Abstract
Background:
The effect of early blood pressure reduction on cognitive function in patients with acute ischemic stroke remains unknown.
Aim:
We tested whether antihypertensive treatment would reduce cognitive impairment in patients with acute ischemic stroke.
Methods:
In the China Antihypertensive Trial in Acute Ischemic Stroke, patients with elevated blood pressure were randomly assigned to receive antihypertensive treatment or to discontinue all hypertensive medications within 48 h of onset. Cognitive function was measured by the Mini-Mental State Examination and Montreal Cognitive Assessment at 3 months after randomization in a subsample of 638 participants.
Results:
Mean systolic blood pressure was reduced by 21.5 mmHg in the antihypertensive treatment group and 13.9 mmHg in the control group within 24 h after randomization (P < 0.001). Mean systolic blood pressure was 134.9 mmHg in the antihypertensive treatment group and 141.6 mmHg in the control group at day 14 after randomization (P < 0.001). Median Mini-Mental State Examination score was 26 and Montreal Cognitive Assessment score was 22 in both the antihypertensive treatment and control groups at 3 months. An Mini-Mental State Examination < 24 was present in 30.9% of patients in the antihypertensive treatment group compared with 29.7% in the control group (odds ratio = 1.06; 95% confidence interval 0.75–1.48; P = 0.75). Likewise, proportions of patients with Montreal Cognitive Assessment < 26 were similar between the antihypertensive treatment (70.6%) and control (70.7%) groups (odds ratio = 0.99; 95% confidence interval 0.70–1.40; P = 0.96).
Conclusions:
These data indicated that early blood pressure reduction with antihypertensive medication in patients with acute ischemic stroke had no effect on cognitive impairment at 3 months.
Keywords: Acute ischemic stroke, antihypertensive therapy, clinical trial, cognitive functions
Introduction
Vascular cognitive impairment and dementia are major complications of stroke.1,2 Approximately 1 in 10 patients with first-ever stroke will develop new dementia, and 1 in 3 will have dementia after a recurrent stroke.2 Prospective cohort studies found that elevated blood pressure (BP) increased the risk of vascular cognitive impairment and dementia.3 Several randomized clinical trials suggested a potential beneficial effect of antihypertensive treatment on dementia in the elderly with hypertension.4–6 However, clinical trials testing the effect of early BP reduction on cognitive function in patients with acute stroke reported inconsistent findings.7,8 The Scandinavian Candesartan Acute Stroke Trial (SCAST) found that antihypertensive treatment did not improve cognitive function7 while the Efficacy of Nitric Oxide in Stroke (ENOS) trial suggested that continuing antihypertensive treatment resulted in significantly lower cognition scores than stopping treatment among acute stroke patients.8 Theoretically, elevated BP might increase cerebral edema or hemorrhagic transformation in ischemic stroke, but lowering BP might reduce cerebral blood flow and increase infarction or perihematomal ischemia.9
The China Antihypertensive Trial in Acute Ischemic Stroke (CATIS) was designed to test whether moderate lowering of BP within the first 48 h after the onset of an acute ischemic stroke would reduce death and major disability.10 In a pre-planned ancillary study, we examined the effects of early BP reduction on cognitive function as measured by the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) at 3 months after randomization among a subgroup of CATIS participants.
Methods
Trial participants
The CATIS study was a multicenter, single-blind, blinded end-points randomized clinical trial conducted among 4071 patients with ischemic stroke and elevated systolic BP. Detailed methods and baseline characteristics have been described elsewhere.10 Eligible participants were ≥ 22 years who had ischemic stroke, confirmed by computed tomography or magnetic resonance imaging within 48 h of symptom onset, and who had an elevated systolic BP between 140 to < 220 mmHg. Approximately, 49.1% of participants were taking antihypertensive medication at baseline. The exclusion criteria were BP levels of ≥ 220/120 mmHg, severe heart failure, acute myocardial infarction or unstable angina, atrial fibrillation, aortic dissection, cerebrovascular stenosis, resistant hypertension, coma, and treatment with intravenous thrombolytic therapy.
