Abstract
In situ revascularization of the subclavian artery can be challenging in the context of emergency situations, a large aortic aneurysm with a posteriorly displaced left subclavian artery, a complex redo procedure or in the presence of an aberrant subclavian artery. A transthoracic aorto-axillary extra-anatomical bypass is a low risk alternative to in situ revascularization or carotid to subclavian bypass. We herein describe the surgical steps during a single-stage surgery complex aortic arch surgery. We report a 95.3% graft patency for 77 consecutive transthoracic aorto-axillary extra-anatomical bypass performed to 66 patients at the mean follow-up of 2.9 ± 2.4 years. We encountered 3 early (before 180 days postop) graft failures and no late graft failure. Graft failure had no clinical significance.
Keywords: Aorta surgery, Axillary revascularization
In situ revascularization of the subclavian artery can be challenging during complex arch reconstruction.
In situ revascularization of the subclavian artery can be challenging during complex arch reconstruction. Emergency situations, reoperations, large aneurysm with posterior displacement of the left subclavian or presence of an aberrant subclavian artery may render difficult in situ revascularization. Simple ligation of the subclavian artery has been proposed as an alternative in these situations, although acute and chronic clinical consequences have been reported [1, 2]. While current guidelines recommend subclavian artery revascularization for elective surgery, we support revascularization in all circumstances including emergency situations. Hence, we describe the use of a transthoracic aorto-axillary extra-anatomical bypass (TAAEB) as an alternative to in situ revascularization and report midterm patency results.
The axillary artery is exposed through a standard mid-subclavicular incision (sec 1). The artery is dissected under the pectoralis minor muscle. The artery is freed proximally, distally and from the adjacent axillary vein (sec 9). A 1-cm hole within the chest wall is performed over the second rib in line with the position of the ascending aorta to avoid kinking or stenosis of the TAAEB (sec 19). These first steps are usually performed prior to the sternotomy. When possible, ligation of the proximal portion of the left subclavian artery should be performed during the aneurysm resection. When inaccessible, the ostium of the left subclavian may be ligated from within the aorta understanding that in such circumstance, the risk of type II endoleak may be increased during zone 0, 1 or 2 frozen elephant trunk procedures. In the presence of an aberrant right subclavian artery, the artery should be ligated in the posterior mediastinum between the superior vena cava and the trachea. The arch reconstruction is achieved according to the surgeon’s usual technique. When using a tripod graft for the arch reconstruction, the third 8-mm branch is tunnelled over the second rib or when using a 4 branch graft or in the presence of an aberrant right subclavian artery, an additional 8-mm Dacron graft is anastomosed to the main aortic graft to perform the TAAEB (sec 25). The distal anastomosis on the axillary artery of the TAAEB can be performed during the rewarming period. Alternatively, to optimize spinal cord protection, the axillary anastomosis may be constructed before initiating cardiopulmonary bypass and perfused during circulatory arrest. Graft should be under slight tension since it will lengthen with sternal closure, hence preventing graft kinking. Through the subclavicular incision, the axillary artery is clamped (sec 32). A longitudinal arteriotomy is then performed (sec 36). A termino-lateral anastomosis using a 5–0 prolene ‘hemoseal’ suture (Johnson-Johnson, New Brunswick, NJ, USA) is performed using a parachute technique for the posterior portion (sec 39). Ideally the anastomosis should lie under the axillary vein to avoid vein constriction and minimize the risk of future complication if percutaneous access of the axillary vein is required. Before sternal closure, coverage the TAAEB graft with pleura or mediastinal fat allows to minimize the risk of graft trauma in the event of a redo sternotomy.
Sixty-six consecutive patients surviving complex aortic arch surgery had 77 TAAEB grafts: 14 right-sided graft, 41 left-sided graft and 11 patients had bilateral grafts. Data were generated by consecutive cases within the Canadian Thoracic Aorta Collaborative multicentre network. Graft patency was evaluated by enhanced chest computed tomography (CT) scan with a mean follow-up time of 2.9 ± 2.4 years. We considered 50% graft lumen narrowing as graft stenosis and complete occlusion as graft thrombosis. Ethics committees did approve this review.
The cohort mean age was 60 years old with 27.2% female. Normal arch vessel anatomy (including bovine arch) was found in 78.8% while 21.2% (14/66) presented an aberrant subclavian artery, including 13 right sided and 1 left sided. Preoperative diagnosis is presented in Table 1. Fifty-five patients had total arch replacement, 3 had hemiarch surgery and 30 had descending aorta replacement either with a hybrid graft (n = 21) or a standard elephant trunk (n = 9). Debranching procedures were performed for zone 0 TEVAR. Twenty-five patients had a concomitant aortic valve procedure.
