Abstract
Purpose:
To investigate the relationship between the rate of retinal nerve fiber layer (RNFL) loss during initial follow-up and the magnitude of associated visual field loss during an extended follow-up period.
Design:
Retrospective cohort study.
Methods:
A total of 1,150 eyes of 839 glaucoma patients extracted from the Duke Glaucoma Registry. Rates of RNFL loss were obtained from global RNFL thickness values of the first 5 optical coherence tomography (OCT) scans. Rates of visual field loss were assessed using standard automated perimetry mean deviation (SAP MD) during the entire follow-up period. Joint longitudinal mixed effects models were used to estimate rates of change. Eyes were categorized as fast, moderate or slow progressors based on rates of RNFL loss, with cutoffs of ≤−2 μm/year, −2 to −1 μm/year and ≥−1 μm/year, respectively. Univariable and multivariable regressions were completed to identify significant predictors of SAP MD loss.
Results:
The rate of RNFL change was −0.76±0.85 μm/y during initial follow-up, which occurred over 3.7±1.5 years. 765 (66%) eyes were slow, 328 (29%) moderate, and 57 (5%) fast progressors, with rates of RNFL thinning of −0.36±0.54 μm/year, −1.34±0.25 μm/year, and −2.87±1.39 μm/year respectively. The rates of SAP MD loss among slow, moderate, and fast OCT progressors were −0.16±0.35 dB/y, −0.32±0.43 dB/y, and −0.71±0.65 dB/y respectively over the extended follow-up period of 6.1±1.9 years (P<0.001). Age, OCT progressor group, and concurrent SAP rate were all significantly associated with the overall rate of SAP MD loss in a multivariable model (all P<0.001).
Conclusion:
Rapid RNFL thinning during an initial follow-up period was predictive of concurrent and subsequent rates of visual field decline over an extended period.
Keywords: Glaucoma, visual field, OCT, perimetry, linear mixed models
Table of Contents statement
Rapid retinal nerve fiber layer thinning during the initial follow-up period of an eye with glaucoma was associated with a large magnitude of visual field loss during the total follow-up period. Compared to slow progressors, eyes with fast progression on optical coherence tomography detected early in the course of follow-up had approximately 4 times faster rates of perimetric loss during the total follow-up period.
Introduction
Optical coherence tomography (OCT) has been widely used to evaluate neural loss in glaucoma, a progressive optic neuropathy that is the leading cause of irreversible blindness in the world. The attractiveness of OCT resides in its ability to quickly obtain quantitative and reproducible measurements of different anatomic regions of the eye affected by glaucoma, such as the retinal nerve fiber layer (RNFL).
Since OCT does not directly represent a patient’s visual function, the use of its measurements in clinical practice relies on the belief that these measurements are predictive of clinically relevant outcomes (i.e., loss of vision). For example, in individuals who are suspected to have glaucoma, the rationale for using OCT as a diagnostic test is to detect structural damage before the appearance of visual field defects on standard automated perimetry (SAP). Similarly, in individuals already diagnosed with glaucoma, progressive loss of neural tissue detected by OCT is believed to be associated with an increased risk of disease progression and worse visual outcomes. As such, abnormalities and change detected on OCT would provide an opportunity to initiate or escalate treatment to prevent irreversible loss of vision.
Several studies have investigated whether OCT measurements are predictive of future functional deterioration. A study by Miki et al.1 demonstrated that RNFL thinning predicts future visual field defects in glaucoma suspects. Each 1-μm/year faster rate of RNFL loss corresponded to a hazard ratio of 2.05 for the risk of visual field loss in their cohort. Similarly, Yu et al.2 showed that progressive RNFL loss had a hazard ratio of 3.81 to predict progressive visual field loss in patients already diagnosed with glaucoma after adjustment for covariates. Although these studies have provided important assessments of the predictive value of OCT, they do not provide easily interpretable quantitative descriptions of how change in OCT is related to change in SAP; hazard ratios do not provide information that can be easily translated to clinical practice. For a given RNFL thinning, the risk of SAP deterioration may be 2 or 3 times higher than a certain baseline but remain relatively small in magnitude. Hazard ratios are relative measures which do not help a clinician decide whether a specific rate of change on OCT actually carries a risk of fast visual field progression for an individual patient. More specifically, it is important to demonstrate that rapid loss observed on OCT during a given follow-up period is associated with concurrent or future risk of fast deterioration on SAP.
The purpose of this study was to investigate the relationship between the magnitude of RNFL thinning seen during an initial period of follow-up of glaucoma patients and the magnitude of concurrent and subsequent visual field loss seen during an extended follow-up period of the same cohort. We hypothesized that fast initial RNFL progression would be associated with fast visual field progression, justifying the rationale for and providing guidance to the use of longitudinal OCT results in assisting clinical decision-making in glaucoma.
