Abstract
Introduction:
Pharmacogenomic testing can indicate which drugs may have limited therapeutic action or lead to adverse effects, hence guiding rational and safe prescribing. However, in the UK and other countries, there are still significant barriers to implementation of testing in primary care.
Objective:
This systematic review presents the barriers and enablers to the implementation of pharmacogenomics in primary care setting.
Materials & methods:
MEDLINE, EMBASE, PsycINFO and CINAHL databases were searched through to July 2020 for studies that reported primary qualitative data of primary care professionals and patient views. Following screening, data extraction and quality assessment, data synthesis was undertaken using meta-aggregation based on the theoretical domain’s framework (TDF). Confidence in the synthesized findings relating to credibility and dependability was established using CONQual. Eligible papers were categorized into six TDF domains – knowledge; social and professional roles; behavioral regulation; beliefs and consequences; environmental context and resources; and social influences.
Results:
From 1669 citations, eighteen eligible studies were identified across seven countries, with a sample size of 504 participants including both primary care professionals and patients. From the data, 15 synthesized statements, all with moderate CONQual rating emerged. These categories range from knowledge, awareness among Primary Care Physicians and patients, professional relationships, negative impact of PGx, belief that PGx can reduce adverse drug reactions, clinical evidence, cost–effectiveness, informatics, reporting issues and social issues.
Conclusion:
Through use of TDF, fifteen synthesized statements provide policymakers with valuable recommendations for the implementation of pharmacogenomics in primary care. In preparation, policymakers need to consider the introduction of effective educational strategies for both PCPs and patients to raise knowledge, awareness, and engagement. The actual introduction of PGx will require reorganization with decision support tools to aid use of PGx in primary care, with a clear delegation of roles and responsibilities between general professionals and pharmacists supplemented by a local pool of experts. Furthermore, policy makers need to address the cost effectiveness of pharmacogenomics and having appropriate infrastructure supporting testing and interpretation including informatic solutions for utilizing pharmacogenomic results.
Keywords: : barriers, enablers, implementation, meta-aggregation, pharmacogenomic testing, primary care, qualitative systematic review
The use of pharmacogenomics (PGx) testing to provide information on drug selection and dosing in routine clinical practice has been steadily increasing [1–4] PGx offers optimization of a patient’s pharmacotherapy by increasing medication effectiveness, reducing drug-related toxicity and reducing healthcare costs [3,5,6]. While significant progress has been made in establishing PGx in secondary and tertiary care settings [3], the implementation of PGx testing in primary care, where the majority of drug prescriptions [7] are written, is less well established [1]. The evidence of the benefits to medicine management in these settings is growing [8] and it is estimated that one in four primary care patients take at least one medication with a genetic variation that could benefit from PGx testing [9]. Countries including the USA, Canada and The Netherlands have more developed systems, however they also report challenges to the widespread utilization of PGx [10,11].
Several studies have investigated the barriers and enablers for implementation of PGx in primary care [10–15]. The most commonly identified barriers in clinical practice were lack of PGx skills and knowledge among HCP [3,12,16–19], lack of decision support tool to aid interpreting results [3,10,12,20–22], lack of clarity of professional roles and responsibilities between primary care clinicians [3] and perceived lack of clinical evidence to support use of PGx testing [3,4,10,12,15–17,19,23,24]. Barriers to policy implementation have also been identified and include lack of evidence for the cost–effectiveness of PGx testing [1,4,8,25], lack of leadership to develop policy and guidance for PGx prescribing [16] ethical, legal and social barriers [3,12,15] and reimbursement issues [15–19]. Enablers identified included a general interest in PGx testing [26], that PGx testing could guide drug choice [26], and recommendations for use from a colleague [26].
More deeper understanding of the barriers and enablers have been exposed by qualitative studies. For example, infrequent experience with personalized medicines [26], professional reliance on personal experience to navigate PGx care pathways [27], and how relationships with genetic specialists and clinics are managed [26]. Systematic reviews of perceived barriers [28,29] have been reported, but these reviews focus upon the provision of genetic services in primary care and not specifically PGx testing. However, a recent structured review by Hayward et al. [30] of existing implementation models for PGx testing provided an insight into the factors which influence PGx testing in primary care. These included pre-test counselling, role of the pharmacist, data integration into the electronic medical record and point-of-care clinical decision support systems (CDSS). Two other systematic reviews of doctor’s and pharmacists’ knowledge, attitude and practice toward PGx [26,31], were limited to survey findings. In this review, we seek to present synthesized statements of the barriers and enablers to the implementation of PGx testing in primary care by conducting a systematic review with meta-aggregation of qualitative studies. Use of a theoretical framework for this systematic review will provide focus for the synthesized evidence-based statements to better inform policy and practice strategies to enhance the uptake of PGx in primary care.
Materials & methods
The methods for this qualitative synthesis are described below. The Enhancing Transparency of Reporting the synthesis of Qualitative research (ENTREQ) [32] checklist for this review has been followed and is presented in Supplementary Table 1.
Search strategy
A comprehensive, systematic search strategy was used to identify all available primary qualitative studies. Systematic searches of the following databases were undertaken (RA and SQ) – Medline, EMBASE, PsycoInfo and CINAHL to identify relevant articles. The full search strategy is available in Supplementary Table 2.
Selection criteria
The PICo mnemonic was used to develop the search strategy.
Population: participants included primary care healthcare professionals including doctors, pharmacists and nurses, policy makers and patients.
Phenomena of interest: the use of PGx testing.
Context: only papers reporting primary qualitative data were included. Questionnaires and surveys were not included in this systematic review. Papers reporting both quantitative and qualitative data were included if the qualitative data could be independently extracted.
Settings: Studies reporting primary qualitative data on the views and perspectives of healthcare professionals, patients and the wider public and service commissioners on the barriers and enablers to utilizing PGx information to aid therapeutic decision making were selected for inclusion. Studies were not restricted for inclusion by country.
A search strategy was developed by the authors in collaboration with an Information Specialist. An initial search for articles was conducted in July 2018, but then updated March 2020 by RA. Studies were limited to those which reported qualitative data on the barriers and enablers for the implementation of PGx in primary care and were eligible for inclusion in the meta-synthesis. Studies which reported surveys or questionnaire data or not primarily based or related to primary care practice were excluded from the meta-synthesis.
Quality appraisal
The Critical Appraisal Skills Program (CASP) [33] qualitative checklist was used to determine the methodological strengths and limitations of the included studies. The checklist contains 10 questions, thus providing rapid evaluation of studies. Questions were scored 0, 1 or 2, reflecting to what extent information from the paper answered each question (0 = no criteria fulfilled or can’t tell; 1 = some criteria fulfilled; 2 = all criteria fulfilled). Papers where then rated low, medium, or high quality based on the following scoring system: high = 18–20; medium = 14–17; low quality ≤14. Details and results of the quality appraisal for all included studies can be found in Supplementary Table 3.
Data extraction
Descriptive and methodological information about each paper was extracted into an excel table devised by LC. Two researchers independently reviewed and extracted all information under the results, discussion and conclusion sections of each paper (SQ, LC) and in the case of ambiguity of data with regards to relevance to the research question, both researchers reached an agreement after full discussion. The emerging barriers and enablers were then coded into 6 out of 14 Theoretical Domains Framework (TDF) domains by the main researchers (SQ) and independently checked by a second researcher (AL). Only six domains were used, as these were the only ones that were supported by the data extracted from our papers.