In a pre-planned ancillary study, 660 CATIS trial participants were systemically selected prior to randomization from seven participating hospitals for cognitive function assessment at their 3-month follow-up visit. Specifically, each of the seven participating hospitals recruited 80–100 patients consecutively. The recruitment completed by November 2012. At the 3-month visit, 15 patients were lost to follow-up and 7 patients were deceased. A total of 638 participants who completed the cognitive function tests at 3 months were included in the present analysis. The exclusion criteria for the ancillary study were visual or hearing impairment substantial enough to hinder performance on cognitive testing. All participants provided written informed consent before the start of the study, and the study protocol was approved by IRBs at Tulane University in the US and Soochow University in China, as well as ethical committees at the participating hospitals.
Intervention
All CATIS participants were randomly assigned to receive antihypertensive treatment or to discontinue all antihypertensive medications during hospitalization. The antihypertensive intervention aimed at lowering systolic BP by 10 – 25% within the first 24 h after randomization, achieving a BP level of < 140/90 mmHg within 7 days, and maintaining that control level during the remainder of hospitalization. Several antihypertensive medications, including intravenous angiotensin-converting enzyme inhibitors (enalapril, first-line), calcium channel blockers (second-line), and diuretics (third-line), were used individually or in combination in the intervention group to achieve the targeted BP reduction according to a pre-specified treatment algorithm.10 Home antihypertensive medications were stopped after randomization. All patients, both in the treatment and control groups, were prescribed antihypertensive medications according to clinical guidelines at their hospital discharge.11
Outcome assessment
The primary outcome for this ancillary study was cognitive impairment at 3 months assessed by trained neurologists unaware of treatment assignment using the MMSE and MoCA in Chinese.12,13 The MMSE contains 20 items that test cognitive performance in domains including orientation, registration, attention and calculation, recall, language, and visual construction.12 The MoCA is a 30-item test that evaluates the following seven cognitive domains: visuospatial/executive functions, naming, memory, attention, language, abstraction, and orientation.13 One point is added for participants with education <12 years. Both MMSE and MoCA have been translated into Chinese and validated as a screening tool for cognitive impairment and dementia in the Chinese population.14,15 Cognitive impairment on the MMSE and MoCA was defined as a score of <24 and <26, respectively.16,17 In addition to using cognitive impairment as a binary variable, MMSE scores were divided into three ordinal categories: 24–30 (no cognitive impairment), 19–23 (mild cognitive impairment), and 0–17 (severe cognitive impairment).17 Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS; scores range from 0 to 42, with higher scores indicating a more severe neurologic deficit) by trained neurologists at baseline.18 BP was measured by trained nurses every 2 h during the first 24 h after randomization, every 4 h between 24 and 72 h, and 3 times daily during days 4 to 14 according to a standard protocol.10
Statistical analysis
We estimated that 660 patients would provide 80% statistical power to detect a 15% reduction in MoCA-cognitive impairment at a two-sided significance level of 0.05. We assumed 70% of patients in the control group would have MoCA-cognitive impairment and 5% of participants would be lost to follow-up at 3 months based on our pilot data. Data were analyzed according to the intention-to-treat principle. Baseline characteristics were compared between the treatment and control groups using χ2 tests, two-sample t tests, or Wilcoxon rank-sum tests when appropriate. The median and interquartile range of MMSE and MoCA scores were calculated, and the between-group difference was compared using the Wilcoxon rank-sum test. Proportions of participants with cognitive impairment were compared between the two treatment groups using a χ2 test. Logistic regression analysis was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for cognitive impairment associated with antihypertensive treatment compared with control. Effects of BP reduction on cognitive impairment severity were analyzed using ordinal logistic regression models. In a sensitivity analysis, ORs were adjusted for baseline age, sex, education levels, and NIHSS scores.
We estimated the heterogeneity of the treatment effect on cognitive impairment in pre-specified subgroups by age, sex, systolic BP at baseline, NIHSS score at baseline, time from onset of stroke to randomization, history of hypertension, antihypertensive treatment, and subtypes of ischemic stroke by adding an interaction term in unadjusted logistic regression models. All analyses were conducted using SAS version 9.3 (SAS Institute Inc.) and Stata version 12 (Stata Corp). Two-sided P value < 0.05 was considered to be significant.
