Abstract
Background
The relationship between postvaccination symptoms and strength of antibody responses is unclear. The goal of this study was to determine whether adverse effects caused by vaccination with the Pfizer/BioNTech BNT162b2 vaccine are associated with the magnitude of vaccine-induced antibody levels.
Methods
We conducted a single-center, observational cohort study consisting of generally healthy adult participants that were not severely immunocompromised, had no history of coronavirus disease 2019, and were seronegative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein before vaccination. Severity of vaccine-associated symptoms was obtained through participant-completed questionnaires. Testing for immunoglobulin G antibodies against SARS-CoV-2 spike protein and receptor-binding domain was conducted using microsphere-based multiplex immunoassays performed on serum samples collected at monthly visits. Neutralizing antibody titers were determined by microneutralization assays.
Results
Two hundred six participants were evaluated (69.4% female, median age 41.5 years old). We found no correlation between vaccine-associated symptom severity scores and vaccine-induced antibody titers 1 month after vaccination. We also observed that (1) postvaccination symptoms were inversely correlated with age and weight and more common in women, (2) systemic symptoms were more frequent after the second vaccination, (3) high symptom scores after first vaccination were predictive of high symptom scores after second vaccination, and (4) older age was associated with lower titers.
Conclusions
Lack of postvaccination symptoms after receipt of the BNT162b2 vaccine does not equate to lack of vaccine-induced antibodies 1 month after vaccination.
Keywords: adverse effects, antibody titer, COVID-19, mRNA vaccine, SARS-CoV-2
We found no correlation between BNT162b2-associated symptom severity and vaccine-induced antibody titers 1 month after vaccination. Adverse effects inversely correlated with age and weight, whereas symptom severity after first vaccination was predictive of that after second vaccination.
The implementation of messenger ribonucleic acid (mRNA)-based severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines is playing a major role in efforts to control the SARS-CoV-2 pandemic. Both the Pfizer/BioNTech BNT162b2 and Moderna mRNA-1273 vaccines induce high-titer anti-SARS-CoV-2 antibodies and confer robust protection against morbidity and mortality from SARS-CoV-2 infection [1–4].
One feature of the SARS-CoV-2 mRNA vaccines is the high level of reactogenicity, with both local and systemic reactions reported by the majority of recipients in Phase 1–3 studies [1–4]. A Centers for Disease Control and Prevention vaccine safety monitoring program of adverse effects (AEs) in the US population has found that injection site pain (79.3%), fatigue (53.5%), myalgia (47.2%), headache (43.4%), chills (30.6%), fever (29.2%), and joint pains (23.5%) are frequent after the second dose of the BNT162b2 vaccine [5].
Reactogenicity to vaccines is typically driven by activation of the innate immune system through ligation of pattern-recognition receptors and subsequent release of inflammatory cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor [6]. Studies suggest type I interferon production elicited by direct mRNA recognition is critical for SARS-CoV-2 control [7–10], and this likely contributes to both immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccines [6]. Adaptive immune pathways also likely play a role in causing vaccine-mediated symptoms, especially during booster vaccinations or vaccination after infection.
During the rollout of coronavirus disease 2019 (COVID-19) vaccines, it has become commonplace for media outlets and medical professionals to state that presence of symptoms means that a vaccine is “working.” Although this statement is fundamentally true because vaccines “work” by inducing inflammatory responses, it also implies incorrectly that lack of symptoms postvaccination may indicate an absence of appropriate antiviral antibody responses. Notably, there is little data demonstrating correlations between vaccine-induced symptoms and antibody titers with any vaccine platforms [6, 11]. The goal of this study was to assess for correlation between AEs caused by BNT162b2 vaccination and the magnitude of SARS-CoV-2 antibody responses 1 month after second vaccination dose.
METHODS
Study Participants
Participants were enrolled in the Prospective Assessment of SARS-CoV-2 (PASS) Study, an observational, longitudinal cohort study of healthcare workers (HCWs) that is evaluating clinical and immunological responses to SARS-CoV-2 infection and vaccination. The cohort consists of generally healthy adults who are ≥18 years old, work at Walter Reed National Military Medical Center, are not severely immunocompromised, and were seronegative for SARS-CoV-2 at time of study enrollment. Details of inclusion and exclusion criteria can be found in the protocol, which has been published [12]. The subset of PASS participants included for analysis in this study also met the following criteria: (1) no history of COVID-19 diagnosis, (2) seronegative for SARS-CoV-2 antispike protein immunoglobulin (Ig)G before vaccination, (3) received 2 vaccinations with the Pfizer/BioNTech BNT162b2 vaccine, and (4) completed 2 vaccination symptom questionnaires by March 24, 2021 (Supplemental Figure 1). The PASS study was initiated in August of 2020 with study participants seen monthly at the Naval Medical Research Center Clinical Trials Center. The study protocol was approved by the Uniformed Services University Institutional Review Board.
Ethics Approval and Consent to Participate
This study protocol was approved by the Uniformed Services University of the Health Sciences Institutional Review Board (FWA 00001628; Department of Defense Assurance P60001) in compliance with all applicable Federal regulations governing the protection of human participants. All participants provided written informed consent for participation.