Methods
The dataset utilized in this study was derived from the Duke Glaucoma Registry developed by the Vision, Imaging and Performance (VIP) Laboratory of Duke University3. Institutional Review Board (IRB) approval was obtained for this analysis, and a waiver of informed consent was provided due to the retrospective nature of this work. All methods adhered to the tenets of Declaration of Helsinki for research involving human participants.
The database contained clinical information from baseline and follow-up visits, including patient diagnostic and procedure codes, medical history and stereoscopic optic disc photographs. The study included patients previously diagnosed with primary open-angle glaucoma (POAG) based on International Classification of Diseases (ICD) codes. Patients were excluded if they presented with any other ocular or systemic disease that could affect the optic nerve or visual field (e.g. retinal detachment, retinal or malignant choroidal tumors, non-glaucomatous disorders of the optical nerve and visual pathways, atrophic and late-stage dry age-related macular degeneration, amblyopia, uveitis and/or venous or arterial retinal occlusion) according to ICD codes. In addition, tests performed after treatment with pan-photocoagulation, according to Current Procedural Terminology (CPT) codes, were excluded. ICD and CPT codes used to construct this database have been detailed in a previous work3. Glaucomatous eyes were required to have an abnormal visual field at baseline (i.e., GHT “outside normal limits” or PSD probability <5%). Intraocular pressure (IOP) measurements were completed using Goldmann applanation or Tonopen tonometry (Reichert Technologies, Depew, NY).
All eligible subjects had imaging with the Spectralis spectral-domain OCT (SD-OCT) system (Heidelberg Engineering, GmbH, Dossenheim, Germany) and SAP tests using the Humphrey Field Analyzer II or III (Carl Zeiss Meditec, Inc., Dublin, CA). The mean global RNFL thickness was calculated by averaging the measurements acquired from a 12-degree (for single circle scans) or a 3.45-mm peripapillary circle scan (for scans from the Glaucoma Module Premium Edition), as described in detail previously4. OCT scans were excluded if the scan quality score was less than 15. In addition, eyes with a baseline RNFL value ≤38 μm were excluded due to the “floor effect,” which would preclude subsequent trend analysis of RNFL thickness during follow-up5. Since manual review of all tests was impractical, we excluded scans that had a global RNFL thickness greater than 130 μm, representing implausible measurements above the higher range of reported RNFL thickness for normal controls.5–7 Values outside this range likely indicate the presence of acquisition or segmentation errors in the presence of otherwise adequate quality scores.8 SAP tests included 24–2 and 30–2 Swedish Interactive Threshold Algorithm (SITA) tests with size III white stimulus. Visual fields were excluded from this analysis if they had more than 33% fixation losses or more than 15% false-positive errors, or if the result of the glaucoma hemifield test (GHT) was “abnormally high sensitivity”. All reliable visual fields from each enrolled eye were utilized, starting with the first visual field within 6 months of the first RNFL OCT visit. Subjects were required to have 5 good-quality OCT scans and at least 5 reliable SAP tests.
Data Analysis
In the present study, we were interested in assessing whether fast progression detected on OCT during the initial follow-up period would be associated with concurrent or subsequent visual field loss. The rationale for this design was based on the concept that if such an association is demonstrated, it would then be justified for clinicians to make clinical decisions based on the initial assessment provided by the OCT, rather than having to wait until irreversible loss of vision occurs as measured by SAP (see Discussion). In order to assess rates of change on OCT during the initial follow-up period, we used global RNFL thickness values of the first 5 valid SD-OCT visits. Rates of visual field loss and magnitude of visual field decline were then measured by SAP mean deviation (MD) during the entire follow-up period, starting with the baseline visit corresponding to the first SD-OCT date through the last available visit. A joint longitudinal linear mixed effects model was used to estimate rates of change for both SD-OCT and SAP, with random effects applied at the eye level. Details about this model have been presented elsewhere9–11. In short, linear mixed models estimate the average rate of change in an outcome variable using a linear function of time, and subject- and eye-specific deviations from this average rate are introduced by random slopes. In joint longitudinal modeling, both OCT and SAP data are modeled concurrently, allows a better determination of the true underlying relationship between the two outcomes by taking into account measurement error.
Eyes were categorized as fast, moderate or slow progressors based on the rates of change in global RNFL as follows: fast OCT progressors were those with a change in global RNFL thickness faster than −2 μm/year, moderate progressors were those with a rate between −2 and −1 μm/year, and slow progressors were those with a rate slower than −1 μm/year12.
