Abstract
Background: The fractional exhaled nitric oxide (FENO) test is a point-of-care test that is used in the assessment of asthma.
Objective: To provide evidence-based clinical guidance on whether FENO testing is indicated to optimize asthma treatment in patients with asthma in whom treatment is being considered.
Methods: An international, multidisciplinary panel of experts was convened to form a consensus document regarding a single question relevant to the use of FENO. The question was selected from three potential questions based on the greatest perceived impact on clinical practice and the unmet need for evidence-based answers related to this question. The panel performed systematic reviews of published randomized controlled trials between 2004 and 2019 and followed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) evidence-to-decision framework to develop recommendations. All panel members evaluated and approved the recommendations.
Main Results: After considering the overall low quality of the evidence, the panel made a conditional recommendation for FENO-based care. In patients with asthma in whom treatment is being considered, we suggest that FENO is beneficial and should be used in addition to usual care. This judgment is based on a balance of effects that probably favors the intervention; the moderate costs and availability of resources, which probably favors the intervention; and the perceived acceptability and feasibility of the intervention in daily practice.
Conclusions: Clinicians should consider this recommendation to measure FENO in patients with asthma in whom treatment is being considered based on current best available evidence.
Keywords: nitric oxide, asthma, inhaled steroids, airway disease, type 2 inflammation
Contents
- Introduction
- Target Audience
- Methods
- Panel Composition
- Conflict-of-Interest Declaration and Management
- Meetings and Conference Calls
- Formulation of Key Questions and Selection of Outcomes of Interest
- Evidence Review and Development of Clinical Recommendations
- Manuscript Preparation
- Peer Review
-
Results
Question: Should patients with asthma in whom treatment is being contemplated undergo FeNO testing?
Discussion
Introduction
Asthma is a heterogeneous disease that causes variable airflow obstruction and features of airway hyperresponsiveness (AHR) in response to a variety of triggers, including respiratory viral infections, environmental allergens, and physical stimuli such as exercise with its accompanying hyperpnea (1, 2). It is generally well accepted that underlying inflammation of the airways acts in conjunction with structural changes collectively known as airway remodeling to form the pathophysiology of the disease (3). Treatment decisions are typically based on measures of airflow obstruction, the frequency of daytime and nighttime symptoms, and the frequency of exacerbations (1, 2). Individuals with persistent symptoms, or with airflow obstruction at lung function testing and/or who experience asthma exacerbations, are typically treated with daily controller therapies such as inhaled corticosteroids (ICSs) with or without a long-acting β-agonist and/or a leukotriene modifier or long-acting muscarinic antagonist (4, 5). In individuals with uncontrolled asthma, biologic therapies targeting specific inflammatory pathways (e.g., monoclonal antibodies targeting type 2 [T2] inflammation) are considered. Treatment is adjusted on the basis of the level of asthma control that is achieved with the selected therapy; however, the response to therapy is heterogeneous. Clinicians are in need of complementary tools that can assist them in making informed treatment decisions about the type and intensity of daily controller therapy.
Once the diagnosis of asthma is established, selecting the type of therapy and selecting the optimal dose of therapy for the patient are challenging decisions faced by clinicians. Therapies such as ICSs improve lung function and asthma control, decrease daytime and nighttime symptoms, and reduce the frequency of asthma exacerbations (6, 7). However, a significant portion of patients do not have a substantive response to therapy, and the response of an individual patient to a given dose of therapy is variable (8–13). Methods that have been developed to further assess the level of airway inflammation to guide corticosteroid responsiveness, such as inducing sputum to measure eosinophils, are not widely available (14–18). Other measures such as AHR (19–21) and peak expiratory flow variability (22) can also provide information that is useful to clinicians but require more involved testing. Recently, measuring biomarkers of T2 inflammation by focusing on peripheral blood eosinophils has been used to direct therapy and has become essential in the era of biologically targeted therapies toward T2 inflammation in asthma (23).
Nitric oxide (NO) is a gas that can be measured in the exhaled breath. Measuring the fraction of this gas during a steady-state exhalation, called the fractional exhaled NO (FeNO), is a standardized and quantitative method for assessing the levels of this gas in exhaled breath (24). The source of FeNO comes from the action of several different NOS (NO synthase) enzymes, but the principal source of the increased levels of NO that are identified in asthma comes from the iNOS2 (inducible NOS 2) enzyme that is induced in the airway epithelium from inflammation (25–27). Functions that have been attributed to NO include actions as a vasodilator, bronchodilator, neurotransmitter, and mediator of inflammation (28). A number of these roles may be protective in nature, which led to trials to augment the levels of NO in the airways through the administration of arginine and citrulline in selected individuals with asthma (29, 30). There are also several homeostatic functions attributed to NO, including bactericidal and cytotoxic effects that play a role in host defense (31). FeNO correlates well with airway eosinophilic inflammation measured in induced sputum, providing a noninvasive way to assess T2 airway inflammation in asthma (32–36). Treatment with ICSs results in a marked decrease in a subset of patients with ongoing inflammation (37–39). There is strong evidence that the levels of FeNO correlate with features of T2 inflammation, particularly the levels of eosinophilia in the peripheral blood and induced sputum (34, 40). In individuals with atopic asthma, FeNO levels tend to correlate with elevated total serum IgE levels or skin prick testing results (41). Although FeNO levels do not correlate well with the degree of baseline airflow obstruction, they tend to correlate well with the severity and features of “indirect” or “endogenous” AHR, such as exercise-induced bronchoconstriction (42–44). Interestingly, FeNO levels change dynamically after bronchoprovocation with an initial decrease during airway narrowing followed by an increase as the airways dilate after the nadir during the late airway response (45, 46).
Notably, an elevated FeNO level is not entirely specific to asthma and levels can be elevated in people with atopy who do not have other features of asthma (47, 48). Recent studies have further delineated the relationship between eosinophilic inflammation and FeNO by suggesting that T2-targeted biological therapy that blocks IL-13/IL-4 leads to a reduction in FeNO levels (49, 50–52) and that therapies targeting eosinophils with anti–IL-5–directed therapies may also lead to a reduction in FeNO levels (53–55).
After the initial development of using FeNO as a test, a clinical practice guideline was developed by the American Thoracic Society (ATS) for the interpretation of FeNO in adults, which included general cut points that represent a low FeNO value as being below 25 ppb and an elevated FeNO value as being above 50 ppb, whereas values between 25 and 50 ppb were considered indeterminant (24). In children, the cut points for FeNO were slightly different: a low FeNO value is below 20 ppb, an elevated FeNO value is above 35 ppb, and values between 20 and 35 ppb were considered indeterminant (24). Subsequent to this initial guideline, several well-conducted studies have been completed that have divided FeNO values into more narrowly defined ranges and have ascertained the utility of this test relative to multiple different outcome measurements relevant to asthma.
Several key questions about the use of FeNO testing in clinical practice include the use of this test as an aid in establishing the diagnosis of asthma, for monitoring the response to therapy, and for making initial treatment decisions in an individual once the diagnosis of asthma is established. Because of this gap in knowledge, the ATS commissioned a multidisciplinary panel to select the single most important and immediate question related to the use of the FeNO test to generate evidence-based recommendations to improve patient-centered outcomes. Several systematic reviews have been published on the use of FeNO testing for asthma (56–60). These analyses informed the panel’s decision regarding the most critical question based on the perceived impact on patient care, and the panel subsequently selected the most important outcome measures to evaluate the use of FeNO testing in clinical practice. These results provide an evidence-based assessment of the utility of the FeNO test in the management of individuals with asthma in whom treatment is being contemplated and are complementary to guidelines by the National Asthma Education and Prevention Program, the Global Initiative for Asthma guidelines, and the Japanese Respiratory Society guidelines (1, 2, 51).
Target Audience
This practice guideline is designed to provide guidance to clinicians who manage adults and children 4 years of age and older with asthma, including adult and pediatric pulmonologists, adult and pediatric allergists, internists, pediatricians, family medicine specialists, and other healthcare providers involved in the care of patients with asthma. The use of this guideline is not designed for the evaluation and management of acute asthma but rather is designed for treatment decisions in ambulatory settings for the ongoing management of this common disease.
Methods
This clinical practice guideline was developed in accordance with ATS policies and procedures.
Panel Composition
This guideline was formulated by using a “top-down” approach, with potential population, intervention, comparator, and outcome questions proposed by the Allergy, Immunology, and Inflammation Assembly of the ATS. Two co-chairs (S.B.K. and T.S.H.) were selected by the ATS Documents Development Committee, and the overall project was approved by the ATS Board of Directors. Seventeen panel members were selected on the basis of their expertise in adult and pediatric asthma. The panel was assisted by a methodology team composed of one senior ATS methodologist (J.M.I.) and two ATS Methodology Scholars (I.S. and A. Barochia).
Conflict-of-Interest Declaration and Management
All potential guideline panelists disclosed their conflicts of interest according to ATS policy, and disclosures were reviewed by ATS staff and the ATS Conflict of Interest Committee. There were no conflicts of interests among any of the panelists, chairs, or methodologists, and all were approved to participate without limitations.
Meetings and Conference Calls
Panel members participated via teleconference. Meetings of all panel members were convened in May, July, and December of 2019 and in January of 2020. Additional meetings were also held by the co-chairs and the methodologists as needed to resolve questions and provide guidance.
