Abstract
HPV vaccination of adolescent girls is the most effective measure to prevent cervical cancer. The World Health Organization recommends that adolescent girls receive two doses of vaccine but only a small proportion of girls from regions with the highest disease burden are vaccinated because of cost and logistical considerations. Our Costa Rica HPV Vaccine trial suggested that one dose of the bivalent HPV vaccine provides robust and lasting protection against persistent HPV infections for over a decade. Data from a post-licensure trial of the quadrivalent vaccine in India also suggested that a single dose may be effective in reducing cervical cancer risk. To formally compare one versus two doses of the bivalent and nonavalent HPV vaccines, we implemented a large, randomized, double-blind trial to investigate the non-inferiority of one compared to two vaccine doses in the prevention of new HPV16/18 infections that persist 6 or more months. Bivalent and nonavalent vaccines will be evaluated separately. The trial enrolled and randomized (1:1:1:1 to 1- and 2-dose arms of the bivalent and nonavalent vaccines) 20,330 girls 12 to 16 years old residing in Costa Rica. Trial participants are followed every 6 months for up to 5 years. We also aim to estimate vaccine efficacy by comparing the rates of 6 month persistent infection in unvaccinated women with the rates in the follow-up visits of trial participants. We included one survey of unvaccinated women at the start of the study (N=4452) and will include another survey concomitant with follow up visits of trial participants at years 4.5 and 5 (planned N=3000). Survey participants attend two visits 6 months appart. Herein, we present the rationale, design, and enrolled study population of the ESCUDDO trial.
ClinicalTrials.gov Identifier:
Keywords: prophylactic HPV vaccine, HPV infection, HPV antibodies, single-dose, cervical cancer prevention
Background
Human papillomavirus (HPV) vaccines are highly efficacious and could prevent most cervical cancers if vaccine uptake is high [1]. Yet as of 2019, only 41% of low- and middle-income countries (LMIC) have introduced HPV vaccination in their national immunization programs compared to 80% of high-income countries (HIC) [2]. Because the LMIC are more populous, this imbalance in HPV vaccination by country results in ~70% of girls globally living in countries that have not yet introduced HPV vaccination [2]. Ninety percent of the cervical cancer burden occurs in these countries; consequently, little progress toward cervical cancer elimination can be expected in the coming decades unless there is dramatic increase in HPV vaccination.
A decade ago, we published a post-hoc analysis of the Costa Rica HPV Vaccine Trial (CVT, ClinicalTrials.gov Identifier: NCT00128661) that showed that four years post vaccination a single-dose of the bivalent HPV vaccine (Cervarix®) had the same vaccine efficacy as two and three doses against incident cervical HPV16/18 infections that persisted a year or more [3]. Recently, we extended that observation, showing that a single dose continues to protect against HPV16/18 infections more than a decade after initial vaccination, addressing concerns about durability of single-dose regimens [4]. We further observed that single-dose HPV vaccine recipients in CVT had a stable systemic antibody response, albeit at lower levels than those induced by three doses, thus necessitating evaluation of virologic endpoints to determine single-dose HPV vaccine efficacy [5]. In 2016, a post-hoc analysis of a vaccine trial in India demonstrated that a single dose of the quadrivalent HPV vaccine (Gardasil) strongly protected against persistent HPV16/18 infections seven years following initial vaccination. Together, these studies suggest that a single dose of either of the licensed HPV vaccines may provide strong and durable protection against persistent HPV infections, a necessary intermediate on the pathway to cervical cancer.
Given the potential public health implication of these findings, we implemented a large, non-inferiority trial evaluating both the bivalent and nonavalent HPV vaccines, as well as concomitant surveys of unvaccinated women, designed to address two policy questions: 1) is one dose non-inferior to the currently-WHO-recommended two doses in protecting adolescents from subsequent acquisition of persistent cervical HPV16/18 infection? and 2) what level of vaccine efficacy does a single-dose provide, an important estimation given the majority of the girls in the world remain unvaccinated? We aim to generate the data needed to motivate policy change, should a single dose demonstrate robust protection against incident persistent HPV infections. Herein, we present the rationale and design of the ESCUDDO trial and describe the enrolled study population.
Methods
Design and objectives of the study
ESCUDDO (NCT03180034) is a randomized trial to compare the efficacy of one- versus two-doses of two HPV vaccines. The study also includes surveys of unvaccinated women to estimate the efficacies of these regimens compared to no vaccination. We aim to evaluate the bivalent HPV16/18 virus-like particle with AS04-adjuvant vaccine (Cervarix®) and HPV6/11/16/18/31/33/45/52/58 virus-like particles with aluminum-containing adjuvant (Gardasil®9). Both vaccines are FDA approved.
The primary objectives of the study are: (1) to compare the infection rates of incident HPV16/18 infections that persist 6 or more months for one- versus two-doses, and (2) to estimate the vaccine efficacy of one-dose, compared to no vaccination, for preventing HPV16/18 infections that persist 6 or more months. The evaluation is done for each vaccine separately.
In addition, multiple secondary and tertiary objectives are envisioned for both vaccines. We will measure the immune response to each regimen, evaluate the vaccine efficacies against any new carcinogenic HPV infections, evaluate the budget impact (i.e., impact on a payer’s budget) and cost-effectiveness of each regimen, and, for Gardasil-9, compare the vaccine efficacies of one- versus two-doses for preventing any vaccine-targeted oncogenic HPV infection (i.e., aggregate HPV 16/18/31/33/45/52/58) and HPV6/11.
The study includes two components: (1) Trial: a controlled, randomized, double-blinded non-inferiority clinical trial; and (2) Surveys: two epidemiologic surveys for HPV status among unvaccinated women.
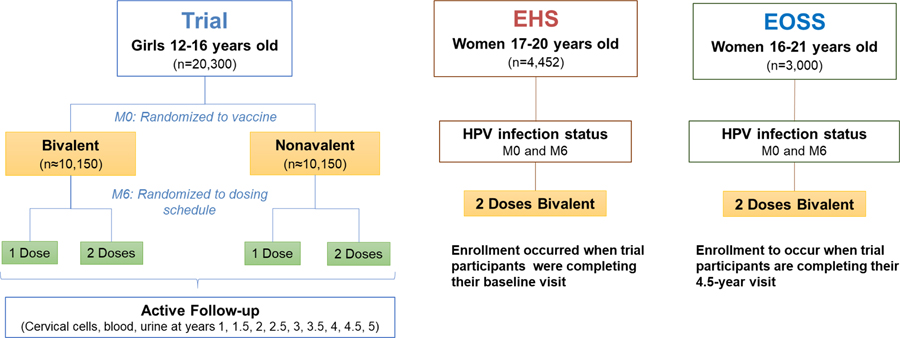
We first consider the Trial. The Trial aimed to enroll 20,000 girls 12 to 16 years old residing in Costa Rica. Girls were enrolled and randomized in two stages into one of four arms (i.e., 1-dose Cervarix, 2-dose Cervarix, 1-dose Gardasil-9, 2-dose Gardasil-9). Girls randomized to the 1-dose arms received an active control (Adacel®, Sanofi Pasteur, Ltd. Tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine, adsorbed) at the time of the second vaccine dose to maintain blinding (Figure 1). After vaccination, girls are followed for 5 years. Girls younger than 15 years are followed once a year and participants aged 15 years and older are followed every six months. For evaluating the primary endpoint, girls 15 years or older provide a self-collected cervicovaginal swab at each visit. For each vaccine, the study will compare the number of incident, 6-month persistent infections that occur in the 1-dose arm with the number of incident, 6-month persistent infections that occur in the 2-dose arm.
Figure 1.
ESCUDDO study design
EHS, Epidemiologic HPV Survey; EOSS, End-of-Study Survey; M, month.
To evaluate a secondary objective of measuring the immune response to one and two doses and its stability after reaching plateau levels, a sub-group of 1400 (350 per arm) 12–14 years old girls were invited to participate in an Immunogenicity Sub-Group (ISG) and a subset of the ISG was invited to the peripheral blood mononuclear cell (PBMC) subgroup. The age restriction for the ISG aims at measuring true vaccination response among girls unexposed to HPV, since girls younger than 15 are less likely to be sexually active. The ISG participants attended a special visit one month following each vaccination (month 1 and month 7) and an 18 month visit to characterize peak and kinetics of antibody responses, and provided additional specimens at their regularly scheduled visits.