We then performed univariable and multivariable regression to evaluate the impact of age, gender, race, mean IOP during the initial follow-up period, OCT progressor group, and baseline MD on the rate of SAP loss. Adjusted rates of SAP loss from the multivariable model were then used in conjunction with the mean total follow-up period in this study to estimate the total magnitude of SAP loss during the study period among OCT progressor groups.
Analyses were performed using R 3.6.1 (R Core Team, Vienna, Austria) within the Protected Analytics Computing Environment (PACE), a highly protected virtual network space developed by Duke University for analysis of identifiable protected health information.
Results
The study included 1,150 eyes of 839 patients with POAG extracted from the Duke Glaucoma Registry. Baseline characteristics are provided in Table 1. Mean age was 66.0 ± 9.9 years, with African-Americans comprising 32.2% of the cohort. Average RNFL thickness at baseline was 73.9 ± 16.7 μm, while baseline MD was −5.44 ± 5.56 dB. The initial follow-up period corresponding to the 5 OCT scans used in this analysis was 3.7 ± 1.5 years. Mean IOP during this initial follow-up period was 14.5±3.2 mmHg. The average total follow-up period was 6.1 ± 1.9 years.
Table 1.
Demographics and clinical characteristics at baseline of the subjects included in the study.
| Characteristic | n = 1,150 eyes of 839 patients |
|---|---|
| Age (years), Mean ± SD | 66.0 ± 9.9 |
| Sex, female (%) | 452 (53.9) |
| Race, (%) Black or African American | 270 (32.2) |
| Mean initial IOP (mmHg) | 14.5 ± 3.2 |
| SD OCT | |
| Number of tests, n | 5,750 |
| Follow-up time, for the first 5 SD OCT tests (years), Mean ± SD Median (IQR) | 3.7 ± 1.5 3.6 (2.3; 4.6) |
| Baseline mean RNFL thickness (μm), Mean ± SD Median (IQR) | 73.9 ± 16.7 73.0 (61.0; 85.0) |
| Baseline mean SD OCT quality, Mean ± SD Median (IQR) | 25.1 ± 4.5 25.0 (22.0; 28.0) |
| SAP | |
| Number of tests, n | 10,259 |
| Total follow-up time (years), Mean ± SD Median (IQR) | 6.1 ± 1.9 5.9 (4.8; 7.5) |
| Number of tests per eye, n (%) Mean ± SD Median (IQR) | 8.9 ± 4.4 7.0 (6.0; 11.0) |
| Baseline SAP MD (dB), Mean ± SD Median (IQR) | −5.44 ± 5.56 −3.61 (−7.37; −1.84) |
| Baseline SAP PSD (dB), Mean ± SD Median (IQR) | 5.50 ± 3.81 3.81 (2.39; 8.16) |
IOP = intraocular pressure; IQR = interquartile range; MD = mean deviation; RNFL = retinal nerve fiber layer; SAP = standard automated perimetry; SD = standard deviation; SD OCT = spectral-domain optical coherence tomography.
The average rate of RNFL loss was −0.76 ± 0.85 μm/year during the first 5 OCT scans. Of the 1,150 eyes, 765 (66%) were classified as slow progressors, 328 (29%) as moderate, and 57 (5%) as fast OCT progressors, with corresponding average rates of global RNFL loss of −0.36 ± 0.54 μm/year, −1.34 ± 0.25 μm/year and −2.87 ± 1.39 μm/year, respectively. For these groups, the corresponding rates of SAP MD loss were −0.16 ± 0.35 dB/year, −0.32 ± 0.43 dB/year and −0.71 ± 0.65 dB/year over the total follow-up period of 6.1 ± 1.9 years (P<0.001; Table 2). Summary of risk factors and other characteristics are presented across levels of OCT progressor status in Table 2. The distributions of the rates of visual field loss in each progressor group are represented by the violin plots shown in Figure 1. Over the entire follow-up period, eyes that had fast initial progression with OCT lost an average of 5.51 ± 4.75 dB in SAP MD. Eyes that had moderate initial progression lost an average of 3.22 ± 3.61 dB, while slow OCT progressors ended up losing only 2.63 ± 2.90 dB on average (P<0.001).
Table 2.