Assessment of Vaccine-Associated Symptoms
Participants completed a structured vaccine-associated symptoms questionnaire at the first monthly visit after each vaccination dose. Questionnaires asked about the presence and severity of 12 symptoms (8 categorized as systemic, 3 categorized as localized to the vaccine site, and 1 categorized as nonlocal and nonsystemic [see Tables 1 and 2]). Severity of each symptom was defined as symptom intensity and measured on a scale of 0–4 (0 = not at all, 1 = a little bit, 2 = somewhat, 3 = quite a bit, 4 = a lot), with scores for each symptom summed for a total symptom severity score of 0–48. The structured questionnaire used for postvaccination adverse effects was based on symptoms queried in published Phase 1 COVID-19 vaccine studies [2, 4]. The severity scale was adopted from that used in Flu-Pro Plus, a validated patient-reported outcome instrument. Participants were also asked to report the total duration of any vaccine-associated symptoms.
Table 1.
Symptoms Experienced After First Vaccination, Ranked by Frequency
| Symptom Score | |||||
|---|---|---|---|---|---|
| Symptom | Presence of Symptoms | 1 | 2 | 3 | 4 |
| Soreness at injection site a | 188 (91.3) | 32 (15.5) | 63 (30.6) | 66 (32.0) | 27 (13.1) |
| Pain at injection site a | 148 (71.8) | 38 (18.4) | 44 (21.4) | 48 (23.3) | 18 (8.7) |
| Weak or tired b | 87 (42.2) | 33 (16.0) | 26 (12.6) | 23 (11.2) | 5 (2.4) |
| Headache b | 61 (29.6) | 22 (10.7) | 16 (7.8) | 18 (8.7) | 5 (2.4) |
| Body aches or pains b | 58 (28.2) | 18 (8.7) | 17 (8.3) | 15 (7.3) | 8 (3.9) |
| Redness at injection site a | 44 (21.4) | 29 (14.1) | 11 (5.3) | 2 (1.0) | 2 (1.0) |
| Joint pains b | 27 (13.1) | 9 (4.4) | 8 (3.9) | 4 (1.9) | 6 (2.9) |
| Felt nauseous b | 19 (9.2) | 10 (4.9) | 5 (2.4) | 3 (1.5) | 1 (0.5) |
| Felt hot b | 19 (9.2) | 7 (3.4) | 8 (3.9) | 2 (1.0) | 2 (1.0) |
| Felt cold b | 18 (8.7) | 6 (2.9) | 8 (3.9) | 3 (1.5) | 1 (0.5) |
| Chills or shivering b | 17 (8.3) | 2 (1.0) | 8 (3.9) | 5 (2.4) | 2 (1.0) |
| Swollen lymph nodes c | 9 (4.4) | 5 (2.4) | 3 (1.5) | 0 (0) | 1 (0.5) |
Local symptoms.
Systemic symptoms.
Nonlocal/nonsystemic symptoms.
Table 2.
Symptoms Experienced After Second Vaccination, Ranked by Frequency
| Symptom Score | |||||
|---|---|---|---|---|---|
| Symptom | Presence of Symptoms | 1 | 2 | 3 | 4 |
| Soreness at injection site a | 169 (82.0) | 58 (28.2) | 54 (26.2) | 38 (18.4) | 19 (9.2) |
| Pain at injection site a | 128 (62.1) | 54 (26.2) | 36 (17.5) | 24 (11.7) | 14 (6.8) |
| Weak or tired b | 128 (62.1) | 34 (16.5) | 32 (15.5) | 35 (17.0) | 27 (13.1) |
| Body aches or pains b | 108 (52.4) | 15 (7.3) | 32 (15.5) | 37 (18.0) | 24 (11.7) |
| Headache b | 104 (50.5) | 32 (15.5) | 32 (15.5) | 19 (9.2) | 21 (10.2) |
| Joint pains b | 64 (31.1) | 17 (8.3) | 16 (7.8) | 23 (11.2) | 8 (3.9) |
| Chills or shivering b | 57 (27.7) | 9 (4.4) | 13 (6.3) | 22 (10.7) | 13 (6.3) |
| Felt hot b | 56 (27.2) | 20 (9.7) | 11 (5.3) | 13 (6.3) | 12 (5.8) |
| Felt cold b | 53 (25.7) | 13 (6.3) | 18 (8.7) | 16 (7.8) | 6 (2.9) |
| Redness at injection site a | 39 (18.9) | 20 (9.7) | 9 (4.4) | 7 (3.4) | 3 (1.5) |
| Felt nauseous b | 33 (16.0) | 15 (7.3) | 9 (4.4) | 4 (1.9) | 5 (2.4) |
| Swollen lymph nodes c | 28 (13.6) | 8 (3.9) | 7 (3.4) | 9 (4.4) | 4 (1.9) |
Local symptoms.
Systemic symptoms.
Nonlocal/nonsystemic symptoms.
Antibody Testing
Immunoglobulin G antibodies against SARS-CoV-2 spike protein and receptor-binding domain (RBD) were measured using microsphere-based multiplex immunoassays (MMIAs) built using Luminex xMAP-based technology as previously described [13] (Supplemental Methods). Our in-house, non-Emergency Use Authorization (EUA) research assay has been tested concurrently with the Mt. Sinai spike/RBD EUA enzyme-linked immunosorbent assay (ELISA), and our thresholds for seropositivity have performed with 99% (334 of 337) concordance for the detection of spike IgG-binding antibodies compared with the Mt. Sinai ELISA [14].
