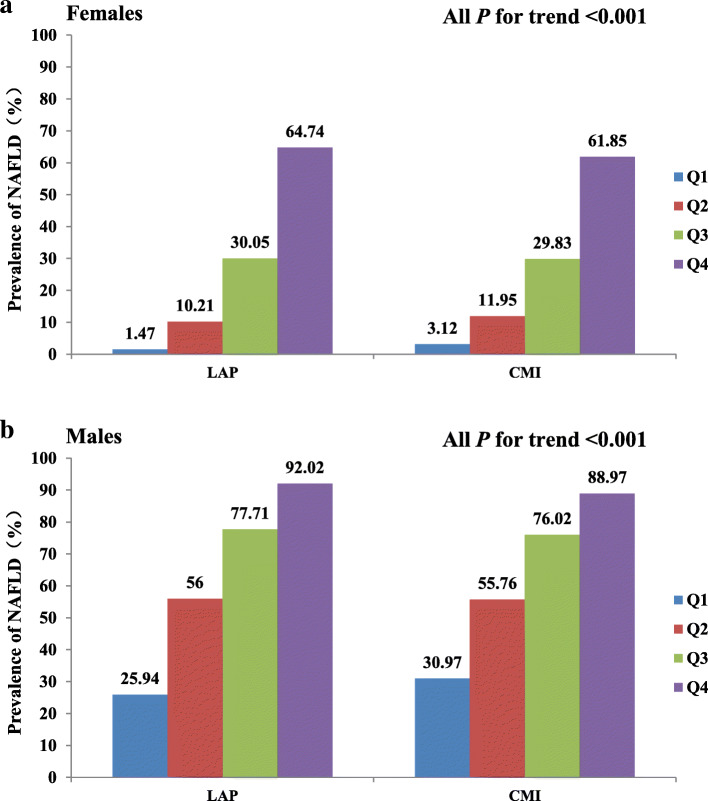
Based on the sex-specific quartile analysis, a dose-response association existed between LAP and CMI with NAFLD risk (Fig.1). Regardless of sex, NAFLD prevalence increased progressively with ascending quartile of LAP and CMI (all p for trend <0.001). In the case of females, NAFLD prevalence was 44.04 and 19.82-folds higher across LAP and CMI quartiles, respectively. In males, NAFLD prevalence was 3.55 and 2.87-folds higher across LAP and CMI quartiles, respectively.
Fig. 1.
Prevalence of NAFLD according to the quartiles of LAP and CMI. Abbreviations: NAFLD, nonalcoholic fatty liver disease; LAP: lipid accumulation product; CMI, cardiometabolic index
Multivariate logistic regression assessing LAP and CMI influence on NAFLD identification
In multivariate logistic regression analyses, LAP and CMI expressed as either continuous variables or quartiles, markedly associated with NAFLD in all models (Table 2).
Table 2.
Multivariate logistic regression of LAP and CMI for NAFLD
| Variables | Odds Ratio (95%CI) | |||||
|---|---|---|---|---|---|---|
| Crude | P value | Model 1 | P value | Model 2 | P value | |
| Females | ||||||
| LAP level (per 1 SD change) | 1.068 (1.065,1.072) | <0.001 | 1.035 (1.031,1.039) | <0.001 | 1.026 (1.022,1.030) | 0.002 |
| Quartiles of LAP | ||||||
| Q1 (≤7.52) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Q2 (7.52–15.12) | 7.639 (5.113,11.412) | <0.001 | 4.275 (2.831,6.456) | <0.001 | 3.437 (2.261,5.225) | <0.001 |
| Q3 (15.12–29.60) | 28.840 (19.609,42.417) | <0.001 | 9.736 (6.481,14.623) | <0.001 | 6.575 (4.327,9.991) | <0.001 |
| Q4 (>29.60) | 123.295 (83.913,181.159) | <0.001 | 24.822 (16.335,37.720) | <0.001 | 13.183 (8.512,20.417) | <0.001 |
| P value for trend | <0.001 | <0.001 | <0.001 | |||
| CMI level (per 1 SD change) | 23.521 (19.410,28.503) | <0.001 | 8.114 (6.642,9.913) | <0.001 | 3.110 (2.579,3.750) | <0.001 |
| Quartiles of CMI | ||||||
| Q1 (≤0.18) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Q2 (0.18–0.30) | 4.211 (3.151,5.628) | <0.001 | 2.841 (2.100,3.843) | <0.001 | 2.246 (1.637,3.082) | <0.001 |
| Q3 (0.30–0.53) | 10.103 (17.371,13.247) | <0.001 | 6.338 (4.758,8.443) | <0.001 | 3.983 (2.946,5.387) | <0.001 |
| Q4 (>0.53) | 38.459 (50.347,65.909) | <0.001 | 18.746 (14.059,24.996) | <0.001 | 8.662 (6.371,11.778) | <0.001 |
| P value for trend | <0.001 | <0.001 | <0.001 | |||
| Males | ||||||
| LAP level (per 1 SD change) | 1.063 (1.059,1.067) | <0.001 | 1.042 (1.038,1.046) | <0.001 | 1.033 (1.029,1.037) | <0.001 |
| Quartiles of LAP | ||||||