Formulation of Key Questions and Selection of Outcomes of Interest
The panel discussed three population, intervention, comparator, and outcome questions proposed by the Allergy, Immunology, and Inflammation Assembly of the ATS. After discussion of the potential questions, the panel formally voted and chose the one question believed to be of the highest priority for this guideline (Table 1). The panel then selected patient-centric outcomes relevant to FeNO testing and voted a priori to rank the importance of the outcomes (Table 2). Outcomes were ranked on a scale of 1–9, with scores of 1–3 considered of limited importance for decision-making for this question, scores of 4–6 considered as important but not critical, and scores of 7–9 considered critical for decision-making (61). Outcomes with a mean score of 7 or above were considered critical and included asthma control as assessed by using any validated questionnaire, the use of oral corticosteroids, the acute asthma exacerbation rate, the frequency of emergency room/urgent care visits, and the frequency of asthma-related hospitalizations. The remaining outcomes, including quality of life measures, symptom-free days, days of work/school missed, daily activities/exercise, use of ICSs, use of rescue medications, patient satisfaction with care, adverse events, medication adherence, cost-effectiveness, lung function measures (FEV1 or FEV1/FVC ratio), blood eosinophils, asthma-related mortality, asthma medication ratios, and inhaler techniques were determined to be important but not critical to decision-making for this question.
Table 1.
Questions Initially Proposed by the Guideline Co-Chairs and Question Selection Results
| Proposed Question | Priority* |
|---|---|
| Should patients with asthma in whom treatment is being contemplated undergo FeNO testing? | 10 |
| Should patients being treated for asthma undergo FeNO monitoring? | 4 |
| Should patients in whom a diagnosis of asthma is being considered undergo FeNO testing? | 3 |
Definition of abbreviation: FeNO = fractional exhaled nitric oxide.
Number of panel members who selected this question as the highest priority.
Table 2.
Prioritization of the Key Outcome Measurements for This Single-Question Guideline
| Outcome Measurement | Mean | SD | Median | IQR |
|---|---|---|---|---|
| Asthma control | 7.94 | 2.49 | 8 | 2 |
| Use of oral corticosteroids | 7.93 | 2.74 | 8 | 0.5 |
| Acute exacerbation rate | 7.69 | 2.64 | 8.5 | 2 |
| ER or urgent care visits | 7.56 | 2.33 | 8 | 2 |
| Hospitalizations due to asthma | 7.25 | 1.99 | 8 | 2.25 |
| Quality of life measures* | 6.88 | 1.79 | 7 | 2 |
| Symptom-free days | 6.69 | 2.11 | 7 | 2 |
| Days of work/school missed | 6.69 | 2.28 | 7 | 2.25 |
| Daily activities/exercise/sports | 6.63 | 2.39 | 7 | 1.25 |
| Use of inhaled corticosteroids | 6.44 | 2.28 | 7 | 2 |
| Use of rescue medications | 6.38 | 1.72 | 6.5 | 3 |
| Patient satisfaction with care | 6.25 | 1.86 | 6.5 | 3 |
| Medication-related adverse events | 6.13 | 1.39 | 6.5 | 2.25 |
| Medication adherence | 6.06 | 1.30 | 6 | 4 |
| Lung function measures† | 5.63 | 2.91 | 5 | 1.25 |
| Blood eosinophils | 4.81 | 1.48 | 4.5 | 3.25 |
| Asthma-related mortality | 4.69 | 1.20 | 4.5 | 4.25 |
| Asthma medication ratio | 4.69 | 0.83 | 4 | 3.25 |
| Inhalation technique | 4.31 | 1.20 | 4.5 | 3.25 |
Definition of abbreviations: AQLQ = Asthma Quality of Life Questionnaire; ER = emergency room; IQR = interquartile range.
Any validated questionnaire or instrument (e.g., AQLQ or St. George’s questionnaire).
FEV1 or FEV1/FVC ratio.
Evidence Review and Development of Clinical Recommendations
The methodologists identified a prior systematic review performed in 2016, which was used in the development of a comparative-effectiveness review by the Agency for Healthcare Research and Quality (AHRQ) (62). In addition to using the results the aforementioned review, an additional review of the literature was performed from 2016 through July 2019 by using the Medline, Embase, and Cochrane Central databases to identify relevant studies about the effect of FeNO-based care compared with usual care on the outcomes of interest (see Table E1 in the online supplement for the search strategy). Titles and abstracts were screened in duplicate by the senior methodologist and an ATS scholar to determine eligibility for inclusion on the basis of predetermined criteria. All full-text articles, including those included in the AHRQ study, were then reviewed by the two ATS Methodology Scholars (I.S. and A. Barochia). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart demonstrates the inclusion and exclusion criteria of the retrieved studies (Figure 1) (63). The co-chairs and the panel members were consulted to confirm selection of the studies and suggested additional studies not captured by the literature review. After extracting relevant data from each study, analysis was performed for each outcome, with a meta-analysis being performed, as appropriate, by using Cochrane Collaboration Review Manager software, version 5.3 (64). If meta-analysis could not be performed, a narrative review of the results was provided.
Figure 1.
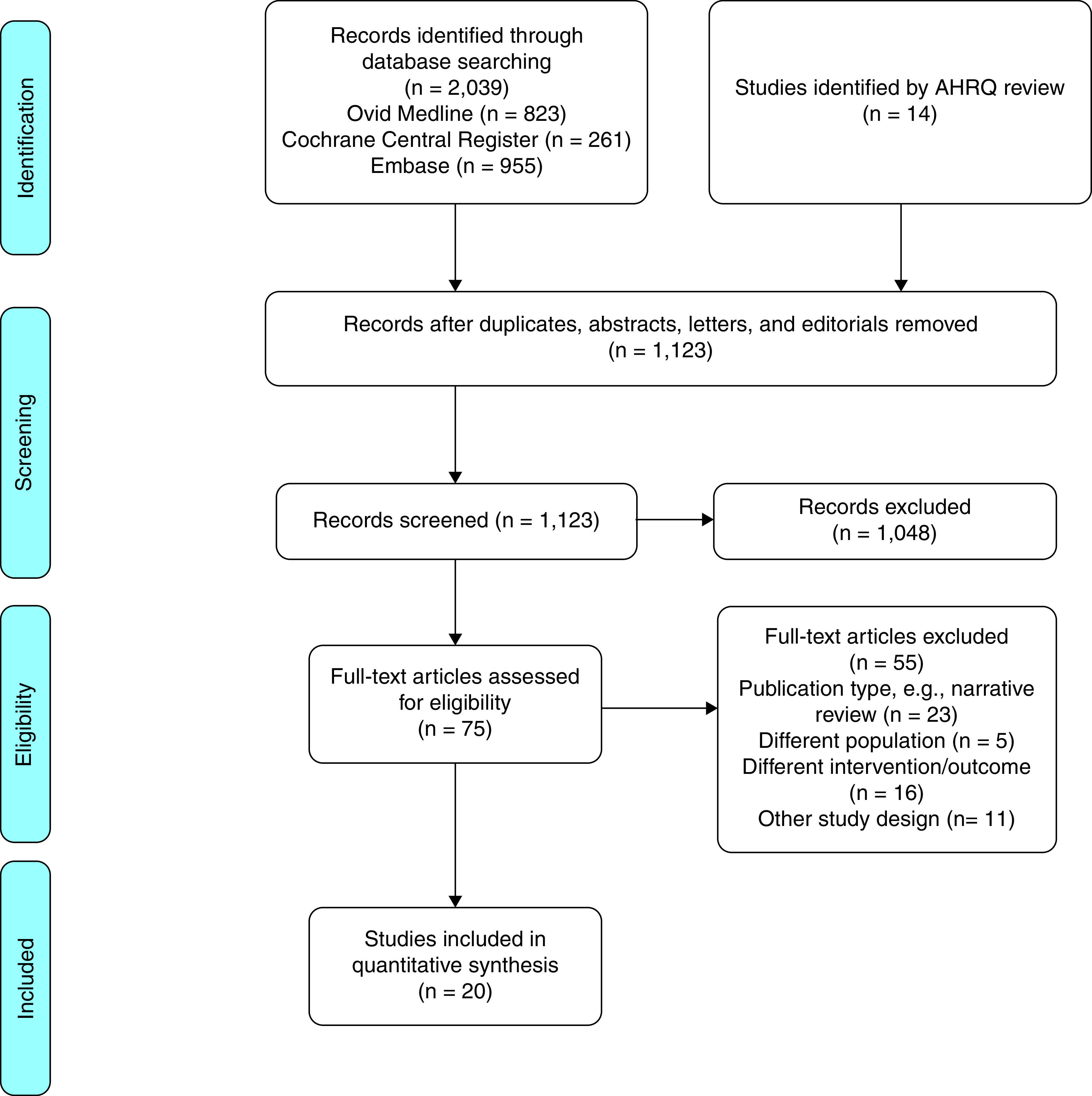
Flowchart summarizing the workflow for the systematic review of the literature to identify studies relevant to this single-question guideline. The methodologists used an existing comparative-effectiveness review as a starting point for the systematic review, which was conducted by using relevant databases. The flowchart that overviews the inclusion and exclusion criteria is presented in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses format. AHRQ = Agency for Healthcare Research and Quality.
A summary of the evidence was prepared on the basis of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach by using the GRADEpro Guideline Development Tool online application (https://gradepro.org/) (65). Risk of bias in the randomized controlled studies selected was assessed by using the Cochrane Collaboration’s risk of bias tool (66). The confidence of the estimates was evaluated for each outcome of interest by following the GRADE approach (67). Certainty of the evidence was categorized into four levels: high, moderate, low, and very low.
The information was summarized in an evidence-to-decision framework that included a description of the desirable and undesirable effects, the certainty of the evidence, assumptions about patients’ values, resource requirements, cost-effectiveness, the potential effect on health equity, the acceptability of the intervention by key stakeholders, and the feasibility of implementation (65).