Characteristics of eyes categorized by optical coherence tomography (OCT) progressor group.
| Slow (n=765) | Moderate (n=328) | Fast (n=57) | p-value | |
|---|---|---|---|---|
| Baseline age (years) | 65.8±10.2 | 66.2±9.8 | 68.2±9.6 | 0.207* |
| Gender (female) | 53.0% | 56.1% | 47.4% | 0.407† |
| Race (African-American) | 31.8% | 35.4% | 35.1% | 0.479† |
| Mean initial IOP (mmHg) | 14.2±3.1 | 14.8±3.2 | 16.5±3.4 | <0.001‡ |
| Baseline SAP MD (dB) | −6.10±6.18 | −4.20±3.92 | −3.80±2.60 | <0.001‡ |
| Mean total follow-up time (years) | 6.2±1.9 | 6.1±1.9 | 6.1±1.9 | 0.813‡ |
| Rate of SAP MD loss (dB/year) | −0.16±0.35 | −0.32±0.43 | −0.71±0.65 | <0.001‡ |
One-way analysis of variance (ANOVA).
Chi-square test of independence.
Linear mixed model.
Mean ± standard deviation.
dB = decibel; IOP = intraocular pressure; MD = mean deviation; SAP = standard automated perimetry.
Figure 1.
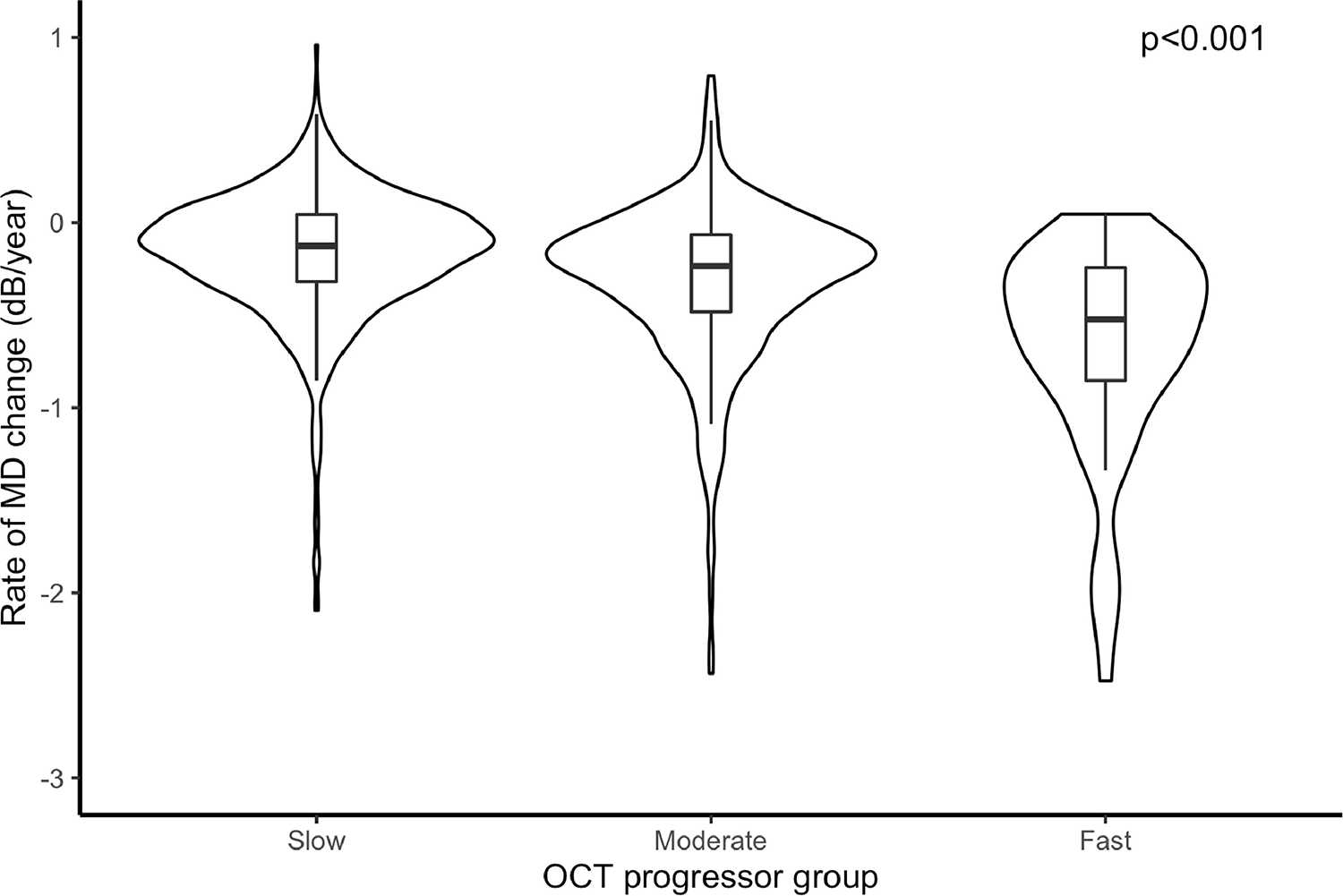
Rates of change in mean deviation (MD) among all slow, moderate, and fast progressors, as defined by the rates of retinal nerve fiber layer thickness loss in the first 5 optical coherence tomography (OCT) tests. * Linear mixed model. Rates of global OCT retinal nerve fiber layer thickness loss: slow (slower than −1.0 μm/year); moderate (between −1.0 and −2.0 μm/year); fast (faster than −2.0 μm/year).