Wild-Type Severe Acute Respiratory Syndrome Coronavirus 2 Microneutralization Assays
Neutralizing antibody titers were determined by microneutralization assays as previously described [15].
Statistical Analyses
The Wilcoxon signed-rank test was used for paired comparisons and the Mann-Whitney test was used for unpaired comparisons. Kruskal-Wallis or analysis of variance was used when comparing multiple groups. Spearman rank analyses were used to assess for correlations. Spearman partial correlations were used to determine whether age, sex, or weight were independently associated with vaccine-associated symptom scores and to adjust for age, sex, and weight when assessing for correlations between vaccine-related symptom scores and antibody titers. P values less than .05 were considered statistically significant. Analyses were conducted using GraphPad Prism Version 9 and SPSS Version 27. Using the University of California, San Francisco (UCSF) sample size calculator for correlation [16], we calculated that, with a power of 0.8 and an alpha of 0.05, a sample size of 196 participants would enable assessment for correlations with a rho of 0.2. Means are reported as ±standard deviation.
RESULTS
Study Participants Evaluated
A total of 206 participants, of 270 enrolled in the PASS study, were seronegative and without a history of COVID-19 diagnosis when they received the first of 2 BNT162b2 vaccinations, and they provided a serum sample at least 3 weeks after final vaccination. Of these, the median age was 41.5 years old (interquartile range 33–51.25) and 69.4% were female. Antispike antibody levels were quantified by MMIA mean fluorescence intensity (MFI) for all participants and by endpoint dilution titers for the first 101 participants for which serum at least 3 weeks after second vaccination dose was available. Demographic information for the study cohort and titers subgroup is in Supplemental Table 1.
Symptom Severity Scores After First and Second Vaccinations
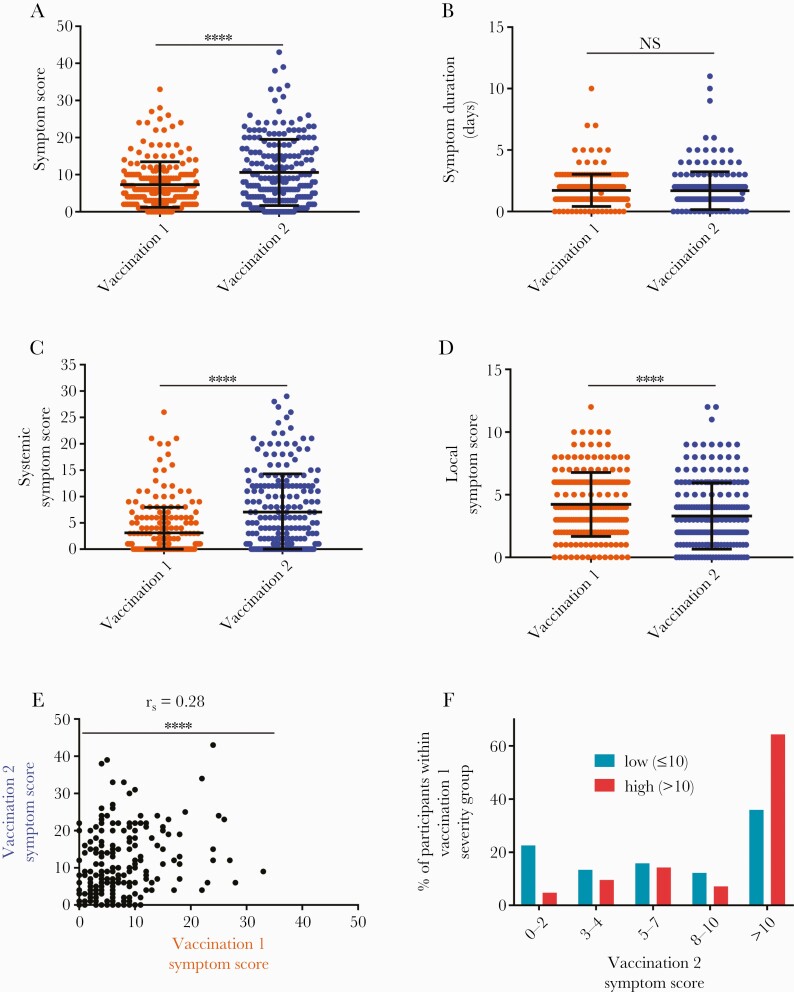
The mean symptom score reported for the second vaccination was significantly greater than that of the first (10.6 ± 8.9 vs 7.3 ± 6.1, P < .0001) (Figure 1A), even though there was no significant difference in the duration of symptoms after either vaccination (Figure 1B). To better understand the observed difference in symptom severity, participant symptom scores were subdivided into systemic (maximum score: 32) and local (maximum score: 12) symptom scores. It is interesting to note that although the mean systemic symptom score after the second vaccination was significantly greater than after the first (7.0 vs 3.1, P < .0001) (Figure 1C), local symptoms displayed an opposing trend with lower severity after the second vaccination (mean 3.3 vs 4.2, P < .0001) (Figure 1D). Overall, there was a positive correlation between vaccination 1 and vaccination 2 symptom scores (rho = 0.28, P < .0001) (Figure 1E). To determine how frequently individuals with substantial symptoms after the first vaccination develop substantial symptoms after the second vaccination, participants were separated into 2 groups: a low symptom severity group made up of individuals with vaccination 1 symptom scores less than or equal to 10 (n = 164, 79.6%) and a high severity group comprising participants with vaccination 1 symptom scores greater than 10 (n = 42, 20.4%). Approximately 35% of participants in the low symptom severity group reported a symptom score greater than 10 after the second vaccination (Figure 1F). This frequency almost doubled in the high severity group, with 64% of those participants having recorded a symptom score greater than 10 after the second vaccination (Figure 1F).