Results
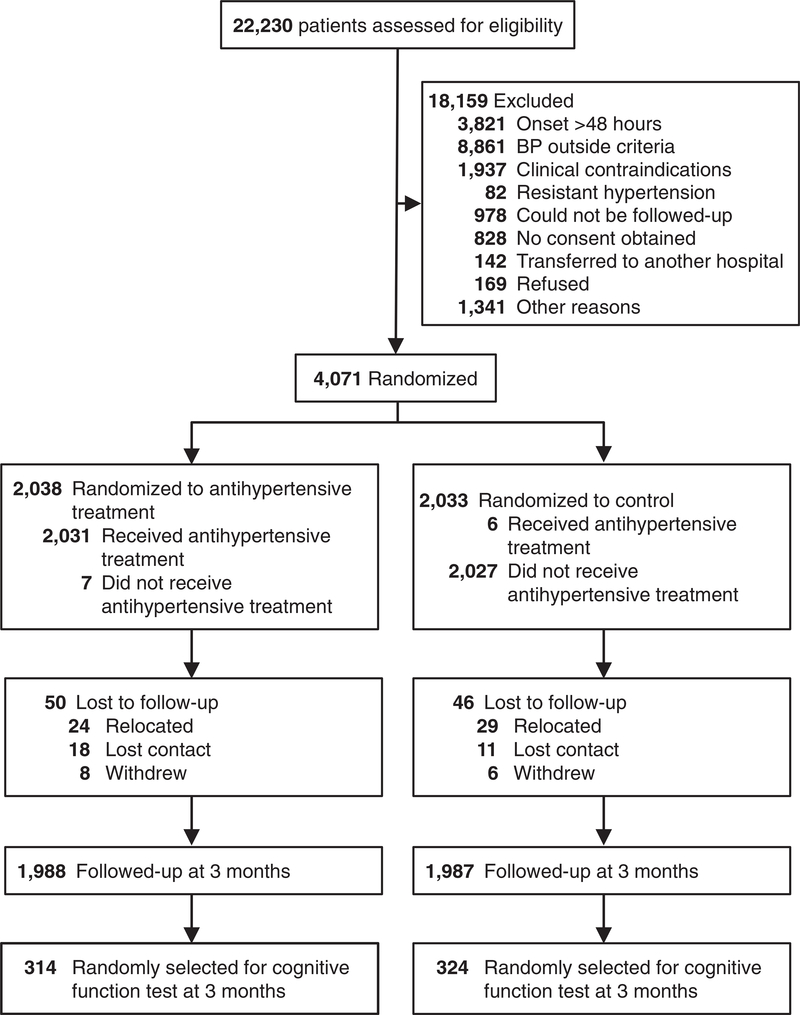
A total of 638 CATIS participants (314 in the treatment group and 324 in the control group) completed cognitive function assessment at the 3-month follow-up visit (Figure 1). Baseline characteristics were well balanced between the two comparison groups (Table 1). In addition, baseline characteristics among individuals in the ancillary study were not significantly different from those among CATIS participants overall.
Figure 1.
Trial participant flow chart.
Table 1.
Baseline characteristics of 638 participants
| Characteristics | Antihypertensive treatment (n = 314) | Control (n = 324) | P value |
|---|---|---|---|
| Age, mean (SD), year | 60.4 (10.1) | 59.8 (10.7) | 0.49 |
| Men, n (%) | 227 (72.3) | 221 (68.2) | 0.26 |
| Education, mean (SD), year | 7.9 (4.2) | 7.4 (4.0) | 0.13 |
| Time from stroke onset to randomization, mean (SD), h | 14.5 (12.2) | 15.0 (12.3) | 0.6 |
| Systolic blood pressure, mean (SD), mmHg | 167.7 (17.0) | 167.1 (16.3) | 0.64 |
| Diastolic blood pressure, mean (SD), mmHg | 97.4 (9.8) | 98.9 (10.2) | 0.06 |
| Body mass index, mean (SD), kg/m2 | 25.0 (3.1) | 24.8 (3.0) | 0.56 |
| NIH stroke scale score, median (IQR) | 4.0 (3.0–7.0) | 4.0 (3.0–7.0) | 0.41 |
| Disease history, n (%) | |||
| Hypertension | 240 (76.4) | 254 (78.4) | 0.55 |
| Hyperlipidemia | 19 (6.1) | 24 (7.4) | 0.49 |
| Diabetes mellitus | 57 (18.2) | 52 (16.1) | 0.48 |
| Coronary heart disease | 29 (9.2) | 39 (12.0) | 0.25 |
| Current cigarette smoking, n (%) | 122 (38.9) | 119 (36.7) | 0.58 |
| Current alcohol drinking, n (%) | 105 (33.4) | 115 (35.5) | 0.56 |
| Ischemic stroke type, n (%) | |||
| Thrombotic | 210 (66.9) | 213 (65.7) | 0.76 |
| Embolic | 8 (2.6) | 15 (4.6) | 0.16 |
| Lacunar | 98 (31.2) | 103 (31.8) | 0.87 |
Data are presented as number of patients with variable present (%), mean (SD), or median (interquartile range).