Panel members reviewed the evidence and discussed the evidence-to-decision framework (Tables E2 and E3) via teleconference in December of 2019 and January of 2020. Subsequently, they formulated the final recommendation and approved it by consensus. As per the GRADE approach, recommendations can be labeled as either “strong” or “conditional.” The panel was instructed to use the words “we recommend” for strong recommendations and “we suggest” for conditional recommendations.
Manuscript Preparation
The initial draft of the manuscript was written by the co-chairs with contributions from all panel members. This draft was then reviewed by the entire panel, providing an opportunity to correct and supplement the manuscript to accurately reflect discussions and explanations for the key components of the recommendation. The wording of the final recommendation was not altered after the recommendation was finalized. After approval of the final manuscript by consensus, it was submitted for external peer review.
Peer Review
The guideline underwent anonymous peer review by four content experts and one methodologist. After multiple cycles of review and revision, the guideline was reviewed and approved by a multidisciplinary board of directors. The guideline will be reviewed by the ATS 3 years after publication, and whether updating is necessary will be determined.
Results
Question: Should patients with asthma in whom treatment is being contemplated undergo FeNO testing?
Background
Our committee identified high-priority outcome measurements to assess the utility of measuring FeNO in patients with asthma in whom treatment is being contemplated. Areas prioritized by the committee reflect outcomes that directly indicate uncontrolled asthma as well as questionnaires that are designed to assess asthma control. These areas were prioritized primarily because they represent the desired outcome of appropriately applied therapy for asthma to complement the more patient-centered outcomes such as quality of life and symptom-free days. In the clinic, asthma control is often assessed by using validated questionnaires, including the Asthma Control Test (ACT) and the 7-item Asthma Control Questionnaire (ACQ-7) (68–70). Such patient-reported outcome measures are considered to be clinically relevant because they are strong predictors of future exacerbations (71, 72). Further evidence of asthma control may also be determined from spirometry or other surrogate lung function measurements. Assessment of FeNO in the clinic may be complementary to these other assessment tools, particularly if there is evidence that the use of FeNO-based care improves specific outcomes that reflect asthma control. In particular, the frequency of exacerbations is a critical endpoint because exacerbations cause significant morbidity, cause mortality in some cases (73, 74), and are associated with increased asthma-related costs (75) and reduced long-term lung function (76–78). Unscheduled visits to the emergency department or urgent care settings, overuse of short-acting β-agonists, and frequent use of oral corticosteroids similarly reflect evidence of poor asthma control. Overall, therapies such as ICSs have been shown to reduce the frequency of exacerbations (6, 7); however, whether an individual patient should be treated with such a therapy and the dose that is adequate to suppress inflammation may not be apparent without additional diagnostic tests because the intensity of the disease varies from individual to individual and the response to therapy can be heterogeneous. The expert panel therefore focused on these key outcome measures and whether the assessment of FeNO during the evaluation of an individual with asthma leads to a significant change in these outcome measures. These outcomes described in the next section are available in Table E4, which includes relevant forest plots that display risk ratios for each outcome.
Validated questionnaires assessing asthma control
The ACT and the ACQ-7 are both validated questionnaires that assess asthma control (68–70). The results of these simple assessment tools were viewed by the panel as critical outcome measurements for the utility of FeNO assessment when treatment is being contemplated. The two different questionnaires could not be combined into a single metric, and the results did not identify evidence that FeNO-based care was associated with significant changes in asthma control on the basis of these questionnaires. Scores on the ACT range from 5 to 25, with higher values (>19) reflecting greater levels of asthma control. The minimal important difference (MID) for the ACT is around 3.0; however, the odds of exacerbation and frequency of bronchodilator use rise steeply when the scores are below approximately 17 (70). Although the mean difference (MD) in the ACT score trended toward favoring FeNO-based care, this difference was not statistically significant (MD, 0.40; 95% confidence interval [CI], −0.49 to 1.28). The trend toward favoring FeNO-based care as assessed by using the ACT was entirely from one of two studies, with no overall difference being demonstrated in one study conducted in adolescents and young adults (79) but an overall difference being demonstrated in the other study that was conducted in children (80). Within the childhood study, the effect was much stronger in the younger children for whom the Childhood ACT was used to monitor asthma control (69), with a 1.8-point difference being demonstrated by using the Childhood ACT (80).
The ACQ-7 is designed to identify uncontrolled asthma, with values ⩾1.5 being associated with poor asthma control and values ⩽0.75 being associated with good control (68). The MID in this outcome is around 0.5 (81). There was no overall difference in the ACQ-7 scores between FeNO-based care and usual care (MD, −0.01; 95% CI, −0.19 to 0.16), and although there was significant heterogeneity between the studies, none of the effect sizes were close to the MID of this instrument. The largest effect favoring FeNO-based care was in a trial that enrolled patients with markedly uncontrolled asthma at the onset of the study, with this study reporting a significant difference in the ACQ-7 results when using FeNO-based care; however, the differences in the ACQ-7 scores between the groups were fairly small relative to the marked improvement in asthma control identified in both treatment groups (82). The two other studies did not reveal a difference with FeNO-based care, although asthma control was much better at baseline in one study (83); in the other study, the score on the ACQ-7 was used as part of the algorithm to adjust asthma care (84).
Number and frequency of asthma exacerbations
Acute asthma exacerbations were viewed as a critical outcome measure. Studies were identified that reported the frequency of exacerbations (per patient per year) and/or reported exacerbations as the number of patients who experienced an exacerbation and as the relative risk (RR). There were 11 total studies that evaluated the exacerbation frequency (39, 80, 83, 85–92), but only 7 of these studies contributed to the analysis because the variance was not reported in all studies (39, 83, 85, 89–92). Overall, there was a reduction in the frequency of asthma exacerbations, favoring FeNO-based care (MD, −0.15; 95% CI, −0.28 to −0.03). The largest effect size within a single study was in a study conducted in children (92); however, the majority of studies contributing to these data reported on either adults alone or adults and children together. The overall certainty for the frequency of exacerbations was considered low.
The rate of exacerbations based on the number of patients in each group was reported in 10 studies with an overall moderate certainty of the evidence (85, 87–95). The overall difference in the number of patients experiencing an exacerbation was significantly lower in the groups that received FeNO-based care (RR, 0.72; 95% CI, 0.56 to 0.93). This result translates on average to 111 fewer exacerbations per 1,000 individuals (95% CI, 175 fewer to 28 fewer). It should be noted that the majority of these studies were conducted in children and that the results favoring a reduction in the number of exacerbations were not as strong in those studies that enrolled adults (85, 89–91).
Oral corticosteroid use
The use of oral corticosteroids was viewed as a critical outcome measure by the panel because it serves as a surrogate measure of exacerbation frequency and severity, and the prevention of oral corticosteroid-associated side effects by dose reduction or omission is likely beneficial to individuals with asthma. A total of six studies were identified that reported on the number of patients who used oral corticosteroids in each treatment arm. In two of the studies, the number was calculated from the percentage (90, 93). The number of patients treated with oral corticosteroids was significantly reduced when FeNO-based care was used (RR, 0.79; 95% CI, 0.65 to 0.95). This translates into 69 fewer individuals using corticosteroids per 1,000 individuals (95% CI, 115 fewer to 16 fewer). The certainty of this finding was considered moderate. It should be noted that a large portion of the weight of the evidence came from one study in children and adults (79) and that four of the studies were conducted entirely in children (93, 94, 96, 97), but all of these studies were relatively small. All of the included studies with the exception of one small study (97) reported results that favored FeNO-based care.
Emergency room and unscheduled healthcare visits
Emergency room visits, unscheduled healthcare visits, and hospitalizations for asthma were viewed by the panel as critical outcome measures, but there were relatively few data available for each of these measures. Therefore, the certainty of evidence was low to moderate. Three studies reported emergency room and unscheduled healthcare visits as the number of patients in each arm (83, 87, 93), revealing a nonsignificant reduction with using FeNO-based care (RR, 0.67; 95% CI, 0.37–1.22). These studies included both children and adults. Hospitalization for asthma is a relatively rare event, and this outcome was only reported in five studies (79, 83, 87, 88, 93); furthermore, the results could only be combined from three studies (79, 83, 87). Overall, there was no significant difference in the frequency of asthma hospitalizations with data derived from both children and adults (RR, 0.78; 95% CI, 0.36–1.70).
Outcome measures that received a lower priority
The panel ranked a number of patient-centered outcomes as lower priority than the five highest-priority measurements. These patient-centered outcomes included quality of life, symptom-free days, days missed from work or school, daily activities including exercise and sports, and the use of rescue medications.
Three studies evaluated quality of life, but data could not be combined because the studies used slightly different outcome measures. None of the studies showed a significant difference in quality of life with the intervention (83, 84, 96). Specifically, in adults, there was no difference in the Asthma Quality of Life Questionnaire scores (MD, 0; 95% CI, −0.29 to 0.29) (83) or in the mini–Asthma Quality of Life Questionnaire scores (MD, 0; 95% CI, −0.49 to 0.49) (84), whereas in studies enrolling children, there was no difference in the Pediatric Asthma Caregivers Quality of Life Questionnaire scores (MD, 0; 95% CI, −0.24 to 0.29) (96). Only two studies reported symptom-free days, and these data could not be pooled. Both studies reported numeric improvements in the frequency of symptom-free days, with one study showing 4.1% more symptom-free days (95% CI, −12.8% to 21%) (87) and the other study showing 69% versus 64% symptom-free days (P = 0.44) (90); however, these results were not statistically significant. One study addressed the frequency of days missed from school or work, identifying an MD of 1.6 fewer days, but this did not reach statistical significance (95% CI, −6.01 to 2.81 d) (83). No data were identified on the effects on daily activity, exercise, or sports participation.