We now consider the Survey component. We note that the Trial by itself does not allow estimation of the vaccine efficacy (e.g., VE = 1 - rate in vaccinated/rate in unvaccinated) and, for ethical reasons, we chose not to enroll 12–16 year-old girls into a placebo arm. We therefore added an “Epidemiologic HPV Survey” (EHS), and aimed to enroll 4000 women between the ages of 17–20 years who lived in the same geographic regions as the trial participants. These young women were enrolled concomitant with trial participants and attended only two study visits six months apart. We can estimate vaccine efficacy by comparing the rates of 6-month persistent infection in this EHS with the rates in the follow-up visits of trial participants. Note that we did not extend the EHS below age 17 because the first two trial visits provided similar information for that younger group. EHS participants were offered HPV vaccination at these visits as a benefit of participation. Importantly, HPV vaccination is understood to have no influence on the persistence of an HPV infection [6, 7].
Modifications to the original design of the study
When the enrollment phase of the study ended, the COVID-19 pandemic began to affect the Costa Rican population. We modified some aspects of the original study design to mitigate its possible effects on the study. For the trial component, the followup of trial participants was extended by one year to a total of 5, comprising two visits in years 4.5 and 5. We were concerned that physical separation and distancing measures due to COVID-19 may modify sexual behavior and reduce exposure and HPV attack rates among trial participants. Extending follow-up when trial participants are between the ages of 17 and 21, peak years for HPV exposure, was expected to generate more events compared to the early years of follow-up.
The COVID-19 pandemic may introduce a period effect and further alter the prevalence of HPV infection over calendar time; we were concerned that if the background HPV infection rate decreased during the assessment of HPV infection towards the end of the trial (when the majority of endpoints in the vaccinated arms are expected to occur), it may inflate estimates of vaccine efficacy. To ensure the validity of the results of the survey component, we plan to conduct a second survey at the end of the study (EOSS). We aim to enroll 3000 unvaccinated participants concurrent with the trial visit at year 4.5.
Randomization and blinding protocol
A custom smartphone app for randomization was developed in collaboration with Information Management Services (IMS) and study investigators.
Participants in the trial component were individually randomized in two stages which resulted in a four-arm randomization with a 1:1:1:1 ratio. For the first randomization at enrollment, for each age stratum (12–14 and 15–16 year olds) and geographic unit, we used blocked randomization (in blocks of four) to successively assign whether a girl received the bivalent or nonavalent HPV vaccine. To create the geographic units for this randomization, neighboring districts were combined, as needed, such that each unit had an estimated population of at least 800 girls 12 to 16 years old according to national census data (2011). For the second randomization at the six-month visit, for each age stratum and HPV vaccine type, we used a blocked randomization (in blocks of four) to successively assign whether a girl received the second HPV vaccine dose or the active control.
Throughout the study, randomization results are held exclusively by IMS and are not made available to the US or Costa Rican investigators or their staff.
Since the study vaccines are commercially available and thus have unavoidable physical differences in the packaging, an independent vaccination team (IVT) not supervised by the investigators was established to effectively blind the vaccine. The IVT performed the randomization, administration and management of the vaccines and did not take part in any other study procedure.
Throughout the study, investigators and participants remain blinded to both the vaccine type and the number of doses received by the participants.
Study population and recruitment
Data from the 2011 national census from the National Institute of Statistics and Census (INEC) were used to define the study catchment area that ensured a sufficient base population to fulfill the study goal of enrolling 20,000 girls 12 to 16 years old. Costa Rica has 472 districts; of these we excluded districts that had fewer than 150 projected resident girls and those located far from headquarters. Ultimately, 202 districts were included in the study.
In ESCUDDO, we will evaluate individual-level rather than population-level protection against HPV (i.e., herd immunity). Consequently, to avoid the induction of herd immunity, we restricted enrollment in each district to 35% or less of the projected population of girls ages 12 to 16 years. Districts are conceptually divided into minimal geo-statistical units (MGUs).We randomly selected a subset of the MGU’s in each district, in order to control the maximun proportion of girls vaccinated in any district, diminishing opportunities for the induction of herd immnunity. The selected MGUs were further grouped into waves so that selected MGUs within the same district were not all released for recruitment at the same time. For each district in the first wave, 30% of the MGUs were released and in the second wave, 33%. All girls ages 12 to 16 years in the randomly-selected MGUs were potentially eligible to participate in the non-inferiority trial.
Trained study outreach workers conducted a census of girls ages 10 to 16 years in each selected MGU. Information was collected on 10- and 11-year old girls, who were expected to reach the eligible study age within the two-year enrollment period. During registration or a subsequent visit, the outreach worker informed potentially eligible girls and their parents about the study, invited them to participate, and scheduled an enrollment appointment at a study clinic. Potential participants were invited at least three times.
The same methods were utilized for recruitment of 17- to 20-year old women in the concurrent EHS group. However, given the smaller accrual targets, outreach workers initially only enumerated and invited EHS women at every fourth household within a given MGU.
The enrollment of trial and EHS participants started on November 29, 2017 and was completed on February 28, 2020 with periodic monitoring of accrual goals by age and study area. On June 21, 2019, registration of girls aged 10 and 11 years was stopped because we had already enrolled comparatively more 12-year old than 16-year old girls. Similarly, several times during the course of the study we adjusted the sampling probability for the EHS group.
The enrollment of 16- to 21-year old women into the EOSS is planned to start in May 2022 when the trial participants are completing their 4.5-year visit; the second visit used to confirm HPV persistence will occur concurrent with the 5-year trial visit. Whereas previously we randomly selected 2/3 of all MGUs in each district of our catchment region for recruitment of the trial and EHS participants, to avoid the invitation of girls who previously refused to participate in the trial, we will use the remaining 1/3 of unused MGUs in those same districts for EOSS recruitment. Identification and invitation of EOSS participants will involve outreach workers and procedures similar to those used during initial enrollment of the EHS.
Organization of the study
The study is conducted using our network of clinic teams serving the districts throughout the catchment area. Twenty-four clinics were established to conduct the study procedures. Each team included clinic staff (a physician, a nurse, a driver, an informed consent administrator, a janitor, a supervisor, and an IVT nurse) and field staff (outreach workers and a field supervisor).
The main headquarters located in Liberia, Guanacaste, serves as the principal logistic center. It houses the vaccine and specimen repositories, document center, supply center, information technology, and quality control department and is supported by two logistic centers. The vaccines were distributed weekly to the logistic centers. Study materials, including paperwork, specimens and vaccines are transported daily to and from the clinics in study vehicles. Receipt, processing (if needed), and interim storage of materials is completed each night at the logistic centers and transported weekly or more frequently to the main headquarters.
Eligibility criteria and informed consent
At the enrollment visit, the study staff first confirmed: participant age, current residence within the study area, and having no plans to move outside of the country in the next six months. A wristband with the name and study ID number of the participant was used to help the staff confirm her identity throughout the study visit. Participants and their parent or legal guardian were asked to watch a video with the contents of their respective assent or consent forms, then the staff completed the consent process. At the selected clinics, trial participants were also invited to participate in the ISG subgroup and administered a separate assent and consent.
Next, all potential participants were asked if they were attending school and the name of it. Potential EHS participants were asked for address history at the district level over the last 4 years, to evaluate, in the future, the comparability with trial participants.
A study physician determined final eligibility, and potential participants were excluded if they had an autoimmune, degenerative or neurological disease; a genetic immunodeficiency; or any other serious chronic disease without treatment and/or adequate control. Also, they were excluded from enrollment if they were allergic to one of the vaccine components, yeast or latex; if they had been vaccinated against HPV or if the investigator considered that there was a reason that precluded participation. A clinician took anthropometric measurements and pulse rate.
If the potential participant was confirmed to be in good general health, she was asked to provide a urine sample and to perform a self-administrated pregnancy test. If negative, she was considered eligible and the remaining study procedures and collection of specimens proceeded (Table 1).