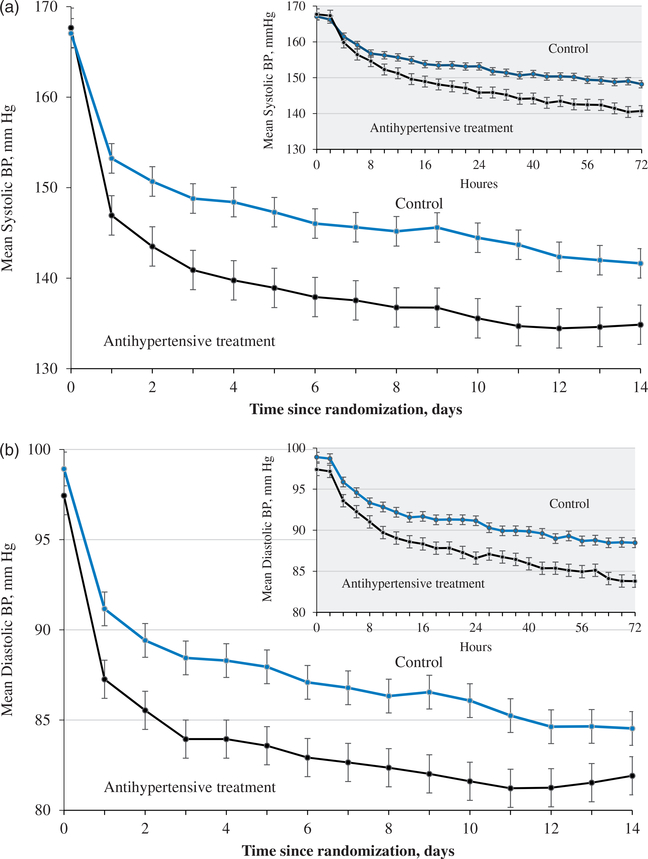
The mean systolic and diastolic BP levels differed significantly between the two comparison groups from 6 h to 14 days after randomization (Figure 1A in the appendix). The mean systolic BP was reduced −21.5 mmHg (from 167.7 to 145.8) in the treatment group and −13.9 mmHg (from 167.1 to 153.2) in the control group within 24 h after randomization (difference −7.6 mmHg; 95% CI −10.1 to −5.1; P < 0.0001; Table 2). The mean systolic BP was 134.9 in the treatment group and 141.6 mmHg in controls at day 14 (difference −6.8 mmHg; 95% CI −9.5 to −4.0; P < 0.0001). At 3 months, the mean systolic BP was 139.7 in the treatment group and 142.1 mmHg in controls (difference −2.5 mmHg; 95% CI −4.5 to −0.4; P = 0.02).
Table 2.
Blood pressure reduction in the antihypertensive treatment and control none
| Variable | Antihypertensive treatment (n = 314) | Control (n = 324) | Difference (95% CI) | P value |
|---|---|---|---|---|
| BP at 24 h after randomization, mmHg | ||||
| Systolic | 145.8 (15.0) | 153.2 (15.0) | −7.3 (−9.7 to −5.0) | <0.0001 |
| Diastolic | 86.6 (8.9) | 91.2 (9.7) | −4.5 (−6.0 to −3.1) | <0.0001 |
| Absolute BP changes from baseline to 24 h after randomization, mmHg | ||||
| Systolic | −21.5 (15.4) | −13.9 (16.1) | −7.6 (−10.1 to −5.1) | <0.0001 |
| Diastolic | −11.0 (8.9) | −7.8 (10.1) | −3.2 (−4.7 to −1.7) | <0.0001 |
| Proportional BP changes from baseline to 24 h after randomization, % | ||||
| Systolic | −12.5 (8.3) | −7.9 (8.7) | −4.6 (−5.9 to −3.2) | <0.0001 |
| Diastolic | −10.8 (8.5) | −7.4 (9.5) | −3.4 (−4.9 to −2.0) | <0.0001 |
| BP at 7 days after randomization, mmHg | ||||
| Systolic | 137.5 (11.3) | 145.6 (13.2) | −8.1 (−10.2 to −6.0) | <0.0001 |
| Diastolic | 82.7 (6.9) | 86.8 (8.2) | −4.1 (−5.4 to −2.9) | <0.0001 |
| BP at 14 days after randomization, mmHg | ||||
| Systolic | 134.9 (9.0) | 141.6 (13.3) | −6.8 (−9.5 to −4.0) | <0.0001 |
| Diastolic | 81.9 (6.4) | 84.5 (8.2) | −2.6 (−4.4 to −0.8) | 0.004 |
| BP at 3 months after randomization, mmHg | ||||
| Systolic | 139.7 (12.1) | 142.1 (13.9) | −2.5 (−4.5 to −0.4) | 0.02 |
| Diastolic | 85.9 (7.6) | 87.9 (9.1) | −2.0 (−3.3 to −0.7) | 0.002 |
Data are presented as mean (SD).