| Q1 (≤16.02) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Q2 (16.02–28.52) | 3.632 (3.142,4.199) | <0.001 | 2.288 (1.925,2.721) | <0.001 | 1.790 (1.491,2.148) | <0.001 |
| Q3 (28.52–50.00) | 9.953 (8.501,11.653) | <0.001 | 5.087 (4.191,6.175) | <0.001 | 3.462 (2.810,4.265) | <0.001 |
| Q4 (>50.00) | 32.922 (26.775,40.481) | <0.001 | 13.048 (10.205,16.683) | <0.001 | 7.544 (5.748,9.902) | <0.001 |
| P value for trend | <0.001 | <0.001 | <0.001 | |||
| CMI level (per 1 SD change) | 9.435 (8.069,11.033) | <0.001 | 6.078 (5.162,7.156) | <0.001 | 3.069 (2.603,3.618) | <0.001 |
| Quartiles of CMI | ||||||
| Q1 (≤0.36) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Q2 (0.36–0.62) | 2.810 (2.442,3.233) | <0.001 | 2.467 (2.100,2.899) | <0.001 | 1.779 (1.492,2.122) | <0.001 |
| Q3 (0.62–1.05) | 7.068 (6.068,8.234) | <0.001 | 5.247 (4.408,6.245) | <0.001 | 2.884 (2.379,3.496) | <0.001 |
| Q4 (>1.05) | 17.978 (14.967,21.595) | <0.001 | 12.278 (9.990,15.089) | <0.001 | 5.400 (4.297,6.786) | <0.001 |
| P value for trend | <0.001 | <0.001 | <0.001 | |||
Abbreviations: NAFLD nonalcoholic fatty liver disease, LAP lipid accumulation product, CMI cardiometabolic index. Crude: no adjustment; Model 1: adjusted for age, current smoking, regularly exercising, BMI. Model 2: adjusted for all the factors in Model 1 and WBC, SBP, TC, LDL-C, FPG, ALT, GGT, UA among males, additionally plus BUN among females
Among women, the ORs for 1 SD elevation of LAP and CMI were 1.068 (95%CI = 1.065–1.072) and 23.521 (95%CI = 19.410–28.503), respectively. Upon adjusting for several possible confounders (Model 2), it was still significant [LAP, OR = 1.026 (95%CI = 1.022–1.030) per 1 SD increment; CMI, OR = 3.110 (95%CI = 2.579–3.750) per 1 SD increment; all P<0.001]. For males, the ORs for 1 SD elevation in LAP and CMI were 1.063 (95%CI = 1.059–1.067) and 9.435 (95%CI = 8.069–11.033), respectively. Upon adjusting for several possible confounders (Model 2), it was still significant [LAP, OR = 1.033 (95%CI = 1.029–1.037) per 1 SD increment; CMI, OR = 3.069 (95%CI = 2.603–3.618) per 1 SD increment; all P<0.001].
Upon dividing LAP and CMI into quartiles, their relationship with NAFLD remained statistically significant. After adjusting for several possible confounders (Model 2), subjects in the largest LAP quartile displayed a 13.183-fold (95%CI = 8.512–20.417) NAFLD risk in females and 7.544-fold (95%CI = 5.748–9.902) NAFLD risk in males. Subjects in the largest CMI quartile displayed an 8.662-fold (95%CI = 6.371–11.778) NAFLD risk in females and 5.400-fold (95%CI = 4.297–6.786) NAFLD risk in males. All P values for this trend were less than 0.001 in both sexes.