This study used the TDF as a framework [34]. TDF is a synthesis of 33 theories of behavior and behavior change clustered into 14 domains [34]. These 14 domains include knowledge, skills, social/professional role and identity, beliefs and capabilities, optimism, beliefs about consequences, reinforcement, intentions, goals, memory, attention and decision processes, environmental context and resources, social influences, emotion and behavioral regulation. To effect change within organizations, there is a requirement for change in the behavior of individuals or systems. Changing behavior requires an understanding of the influences on behavior in the context in which they occur [35]. This framework has been applied across a range of healthcare systems to influence healthcare behaviors [35]. The following six domains have been used to categorize the extracted data for this systematic review:
-
1.
Knowledge
-
2.
Social and professional roles
-
3.
Bhavioral regulation
-
4.
Beliefs about consequences
-
5.
Environmental context and resources
-
6.
Social influences
Data synthesis
The data was synthesized through use the use of meta-aggregation, which involves aggregation of findings to generate a set of statements. This was achieved through assembling the findings and categorizing these findings based on similarity in meaning. These categories were then subjected to a synthesis to produce a set of findings.
Confidence in findings
We used the CONQUal approach to rate confidence in the synthesized findings. Based on the answers to the five Joanna Briggs Institute [36] questions we rated each paper as high, moderate, low or very low [37]. Initially each synthesized finding was ranked as high and was downgraded based on assessments of dependability and credibility.
Dependability [37] – the dependability score was based on whether the critical appraisal scores fulfilled the following five dependability domains: Congruity between the research methodology and the research question or objectives, the methods used to collect data and the representation and analysis of data. A statement locating the researcher culturally or theoretically. The influence of the researcher on the research, and vice versa.
All studies started with a ‘high’ ranking. No downgrading was performed when 4–5 of the domains were met. Downgrading by one level occurred when the included studies met 2–3 of the domains (from high to moderate). Downgrading by two levels occurred when only 0–1 of these domains were met in other words, from high to low.
Credibility [37] – assesses the ‘findings’. Credibility assesses the congruency between the author’s interpretation and the supporting data. Each finding extracted from the paper was evaluated with a level of credibility based on the following ranking scale:
Unequivocal (findings accompanied by an illustration that is beyond reasonable doubt and therefore not open to challenge)
Equivocal (findings accompanied by an illustration lacking clear association with it and therefore open to challenge)
Unsupported (findings are not supported by the data)
We quality scored each paper using the CASP rating and the five Joanna Briggs Institute questions for dependability. The credibility was rated through assessing whether each extracted finding was accompanied by an interpretation which was either illustrated by the findings, lacked clear association, or was not supported [37].
Results
Through our search strategy, 1669 unique citations were generated. After title and abstract review, 147 citations underwent full-text article review, independently by three reviewers (SQ, LC, RA), to assess their eligibility based on the pre-specified inclusion criteria. A total of 129 papers were excluded at this stage, common reasons for rejection included the paper reported survey data, only conference abstracts were available, it was not possible to extract data, the paper focused upon direct-to-consumer testing kits, the paper reported on other aspects of genetic testing, or the paper did not specifically relate to primary care PGx testing. A total of 18 studies, which met the inclusion criteria were included in the qualitative synthesis (Figure 1). At each stage any disagreement between the three researchers was discussed and consensus reached (SQ, LC and RA).
Figure 1. . Preferred reporting items for systematic reviews and meta-analyses flow diagram.
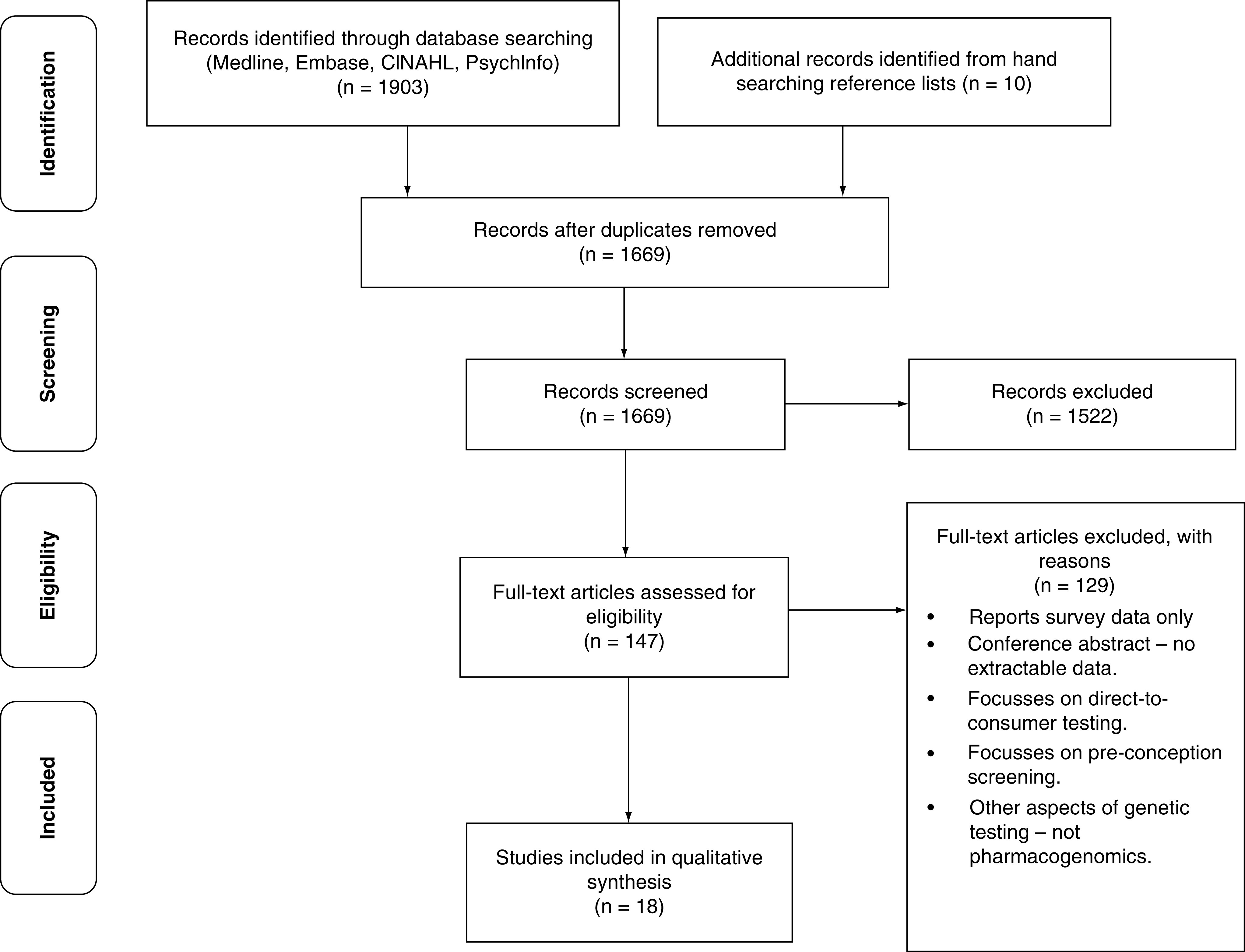
Pharmacogenomic testing.
Quality appraisal
The CASP quality appraisal scores for the 18 papers ranged from 14 to 19 out of 20, with six papers rated as high and 12 as medium (Supplementary Table 3). All studies included a clear statement of the aims of the qualitative studies and explicit statement of findings, including linking the results to the original research question.