Figure 1.
Symptom severity after the first vaccination with BNT162b2 correlates with severity of symptoms after the second vaccination. (A) Total symptom severity scores (range 0–48), (B) duration of symptoms, (C) systemic symptom severity scores (range 0–32), and (D) local symptom severity scores (range 0–12) after the first (orange) and second (blue) vaccinations. (E) Correlation of vaccination 1 and vaccination 2 symptom scores. (F) Percentage of subjects categorized as having low (≤10, teal) or high (>10, red) total symptom scores after vaccination 1 that exhibited symptom scores of 0–2, 3–4, 5–7, 8–10, or >10 after vaccination 2. N = 206 for all panels. ∗∗∗∗P < .0001. NS = not significant, significance assessed by Wilcoxon signed-rank test for A–D and by Spearman correlation analysis for E. Bars represent mean and standard deviation in A–D.
Frequency of Specific Symptoms Experienced After First and Second Vaccine Doses
Soreness at the injection site (vaccination 1: 91.3%, vaccination 2: 82.0%), pain at the injection site (vaccination 1: 71.8%, vaccination 2: 62.1%), and the feeling of being weak or tired (vaccination 1: 42.2%, vaccination 2: 62.1%) were the 3 most common symptoms reported after receiving the first (Table 1) and second (Table 2) doses of the Pfizer-BioNTech BNT162b2 vaccine. Except for the local symptoms of soreness, pain, or redness at the injection site, all symptoms listed on the questionnaire increased in frequency from the first vaccination to the second. For example, although 28.2% of participants experienced body aches or pains after the first vaccination, 52.4% reported this symptom after the second vaccination. This increase was present for even the least common symptom of swollen lymph nodes, which increased from 4.4% to 13.6% of participants after the first and second doses, respectively.
Relationship Between Vaccine Symptoms, Age, Sex, and Weight
Younger age, female sex, and lower weight were all associated with higher symptom scores when evaluated individually. There was a modest, yet significant, negative correlation between age and symptom severity for both the first (rho = −0.17, P = .02) (Figure 2A) and second (rho = −0.17, P = .01) (Figure 2B) vaccine doses, representing a 1.7 value decrease in symptom score for every decade lived (Supplemental Table 2). Female participants reported significantly higher symptom scores than males after the first vaccination (mean 8.0 ± 6.4 vs 5.7 ± 5.2, P = .006) (Figure 2C). Females also had higher symptom scores after the second vaccination compared to males (mean 11.3 ± 9.2 vs 9.1 ± 8.2), but this difference was not statistically significant (P = .11) (Figure 2D). Spearman partial correlation analysis determined age to be an independent predictor of total symptom scores after both first (partial rho = −0.17, P = .018) and second (partial rho = −0.17, P = .018) vaccinations after adjusting for sex and weight (Supplemental Table 2). Although not statistically significant, female sex was consistently found to positively correlate with symptom scores and weight was found to negatively correlate with symptom scores when analyzed with partial Spearman correlations (Supplemental Table 2). No differences in symptom scores based on race for either first or second vaccination were noted (Supplemental Figure 2).
Figure 2.
Younger age, female sex, and lower weight are associated with higher symptom severity. Total symptom severity scores after vaccination 1 (orange) and vaccination 2 (blue) graphed against age (A and B), sex (C and D), and weight (E and F). Correlations were assessed by Spearman rank analysis for age and weight. Mann-Whitney analysis was used to assess for significance between males and females. N = 206 for all panels. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. NS is defined as not significant. Bars represent median and interquartile range in C and D.