Multiple studies reported ICS use; however, the different medications and doses that were administered did not allow pooling of the data. The majority of the studies showed no difference in ICS use (80, 83–86, 91, 94–96), but there was evidence in some studies of increased ICS use (39, 87–89), and a few studies showed less ICS use (90, 98). Two studies evaluated rescue inhaler use, and neither the individual studies nor the combined data identified a significant effect on rescue inhaler use (MD, 0.07; 95% CI, −0.16 to 0.30) (80, 90). The committee had identified the asthma medication ratio as a key outcome measure (i.e., the ratio of ICSs to short-acting β-agonists), but no data could be identified. One study reported medication adherence as an outcome measure but did not identify a significant difference in adherence (MD, −0.2; 95% CI, −0.34 to 0.06) (83). Two other studies reported the percentage of adherence in each group, but neither identified a significant difference (80, 90).
Although the panel generally ranked lung function measurements as being lower in priority than other measures, 11 studies reported spirometry testing (79, 80, 82–84, 86–88, 90, 96), and the results of nine of these studies could be pooled for analysis that used the percent change in the FEV1 as the primary outcome (79, 80, 82–84, 86, 87, 90, 96). The results demonstrate an overall improvement in lung function when FeNO-based care was used, but the effect size was small (MD, 1.11%; 95% CI, 0.02–2.21%). There was some heterogeneity in the studies, with one pediatric study showing an MD of 4.9% (84); there was no obvious difference overall between pediatric and adult studies, which were equally represented among the results. Although the effect size was small and it is uncertain whether this difference is meaningful to individual patients with asthma, these results may provide additional evidence that FeNO-based care can be used to optimize the dose of ICSs, a class of drugs known to improve baseline lung function in asthma (99).
Patient satisfaction with care and adverse events related to medications both received moderate priority by the panel. One study that focused on home monitoring of FeNO, spirometry, and symptoms to facilitate tapering of oral corticosteroids reported that satisfaction with care on a scale of 1–7 was actually lower in the intervention group receiving FeNO testing, but this difference did not reach statistical significance (MD, 0.5; 95% CI, −1.03 to 0.03). Adverse events were reported in four studies, but adverse events were not reported in a manner that allowed the data to be pooled. Two studies reported adverse events in a manner that could be pooled, but the frequency was low and not necessarily related to the intervention (RR, 0.20; 95% CI, 0.04 to 0.98) (39, 80). The other two studies reported no significant difference in adverse events between the groups (79, 96). Three studies reported the percentage of blood eosinophils, but results could not be combined because of differences in reporting; however, no differences in the peripheral blood eosinophil count were identified (39, 82, 84).
Cost-effectiveness
We conducted a limited pragmatic review of studies addressing cost-effectiveness of the use of FeNO-based care to inform treatment decisions. Eight studies were identified that addressed cost-effectiveness (83, 100–106). Although an initial evaluation of cost-effectiveness concluded that the potential costs outweigh the benefits (106), subsequent analyses of the potential costs relative to the benefits have generally favored the cost-effectiveness of FeNO-based care (102), particularly in individuals who experience exacerbations (101). Importantly, it should be noted that a number of the studies addressing costs also used FeNO testing to monitor disease (83, 103, 105), rather than to answer the specific question addressed in this guideline, but generally found that FeNO-based care was cost-effective or at least comparable with other strategies (104). One study reported that the use of FeNO testing could reduce the costs by identifying potential responders to a T2 biologic (100). Although these data are relatively limited, the panel concluded that the available evidence probably favors the intervention from a cost-effectiveness standpoint.
Panel judgment
In patients with asthma in whom treatment is being contemplated, the panel believed that the evidence favored the assessment of FeNO during evaluation of an individual with asthma in addition to usual care. The problem outlined in this question was believed to be a priority by ATS leadership as well as by the authors of guidelines on asthma (1, 2). Further interest in this area was reinforced by the frequency of citations of the initial guideline for the use of FeNO testing (24) as well as of the recent analyses conducted by the U.S. AHRQ (62) and a recent systematic review conducted in Europe (107). Although the desirable effects of the intervention were relatively modest in magnitude, the outcome measurements that revealed a desirable effect were prioritized by the panel before the analysis and demonstrated reduced exacerbation frequency (28.9% vs. 39.7%; RR, 0.73 [95% CI, 0.62–0.86]) and oral corticosteroid use (26.0% vs. 32.8%; RR, 0.79 [95% CI, 0.65–0.95]), two critical outcomes of asthma management. The panel also noted that in some of these critical outcomes, the results favoring FeNO testing were generally stronger in the pediatric trials.
The panel also noted that there were no differences in a number of patient-reported outcomes, including measures of quality of life, symptom-free days, missed days of work, and patient satisfaction with care, although the studies were underpowered for these outcomes. Furthermore, there were no differences in ICS use, rescue inhaler use, medication adherence, or blood eosinophil levels; however, the judgment of the panel was that these measurements would not necessarily be altered by the intervention, as the intervention is primarily designed to individualize treatment decisions. With regard to the dose of ICSs, the majority of studies showed no overall difference in the dose of ICSs. Some studies reported that the overall ICS dose actually increased, whereas a small minority of studies reported an overall ICS dose decrease. When considering the certainty of the evidence, the effects on exacerbation frequency and oral corticosteroid use were graded as moderate, whereas other outcome measures that also reflect asthma control were graded as having low certainty.
The undesirable effects of the intervention were generally deemed to be trivial, with a low overall rate of adverse events (RR, 0.20; 95% CI, 0.04 to 0.98) and absolute effect of 55 fewer per 1,000 (95% CI, 66 fewer to 1 fewer) for FeNO-based care compared with usual care being demonstrated. Although the availability of this diagnostic test may be variable among institutions, the resources required for this test appear to be moderate in cost, although resources such as operator training may be a significant consideration depending on the size of the center and the frequency with which the test is conducted. The cost of the test was another significant consideration; however, our preliminary analysis of cost-effectiveness probably favors the intervention of FeNO-based care, although study methodologies and statistical modeling approaches varied and certain subgroups in which FeNO testing may not be effective were not delineated. The intervention was believed to be acceptable to key stakeholders. Primary care asthma clinics found it feasible and acceptable among adults and children above the age of 4 years, as did healthcare providers managing pregnant patients with asthma (108). Overall, the balance between desirable and undesirable effects probably favored the intervention, with the relative absence of a downside and the potential to determine and reduce exacerbations and reduce oral corticosteroid use being shown.
ATS recommendation
In patients with asthma in whom treatment is being considered, we suggest the use of FeNO testing in addition to usual care over usual care alone (conditional recommendation, low confidence in estimates of effect).
Remarks
Despite nearly 10 years of research since the initial guidelines in this area were published, the available data from randomized control trials remain fairly limited, and some of the selected outcome measures may not have had adequate power to detect a difference. The consensus of the group was that FeNO-based care provides the clinician with a simple and noninvasive point-of-care test that provides complementary information over usual care and that the undesirable effects were small relative to the potential benefit. Given the relatively modest effect sizes and statistical significance, it is reasonable for individual groups of providers to weigh the potential costs and administrative burden relative to the benefits outlined in this guideline. In addition to providers, stakeholders such as individuals with asthma, payors, and the Food and Drug Administration could reasonably grade other outcomes such as quality of life and symptom-free days as a higher priority.
The recommendation places a high value on asthma exacerbations and reduction in need for oral corticosteroid use, which were categorized as critical outcome measures by this panel and other groups (1, 2). Although the balance of desirable and undesirable effects probably favored the intervention, it was noted by the group that usual asthma care has evolved over the past few years. Although phenotyping and endotyping asthma have become paramount in asthma management, there have been no studies to look at the additional benefit of adding FeNO testing once an asthma phenotype has already been classified, although there is some evidence that FeNO testing can be used as an assessment tool for the use of biologics. Furthermore, it has not been determined whether there are subgroups in which FeNO measurement should not be used because the intervention may not be cost-effective or beneficial for achieving the desired outcomes.
Future research opportunities
There are several limitations identified in this report and other contemporary systematic reviews regarding the use of FeNO testing that should be further evaluated. Larger pragmatic randomized controlled trials that are powered on the basis of the available evidence should be conducted to more clearly delineate the benefit of FeNO-based care in patients with asthma in whom treatment is being considered. There is similarly a need for larger trials that evaluate the use of FeNO testing to monitor therapy with serial measurements once the FeNO level is established, and there is a need to better delineate the potential diagnostic accuracy of FeNO testing as a tool to establish the diagnosis of asthma. Timing of the initial assessment of FeNO is also uncertain because there are anticipated differences between the values when individuals with asthma are medication naive and the values when establishing the initial FeNO value while individuals are on a stable dose of therapy, as was the case in the majority of trials identified in this report.
Another area that requires further investigation is specific subgroups with asthma and the need to adequately power future clinical studies and trials specifically for analyses of these subgroups. Pertinent subgroups include individuals with T2-predominant asthma as well as individuals with allergic sensitization, in whom the value may be greater; however, the value added to other currently used tests such as those measuring peripheral blood eosinophils and allergen-specific IgE needs further delineation. Studies should also further evaluate subgroups in which FeNO-based care may be less helpful, as there are lower anticipated levels in subgroups such as individuals with obesity-associated asthma (109) and cigarette smokers (110–112). There were also notable differences between children and adults in the studies evaluated for this guideline, indicating that larger studies are needed to further define the benefits within these different populations.