Characteristics of included studies
Approximately 504 participants were included from the 18 studies; this is because two studies did not state a definitive sample size [7,14] with the latter study stating, “an average of 7 primary care clinicians included at the five sites.” We have taken this to be 35 participants for this study [7]. Of the reported participants the majority (270 [54%]) comprised primary care professionals (PCPs), with 186 (37%) patient’s participants and 48 (9%) pharmacists were included. Not all studies reported the male to female breakdown of participants; but from those reported, males comprised 139 and females 210. Ten studies originated from the USA, three from Canada, one UK, one Australia, two The Netherland and one was a multicenter study based in four European countries. Seven of the 18 included studies reported patients views on pharmacogenomic testing. Full characteristics of the included papers and samples are presented in Table 1.
Table 1. . Characteristics of included studies and Joanna Briggs Institute and Critical Appraisal Skills Program quality assessment.
| Citation | Context | Data collection method | Country | Participant’s characteristics | Sample size | Sample characteristics | Setting | JBI quality assessment | Overall score | CASP rating | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||||||
| Park et al. (2006) | Exploration of primary care physician’s attitudes about the strengths of and barriers to using genetic testing to match patients to optimal nicotine replacement therapy. | Focus groups | USA | Primary Care Physicians | 27 | 16 M & 11 F. Mean age 36 years (Range: 29–57 yrs) | Academic medical centers, Primary care academic faculty meetings | Y | Y | Y | N | N | Moderate | Medium | [38] |
| Dressler et al. (2019) | To reveal additional ‘real-life’ barriers to the implementation of PGx, that have not already been identified (cost, education and training were addressed in the study) | Phone interviews | USA | Primary Care Physicians | 4 PCPs = 4 | Not stated | Rural primary care practices | Y | Y | U | N | N | Moderate | Medium | [39] |
| Williams et al. (2016) | To assess primary care providers interest in using a genetic test to inform treatment of alcohol use disorder with pharmacotherapy at Veterans Health Administration clinics | Interviews | USA | Primary Care Clinicians with prescribing privileges | 24 MD = 19, Dr Osteopathy = 1, nurse practitioners = 4 | 11 M and 13 F. Mean age 48 years (range: 29–65 years) | 5 primary care clinics associated with large VA medical facility | Y | Y | Y | U | N | Moderate | High | [40] |
| Lemke et al. (2017) | To explore PCP views of the utility and delivery of direct access to PGx testing in a community health system. Direct access – provider-ordered PGx test, mailed to the pt and results returned by PCP by telephone, in-person, mail etc. | Interviews | USA | Primary Care Physicians | 15 PCPs = 15 | 6 M and 9 F. Mean age: not stated | NorthShore University Health System - four hospital community health system in north suburban Chicago | Y | Y | Y | Y | Y | High | High | [41] |
| Frigon et al. (2019) | To understand the perceptions of PCPs, pharmacists and pts on the implementation of PGx testing in clinical practice | Focus groups | Canada | Primary Care Physicians, pharmacists and patients | 64 PCP = 23, Pharm = 11 pts = 30 | PCPs: 6 M and 17 F Mean age: not asked Pharmacists: 2 M and 9 F Mean age: not asked Patients: 9 M and 21 F Mean age: 50.3 years (range 19–78 years) |
PCPs invited through regional department of general medicine. Community pharmacists invited through Regional Department of Pharmaceutical Services and convenience sampling and snowball recruitment strategies. Patients through same regions general population through convenience sampling following public announcements through social media and leaflets. |
Y | U | Y | N | N | Moderate | Medium | [42] |
| Carroll et al. (2016) | To assess primary care providers’ experiences with, perceptions of, and desired role in personalized medicine, with a focus on cancer. | Focus groups | Canada | Family practitioners, nurses, nurse practitioners, physician assistants, family medicine resident other | 51 PCPs = 51 | 11 M and 34 F. Mean age 44 years (range: 23–65 years) | Urban and rural interprofessional primary care team practices in Alberta and Ontario. | Y | Y | Y | N | N | Moderate | Medium | [43] |
| Chase et al. (2017) | Examines some of the barriers to clinical decision support system for precision medicine | Interviews | USA | Primary Care Clinicians | ≈35 | Not stated | 5 primary care sites varying between community clinics to large academic medical centers. | Y | Y | Y | Y | U | High | High | [44] |
| Unertl et al. (2015) | To describe the knowledge and attitudes of clinicians participating in a large PGx implementation program | Interviews | USA | Primary Care Providers Cardiologist | 15 PCP = 6, cardiologist = 9 | Not stated | Vanderbilt University Medical Center | Y | Y | Y | N | N | Moderate | Medium | [45] |
| Haga et al. (2012) | To explore each group’s attitudes about the use of PGx testing, potential for ancillary information, role of genetics experts and sharing of PGx information among healthcare professionals | Focus groups | USA | Primary Care Professionals Physician assistant, nurse practitioners, family medicine (MDs) Internists, medical geneticists, genetic counselors |
21 | 6 M and 15 F. Mean age: not recorded | Physicians from Duke-affiliated primary care clinics invited. Geneticists practicing at Duke uni medical center also invited. |
Y | Y | Y | N | N | Moderate | Medium | [46] |
| Harding et al. (2019) | The purpose of this study was to explore genetics in primary care from the perspective of both rural and urban PCPs | Interviews Focus groups | Canada | Primary Care Providers | 29 n = 10 interview n = 19 focus group Interview group: - healthcare administrator = 1, clinical geneticist = 1, nurse practitioner = 1, public health administrator = 1, genetic counselors = 2, and PCPs = 4 Focus group: -urban PCPs = 5 rural PCPs = 14 |
Interview group – not stated Focus group - age range 30–60 years | Rural and urban Canadian PCP | Y | Y | Y | N | N | Moderate | Medium | [47] |
| Lee et al. (2017) | To explore the attitudes and perceptions of pharmacogenomics among genotyped patients actively participating in an institutional pharmacogenomic implementation project, compared with that of a control group receiving traditional care | Focus groups | USA | Patients | 22 | 11 M and 11 F. Mean age 59.5 years (range: 40–77 years) | Institutional pharmacogenomic implementation study | Y | Y | Y | N | N | Moderate | High | [48] |
| Rafi et al. (2020) | To identify potential barriers, challenges and opportunities to implementation of PGx into UK General Practice | Interviews | UK | GPs and nonmedic | 18 GPs = 16 scientific curator = 1 Public health med researcher = 1 |
M and F – not stated GP modal age 50–59 years Scientific curator age 30–39 years Public health Med researcher age 50–59 years |
Primary care | Y | U | Y | N | N | Moderate | medium | [49] |
| Rigter et al. (2020) | To define actions, roles and responsibilities for implementation of pharmacogenetics by conducting a multi-phased stakeholder study. Stakeholders such as pharmacists, primary care physicians, patients, scientists and policy makers were invited to discuss thresholds and opportunities for next steps in the implementation of pharmacogenetics in primary care in the Netherlands. Mixed method | Reporting only Focus groups Interviews | The Netherlands | GPs Pharmacists Patients | 49 GP = 8, Pharmacist = 22 Pts = 19 | 28 M and 21 F. Mean age 46.7 years (range: 17–68 years) | Primary care – comprising an urban environment, a rural environment and a ‘mixed’ region. | Y | U | Y | Y | N | Moderate | Medium | [50] |
| Barr (2008) | To explore the range of factors that may impinge upon public and service user acceptability of the pharmacogenomics of antidepressants | Focus groups | Multi-centered UK, Poland, Denmark Germany | Patients | Not stated | Not stated | General public and mental health service users from four European sites | Y | Y | Y | Y | Y | High | Medium | [51] |
| Issa et al. (2009) | Examining patients’ understanding and knowledge of personalized medicine and the process of decision-making regarding pharmacogenomics and targeted therapeutics and how patients value receiving pharmacogenomics-based personalized healthcare relative to the standard models of diagnosing and prescribing treatments. | Focus groups | USA | Patients | 32 | 17 M and 15 F. Mean age: not stated (range: 25–64 years) | Outpatient clinics at The Methodist Hospital | Y | U | Y | N | N | Moderate | Medium | [52] |
| De Marco et al. (2010) | Views on Personalized Medicine: do the attitudes of African American and white prescription drug consumers differ? | Focus groups | USA | Patients | 48 | 2 clinics and a family practice center at a large public medical center in a central North Carolina city | Y | Y | U | Y | N | Moderate | Medium | [53] | |
| Haddy et al. (2010) | To investigate the current opinions and experiences of consumers with regard to medication use and side effects. It also explored what they understood by the term ‘Personalized Medicine’ and whether they had any concerns regarding the use of genetics to determine medication selection. Consumers’ opinions on the storage of medical and genetic information were also investigated. | Focus groups | Australia | Patients | 35 | 9 M and 26 F. Mean age: not stated (range: 18–>60 years) | Members of the general public | Y | Y | Y | Y | N | High | High | [54] |
| Van Der Wouden et al. (2020) | The primary aim was to identify pharmacists perceived remaining barriers preventing and enablers facilitating implementation of pharmacist initiated PGx in primary care. | Interviews | The Netherlands | Pharmacists (involved in PREPARE study) | 15 | 7M and 8 F Mean age 38.5 (Range: 25–59) | Community pharmacy | Y | Y | Y | N | U | Moderate | High | [55] |
ConQual criteria for assessing confidence. The following five questions to confirm the dependability of the results.
