Abstract
Rationale: The Southeast Asian tuberculosis burden is high, and it remains unclear if urban indoor air pollution in this setting is exacerbating the epidemic.
Objectives: To determine the associations of latent tuberculosis with common urban indoor air pollution sources (secondhand smoke, indoor motorcycle emissions, and cooking) in Southeast Asia.
Methods: We enrolled child household contacts of patients with microbiologically confirmed active tuberculosis in Vietnam, from July 2017 to December 2019. We tested children for latent tuberculosis and evaluated air pollution exposures with questionnaires and personal aerosol sampling. We tested hypotheses using generalized estimating equations.
Measurements and Main Results: We enrolled 72 patients with tuberculosis (27% with cavitary disease) and 109 of their child household contacts. Latent tuberculosis was diagnosed in 58 (53%) household contacts at baseline visit. Children experienced a 2.56-fold increased odds of latent tuberculosis for each additional household member who smoked (95% confidence interval, 1.27–5.16). Odds were highest among children exposed to indoor smokers and children <5 years old exposed to household smokers. Each residential floor above street-level pollution decreased the odds of latent tuberculosis by 36% (adjusted odds ratio, 0.64; 95% confidence interval, 0.42–0.96). Motorcycles parked inside children’s homes and cooking with liquid petroleum gas compared with electricity increased the odds of latent tuberculosis, whereas kitchen ventilation decreased the effect, but these findings were not statistically significant.
Conclusions: Common urban indoor air pollution sources were associated with increased odds of latent tuberculosis infection in child household contacts of patients with active tuberculosis.
Keywords: tobacco smoke pollution, motorcycles, built environment, cooking, smoking water pipes
At a Glance Commentary
Scientific Knowledge on the Subject
Only a fraction of children exposed to a household member with active tuberculosis (TB) develops TB infection from this close contact, and it remains unclear if commonly inhaled indoor air pollutants may increase risk for transmission.
What This Study Adds to the Field
In this cohort study of healthy Vietnamese children exposed to household members with active TB, secondhand smoke exposures significantly increased the odds of latent TB infection, whereas indoor motorcycle emissions and cooking with liquid petroleum gas require further investigation. Our study identifies mitigatable environmental factors in the Southeast Asian setting that might increase TB transmission.
Mycobacterium tuberculosis causes an asymptomatic infection known as latent tuberculosis infection (LTBI) in nearly a quarter of the world’s population (1) and active tuberculosis (TB) disease in 10 million people annually (2). Nearly 50% of active TB cases diagnosed in 2019 were from Asian countries, and Vietnam is one of 30 high-TB-burden countries worldwide (2). TB remains a global public health threat despite the widespread availability of effective treatment, and approaches to identifying and remediating environmental risk factors are needed.
Tuberculosis transmission is greatest for close contact with patients with highly infectious TB: those with cavitary pulmonary TB and those with a high sputum smear Mycobacterium load (smear grade) (3). Environmental variables could also be important risk factors. For instance, indoor air pollution has been linked to LTBI and active TB in some observational studies (4–6), whereas other studies have failed to find statistically significant associations after controlling for socioeconomic variables (7–9). Furthermore, these studies have focused on indoor solid fuel combustion and are therefore not generalizable to low- and middle-income country urban populations that have transitioned to cleaner cooking and heating fuels such as liquid petroleum gas (LPG) and electricity.
In Southeast Asian urban settings, motorcycle emissions constitute an important source of indoor air pollution. In Hanoi, Vietnam’s capital city of >7 million population and the setting for our study, motorcycles make up >95% of the Hanoi vehicle fleet (10) and are the predominant source of ambient air pollution (11, 12). Residents typically live on motorcycle-congested roadways and alleys, with exhaust emissions entering homes through open windows and doors. In addition, motorcycles are routinely parked inside residential living areas after turning them off, for lack of garage and street parking, further exposing household members to motorcycle evaporative emissions (13). There is a lack of data evaluating the associations of indoor motorcycle emissions with TB outcomes.
Secondhand smoke (SHS) is another major source of indoor air pollution in East and Southeast Asia. In Vietnam, 47% of adult men smoke (but only 1.4% of women) and more than 70% of adults and teenagers report weekly exposure to SHS (14, 15). Whereas active cigarette smoking is a robust risk factor for LTBI and active TB (16, 17), findings for SHS as a TB risk factor are heterogeneous (18); some studies support statistically significant associations between SHS and LTBI (19, 20), whereas others do not (21, 22). Furthermore, most of these studies do not differentiate types of smoking behaviors. In Vietnam, for instance, 13% of men report smoking tobacco from the traditional bamboo waterpipe “điếu cày” (23), popularly believed to be safer than cigarette smoking. Further investigations are needed to evaluate the effects of culturally specific smoking behaviors on TB outcomes (24).
Our study objective is to determine the effects of indoor air pollution on LTBI in children living in Southeast Asian urban settings. Our hypothesis is that SHS, motorcycle, and LPG emissions inside children’s homes in Hanoi, Vietnam, are associated with increased LTBI. To test this hypothesis, we are conducting a prospective cohort household contacts study of children living with patients with active pulmonary TB. Some of the results of these studies have been previously reported in the form of abstracts (25, 26).
Methods
Study Design and Ethics Approval
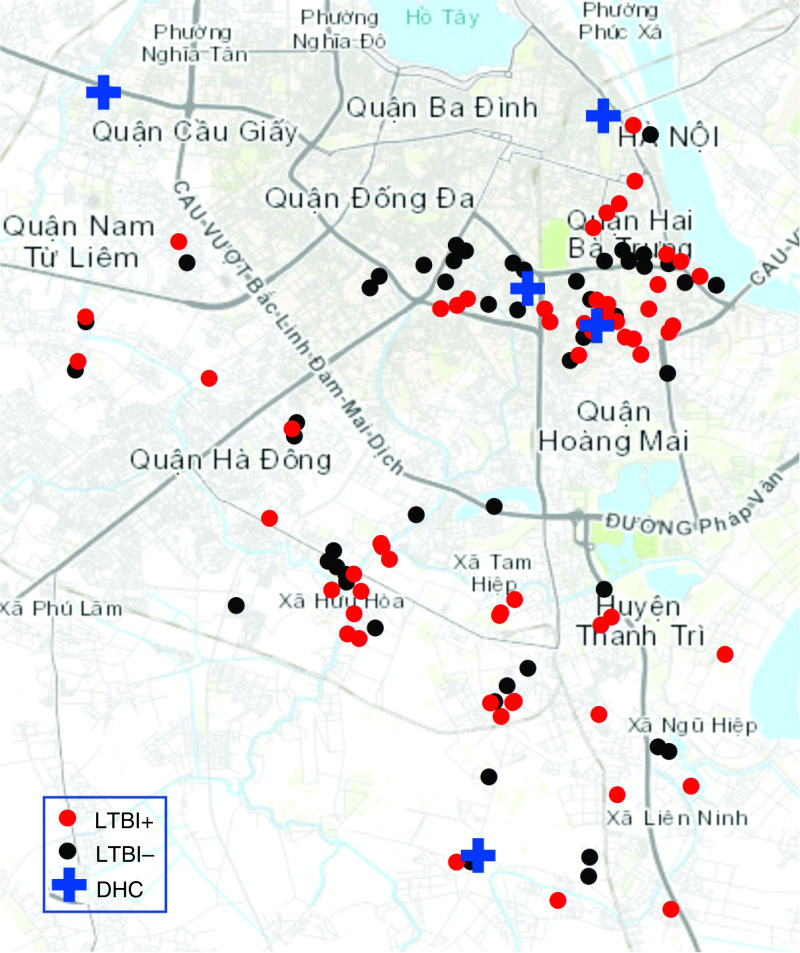
We performed a cross-sectional analysis of baseline data collected from an ongoing prospective cohort study of children (household contacts) living with patients with active TB (index cases). Medical doctors identified, recruited, and enrolled consecutive patients with active TB from the five participating District Health Centers (Figure 1). We then identified and enrolled children living with the index cases, providing written informed consent in Vietnamese. The study was approved by institutional review boards at the Vietnam Ministry of Health; University of California, San Francisco; and University of Iowa.