Table 1.
Timing of vaccination and specimen collection in the ESCUDDO study.
| Procedure | Study Visit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| M0 (yr-0) | M1* | M6 | M7* | M12 (yr-1) | M18 (yr-1.5) | M24 (yr-2) | M30 (yr-2.5) | M36 (yr-3) | M42 (yr-3.5) | M48 (yr-4) | M54 (yr-4.5) | M60 (yr-5) | |
|
| |||||||||||||
| Trial EHS | Trial | Trial EHS | Trial | Trial | Trial | Trial | Trial | Trial | Trial | Trial | Trial EOSS | Trial EOSS | |
|
| |||||||||||||
| Vaccination | • | • | • | • | |||||||||
|
| |||||||||||||
| Specimen collections for all participants | |||||||||||||
| Urine for HPV test | • | • | • | • | • | • | • | • | • | • | • | • | |
| 10 mL blood for serum | • | • | • | • | • | • | • | • | • | • | |||
| Self-collected cervicovaginal sample (15+ years only) | • | • | • | • | • | • | • | • | • | • | • | ||
|
| |||||||||||||
| Additional samples collected from ISG | |||||||||||||
| Urine for other tests | • | • | • | ||||||||||
| Spit | • | • | • | • | • | • | • | ||||||
| 10 mL blood for serum | • | • | • | • | • | • | • | • | |||||
| Blood for PBMC isolation | • | • | • | • | • | • | • | • | |||||
M, Month; yr; year; EHS, Epidemiologic HPV Survey; EOSS, End-of-Study Survey; ISG, Immunogenicity Subgroup; PBMC, Peripheral Blood Mononuclear Cells.
Visits conducted only among ISG participants.
Enrollment was deferred if a participant had a positive pregnancy test, if she had an acute condition that precluded vaccination, or if she was being evaluated to rule out a diagnosis of a chronic disease, if she received immunoglobulins withing 90 days, or if she received a registered vaccine within 30 days of enrollment except for Meningococcal, Hepatitis B, Influenza, and Diphtheria/Tetanus vaccines up to eight days before any dose of study vaccine.
Clinical procedures and specimen collection at enrollment
Participants collected 15 mL of first-catch urine (initial urine stream) and were instructed on how to perform the pregnancy test. Then, the clinician prepared two 2 mL urine aliquots for HPV testing and placed them in vials containing 1 mL of PreservCyt solution (Hologic Corp). For participants in the ISG subgroup, two additional urine 5 mL aliquots for hormone testing were prepared and placed in vials without preservative.
Participants were asked to complete a self-administered questionnaire on transportation used and time expended to attend the visit (for cost analysis), education, pubertal development, menstrual period characteristics, cigarette smoking and, among participants 15 years or older, sexual activity.
Participants 15 years or older were asked to provide a self-collected cervicovaginal swab, irrespective of initiation of sexual activity. The physician verbally explained to the participant how to collect the cervicovaginal sample, showed a pictorial diagram, and answered any questions before guiding participants to the bathroom to conduct the procedure. Participants were instructed to insert a sterile Dacron swab into the vagina and to firmly rotate the swab 5 times 360°C around the cervix, and then place the swab in a vial containing 2 mL of PreservCyt solution. Once the procedure was completed, the physician retrieved the sample and vigorously rinsed the swab in the medium, cut the handle of the swab at the level of the vial height and placed the cap on the vial.
Next, all participants were asked to provide a blood sample to obtain serum. Among the consenting ISG subgroup, participants provided an additional serum sample and, in selected cases, four additional blood samples were collected in acid citrate dextrose (ACD) solution for cryopreservation of PBMCs. ISG participants were also asked to provide a saliva sample for immunologic assays to evaluate the mucosal immune response. Finally, the staff escorted the participant to the vaccination room.
The urine aliquots in PreservCyt, cervicovaginal self-collected specimens, and blood samples for serum were stored in cold boxes in the clinics at 2–10°C, blood samples in tubes containing Acid Citrate Dextrose (ACD) were stored in cold boxes at 20–24°C. The ISG-only urine aliquots (without preservative) and the saliva samples were frozen immediately in liquid nitrogen (LN) vapor phase at the clinics. All samples were transported to the logistic centers at the end of each clinic day.
Randomization and vaccination of the participant
The IVT nurse first verified the identity of the participant asking her the date of birth and entering the information in the randomization app and verified the information displayed against the name and the participant ID written in the wristband.
Then, the IVT nurse went into a separate controlled-access area within the vaccination room where the vaccines were stored at 2–8°C in a cold box. There, the IVT nurse used the smartphone app to perform the randomization procedure. If the participant was part of the trial component, the app randomized her to one of two of the HPV vaccine products. If she was part of the EHS, the app assigned her to the Cervarix vaccine. Then, the IVT nurse retrieved the corresponding vaccine vial type and loaded a standard generic syringe with the content of the assigned vaccine.
The IVT nurse took the syringe into the vaccination room and vaccinated the participant intramuscularly in the deltoid muscle (upper arm), typically on the non-dominant arm. The nurse registered the type of vaccine administered and indicated which arm was vaccinated in the app.
The participant was kept under observation for 15 minutes after vaccination to monitor her response to the vaccine and observe any side effects or adverse reactions. The enrollment visit finished by assigning the next study visit, removing the wristband, and offering transportation in the study vehicles or reimbursement of travel expenses.
Six months vaccination visit
The second vaccination visit occurred approximately six months following the enrollment visit. The study physician assessed eligibility for the second vaccine dose, including pregnancy test, occurrence of serious adverse event reactions or newly diagnosed chronic conditions after the first vaccine. Participants with positive pregnancy tests were deferred, but if a participant refused or was ineligible for the second vaccine, she was excluded from vaccination but permitted to continue in the study. Study procedures at the second visit included a self-administered questionnaire, blood specimen collection, and a self-collected cervicovaginal sample if 15 years or older (Table 1). For ISG participants, a second set of urine aliquots was prepared, and a saliva sample and additional serum tube were collected (Table 1).
At this visit, participants were randomized to the number of vaccine doses (one or two doses). Girls randomized to the two-dose arm received a second dose of the HPV vaccine (whichever they received at enrollment), whereas girls randomized to the one dose arm received an active control (Adacel). Participants in the EHS received the second dose of Cervarix. Vaccination procedures were completed by the IVT nurse and participants were monitored for adverse events the same way as in the enrollment visit. If the participant did not receive the second vaccine dose at this visit, the vaccine was not provided in any further study visit.
Active follow-up year 1 through 5 and enrollment of EOSS
The follow-up visits are conducted at the clinic or at the participant’s home and include a self-administered questionnaire, urine sample, and for those aged 15 years or older, an inquiry on self-reported genital warts and a self-collected cervicovaginal specimen (unless pregnant), and annual blood sample collection (Table 1). Participants from the ISG are asked to provide saliva samples at 1.5, 2 and 3 year visits and additional blood samples for cryopreservation of PBMC at the 2 and 3 year visits (Table 1).
Enrollment of the EOSS participant will occur concurrent with the trial visit at year 4.5. Participant in the EOSS will undergo enrollment visit procedures similar to those performed at enrollment of the initial EHS (Table 1). Also, inclusion criteria for the EOSS are similar as in the trial and EHS apart from the age range (16–21 years old), but we will not exclude EOSS women from participating on the basis of having a chronic condition, or medical condition for which vaccination is contraindicated. This change is in order to make the EOSS participants more similar to the trial participants who are attending their 4.5- and 5-year visits, some of whom may have been diagnosed with a chronic disease or other condition during study follow-up. EOSS will be asked to complete a follow-up visit 6 months later, concurrent with the trial 5-year visit. EOSS participants will be offered HPV vaccination as a benefit of participation.
Vaccine handling
The vaccines were received and stored in the main headquarter, then transported weekly in cold boxes to the local logistic centers. At all levels of storage and distribution, a strict cold chain control was followed. The traceability of each vaccine dose was tracked by a unique code label. The IVT staff was in charge of the investigational product custody, inventory, and final accountability. All electronic and paper documents related to the vaccine were kept separately by the IVT.