1. Is there congruity between the research methodology and the research question or objectives?
2. Is there congruity between the research methodology and the methods used to collect data?
3. Is there congruity between the research methodology and the representation and analysis of data?
4. Is there a statement locating the researcher culturally or theoretically?
5. Is the influence of the researcher on the research, and vice versa, addressed?
The letter denotes the ratings for each study (N = No, U = Unclear, Y = Yes).
CASP: Critical Appraisal Skills Program; JBI: Joanna Briggs Institute; M: Male; F:Female; GP: General Practitioner; PCP: Primary care professional; PGx: Pharmacogenomics.
Data taken from [37].
Synthesized findings
We used the TDF to organize the barriers and enablers through an iterative process. All qualitative data from the 18 papers were initially extracted and categorized into barriers and enablers (Supplementary Table 4). The data was then coded into six theoretical domains of the TDF: knowledge; social and professional roles; behavioral regulation; beliefs and consequences; environmental context and resources and social influences. Table 2 shows the extracted barriers (B) and enablers (E) categorized as per the selected TDF domains. The extracted findings from each category were then analyzed by two researchers (SQ and AL) to produce 15 synthesized findings.
Table 2. . Categories and findings including barriers and enablers across the included papers.
| Category | Finding | Ref. |
|---|---|---|
| Knowledge (an awareness of the existence of something) | ||
| Lack of genetic knowledge (B) | Lack of knowledge and awareness | [39,42,43,47,49,50] |
| HCP PGx knowledge and awareness | [55] | |
| Profound lack of knowledge of direct-to-consumer genetic tests | [43] | |
| Limited experience with PGx (B) | Personal unfamiliarity with genomic medicine | [44] |
| Limited encounters with genetics in practice | [47] | |
| Limited experience with personalized medicine | [43] | |
| Level of comfort with genetic testing | [47] | |
| Varying level of knowledge | [44,45] | |
| Preparation and knowledge | [45] | |
| Training and education (B) | Lack of genomic education | [42] |
| PGx/Genetic education | [41,47,49] | |
| Education | [47] | |
| Resources/support | [47] | |
| Rapidly changing PGx knowledge and need for continuing education | [45] | |
| Patient and provider education material | [41] | |
| Patient education material – for frequent Q&As | [45] | |
| Policies for responsibilities and ownership of PGx data | [45] | |
| Patients lack of genetic knowledge (B) | Unfamiliar with term PGx | [52] |
| General interest in PGx testing (E) | Greater role for genetics | [47] |
| Shifting patterns of work to allow new advances | [47] | |
| General interest in PGx testing | [48] | |
| Potential of using PGx | [49] | |
| Positive attitude toward PGx | [51] | |
| PGx test results rapidly obtained to be valuable | [42] | |
| Perceived role in delivering PGx | [55] | |
| Social and professional roles (a coherent set of behaviors and displayed personal qualities of an individual in a social or work setting) | ||
|---|---|---|
| Skill mix (B) | More access for pharmacists (and other HCP) to genetic information | [54] |
| Pharmacists to have major role in PGx | [42] | |
| Division of responsibility | [50] | |
| PCP’s role in personalized medicine | [43] | |
| PCP role – education, counselling, testing and referrals to specialists | [47] | |
| Pool of experts (B) | Pool of experts in general practice | [49] |
| Need for buddy or connection into a genetic service | [43] | |
| Professional relationships (E) | Relationship with healthcare professional | [48] |
| High regard for physicians who adopted pharmacogenomics | [48] | |
| Relationship with healthcare professional | [48] | |
| Opportunities for pharmacists | [50] | |
| Patient–doctor relationship | [39,43] | |
| Acting upon PGx and reporting to patients | [55] | |
| Pharmacist added value and learning by doing | [55] | |
| Professional interaction improvement | [55] | |
| Behavioral regulation (anything aimed at managing or changing objectively observed or measured actions) | ||
|---|---|---|
| Negative impact of PGx (B) | Adverse impact resulting from negative results | [40] |
| Repercussions of positive test result – labeled, stigmatized, develop fatalistic perceptions | [38] | |
| Anxiety about genetic information | [42] | |
| Ambivalence – depression and genetic research (targeted PGx research and meds designed to treat) | [51] | |
| Impact on patient perspectives and shared decision-making | [49] | |
| Patient views (B) | Consumer demand | [52] |
| Conflation of disease risk and drug reaction | [51] | |
| Concerns when starting a new medication | [48] | |
| Therapeutic benefit | [48] | |
| Behavioral change (B) | Patients use a positive test result as rationalization for giving up | [38] |
| Managing results expectations | [41] | |
| Reluctant to change current practice | [50] | |
| Reliance on genetic testing (B) | Reliance on genetic test rather than patient history | [38] |
| Undermining the importance of psychological and behavioral determinants of both smoking/quitting | [38] | |
| Incentive to use medicines instead of conversation therapy | [51] | |
| Medical mistrust (B) | Medical mistrust by marginalized population (pt. view) | [53] |
| Beliefs about consequences (acceptance of the truth, reality or validity about outcomes of a behavior in a given situation) | ||
|---|---|---|
| Reduces adverse drug reactions (E) | Avoid adverse drug reactions | [41] |
| Reduce side effects | [41] | |
| Improve compliance through less side effects | [41] | |
| Reduction of adverse events | [42] | |
| Concept of individualized medicine | [44] | |
| Adverse effects | [46] | |
| Tolerate adverse effects | [48] | |
| Value of PGx testing in primary care | [49] | |
| Reduce adverse effects | [51] | |
| Reduction of adverse drug effects | [52] | |
| Reduces adverse effects | [53] | |
| Pharmacotherapy improvement | [55] | |
| Reduces trial and error (E) | Aid in therapeutic choice | [40] |
| Increase patient’s confidence in their care | [40] | |
| Reduce trial and error | [53] | |
| Improved effectiveness | [52] | |
| Patient benefit (E) | Patient motivation | [38,40] |
| Benefit patients who had exhausted other treatment options | [38] | |
| Improve patient adherence to treatment | [40] | |
| Relieve patients of personal blame | [38] | |
| Use as preventative tool through raising patient awareness | [38] | |
| Create a placebo effect for patients | [40] | |
| Quick access to results, cost-effective options | [38] | |
| Implications for future medication management | [39] | |
| Competitive edge | [39] | |
| Environmental context and resources (any circumstance of a person’s situation or environment that discourages or encourages the development of skills and abilities, independence, social competence and adaptive behavior) | ||
|---|---|---|
| EHR implementation (B) | Priority for EHR implementation | [44] |
| Clinical decision support in EMR | [39] | |
| Workflow issues (B) | Translating results into clinical decisions | [45] |
| PGx integrated into EMR – integrating electronic alerts | [42] | |
| Workflow issues for CDS, unwilling to have interruptions on their workflow | [44] | |
| Reporting results (B) | Clearer layout | [41] |
| Information overload | [45] | |
| Electronic capture of genomic information | [49] | |
| Ordering/interpreting tests (B) | Ordering and interpreting tests | [38,45] |
| Ability to understand and explain PGx test results | [41,45,46] | |
| Specific training to report PGx results | [41] | |
| Interpreting genetic information | [38] | |
| Unclear procedures outside of the study | [55] | |
| Cost concerns (B) | Cost of PGx testing | [39–42,44,45,47,48,50,52,53] |
| Cost–effectiveness | [47,49] | |
| Who pays? | [44,45,52,54] | |
| Insurance coverage | [41,42] | |
| Insurance loading (paying extra premiums based on personal medical data) | [49] | |
| Insurability and costs | [52] | |