Diagnostic capacity of anthropometric indices for predicting NAFLD
Table 3 summarized the AUCs of various adiposity NAFLD markers by sex. In females, LAP displayed the largest AUC (0.860, 95%CI = 0.852–0.867) followed by CMI (0.833, 95%CI = 0.825–0.842), WHtR (0.826, 95%CI = 0.817–0.834), BMI (0.820, 95%CI = 0.814–0.825) and WC (0.819, 95%CI = 0.810–0.827). In males, LAP displayed the largest AUC (0.816, 95%CI = 0.806–0.825), followed by CMI (0.779, 95%CI = 0.769–0.789), BMI (0.777, 95%CI = 0.771–0.783), WC (0.772, 95%CI = 0.762–0.782) and WHtR (0.764, 95%CI = 0.753–0.774). Notably, in both sexes, LAP and CMI were more accurate than other obesity-related indexes in discriminating the presence of NAFLD.
Table 3.
AUCs of various indexes for discriminating NAFLD by sex
| Variables | AUC (95%CI) | P value | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Females | |||||
| LAP (cm.mmol/L) | 0.860 (0.852,0.867) | <0.001 | 19.2 | 82.32 | 74.43 |
| CMI | 0.833 (0.825,0.842) | <0.001 | 0.34 | 81.92 | 68.69 |
| WHtR | 0.826 (0.817,0.834) | <0.001 | 0.47 | 80.54 | 69.57 |
| BMI (Kg/m2) | 0.820 (0.814,0.825) | <0.001 | 23.94 | 78.19 | 70.38 |
| WC (cm) | 0.819 (0.810,0.827) | <0.001 | 75 | 78.28 | 70.11 |
| Males | |||||
| LAP (cm.mmol/L) | 0.816 (0.806, 0.825) | <0.001 | 27.86 | 68.82 | 78.72 |
| CMI | 0.779 (0.769, 0.789) | <0.001 | 0.56 | 69.97 | 72.39 |
| WHtR | 0.764 (0.753, 0.774) | <0.001 | 0.48 | 74.38 | 65.91 |
| BMI (Kg/m2) | 0.777 (0.771,0.783) | <0.001 | 25.08 | 75.36 | 68.23 |
| WC (cm) | 0.772 (0.762, 0.782) | <0.001 | 83 | 73.99 | 66.79 |
Abbreviations: AUC area under the ROC curve, 95% CI 95% confidence interval, LAP lipid accumulation product, BMI body mass index, CMI cardiometabolic index, WC waist circumference
Discussion
Among the populous Chinese study population, the findings were as follows. First, the role of LAP was confirmed in the identification of NAFLD, after adjusting for major confounders in both sexes. Second, for the first time, CMI was found to be significantly associated with NAFLD prevalence, after adjusting for major confounders in both sexes. Thus, CMI appears to be a simple and promising tool in the early monitoring and targeted intervention of NAFLD. Third, both LAP and CMI exhibited a stronger correlation with NAFLD in females than in males. The tends of NAFLD risk to increase in females, particularly around the age of 55 years old, at which point most women underwent menopause. Post menopause, estrogen levels plummet and body fat distribution shifts to the abdominal region [9]. Estrogen is known to modulate lipid metabolism while suppressing vascular, inflammation, cell growth, and plaque advancement in premenopausal women. Menopausal initiates a cascade of biological and physiological alterations, which includes fat redistribution (i.e., accumulation of visceral fat), dyslipidemia and glucose intolerance, which are strongly correlated with enhanced IR, cardiovascular disease and NAFLD [20].
LAP, established by the National Nutrition Survey, is well accepted as a novel obesity-related index [4]. Compared to traditional obesity-related indexes, LAP comprehensively evaluates excessive lipid accumulation. In addition, LAP performed better than BMI in predicting the diabetes risk [21], and was a reliable predictor of insulin resistance. LAP also had a higher diagnostic accuracy in terms of MS, compared to BMI, WC and WHtR [22, 23]. In a cross-sectional study involving 40,459 subjects from southern China, LAP was shown to strongly associate with the diagnosis and severity of NAFLD [24], which was similar to this current findings from northern China. However, the best cut-off values for LAP in predicting NAFLD were a bit different between northern and southern Chinese population. This might be due to potential heterogeneity in geographical environment, regional climate, dietary and living habits, and prevalence of overweight and obese population between these two regions.
