Abstract
Background
Normal swallowing function is divided into oral, pharyngeal, and oesophageal phases. The anatomy and physiology of the oral cavity facilitates an oral preparatory phase of swallowing, in which food and liquid are pushed towards the pharynx by the tongue. During pharyngeal and oesophageal phases of swallowing, food and liquid are moved from the pharynx to the stomach via the oesophagus. Our understanding of swallowing function in health and disease has informed our understanding of how muscle weakness can disrupt swallowing in people with muscle disease. As a common complication of long‐term, progressive muscle disease, there is a clear need to evaluate the current interventions for managing swallowing difficulties (dysphagia). This is an update of a review first published in 2004.
Objectives
To assess the effects of interventions for dysphagia in people with long‐term, progressive muscle disease.
Search methods
On 11 January 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AMED, LILACS, and CINAHL. We checked references in the identified trials for additional randomised and quasi‐randomised controlled trials. We also searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform on 12 January 2016 for ongoing or completed but unpublished clinical trials.
Selection criteria
We included randomised and quasi‐randomised controlled trials that assessed the effect of interventions for managing dysphagia in adults and children with long‐term, progressive muscle disease, compared to other interventions, placebo, no intervention, or standard care. Quasi‐randomised controlled trials are trials that used a quasi‐random method of allocation, such as date of birth, alternation, or case record number. Review authors previously excluded trials involving people with muscle conditions of a known inflammatory or toxic aetiology. In this review update, we decided to include trials of people with sporadic inclusion body myositis (IBM) on the basis that it presents as a long‐term, progressive muscle disease with uncertain degenerative and inflammatory aetiology and is typically refractory to treatment.
Data collection and analysis
We applied standard Cochrane methodological procedures.
Main results
There were no randomised controlled trials (RCTs) that reported results in terms of the review's primary outcome of interest, weight gain or maintenance. However, we identified one RCT that assessed the effect of intravenous immunoglobulin on swallowing function in people with IBM. The trial authors did not specify the number of study participants who had dysphagia. There was also incomplete reporting of findings from videofluoroscopic investigations, which was one of the review's secondary outcome measures. The study did report reductions in the time taken to swallow, as measured using ultrasound. No serious adverse events occurred during the study, although data for the follow‐up period were lacking. It was also unclear whether the non‐serious adverse events reported occurred in the treatment group or the placebo group. We assessed this study as having a high risk of bias and uncertain confidence intervals for the review outcomes, which limited the overall quality of the evidence. Using GRADE criteria, we downgraded the quality of the evidence from this RCT to 'low' for efficacy in treating dysphagia, due to limitations in study design and implementation, and indirectness in terms of the population and outcome measures. Similarly, we assessed the quality of the evidence for adverse events as 'low'. From our search for RCTs, we identified two other non‐randomised studies, which reported the effects of long‐term intravenous immunoglobulin therapy in adults with IBM and lip‐strengthening exercises in children with myotonic dystrophy type 1. Headaches affected two participants treated with long‐term intravenous immunoglobulin therapy, who received a tailored dose reduction; there were no adverse events associated with lip‐strengthening exercises. Both non‐randomised studies identified improved outcomes for some participants following the intervention, but neither study specified the number of participants with dysphagia or demonstrated any group‐level treatment effect for swallowing function using the outcomes prespecified in this review.
Authors' conclusions
There is insufficient and low‐quality RCT evidence to determine the effect of interventions for dysphagia in long‐term, progressive muscle disease. Clinically relevant effects of intravenous immunoglobulin for dysphagia in inclusion body myositis can neither be confirmed or excluded using the evidence presented in this review. Standardised, validated, and reliable outcome measures are needed to assess dysphagia and any possible treatment effect. Clinically meaningful outcomes for dysphagia may require a shift in focus from measures of impairment to disability associated with oral feeding difficulties.
Plain language summary
Interventions for swallowing difficulty in long‐term, progressive muscle disease
Review question
What is the effect of interventions for dysphagia in people with long‐term, progressive muscle disease?