| Undetermined reimbursement for test and consult | [55] | |
| Limitations (B) | Limitations/implications of genetic testing | [47] |
| Concerns about consenting to PGx test | [48] | |
| Population level benefits limited by reducing target population | [40] | |
| Ancillary findings (B) | Dealing with ancillary findings | [46,48] |
| Technical issues (B) | Restricted time constraints | [38,41,46] |
| Accessibility of PGx test results/easily accessible personalized med tools | [43,46] | |
| Flexible testing options | [41] | |
| Turnaround times | [40,41] | |
| When and whom to test? | [50] | |
| Access to testing (pt. view) | [52] | |
| Pre-emptive vs reactive | [39] | |
| Pre-emptive | [50] | |
| Technical issues | [41] | |
| Clinical utility (B) | Lack of evidence – clinical utility | [50,55] |
| Need for evidence | [44] | |
| Utility dependent on prognostic accuracy | [40] | |
| No incremental utility over standard care | [40] | |
| Clinical utility of tests | [40,52] | |
| Accuracy of the test | [48] | |
| Guideline development/accessibility (B) | Accessible PGx guideline | [42] |
| Lack of genetic referral guidelines | [43] | |
| Guidance document | [39] | |
| Infrastructure inefficiencies (guideline factors, incentives and resources) | [55] | |
| Decision-making (E) | Another aspect of clinical decision making | [40] |
| Guiding primary care medical decision-making | [41] | |
| Individualize medication treatments | [41] | |
| Informed decision making | [41] | |
| Efficient decision making | [41] | |
| Increased patient autonomy | [41] | |
| Follow-up | [55] | |
| Less fear and anxiety about trying a new medication | [41] | |
| Valuable tool in the future | [41] | |
| Social influences (those interpersonal processes that can cause individuals to change their thoughts, feelings or behaviors) | ||
|---|---|---|
| Employment discrimination (B) | Genetic information not shared with employers | [54] |
| Insurance, employment discrimination | [53] | |
| Genetic discrimination and confidentiality | [38] | |
| Health insurance, employment discrimination and stigma | [38] | |
| Confidentiality/privacy of data (B) | Information stored in a confidential manner | [42,54] |
| Storage and future use of information | [52] | |
| Disclosure, privacy and confidentiality | [52] | |
| Data and privacy concerns | [41] | |
| Privacy and personal pharmacogenomic information (pt. view) | [48] | |
| Data ownership responsibility and liability | [45] | |
| Abuse of test results (B) | Test information not used in a harmful manner to patients | [38] |
| Use of information over time | [39] | |
| Social inequalities (B) | Social inequalities | [42] |
B: Barrier; E: Enabler; EHR: Electronic health record; HCP: Healthcare professional; PCP: Primary care professional; PGx: Pharmacogenomics.
The summary of the extracted findings, categories and credibility ratings are included in Supplementary Table 5. A summary of the synthesized findings is presented in Table 3- CONQUal summary of findings.
Table 3. . CONQUal summary of findings.
| Synthesized findings | Type of research | Dependability | Credibility | CONQual score | |
|---|---|---|---|---|---|
| Knowledge | If pharmacogenomic knowledge, awareness and engagement of primary care professionals can be improved through effective undergraduate and postgraduate education programs and if patients are appropriately engaged with the process, then primary care pharmacogenomic testing uptake is likely to increase. | Qualitative | Moderate | Equivocal | Moderate |
| Social and professional roles | Better engagement with pharmacogenomics testing can be achieved if there is a clear division of responsibility between Primary Care Professionals – GPs making the diagnosis and the pharmacist choosing the appropriate drug through use of pharmacogenomics testing. If a more comprehensive model for GP–pharmacist responsibility and engagement with patients is developed, then this could improve the uptake of pharmacogenomics testing in primary care. Furthermore, the process can be facilitated by a committee of local pharmacogenomics experts to guide decision making. |
Qualitative | Moderate | Equivocal | Moderate |
| Behavioral regulation | If learning about the potential benefits and limitations of pharmacogenomics testing is made clearer for Primary Care Professionals and if patient expectations are managed effectively, then the benefits of pharmacogenomics testing in primary care can be more easily realized. | Qualitative | Moderate | Equivocal | Moderate |
| If there is greater awareness and understanding between Primary Care Professionals that pharmacogenomics testing is complimentary rather than a substitute for current clinical decision-making, then this will increase their confidence and competence in using pharmacogenomics testing in primary care. | Qualitative | Moderate | Unequivocal | Moderate | |
| Beliefs about consequences | If patients and Primary Care Professionals recognized the potential for pharmacogenomics testing to reduce adverse drug reactions and trial-and-error aspect of prescribing, then this will facilitate the uptake of pharmacogenomics testing in primary care. | Qualitative | Moderate | Equivocal | Moderate |
| If the perceived benefit for the use of pharmacogenomics among primary care patients is promoted, then this will facilitate the uptake of pharmacogenomics testing in primacy care. | Qualitative | Moderate | Equivocal | Moderate | |
| Environmental context and resources | If pharmacogenomics results can be implemented and incorporated into the normal workflow patterns at the point of prescribing and if results are easily comprehensible, then uptake by Primary Care Professionals in primary care can be improved. | Qualitative | Moderate | Equivocal | Moderate |
| If policy makers and commissioners invest in cost-effective models for pharmacogenomics testing (where the benefit of testing outweighs the cost of the test), then this will help minimize inequities between low and high socio-economic patient groups and facilitate the uptake of pharmacogenomics testing in primacy care. | Qualitative | Moderate | Equivocal | Moderate | |
| If pharmacogenomics test results, which offer valid and reliable information are used to aid decision-making during prescribing, then this will facilitate the uptake of pharmacogenomics testing in primary care. | Qualitative | Moderate | Unequivocal | Moderate | |
| If the infrastructure around pharmacogenomics testing is strengthened such that results can be accessed in a timely manner and a prompt alerts the clinician to the availability of the results, then the uptake of pharmacogenomics in primacy care can be increased. | Qualitative | Moderate | Unequivocal | Moderate | |
| If more specific guidance is produced for Primary Care Professionals, highlighting when pharmacogenomics testing is appropriate. For example, if guidelines were produced by national bodies such as NICE or MHRA, which build upon international guidance produced by CPIC or DPWG, then Primary Care Professionals would be reassured of the evidence base for pharmacogenomics recommendations, and this could lead to increase uptake of pharmacogenomics testing in primacy care. | Qualitative | Moderate | Equivocal | Moderate | |
| If Primary Care Professionals have successfully used pharmacogenomics or have personal experience of undertaking a pharmacogenomics test, then they are more likely to use pharmacogenomics testing for their patients in the future. | Qualitative | Moderate | Equivocal | Moderate | |
| Social influences | One of the benefits of genetic information collated for pharmacogenomics testing is related to whether a person will be susceptible to adverse drug reactions or not. Reassurance that genetic information is kept confidential, and this would not adversely affect a person’s employment or insurance rights, then the patients are going to be more receptive to pharmacogenomics testing. |
Qualitative | Moderate | Equivocal | Moderate |
GP: General practitioner; PGx: Pharmacogenomics.