Figure 1.
Study catchment area in Hanoi, Vietnam. Shown are participant residences by LTBI diagnosis, together with DHC recruitment sites. Residences are jittered randomly up to 300 m in any direction, to protect confidentiality. DHC = District Health Center; LTBI = latent tuberculosis infection.
Participants
Index cases were patients with active pulmonary TB diagnosed within 2 months of enrollment and living with children <16 years old. World Health Organization (WHO) criteria for microbiologically confirmed TB cases were used as inclusion criteria: clinical and/or radiographic evidence of pulmonary TB plus at least one sputum smear positive for acid-fast bacilli (AFB) or GeneXpert PCR positive for M. tuberculosis.
Household contacts were children <16 years old living with an index case within the 2 months before enrollment. Children with a prior history of positive tuberculin skin test (TST) or a known adverse reaction to TST before enrollment were excluded.
Measurements and Data Collection
We evaluated sources of indoor air pollution exposures—SHS, motorcycle emissions, and cooking emissions—through a questionnaire administered at the time of enrollment. We developed the questionnaire using an iterative approach, translating the format into Vietnamese and back-translating into English to test for accuracy of translation and then testing the questionnaire in multiple rounds with native Vietnamese speakers to ensure culturally appropriate and accurate assessments before its deployment. Clinical data were also obtained from the medical record at the time of enrollment.
Secondhand smoke exposures were assessed by determining the smoking status of all members of the household, whether each household member smoked inside the house, type of smoking (manufactured or home-rolled cigarettes, Vietnamese waterpipe, cigar, e-cigarette, or marijuana), and average number of smoking events per day. Cooking exposure was determined by the primary cooking fuel and average daily cooking hours by fuel type. Active ventilation of the kitchen was defined as a functioning ventilation hood over the stove or a fan in the kitchen window venting to the outside. Our surrogate for indoor exposure to street-level motorcycle exhaust emissions was the residential floor levels that participants lived and slept on, with higher residential floors correlating with lower motorcycle exhaust exposures under typical meteorological conditions as found in prior studies (27, 28). We defined exposure to indoor motorcycle evaporative emissions (from parked vehicles after they are turned off) as the average number of motorcycles parked inside the residential living area.
Nutritional status was determined by baseline height and weight measurements compared with age, using WHO Multicentre Growth Reference Study data (29) and WHO definitions (30, 31): underweight, body mass index (BMI) z-score <2 SD; overweight, BMI z-score 1–2 SD (children ⩾5 years old) or weight for height z-score 2–3 SD (children <5 yr); obese, BMI z-score >2 SD (⩾5 years old) or weight for height z-score >3 SD (<5 yr).
We measured personal particulate matter ⩽2.5 μm in aerodynamic diameter (PM2.5) exposure with ultrasonic personal aerosol samplers (UPAS; Access Sensor Technologies) (32). At the time of enrollment, children were instructed to carry the UPAS with them in the outer mesh pocket of a backpack during the day and at the bedside during sleep over a 48- to 72-hour period. PM2.5 was collected on polytetrafluoroethylene (Teflon) filters (Measurement Technology Laboratories). PM2.5 mass was determined by standard gravimetric methods within a climate-controlled facility and flow determined via UPAS internal flow meter, calibrated against a gold-standard mass flow meter (Alicat Whisper) before each run.
Our primary outcome was LTBI diagnosed in household contacts at baseline visit. We diagnosed LTBI using TST as recommended by the WHO. A certified healthcare worker administered 5 units of tuberculin extract (Sanofi Pasteur) by intradermal injection at the time of enrollment and measured the diameter of skin induration after 48–72 hours. A positive TST was defined as an induration ⩾10 mm diameter. Exposure data were collected before the ascertainment of outcome, such that respondents were unaware of TST status at the time of the interview and deployment of personal monitoring devices.
Statistical Analyses
Chi-square and t tests were used to describe baseline characteristics by LTBI status. To evaluate the extent of clustering of LTBI outcomes by household, we used ANOVA to calculate the intraclass correlation coefficient. We compared within-household correlation of outcome with between-household correlation of outcome, finding a significant intraclass correlation coefficient of 0.40 (95% confidence interval [CI], 0.13–0.67). We accounted for this hierarchical structure of the data by using generalized estimating equations (GEE) to test our hypotheses that sources of indoor air pollution were associated with increased LTBI. We fit a separate GEE model for each indoor air pollution exposure as a primary predictor and LTBI as the outcome. We specified an exchangeable correlation structure, a binomial distribution of outcomes, a logistic model for relating predictors to outcome, and “household” as the grouping variable. We selected a full set of potential confounders a priori by using directed acyclic graphing and by recognizing the biological plausibility of potential associations (see Figure E1 in the online supplement) (33). This full set included the following dichotomous or continuous covariates: age, sex, BMI z-score, highest education achieved by the most educated household member, proportion of adult household members who were employed, and occupant density (number of household members per 50 m2 of residential floor area). We then used a backward selection approach to eliminate duplicative covariates and covariates that did not substantively impact primary predictor estimates (34). A final GEE model for each primary predictor of interest was then adjusted for four covariates: age, sex, household employment, and BMI z-score (Figure E1).
To reduce the risk of overfitting GEE models and introducing bias, we limited our events-per-variable (EPV) ratio to no less than 5–10 (35). Given a total of 47 households with at least one event, we thus limited each GEE model to one primary predictor and four covariates for an EPV of 9.4 for models with a dichotomous or continuous primary predictor and an EPV of 6.7 for models with a 3-level categorical primary predictor. Data regarding index case infectivity were not known for all households. We therefore conducted an additional set of analyses including only the subgroup in which these data were available (Figure E2). In these subgroup analyses, in addition to the aforementioned primary predictors and covariates, we separately adjusted for each of two measures of index case infectivity: 1) cavitation on chest radiograph and 2) sputum AFB smear grade at the time of diagnosis, using WHO definitions as detailed in Table 1 but dichotomizing the covariate with a cut-point between “Scant” and “1+.” The effect modification of age, sex, and kitchen ventilation was determined by fitting the above GEE models with interaction terms between the dichotomized effect modifier and the indoor air pollution predictor of interest. All indoor air pollution exposures were considered separately as both categorical and continuous variables where possible. Categorical exposure variables exhibiting nonlinear associations with LTBI (parked motorcycles and PM2.5) were not considered as continuous variables. Categorical predictors were further compared with the dichotomous LTBI outcome using chi-square tests or, alternatively, the Fisher exact test for conditions involving a cell size <5. Software for analyses included Stata SE 15.1 for statistical analyses; ArcGIS 10.6 (ESRI) for mapping; and Prism 9.0 (GraphPad) and DAGitty (GNU public software) for figures.
Table 1.
Baseline Characteristics of Household Contacts by Latent TB Infection Diagnosis (N = 109)
| Baseline Characteristic | Latent TB Infection |
P Value | |
|---|---|---|---|
| Negative (n = 51) | Positive (n = 58) | ||
| Demographic and socioeconomic | |||
| Age, yr, median (IQR) | 6.5 (3.5–10.0) | 6.2 (4.1–9.6) | 0.91 |
| Sex, F | 18 (35) | 23 (40) | 0.64 |
| Urban residence (vs. suburban) | 27 (53) | 27 (47) | 0.51 |
| Household education* | 0.40 | ||
| Secondary school or lower | 24 (47) | 32 (55) | |
| University or polytechnical | 27 (53) | 26 (45) | |
| Occupants, number/household, mean (SD) | 5.1 (1.4) | 5.8 (2.0) | 0.04 |
| Residential floor area, m2, median (IQR) | 60 (44–100) | 70 (47–120) | 0.96 |
| Occupant density, persons/50 m2, mean (SD) | 4.5 (3.08) | 4.8 (3.27) | 0.59 |
| Bedrooms, number/household, mean (SD) | 2.8 (1.4) | 2.8 (1.0) | 0.99 |
| Employed, proportion of adult household members, mean (SD) | 0.67 (0.27) | 0.65 (0.27) | 0.73 |
| Kitchen ventilation† | 0.67 | ||
| No ventilation | 0 | 2 (3.5) | |
| Passive ventilation | 24 (47) | 27 (47) | |
| Active ventilation | 27 (53) | 29 (50) | |
| Air conditioning installed | 46 (90) | 55 (95) | 0.36 |
| Clinical | |||
| BCG vaccination at birth, household contact | 48 (94) | 53 (91) | 0.58 |
| Exposed to index case with cavitary TB‡ | 11 (24) | 14 (25) | 0.91 |
| Smear grade of index case§ | 0.10 | ||
| Negative | 5 (11) | 1 (1.9) | |
| Scant (1–9 AFB in 100 HPF) | 9 (20) | 4 (7.7) | |
| 1+ (10–99 AFB in 100 HPF) | 19 (41) | 24 (46) | |
| 2+ (1–10 AFB in each of 50 HPFs) | 8 (17) | 16 (31) | |
| 3+ (>10 AFB in each of 20 HPFs) | 5 (11) | 7 (13) | |
| Percentage contact with index case, mean (SD)ǁ | 92 (19) | 91 (19) | 0.59 |
| Overweight or obese§ | 16 (31) | 20 (34) | 0.73 |
Definition of abbreviations: AFB = acid-fast bacilli; BCG = bacillus Calmette-Guérin vaccine; HPF = high-powered field (1,000×); index case = a patient with active tuberculosis to whom household contacts have been exposed; IQR = interquartile range; TB = tuberculosis.