NAFLD pathogenesis may be attributed to abdominal obesity and high TG levels. In patients with abdominal obesity, visceral adipocytes induce synthesis of a variety of cytokines like interleukim-6 and tumor necrosis factor-α, which promote macrophage infiltration and chronic inflammation [25]. Simultaneously, adipocytes secrete adipose factor chemokine, which regulates carbohydrate and lipid metabolism [26]. Chronic inflammation can affect the signal transduction pathway of surrounding cells such as T cells (including invariant natural killer cells), eosinophils, B-regulatory cells (Bregs)12 and macrophages, leading to insulin resistance, liver steatosis, and eventually promotes NAFLD development [27]. In addition, omega-3 fatty acids, usage can usually lower liver steatosis by reducing TG levels, thus supporting the role of elevated TG levels in the development of NAFLD [28].
CMI is a recently developed index, based on TG/HDL-C and WHtR values that could easily be achieved during health check-ups [10]. As previously mentioned, multiple studies suggested that CMI was strongly associated with obesity-related metabolic diseases, such as diabetes and cardiovascular disease [12–15]. All components of CMI are also considered in the criteria for MS, including abdominal obesity and dyslipidemia. WHtR is an abdominal obesity measurement index that is strongly associated with lipid content and lipid distribution, and is superior to WC and BMI in the assessment of NAFLD [29]. Additionally, previous studies confirmed that TG/HDL-C was closely related to insulin resistance (IR), obesity and metabolic disorders and had a good predictive value for NAFLD diagnosis [30–32]. In this study, it was found that a larger CMI quartile was markedly and independently correlated with an enhanced NAFLD risk, based on a graded mode regardless of sex. In addition, the ROC analyses showed that CMI presented an adequate diagnostic performance.
In a relatively recent study, NAFLD was shown to be mutual and bi-directional related to MS [33]. Multiple essential metabolic indicators that make up the CMI also participate in regulating fatty liver disease. Moreover, the theory of IR is the core of NAFLD pathogenesis [34, 35]. The relationship between CMI and NAFLD is somewhat unclear. Based on the present findings, IR may mediate the connection [36]. Prior studies confirmed that the abdominal fat and TG/HDL-C ratio were closely related to IR. Patients with abdominal obesity exhibit high levels of glucose and lipid oxidation, and releases free fatty acid (FFA). Once FFA exceeds the buffer capacity of the peripheral fat storage library, liver fat accumulation can accelerate development of IR and NAFLD [37, 38]. Other studies revealed that IR promoted secretion of very low-lipoprotein (VLDL) and TG, and reduced HDL-C levels [39, 40]. In addition, some researchers reported that IR can promote NAFLD development by inducing TG decomposition of within adipose tissue and simultaneously enhancing TG synthesis in the liver [41, 42]. On contrary, IR emergence accelerates sugar decomposition, leading to an enhancement in blood glucose and VLDL, which then promotes the release of excessive TC into the blood, thus raising serum TC levels [43, 44].
Study strength and limitations
In this study, it was confirmed the role of LAP in identifying NAFLD, after adjusting for major confounders in both sexes. In addition, it was the first time to find that CMI was significantly associated with NAFLD prevalence, after adjusting for major confounders in both sexes. Third, both LAP and CMI exhibited a stronger correlation with NAFLD in females than in males. There were also some limitations in this study that deserved mention. First, the cross-sectional nature of this study only provided the correlation between LAP, CMI and NAFLD, but the cause of this association needs exploration via longitudinal investigations. Second, the patients were selected from the health check-up populations of Chinese adults. Thus, the conclusions might not be appropriate to people of other ethnicities or races. Thirdly, NAFLD was diagnosed via ultrasonography, which has limited sensitivity and unreliable detection of <5% liver fat infiltration [45]. Fourth, this study did not include subgroup analysis involving female menopausal status. Finally, there was no detailed division of various degrees of fatty liver, so it was impossible to evaluate the diagnostic value of CMI in nonalcoholic steatohepatitis and liver fibrosis. At present, liver biopsy, being an invasive test, is not typically required for NAFLD diagnosis [46]. Instead, scientists designed noninvasive approaches like computed tomography and magnetic resonance imaging to detect NAFLD. However, these approaches are both costly and time-consuming, and are not suitable for the screening and application of large-scale population [47]. Thus, simple and convenient new synthetic biological indexes, namely LAP and CMI, are of great significance to the general screening of NAFLD.