Lack of Correlation Between Vaccine-Associated Symptoms and Antibody Titers
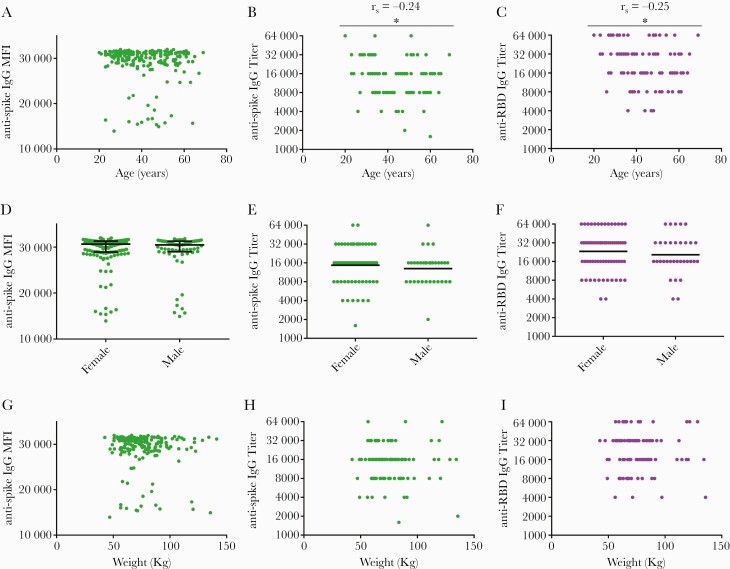
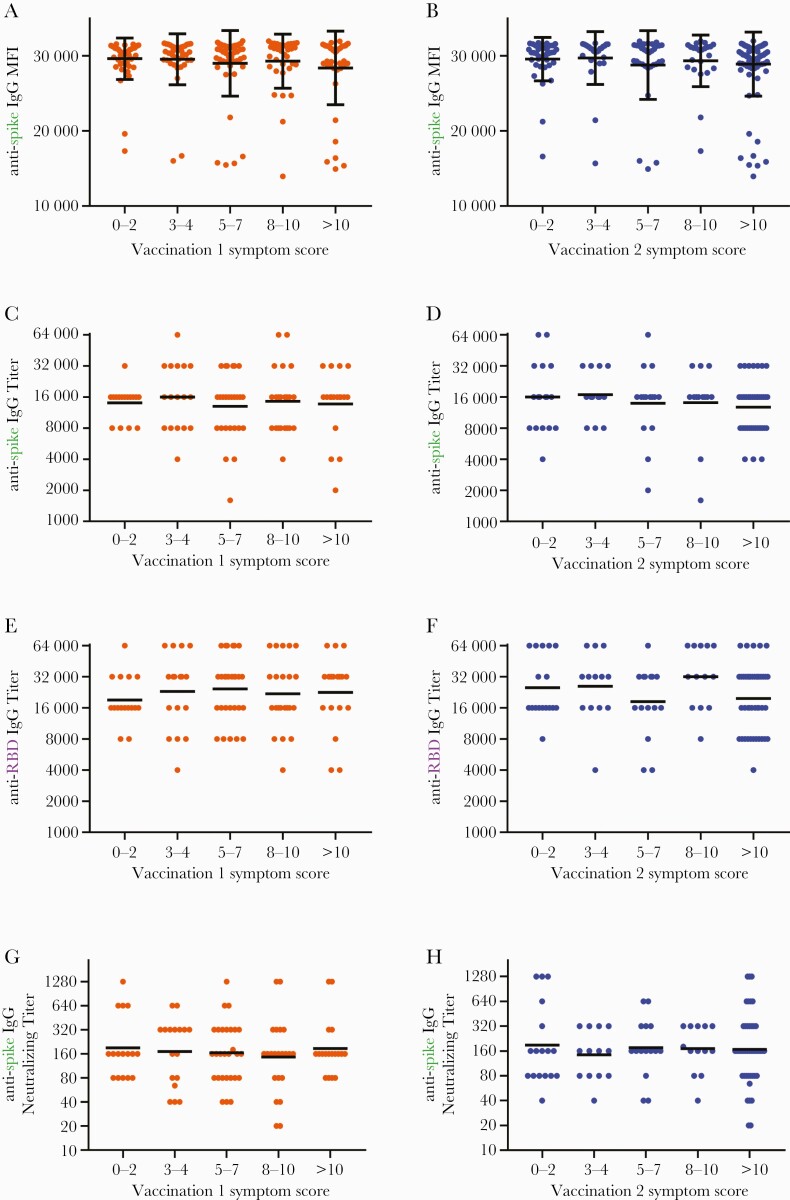
Time between final vaccination and serum sampling was a mean of 36.8 ± 10.0 days (range 22–104) for the entire cohort and 33.9 ± 6.8 days (range 23-51) for the titers subgroup. Older age, but not sex, weight, or race, was negatively associated with vaccine-induced antibody levels (Figure 3 and Supplemental Figure 3). No correlation between symptom severity after the first or second vaccine doses and IgG reactivity with spike protein was noted (Figure 4A and B). Endpoint dilution assays also exhibited no correlation between vaccine symptom scores and endpoint titers of antispike IgG (Figure 4C and D) or anti-RBD IgG (Figure 4E and F). Lack of correlation was observed with both Spearman rank analyses and with partial Spearman correlations analyses after adjusting for age and sex. In addition, no correlation was observed between vaccine symptoms scores and neutralizing titers (Figure 4G and H). Secondary analyses also revealed no associations between systemic symptoms or lymph node swelling and antispike and anti-RBD titers (data not shown). Analysis of total symptom duration after first or second vaccination revealed no association with antispike MFI levels or anti-RBD IgG titers, although a significant negative correlation was observed with duration of symptoms after second vaccination and antispike IgG titers (Supplemental Figure 4).
Figure 3.