Data are presented as n (%) unless otherwise indicated.
Highest education level attained by a household member.
Kitchen ventilation categories: no ventilation during cooking; passive ventilation through an open window or door to the outside; or active ventilation with a hood above the cooking stove or a fan in the kitchen window venting to the outside.
Missing data: chest radiograph not obtained or results not available for index cases of nine household contacts; AFB smear grade was not performed or unavailable for index cases of 11 household contacts.
As defined by the World Health Organization (30).
Percentage of days that household contact was living with index case in the 2 months before enrollment.
Results
From July 2017 through December 2019, 73 index cases with microbiologically confirmed active pulmonary TB were consecutively enrolled from five District Health Center enrollment sites in Hanoi, Vietnam, together with 112 child household contacts. One family moved from Hanoi soon after enrollment, whereas the remaining 72 index cases and 109 household contacts completed baseline procedures and were included in the cross-sectional analysis. One patient with active TB lived in each household: there were no households with multiple concurrent active TB cases. Index cases were predominately male (61%). Cavitary TB was diagnosed in 18 (27%) of the 67 index cases who had chest radiograph results at the time of diagnosis. AFB smear grade was known for 66 index cases: negative, n = 4; scant, n = 9; 1+, n = 31; 2+, n = 13; and 3+, n = 9. None of the women smoked, whereas 30 (68%) of male index cases were active smokers. Twenty-seven smoked manufactured cigarettes, seven smoked waterpipe, four smoked both, and none reported smoking of cigar, home-rolled cigarettes, e-cigarettes, or marijuana. Index case age ranged from 15 to 83 years (median age, 41), and household contact age ranged from 9 months to 15 years (median, 6.2 years old) (Table 1). Household contacts were all of the predominant Kinh ethnicity, and none smoked. Nearly half of households included someone who had attended university or polytechnical training, and two-thirds of adult household members were employed. Nearly all children had received the bacillus Calmette-Guérin vaccination at birth (93%). Household contacts reported no medical comorbidities; only one child was underweight, 21 were overweight, and 15 were obese. Children with LTBI lived with more household members (P = 0.04) and lived with index cases who had higher smear grades (P = 0.10) compared with children with no LTBI (Table 1).
Of the three indoor air pollution sources investigated—SHS, motorcycle emissions, and LPG cooking emissions—SHS was the most robustly associated with increased LTBI among household contacts. We determined associations with LTBI for both categorical (Table 2) and continuous (Figure 2) exposure variables. Sixty-seven (61%) household contacts lived with smokers in the household. Compared with households with no smokers, each additional smoker living in the household increased the odds of LTBI in the household contacts by 2.56-fold (95% CI, 1.27–5.16) (Figure 2). The findings were similar after additional adjustment for the following measures of index case infectivity: index case cavitary TB (adjusted odds ratio [aOR], 2.71; 95% CI, 1.25–5.87) and index case AFB smear grade (aOR, 2.67; 95% CI, 1.23–5.80) (Figure E2). In addition, a dose–response was evident when total smokers were considered as a categorical variable: there was a 3.17-fold higher odds of LTBI in children exposed to one household smoker (aOR, 3.17; 95% CI, 1.22–8.26) and a 5.36-fold higher odds of LTBI in children exposed to two household smokers (OR, 5.36; 95% CI, 1.23–23.5) (Table 2). Furthermore, indoor SHS was more deleterious than outdoor SHS. We stratified household smokers by where they smoked, finding that household members smoking inside the home increased the odds of LTBI in their household contacts, whereas smokers who smoked exclusively outside the house did not significantly increase LTBI odds (Table 2 and Figures 2 and E2). The odds of LTBI were highest in children <5 years old who were exposed to household smokers: aOR of 6.05 compared with an odds of 1.94 for children 5–15 exposed to household smokers (Figure 2). Manufactured cigarette SHS increased the odds of LTBI to a greater extent than waterpipe SHS, but confidence intervals overlapped widely and our power to detect statistically significant waterpipe SHS associations was limited by only 13 children exposed to a waterpipe. Finally, index case smoking was independently associated with increased odds of LTBI among household contacts (aOR, 2.68; 95% CI, 1.08–6.64), a magnitude of association similar to one household member smoking (Table 2).
Table 2.
Odds of Latent TB Infection by Indoor Air Pollution Categorical Exposure Variables (N = 109)
| Exposure | Latent TB Infection [n (%)] |
P Value* | Unadjusted OR (95% CI)† | Adjusted OR (95% CI)‡ | |
|---|---|---|---|---|---|
| No (n = 51) | Yes (n = 58) | ||||
| Total household smokers | 51 (100) | 58 (100) | 0.01 | ||
| 0 | 27 (53) | 15 (26) | 1.00 (ref) | 1.00 (ref) | |
| 1 | 20 (39) | 31 (53) | 2.54 (1.03–6.28) | 3.17 (1.22–8.26) | |
| 2 | 4 (7.8) | 12 (21) | 4.34 (1.07–17.7) | 5.36 (1.23–23.5) | |
| Householders smoking in home | 51 (100) | 58 (100) | 0.007 | ||
| 0 | 42 (82) | 34 (59) | 1.00 (ref) | 1.00 (ref) | |
| 1–2 | 9 (18) | 24 (41) | 3.24 (1.20–8.75) | 3.80 (1.38–10.5) | |
| Householders smoking only outside | 51 (100) | 58 (100) | 0.71 | ||