Discussion
The recommendations in this single-question guideline were derived from our systematic review of the available evidence and our interpretation of how the evidence may be applied in clinical practice. We gave a conditional recommendation for the use of FeNO testing in addition to usual care in patients with asthma in whom treatment is being considered. This single-question guideline incorporated a decision structure in which experts in the FeNO field initially made a determination about the question with greatest potential impact. An expert panel including individuals with diverse backgrounds in various practice settings and in different regions of the world was assembled to provide input about the selection of the most important single question and to identify the key outcome measures. This guideline is not meant to be all inclusive regarding the use of FeNO testing in clinical practice but was designed to answer this single question in a timely manner by using rigorous statistical methods to evaluate the evidence.
The decision that was reached was based primarily on the supportive evidence of effects on the frequency and percentage of asthma exacerbations as well as the need for treatment with oral corticosteroids, which were deemed by the panel a priori to be critical outcome measures. It should also be noted that the summation of the evidence for several other outcome measures that were deemed a high priority by the panel—asthma control as assessed by using validated questionnaires and acute care visits for asthma, including emergency department visits, urgent care visits and hospitalizations—did not reach statistical significance, but each of these outcomes had mean values that favored FeNO-based care. The conditional recommendation means that there is some uncertainty that the desirable consequences of the intervention outweigh the undesirable consequences. This decision means that most well-informed patients would choose FeNO-based care but that a substantial minority of individuals with asthma may not desire this test and that the decision to conduct this test in an individual patient should be the result of an informed discussion between that individual and the provider.
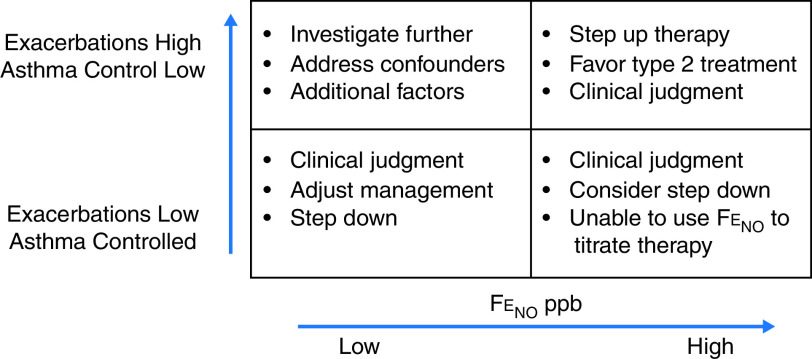
An important caveat about the results of our recommendation to use FeNO testing when treatment is considered for an individual with asthma is that the precise FeNO value that should initiate a change in decision-making regarding therapy for asthma was not ascertained by the available evidence. In the initial guidelines in this area, a low FeNO value of 25 ppb (20 ppb in children) was considered evidence that a response to the corticosteroids was unlikely, a high FeNO value above 50 ppb (35 ppb in children) was considered evidence of a likely corticosteroid response, and values between these two boundaries were considered indeterminate. Subsequent studies have used much more narrowly defined ranges for FeNO values, with various algorithms being used to make treatment decisions on the basis of the results. Because of the variability in these studies as well as the inherent phenotypic variability of asthma, it was the consensus of the committee that the available evidence did not provide enough data to recommend specific cut points associated with specific actions such as starting or increasing the dose of an ICS. Although we did not reach this aspirational goal, we believe that the level of FeNO should be combined with other measures that are used to assess asthma control and that the level of FeNO should be interpreted within this context of the pretest probability (Figure 2). This framework is similar to that of other tests such as bronchoprovocation tests, in which the pretest probability combined with the degree of AHR act together to establish the likelihood of asthma (113). The decision of an individual clinician to use a lower FeNO value to identify persistent inflammation means that the sensitivity to detect inflammation is being prioritized over the specificity that a change in treatment will make a difference. Similarly, a decision to use a higher FeNO value assigns greater priority to the specificity of the finding at the cost of reducing sensitivity (62). The consensus of the panel was to give the clinician latitude within their own practice to use this framework to make treatment decisions.
Figure 2.
Conceptual framework for the use of fractional exhaled nitric oxide (FeNO) testing to guide treatment decisions for individuals with asthma. The decision to act on an individual FeNO value in an individual patient requires that the clinician combine clinical judgments based on the perceived probability of benefit, with particular attention being given to the key outcome measures such as exacerbation risks that were assessed in this guideline. As the level of FeNO increases in value, the specificity for a step up in therapy increases, whereas accepting lower values of FeNO to make treatment decisions places a higher value on the sensitivity to detect the possibility that a step up in therapy may impact asthma control.
The panel also recognizes that the landscape of asthma management is in continual evolution. The majority of the patients considered within these studies were already being treated with a stable ICS dose at study enrollment, meaning that the question that was examined is most pertinent to the decision-making that is often encountered during subspecialty consultation for an individual with asthma. Furthermore, it is now common to evaluate individuals for evidence of T2 biomarkers such as peripheral blood eosinophils and serum total and allergen-specific IgE (41). The value added for the use of FeNO testing in an individual who has already undergone phenotyping for T2 asthma or other advanced testing such as bronchoprovocation or assessment of induced sputum eosinophils was not addressed in a manner that was amenable to systematic review. Furthermore, there is evidence that some subgroups such as individuals with asthma associated with obesity and individuals who smoke cigarettes have lower FeNO values and that the value of FeNO-based care may be limited in these individuals (109–112). It should also be noted that if such individuals with low FeNO values were specifically excluded from the analysis, the results could be more strongly in favor of the use of FeNO testing when making treatment decisions in an individual with asthma.
Finally, we would like to reiterate that we selected the single question with the greatest significance to clinical practice as perceived by our panel. Leadership in the ATS also identified two additional questions relevant to the use of FeNO testing that were not addressed in this guideline. In this regard, we view our analysis as being complementary to other broader guidelines on this topic.
The recommendations in this guideline were reviewed by the ATS Quality Improvement and Implementation Committee and none are considered suitable for performance measure development.
Acknowledgments
This official clinical practice guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Allergy, Immunology, and Inflammation.
Members of the subcommittee are as follows:
Sumita B. Khatri, M.D., M.S. (Co-Chair)1
Teal S. Hallstrand, M.D., M.P.H. (Co-Chair)2,3
Praveen Akuthota, M.D.4
Amisha Barochia, M.D.5
Anna Brady, M.D.6
Ronina A. Covar, M.D.7
Jason S. Debley, M.D., M.P.H.8
Zuzana Diamant, M.D., Ph.D.9,10,11,12
Anne M. Fitzpatrick, Ph.D., M.S.C.R., A.P.R.N.13
Jonathan M. Iaccarino, M.D., M.S.14
David A. Kaminsky, M.D.15
Nicholas J. Kenyon, M.D.16,17
Sandhya Khurana, M.D.18
Brian J. Lipworth, M.D.19
Kevin McCarthy, R.P.F.T.20
Michael Peters, M.D.21
Loretta G. Que, M.D.22
Kristie R. Ross, M.D., M.S.23
Elena K. Schneider-Futschik, Pharm.D., Ph.D.24
Israa Soghier, M.D.25
Christine A. Sorkness, Pharm.D., R.Ph.26
1Asthma Center, Respiratory Institute, Cleveland Clinic, Cleveland, Ohio; 2Division of Pulmonary, Critical Care and Sleep Medicine and 3Center for Lung Biology, Department of Medicine, University of Washington, Seattle, Washington; 4Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, University of California San Diego, San Diego, California; 5Pulmonary Branch, NHLBI, Bethesda, Maryland; 6Division of Pulmonary and Critical Care, Department of Medicine, Oregon Health and Sciences University, Portland, Oregon; 7Division of Pediatric Allergy and Clinical Immunology, Department of Pediatrics, School of Medicine, University of Colorado Denver, Denver, Colorado; 8Division of Pulmonary and Sleep Medicine, Department of Pediatrics, Seattle Children’s Hospital and University of Washington, Seattle, Washington; 9Department of Microbiology, Immunology and Transplantation, Catholic University of Leuven, Leuven, Belgium; 10Department of Respiratory Medicine and Allergology, Institute for Clinical Science, Skåne University Hospital, Lund University, Lund, Sweden; 11Department of Respiratory Medicine, First Faculty of Medicine, Charles University and Thomayer Hospital, Prague, Czech Republic; 12Department of Clinical Pharmacy and Pharmacology, University of Groningen, Groningen, the Netherlands; 13Division of Pulmonology, Allergy and Immunology, Department of Pediatrics, Emory University, Atlanta, Georgia; 14Pulmonary, Allergy, Sleep, and Critical Care Section of Boston University, Boston, Massachusetts; 15Division of Pulmonary and Critical Care, Department of Medicine, Larner College of Medicine, University of Vermont, Burlington, Vermont; 16Division of Pulmonary, Critical Care, and Sleep, Department of Medicine, University of California Davis, Davis, California; 17Veterans Affairs Northern California Health Care System, Mather, California; 18Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Rochester Medical Center, Rochester, New York; 19Scottish Centre for Respiratory Research, University of Dundee, Scotland, United Kingdom; 20ExpertPFT, LLC, Matthews, North Carolina; 21Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of California San Francisco, San Francisco, California; 22Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, Duke University, Durham, North Carolina; 23Division of Pulmonary, Allergy/Immunology, and Sleep Medicine, Department of Pediatrics, Case Western Reserve University, Cleveland, Ohio; 24Department of Pharmacology and Therapeutics, Lung Health Research Centre, School of Biomedical Sciences, Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Parkville, Victoria, Australia; 25Department of Pulmonary Medicine, Albert Einstein College of Medicine, New York, New York; and 26Division of Allergy, Pulmonary, and Critical Care Medicine, Department of Medicine, School of Pharmacy and School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wisconsin
Acknowledgment
The co-chairs of the committee thank Kevin Wilson for his guidance on this new practice guideline creation; their methodologists Drs. Iaccarino, Barochia, and Soghier; and all members of their highly engaged international team of asthma specialists, clinicians, and scientists who contributed significantly to their efforts to provide a succinct document that guides clinicians on the use of a exhaled nitric oxide to guide the treatment of asthma and highlight future directions for evaluation and research.