Background
People with progressive muscle disease often develop swallowing difficulties (dysphagia) as a result of weakness. These changes in swallowing function can lead to weight loss or inability to gain weight, as well as breathing problems due to food inhalation into the airways and recurrent respiratory infections. Fear or embarrassment about symptoms such as choking, coughing, or spluttering while eating and long meal times can also lead to psychological and social difficulties for those living with dysphagia. We wanted to find out how effective a range of different interventions are for treating dysphagia in people with long‐term, progressive muscle disease.
Study characteristics
This review included one trial (22 participants), which compared the effect of three months' intravenous immunoglobulin (IVIg) therapy with placebo. Three participants were also treated with prednisone during the trial. Some limitations in the design, conduct, and reporting of the study might have affected the results. We were most interested in the degree to which treatment affected weight, in terms of either halting weight loss or producing weight gain.
Key results and quality of the evidence
We identified only one randomised controlled trial of intervention for managing dysphagia in one muscle disease, inclusion body myositis. There was not enough evidence for or against any specific intervention for dysphagia. Clinically relevant effects of IVIg for dysphagia in inclusion body myositis can neither be confirmed nor ruled out using the evidence in this review. This trial did not assess weight gain or maintenance or fully report effects of IVIg on swallowing, which the investigators measured using a self report questionnaire and videofluoroscopy (a moving X‐ray of swallowing). Any harmful effects were not fully reported. Overall quality of the evidence was low due to limitations in study design and reporting.
The evidence is up to date to January 2016.
Background
Description of the condition
Normal swallowing function is divided into oral, pharyngeal, and oesophageal phases. During the oral phase, food is prepared by the lips, tongue, and teeth to form a bolus which is propelled backwards by the tongue. In the pharyngeal phase, the tongue base retracts to push the formed bolus into the pharynx. Airway protection is achieved mainly by closure of the larynx; the associated upwards and forwards movement is also protective and serves to pull open the relaxed upper oesophageal sphincter, the main muscle of which is the cricopharyngeus. A drop in cricopharyngeal pressure created by the elevation of the hyoid and larynx pulling open the cricopharyngeus is understood to be the main source of pressure change propelling the bolus into the oesophagus. Top‐to‐bottom, sequential contraction of the pharyngeal constrictor muscles also squeezes the bolus downwards. Only the oral phase of swallowing is completely under voluntary control. During the oesophageal phase, the bolus moves towards the stomach by peristalsis, a movement which is regulated entirely by the autonomic nervous system.
Dysphagia can be defined on the basis of a symptom, a clinical sign, a radiological sign, or as a cause or an otherwise unexplained nutritional or respiratory problem. Feeding problems often develop insidiously, at first allowing the person to compensate for swallowing that may be assessed as abnormal when measured objectively. In conditions such as oculopharyngeal muscular dystrophy (OPMD), dysphagia forms part of a symptom complex and therefore occurs, to some degree, in all those affected by the condition. Dysphagia is also frequently under‐reported in more generalised muscular dystrophies, where feeding difficulties are one aspect of a multi‐system condition. An important complication of impaired swallowing is entry of the bolus into the airway, which is usually described by the extent of penetrance and can be divided into (i) laryngeal penetration, which is the passage of the bolus into the laryngeal vestibule but not further than the vocal cords, and (ii) aspiration, which is defined as passage of the bolus beyond the vocal cords.
Dysphagia in long‐term, progressive muscle disease is primarily due to muscle weakness. Weakness of the tongue, face, and jaw or abnormal mouth architecture can impair the ability to prepare a bolus adequately and retrieve bolus particles. Palatal weakness may predispose to nasal regurgitation, and a weakness of the suprahyoid musculature can lead to impaired upper oesophageal sphincter opening; this in turn leads to impaired bolus transit, pooling in the pharynx, and an increased risk of aspiration. Muscle weakness can also compromise laryngeal function, affecting laryngeal closure and coughing. Furthermore, any ventilatory muscle weakness may be expected to compromise effective coughing and airway clearance, particularly in neuromuscular conditions such as myotonic dystrophy.