Specimen handling
Samples collected at the clinic are placed in certified cold boxes equipped with thermometer and alarm system. On the same day, lab technicians prepare aliquots from the blood (serum) and the self-collected specimens. Biospecimens are frozen and temporarily stored at −80°C at the logistic centers. Logistic centers receive and inventory saliva and urine aliquots prepared at the clinics. Tubes containing ACD from the ISG are processed on the same day to isolate the PBMCs using the ficoll-hypaque gradient method and are frozen with liquid nitrogen under controlled conditions, a process completed exclusively in the repository at the main headquarters. About every two months, biospecimens are sent to the NCI Frederick Central Repository for long-term storage using large capacity dry vapor liquid nitrogen shippers. One aliquot of the self-collected cervicovaginal sample and one aliquot of the ISG serum sample are retained in the biorepository in Costa Rica. Samples and the aliquots produced are tracked using the NCI biospecimen inventory system; BSI-II (IMS, MD).
Monitoring of adverse events
There is considerable accumulated data on the safety of the HPV vaccines used in this study. Nevertheless, all reactogenicity symptoms are documented and followed through resolution. Also, all serious and non-serious adverse events (unfavorable occurrences or medical events that happen to a participant) are reported to the study physician and followed through resolution, regardless of their possible relationship to vaccination. After each vaccination, participants were evaluated at the end of their 15 minutes observation period by the clinic doctor, who documented any reactogenicity symptoms (e.g., pain at the injection site, dizziness, headache) or adverse events (e.g., fainting, difficulty breathing). All participants were instructed to report any additional symptoms or adverse events, mainly in the month after each vaccine administration, regardless of possible relation to the vaccine. At the visit following each vaccination, participants were asked directly if they had experienced any new disease diagnoses or been hospitalized for one or more days since their last visit. Subsequently, adverse events are documented whenever they are spontaneously reported by a participant. The reactogenicity symptoms were reported weekly to the local Institutional Review Boards (IRB). The related and unrelated non serious adverse events are reported monthly to the local IRB. All deaths are documented and reported to the IRB irrespective of timing between the occurrence of the event and vaccination. All study staff, including non-clinical staff are trained to initiate adverse event reports.
Because of the young age-range of the study participants, all pregnancies are documented and followed up to assure they receive prenatal care and to document the outcome. All adverse events related to the pregnancy are documented and reported.
Regulatory supervision and quality control
The study was approved by the IRBs from Costa Rica and the United States. The primary IRB is the Instituto Costarricense de Investigaciones Clínicas IRB (CEC-ICIC). A data and safety monitoring board (DSMB) with members from Costa Rica and the US carries out periodic evaluations of participant safety and study progress. In addition, an independent Scientific Working Group advises the study investigators on scientific and policy issues surrounding the trial.
The study is conducted under a quality management system to assure custody of study documents, compliance with internal and external audits plans, and compliance with the monitoring plan for data collection, specimens and vaccine management. All the information from the participants is collected using secure electronic case report forms in an electronic system developed in collaboration with IMS.
HPV testing
Once the self-collected cervicovaginal specimen is received at the logistic center, the lab technician vigorously rinses the swab in the medium using a vortex and prepares and then freezes three aliquots following PCR-safe procedures for type-specific HPV detection. HPV genotyping will be performed using the NCI-developed TypeSeq assay and run at the Next-Generation Sequencing (NGS) lab at ACIB-FUNIN using the TypeSeq 3-stage PCR workflow [8, 9]. This assay detects 51 HPV genotypes by next-generation sequencing (NGS) and consists of three PCR steps that normalize viral load and each type’s amplicon copies. Genotyping is done by Ion S5 NGS followed by custom Torrent Suite plugin analysis (Thermo Fisher Scientific, Waltham, MA, USA). A binary result of positive or negative is reported for the human positive control and for each of the 51 HPV types detected by the assay [8]. All cervical samples will be tested.
HPV serology testing
Once serum samples are received and inventoried at the NCI Frederick Central Repository, they are transferred to the DNA Extraction and Staging Laboratory (DESL) to perform the blinded aliquot process and to include blinded replicates. These blinded samples are shipped to the Serology Laboratory for HPV serology testing. HPV-specific serum antibodies will be measured using a 9-plex Luminex-bead based immunoassay. The HPV type-specific virus like particles (HPV-6, -11, -16, -18, -31, -33, -45, - 52, and -58) are coupled to separate Luminex bead sets. Serum is incubated with the Luminex beads, washed, and incubated with goat anti-human IgG antibody tagged with R-Phycoerythrin to detect IgG antibody interactions with each HPV type-specific virus-like particle. Following a final wash procedure, the fluorescent signal is measured on a Luminex instrument and the fluorescent signal is proportional to the concentration of the HPV type-specific IgG antibodies within the sera. An internal reference standard (contains a specific concentration of anti-HPV type-specific antibodies), two levels of positive control (each contain a specific concentration of anti-HPV type-specific antibodies), and negative control (lack anti-HPV type-specific antibodies) are tested on each plate to assess plate acceptability, quantitate a relative amount of HPV type-specific IgG antibodies within the sera. In addition, antibody levels are presented as International Units per milliliter. Of note, HPV16/18 antibodies (for our secondary objective) as well as the additional five carcinogenic HPV types in the nonavalent HPV vaccine (ancillary objective) for all samples in the ISG group will be primarily analyzed for the study. Tests for neutralizing antibodies will also be conducted. We will monitor immune response to vaccination, both humoral (i.e., antibody; B-cells) and cell-mediated (i.e., T-cells) using samples from the ISG PBMC subgroup.
Statistical methods
The statistical methods have been previously described [10] and will only be briefly reviewed here.
Our first objective is to compare the incidence (π1) of persistent infection during the trial in the 1-dose group with the incidence (π2) of persistent infection during the trial in the 2-dose group for each vaccine separately. Specifically, we aim to show that their difference Δ = π1 ― π2 does not exceed a specified threshold, ΔN. In other words, we will test the null hypothesis H0:Δ > ΔN. Our proposed method [10] for estimating Δ and testing H0 accounts for the fact that participants are expected to miss some visits. Because of updated assumptions of sexual activity based on ESCUDDO’s baseline demographic data and extension of the follow-up of the trial from 4 to 5 years, the value ΔN has been updated from 0.00986 to 0.012 since writing the statistical design paper [10].
Our second objective is to estimate the Vaccine Efficacy (VE) against incident persistent infection for each of the four regimens (i.e., 1-dose Cervarix, 2-dose Cervarix, 1-dose Gardasil-9, 2-dose Gardasil-9). We note that we could get an approximate VE of a regimen by comparing the rate (rT) of persistent infection in the last two trial visits to the rate (rs) of persistent infection in the two survey visits (i.e., VE ≈ 1 - rT/rs). However, in our statistical design paper [10], we describe a more accurate and robust analysis that can (i) use incident persistent infections instead of all persistent infections in the trial arm and (ii) account for differences in covariates between trial and survey participants. Furthermore, we noted the possibility of increasing power by using information from additional trial visits, and have ultimately decided to use all trial visits starting with the year 2 visit.
Micro-costing and cost-effectiveness study
Even if one-dose protection is found to be lower than the protection associated with two doses, we expect that vaccinating with one dose would yield cervical cancer cases and deaths averted compared to no vaccination and may yield greater benefits compared to two doses if higher coverage can be achieved. To quantify the potential tradeoffs between resource utilization, simplicity of implementation and administration, and effectiveness associated with each regimen, we plan to 1) quantify the financial budget impact of each regimen, and 2) estimate the lifetime cost-effectiveness (i.e., value) of each regimen. We will apply standard micro-costing methods to quantify resources that would be used for each regimen within Costa Rica’s national vaccination program. These resources include direct medical costs (e.g., vaccine doses, supplies), direct non-medical costs (e.g., transportation costs), and programmatic costs (e.g., patient outreach, personnel training). Cost data will then be used to estimate the budget impact of one-dose HPV vaccination and assess the cost-effectiveness of one-dose vaccination versus two-dose or no vaccination. Health decision models— including a dynamic transmission model [11] that simulates sexual transmission of HPV infections between men and women and an individual-based microsimulation model [12] that reflects cervical carcinogenesis in women— will then incorporate cost and vaccine effectiveness data to estimate the cost-effectiveness of each regimen in the context of Costa Rica’s cervical cancer screening program. We will perform in-depth sensitivity analyses to examine the robustness of findings under different scenarios of costs, vaccine uptake, and long-term efficacy.