A multivariable model was created to account for age, gender, race, mean IOP during the initial period, and baseline MD (i.e., baseline glaucoma severity) (Table 3). The concurrent SAP rate (i.e., the SAP rate during the same period of OCT tests) was also included as a covariate in order to assess whether OCT progressor status significantly influenced future rates of SAP loss even when accounting for concurrent rates of SAP change. Older age (P<0.001), concurrent SAP rate (P<0.001), and OCT progressor group (P<0.001) were all associated with faster rates of overall SAP loss. OCT progressor group remained statistically significant regardless of the concurrent rate of SAP change. When adjusted for these variables, overall rates of SAP loss were −0.17 ± 0.02 dB/year, −0.32 ± 0.03 dB/year, and −0.66 ± 0.05 dB/year for slow, moderate, and fast OCT progressors respectively (P<0.001). These rates were computed for a patient with the average values of age, baseline MD, mean initial IOP, and mean rate of concurrent SAP loss in the sample. Adjusted total magnitude of SAP loss over the average follow-up period of 6.1 years was −1.05 ± 0.10 dB, −1.96 ± 0.12 dB, and −4.01 ± 0.24 dB for slow, moderate, and fast OCT progressors respectively (P<0.001).
Table 3.
Coefficients from univariable and multivariable linear regression models estimating rates of standard automatic perimetry mean deviation loss. Reference for the multivariable model is a patient classified as a slow optical coherence tomography (OCT) progressor.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Coefficient | p-value* | Coefficient | p-value* | |
| Intercept | - | - | 0.250 | - |
| Baseline age (years) | −0.005 | <0.001 | −0.004 | <0.001 |
| Gender (female) | 0.030 | 0.253 | 0.020 | 0.294 |
| Race (African-American) | 0.037 | 0.190 | −0.005 | 0.788 |
| Mean initial IOP (mmHg) | −0.006 | 0.107 | −0.005 | 0.082 |
| Baseline SAP MD (dB) | 0.004 | 0.061 | −0.002 | 0.184 |
| Concurrent SAP rate (dB/year) | 0.695 | <0.001 | 0.668 | <0.001 |
| OCT progressor group | ||||
| Intercept (baseline: slow) | −0.158 | <0.001 | - | - |
| Moderate – slow | −0.165 | <0.001 | −0.103 | <0.001 |
| Fast – slow | −0.487 | <0.001 | −0.254 | <0.001 |
Linear mixed models.
dB = decibel; IOP = infraocular pressure; MD = mean deviation; OCT: optical coherence tomography; SAP = standard automated perimetry.
Discussion
In this study, we have demonstrated that rapid OCT RNFL thinning during the initial follow-up period of an eye with glaucoma was associated with a large magnitude of visual field loss during the total follow-up period; a clinician who observes rapid RNFL thinning is justified in believing that this thinning will likely be accompanied by concurrent or future rapid visual field loss. This finding has clinical implications, as those patients classified as fast progressors by OCT may be at higher risk for developing subsequent functional disability. In addition, this finding may be used to identify eyes at risk for fast visual field progression when designing clinical trials in glaucoma.
Our findings showed that eyes with fast OCT progression detected early in the course of follow-up had approximately 4 times faster rates of SAP loss during the extended follow-up period compared to eyes that had slow OCT progression (−0.71 dB/year versus −0.16 dB/year). In terms of total magnitude of SAP loss, eyes with rapid initial OCT progression ended up losing an average of 5.51 dB over the extended follow-up period, over twice the amount in eyes that were deemed to have slow OCT progression initially. These differences remained significant even when adjusting for potential confounders, including age and concurrent rate of SAP loss. Even after adjusting for confounders, the total magnitude of SAP loss in fast OCT progressors was approximately 4 times greater than in slow OCT progressors.