Table 1:
Aortic pathologies treated
| Pathologies treated | |
|---|---|
| Dissection | 14 |
| Acute type A | 3 |
| Acute type B | 1 |
| Chronic type B | 10 |
| Aneurysm | 60 |
| Intramural haematoma | 4 |
| Kommerell diverticulum | 8 |
| Occlusion/atherosclerotic | 5 |
| Traumatic aortic rupture | 1 |
Of the 77 TAAEB performed, 1 graft stenosis (1.5%) was identified at 162 days postoperative. Two TAAEB were found occluded (both left sided) on postoperative days 30 and 90 with no clinical consequences. No late graft failure (>180 days) was encountered.
Axillary revascularization using a carotid to subclavian bypass or transposition yields a 5-year patency between 96% and 100% [3, 4]. Carotid-axillary bypass is also a good alternative for the revascularization of LSA [5]. We report a similar patency rate of 95.3% at 3 years (Fig. 1). Reported graft failures occurred early after surgery, suggesting technical issues as the main failure mechanism. TAAEB is especially useful in emergency situations such as frozen elephant trunk procedures in type A dissection. Furthermore, complex reoperative arch procedures, a posteriorly displaced left subclavian artery or an aberrant subclavian artery may be indications to consider as an alternative to a preoperative carotid-subclavian bypass procedure. In addition, TAAEB lessens the risk of phrenic or recurrent nerve injury.
Figure 1:
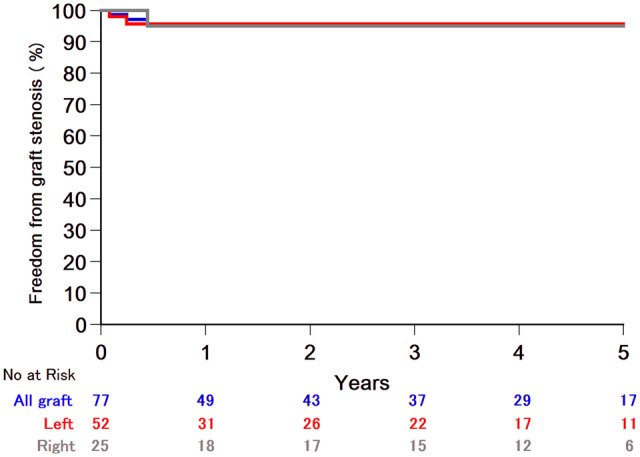
Transthoracic aorto-axillary extra-anatomical bypass graft patency.
In conclusion, TAAEB grafts are safe and yield high mid-term patency. They should be considered a valuable alternative when subclavian arteries are difficult to revascularize in situ especially in emergency situations.
Conflict of interest: none declared.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Luca Bertoglio, Mario Lescan and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1.Waterford SD, Chou D, Bombien R, Uzun I, Shah A, Khoynezhad A.. Left subclavian arterial coverage and stroke during thoracic aortic endografting: a systematic review. Ann Thorac Surg 2016;101:381–9. [DOI] [PubMed] [Google Scholar]
- 2.Sobocinski J, Patterson BO, Karthikesalingam A, Thompson MM.. The effect of left subclavian artery coverage in thoracic endovascular aortic repair. Ann Thorac Surg 2016;101:810–7. [DOI] [PubMed] [Google Scholar]
- 3.Duran M, Grotemeyer D, Danch MA, Grabitz K, Schelzig H, Sagban TA.. Subclavian carotid transposition: immediate and long-term outcomes of 126 surgical reconstructions. Ann Vasc Surg 2015;29:397–403. [DOI] [PubMed] [Google Scholar]
- 4.Takach TJ, Duncan JM, Livesay JJ, Ott DA, Cervera RC, Cooley DA.. Contemporary relevancy of carotid subclavian bypass defined by an experience spanning five decades. Ann Vasc Surg 2011;25:895–901. [DOI] [PubMed] [Google Scholar]
- 5.Bartos O, Mustafi M, Andic M, Grözinger G, Artzner C, Schlensak C. et al. Carotid-axillary bypass as an alternative revascularization method for zone II thoracic endovascular aortic repair. J Vasc Surg 2020;72:1229–36. [DOI] [PubMed] [Google Scholar]