Results
Recruitment, demographics and initial compliance with protocol follow-up
Trial:
Our Trial is conducted across a large region of Costa Rica (Supplementary Figure 1). Of the 472 districts in the country (Table 2), our catchment region included 202 districts. Those 202 districts include 20,336 MGUs and, according to projected estimates from the 2011 national census, 93,749 girls who will be 12–16 years during our calendar period of interest, accounting for 50.6% of all MGUs and 54.3% of all 12–16 year old girls in the country.
Table 2.
Description of the country’s geographical limits, target population size, and the study catchment area, according to the 2011 census
| Description | Country N | Catchment Region N (%A) | Released N (%B) |
|---|---|---|---|
| Districts | 472 | 202 (42.8) | |
| MGUs | 40,211 | 20,336 (50.6) | 13,350 (65.6) |
| 12–16 year olds | 172,740 | 93,749 (54.3) | 61,759 (65.9) |
| 17–20 year olds | 153,631 | 83,192 (54.2) | 54,773 (65.8) |
MGUs, Minimal geo-statistical units; N, total number derived from the 2011 Census
Percentage of total country
Percentage of catchment region
We recruited participants from the 13,350 (nearly 2/3 × 20,336) released MGUs. The census counted 495,933 households in these MGUs of which we identified 96.7% (n=479,751 households) (Table 3). The census counted 61,759 12–16 year old girls in these MGUs and we identified approximately 33,174 girls (Table 3). Therefore, we identified approximately 53.7% of the eligible population.
Table 3.
Proportion of households and potential participants identified by ACIB staff compared to the national census, for both the trial and survey, within the primary catchment area (districts = 202/472)
| Census | Trial | Census (weighted)A | EHS | |
|---|---|---|---|---|
| Households | 495,933 | 479,751 (96.7%) | 100,019 | 96,755 (96.7%) |
| Girls 12–16 years of age | 61,759 | 33,174 † (53.7%) | 11,046 | 6,926 (62.7%) |
MGUs, Minimal geo-statistical units; EHS, Epidemiologic HPV Survey
Whereas the raw census numbers were used to compute the proportion of households identified and potentially-eligible girls for the trial, we needed to weight census counts for the survey because, by design, we only approached a subset of the households and girls (roughly 20% because we needed about to enroll about 4000 survey participants compared to 20,000 trial participants (or ∼20%). Thus, the census (weighted) counts are the raw census numbers multiplied by the following weighting factor: number of survey houseolds identified divided by the number of trial households identified = 96,755/479,751 ≈ 1/4.96 or 20.2%. This adjustment to the census enables a more accurate denominator for the calculation proportion of households and potential participants identified for our study.
29,734 registered when they were 12–16 years old; 3,440 girls registered when they were 10–11 years.
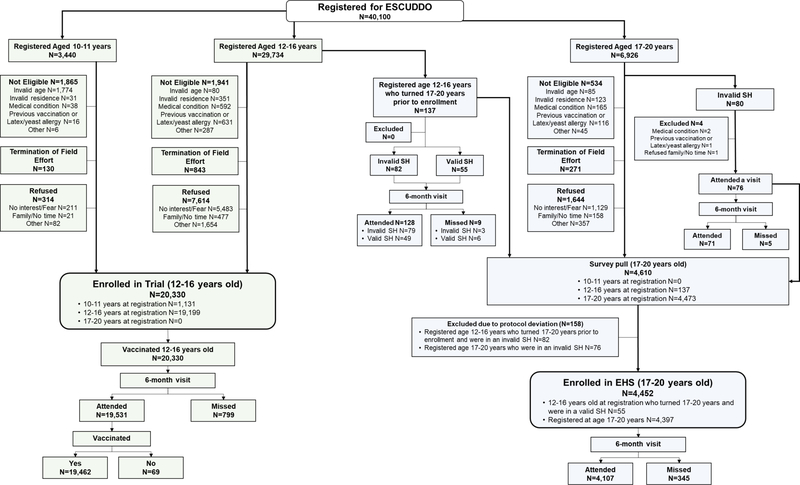
Among the 33,174 registered girls, 20,330 girls were successfully enrolled into the trial and were randomized and vaccinated (Figure 2); the recruitment phase ended just prior to COVID-19 pandemic in Costa Rica. The main reasons for not enrolling included refusal, termination of field efforts when 35% of the projected population of girls in the district was enrolled, or failure to meet eligibility requirements (Figure 2). Therefore, we provided at least one vaccination to 21.7% (=100 × 20,330/93,749) of the girls in the eligible population (Table 4). This percentage varied by district (supplementary Figure 2). The maximum vaccination proportion was 34.1% (below our pre-specified herd immunity threshold of 35%) and 74.8% of all districts vaccinated less than 30.0% of girls in the age groups of interest.
Figure 2.
CONSORT diagram of the ESCUDDO study.
SH, survey house. Invalid SH were houses selected during fieldwork but were not within the established selection fraction. Valid SH were houses selected during fieldwork within the established selection fraction.
EHS, Epidemiologic HPV Survey.
Table 4.
Different measures of response rates in Trial participants
| Description | Overall |
|---|---|
| Census 12–16 year old girls in catchment area | 93,749 |
| Census 12–16 year old girls in released MGUs | 61,759 |
| Registered 10–16 year old girls | 33,174a |
| Registered and eligible 10–16 year old girls | 28,258b |
| Enrolled 12–16 year old girls | 20,330 |
| Yield (enrolled/Census in released MGUs) | 32.9% |
| Response Rate (enrolled / Registered and eligible) | 71.9% |
| Vaccine uptake (enrolled / Census in catchment region) | 21.7% |
MGUs, Minimal geo-statistical units
29,734 registered when they were 12–16 years old; 3,440 girls registered when they were 10–11 years.
This excludes girls who were found to be ineligible, were not enrolled prior to the termination of the field effort in their MGU, or were not enrolled prior to reaching 17 years old.
The demographic characteristics of the 20,330 girls are presented in Table 5. Twelve-year-olds accounted for 23.9% of the cohort while 16 year-olds only accounted for 17.9% of the cohort. Most participants were living in the Greater Metropolitan Area (GAM) East, GAM West and Pacific regions (38.5% and 31.0%, 24.5%, respectively). The majority of girls were never-smokers, had a normal BMI, and were not using hormonal contraceptives. Among participants queried about sexual behaviors (i.e., age ≥ 15 years old), 67.7% had never had sex with a man and 19.4%, 6.2%, and 5.6% had one, two, and three or more lifetime partners, respectively.
Table 5.