| 0 | 36 (71) | 39 (67) | 1.00 (ref) | 1.00 (ref) | |
| 1–2 | 15 (29) | 19 (33) | 0.97 (0.39–2.40) | 0.91 (0.35–2.38) | |
| Household cigarettes smoked per day | 51 (100) | 58 (100) | 0.02 | ||
| 0 | 29 (57) | 18 (31) | 1.00 (ref) | 1.00 (ref) | |
| 1–19 | 13 (25) | 22 (38) | 2.42 (0.91–6.43) | 2.81 (1.01–7.78) | |
| 20–60 | 9 (18) | 18 (31) | 2.65 (0.90–7.82) | 2.99 (0.96–9.33) | |
| Index case is a smoker | 51 (100) | 58 (100) | 0.03 | ||
| No | 36 (71) | 29 (50) | 1.00 (ref) | 1.00 (ref) | |
| Yes | 15 (29) | 29 (50) | 2.25 (0.93–5.40) | 2.68 (1.08–6.64) | |
| Household waterpipes smoked per day | 51 (100) | 58 (100) | 0.52 | ||
| 0 | 46 (90) | 50 (86) | 1.00 (ref) | 1.00 (ref) | |
| 1–30 | 5 (10) | 8 (14) | 1.18 (0.32–4.35) | 1.25 (0.32–4.83) | |
| Daytime residential floor level§ | 51 (100) | 58 (100) | 0.01 | ||
| Street-level (first) | 15 (29) | 28 (48) | 1.00 (ref) | 1.00 (ref) | |
| Second and third floor | 28 (55) | 29 (50) | 0.49 (0.20–1.20) | 0.35 (0.13–0.94) | |
| Fourth to 11th floor | 8 (16) | 1 (1.7) | 0.07 (0.008–0.64) | 0.05 (0.005–0.46) | |
| Bedroom floor level | 51 (100) | 58 (100) | 0.04 | ||
| Street-level (first) | 10 (20) | 20 (34) | 1.00 (ref) | 1.00 (ref) | |
| Second and third floor | 33 (65) | 36 (62) | 0.48 (0.18–1.28) | 0.32 (0.11–0.93) | |
| Fourth to 11th floor | 8 (16) | 2 (3.5) | 0.13 (0.02–0.75) | 0.08 (0.01–0.52) | |
| Motorcycles parked inside houseǁ | 51 (100) | 58 (100) | 0.01 | ||
| 0 | 26 (51) | 24 (41) | 1.00 (ref) | 1.00 (ref) | |
| 1–2 | 18 (35) | 12 (21) | 0.83 (0.31–2.24) | 0.82 (0.30–2.23) | |
| 3–7 | 7 (14) | 22 (38) | 3.44 (1.11–10.6) | 3.53 (1.12–11.1) | |
| Primary cooking fuel | 50 (98)¶ | 58 (100) | 0.047 | ||
| Electricity | 19 (38) | 12 (21) | 1.00 (ref) | 1.00 (ref) | |
| LPG | 31 (62) | 46 (79) | 2.29 (0.88–5.96) | 2.49 (0.93–6.67) | |
| LPG with ventilation** | 14 (28) | 18 (31) | 1.49 (0.45–4.89) | 1.53 (0.46–5.10) | |
| LPG, no ventilation** | 17 (34) | 28 (48) | 7.53 (0.78–73.2) | 8.91 (0.85–92.9) | |
| Personal PM2.5, μg/m3†† | 44 (85) | 52 (90) | 0.11 | ||
| 1.00–24.9 | 13 (30) | 20 (38) | 1.00 (ref) | 1.00 (ref) | |
| 25.0–40.9 | 19 (43) | 12 (23) | 0.44 (0.16–1.18) | 0.43 (0.15–1.22) | |
| 41.0–284 | 12 (27) | 20 (38) | 1.02 (0.36–2.94) | 1.01 (0.34–2.99) | |
Definition of abbreviations: CI = confidence interval; GEE = generalized estimating equation; index case = a patient with active tuberculosis to whom household contacts have been exposed; LPG = liquid petroleum gas; OR = odds ratio; PM2.5 = particulate matter ⩽2.5 μm in aerodynamic diameter; ref = referent; TB = tuberculosis.
We determined P value for comparisons between each indoor exposure and latent TB infection using chi-square analyses, or Fisher exact test for cell size <5.
We fit a separate unadjusted GEE regression model for each indoor exposure variable, with latent TB infection as the outcome.
We fit a separate multivariable GEE regression model for each indoor exposure variable, with latent TB infection as the outcome and adjusted for age, sex, body mass index z-score, and household employment.
Residential floor level where household contact spends most of her/his time during the day.
Number of motorcycles typically parked (after turning them off) inside the participant’s residential living area.
Excluded one participant whose household used wood as primary fuel instead of electric or LPG.
Interaction analysis, same GEE regression models used, unadjusted or adjusted, fitting an interaction term between active kitchen ventilation and primary cooking fuel: unadjusted model P-interaction, 0.22; adjusted model P-interaction, 0.19. Active kitchen ventilation was defined as a hood above the cooking stove or a fan in the kitchen window venting to the outside.
Mean airborne concentration of PM2.5 using personal aerosol samplers over a period of 2–3 days at the time of enrollment. Thirteen participants were unable to perform the procedure.
Figure 2.
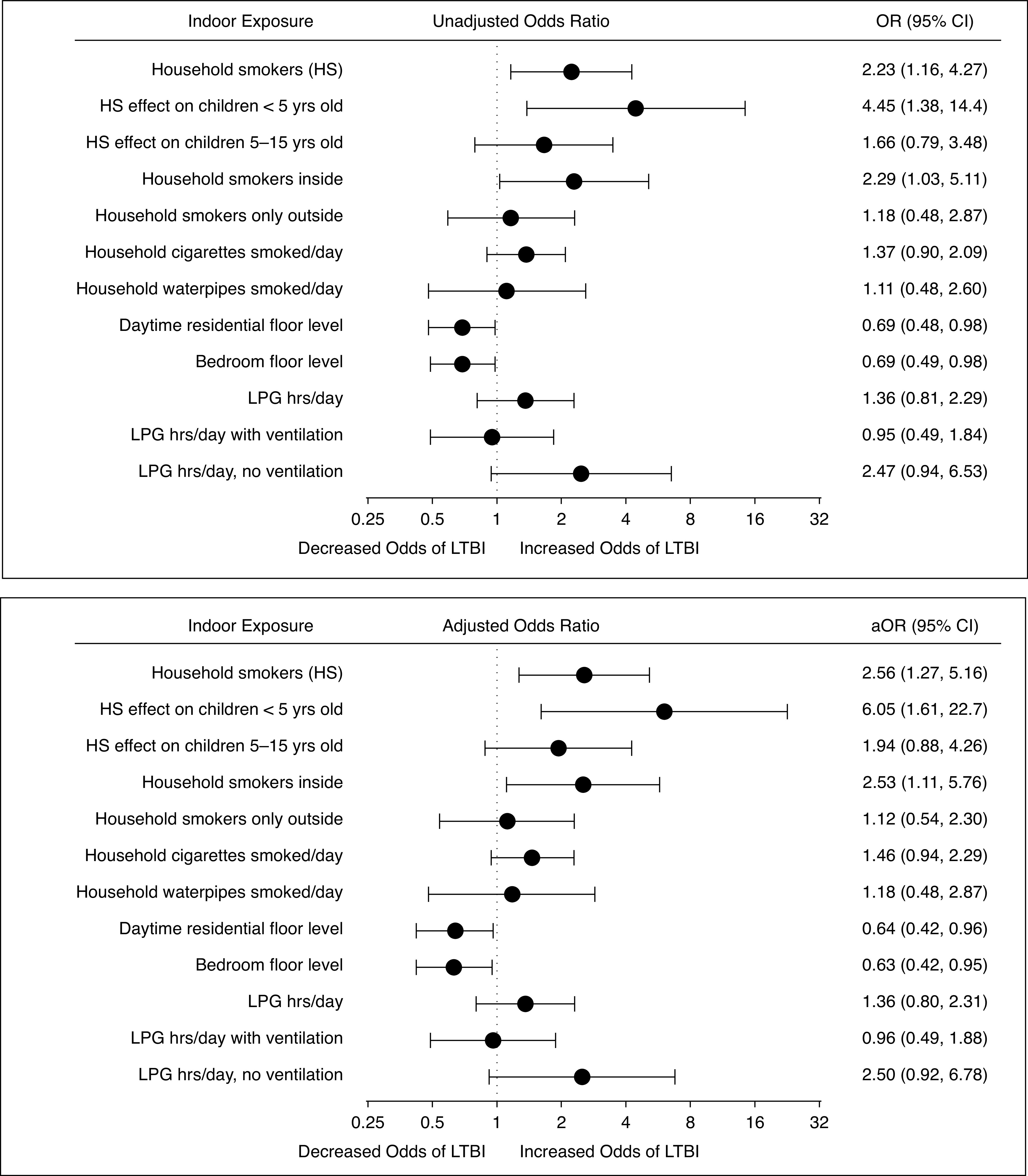
Unadjusted odds (top) and adjusted odds (bottom) of LTBI by air pollution continuous exposure variables (n = 109). We fit a separate generalized estimating equation model for each indoor exposure, with LTBI as the outcome and each model adjusted for age, sex, body mass index z-score, and household employment. Two interactions were also tested with this model: 1) the interaction between HS and age of household contacts, P-interaction 0.12; and 2) the interaction between LPG cooking hours per day and active kitchen ventilation (hood above the cooking stove or fan in the kitchen window), P-interaction also 0.12. Units were expressed in number of HS; number of cigarettes or waterpipes smoked per day (in increments of 10); floor level lived and slept on; and hours of LPG use per day. Zero was the referent value for all variables except floor level, where ground (first floor) was the referent. The aOR represented the adjusted odds of LTBI for each unit increase of continuous exposure variable. For instance, for each additional household member who was a smoker, there was a 2.56-fold higher odds of LTBI in the household contact; and for each higher floor the household contact’s bedroom was located on, there was a 37% lower odds of LTBI. Continuous models should not be interpreted outside the range of observed exposure values (see Table 2 for observed exposure value ranges). aOR = adjusted odds ratio; CI = confidence interval; HS = household smokers; LPG = liquid petroleum gas; LTBI = latent tuberculosis infection; OR = odds ratio.