A recent analysis has shown that an average loss of 3 dB in SAP MD is associated with a significant decline in quality of life (QoL) if occurring in the better seeing eye of an individual with glaucoma.13 Among eyes classified as having initial fast progression on OCT, 35 eyes (61%) had loss equal to or greater than 3 dB in the visual field during the entire follow-up period, indicating that a substantial proportion were at risk for decrease in QoL. Even among moderate OCT progressors, 135 eyes (41%) had a loss equal or greater to 3 dB. For slow progressors, 247 eyes (32%) had such a decline in visual field. While fast rates of OCT during the initial period are more likely to lead to larger SAP loss and visual disability, our results also show that a slow rate of progression on OCT does not completely eliminate the possibility of significant visual field loss. This finding confirms the utility of both OCT and SAP in monitoring glaucoma patients. Figure 2 provides examples of both strong and weak correlation between initial OCT loss and concurrent or subsequent SAP loss.
Figure 2.
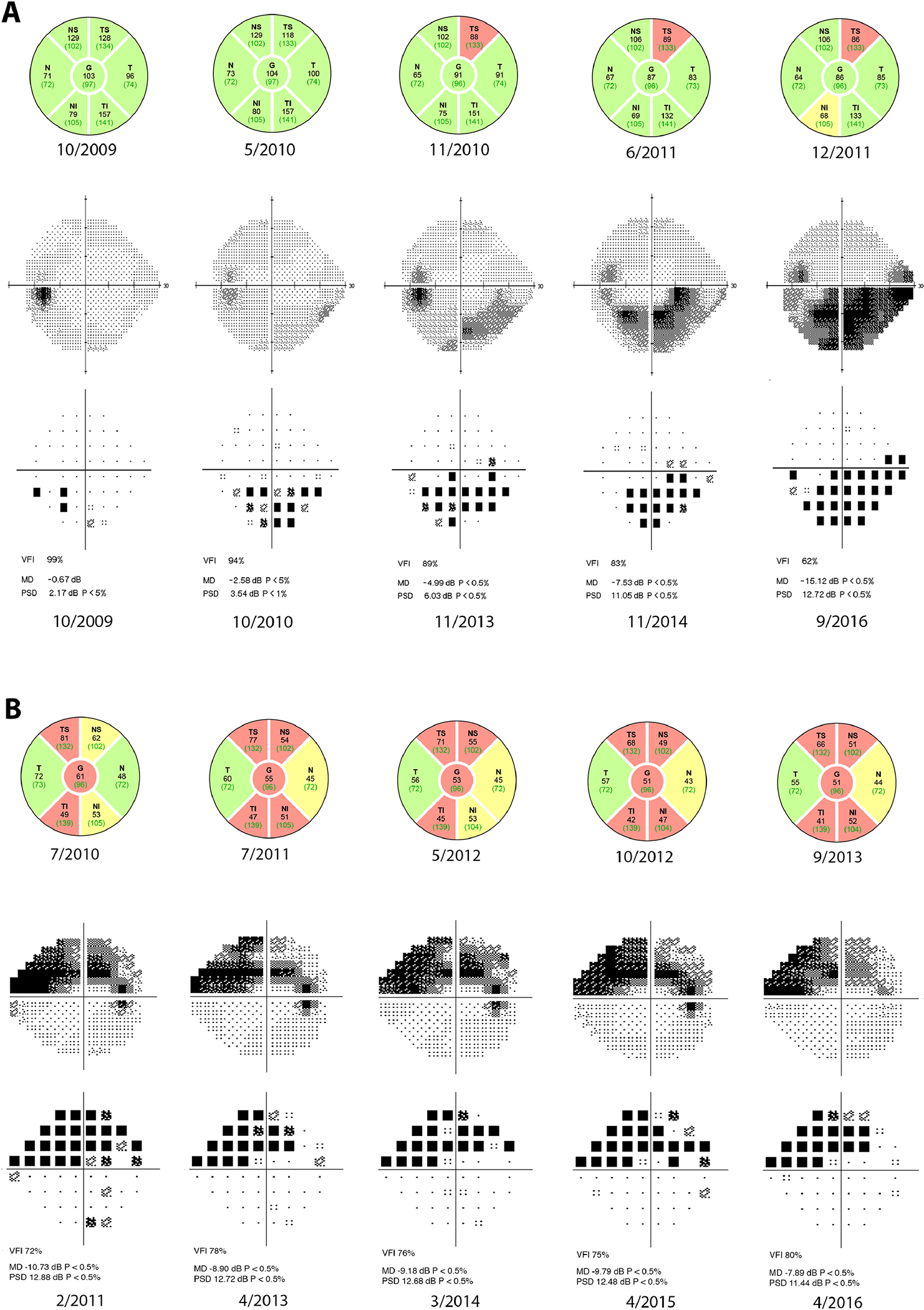
Case examples of strong and weak correlation between initial changes on optical coherence tomography (OCT) and concurrent or subsequent changes in standard automated perimetry (SAP). The five OCTs used in the study are displayed, while representative visual fields from the total follow-up period are presented. Respective dates are listed. (A) Testing from a 48-year old African-American male. The estimated retinal nerve fiber layer (RNFL) OCT rate of loss was −4.02 μm/year and estimated SAP mean deviation (MD) rate of loss was −1.19 dB/year. Initial follow-up period for OCT was 2.1 years, while total follow-up period for SAP was 8.5 years. (B) Testing from a 56-year old Caucasian female. The estimated RNFL OCT rate of loss was −1.71 μm/year, but estimated SAP MD rate of loss was only −0.02 dB/year. Initial follow-up period for OCT was 3.1 years, while total follow-up period for SAP was 5.1 years.