Demographic and descriptive characteristics of Trial participants at enrollment
| Characteristics | N=20,330 | %‡ |
|---|---|---|
|
| ||
| Age (years) | ||
| 12 | 4857 | 23.9 |
| 13 | 4026 | 19.8 |
| 14 | 3958 | 19.5 |
| 15 | 3855 | 19.0 |
| 16 | 3634 | 17.9 |
|
| ||
| Region † | ||
| GAM East | 7826 | 38.5 |
| GAM West | 6307 | 31.0 |
| Pacific | 4983 | 24.5 |
| North | 1214 | 6.0 |
|
| ||
| Highest level of education completed | ||
| Primary or lower | 8437 | 41.9 |
| Secondary | 11709 | 58.1 |
| University | 1 | 0.0 |
| Technical School | 12 | 0.1 |
| DK/Refuse/Missing | 171 | |
|
| ||
| Smoking | ||
| Never | 17853 | 88.2 |
| Ever | 2388 | 11.8 |
| DK/Refuse/Missing | 89 | |
|
| ||
| BMI-for-age †† | ||
| Thinness/wasting (Z-score < −2SD) | 309 | 1.5 |
| Normal (Z-score between ≥−2 SD and ≤+1 SD) | 13028 | 64.1 |
| Overweight/obese (Z-score >+1 SD) | 6993 | 34.4 |
|
| ||
| Age at menarche (years) | ||
| Menses have not started | 2071 | 10.4 |
| 10 or under | 3325 | 16.6 |
| 11 | 5420 | 27.1 |
| 12 | 6142 | 30.8 |
| 13 | 2310 | 15.2 |
| 14+ | 705 | 6.3 |
| DK/Refuse/Missing | 357 | |
|
| ||
| Ever pregnant | ||
| No | 20084 | 98.8 |
| Yes | 246 | 1.2 |
|
| ||
| Hormonal contraceptive use | ||
| Never | 18843 | 92.7 |
| Ever | 1487 | 7.3 |
|
| ||
| Ever had genital warts | ||
| Not queried (<15 years old) | 12841 | |
| No | 7414 | 99.0 |
| Yes | 74 | 1.0 |
| DK/Refuse/Missing | 1 | |
|
| ||
| Ever had sex with a man | ||
| Not queried (<15 years old) | 12838 | |
| Never | 4969 | 67.7 |
| Ever | 2372 | 32.3 |
| DK/Refuse/Missing | 151 | |
|
| ||
| Age at sexual debut | ||
| Not queried (<15 years old) | 12838 | |
| No sexual debut | 4969 | 68.0 |
| ≤14 | 1040 | 14.2 |
| 15 | 957 | 13.1 |
| 16 | 343 | 9.7 |
| DK/Refuse/Missing | 183 | |
|
| ||
| Lifetime number of sexual partners among sexually active | ||
| Not queried (<15 years old) | 12838 | |
| 0 | 4969 | |
| 1 | 1423 | 62.1 |
| 2 | 455 | 19.9 |
| 3+ | 414 | 18.1 |
| DK/Refuse/Missing | 231 | |
|
| ||
| Number of sexual partners in the last 12 months among sexually active | ||
| Not asked (<15 years old) | 12838 | |
| No sexual debut | 4969 | |
| None | 452 | 19.5 |
| 1 | 1431 | 61.9 |
| 2 | 293 | 12.7 |
| 3+ | 137 | 5.9 |
| DK/Refuse/Missing | 210 | |
GAM, Greater Metropolitan Area; DK, Don’t know; BMI, Body mass index.
Don’t know, refuse and missing values are not taken into account for the calculation of the percentages.
GAM East includes districts from Cartago and San Jose provinces; GAM West includes districts from Alajuela and Heredia provinces; Pacific includes districts from Guanacaste, parts of Puntarenas provinces and from Upala canton; North includes districts from San Carlos canton.
BMI-for-age Z-scores calculated based on the WHO standards for children and adolescents between 5 and 19 years.
The demographics of the ISG and PBMC subcohorts are provided in Supplementary Table 1. These subcohorts were restricted to 12–14 year olds and the PBMC subcohort was further restricted to girls from the Pacific region; otherwise, characteristics reflected those of the overall study population.
Among the 20,330 enrolled girls, 19,462 girls (95.7%) attended their 6-month visit and received their second vaccination (Figure 2). In addition to high retention, participants overwhelmingly provided the requested specimens at the study visits (Table 6). At the enrollment visit, 98.1% of participants provided blood samples and 93.3% of the eligible participants (15 years or older) provided the self-collected cervicovaginal swab. Urine samples were a requirement for enrollment (to conduct the pregnancy test) and thus compliance was 100%. At the 6-month visit, 99.8%, 98.4%, and 94.5% of participants provided urine, blood, and cervical samples. We observed similarly high compliance with urine (97.5–100%), saliva (99.3–99.5%), blood (98.6–99.4%) and blood for PBMC (95.7–98.1%) collections from the ISG (Supplementary Table 2). Of note, ISG girls are under the age of 15 and therefore we do not ask them to provide a self-collected cervical cell sample.
Table 6.
Compliance with specimen collection during vaccination
| Sample type | Trial (12–16 years
old) |
EHS (17–20 years
old) |
||||
|---|---|---|---|---|---|---|
| Expected | Collected | % | Expected | Collected | % | |
|
| ||||||
| Enrollment visit | ||||||
| Urine | 20330 | 20328 | 100.0 | 4452 | 4452 | 100.0 |
| Blood | 20330 | 19938 | 98.1 | 4452 | 4380 | 98.4 |
| Self-collected cervical† | 7489 | 6984 | 93.3 | 4452 | 4271 | 95.9 |
|
| ||||||
| 6-month visit | ||||||
| Urine | 19598 | 19557 | 99.8 | 4134 | 4122 | 99.7 |
| Blood | 19598 | 19278 | 98.4 | 4134 | 4069 | 98.4 |
| Self-collected cervical† | 9307 | 8796 | 94.5 | 4134 | 3966 | 95.9 |
EHS, Epidemiologic HPV Survey
Only for participants 15 years or older.
Survey:
We conducted the EHS in the same catchment region that was used for the trial. According to the 2011 census, this area included 83,192 women aged 17–20 years (54.2% of the total population, Table 2). We recruited EHS participants in approximately 1-in-4.96 households within the 13,350 (nearly 2/3 × 20,336) released MGUs. According to the census, we expected approximately 11,046 individuals (nearly 83,192 × 2/3 × 1/4.95) in these households and we identified 6,926 women (Table 3). we identified 62.7% of the eligible population, a slightly higher percentage than in the trial.
Among those 6,926 registered women, we successfully enrolled 4,397 women into the EHS (Figure 2). Moreover, we enrolled 55 women from the 12–16 year age group at registration who aged into the 17–20 year age group at the enrollment visit (and thus were no longer eligible for trial enrollment), therefore we enrolled a total of 4,452 women (=4,397 + 55) into the EHS. We note that enrollment into the EHS did not require vaccination.
The demographics of the 4,452 EHS women are provided in Table 7. We recruited more women in the younger age range, with 29.1%, 30.3%, 23.4%, and 17.3% of the cohort being respectively 17, 18, 19, and 20 years old at enrollment. The majority of women were never-smokers, had a normal BMI, and were not using hormonal contraceptives. Among all participants, 31.0% never have had sex with a man and among sexually active women 41.3%, 23.0%, and 35.8% had one, two, and three or more partners, respectively. For most characteristics, it would be inappropriate to compare characteristics between trial and EHS participants due to the age difference (by design), but we do note that their geographic characteristics were broadly similar.
Table 7.