Footnotes
This official clinical practice guideline of the American Thoracic Society was approved September 2021
Supported by the American Thoracic Society.
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/rccm.202109-2093ST.
This document has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author Disclosures: P.A. served on an advisory committee for AstraZeneca and GlaxoSmithKline; served as a consultant for Advance Medical, AstraZeneca, and GlaxoSmithKline; served as a speaker for Advancing Knowledge in Healthcare Inc., AstraZeneca, GlaxoSmithKline, Medscape/WebMD, MJH Life Sciences, Prime CME, Projects in Knowledge, Rockpointe, and Vindico CME; received research support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, NIH, Novartis, and Regeneron; and received royalties from UpToDate. R.A.C. served as a consultant for Cohero Health; and received research support from the American Lung Association ACRC, Avillion, GlaxoSmithKline, and NHLBI. Z.D. served as a speaker for Sanofi. D.A.K. served as a consultant for Spiration; served on a data and safety monitoring board for Acorda; and served as a speaker for MGC Pharmaceuticals. S.K. received research support from GlaxoSmithKline and Sanofi; and provided writing support for Boehringer Ingelheim. B.J.L. served on an advisory committee for AstraZeneca, Chiesi, Circassia, Novartis, Sanofi, and Teva; served as a consultant for AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Circassia, Genentech, Lupin, Sanofi, Teva, and Vectura; served as a speaker for AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Sanofi, Teva, and Thorasys; received research support from AstraZeneca, Boehringer Ingelheim, Chiesi, Sanofi, and Teva; received travel support from AstraZeneca, Chiesi, and Teva; and received equipment from GlaxoSmithKline and Thorasys. S.B.K., T.S.H., A. Barochia, A. Brady, J.S.D., A.M.F., J.M.I., N.J.K., K.M., M.P., L.G.Q., K.R.R., E.K.S.-F., I.S., and C.A.S. reported no commercial or relevant noncommercial interests.
Contributor Information
on behalf of the American Thoracic Society Assembly on Allergy, Immunology, and Inflammation:
Sumita B. Khatri, Teal S. Hallstrand, Praveen Akuthota, Amisha Barochia, Anna Brady, Ronina A. Covar, Jason S. Debley, Zuzana Diamant, Anne M. Fitzpatrick, Jonathan M. Iaccarino, David A. Kaminsky, Nicholas J. Kenyon, Sandhya Khurana, Brian J. Lipworth, Kevin McCarthy, Michael Peters, Loretta G. Que, Kristie R. Ross, Elena K. Schneider-Futschik, Israa Soghier, and Christine A. Sorkness
References
- 1.Global strategy for asthma management and prevention Fontana, WI: Global Initiative for Asthma; 2019. [accessed 2020 Apr 1]. Available from: https://ginasthma.org/ [Google Scholar]
- 2.National Asthma Education and Prevention Program Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma: summary report 2007 J Allergy Clin Immunol 2007120S94–S138.. [Published erratum appears in J Allergy Clin Immunol 121:1330]. [DOI] [PubMed] [Google Scholar]
- 3. Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol . 2014;205:621–631. doi: 10.1083/jcb.201401050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beasley R, Braithwaite I, Semprini A, Kearns C, Weatherall M, Pavord ID. Optimal asthma control: time for a new target. Am J Respir Crit Care Med . 2020;201:1480–1487. doi: 10.1164/rccm.201910-1934CI. [DOI] [PubMed] [Google Scholar]
- 5. King-Biggs MB. Asthma. Ann Intern Med . 2019;171:ITC49–ITC64. doi: 10.7326/AITC201910010. [DOI] [PubMed] [Google Scholar]
- 6. Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. START Investigators Group. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet . 2003;361:1071–1076. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 7. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax . 2002;57:880–884. doi: 10.1136/thorax.57.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Childhood Asthma Research and Education Network of the NHLBI. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol . 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 9. Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Childhood Asthma Research and Education Network of the NHLBI. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol . 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 10. Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol . 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 11. Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Asthma Clinical Research Network of the National Heart Lung, and Blood Institute. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol . 2002;109:410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 12. Busse W, Raphael GD, Galant S, Kalberg C, Goode-Sellers S, Srebro S, et al. Fluticasone Propionate Clinical Research Study Group. Low-dose fluticasone propionate compared with montelukast for first-line treatment of persistent asthma: a randomized clinical trial. J Allergy Clin Immunol . 2001;107:461–468. doi: 10.1067/mai.2001.114657. [DOI] [PubMed] [Google Scholar]
- 13. Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Piñeiro A, Wei LX, et al. Montelukast/Beclomethasone Study Group. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma: a randomized, controlled trial. Ann Intern Med . 1999;130:487–495. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 14. Cowan DC, Taylor DR, Peterson LE, Cowan JO, Palmay R, Williamson A, et al. Biomarker-based asthma phenotypes of corticosteroid response. J Allergy Clin Immunol . 2015;135:877–883, e1. doi: 10.1016/j.jaci.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demarche SF, Schleich FN, Paulus VA, Henket MA, Van Hees TJ, Louis RE. Asthma control and sputum eosinophils: a longitudinal study in daily practice. J Allergy Clin Immunol Pract . 2017;5:1335–1343, e5. doi: 10.1016/j.jaip.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 16. Deykin A, Lazarus SC, Fahy JV, Wechsler ME, Boushey HA, Chinchilli VM, et al. Asthma Clinical Research Network, NHLBI/NIH. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol . 2005;115:720–727. doi: 10.1016/j.jaci.2004.12.1129. [DOI] [PubMed] [Google Scholar]
- 17. Fleming L, Wilson N, Regamey N, Bush A. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax . 2012;67:193–198. doi: 10.1136/thx.2010.156836. [DOI] [PubMed] [Google Scholar]
- 18. Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemière C, Pizzichini E, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J . 2006;27:483–494. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 19. Leuppi JD, Salome CM, Jenkins CR, Anderson SD, Xuan W, Marks GB, et al. Predictive markers of asthma exacerbation during stepwise dose reduction of inhaled corticosteroids. Am J Respir Crit Care Med . 2001;163:406–412. doi: 10.1164/ajrccm.163.2.9912091. [DOI] [PubMed] [Google Scholar]
- 20. Clearie KL, Jackson CM, Fardon TC, Williamson PA, Vaidyanathan S, Burns P, et al. Supervised step-down of inhaled corticosteroids in the community: an observational study. Respir Med . 2011;105:558–565. doi: 10.1016/j.rmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21. Lipworth BJ, Short PM, Williamson PA, Clearie KL, Fardon TC, Jackson CM. A randomized primary care trial of steroid titration against mannitol in persistent asthma: STAMINA trial. Chest . 2012;141:607–615. doi: 10.1378/chest.11-1748. [DOI] [PubMed] [Google Scholar]
- 22. Higgins BG, Britton JR, Chinn S, Cooper S, Burney PG, Tattersfield AE. Comparison of bronchial reactivity and peak expiratory flow variability measurements for epidemiologic studies. Am Rev Respir Dis . 1992;145:588–593. doi: 10.1164/ajrccm/145.3.588. [DOI] [PubMed] [Google Scholar]
- 23. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med . 2019;199:433–445. doi: 10.1164/rccm.201810-1944CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med . 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lane C, Knight D, Burgess S, Franklin P, Horak F, Legg J, et al. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax . 2004;59:757–760. doi: 10.1136/thx.2003.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roos AB, Mori M, Grönneberg R, Österlund C, Claesson HE, Wahlström J, et al. Elevated exhaled nitric oxide in allergen-provoked asthma is associated with airway epithelial iNOS. PLoS One . 2014;9:e90018. doi: 10.1371/journal.pone.0090018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo FH, Comhair SA, Zheng S, Dweik RA, Eissa NT, Thomassen MJ, et al. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol . 2000;164:5970–5980. doi: 10.4049/jimmunol.164.11.5970. [DOI] [PubMed] [Google Scholar]
- 28. van der Vliet A, Eiserich JP, Cross CE. Nitric oxide: a pro-inflammatory mediator in lung disease? Respir Res . 2000;1:67–72. doi: 10.1186/rr14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liao SY, Showalter MR, Linderholm AL, Franzi L, Kivler C, Li Y, et al. l-Arginine supplementation in severe asthma. JCI Insight . 2020;5:137777. doi: 10.1172/jci.insight.137777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holguin F, Grasemann H, Sharma S, Winnica D, Wasil K, Smith V, et al. l-Citrulline increases nitric oxide and improves control in obese asthmatics. JCI Insight . 2019;4:e131733. doi: 10.1172/jci.insight.131733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest . 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax . 1998;53:91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brightling CE, Symon FA, Birring SS, Bradding P, Wardlaw AJ, Pavord ID. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax . 2003;58:528–532. doi: 10.1136/thorax.58.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Silkoff PE, Lent AM, Busacker AA, Katial RK, Balzar S, Strand M, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol . 2005;116:1249–1255. doi: 10.1016/j.jaci.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 35. Wagener AH, de Nijs SB, Lutter R, Sousa AR, Weersink EJ, Bel EH, et al. External validation of blood eosinophils, FeNO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax . 2015;70:115–120. doi: 10.1136/thoraxjnl-2014-205634. [DOI] [PubMed] [Google Scholar]