BP: blood pressure.
The median scores of MMSE and MoCA were identical between the treatment and control groups (Table 3). Likewise, the median score for subdomains of MMSE and MoCA were also very similar between the two comparison groups (Table A1 in the appendix). In addition, the proportion of cognitive impairment was similar between the antihypertensive treatment and control groups (Table 3). In all, 30.9% of patients in the treatment group and 29.7% in the control had cognitive impairment defined by MMSE <24 points (OR 1.06; 95% CI 0.75–1.48; P=0.75). Similarly, 70.6% of patients in the treatment group and 70.7% of controls had cognitive impairment defined by MoCA< 26 points (OR 0.99; 95% CI 0.70–1.40; P = 0.96). The ordinal regression analysis showed no significant difference between the distribution of MMSE scores in the two comparison groups (OR 1.05; 95% CI 0.75–1.46; P = 0.80). In the sensitivity analysis, all of the results remained nonsignificant after adjustment for age, sex, education levels, and NIHSS scores at baseline.
Table 3.
Effects of antihypertensive treatment on cognitive performance on MMSE and MoCA
| Variable | Antihypertensive treatment (n = 314) | Control (n = 324) | OR (95%CI) | P value | Adjusted ORa (95% CI) | P value |
|---|---|---|---|---|---|---|
| MMSE score, median (IQR) | 26 (22–29) | 26 (22–29) | 0.50 | |||
| MMSE <24, n (%) | 96 (30.9) | 96 (29.7) | 1.06 (0.75–1.48) | 0.75 | 1.09 (0.76–1.56) | 0.64 |
| MMSE category, n (%) | ||||||
| 24–30 | 215 (69.1) | 227 (70.3) | 1.05 (0.75–1.46) | 0.80 | 1.07 (0.75–1.51) | 0.71 |
| 18–23 | 63 (20.3) | 61 (18.9) | ||||
| 0–17 | 33 (10.6) | 35 (10.8) | ||||
| MoCA score, median (IQR) | 22 (18–26) | 22 (18–26) | 0.57 | |||
| MoCA <26, n (%) | 218 (70.6) | 227 (70.7) | 0.99 (0.70–1.40) | 0.96 | 0.97 (0.68–1.38) | 0.85 |
Data are presented as median (interquartile range) and number (percentages). MMSE < 24 or MoCA < 26 indicate cognitive impairment.
Adjusted for age, sex, education levels, and NIHSS score at baseline.
MMSE: Mini Mental State Examination; MoCA: Montreal Cognitive Assessment.