TDF Domain 1: Knowledge
Twenty-five findings were categorized in the knowledge domain that informed five categories (Supplementary Table 5). These five categories include lack of genetic knowledge, limited experience with PGx, education and training, patient’s lack of genetic knowledge and general interest in PGx testing. These five categories informed synthesized finding 1.
Synthesized finding 1 mapped to the knowledge domain.
If pharmacogenomic knowledge, awareness and engagement of primary care professionals can be improved through effective undergraduate and postgraduate education programs and if patients are appropriately engaged with the process, then primary care pharmacogenomic testing uptake is likely to increase.
TDF Domain 2: social & professional roles
Fifteen findings were categorized in the social and professional roles domain that informed three categories (Supplementary Table 5). These include skill mix, pool of experts and professional relationships. These three categories informed synthesized findings 2 and 3.
Synthesized findings 2 and 3 regarding professional role and responsibilities, mapped to social and professional roles domain.
Better engagement with pharmacogenomics testing can be achieved if there is a clear division of responsibility between Primary Care Professionals – one of the potential ways forward could be GPs making the diagnosis and the pharmacist choosing the appropriate drug through use of pharmacogenomics testing. This finding is presented as one possible solution for professional roles and responsibilities.
If a more comprehensive model for GP-pharmacist responsibility and engagement with patients is developed, then this could improve the uptake of pharmacogenomics testing in primary care. Furthermore, the process can be facilitated by a committee of local pharmacogenomics experts to guide decision making.
TDF Domain 3: behavioral regulation
Sixteen findings were categorized in the behavioral regulation domain that informed five categories (Supplementary Table 5). These include negative impact of PGx, patient views, behavioral change, reliance on genetic testing and medical mistrust. These five categories informed synthesized findings 4 and 5.
Synthesized findings 4 and 5 regarding ‘countering negative concerns about pharmacogenomics’, mapped to behavioral regulation domain.
If learning about the potential benefits and limitations of pharmacogenomics testing is made clearer for Primary Care Professionals and if patient expectations are managed effectively, then the benefits of pharmacogenomics testing in primary care can be more easily realized.
If there is greater awareness and understanding between Primary Care Professionals that pharmacogenomics testing is complimentary rather than a substitute for current clinical decision-making, then this will increase their confidence and competence in using pharmacogenomics testing in primary care.
TDF Domain 4: beliefs about consequences
Twenty-five findings were categorized in the beliefs about consequences domain that informed three categories (Supplementary Table 5). These include reduces adverse drug reactions and reduces trial and error. These two categories informed synthesized finding 6.
Synthesized finding 6 and 7 regarding positive impact of pharmacogenomics mapped to ‘beliefs about consequences’ domain.
If patients and Primary Care Professionals recognized the potential for pharmacogenomics testing to reduce adverse drug reactions and trial-and-error aspect of prescribing, then this will facilitate the uptake of pharmacogenomics testing in primary care.
One of the benefits of genetic information collated for pharmacogenomics testing is related to whether a person will be susceptible to adverse drug reactions or not.
TDF Domain 5: environmental context & resources
Fifty-two findings were categorized in the environmental context and resources domain that informed eleven categories (Supplementary Table 5). These include electronic health record implementation, workflow issues, reporting results, ordering/ interpreting tests, cost concerns, limitations, ancillary findings, technical issues, clinical utility, guideline development/accessibility and decision making. These eleven categories informed synthesized finding 7, 8, 9, 10, 11, 12 and 13.
Synthesized findings 8, 9, 10, 11, 12 and 13 regarding the practical implementation of pharmacogenomics mapped to environmental context and resources domain.
If pharmacogenomics results can be implemented and incorporated into the normal workflow patterns at the point of prescribing and if results are easily comprehensible, then uptake by Primary Care Professionals in primary care can be improved.
If policy makers and commissioners invest in cost-effective models for pharmacogenomics testing (where the benefit of testing outweighs the cost of the test), then this will help minimize inequities between low and high socio-economic patient groups and facilitate the uptake of pharmacogenomics testing in primacy care.
If pharmacogenomics test results, which offer valid and reliable information are used to aid decision-making during prescribing, then this will facilitate the uptake of pharmacogenomics testing in primary care.
If the infrastructure around pharmacogenomics testing is strengthened such that results can be accessed in a timely manner and a prompt alerts the clinician to the availability of the results, then the uptake of pharmacogenomics in primacy care can be increased.
If more specific guidance is produced for Primary Care Professionals, highlighting when pharmacogenomics testing is appropriate. For example, if guidelines were produced by national bodies such as NICE or MHRA, which build upon international guidance produced by CPIC or DPWG, then Primary Care Professionals would be reassured of the evidence base for pharmacogenomics recommendations, and this could lead to increase uptake of pharmacogenomics testing in primacy care.
If Primary Care Professionals have successfully used pharmacogenomics or have personal experience of undertaking a pharmacogenomics test, then they are more likely to use pharmacogenomics testing for their patients in the future.
TDF Domain 6: social influences
Thirteen findings were categorized in the social influence’s domain that informed four categories (Supplementary Table 5). These include employment discrimination, confidentiality/privacy of data, abuse of test results and social inequities. These four categories informed synthesized finding 14 and 15.
Synthesized findings 14 and 15 regarding ethico-legal implications of pharmacogenomics mapped to the social influence’s domain.
Reassurance that genetic information is kept confidential, and this would not adversely affect a person's employment or insurance rights, then the patients are going to be more receptive to pharmacogenomics testing.
If the perceived benefit for the use of pharmacogenomics among primary care patients is promoted, then this will facilitate the uptake of pharmacogenomics testing in primacy care.
Discussion
This study utilizes synthesized findings from qualitative PGx primary care studies. Our systemic review produced fifteen synthesized statements from 18 papers, assimilating the barriers and enablers to the implementation of PGx testing in primary care to formulate actionable policy recommendations. These include the use of effective educational strategies to raise the knowledge, awareness and engagement of PCPs in PGx testing. Further, educational strategies should explain the benefits of using PGx testing – including reduction of adverse drug reactions, reduction of the trial-and-error model of prescribing and the knowledge that PGx testing is complimentary rather than a substitute for current decision-making in the prescribing process. Development of a comprehensive model with a division of responsibility between the GP and the pharmacist, supplemented by a local pool of experts is seen as a facilitator. Furthermroe, important elements for the uptake of PGx testing included the cost of the test, so as not to disadvantage lower socio-economic groups. Furthermore, implementation will be enhanced by confidence in the evidence base for PGx clinical guidelines. Finally, the infrastructure supporting results turnaround times is an important element to incorporate PGx test results into their normal workflow patterns, with results presented in an easily understandable format. The barriers and enablers highlighted in this study have been stated in previous studies [14,23,56]. However, it is poignant to note that despite the advances in pharmacogenomic technology, the same barriers (e.g., knowledge gap) are still pivotal to preventing PGx uptake.