Demographic and descriptive characteristics of the Epidemiologic HPV Survey participants at baseline
| Characteristics | N=4452 | %‡ |
|---|---|---|
|
| ||
| Age (years) | ||
| 17 | 1295 | 29.1 |
| 18 | 1349 | 30.3 |
| 19 | 1040 | 23.4 |
| 20 | 768 ( | 17.3 |
|
| ||
| Region † | ||
| GAM East | 2062 | 46.3 |
| GAM West | 1180 | 26.5 |
| Pacific | 987 | 22.2 |
| North | 223 | 5.0 |
|
| ||
| Highest level of education completed | ||
| Primary or lower | 359 | 8.1 |
| Secondary | 3212 | 72.3 |
| University | 628 | 14.1 |
| Technical School | 241 | 5.4 |
| DK/Refuse/Missing | 12 | |
|
| ||
| Smoking | ||
| Never | 2819 | 63.5 |
| Ever | 1623 | 36.5 |
| DK/Refuse/Missing | 10 | |
|
| ||
| BMI | ||
| Low (<18.5 kg/m2) | 437 | 9.8 |
| Normal (18.5 to <25 kg/m2) | 2491 | 56.0 |
| High (25+ kg/m2) | 1524 | 34.2 |
|
| ||
| Age at menarche (years) | ||
| ≤10 | 648 | 14.9 |
| 11 | 947 | 21.8 |
| 12 | 1552 | 35.7 |
| 13 | 669 | 15.4 |
| 14+ | 533 | 12.3 |
| DK/Refuse/Missing | 103 | |
|
| ||
| Ever pregnant | ||
| No | 3688 | 82.8 |
| Yes | 764 | 17.2 |
|
| ||
| Hormonal contraceptive use | ||
| Never | 2385 | 53.6 |
| Ever | 2067 | 46.4 |
|
| ||
| Ever had genital warts | ||
| No | 4285 | 96.2 |
| Yes | 167 | 3.8 |
|
| ||
| Ever had sex with a man | ||
| Never | 1360 | 31.0 |
| Ever | 3030 | 69.0 |
| DK/Refuse/Missing | 62 | |
|
| ||
| Age at sexual debut | ||
| No sexual debut | 1360 | 31.2 |
| ≤14 | 629 | 14.4 |
| 15 | 689 | 15.8 |
| 16 | 634 | 14.5 |
| 17 | 561 | 12.9 |
| 18 | 355 | 11.5 |
| 19 | 100 | 5.7 |
| 20 | 30 | 4.0 |
| DK/Refuse/Missing | 94 | |
|
| ||
| Lifetime number of sexual partners among sexually active | ||
| 0 | 1360 | |
| 1 | 1214 | 41.3 |
| 2 | 676 | 23.0 |
| 3 or more | 1053 | 35.8 |
| DK/Refuse/Missing | 149 | |
|
| ||
| Number of sexual partners in the last 12 months among sexually active | ||
| No sexual debut | 1360 | |
| None | 399 | 13.4 |
| 1 | 1860 | 62.4 |
| 2 | 433 | 14.5 |
| 3 or more | 290 | 9.7 |
| DK/Refuse/Missing | 110 | |
|
| ||
| Age of the sexual partner at time of sexual debut among sexually active (years) | ||
| No sexual debut | 1360 | |
| ≤16 | 631 | 21.7 |
| 17 | 556 | 19.1 |
| 18 | 486 | 16.7 |
| 19 | 297 | 10.2 |
| 20 | 293 | 10.1 |
| 21+ | 644 | 22.2 |
| DK/Refuse/Missing | 185 | |
GAM, Greater Metropolitan Area; DK, Don’t know; BMI, Body mass index.
Don’t know, refuse and missing values are not taken into account for the calculation of the percentages.
GAM East includes districts from Cartago and San Jose provinces; GAM West includes districts from Alajuela and Heredia provinces; Pacific includes districts from Guanacaste, parts of Puntarenas provinces and from Upala canton; North includes districts from San Carlos canton.
Among the 4,452 enrolled women, 4,107 women (92.3%) attended their 6-month (Figure 2). In addition to high retention, participants overwhelmingly provided requested specimens (Table 6). At the enrollment visit, 98.4% participants provided blood samples and 95.9% provided the self-collected cervicovaginal swab. At the 6-month visit, 99.7%, 98.4%, and 95.9% of participants provided urine, blood, and cervical samples, respectively.
Discussion
In seeing the promise and public health potential of single-dose administration of the HPV vaccines, we designed and implemented a large-scale, non-inferiority trial of two HPV vaccines (bivalent and nonavalent), to determine if a single dose is non-inferior to two doses in the prevention of persistent HPV infection, and embedded in the trial a study to quantitate the HPV vaccine efficacy compared to no vaccination.
Our study had several important design considerations that were informed by global context. For example, two co-primary aims were selected because, while we understood recommending bodies would evaluate the policy question of how single-dose compares to the recommended two dose schedule we were also aware that more than half of all countries did not include HPV vaccination in the national vaccine programs, elevating the relevance of the evaluation of efficacy relative to no doses. As example The Costa Rican national vaccination program initiated their HPV vaccine campaign targeting 10-year old girls in 2019. The primary endpoint of our trial is virologic, because based on current evidence, a single HPV vaccine dose may provide adequate protection against HPV infection despite the induction of significantly lower antibody levels compared to multi-dose regimens. Histologic endpoints were considered unnecessary given HPV persistence is on the causal pathway and is considered a valid endpoint by WHO and other groups [13]. The resulting need to compare HPV incidence in the one- and two-dose arms necessitated a large sample size for adequate power. We considered it ethically important to offer HPV vaccination to all trial participants. We also viewed it as critical to evaluate individual-level protection and to avoid interference with our interpretation by herd immunity. Consequently, we designed our trial to vaccinate less than 35 percent of any district within the study catchment area, safeguarding against the introduction of appreciable herd effects and facilitating generalizability beyond the local HPV vaccine context.
Another key design decision of our trial was the selection of the non-inferiority margin, ΔN. This parameter choice was driven by considerations of vaccine efficacy, which is about 95% for two doses. We judged that if we could rule out a vaccine efficacy less than 80% for one dose, then one dose would be “non-inferior” to two doses. This criterion plus an estimate of the rate of incident persistent infection in unvaccinated women translated into a value of ΔN as described in [12]. However, we strongly believe, and think it is important to emphasize prior to our trial results becoming available, that a widely administered single-dose of HPV vaccine with efficacy even less than 80% could still avert great numbers of cervical cancer cases, compared to no HPV vaccination. Individual countries and regions will be able to use our data to determine how they define ‘sufficient’. Modeling efforts to inform that threshold in different scenarios and country-contextualized cost-effectiveness analyses will be important. The Costa Rican national vaccination program initiated their HPV vaccine campaign targeting 10-year old girls in 2019, as soon as the results of our trial are available, they will be presented to the National Immunization Commission for consideration.
Our careful and detailed approach to field work, as well as the enthusiasm and commitment from our staff and the prioritization of community engagement, resulted in the accrual of more than 20000 girls, individual randomization first to the HPV vaccine product and then to the number of doses by either receiving a second dose of HPV vaccine or the control vaccine, and excellent compliance with study visits and specimen collection. Our field effort was complex—the sample size was large, it involved girls and young adults and required the establishment of tens of clinics all over Costa Rica, as well as the selection and sophisticated training of hundreds of fully-dedicated study staff. Furthermore, we restricted enrollment to only a small proportion of any district, which in turn considerably increased the catchment area (the geographic area in which we worked) and the difficulty of the field work. The SARS-CoV-2 pandemic then interrupted the field effort, just after the completion of the enrollment phase and towards the end of the six-month vaccination visit, sometimes resulting in full shutdowns and then intermittent pausing of some clinics in high-transmission regions. We reduced the number of participants seen per day in our clinics to accommodate physical distancing while prioritizing the six-month vaccination visit which was essential for the study and had the benefit of vaccination. While the six-month visit was completed with ~ 95% retention, the consequence will likely be missed study visits in the followup phase when endpoints are assessed. Thus, the pandemic could potentially impact the science of our study in three ways: 1) missed study visits in the followup phase could reduce opportunities to observe endpoints, and 2) physical distancing could reduce opportunities for sexual behavior and thus lower the HPV attack rate, and 3) physical distancing could also result in temporal changes in HPV acquisition (i.e., a period effect) thus invalidating comparisons between HPV incidence in the beginning and end of the study (as was originally planned to address the second co-primary aim, comparison of endpoints between the EHS and trial to yield vaccine efficacy estimates). To mitigate the potential impact of the pandemic, we modified our study in two ways: 1) followup was extended by one year, adding two additional study visits in years 4.5 and 5 to address concerns about lower endpoints reducing study power, and 2) an EOSS was added avoid a possible period effect. The extension of our trial to five years roughly doubles the anticipated endpoints (virologic endpoints accrue towards the end of followup given the increasing age of the participants). The new EOSS will account for temporal changes in HPV incidence and render more valid estimates of vaccine efficacy. These modifications will ensure that ESCUDDO will remain the well-powered and valid trial that it was designed to be.
There are several additional research questions that ESCUDDO alone does not address. We question whether results from a trial in Costa Rica will generalize to other populations, particularly in countries with additional comorbidities that may compromise immune responses, such as HIV, parasites and malnutrition. Thus, the ESCUDDO trial formally has a sister study, the DoRIS trial (NCT02834637), an immunogenicity study of 9 to 14 year old girls in Tanzania that aims to evaluate the immune response for 1, 2, and 3 doses of the bivalent and nonavalent HPV vaccine [14], and will immunobridge to the efficacy results of ESCUDDO. Other ongoing single-dose trials aim to also address populations in Africa, including the HANDS (Gambia, NCT03832049) and KEN-SHE (Kenya, NCT03675256) [15] trials, which have the potential to expand the possible age range for single-dose HPV vaccine administration to 4 and 20 years, respectively. Special populations of interest, such as people living with HIV, will require additional research. At present, ESCUDDO will measure the durability of protection to 5 years; most trial followup ranges from three to five years whereas the modeling work suggests that 20 years of protection is critical to reduce cervical cancer incidence. In preparation for such questions, the non-randomized CVT and India (NCT00923702) [16] studies will continue to provide long-term durability data on the antibody response and efficacy of a single dose.