Participants were exposed to indoor motorcycle exhaust emissions entering the home from the street. We assumed this exposure to be greatest for children living and sleeping on the ground floor and that indoor exhaust emissions entering from the street decreased as residential floor level increased, as determined in prior urban vertical gradient exposure assessments (27, 28). The primary daytime residential floor was on street-level for 39% of household contacts, and 28% slept on the ground floor (Table 2). Whereas 84% of children lived on the same floor as they slept, 16% slept on a higher floor than they lived on during the daytime. Children living and sleeping on the ground floor experienced the highest odds of LTBI, and this risk decreased in a dose–response relationship as residential floor level increased for both daytime floor and bedroom floor in both unadjusted analyses and after controlling for socioeconomic, demographic, and clinical factors (Table 2). The associations were also statistically significant when considering floor level as a continuous variable (Figure 2) and with the addition of index case infectivity to the models (Figure E2). Designating ground floor as referent, each additional daytime residential floor above street-level predicted a 36% decreased odds of LTBI (aOR, 0.64; 95% CI, 0.42–0.96) and each bedroom floor above street-level predicted a 37% decreased odds of LTBI.
Motorcycles continue to emit harmful pollutants (evaporative emissions) after the vehicles are turned off and parked inside the house (13), and 54% of children in our cohort lived with motorcycles parked inside the living areas of their homes. Living with three or more motorcycles parked inside the residential living area increased the odds of LTBI by 3.53-fold (95% CI, 1.12–11.1), although the association was nonlinear (Table 2). All indoor motorcycles were parked on the ground floor. When adjusting for number of motorcycles parked on the ground floor in the association between floor level and LTBI, we found that inclusion of motorcycle number in the model did not significantly impact the magnitude of association or level of significance of floor level on LTBI (data not shown).
All households in our urban cohort used either LPG or electricity as their primary cooking fuel except for one household that used primarily wood. LPG fuel usage increased the odds of LTBI when compared with electric (aOR, 2.49; 95% CI, 0.93–6.67), although the association was not statistically significant (Table 2). Kitchen ventilation modified the effect of LPG on LTBI: children exposed to LPG cooking with no active kitchen ventilation experienced an 8.9-fold increased odds of LTBI compared with a 1.5-fold increase odds of LTBI among children exposed to LPG cooking with active kitchen ventilation (Table 2). However, the P value for interaction was not statistically significant (P = 0.19) and the aOR confidence intervals were wide. Similar trends were evident when analyzing LPG usage as a continuous variable (LPG cooking hours per day) (Figure 2) and when adjusting for index case infectivity (Figure E2). These findings are supported by personal PM2.5 monitoring, which was significantly lower in kitchens with active ventilation (Table 3).
Table 3.
Effect of Indoor Air Pollution Source on Personal PM2.5 Exposure (N = 96)
| Variable | PM2.5 Personal Exposure (μg/m3)* |
||
|---|---|---|---|
| n | Mean (SD) | P Value | |
| Household smokers | 96 | 0.35 | |
| No | 37 | 37 (23) | |
| Yes | 59 | 44 (40) | |
| Householders smoking in home | 96 | 0.45 | |
| No | 69 | 40 (37) | |
| Yes | 27 | 46 (28) | |
| Daytime residential floor level | 96 | 0.25 | |
| Street-level (first) | 39 | 47 (45) | |
| Second floor and up | 57 | 38 (25) | |
| Bedroom floor level | 96 | 0.31 | |
| Street-level (first) | 26 | 47 (52) | |
| Second floor and up | 70 | 39 (25) | |
| Motorcycles parked inside house | 96 | 0.11 | |
| No | 45 | 36 (22) | |
| Yes | 51 | 47 (42) | |
| Primary cooking fuel | 95† | 0.37 | |
| Electricity | 27 | 36 (24) | |
| LPG | 68 | 44 (38) | |
| Kitchen active ventilation | 96 | 0.002 | |
| No | 45 | 52 (45) | |
| Yes | 51 | 32 (18) | |
| Air conditioning | 96 | <0.001 | |
| No | 7 | 91 (88) | |
| Yes | 89 | 38 (23) | |
Definition of abbreviations: LPG = liquid petroleum gas; PM2.5 = particulate matter ⩽2.5 μm in aerodynamic diameter.
Mean airborne concentration of PM2.5 using personal aerosol samplers over a period of 2–3 days at the time of enrollment. Procedure not performed for 13 participants.
Excluded one participant whose household used wood as primary fuel instead of electricity or LPG.
Personal PM2.5 measurements were associated with questionnaire-determined indoor air pollution sources (Table 3). Household smokers, smoking inside the home versus outside, motorcycles parked inside the home, and LPG cooking fuel usage were all associated with higher mean PM2.5 exposures, whereas higher residential floor levels, active kitchen ventilation, and air conditioning were associated with lower mean PM2.5 exposures, although many of these associations were not statistically significant. Furthermore, personal PM2.5 was not found to be independently associated with LTBI (Table 2) in this study.
Discussion
This study addresses indoor environmental factors that could facilitate transmission of M. tuberculosis from patients with active TB to their household contacts. We found that secondhand smoke was robustly associated with LTBI. The effects of residential floor level, indoor motorcycle emissions, cooking with LPG, and active kitchen ventilation were intriguing yet inconclusive.
Our study contributes to the evidence that SHS increases risk for LTBI in children living with a patient with active TB. Other studies have suggested similar associations (19, 20, 36, 37). Our study is distinct in that it evaluates detailed SHS exposures and LTBI in the context of other indoor exposures and within the Southeast Asian cultural context including Vietnamese traditional waterpipe usage (23). Household smokers increased LTBI odds among household contacts in a dose–response relationship, and indoor smoking increased LTBI odds to a greater extent than outdoor, further strengthening the argument for a causal association. Sixty-eight percent of male index cases were smokers in this cohort, consistent with national surveys finding that 47% of Vietnamese men smoke (14) and multiple studies linking active smoking with TB (17). Our study identifies a public health opportunity: smoking cessation interventions in patients with active TB not only could improve TB treatment outcomes (38, 39) but also might have the added benefit of decreasing risk for LTBI among household contacts. Children <5 years old were found to be most susceptible to the effects of SHS, perhaps because young children spend more time around smoking household caregivers or because young children might be more susceptible to the immunotoxic effects of SHS.
Manufactured cigarette SHS seemed to be more immunotoxic than waterpipe SHS, although the small number of waterpipe smokers limited the interpretation of this analysis. The Vietnamese waterpipe is distinct from the more widely studied Arabic waterpipe: no charcoal filtration is used, and smoking sessions are brief. Furthermore, the minimally processed Nicotiana rustica smoked in the Vietnamese waterpipe is distinct from the manufactured cigarette’s highly processed N. tabacum (40). Locals believe that waterpipe smoking is safer than cigarettes, but scant evidence supports (24) or refutes (40, 41) this assertion. Vietnamese waterpipe emissions and health studies are greatly needed.