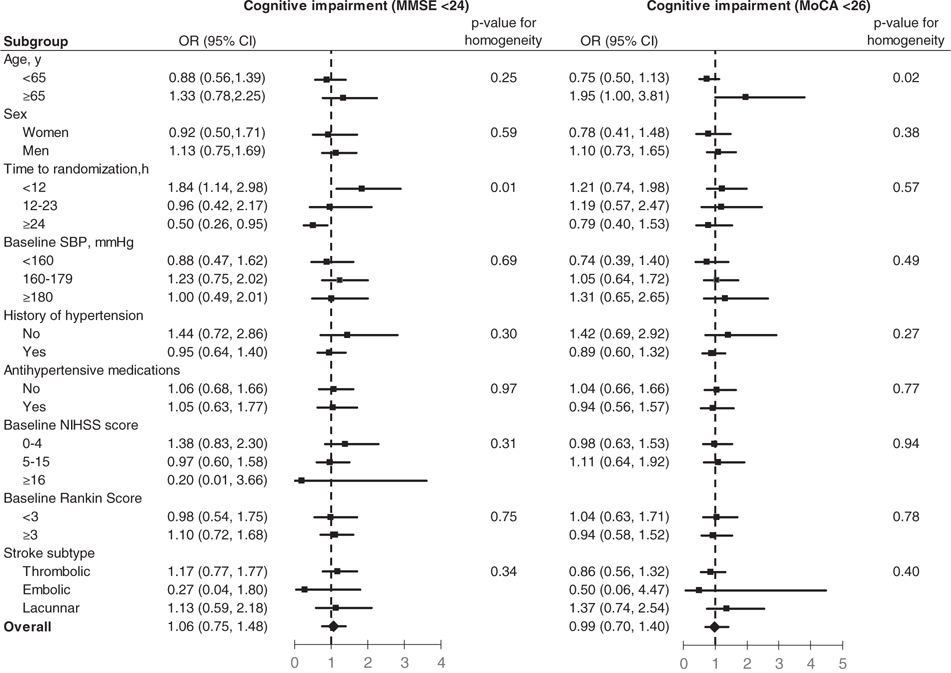
The effects of antihypertensive treatment on the primary outcome were consistent across all pre-specified subgroups, except for age and time from stroke onset to treatment (Figure 2). In patients aged≥65 years, BP lowering was associated with a significant risk of cognitive impairment defined by MoCA < 26 (OR 1.95; 95% CI 1.00–3.81; P for homogeneity = 0.02). However, we did not find a similar interaction between antihypertensive treatment and age with respect to cognitive impairment defined by MMSE < 24. Among patients who received or discontinued antihypertensive treatment within 12 h after stroke onset, BP reduction increased the risk of cognitive impairment defined by MMSE < 24 (OR 1.84; 95% CI 1.14–2.98), while it decreased cognitive impairment risk in those patients who were randomized between 24 and 48 h after stroke onset (OR 0.50; 95% CI 0.26–0.95; P for homogeneity=0.006).
Figure 2.
Effect of antihypertensive treatment on cognitive impairment at 3-month follow-up visit according to pre-specified subgroups. Data markers indicate point estimates (with the area of the square proportional to the number of events), error bars indicate 95% confidence intervals.
Discussion
In this ancillary study of a randomized controlled trial, early antihypertensive treatment in patients with acute ischemic stroke and elevated BP had no effect on cognitive impairment at 3 months after treatment, despite the achievement of a fairly large reduction (7.6 mmHg) in systolic BP within 24 h after randomization. Proportions of cognitive impairment using either MMSE or MoCA were similar between the treatment and control groups. Neither the median MMSE score nor the median MoCA score was different between the two comparison groups. These findings suggest that early BP reduction with antihypertensive medications in patients with acute ischemic stroke does not reduce the risk of cognitive impairment at 3 months after treatment.
Randomized clinical trials have documented that antihypertensive treatment reduced the risk of cognitive decline and dementia in hypertensive patients without a history of stroke.4–6,19 However, the effect of BP reduction on cognitive function was inconsistent in patients with a history of stroke.20,21 In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, 2916 patients with recent (<6 months) lacunar stroke were randomly assigned to target levels of systolic BP 130–149 vs. <130 mmHg.20 The study found that cognitive function was not significantly different between two treatment groups, although mean difference in systolic BP was 11 mmHg between them. However, the Perindopril Protection Against Recurrent Stroke Study (PROGRESS) conducted among 6105 people with a history of stroke or transient ischemic attack within the previous 5 years, showed that active BP lowering was associated with a non-significant 12% reduction (P = 0.2) of dementia and a significant 19% reduction (P = 0.01) in cognitive decline.21
The SCAST trial tested the effects of the angiotensin receptor blocker candesartan on cognitive function at 6 months in patients with acute ischemic or hemorrhagic stroke (within 30 h of onset) and systolic BP ≥140 mmHg.7 It showed that candesartan did not improve cognitive function as compared with control when measured by the MMSE questionnaire. The ENOS trial randomly allocated 1053 patients with acute stroke within 48 h of onset to continue antihypertensive treatment and 1044 patients to stop treatment.8 The results suggested that continued antihypertensive treatment resulted in significantly lower cognition scores (P = 0.013) compared with stopping treatment among acute stroke patients at 90 days.8 The present study showed that a BP reduction strategy to achieve a target level of <140/90 mmHg during hospitalization has no effect on cognitive impairment as measured by either MMSE or MoCA in patients with acute ischemic stroke.