To control cervical cancer, WHO and other bodies recommend routine vaccination of adolescent girls with two doses of the HPV vaccine. Many countries have yet to include HPV vaccination in their national vaccine programs because of financial or logistical barriers to delivering two doses outside the Expanded Immunization Program (EPI) [17]. We designed and implemented the ESCUDDO trial to generate robust data on single-dose efficacy, in support of all girls and women having access to cervical cancer prevention in their lifetimes. All women have a fundamental and equal right to health-- inequity anywhere is a harm to humanity everywhere.
Supplementary Material
Highlights:
1-dose HPV vaccination may offer robust protection against HPV16/18 infections
ESCUDDO aims to evaluate 1 vs. 2 HPV vaccine doses to prevent HPV infection
Goal is to generate high-quality data to be used to change policy, if successful
Successfully enrolled the ESCUDDO study and are now following participants
Acknowledgments
We dedicate this work to the memory of our beloved colleague and friend Paula Gonzalez, the initial principal investigator of the ESCUDDO study. We extend a special thanks to the young women and girls of Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the staff involved in this project; we would like to specifically acknowledge the meaningful contributions by Carlos Avila, Manuel Barrantes, Gloriana Barrientos, Cynthia Castillo, Arianne Castrillo, Loretto Carvajal, Karla Coronado, Blanca Cruz, Cristian Montero, Rebeca Ocampo, and Josias Solis. In the United States, we extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data systems used in the trial and who serve as the data management center for this effort, especially Jean Cyr, Bran Handly, Scott Hostovich, Adam Vaughn, Dan Watson, Brian Befano, Dave Ruggieri and John Schussler. We thank study coordinator Kate Torres of Westat. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interests of participants (Kathy Edwards, Chair, Steve Self, former Chair, Ron Brookmeyer, Willem Bujan, Holly Janes, Ruth Karron, Fabian Madrigal-Leer, and Elizabeth Woods) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Henriette Raventós, Chair, Joanna Cain, Diane Davey, Gypsyamber D’Souza, Elizabeth Fontham, Anne Gershon, Elizabeth Holly, Silvia Lara, Wasima Rida, Richard Roden, Maria del Rocío Sáenz Madrigal, and Margaret Stanley).
Role of the funding source
ESCUDDO is part of a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored by the NCI Division of Cancer Epidemiology and Genetics, with funding from the Cancer Moonshot Initiative in the Intramural Research Program of the NIH, and with support from the National Institutes of Health Office of Research on Women’s Health. This work was supported, in part, by the Bill & Melinda Gates Foundation (OPP1130259). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Dr. Schiller is an inventor on U.S. government-owned patents that were licensed to Merck and GlaxoSmithKline, the makers of the commercial HPV vaccines. As such, he was entitled to limited royalties as specified by U.S. law. These licenses are now expired. Dr. Lowy is an inventor on U.S. government-owned patents that were licensed to Merck and GlaxoSmithKline, the makers of the commercial HPV vaccines. As such, he was entitled to limited royalties as specified by U.S. law. These licenses are now expired. John T. Schiller and Douglas R. Lowy report that they are named inventors on US Government-owned HPV vaccine patents that are licensed to GlaxoSmithKline and Merck and for which the National Cancer Institute received licensing fees. They were entitled to limited royalties as specified by federal law. The other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Global Health. 2016;4:E453–E63. doi: 10.1016/S2214-109X(16)30099-7 [DOI] [PubMed] [Google Scholar]
- [2].Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:106399. doi: 10.1016/j.ypmed.2020.106399 [DOI] [PubMed] [Google Scholar]
- [3].Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–51. doi: 10.1093/jnci/djr319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kreimer AR, Sampson JN, Porras C, Schiller JT, Kemp T, Herrero R, et al. Evaluation of durability of a single-dose of the bivalent HPV vaccine: the CVT Trial. J Natl Cancer Inst. 2020;112:1038–46. doi: 10.1093/jnci/djaa011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Safaeian M, Sampson JN, Pan YJ, Porras C, Kemp TJ, Herrero R, et al. Durability of Protection Afforded by Fewer Doses of the HPV16/18 Vaccine: The CVT Trial. J Natl Cancer Inst. 2018;110. doi: 10.1093/jnci/djx158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hildesheim A, Gonzalez P, Kreimer AR, Wacholder S, Schussler J, Rodriguez AC, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. American journal of obstetrics and gynecology. 2016;215:212.e1–e15. doi: 10.1016/j.ajog.2016.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. Jama. 2007;298:743–53. doi: 10.1001/jama.298.7.743 [DOI] [PubMed] [Google Scholar]
- [8].Wagner S, Roberson D, Boland J, Kreimer AR, Yeager M, Cullen M, et al. Evaluation of TypeSeq, a Novel High-Throughput, Low-Cost, Next-Generation Sequencing-Based Assay for Detection of 51 Human Papillomavirus Genotypes. J Infect Dis. 2019;220:1609–19. doi: 10.1093/infdis/jiz324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wagner S, Roberson D, Boland J, Yeager M, Cullen M, Mirabello L, et al. Development of the TypeSeq Assay for Detection of 51 Human Papillomavirus Genotypes by Next-Generation Sequencing. J Clin Microbiol. 2019;57:e01794–18. doi: 10.1128/JCM.01794-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sampson JN, Hildesheim A, Herrero R, Gonzalez P, Kreimer AR, Gail MH. Design and statistical considerations for studies evaluating the efficacy of a single dose of the human papillomavirus (HPV) vaccine. Contemp Clin Trials. 2018;68:35–44. doi: 10.1016/j.cct.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burger EA, Portnoy A, Campos NG, Sy S, Regan C, Kim JJ. Choosing the optimal HPV vaccine: The health impact and economic value of the nonavalent and bivalent HPV vaccines in 48 Gavi-eligible countries. Int J Cancer. 2021;148:932–40. doi: 10.1002/ijc.33233 [DOI] [PubMed] [Google Scholar]
- [12].Kim JJ, Burger EA, Regan C, Sy S. Screening for Cervical Cancer in Primary Care: A Decision Analysis for the US Preventive Services Task Force. Jama. 2018;320:706–14. doi: 10.1001/jama.2017.19872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lowy DR, Herrero R, Hildesheim A. Primary endpoints for future prophylactic human papillomavirus vaccine trials: towards infection and immunobridging. Lancet Oncol. 2015;16:e226–33. doi: 10.1016/s1470-2045(15)70075-6 [DOI] [PubMed] [Google Scholar]
- [14].Baisley KJ, Whitworth HS, Changalucha J, Pinto L, Dillner J, Kapiga S, et al. A dose-reduction HPV vaccine immunobridging trial of two HPV vaccines among adolescent girls in Tanzania (the DoRIS trial) - Study protocol for a randomised controlled trial. Contemporary clinical trials. 2021;101:106266–. doi: 10.1016/j.cct.2021.106266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barnabas R, Brown E, Onono M, Bukusi EA, Njoroge BW, Winer RL, et al. Single-dose HPV Vaccination Efficacy Among Adolescent Girls and Young Women in Kenya (the KEN SHE Study): Study Protocol for a Randomized Controlled Trial. 2021. [DOI] [PMC free article] [PubMed]
- [16].Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17:67–77. doi: 10.1016/S1470-2045(15)00414-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Prem K, Choi YH, Bénard É, Burger EA, Hadley L, Laprise J-F, et al. Global impact and cost-effectiveness of one-dose versus two-dose human papillomavirus vaccination schedules: A comparative modelling analysis. MedRxiv. 2021. doi: 10.1101/2021.02.08.21251186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.