Motorcycle emissions are the prevailing source of air pollution for city dwellers living throughout Vietnam (12), and in Hanoi, 96% of the vehicle fleet is composed of motorcycles (10). In recent source apportionment studies, the four-stroke motorcycle engine without a catalytic converter most prevalent on the streets of Hanoi (42) produced a laboratory-based emission spectra highly correlated with Hanoi ambient samplings (11). Hanoi motorcycles account for 70% of traffic-related total suspending particles and >95% of traffic-related volatile organic compounds (VOCs) (10). Motorcycles congest the residential streets outside people’s homes, exposing residents to exhaust emissions that enter homes through open windows and doors. From air pollution vertical gradient studies with street-level predominant air pollution sources, indoor exposures to vehicular exhaust are highest on the ground floor and dissipate as residential floors increase and distance from motorcycle traffic increases (27, 28). Although vertical pollution gradients are altered by atmospheric temperature inversion events, these inversions are infrequent occurrences in Hanoi (43, 44). Furthermore, vertical gradient health outcomes studies have found decreased risk for pneumonia and all-cause mortality with higher residential floors, although socioeconomic factors were important covariates (45, 46). We found an inverse dose–response relationship between residential floor lived and slept on and odds of LTBI. Children living on higher floors, farther from street-level pollution, experienced lower odds of LTBI, findings that were robust across multiple models. However, PM2.5 was not significantly lower on higher floors, raising the possibility that the “protective” effect of living on higher floors was not primarily mediated through PM2.5 but through unmeasured pollutants or socioeconomic factors. In addition, the majority of children in this cohort were exposed to motorcycles parked inside their homes, and this practice increased LTBI odds, although the association was nonlinear. Parked motorcycles expose residents to evaporative emissions from the vehicles’ fuel systems plus any residual exhaust emission remaining in the tailpipe after the vehicle is turned off (13, 47). Larger studies are needed to confirm these findings, and interventions should be designed to decrease motorcycle exposures in household contact cohorts.
Although LPG burns cleaner than solid fuels (48), the health benefits of LPG cooking interventions remain unclear (49), and LPG emissions can exceed WHO standards for NO2 in poorly ventilated kitchens (50). For this reason the WHO has recommended that LPG cooking be actively ventilated and that further investigations into LPG health effects be conducted (51). Although our LPG findings were not statistically significant, the direction of association was consistent across models, with attenuation of risk for actively ventilated kitchens. Indoor air pollution and TB studies to date have not compared LPG with electricity, but a recent large Nepali case–control study found LPG to increase odds of active TB (52) but not LTBI (9) when compared with solid fuel use. Further LPG ventilation health studies are needed to contribute to cost–benefit analyses as governments consider requiring active kitchen ventilation installations with new constructions and upgrades and as governments consider transitioning from LPG and natural gas to zero emissions renewable electricity sources. In addition to being used for cooking, fuel can also be burned indoors for heating and lighting. In the Albers Nepali study, for instance, lighting with candles and oil lamps was associated with increased risk for LTBI (9). In our urban cohort, none of our participants relied on nonelectrical lighting and only 17 had heating in their homes (15 used electric space heaters and 2 used LPG space heaters).
Personal PM2.5 exposures were associated with indoor air pollution sources, providing a degree of validity to our questionnaire, although many of these PM2.5 associations were not statistically significant. However, PM2.5 did not appear to be independently associated with LTBI as we had hypothesized. This suggests that the effects of indoor sources were not principally mediated through PM2.5 but rather perhaps through a mixture of unmeasured toxicants such as VOCs and nitrogen oxides (NOx) in addition to PM2.5. Second, PM2.5 monitoring represented a composite exposure to multiple indoor and outdoor sources of potentially variable toxicities, perhaps diluting any effect on LTBI. Furthermore, personal PM2.5 aerosol sampling was of limited duration, not likely an adequate reflection of overall particulate exposures, and not all children were able to complete this procedure, thereby introducing a degree of nondifferential misclassification of exposure and selection bias.
The findings from this observational study are biologically plausible and supported by mechanistic studies. Secondhand smoke (53, 54), motorcycle emissions (13, 42, 55), and LPG emissions (48) are composed of chemical constituents known to be potentially immunotoxic (18, 56, 57) including VOCs, polycyclic aromatic hydrocarbons, carbonyls, respirable particulate matter, NOx, and sulfur oxides. Airborne toxicants have been shown to interfere with early immune responses that are likely important in preventing TB infection in household contacts. For instance, particulate matter has been shown in in vitro studies to inhibit airway epithelial cell antimicrobial peptide activity against M. tuberculosis (58) and alter macrophage phagocytosis (59). Recent studies have demonstrated that nonconventional T cells (γδ T cells, natural killer T cells, mucosal-associated invariant T cells) (60–62) and innate lymphoid cells (specifically natural killer cells and innate lymphoid cells type 3) (63, 64) have resident lung populations and may play early roles against TB infection, yet the effects of air pollution on these immune responses against M. tuberculosis remain unclear, representing a needed area of research. Air pollution could also increase M. tuberculosis transmission through nonimmune mechanisms. Several airborne toxicants are airway irritants causing increased cough frequency and force (65). Increased coughing of the patient with active TB could then increase transmission of M. tuberculosis from index case to household contacts (66). However, the direct role of indoor air pollution increasing cough and transmission has not been adequately studied.
We limited this study to a small sample size, such that we could expend resources on a more detailed household exposure assessment. Bacillus Calmette-Guérin vaccination can result in false-positive tuberculin skin tests, and because an equal proportion of children with and without LTBI received the vaccination (Table 1), this would be expected to introduce nondifferential misclassification of outcome, which could decrease our power to detect true associations. In our consecutive sampling of eligible households, several families did not ultimately agree to participate, thereby introducing possible selection bias. Although we did measure PM2.5 intermittently, we were not able to measure other important indoor pollutants such as VOCs and NOx. The cross-sectional study design limits our ability to draw conclusions about causality. Specifically, we do not know the precise timing of index case progression to active TB and household contact becoming infected with TB regarding exposure time windows. To address this study design limitation, we have selected relatively time-invariant predictors, making the assumptions that household smoking, cooking practices, and motorcycle usage remained relatively constant over time.
Conclusions
Secondhand smoke increased the odds of LTBI in this urban cohort of Vietnamese children, whereas linkages between other indoor air pollution sources and LTBI remained inconclusive. Larger observational studies are needed to confirm associations of indoor motorcycle emissions and LPG with LTBI, coupling questionnaire data to indoor monitoring of VOCs, NOx, and PM2.5. These findings could inform interventional studies designed to mitigate TB risk through smoking cessation counseling, motorcycle emissions controls, and active kitchen ventilation.
Acknowledgments
Acknowledgment
The authors thank the following teams at the National Lung Hospital: Dr. Nguyen Thi Ngoan, Nurses Thuy Anh, Thuy Hoang, Tho Nguyen, Ngoc Anh, and Thao Ha, and the Pediatric Department; Dr. Phuong Anh, Mr. Tam, and the Respiratory Testing & Rehabilitation Department; and Dr. Vo Thanh, Ms. Dung, Ms. Thao Tran, and the Hematological & Blood Transfusion Department for study design and implementation. They thank the District Health Center medical doctors and staff at Dong Da, Hai Ba Trung, Thanh Tri, Nam Tu Liem, and Hoan Kiem Districts for enrolling participants and performing study procedures. They also thank Dr. Hanh Nguyen, Mr. Nam Pham, and staff at the Vietnam National Tuberculosis Program–University of California San Francisco Research Collaboration Unit for provision of clinical research infrastructure. Finally, they thank the participants, who have graciously volunteered hours of their time to participate in this public health study.
Footnotes
Supported by National Institute of Environmental Health Sciences grants K23ES025807 (R.J.B.) and P30 ES005605 (National Institute of Environmental Health Sciences funding through the University of Iowa Environmental Health Sciences Research Center).