Age, but neither sex nor weight, correlates with BNT162b2-induced anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody titers. Samples collected ~1 month after participants received the second vaccine dose were tested for antibodies against SARS-CoV-2 spike protein (green) and receptor-binding domain ([RBD] purple) and plotted against age (A–C), sex (D–F), and weight (G–I). Antibody reactivity against SARS-CoV-2 spike protein in samples diluted 1:400 was assessed in 206 subjects and reported as mean fluorescence intensity (MFI). Endpoint dilution titers were measured in a subset of 101 subjects for both antispike immunoglobulin (Ig)G and anti-RBD IgG. Correlations were assessed by Spearman rank analysis for age and weight, and Mann Whitney analysis was used to assess for significance between males and females. N = 206 for MFI values; n = 101 for antibody titers. ∗P < .05. Bars indicate median and interquartile range (D) or geometric mean (E and F). Titers recorded as >32000 are plotted as 64000.
Figure 4.
Severity of symptoms after vaccination correlates with neither vaccine-induced antispike immunoglobulin (Ig)G reactivity nor with titers of antispike and anti-receptor-binding domain (RBD) IgG antibodies. Samples collected ~1 month (mean 36.8 days for A-B and 33.9 days for C-H) after participants received the second vaccine dose of BNT162b2 were tested for antibodies against SARS-CoV-2 spike and RBD proteins using the Luminex microsphere-based multiplex immunoassay. (A and B) Levels of antispike IgG antibodies, as measured by mean fluorescence intensity (MFI), were plotted against symptom scores reported after first (orange) and second (blue) vaccination (N = 206). (C and D) Titers of antispike IgG antibodies, (E and F) anti-RBD IgG antibodies, and (G and H) neutralizing antibodies were plotted against symptom scores reported after first and second vaccination (n = 101). Bars indicate mean and standard deviation (A and B) or geometric mean (C–F). Titers recorded as >32000 are plotted as 64000. Assessments for correlations were conducted by both Kruskal-Wallis analysis with subjects binned into categories of symptom score ranges and by Spearman rank analysis evaluating antibody levels against symptom scores as a continuous variable. All showed no significant correlations.
DISCUSSION
Local and systemic symptoms often occur after vaccination and are predominantly due to activation of inflammatory pathways [6]. In this study, we evaluated vaccine-related AEs that occur in response to the Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine in a cohort of HCWs. It is notable that we found no correlation between vaccine-related symptom severity and vaccine-induced antibody titers. This lack of correlation was observed even when adjusting for age, weight, and sex. In addition, we observed that (1) symptoms were modestly more common in women and inversely correlated with age and weight, (2) systemic symptoms were more frequent after the second vaccination, (3) high symptom scores after first vaccination were associated with high symptom scores after second vaccination, and (4) older age was associated with slightly lower vaccine-induced antibody titers.
We did not observe a significant correlation between vaccine-related AEs and the magnitude of vaccine-induced antibody titers. Individuals with both high and low symptom scores had (1) similar levels of spike-specific IgG antibodies when measured by MFI and (2) similar endpoint dilution titers of spike-specific IgG antibodies, RBD-specific IgG antibodies, and neutralizing antibodies. This lack of correlation was maintained even when controlling for age and sex. Although it would seem logical that vaccine-associated AEs could be predictive of antibody titers, there is little evidence for such a relationship [6]. One study that evaluated different adjuvants for hepatitis B vaccination found a modest association of symptoms after first vaccination with CD4+ T-cell responses [17]. However, the same study found no association between first vaccination AEs and antibody responses and no associations between symptoms after second vaccination and either CD4+ T-cell or antibody responses [17]. Consistent with our study, Muller et al [18] did not find an association between symptoms induced by BNT162b2 vaccination and antibody titers.
The lack of correlation between vaccine-associated symptoms and antibody titers has 2 important implications for mRNA SARS-CoV-2 vaccines. First, individuals that exhibit few symptoms after vaccination can be reassured that this does not mean the vaccine “did not work.” Indeed, in this cohort, individuals with few to no symptoms were just as likely to have developed strong antibody responses as individuals that exhibited substantial symptoms. Second, the immunological pathways responsible for mRNA vaccine-induced AEs may not be required for development of robust antibody responses.
The mRNA vaccines induce inflammation through multiple pathways, including ligation of innate immune receptors, release of inflammatory cytokines and chemokines, and activation of antigen-presenting cells, natural killer cells, and antigen-specific T and B cells [19–24]. Given the lack of association between symptoms and antibody titers, we speculate that some pathways may be expendable for development of robust adaptive immune responses. If such pathways can be defined, then efforts on developing mRNA vaccines that minimally activate such pathways could be advantageous.
In regards to vaccination reactogenicity, as with the BNT162b2 clinical trials [3, 4], we observed greater symptom severity after the second vaccination. It is notable that individuals that had a high symptom score after the first vaccination were almost twice as likely to have substantial symptoms after second vaccination compared to those with a low symptom score from the first vaccination. Nevertheless, individuals with few symptoms after the first vaccination still had a 35% chance of having substantial symptoms (total symptom score >10) after second vaccination.
Local symptoms were more frequent than systemic symptoms after both first and second vaccinations, with pain, soreness, and redness reported in 91%, 72%, and 21% of participants, respectively, after first vaccination and by 82%, 62%, and 19% of participants, respectively, after the second vaccination. These frequencies paralleled those observed in the clinical trials of BNT162b2 [3, 4]. Systemic symptoms were common with symptoms of feeling weak or tired, having body aches or pains, or having joint pains reported by 42%, 28%, and 13% of participants, respectively, after first vaccination and by 62%, 52%, and 31%, respectively, after the second vaccination. Again, these frequencies were similar to clinical trial reports [3].