Conclusions
In conclusion, LAP and CMI positively and independently correlated with NAFLD risk, and had a stronger correlation in women. The novel and clinically effective markers LAP and CMI offer simple and easy approach to the early identification of people at an elevated risk of NAFLD. The conclusions emphasized the significance of personalized treatment plans, using early detection markers LAP and CMI and their sex-specific qualities in preventing NAFLD.
Acknowledgements
The authors are grateful to all the participants for their participation.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- BMI
body mass index
- WC
waist circumference
- WHtR
waist-to-height
- LAP
lipid accumulation product
- CMI
cardiometabolic index
- WBC
white blood cell count
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- TG
triglyceride
- TC
total cholesterol
- HDL-C
high density lipoprotein cholesterol
- LDL-C
low density lipoprotein cholesterol
- FPG
fasting blood glucose
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GGT
γ-glutamyltransferase
- BUN
blood urea nitrogen
- Scr
serum creatinine
- UA
uric acid
Authors’ contributions
Wei Wang conceived, designed and supervised the study. Yiting Liu collected the data and wrote the paper; and Wei Wang critically reviewed and edited the draft. All authors have read and approved the final manuscript.
Funding
This work was supported by the Doctor Initiation Fund of Natural Science Foundation Program [grant no.2021-BS-097].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent participate
The study was approved by the Ethics Committee of China Medical University (approval number: 2019–77). The informed consent requirement was exempted due to the retrospective study design.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huanan C, Sangsang L, Amoah AN, Yacong B, Xuejiao C, Zhan S, Guodong W, Jian H, Songhe S, Quanjun L. Relationship between triglyceride glucose index and the incidence of non-alcoholic fatty liver disease in the elderly: a retrospective cohort study in China. BMJ Open. 2020;10(11):e039804. doi: 10.1136/bmjopen-2020-039804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Zheng R, Li J, Feng S, Wang L, Huang Z. Association between triglyceride glucose-body mass index and non-alcoholic fatty liver disease in the non-obese Chinese population with normal blood lipid levels: a secondary analysis based on a prospective cohort study. Lipids Health Dis. 2020;19(1):229. doi: 10.1186/s12944-020-01409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu Y, Cai J, She Z, Li H. Insights into the epidemiology, pathogenesis, and therapeutics of nonalcoholic fatty liver diseases. Adv Sci (Weinh) 2019;6(4):1801585. doi: 10.1002/advs.201801585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn HS. The "lipid accumulation product" performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5(1):26. doi: 10.1186/1471-2261-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding YS, Li Y, Zhang XH, Ma RL, Guo H, Ma L, Liu T, Yao P. The improved lipid accumulation product is an accurate index for predicting metabolic syndrome in the Xinjiang population. Biomed Environ Sci. 2021;34(6):503–507. doi: 10.3967/bes2021.070. [DOI] [PubMed] [Google Scholar]
- 6.Dong L, Lin M, Wang W, Ma D, Chen Y, Su W, Chen Z, Wang S, Li X, Li Z, Liu C. Lipid accumulation product (LAP) was independently associatedwith obstructive sleep apnea in patients with type 2 diabetes mellitus. BMC Endocr Disord. 2020;20(1):179. doi: 10.1186/s12902-020-00661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HJ, Jo HN, Kim YH, Kim SC, Joo JK, Lee KS. Predictive value of lipid accumulation product, fatty liver index, visceral adiposity index for metabolic syndrome according to menopausal status. Metab Syndr Relat Disord. 2018;16(9):477–482. doi: 10.1089/met.2018.0019. [DOI] [PubMed] [Google Scholar]
- 8.Mazidi M, Kengne AP, Katsiki N, Mikhailidis DP, Banach M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J Diabetes Complicat. 2018;32(3):266–270. doi: 10.1016/j.jdiacomp.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, Suzuki A. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457–1469. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakabayashi I, Daimon T. The "cardiometabolic index" as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin Chim Acta. 2015;438:274–278. doi: 10.1016/j.cca.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Shi WR, Wang HY, Chen S, Guo XF, Li Z, Sun YX. Estimate of prevalent diabetes from cardiometabolic index in general Chinese population: a community-based study. Lipids Health Dis. 2018;17(1):236. doi: 10.1186/s12944-018-0886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Chen Y, Sun G, Jia P, Qian H, Sun Y. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad Med. 2018;130(3):325–333. doi: 10.1080/00325481.2018.1444901. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Sun Y, Wang S, Qian H, Jia P, Chen Y, Li Z, Zhang L. Body adiposity index, lipid accumulation product, and cardiometabolic index reveal the contribution of adiposity phenotypes in the risk of hyperuricemia among Chinese rural population. Clin Rheumatol. 2018;37(8):2221–2231. doi: 10.1007/s10067-018-4143-x. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Chen Y, Guo X, Chang Y, Sun Y. Usefulness of cardiometabolic index for the estimation of ischemic stroke risk among general population in rural China. Postgrad Med. 2017;129(8):834–841. doi: 10.1080/00325481.2017.1375714. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Sun Y, Li Z, Guo X, Chen S, Ye N, Tian Y, Zhang L. Gender-specific contribution of cardiometabolic index and lipid accumulation product to left ventricular geometry change in general population of rural China. BMC Cardiovasc Disord. 2018;18(1):62. doi: 10.1186/s12872-018-0798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakabayashi I, Marumo M, Kubota Y, Higashiyama A, Miyamoto Y, Okamura T. Cardiometabolic index as a useful discriminator for the risk of increased arterial stiffness. Clin Chim Acta. 2018;486:42–43. doi: 10.1016/j.cca.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Farrell GC, Chitturi S, Lau GK, Sollano JD. Asia-Pacific working party on N. guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22(6):775–777. doi: 10.1111/j.1440-1746.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan RC, Baldoni PL, Strizich GM, Perez-Stable EJ, Saccone NL, Peralta CA, Perreira KM, Gellman MD, Williams-Nguyen JS, Rodriguez CJ, et al. Current smoking raises risk of incident hypertension: Hispanic community health study-study of Latinos. Am J Hypertens. 2021;34(2):190–197. doi: 10.1093/ajh/hpaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rominger C, Papousek I, Fink A, Perchtold CM, Lackner HK, Weiss EM, Schwerdtfeger AR. Creative challenge: regular exercising moderates the association between task-related heart rate variability changes and individual differences in originality. PLoS One. 2019;14(7):e0220205. doi: 10.1371/journal.pone.0220205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barton M. Cholesterol and atherosclerosis: modulation by oestrogen. Curr Opin Lipidol. 2013;24(3):214–220. doi: 10.1097/MOL.0b013e3283613a94. [DOI] [PubMed] [Google Scholar]
- 21.Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care. 2006;29(1):151–153. doi: 10.2337/diacare.29.1.151. [DOI] [PubMed] [Google Scholar]
- 22.Nascimento-Ferreira MV, Rendo-Urteaga T, Vilanova-Campelo RC, Carvalho HB, da Paz OG, Paes Landim MB, Torres-Leal FL. The lipid accumulation product is a powerful tool to predict metabolic syndrome in undiagnosed Brazilian adults. Clin Nutr. 2017;36(6):1693–1700. doi: 10.1016/j.clnu.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Karatas S, Beysel S. Visceral adiposity index, triglyceride/high-density lipoprotein ratio, and lipid accumulation product index to discriminate metabolic syndrome among adult type 1 diabetes patients. Metab Syndr Relat Disord. 2021;19(9):507–512. doi: 10.1089/met.2021.0047. [DOI] [PubMed] [Google Scholar]
- 24.Dai H, Wang W, Chen R, Chen Z, Lu Y, Yuan H. Lipid accumulation product is a powerful tool to predict non-alcoholic fatty liver disease in Chinese adults. Nutr Metab (Lond) 2017;14:49. doi: 10.1186/s12986-017-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tantanavipas S, Vallibhakara O, Sobhonslidsuk A, Phongkitkarun S, Vallibhakara SA, Promson K, et al. Abdominal obesity as a predictive factor of nonalcoholic fatty liver disease assessed by ultrasonography and transient Elastography in polycystic ovary syndrome and healthy women. Biomed Res Int. 2019;9047324. 10.1155/2019/9047324. [DOI] [PMC free article] [PubMed]
- 26.Silaghi CA, Silaghi H, Craciun AE, Farcas A, Colosi HA, Cosma DT, Pais R, Hancu N, Georgescu CE. Age, abdominal obesity, and glycated hemoglobin are associated with carotid atherosclerosis in type 2 diabetes patients with nonalcoholic fatty liver disease. Med Ultrason. 2015;17(3):300–307. doi: 10.11152/mu.2013.2066.173.cas. [DOI] [PubMed] [Google Scholar]