Author Contributions: R.J.B. wrote the manuscript. R.J.B., P.N., N.V.N., J.B., and H.P. conceived the study and designed the experiments. C.M., H.P., T.T., H.D., P.N., and R.J.B. implemented the study and collected the data. M.R.S., M.Z., A.P.C., E.M.S., J.Z., and R.J.B. analyzed and interpreted the data. All authors contributed to revising the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202101-0136OC on August 3, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med . 2016;13:e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Global tuberculosis report 2020. 2020. [accessed 2020 Dec 01]. Available from: https://www.who.int/teams/global-tuberculosis-programme/data
- 3. Marks SM, Taylor Z, Qualls NL, Shrestha-Kuwahara RJ, Wilce MA, Nguyen CH. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med . 2000;162:2033–2038. doi: 10.1164/ajrccm.162.6.2004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabbani U, Sahito A, Nafees AA, Kazi A, Fatmi Z. Pulmonary tuberculosis is associated with biomass fuel use among rural women in Pakistan: an age- and residence-matched case-control study. Asia Pac J Public Health . 2017;29:211–218. doi: 10.1177/1010539517696554. [DOI] [PubMed] [Google Scholar]
- 5. Sumpter C, Chandramohan D. Systematic review and meta-analysis of the associations between indoor air pollution and tuberculosis. Trop Med Int Health . 2013;18:101–108. doi: 10.1111/tmi.12013. [DOI] [PubMed] [Google Scholar]
- 6. Pokhrel AK, Bates MN, Verma SC, Joshi HS, Sreeramareddy CT, Smith KR. Tuberculosis and indoor biomass and kerosene use in Nepal: a case-control study. Environ Health Perspect . 2010;118:558–564. doi: 10.1289/ehp.0901032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jafta N, Jeena PM, Barregard L, Naidoo RN. Association of childhood pulmonary tuberculosis with exposure to indoor air pollution: a case control study. BMC Public Health . 2019;19:275. doi: 10.1186/s12889-019-6604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hystad P, Duong M, Brauer M, Larkin A, Arku R, Kurmi OP, et al. on behalf of Prospective Urban and Rural Epidemiological (PURE) Study investigators. Health effects of household solid fuel use: findings from 11 countries within the Prospective Urban and Rural Epidemiology Study. Environ Health Perspect . 2019;127:57003. doi: 10.1289/EHP3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albers AE, Pope K, Sijali TR, Subramanya SH, Verma SC, Bates MN. Household fuel use and latent tuberculosis infection in a Nepali population. Environ Res . 2019;173:69–76. doi: 10.1016/j.envres.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hieu VV, Quynh LX, Ho PN, Hens L. Health risk assessment of mobility-related air pollution in Ha Noi, Vietnam. J Environ Prot (Irvine Calif) . 2013;04:1165–1172. [Google Scholar]
- 11. Sakamoto Y, Shoji K, Bui MT, Phạm TH, Vu TA, Ly BT, et al. Air quality study in Hanoi, Vietnam in 2015–2016 based on a one-year observation of NO x, O 3, CO and a one-week observation of VOCs. Atmos Pollut Res . 2018;9:544–551. [Google Scholar]
- 12. Hien PD, Men NT, Tan PM, Hangartner M. Impact of urban expansion on the air pollution landscape: a case study of Hanoi, Vietnam. Sci Total Environ . 2020;702:134635. doi: 10.1016/j.scitotenv.2019.134635. [DOI] [PubMed] [Google Scholar]
- 13. Li L, Ge Y, Wang M, Peng Z, Song Y, Zhang L, et al. Exhaust and evaporative emissions from motorcycles fueled with ethanol gasoline blends. Sci Total Environ . 2015;502:627–631. doi: 10.1016/j.scitotenv.2014.09.068. [DOI] [PubMed] [Google Scholar]
- 14.GATS 2015. [accessed 2020 Nov 18]. Available from: https://www.tobaccofreekids.org/assets/global/pdfs/en/GATS_2015_Vietnam_Report.pdf.
- 15.GSHS 2013. [accessed 2020 Nov 18]. Available from: https://extranet.who.int/ncdsmicrodata/index.php/catalog/482/related-materials
- 16. Horne DJ, Campo M, Ortiz JR, Oren E, Arentz M, Crothers K, et al. Association between smoking and latent tuberculosis in the U.S. population: an analysis of the National Health and Nutrition Examination Survey. PLoS One . 2012;7:e49050. doi: 10.1371/journal.pone.0049050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med . 2007;4:e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai X, Aerts SL, Verma D, Ordway DJ, Chan ED. Epidemiologic evidence of and potential mechanisms by which second-hand smoke causes predisposition to latent and active tuberculosis. Immune Netw . 2018;18:e22. doi: 10.4110/in.2018.18.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ganmaa D, Khudyakov P, Buyanjargal U, Jargalsaikhan B, Baigal D, Munkhjargal O, et al. Prevalence and determinants of QuantiFERON-diagnosed tuberculosis infection in 9810 Mongolian schoolchildren. Clin Infect Dis . 2019;69:813–819. doi: 10.1093/cid/ciy975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patra J, Bhatia M, Suraweera W, Morris SK, Patra C, Gupta PC, et al. Exposure to second-hand smoke and the risk of tuberculosis in children and adults: a systematic review and meta-analysis of 18 observational studies PLoS Med 201512e1001835. [Discussion, e1001835.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindsay RP, Shin SS, Garfein RS, Rusch MLA, Novotny TE. The Association between active and passive smoking and latent tuberculosis infection in adults and children in the united states: results from NHANES. PLoS One . 2014;9:e93137. doi: 10.1371/journal.pone.0093137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dogar OF, Pillai N, Safdar N, Shah SK, Zahid R, Siddiqi K. Second-hand smoke and the risk of tuberculosis: a systematic review and a meta-analysis. Epidemiol Infect . 2015;143:3158–3172. doi: 10.1017/S0950268815001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xuan TT, Van Minh H, Giang KB, Nga PT, Hai PT, Minh NT, et al. Prevalence of waterpipe tobacco smoking among population aged 15 years or older, Vietnam, 2010. Prev Chronic Dis . 2013;10:E57. doi: 10.5888/pcd10.120100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petersen AB, Muffley N, Somsamouth K, Singh PN. Smoked tobacco, air pollution, and tuberculosis in Lao PDR: findings from a national sample. Int J Environ Res Public Health . 2019;16:3059. doi: 10.3390/ijerph16173059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blount RJ, Hung NV, Nhung NV, Phan H, Lien LT, Balmes J, et al. “Effects of PM2.5 on tuberculosis infection in Vietnam: preliminary findings.” Poster presentation at the NIEHS Core Centers Meeting, July 2018University of California, David; Davis, CA [Google Scholar]
- 26. Blount RJ, Phan H, Trinh T, McLaughlin R, Huy Han D, Zabner J, et al. Am J Respir Crit Care Med . 2020;201:A6380. [Google Scholar]
- 27. Li C, Fu J, Sheng G, Bi X, Hao Y, Wang X, et al. Vertical distribution of PAHs in the indoor and outdoor PM2.5 in Guangzhou, China. Build Environ . 2005;40:329–341. [Google Scholar]
- 28. Chen H-L, Li C-P, Tang C-S, Lung S-CC, Chuang H-C, Chou D-W, et al. Risk assessment for people exposed to PM2.5 and constituents at different vertical heights in an urban area of Taiwan. Atmosphere . 2020;11:1145. [Google Scholar]
- 29. de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull . 2004;25(1 Suppl):S15–S26. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Obesity and overweight [accessed 2021. Jan 17]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=Overweight%20and%20obesity%20are%20defined%20as%20follows%20for%20children%20aged,the%20WHO%20Growth%20Reference%20median
- 31. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ . 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Volckens J, Quinn C, Leith D, Mehaffy J, Henry CS, Miller-Lionberg D. Development and evaluation of an ultrasonic personal aerosol sampler. Indoor Air . 2016;27:409–416. doi: 10.1111/ina.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol . 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 34.Vittinghoff E, Glidden D, Shiboski SC, McCulloch C. Regression methods in biostatistics. 2012. [Google Scholar]