Partial correlation analysis demonstrated that age was an independent predictor of vaccine-related AEs, with age exhibiting a rho factor of −0.17 for total symptom scores after both vaccine doses in bivariate analyses after controlling for sex and weight. The finding that older age is associated with lower postvaccination symptoms is similar to results of BNT162b2 clinical trials and to findings reported in a comparison study evaluating vaccine responses in individuals greater than 80 years old and less than 60 years old [3, 4, 18].
Although both women and individuals with lower weights were found to have greater vaccine-related symptoms in our study, neither sex nor weight was found to be an independent predictor of symptom scores. Our study may have been insufficiently powered to separate the independent contributions of sex and weight to vaccine-associated symptoms, although sex and weight may simply not be independent predictors. Consistent with our findings, women in a large-scale United Kingdom study were found to have more symptoms than men after BNT162b2 vaccination [25]. In addition, women have been shown to have greater reactogenicity to other vaccines, including measles/mumps/rubella, hepatitis B, influenza, and yellow fever [26, 27]. The determination of whether women and/or low weight individuals have greater BNT162b2 AEs may be informed by additional cohort studies.
The mechanisms by which younger individuals, or women, exhibit greater vaccine-related AEs is unclear, but they may be due to differences in innate immune function. Dendritic cells of older individuals have been shown to release decreased quantities of proinflammatory cytokines when stimulated through pattern recognition receptors [28, 29]. For women, increased AEs may be due to increased responsiveness of innate immune pathways, although differences in anatomy at injection sites, sex hormones, and adaptive immune function may also play a role [30, 31].
Of note, in addition to being associated with lower vaccine-associated symptom scores, age was also found to be significantly correlated with lower titers of vaccine-induced IgG antibodies against spike protein and RBD. Reduced titers were also observed in elderly individuals in both the Phase 1 clinical trial of BNT162b2 and in a recent study evaluating BNT162b2 responses in individuals greater than 80 years old [3, 18]. The mechanisms underlying reduced antibody responses in elderly individuals are not yet fully elucidated, but they likely include factors such as reductions in T-cell receptor signaling, predilection for naive T cells to differentiate into effector rather than memory T cells, decreased function of follicular helper T cells, and lower antibody production by plasma cells [32].
The present study is limited to antibody responses. It is possible that vaccine reactogenicity, although it does not impact antibody response magnitude, might correlate with vaccine-induced, antigen-specific, T-cell responses. In addition, whether reactogenicity impacts durability of vaccine-induced immune responses will be an important area to explore in future studies. Notably, we did find that symptom duration after second vaccination negatively correlated with vaccine-induced antispike IgG titers but not with antispike MFI levels or anti-RBD titers. Although this may reflect a true association, we suspect it is an artifact due to conducting multiple secondary analyses. Another limitation is that the cohort consisted of healthy volunteers without substantial immunocompromising conditions.
CONCLUSIONS
In conclusion, this study demonstrates that BNT162b2 vaccinations are commonly associated with both local and systemic symptoms. Symptoms are greater after second vaccination, are more common in younger individuals, and do not correlate with vaccine-induced antiviral IgG titers. These findings suggest that patients receiving the BNT162b2 vaccine should be reassured that lack of symptoms does not necessarily equate to lack of desired vaccine function. This study also suggests that it may be possible to design future mRNA vaccines that confer robust antibody responses with lower frequencies of vaccine-associated symptoms. Indeed, emerging studies suggest the balance between vaccine immunogenicity and reactogenicity can be better tuned for COVID-19 mRNA-based vaccines as well [33].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Camille Estupigan, Dr. Mark Simons, and Dr. Kimberly Edgel for providing insightful comments on the manuscript.
Author contributions. S. A. C., E. D. L., C. H. O., E. G., B. M. J.-T., S. D. P., D. R. T., L. I., M. H.-P., C. A. D., A. L. S., A. M. W. M., J. H. P., T. H. B., C. C. B., and E. M. conceived and designed the analysis. S. A. C., E. G., M. M., B. M. J.-T., E. C. S., S. E. M., C. A. D., K. F. R., A. E. R., Y. A., M. A. W., and G. W. collected the data. S. A. C., E. D. L., C. H. O., E. C. S., J. D., O. O., E. P., J. H. P., K. L. S., and D. E. contributed data or analysis tools. S. A. C., C. H. O., and E. M. performed the analysis. S. A. C., E. D. L., C. H. O., E. G., S. D. P., D. R. T., C. A. D., A. R. L., A. L. S., A. M. W. M., A. G. L., J. H. P., C. C. B., and E. M. wrote the paper.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University of the Health Sciences, Walter Reed National Military Medical Center, Naval Medical Research Center, the Department of Navy, the Department of Defense, the Henry M. Jackson Foundation for the Advancement of Military Medicine, or the U.S. Government. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government. The funding bodies have had no role in the study design or the decision to submit the manuscript for publication.
Financial support. This study was executed by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USUHS) through a cooperative agreement by the Henry M. Jackson Foundation (HJF) for the Advancement of Military Medicine, Inc. This work was funded by the Defense Health Program, U.S. Department of Defense, under awards HU00012120067 and HU00012120094. Project funding for J. H. P. was in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E.