Many people with muscle disease are observed to develop dysphagia, although this is not necessarily a late‐onset feature. According to Jaradeh 2006, OPMD and myotonic dystrophy (MD) are the forms of muscular dystrophy most commonly associated with dysphagia. OPMD is a late‐onset autosomal dominant or autosomal recessive inherited disease related to expanded GCG repeats in the PABPN1 gene and with rimmed vacuoles in the muscle biopsy; voluntary swallowing muscles including the tongue are affected in this condition (Palmer 2010), and in rare cases there is evidence of peripheral nervous system involvement (Abu‐Baker 2007). Adult‐onset oculopharyngodistal myopathy (OPDM) is considered a distinct hereditary muscle disease also characterised by oropharyngeal dysphagia of myopathic origin, although the genetic defect is unknown (Durmus 2011). Oropharyngeal dysphagia is a common feature of MD type I, the most prevalent inherited neuromuscular disease; MD type I is associated with expanded CTG repeats in the DMPK gene. Oropharyngeal dysphagia is less common in MD type II, which is associated with mutations in the CCTG repeat sequence in the ZNF9 gene (Tieleman 2009). The presentation of oropharyngeal dysphagia in MD has been attributed to myopathic weakness in the control and formation of the bolus, myotonia of muscles with a warm‐up phenomenon, as well as possible central nervous system involvement in delayed swallow reflexes (Ertekin 2001). Other untreatable chronic muscle diseases that are often associated with dysphagia include inclusion body myositis (IBM), advanced Duchenne muscular dystrophy (DMD), and some types of metabolic myopathy. Congenital myopathies are less commonly associated with swallowing difficulties (Jaradeh 2006). The cause of dysphagia in DMD has been attributed to oral muscle weakness and facial and dental morphology changes (Straathof 2014). A combination of impaired cricopharyngeal muscle function and impaired sphincter opening has been implicated in cases of dysphagia in IBM (Cox 2009); impaired sphincter function in IBM, a late‐onset non‐hereditary rimmed vacuolar myopathy with inflammation and degeneration of muscles, could also be attributed to ageing processes (Cox 2009). It is further postulated that an individual's sense of effort in engaging appropriate submaximal swallowing pressures may not be adjusted for age‐related muscle weakness and progressive muscle disease (Palmer 2010). Precise estimates of the prevalence of dysphagia remain difficult to determine across different muscle diseases, due, in part, to a lack of standardised assessment procedures (Archer 2013). Also, the swallowing effort perception may not be adequately evaluated in these assessments.
An early questionnaire‐based study estimated a prevalence of dysphagia of 34.9% in people with inflammatory and progressive neuromuscular disorders (Willig 1994). However, the relative prevalence of dysphagia symptoms were not given for an apparently healthy population. Even respondents with facioscapulohumeral muscular dystrophy, which is not typically associated with swallowing difficulties, reported occasional choking episodes. Inconsistency in the definition and interpretation of dysphagia is perhaps also reflected in the highly variable estimated prevalence of dysphagia (approximately 40% to 80%) in people with IBM (Cox 2009). In a later study, Cox 2011 found that more than half of their sample population with IBM and dysphagia specified having a combination of obstruction‐related symptoms (such as food becoming stuck in throat) and aspiration (choking). While questionnaires on swallowing function may be important for identifying dysphagia as a collection of symptoms or syndrome, the diagnostic accuracy of self reporting is yet to be established. In addition to a lack of standardised definition and assessment, the rate of change in swallowing indices is also not yet fully understood in muscle disease; this limits the identification of a clinically and statistically significant treatment effect size in clinical trials.
From a clinical and patient perspective, dysphagia can result in failure to maintain adequate nutritional intake and respiratory complications, which can lead to a number of physical, social, and psychological consequences, including (i) weight loss or failure to gain weight, (ii) increased susceptibility to chest infections due to aspiration, (iii) breathing problems due to upper or lower airway blockage secondary to aspiration into the larynx, and (iv) poorer quality of life that may be associated with a loss of enjoyment of eating, fear of choking, embarrassment, and/or social isolation secondary to coughing, spluttering, and prolonged feeding times.
Description of the intervention
Despite the manifest importance of maintaining an adequate nutritional intake and preventing respiratory complications, there is no established best practice for managing dysphagia in chronic muscle disease.
Currently, the main interventions for managing oral feeding difficulties that can lead to dysphagia include dietary manipulation, adoption of safe swallowing techniques, surgical intervention, and enteral feeding. Generally, the first step is to alter the consistency of food and liquids and add appropriate dietary supplements (Ganger 1990; Martin 1991; O'Gara 1990). Manoeuvres to improve the safety of the swallow include changing posture during swallowing, effortful swallowing, and double swallows (Bülow 2001; Drake 1997; Ertekin 2001; Logemann 1994). Much of the supportive evidence for swallowing manoeuvres has been obtained from studies of stroke and other conditions affecting the brain and brain stem; it has not as yet been established whether these interventions are effective in muscle disease.