This study has important implications for policymakers and healthcare professionals when considering what actions are needed for the implementation of PGx testing in primary care. This study also has important implications for primary care patients, which include raising their knowledge and awareness of testing through engagement.
Strengths & limitations
The main strength of this systematic review and meta-aggregation are the clear evidence-based statements which have been synthesized to inform policy and practice. Furthermore, all 18 papers were either classed as moderate or high as per the CASP quality rating.
A limitation of this review is that most included studies were from USA, where the healthcare system operates under an insurance-based system; therefore, these findings may not be transferable to other healthcare setting. Three studies in the review were from the perspective of a nationally funded healthcare system, one from the UK and two from The Netherlands. Although both Dutch and UK have nationally funded healthcare systems, PGx has been integrated into The Netherlands in primary care, while PGx strategies are still emerging in UK, and so careful consideration of the results would be needed for countries which have emerging PGx strategies. Furthermore, a limitation was no data from low- or middle-income countries was included in this review, therefore transferability to such setting may not be applicable. Also, none of the included studies utilized ethnography as a method of qualitative data collection.
Future research should include more qualitative studies from low- and middle- income countries and utilize ethnography methodology in developed primary care systems, thereby producing rich qualitative data. A further area for research should include the development of appropriate CDSS that facilitates use of PGx information at the point of prescribing and, ideally, integrating this information with other factors routinely considered in the prescribing decision-making process, such as co-medications and comorbidities. In the UK at least, the widespread and early adoption of electronic medical patient records in primary care, which are provided by only a handful of service providers (e.g., EMIS and SystemOne), provide a pre-existing technological infrastructure to build pharmacogenomic CDSS into.
Conclusion
This qualitative systematic review presents fifteen synthesized statements for policymakers with valuable recommendations that need addressing prior to implementation of PGx in primary care.
In this review we have identified a pathway for the implementation of PGx in primary care. In preparation, policymakers need to consider the introduction of effective educational strategies for both PCPs and patients to raise knowledge, awareness, and engagement. The actual introduction of PGx will require reorganization with decision support tools to aid use of PGx in primary care, with a clear delegation of roles and responsibilities between PGs and pharmacists supplemented by a local pool of experts. Furthermore, policy makers need to address the cost–effectiveness of PGx and having appropriate infrastructure supporting testing and interpretation including IT solutions for utilizing pharmacogenomic results.
Future perspective
One of the biggest challenges will involve training the current and next generation of healthcare professionals, with a particular focus of dissemination of knowledge that will be compatible with work-life balance.
Pharmacogenomic testing will be an additional test ordered ahead of prescribing (similar to renal and liver function tests) and Pharmacogenomic experts including GPs and Pharmacists will be integrated in primary care teams in the future.
Useable clinical decision support tools which easily convey the pharmacogenomic results for the clinician will be an essential element for implementing.
Summary points.
Policymakers need to consider the introduction of effective educational strategies for both primary care professionals (PCPs) and patients to raise knowledge, awareness and engagement.
The potential benefits of pharmacogenomic testing through a reduction in adverse drug reactions and reducing the trial-and-error aspect of prescribing should be raised among PCPs and patients.
Implementation of pharmacogenomics will require the reorganization of clinical decision support tools to aid the use of pharmacogenomics in primary care.
Policy makers need to address the cost effectiveness of pharmacogenomics and having appropriate infrastructure supporting testing and interpretation including informatic solutions for utilizing pharmacogenomic results.
Policymakers need to consider PCPs confidence in the evidence base for the clinical utility of pharmacogenomics tests particularly if national bodies such NICE or MHRA are involved in guideline production.
A clear delegation of roles and responsibilities between general practitioners and pharmacists responsible for pharmacogenomic testing, supplemented by a local pool of pharmacogenomics experts to aid implementation.
Supplementary Material
Acknowledgments
The authors thank Nia Roberts, Information Specialist with the University of Oxford, for her tremendous support and guidance in developing the search strategies for the various databases and J Kai for comments on final version.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/pgs-2021-0131
Financial & competing interests disclosure
This study is funded by the National Institute for Health Research (NIHR) School for Primary Care Research. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Caudle KT, Klein TE, Hoffman JM et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline Development Process. Curr. Drug Metab. 15(2), 209–217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cecchin E, Roncato R, Guchelaar HJ, Toffoli G. Ubiquitous Pharmacogenomics Consortium. Ubiquitous Pharmacogenomics (U-PGx): the time for implementation is now. An Horizon2020 Program to drive pharmacogenomics into clinical practice. Curr. Pharm. Biotechnol. 18(3), 204–209 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Rollinson V, Turner R, Pirmohamed M. Pharmacogenomics for primary care: an overview. Genes 11, 1337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawes M. Pharmacogenetics in primary care. Healthcare Manag. Forum 33(3), 97–101 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Verbelen M, Weale M, Lewis C. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J. 17, 395–402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suppiah V, Lim CX, Hotham E. Community pharmacists and their role in pharmacogenomics testing: an Australian perspective drawing on international evidence. Aust. J. Primary Health 24(6), 441–447 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Ewbank L, Omojomolo D, Sullivan K, McKenna H. The rising cost of medicines to the NHS. What's the story? The King’s Fund Briefing (2018). https://www.kingsfund.org.uk/sites/default/files/2018-04/Rising-cost-of-medicines.pdf [Google Scholar]
- 8.Bank PCD, Swen JJ, Schaap RD et al. A pilot study of the implementation of pharmacogenomic pharmacist initiated pre-emptive testing in primary care. Eur. J. Hum. Genet. 27, 1532–1541 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grice GR, Seaton TL, Woodland AM, McLeod HL. Defining the opportunity for pharmacogenetic intervention in primary care. Pharmacogenomics 7(1), 61–65 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Volpi S, Bult CJ, Chisholm RL et al. Research directions in the clinical implementation of pharmacogenomics: an overview of US programs and projects. Clin. Pharmacol. Ther. 103(5), 778–786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JA. Pharmacogenetics in clinical practice: how far have we come and where are we going? Pharmacogenomics 14(7), 835–843 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein ME, Parvez MM, Shin JG. Clinical implementation of pharmacogenomics for personalized precision medicine: barriers and solutions. J. Pharm. Sci. 106(9), 2368–2379 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Wang YT, Merl MY, Yang J et al. Opportunities for pharmacists to integrate pharmacogenomics into clinical practice. Pharmacogenomics J. 20, 169–178 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Carroll JC, Allanson J, Morrison S et al. Informing integration of genomic medicine into primary care: an assessment of current practice, attitudes, and desired resources. Front. Genet. 