- 36. Gao J, Wu F. Association between fractional exhaled nitric oxide, sputum induction and peripheral blood eosinophil in uncontrolled asthma. Allergy Asthma Clin Immunol . 2018;14:21. doi: 10.1186/s13223-018-0248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silkoff PE, McClean P, Spino M, Erlich L, Slutsky AS, Zamel N. Dose-response relationship and reproducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest . 2001;119:1322–1328. doi: 10.1378/chest.119.5.1322. [DOI] [PubMed] [Google Scholar]
- 38. Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med . 2005;172:453–459. doi: 10.1164/rccm.200411-1498OC. [DOI] [PubMed] [Google Scholar]
- 39. Calhoun WJ, Ameredes BT, King TS, Icitovic N, Bleecker ER, Castro M, et al. Asthma Clinical Research Network of the NHLBI. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA . 2012;308:987–997. doi: 10.1001/2012.jama.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Longitudinal relationship between sputum eosinophils and exhaled nitric oxide in children with asthma. Am J Respir Crit Care Med . 2013;188:400–402. doi: 10.1164/rccm.201212-2156LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simpson A, Custovic A, Pipis S, Adisesh A, Faragher B, Woodcock A. Exhaled nitric oxide, sensitization, and exposure to allergens in patients with asthma who are not taking inhaled steroids. Am J Respir Crit Care Med . 1999;160:45–49. doi: 10.1164/ajrccm.160.1.9809091. [DOI] [PubMed] [Google Scholar]
- 42. Buchvald F, Hermansen MN, Nielsen KG, Bisgaard H. Exhaled nitric oxide predicts exercise-induced bronchoconstriction in asthmatic school children. Chest . 2005;128:1964–1967. doi: 10.1378/chest.128.4.1964. [DOI] [PubMed] [Google Scholar]
- 43. ElHalawani SM, Ly NT, Mahon RT, Amundson DE. Exhaled nitric oxide as a predictor of exercise-induced bronchoconstriction. Chest . 2003;124:639–643. doi: 10.1378/chest.124.2.639. [DOI] [PubMed] [Google Scholar]
- 44. Manoharan A, Lipworth BJ, Craig E, Jackson C. The potential role of direct and indirect bronchial challenge testing to identify overtreatment of community managed asthma. Clin Exp Allergy . 2014;44:1240–1245. doi: 10.1111/cea.12352. [DOI] [PubMed] [Google Scholar]
- 45. Boot JD, Tarasevych S, Sterk PJ, Schoemaker RC, Wang L, Amin D, et al. Reversal of the late asthmatic response increases exhaled nitric oxide. Respir Med . 2005;99:1591–1594. doi: 10.1016/j.rmed.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 46. Khatri SB, Hammel J, Kavuru MS, Erzurum SC, Dweik RA. Temporal association of nitric oxide levels and airflow in asthma after whole lung allergen challenge. J Appl Physiol (1985) . 2003;95:436–440, discussion 435. doi: 10.1152/japplphysiol.01127.2002. [DOI] [PubMed] [Google Scholar]
- 47. Kurukulaaratchy RJ, Zhang H, Patil V, Raza A, Karmaus W, Ewart S, et al. Identifying the heterogeneity of young adult rhinitis through cluster analysis in the Isle of Wight birth cohort. J Allergy Clin Immunol . 2015;135:143–150. doi: 10.1016/j.jaci.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scott M, Raza A, Karmaus W, Mitchell F, Grundy J, Kurukulaaratchy RJ, et al. Influence of atopy and asthma on exhaled nitric oxide in an unselected birth cohort study. Thorax . 2010;65:258–262. doi: 10.1136/thx.2009.125443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Diamant Z, Vijverberg S, Alving K, Bakirtas A, Bjermer L, Custovic A, et al. Toward clinically applicable biomarkers for asthma: an EAACI position paper. Allergy . 2019;74:1835–1851. doi: 10.1111/all.13806. [DOI] [PubMed] [Google Scholar]
- 50. Arron JR, Choy DF, Scheerens H, Matthews JG. Noninvasive biomarkers that predict treatment benefit from biologic therapies in asthma. Ann Am Thorac Soc . 2013;10:S206–S213. doi: 10.1513/AnnalsATS.201303-047AW. [DOI] [PubMed] [Google Scholar]
- 51. Matsunaga K, Kuwahira I, Hanaoka M, Saito J, Tsuburai T, Fukunaga K, et al. Japanese Respiratory Society Assembly on Pulmonary Physiology. An official JRS statement: the principles of fractional exhaled nitric oxide (FeNO) measurement and interpretation of the results in clinical practice. Respir Investig . 2021;59:34–52. doi: 10.1016/j.resinv.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 52. Voraphani N, Gladwin MT, Contreras AU, Kaminski N, Tedrow JR, Milosevic J, et al. An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol . 2014;7:1175–1185. doi: 10.1038/mi.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sposato B, Camiciottoli G, Bacci E, Scalese M, Carpagnano GE, Pelaia C, et al. Mepolizumab effectiveness on small airway obstruction, corticosteroid sparing and maintenance therapy step-down in real life. Pulm Pharmacol Ther . 2020;61:101899. doi: 10.1016/j.pupt.2020.101899. [DOI] [PubMed] [Google Scholar]
- 54. Numata T, Nakayama K, Utsumi H, Kobayashi K, Yanagisawa H, Hashimoto M, et al. Efficacy of mepolizumab for patients with severe asthma and eosinophilic chronic rhinosinusitis. BMC Pulm Med . 2019;19:176. doi: 10.1186/s12890-019-0952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Caminati M, Cegolon L, Vianello A, Chieco Bianchi F, Festi G, Marchi MR, et al. Mepolizumab for severe eosinophilic asthma: a real-world snapshot on clinical markers and timing of response. Expert Rev Respir Med . 2019;13:1205–1212. doi: 10.1080/17476348.2019.1676734. [DOI] [PubMed] [Google Scholar]
- 56. Bjermer L, Alving K, Diamant Z, Magnussen H, Pavord I, Piacentini G, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med . 2014;108:830–841. doi: 10.1016/j.rmed.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 57. Petsky HL, Cates CJ, Kew KM, Chang AB. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a systematic review and meta-analysis. Thorax . 2018;73:1110–1119. doi: 10.1136/thoraxjnl-2018-211540. [DOI] [PubMed] [Google Scholar]
- 58. Song WJ, Kim HJ, Shim JS, Won HK, Kang SY, Sohn KH, et al. Diagnostic accuracy of fractional exhaled nitric oxide measurement in predicting cough-variant asthma and eosinophilic bronchitis in adults with chronic cough: a systematic review and meta-analysis. J Allergy Clin Immunol . 2017;140:701–709. doi: 10.1016/j.jaci.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 59. Tang S, Xie Y, Yuan C, Sun X, Cui Y. Fractional exhaled nitric oxide for the diagnosis of childhood asthma: a systematic review and meta-analysis. Clin Rev Allergy Immunol . 2019;56:129–138. doi: 10.1007/s12016-016-8573-4. [DOI] [PubMed] [Google Scholar]
- 60. Karrasch S, Linde K, Rücker G, Sommer H, Karsch-Völk M, Kleijnen J, et al. Accuracy of FeNO for diagnosing asthma: a systematic review. Thorax . 2017;72:109–116. doi: 10.1136/thoraxjnl-2016-208704. [DOI] [PubMed] [Google Scholar]
- 61. Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol . 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Pianosi P, Keogh K, Zaiem F, Alsawas M, Alahdab F, et al. Rockville, MD: Agency for Healthcare Research and Quality; 2017. [PubMed] [Google Scholar]
- 63. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ . 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Review Manager (RevMan). Version 5.3. Copenhagen, Denmark: Nordic Cochrane Centre, Cochrane Collaboration; 2014. [accessed 2020 Aug 13]. Available from: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman [Google Scholar]
- 65.Evidence Prime, Inc Hamilton, ON, Canada: McMaster University; 2015https://gradepro.org/. [Google Scholar]
- 66. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ . 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol . 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 68. Juniper EF, Bousquet J, Abetz L, Bateman ED. GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med . 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 69. Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol . 2007;119:817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 70. Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol . 2009;124:719–723, e1. doi: 10.1016/j.jaci.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 71. Meltzer EO, Busse WW, Wenzel SE, Belozeroff V, Weng HH, Feng J, et al. Use of the Asthma Control Questionnaire to predict future risk of asthma exacerbation. J Allergy Clin Immunol . 2011;127:167–172. doi: 10.1016/j.jaci.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 72. Bateman ED, Reddel HK, Eriksson G, Peterson S, Ostlund O, Sears MR, et al. Overall asthma control: the relationship between current control and future risk. J Allergy Clin Immunol . 2010;125:600–608, e1–e6. doi: 10.1016/j.jaci.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 73. Bourdin A, Bjermer L, Brightling C, Brusselle GG, Chanez P, Chung KF, et al. ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. Eur Respir J . 2019;54:1900900. doi: 10.1183/13993003.00900-2019. [DOI] [PubMed] [Google Scholar]