The subgroup analyses found a weak interaction between age and BP reduction on cognitive impairment. Early antihypertensive treatment might increase cognitive impairment in patients aged ≥65 years with acute ischemic stroke but reduce risk among patients <65 years. Most of the patients in our study are <65 years, which is similar to the China National Stroke Registry data (average age of 63.8 years),22 but much lower than the average age of patients in western populations.23 This subgroup analysis suggests there is a need to be additionally cautious in considering early BP lowering among older patients with acute ischemic stroke. In addition, the subgroup analysis indicated that very early antihypertensive treatment (<12h after onset) might increase risk of cognitive impairment.
Our study has several strengths. The randomized clinical trial design provides an unbiased assessment of the relationship between BP and cognitive function among acute stroke patients. In addition, both MMSE and MoCA tests were used to assess cognitive performance. The MMSE showed high levels of sensitivity for moderate-to-severe cognitive impairment but lower levels for mild degrees of impairment, whereas MoCA is more sensitive to mild cognitive impairment.24 Furthermore, treated BP levels were substantially separated between the treatment and control groups in our study.
Several limitations of our study need to be considered. First, data on cognitive function were only collected in a random sample of CATIS participants. The follow-up period of 3 months is relatively short. Statistical power to detect a small difference in the cognitive outcomes in our study may be limited. In addition, our trial only achieved a difference in systolic BP reduction of −7.6 mmHg, which might not be sufficient to affect cognitive function. Future large long-term clinical trials are needed to detect the effects of early BP reduction on cognitive function among patients with acute ischemic stroke. Furthermore, the MMSE and MOCA are coarse measures and likely miss patients with attentional and executive deficits. Our study did not include objective measures of vascular cognitive impairment and dementia, such as brain magnetic resonance imaging.25 However, MMSE and MoCA have been widely used in clinical patient care and research. Another limitation is the lack of cognitive function tests at baseline, so changes in cognitive function during antihypertensive treatment cannot be assessed.
In conclusion, among patients with acute ischemic stroke and elevated BP, early antihypertensive treatment using a single drug or a combination of multiple drug classes to achieve a target level of <140/90 mmHg during hospitalization did not significantly affect cognitive impairment at 3 months post-treatment follow-up compared with the absence of hypertensive medications.
Acknowledgments
We thank the clinical staff at all participating hospitals for their support and contribution to this project and Miss Katherine Obst for editorial assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Tulane University and Collins C. Diboll Private Foundation, both in New Orleans, LA; Soochow University, a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the National Natural Science Foundation of China (Grant No. 81320108026), all in China. Dr. Xiaoqing Bu was supported by a research training grant (D43TW009107) from the NIH Fogarty International Center, Bethesda, MD. We acknowledge that the Changzhou Pharmaceutical Factory provided the study drug (Enalapril) for this trial.
Appendix
Figure 1A.
Mean Systolic (a) and Diastolic (b) Blood Pressure Since Randomization, by Treatment Group.
Table 1A.
Effects of antihypertensive treatment on sub-domains of MMSE and MoCA
| Variable | Antihypertensive treatment (n = 314) | Control (n = 324) | P value |
|---|---|---|---|
| MMSE assessment | |||
| Orientation | 10 (9–10) | 10 (8–10) | 0.36 |
| Registration | 3 (3–3) | 3 (3–3) | 0.53 |
| Attention and calculation | 4 (3–5) | 5 (3–5) | 0.60 |
| Recall | 3 (2–3) | 3 (2–3) | 0.25 |
| Language | 7 (6–8) | 7 (6–8) | 0.76 |
| Visuospatial | 1 (0–1) | 1 (0–1) | 0.56 |
| MoCA assessment | |||
| Visuospatial/executive | 3 (2–5) | 3 (2–5) | 0.52 |
| Naming | 3 (2–3) | 3 (2–3) | 0.67 |
| Memory | 3 (1–5) | 3 (1–5) | 0.33 |
| Attention | 4 (3–5) | 4 (3–6) | 0.14 |
| Language | 1 (0–2) | 1.5 (0–2) | 0.19 |
| Abstraction | 2 (1–2) | 2 (1–2) | 0.96 |
| Orientation | 6 (5–6) | 6 (5–6) | 0.44 |
Data are presented as median (interquartile range).