10, 1189 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Wouden CH, Cambon-Thomsen A, Cecchin E et al. Ubiquitous Pharmacogenomics Consortium. Implementing pharmacogenomics in Europe: design and implementation strategy of the Ubiquitous Pharmacogenomics Consortium. Clin. Pharmacol. Ther. 101(3), 341–358 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Hippman C, Nislow C. Pharmacogenomic testing: clinical evidence and implementation challenges. J. Pers. Med. 9(3), 40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deverka PA, Doksum T, Carlson RJ. Integrating molecular medicine into the US health-care system: opportunities, barriers, and policy challenges. Clin. Pharmacol. Ther. 82(4), 427–434 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Haga SB. Integrating pharmacogenetic testing into primary care. Expert Rev. Precis. Med. Drug Dev. 2(6), 327–336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature 526(7573), 343–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbasi J. Getting pharmacogenomics into the clinic. JAMA 316(15), 1533–1535 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Bell GC, Crews KR, Wilkinson MR et al. Development and use of active clinical decision support for preemptive pharmacogenomics. J. Am. Med. Inform. Assoc. 21(e1), e93–e99 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hicks JK, Stowe D, Willner MA et al. Implementation of clinical pharmacogenomics within a large health system: from electronic health record decision support to consultation services. Pharmacotherapy 36(8), 940–948 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Borden BA, Galecki P, Wellmann R et al. Assessment of provider-perceived barriers to clinical use of pharmacogenomics during participation in an institutional implementation study. Pharmacogenet. Genomics 29(2), 31–38 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Ellingrod VL, Moline J. Incorporating pharmacogenomics into practice. J. Pharm. Pract. 20(3), 277–282 (2007). [Google Scholar]
- 25.Petit C, Croisetière A, Chen F, Laverdière I. Are pharmacists from the province of Quebec ready to integrate pharmacogenetics into their practice. Pharmacogenomics 21(4), 247–256 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Yau A, Husain RB, Haque M. Systematic review of knowledge, attitude and practice towards pharmacogenomics among doctors. Int. J. Pharmaceut. Res. 7(1), Jan–Mar (2015). [Google Scholar]
- 27.O'Brien TJ, LeLacheur S, Ward C, Lee NH, Callier S, Harralson AF. Impact of a personal CYP2D6 testing workshop on physician assistant student attitudes toward pharmacogenetics. Pharmacogenomics 17(4), 341–352 (2016) [DOI] [PubMed] [Google Scholar]
- 28.Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers' perceived barriers to integration of genetics services: a systematic review of the literature. Genet. Med. 17(3), 169–176 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Suther S, Goodson P. Barriers to the provision of genetic services by primary care physicians: a systematic review of the literature. Genet Med. 5(2), 70–76 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Hayward J, McDermott J, Qureshi N, Newman W. Pharmacogenomic testing to support prescribing in primary care: a structured review of implementation models. Pharmacogenomics 22(12), 761–776 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yau A, Aziz ABA, Haque M. Knowledge, attitude and practice concerning pharmacogenomics among pharmacists: a systematic review. J. Young Pharmacists 7(3), Jul–Sep (2015). [Google Scholar]
- 32.Tong A, Flemming K, McInnes E et al. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med. Res. Methodol. 12, 181 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Critical appraisal skills programme. CASP (Qualitative) Checklist (2018). http://casp-uk.net [Google Scholar]
- 34.Cane J, O'Connor D, Michie S. Validation of the theoretical domains framework for use in behavior change and implementation research. Implementation Sci. 7, 37 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins L, Francis J, Islam R et al. A guide to using the Theoretical Domains Framework of behavior change to investigate implementation problems. Implementation Sci. 12, 77 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joanna Briggs Institute. Critical appraisal tools. https://jbi.global/critical-appraisal-tools
- 37.Munn Z, Porritt K, Lockwood C, Aromataris E, Pearson A. Establishing confidence in the output of qualitative research synthesis: the ConQual approach. BMC Med. Res. Methodol. 14, 108 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park ER, Kleimann S, Pelan JA, Shields AE. Anticipating clinical integration of genetically tailored tobacco dependence treatment: perspectives of primary care physicians. Nicotine Tob. Res. 9(2), 271–279 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Dressler LG, Bell GC, Abernathy PM, Ruch K, Denslow S. Implementing pharmacogenetic testing in rural primary care practices: a pilot feasibility study. Pharmacogenomics 20(6), 433–446 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Williams EC, Young JP, Achtmeyer CE, Hendershot CS. Primary care providers' interest in using a genetic test to guide alcohol use disorder treatment. J. Substance Abuse Treat. 70, 14–20 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Lemke AA, Hutten Selkirk CG, Glaser NS et al. Primary care physician experiences with integrated pharmacogenomic testing in a community health system. Per. Med. 14(5), 389–400 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Frigon MP, Blackburn MÈ, Dubois-Bouchard C, Gagnon AL, Tardif S, Tremblay K. Pharmacogenetic testing in primary care practice: opinions of physicians, pharmacists and patients. Pharmacogenomics 20(8), 589–598 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Carroll JC, Makuwaza T, Manca DP et al. Primary care providers' experiences with and perceptions of personalized genomic medicine. Can. Fam. Physician 62(10), e626–e635 (2016). [PMC free article] [PubMed] [Google Scholar]
- 44.Chase DA, Baron S, Ash JS. Clinical decision support and primary care acceptance of genomic medicine. Stud. Health Technol. Inform. 245, 700–703 (2017). [PubMed] [Google Scholar]
- 45.Unertl KM, Jaffa H, Field JR, Price L, Peterson JF. Clinician perspectives on using pharmacogenomics in clinical practice. Per. Med. 12(4), 339–347 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haga SB, Tindall G, O'Daniel JM. Professional perspectives about pharmacogenetic testing and managing ancillary findings. Genet. Test Mol. Biomarkers 16(1), 21–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harding B, Webber C, Ruhland L et al. Primary care providers' lived experiences of genetics in practice. J. Community Genet. 10(1), 85–93 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee YM, McKillip RP, Borden BA, Klammer CE, Ratain MJ, O'Donnell PH. Assessment of patient perceptions of genomic testing to inform pharmacogenomic implementation. Pharmacogenet. Genomics 27(5), 179–189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rafi I, Crinson I, Dawes M, Rafi D, Pirmohamed M, Walter FM. The implementation of pharmacogenomics into UK general practice: a qualitative study exploring barriers, challenges and opportunities. J. Commun. Genet. 11(3), 269–277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rigter T, Jansen ME, de Groot JM, Janssen SWJ, Rodenburg W, Cornel MC. Implementation of pharmacogenetics in primary care: a multi-stakeholder perspective. Front. Genet. 11, 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barr M, Rose D. The great ambivalence: factors likely to affect service user and public acceptability of the pharmacogenomics of antidepressant medication. Social Health Illn. 30(6), 944–958 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Issa AM, Tufail W, Hutchinson J, Tenorio J, Baliga MP. Assessing patient readiness for the clinical adoption of personalized medicine. Public Health Genomics 12(3), 163–169 (2009). [DOI] [PubMed] [Google Scholar]
- 53.De Marco M, Cykert S, Coad N et al. Views on personalized medicine: do the attitudes of African American and white prescription drug consumers differ? Public Health Genomics 13(5), 276–283 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haddy CA, Ward HM, Angley MT, McKinnon RA. Consumers' views of pharmacogenetics – a qualitative study. Res. Social Adm. Pharm. 6(3), 221–231 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Van der Wouden CH, Paasman E, Teichert M, Crone MR, Guchelaar HJ, Swen JJ. Assessing the implementation of pharmacogenomic panel-testing in primary care in the Netherlands utilizing a theoretical framework. J. Clin. Med. 9(3), 814 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haga SB. First responder to genomic information: a guide for primary care providers. Mol. Diagn. Ther. 23(4), 459–466 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med. Res. Methodol. 8, 45 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