Potential conflicts of interest. S. D. P., T. H. B., and D. R. T. report that the Uniformed Services University Infectious Diseases Clinical Research Program (IDCRP), a US Department of Defense institution, and the HJF were funded under a Cooperative Research and Development Agreement to conduct an unrelated Phase 3 coronavirus disease 2019 (COVID-19) monoclonal antibody immunoprophylaxis trial sponsored by AstraZeneca. The HJF, in support of the USU IDCRP, was funded by the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense to augment the conduct of an unrelated Phase 3 vaccine trial sponsored by AstraZeneca. Both of these trials were part of the US Government COVID-19 response. Neither is related to the work presented here. E. M., E. D. L., C. C. B., A. L. S., A. M. W. M., M. H.-P., A. G. L., C. A. D., K. L. S., and T. H. B. are military Service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17, U.S.C., §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C., §101 defines a U.S. Government work as a work prepared by a military Service member or employee of the U.S. Government as part of that person’s official duties.
References
- 1. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson LA, Anderson EJ, Rouphael NG, et al. ; mRNA-1273 Study Group. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med 2020; 383:1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walsh EE, Frenck RW Jr, Falsey AR, et al. . Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020; 383:2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gee J, Marquez P, Su J, et al. . First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hervé C, Laupèze B, Del Giudice G, et al. . The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019; 4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Q, Bastard P, Liu Z, et al. . Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020; 370:eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bastard P, Rosen LB, Zhang Q, et al. . Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020; 370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Acharya D, Liu G, Gack MU.. Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol 2020; 20:397–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hadjadj J, Yatim N, Barnabei L, et al. . Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020; 369:718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell TC, Casella CR.. No pain no gain? Adjuvant effects of alum and monophosphoryl lipid A in pertussis and HPV vaccines. Curr Opin Immunol 2017; 47:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson-Thompson BM, Goguet E, Laing ED, et al. . Prospective Assessment of SARS-CoV-2 seroconversion (PASS) study: an observational cohort study of SARS-CoV-2 infection and vaccination in healthcare workers. BMC Infect Dis 2021; 21:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laing ED, Sterling SL, Richard SA, et al. . Antigen-based multiplex strategies to discriminate SARS-CoV-2 natural and vaccine induced immunity from seasonal human coronavirus humoral responses [preprint]. medRxiv 2021. [Google Scholar]
- 14. Clifton GT, Pati R, Krammer F, et al. . SARS-CoV-2 infection risk among active duty military members deployed to a field hospital - New York City, April 2020. MMWR Morb Mortal Wkly Rep 2021; 70:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laing E, Epsi N, Richard S, et al. . SARS-CoV-2 antibodies remain detectable 12 months after infection and antibody magnitude is associated with age and COVID-19 severity [preprint]. medRxiv 2021. [Google Scholar]
- 16. Institute UCaTS. Sample Size Calculators for Designing Clinical Research. 2020; Available at: https://www.sample-size.net/sample-size-conf-interval-proportion/. Accessed 12 April 2020.
- 17. Burny W, Callegaro A, Bechtold V, et al. ; ECR-002 Study Group. Different adjuvants induce common innate pathways that are associated with enhanced adaptive responses against a model antigen in humans. Front Immunol 2017; 8:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muller L, Andree M, Moskorz W, et al. . Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pardi N, Hogan MJ, Naradikian MS, et al. . Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med 2018; 215:1571–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liang F, Lindgren G, Lin A, et al. . Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol Ther 2017; 25:2635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teijaro JR, Farber DL.. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 2021; 21:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pardi N, Hogan MJ, Porter FW, Weissman D.. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 2018; 17:261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scheel B, Teufel R, Probst J, et al. . Toll-like receptor-dependent activation of several human blood cell types by protamine-condensed mRNA. Eur J Immunol 2005; 35:1557–66. [DOI] [PubMed] [Google Scholar]
- 24. Kowalczyk A, Doener F, Zanzinger K, et al. . Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine 2016; 34:3882–93. [DOI] [PubMed] [Google Scholar]
- 25. Menni C, Klaser K, May A, et al. . Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 2021; 21:939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klein SL, Marriott I, Fish EN.. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 2015; 109:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fischinger S, Boudreau CM, Butler AL, et al. . Sex differences in vaccine-induced humoral immunity. Semin Immunopathol 2019; 41:239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Allen JC, Toapanta FR, Chen W, Tennant SM.. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine 2020; 38:8264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panda A, Qian F, Mohanty S, et al. . Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol 2010; 184:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flanagan KL, Fink AL, Plebanski M, Klein SL.. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol 2017; 33:577–99. [DOI] [PubMed] [Google Scholar]
- 31. Cook IF. Sex differences in injection site reactions with human vaccines. Hum Vaccin 2009; 5:441–9. [DOI] [PubMed] [Google Scholar]
- 32. Gustafson CE, Kim C, Weyand CM, Goronzy JJ.. Influence of immune aging on vaccine responses. J Allergy Clin Immunol 2020; 145:1309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kobiyama K, Imai M, Jounai N, et al. . Optimization of an LNP-mRNA vaccine candidate 1 targeting SARS-CoV-2 receptor-binding domain [preprint]. bioRxiv 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.