Rates of OCT change in our study were calculated based only on the first 5 OCT tests while rates and magnitude of SAP change used all visual fields during follow-up. While this design may appear counterintuitive, it simulates a common scenario in clinical practice and addresses an important clinical question. One could consider an alternative design in which all OCT and visual fields would be used for each patient. However, while this design would allow assessing the structure-function correlation during the entire follow-up, it would not assess the impact of initial OCT changes on the magnitude of subsequent visual field damage. In practice, a clinician wants to decide whether he or she needs to act on OCT findings identified during follow-up without having to wait until irreversible visual field loss is observed on SAP. Another potential design could have considered only the visual field tests acquired after the initial OCT follow-up period. This design has been used in several previous studies. However, from a clinical decision-making standpoint, there is no reason to exclude information provided by the visual fields acquired during the initial follow-up period, as these data would be available to the clinician. Given the greater variability of visual fields, it is conceivable that substantial changes may be deciphered only after a larger number of tests have been acquired during follow-up.14 Using all available visual fields lets one estimate the true magnitude of visual field loss that occurred during follow-up with greater precision. Of note, even when accounting for the concurrent (i.e., during the same OCT period) rate of SAP loss in the multivariable model, OCT progressor status was still significantly predictive of the overall rate of SAP loss. This finding demonstrates the importance of OCT progression rate in independently predicting visual field changes. It should be noted, however, that while our data provides evidence to support treatment initiation or augmentation by a clinician in response to an early large rate of loss in RNFL thickness alone, additional factors need to be taken into account in decision-making, such as patient age, life-expectancy, and potential side-effects of treatment.
Several large-scale studies have previously evaluated structure-function correlation between OCT and SAP, particularly the rate of RNFL loss and the risk of progression by SAP. Diniz-Filho et al demonstrated that RNFL thinning is faster among progressing eyes versus non-progressing eyes as defined by SAP guided progression analysis15. Miki et al demonstrated a 2.05-times higher risk of developing visual field defects per 1 additional μm/year increase in the rate of RNFL loss1. Yu et al showed that glaucoma patients with progressive RNFL loss were more likely to develop perimetric progression, with hazard ratios of 3.81 to 8.44 depending on the methodology used to gauge visual field progression2. In the Advanced Imaging for Glaucoma study, Sehi et al demonstrated that the rate of VFI loss was significantly greater in those eyes that had concurrent RNFL loss; for every 10 μm lost in RNFL thickness, the risk of visual field progression was 38% higher16. These studies suggest that the greater the magnitude of RNFL loss, the greater the probability of perimetric loss. However, these reports compared RNFL and SAP losses over a concurrent period and only commented on the risk of SAP progression in terms of hazard ratios, which are difficult to apply to individual patients. While these studies demonstrated structure-function correlation, they did not provide interpretable quantitative descriptions of how the magnitude of change in OCT is related to the magnitude of change in SAP.
The present study had some limitations. Patients were treated at the discretion of the ophthalmologist, which may have led to intensification of IOP-lowering treatment in response to perceived RNFL thinning. This change may have reduced the magnitude and rates of subsequent SAP loss. In addition, the degree of baseline disease may have influenced treatment intensity – patients with more severe disease at baseline or with faster OCT changes may have been treated more aggressively, leading to subsequent slower rates of visual field loss. (Table 2). Therefore, our results may be seen as conservative; it is possible that eyes with significant progression on OCT may have had even greater changes on visual fields during follow-up if there had been no change in therapy. Given the widespread use of OCT in clinical practice, this limitation is inherent to retrospective cohort analyses such as ours. It should be noted also that such a limitation also applies to previously reported prospective studies that did not use a fixed-treatment protocol. In addition, patients were initially identified using ICD codes, which were assigned by attending physicians at their own discretion.