MMSE: Mini Mental State Examination; MoCA: Montreal Cognitive Assessment.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Brainin M, Tuomilehto J, Heiss WD, et al. Post-stroke cognitive decline: An update and perspectives for clinical research. Eur J Neurol 2015; 22: 229–238. [DOI] [PubMed] [Google Scholar]
- 2.Pendlebury ST and Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 3.Rouch L, Cestac P, Hanon O, et al. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs 2015; 29: 113–130. [DOI] [PubMed] [Google Scholar]
- 4.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352: 1347–1351. [DOI] [PubMed] [Google Scholar]
- 5.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the hypertension in the very elderly trial cognitive function assessment (HYVET-COG): A double-blind, placebo controlled trial. Lancet Neurol 2008; 7: 683–689. [DOI] [PubMed] [Google Scholar]
- 6.The SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated hypertension. JAMA 1991; 265: 3255–3264. [PubMed] [Google Scholar]
- 7.Hornslien AG, Sandset EC, Bath PM, et al. Effects of candesartan in acute stroke on cognitive function and quality of life: Results from the Scandinavian Candesartan Acute Stroke Trial. Stroke 2013; 44: 2022–2024. [DOI] [PubMed] [Google Scholar]
- 8.ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet 2015; 385: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan JD and Powers WJ. Cerebral autoregulation and acute ischemic stroke. Am J Hypertens 2012; 25: 946–950. [DOI] [PubMed] [Google Scholar]
- 10.He J, Zhang Y, Xu T, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke the CATIS randomized clinical trial. JAMA 2014; 311: 479–489. [DOI] [PubMed] [Google Scholar]
- 11.Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE and McHugh PR. Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 14.Xu G, Meyer JS, Huang Y, et al. Adapting mini-mental state examination for dementia screening among illiterate or minimally educated elderly Chinese. Int J Geriatr Psychiatry 2003; 18: 609–616. [DOI] [PubMed] [Google Scholar]
- 15.Wen HB, Zhang ZX, Niu FS, et al. The application of Montreal cognitive assessment in urban Chinese residents of Beijing (Chinese). Zhonghua Nei Ke Za Zhi 2008; 47: 36–39. [PubMed] [Google Scholar]
- 16.Lees R, Selvarajah J, Fenton C, et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 2014; 45: 3008–3018. [DOI] [PubMed] [Google Scholar]
- 17.Tombaugh TN and McIntyre NJ. The mini-mental state examination: A comprehensive review. J Am Geriatr Soc 1992; 40: 922–935. [DOI] [PubMed] [Google Scholar]
- 18.Lyden P, Raman R, Liu L, et al. NIHSS training and certification using a new digital video disk is reliable. Stroke 2005; 36: 2446–2449. [DOI] [PubMed] [Google Scholar]
- 19.Levi Marpillat N, Macquin-Mavier I, Tropeano AI, et al. Antihypertensive classes, cognitive decline and incidence of dementia: a network meta-analysis. J Hypertens 2013; 31: 1073–1082. [DOI] [PubMed] [Google Scholar]
- 20.Pearce LA, McClure LA, Anderson DC, et al. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: A secondary analysis from the SPS3 randomised trial. Lancet Neurol 2014; 13: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Cui L, Ji X, et al. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke 2011; 6: 355–361. [DOI] [PubMed] [Google Scholar]
- 23.Cadilhac DA, Kilkenny MF, Srikanth V, et al. Do cognitive, language, or physical impairments affect participation in a trial of self-management program for stroke? Int J Stroke 2016; 11: 77–84. [DOI] [PubMed] [Google Scholar]
- 24.Pendlebury ST, Cuthbertson FC, Welch SJ, et al. Underestimation of cognitive impairment by mini-mental state examination versus the Montreal cognitive assessment in patients with transient ischemic attack and stroke: A population-based study. Stroke 2010; 41: 1290–1293. [DOI] [PubMed] [Google Scholar]
- 25.Bigler ED. Magnetic resonance imaging in the evaluation of cognitive function. Pediatr Blood Cancer 2014; 61: 1724–1728. [DOI] [PubMed] [Google Scholar]