- 74. Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol . 2008;122:662–668. doi: 10.1016/j.jaci.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 75. Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol . 2012;129:1229–1235. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 76. Calhoun WJ, Haselkorn T, Miller DP, Omachi TA. Asthma exacerbations and lung function in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol . 2015;136:1125–1127, e4. doi: 10.1016/j.jaci.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 77. O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. START Investigators Group. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med . 2009;179:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 78. Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J . 2007;30:452–456. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 79. Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet . 2008;372:1065–1072. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Voorend-van Bergen S, Vaessen-Verberne AA, Brackel HJ, Landstra AM, van den Berg NJ, Hop WC, et al. Monitoring strategies in children with asthma: a randomised controlled trial. Thorax . 2015;70:543–550. doi: 10.1136/thoraxjnl-2014-206161. [DOI] [PubMed] [Google Scholar]
- 81. Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med . 2005;99:553–558. doi: 10.1016/j.rmed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 82. Wang X, Wu L, Zhang Z, Kong Q, Qi H, Lei H. The reliability of adjusting stepped care based on FeNO monitoring for patients with chronic persistent asthma. Open Med (Wars) . 2019;14:217–223. doi: 10.1515/med-2019-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Honkoop PJ, Loijmans RJ, Termeer EH, Snoeck-Stroband JB, van den Hout WB, Bakker MJ, et al. Asthma Control Cost-Utility Randomized Trial Evaluation (ACCURATE) Study Group. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol . 2015;135:682–688, e11. doi: 10.1016/j.jaci.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 84. Bernholm KF, Homøe AS, Meteran H, Jensen CB, Porsbjerg C, Backer V. FeNO-based asthma management results in faster improvement of airway hyperresponsiveness. ERJ Open Res . 2018;4:00147-2017. doi: 10.1183/23120541.00147-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Garg Y, Kakria N, Katoch CDS, Bhattacharyya D. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India . 2020;76:17–22. doi: 10.1016/j.mjafi.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Malerba M, Radaeli A, Olivini A, Ragnoli B, Ricciardolo F, Montuschi P. The combined impact of exhaled nitric oxide and sputum eosinophils monitoring in asthma treatment: a prospective cohort study. Curr Pharm Des . 2015;21:4752–4762. doi: 10.2174/1871524915666150710123415. [DOI] [PubMed] [Google Scholar]
- 87. Peirsman EJ, Carvelli TJ, Hage PY, Hanssens LS, Pattyn L, Raes MM, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol . 2014;49:624–631. doi: 10.1002/ppul.22873. [DOI] [PubMed] [Google Scholar]
- 88. Petsky HL, Li AM, Au CT, Kynaston JA, Turner C, Chang AB. Management based on exhaled nitric oxide levels adjusted for atopy reduces asthma exacerbations in children: a dual centre randomized controlled trial. Pediatr Pulmonol . 2015;50:535–543. doi: 10.1002/ppul.23064. [DOI] [PubMed] [Google Scholar]
- 89. Shaw DE, Berry MA, Thomas M, Green RH, Brightling CE, Wardlaw AJ, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med . 2007;176:231–237. doi: 10.1164/rccm.200610-1427OC. [DOI] [PubMed] [Google Scholar]
- 90. Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med . 2005;352:2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 91. Syk J, Malinovschi A, Johansson G, Undén AL, Andreasson A, Lekander M, et al. Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a randomized, controlled trial. J Allergy Clin Immunol Pract . 2013;1:639–648, e1–e8. doi: 10.1016/j.jaip.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 92. Verini M, Consilvio NP, Di Pillo S, Cingolani A, Spagnuolo C, Rapino D, et al. FeNO as a marker of airways inflammation: the possible implications in childhood asthma management. J Allergy (Cairo) . 2010;2010:691425. doi: 10.1155/2010/691425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Morphew T, Shin HW, Marchese S, Pires-Barracosa N, Galant SP. Phenotypes favoring fractional exhaled nitric oxide discordance vs guideline-based uncontrolled asthma. Ann Allergy Asthma Immunol . 2019;123:193–200. doi: 10.1016/j.anai.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 94. Pijnenburg MW, Bakker EM, Hop WC, De Jongste JC. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med . 2005;172:831–836. doi: 10.1164/rccm.200503-458OC. [DOI] [PubMed] [Google Scholar]
- 95. Pike K, Selby A, Price S, Warner J, Connett G, Legg J, et al. Exhaled nitric oxide monitoring does not reduce exacerbation frequency or inhaled corticosteroid dose in paediatric asthma: a randomised controlled trial. Clin Respir J . 2013;7:204–213. doi: 10.1111/j.1752-699X.2012.00306.x. [DOI] [PubMed] [Google Scholar]
- 96. de Jongste JC, Carraro S, Hop WC, Baraldi E. CHARISM Study Group. Daily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthma. Am J Respir Crit Care Med . 2009;179:93–97. doi: 10.1164/rccm.200807-1010OC. [DOI] [PubMed] [Google Scholar]
- 97. Fritsch M, Uxa S, Horak F, Jr, Putschoegl B, Dehlink E, Szepfalusi Z, et al. Exhaled nitric oxide in the management of childhood asthma: a prospective 6-months study. Pediatr Pulmonol . 2006;41:855–862. doi: 10.1002/ppul.20455. [DOI] [PubMed] [Google Scholar]
- 98. Hashimoto S, Brinke AT, Roldaan AC, van Veen IH, Möller GM, Sont JK, et al. Internet-based tapering of oral corticosteroids in severe asthma: a pragmatic randomised controlled trial. Thorax . 2011;66:514–520. doi: 10.1136/thx.2010.153411. [DOI] [PubMed] [Google Scholar]
- 99. Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, et al. NHLBI’s Asthma Clinical Research Center. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol . 2007;119:73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brooks EA, Massanari M, Hanania NA, Weiner DJ. Cost-effectiveness of fractional exhaled nitric oxide (FeNO) measurement in predicting response to omalizumab in asthma. Clinicoecon Outcomes Res . 2019;11:301–307. doi: 10.2147/CEOR.S177207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Brooks EA, Massanari M. Cost-effectiveness analysis of monitoring fractional exhaled nitric oxide (FeNO) in the management of asthma. Manag Care . 2018;27:42–48. [PubMed] [Google Scholar]
- 102. Arnold RJ, Massanari M, Lee TA, Brooks E. A review of the utility and cost effectiveness of monitoring fractional exhaled nitric oxide (FeNO) in asthma management. Manag Care . 2018;27:34–41. [PubMed] [Google Scholar]
- 103. Sabatelli L, Seppälä U, Sastre J, Crater G. Cost-effectiveness and budget impact of routine use of fractional exhaled nitric oxide monitoring for the management of adult asthma patients in Spain. J Investig Allergol Clin Immunol . 2017;27:89–97. doi: 10.18176/jiaci.0103. [DOI] [PubMed] [Google Scholar]
- 104. Beerthuizen T, Voorend-van Bergen S, van den Hout WB, Vaessen-Verberne AA, Brackel HJ, Landstra AM, et al. Cost-effectiveness of FeNO-based and web-based monitoring in paediatric asthma management: a randomised controlled trial. Thorax . 2016;71:607–613. doi: 10.1136/thoraxjnl-2015-207593. [DOI] [PubMed] [Google Scholar]
- 105. LaForce C, Brooks E, Herje N, Dorinsky P, Rickard K. Impact of exhaled nitric oxide measurements on treatment decisions in an asthma specialty clinic. Ann Allergy Asthma Immunol . 2014;113:619–623. doi: 10.1016/j.anai.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 106. Franklin PJ, Stick SM. The value of FeNO measurement in asthma management: the motion against FeNO to help manage childhood asthma: reality bites. Paediatr Respir Rev . 2008;9:122–126. doi: 10.1016/j.prrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 107. Essat M, Harnan S, Gomersall T, Tappenden P, Wong R, Pavord I, et al. Fractional exhaled nitric oxide for the management of asthma in adults: a systematic review. Eur Respir J . 2016;47:751–768. doi: 10.1183/13993003.01882-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pijnenburg MW, Lissenberg ET, Hofhuis W, Ghiro L, Ho WC, Holland WP, et al. Exhaled nitric oxide measurements with dynamic flow restriction in children aged 4-8 yrs. Eur Respir J . 2002;20:919–924. doi: 10.1183/09031936.02.01282002. [DOI] [PubMed] [Google Scholar]
- 109. Holguin F, Comhair SA, Hazen SL, Powers RW, Khatri SS, Bleecker ER, et al. An association between l-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. Am J Respir Crit Care Med . 2013;187:153–159. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. McSharry CP, McKay IC, Chaudhuri R, Livingston E, Fraser I, Thomson NC. Short and long-term effects of cigarette smoking independently influence exhaled nitric oxide concentration in asthma. J Allergy Clin Immunol . 2005;116:88–93. doi: 10.1016/j.jaci.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 111. Michils A, Louis R, Peché R, Baldassarre S, Van Muylem A. Exhaled nitric oxide as a marker of asthma control in smoking patients. Eur Respir J . 2009;33:1295–1301. doi: 10.1183/09031936.00154008. [DOI] [PubMed] [Google Scholar]
- 112. Nadif R, Matran R, Maccario J, Bechet M, Le Moual N, Scheinmann P, et al. Passive and active smoking and exhaled nitric oxide levels according to asthma and atopy in adults. Ann Allergy Asthma Immunol . 2010;104:385–393. doi: 10.1016/j.anai.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 113. Coates AL, Wanger J, Cockcroft DW, Culver BH, Carlsen K-H, Diamant Z, et al. Bronchoprovocation Testing Task Force. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J . 2017;49:1601526. doi: 10.1183/13993003.01526-2016. [DOI] [PubMed] [Google Scholar]