- 35. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol . 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 36. du Preez K, Mandalakas AM, Kirchner HL, Grewal HM, Schaaf HS, van Wyk SS, et al. Environmental tobacco smoke exposure increases Mycobacterium tuberculosis infection risk in children. Int J Tuberc Lung Dis . 2011;15:1490–1496, i. doi: 10.5588/ijtld.10.0759. [DOI] [PubMed] [Google Scholar]
- 37. Chan ED, Keane J, Iseman MD. Should cigarette smoke exposure be a criterion to treat latent tuberculous infection? Am J Respir Crit Care Med . 2010;182:990–992. doi: 10.1164/rccm.201006-0861ED. [DOI] [PubMed] [Google Scholar]
- 38. Jee SH, Golub JE, Jo J, Park IS, Ohrr H, Samet JM. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am J Epidemiol . 2009;170:1478–1485. doi: 10.1093/aje/kwp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maciel EL, Brioschi AP, Peres RL, Guidoni LM, Ribeiro FK, Hadad DJ, et al. Smoking and 2-month culture conversion during anti-tuberculosis treatment. Int J Tuberc Lung Dis . 2013;17:225–228. doi: 10.5588/ijtld.12.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai HT, Koriyama C, Tokudome S, Tran HH, Tran LT, Nandakumar A, et al. Waterpipe tobacco smoking and gastric cancer risk among Vietnamese men. PLoS One . 2016;11:e0165587. doi: 10.1371/journal.pone.0165587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. She J, Yang P, Wang Y, Qin X, Fan J, Wang Y, et al. Chinese water-pipe smoking and the risk of COPD. Chest . 2014;146:924–931. doi: 10.1378/chest.13-1499. [DOI] [PubMed] [Google Scholar]
- 42. Kim Oanh NT, Thuy Phuong MT, Permadi DA. Analysis of motorcycle fleet in Hanoi for estimation of air pollution emission and climate mitigation co-benefit of technology implementation. Atmos Environ . 2012;59:438–448. [Google Scholar]
- 43. Wang Q, Sun Y, Xu W, Du W, Zhou L, Tang G, et al. Vertically resolved characteristics of air pollution during two severe winter haze episodes in urban Beijing, China. Atmos Chem Phys . 2018;18:2495–2509. [Google Scholar]
- 44. Trinh TT, Trinh TT, Le TT, Nguyen TDH, Tu BM. Temperature inversion and air pollution relationship, and its effects on human health in Hanoi City, Vietnam. Environ Geochem Health . 2019;41:929–937. doi: 10.1007/s10653-018-0190-0. [DOI] [PubMed] [Google Scholar]
- 45. Chang J, Liu W, Huang C. Residential ambient traffic in relation to childhood pneumonia among urban children in Shandong, China: a cross-sectional study. Int J Environ Res Public Health . 2018;15:1076. doi: 10.3390/ijerph15061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panczak R, Galobardes B, Spoerri A, Zwahlen M, Egger M. High life in the sky? Mortality by floor of residence in Switzerland. Eur J Epidemiol . 2013;28:453–462. doi: 10.1007/s10654-013-9809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Graham LA, Noseworthy L, Fugler D, O’Leary K, Karman D, Grande C. Contribution of vehicle emissions from an attached garage to residential indoor air pollution levels. J Air Waste Manag Assoc . 2004;54:563–584. doi: 10.1080/10473289.2004.10470931. [DOI] [PubMed] [Google Scholar]
- 48. Bilsback KR, Dahlke J, Fedak KM, Good N, Hecobian A, Herckes P, et al. A laboratory assessment of 120 air pollutant emissions from biomass and fossil fuel cookstoves. Environ Sci Technol . 2019;53:7114–7125. doi: 10.1021/acs.est.8b07019. [DOI] [PubMed] [Google Scholar]
- 49. Checkley W, Williams KN, Kephart JL, Fandiño-Del-Rio M, Steenland NK, Gonzales GF, et al. CHAP Trial Investigators. Effects of a cleaner energy intervention on cardiopulmonary outcomes in Peru: a randomized controlled trial. Am J Respir Crit Care Med . 2021;203:1386–1397. doi: 10.1164/rccm.202006-2319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasanudin U, Haryanto A, Romero J.Effect of stove types on in-kitchen air quality: case study at Way Isem village, Lampung province, Indonesia J Sustain Energy Environ 20112181–186.. [Google Scholar]
- 51.WHO. 2014. https://www.who.int/publications/i/item/9789241548885
- 52. Bates MN, Pope K, Sijali TR, Pokhrel AK, Pillarisetti A, Lam NL, et al. Household fuel use and pulmonary tuberculosis in western Nepal: A case-control study. Environ Res . 2019;168:193–205. doi: 10.1016/j.envres.2018.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1, Composition, exposure and regulations. IARC monographs on the evaluation of carcinogenic risks to humans, No. 83. Tobacco smoke and involuntary smoking. pp. 59–94. 2004. [PMC free article] [PubMed]
- 54. Avino P, Scungio M, Stabile L, Cortellessa G, Buonanno G, Manigrasso M. Second-hand aerosol from tobacco and electronic cigarettes: Evaluation of the smoker emission rates and doses and lung cancer risk of passive smokers and vapers. Sci Total Environ . 2018;642:137–147. doi: 10.1016/j.scitotenv.2018.06.059. [DOI] [PubMed] [Google Scholar]
- 55. Hoang AT, Tran QV, Al-Tawaha ARMS, Pham VV, Nguyen XP. Comparative analysis on performance and emission characteristics of an in-Vietnam popular 4-stroke motorcycle engine running on biogasoline and mineral gasoline. Renew Energy Focus . 2019;28:47–55. [Google Scholar]
- 56. Suzuki T, Hidaka T, Kumagai Y, Yamamoto M. Environmental pollutants and the immune response. Nat Immunol . 2020;21:1486–1495. doi: 10.1038/s41590-020-0802-6. [DOI] [PubMed] [Google Scholar]
- 57. Vargas Buonfiglio LG, Borcherding JA, Frommelt M, Parker GJ, Duchman B, Vanegas Calderón OG, et al. Airway surface liquid from smokers promotes bacterial growth and biofilm formation via iron-lactoferrin imbalance. Respir Res . 2018;19:42. doi: 10.1186/s12931-018-0743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rivas-Santiago CE, Sarkar S, Cantarella P, IV, Osornio-Vargas Á, Quintana-Belmares R, Meng Q, et al. Air pollution particulate matter alters antimycobacterial respiratory epithelium innate immunity. Infect Immun . 2015;83:2507–2517. doi: 10.1128/IAI.03018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rylance J, Fullerton DG, Scriven J, Aljurayyan AN, Mzinza D, Barrett S, et al. Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. Am J Respir Cell Mol Biol . 2015;52:584–593. doi: 10.1165/rcmb.2014-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wong EB, Gold MC, Meermeier EW, Xulu BZ, Khuzwayo S, Sullivan ZA, et al. TRAV1-2+ CD8+ T-cells including oligoconal expansions of MAIT cells are enriched in the airways in human tuberculosis. Commun Biol . 2019;2:203. doi: 10.1038/s42003-019-0442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Y, Wang X, Teng D, Chen H, Wang M, Wang J, et al. Identification of the ligands of TCRγδ by screening the immune repertoire of γδT cells from patients with tuberculosis. Front Immunol . 2019;10:2282. doi: 10.3389/fimmu.2019.02282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verrall AJ, Schneider M, Alisjahbana B, Apriani L, van Laarhoven A, Koeken V, et al. Early clearance of Mycobacterium tuberculosis is associated with increased innate immune responses J Infect Dis 20202211342–1350.. [DOI] [PubMed] [Google Scholar]
- 63. Khader SA, Divangahi M, Hanekom W, Hill PC, Maeurer M, Makar KW, et al. Bill and Melinda Gates Foundation Collaboration for TB Vaccine Discovery Innate Immunity Working Group18. Targeting innate immunity for tuberculosis vaccination. J Clin Invest . 2019;129:3482–3491. doi: 10.1172/JCI128877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ardain A, Domingo-Gonzalez R, Das S, Kazer SW, Howard NC, Singh A, et al. Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis. Nature . 2019;570:528–532. doi: 10.1038/s41586-019-1276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Joad JP, Sekizawa S, Chen C-Y, Bonham AC. Air pollutants and cough. Pulm Pharmacol Ther . 2007;20:347–354. doi: 10.1016/j.pupt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 66. Turner RD, Birring SS, Darmalingam M, Hooper RL, Kunst H, Matos S, et al. Daily cough frequency in tuberculosis and association with household infection. Int J Tuberc Lung Dis . 2018;22:863–870. doi: 10.5588/ijtld.17.0652. [DOI] [PubMed] [Google Scholar]