- 27.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 28.Antraco VJ, Hirata BKS, de Jesus Simao J, Cruz MM, da Silva VS, da Cunha de Sa RDC, Abdala FM, Armelin-Correa L, Alonso-Vale MIC.Omega-3 Polyunsaturated Fatty Acids Prevent Nonalcoholic Steatohepatitis (NASH) and Stimulate Adipogenesis. Nutrients. 2021;13(2):622. 10.3390/nu13020622. [DOI] [PMC free article] [PubMed]
- 29.Lin IT, Lee MY, Wang CW, Wu DW, Chen SC. Gender differences in the relationships among metabolic syndrome and various obesity-related indices with nonalcoholic fatty liver disease in a Taiwanese population. Int J Environ Res Public Health. 2021;18(3):857. doi: 10.3390/ijerph18030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Wang B, Yuan H, Li X, Mg KB. Triglycerides to High-Density Lipoprotein Cholesterol Ratio Is the Best Surrogate Marker for Insulin Resistance in Nonobese Middle-Aged and Elderly Population: A Cross-Sectional Study. Int J Endocrinol. 2021;2021:6676569. doi: 10.1155/2021/6676569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quispe R, Martin SS, Jones SR. Triglycerides to high-density lipoprotein-cholesterol ratio, glycemic control and cardiovascular risk in obese patients with type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):150–156. doi: 10.1097/MED.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 32.Fan N, Peng L, Xia Z, Zhang L, Song Z, Wang Y, Peng Y. Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis. 2019;18(1):39. doi: 10.1186/s12944-019-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonardo A, Leoni S, Alswat KA, Fouad Y. History of nonalcoholic fatty liver disease. Int J Mol Sci. 2020;21(16):5888. doi: 10.3390/ijms21165888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armandi A, Rosso C, Caviglia GP, Bugianesi E. Insulin resistance across the Spectrum of nonalcoholic fatty liver disease. Metabolites. 2021;11(3):155. doi: 10.3390/metabo11030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. 2017;9(4):387. doi: 10.3390/nu9040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5(5):1544–1560. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin Y, Wang Y, Chi J, Zhu X, Zhao H, Zhao S, Wang Y. Elevated free fatty acid level is associated with insulin-resistant state in nondiabetic Chinese people. Diabetes Metab Syndr Obes. 2019;12:139–147. doi: 10.2147/DMSO.S186505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medina-Urrutia A, Posadas-Romero C, Posadas-Sanchez R, Jorge-Galarza E, Villarreal-Molina T, Gonzalez-Salazar Mdel C, Cardoso-Saldana G, Vargas-Alarcon G, Torres-Tamayo M, Juarez-Rojas JG. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc Diabetol. 2015;14(1):20. doi: 10.1186/s12933-015-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32(9):2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 40.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011;22(9):353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Du T, Zhang J, Lu H, Lin X, Xie J, Yang Y, Yu X. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16(1):15. doi: 10.1186/s12944-017-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berk PD, Verna EC. Nonalcoholic fatty liver disease: lipids and insulin resistance. Clin Liver Dis. 2016;20(2):245–262. doi: 10.1016/j.cld.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ballestri S, Nascimbeni F, Romagnoli D, Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res. 2016;46(11):1074–1087. doi: 10.1111/hepr.12656. [DOI] [PubMed] [Google Scholar]
- 44.Makaridze Z, Giorgadze E, Asatiani K. Association of the apolipoprotein b/apolipoprotein a-I ratio, metabolic syndrome components, total cholesterol, and low-density lipoprotein cholesterol with insulin resistance in the population of georgia. Int J Endocrinol. 2014;2014:925650. doi: 10.1155/2014/925650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballestri S, Mantovani A, Byrne CD, Lonardo A, Targher G. Diagnostic accuracy of ultrasonography for the detection of hepatic steatosis: an updated meta-analysis of observational studies. Metab Target Organ Damage. 2021;1:7. 10.20517/mtod.2021.05.
- 46.Farrell GC, Chitturi S, Lau GK, Sollano JD. Asia-Pacific working party on NAFLD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia–Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22(6):775–777. doi: 10.1111/j.1440-1746.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- 47.Schattenberg JM, Anstee QM, Caussy C, Bugianesi E, Popovic B. Differences between current clinical guidelines for screening, diagnosis and management of non-alcoholic fatty liver disease and real-world practice: a targeted literature review. Expert Rev Gastroenterol Hepatol. 2021;15(11):1253–1266. doi: 10.1080/17474124.2021.1974295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.