In conclusion, rapid RNFL thinning during an initial follow-up period was significantly predictive of concurrent and subsequent rates of visual field decline over an extended period. These findings are of value to the clinician, as fast rates of RNFL thinning portend rapid future worsening in SAP. Intensifying treatment based on rapid structural loss in order to mitigate future functional loss may be warranted.
Acknowledgments
Funding/Support
Supported in part by National Institutes of Health/National Eye Institute grant EY029885 (FAM). The funding organizations had no role in the design or conduct of this research.
Biography
Swarup S. Swaminathan, MD is an Assistant Professor of Ophthalmology at the Bascom Palmer Eye Institute in Miami, Florida. His research interests focus on improving testing modalities used to diagnose and monitor glaucoma patients, including the use of novel imaging metrics, risk factor assessment, and higher-order statistical modeling.
Footnotes
Disclosures
SSS: Sight Sciences (C), Ivantis (C), Heidelberg Engineering (C); AAJ: none; SIB: none; FAM: Alcon Laboratories (C, L, S), Allergan (C, L), Bausch&Lomb (F), Carl Zeiss Meditec (C, L, S), Heidelberg Engineering (L), Merck (L), nGoggle Inc. (P), Sensimed (C), Topcon (C), Reichert (C, S), National Institutes of Health/National Eye Institute (S).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology 2014;121:1350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu M, Lin C, Weinreb RN, Lai G, Chiu V, Leung CK. Risk of Visual Field Progression in Glaucoma Patients with Progressive Retinal Nerve Fiber Layer Thinning: A 5-Year Prospective Study. Ophthalmology 2016;123:1201–10. [DOI] [PubMed] [Google Scholar]
- 3.Jammal AA, Thompson AC, Mariottoni EB, et al. Rates of Glaucomatous Structural and Functional Change From a Large Clinical Population: The Duke Glaucoma Registry Study. Am J Ophthalmol 2020;222:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leite MT, Rao HL, Zangwill LM, Weinreb RN, Medeiros FA. Comparison of the diagnostic accuracies of the Spectralis, Cirrus, and RTVue optical coherence tomography devices in glaucoma. Ophthalmology 2011;118:1334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowd C, Zangwill LM, Weinreb RN, Medeiros FA, Belghith A. Estimating Optical Coherence Tomography Structural Measurement Floors to Improve Detection of Progression in Advanced Glaucoma. Am J Ophthalmol 2017;175:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fiber layer thickness in normal latinos. Invest Ophthalmol Vis Sci 2003;44:3369–73. [DOI] [PubMed] [Google Scholar]
- 7.Patel NB, Lim M, Gajjar A, Evans KB, Harwerth RS. Age-associated changes in the retinal nerve fiber layer and optic nerve head. Invest Ophthalmol Vis Sci 2014;55:5134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asrani S, Essaid L, Alder BD, Santiago-Turla C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol 2014;132:396–402. [DOI] [PubMed] [Google Scholar]
- 9.Crowther MJ, Lambert PC, Abrams KR. Adjusting for measurement error in baseline prognostic biomarkers included in a time-to-event analysis: a joint modelling approach. BMC Med Res Methodol 2013;13:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medeiros FA, Zangwill LM, Alencar LM, Sample PA, Weinreb RN. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol 2010;149:908–15. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining structural and functional measurements to improve detection of glaucoma progression using Bayesian hierarchical models. Invest Ophthalmol Vis Sci 2011;52:5794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jammal AA, Thompson AC, Mariottoni EB, et al. Impact of Intraocular Pressure Control on Rates of Retinal Nerve Fiber Layer Loss in a Large Clinical Population. Ophthalmology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe RY, Diniz-Filho A, Costa VP, Wu Z, Medeiros FA. Predicting Vision-Related Disability in Glaucoma. Ophthalmology 2018;125:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Saunders LJ, Daga FB, Diniz-Filho A, Medeiros FA. Frequency of Testing to Detect Visual Field Progression Derived Using a Longitudinal Cohort of Glaucoma Patients. Ophthalmology 2017;124:786–792. [DOI] [PubMed] [Google Scholar]
- 15.Diniz-Filho A, Abe RY, Zangwill LM, et al. Association between Intraocular Pressure and Rates of Retinal Nerve Fiber Layer Loss Measured by Optical Coherence Tomography. Ophthalmology 2016;123:2058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehi M, Zhang X, Greenfield DS, et al. Retinal nerve fiber layer atrophy is associated with visual field loss over time in glaucoma suspect and glaucomatous eyes. American Journal of Ophthalmology 2013;155:73–82.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]