Indications for surgical intervention and enteral feeding typically include oral feeding difficulties and the prevention of recurrent aspiration. Percutaneous endoscopic gastrostomy (PEG) is often the preferred method for delivering nutritional support, but the current evidence for PEG insertion over nasogastric feeding in preventing aspiration is based mostly on stroke‐related dysphagia, where consciousness level is sometimes reduced (Norton 1996). These findings may not apply to dysphagia in chronic muscle disease (Hill 2002b).
In a previous version of this review, the review authors reported findings from uncontrolled trials that suggested that both cricopharyngeal myotomy and upper oesophageal dilatation could offer symptom relief in cases of moderate to severe dysphagia secondary to OPMD (Hill 2004). However, there was limited information on people who were not offered the procedure, and no attempt was made to compare these interventions with each other, or with other interventions. It has therefore not been possible to determine whether cricopharyngeal myotomy and upper oesophageal dilation are safe and effective interventions in OPMD. Similarly, positive outcomes have been reported for cricopharyngeal myotomy in people with IBM, but there is a lack of available data on the long‐term outcome (Darrow 1992; Wintzen 1988). Meanwhile, botulinum toxin injection of the cricopharyngeus muscle as treatment for dysphagia has gained popularity in the management of a variety of conditions. It is not yet clear what role, if any, botulinum toxin injection should have in managing dysphagia secondary to chronic muscle disease because no case‐controlled or long‐term follow‐up studies are available. Some clinicians may have concerns about prescribing a substance that causes weakness if the dysphagia itself results from weakness.
Few studies have attempted to determine the benefit or otherwise of PEG feeding in people with primary muscle disease. Review authors previously identified one uncontrolled study of the introduction of gastrostomy feeding in six children with congenital myopathy (Philpot 1999). Following intervention, there was a reduction in respiratory infections and accelerated weight gain, but treated children appeared to be at increased risk of symptomatic gastro‐oesophageal reflux. Seguy 2002 studied the nutritional effects and tolerance of PEG and surgical gastrostomy in 12 children and young adults with different types of neuromuscular disease (muscular dystrophy, spinal muscular atrophy, congenital myopathy, and polyradiculoneuritis). In this study, the indications for nutritional support by gastrostomy were undernutrition or swallowing disorders. The study authors found a significant improvement in z‐scores for age‐ and height‐adjusted weight, which was maintained in 10 participants followed up after one year. However, as in Philpot 1999, the trial authors highlighted possible occurrence or worsening of gastro‐oesophageal reflux following intervention. Seguy 2002 emphasised that non‐invasive techniques and orthopaedic supports need to be considered before gastrostomy.
Why it is important to do this review
This review aims to (i) assess the available RCT evidence for optimal management of swallowing function, and (ii) identify key considerations for future clinical trials of dysphagia in long‐term, progressive muscle disease. This is an update of a review first published in 2004 (Hill 2004).
Objectives
To assess the effects of interventions for dysphagia in people with long‐term, progressive muscle disease.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials (RCTs) and quasi‐RCTs examining the effectiveness of different interventions to manage dysphagia in people with long‐term, progressive muscle disease. From these searches we also identified non‐randomised controlled trials that otherwise met our selection criteria for inclusion; we have provided details in the Discussion section.
Types of participants
All participants were adults or children with long‐term, progressive primary muscle disease, including Duchenne muscular dystrophy (DMD), myotonic dystrophy (MD), oculopharyngeal muscular dystrophy (OPMD), oculopharyngodistal myopathy (OPDM), inclusion body myositis (IBM), metabolic myopathy, and congenital myopathy. Authors of a previous version of this review excluded trials involving people with muscle conditions of a known inflammatory or toxic aetiology. In this review update, we decided to include trials of people with sporadic IBM on the basis that it presents as a long‐term, progressive muscle disease with uncertain degenerative and inflammatory aetiology and is typically refractory to treatment.
Types of interventions
We searched for studies investigating a range of interventions to manage swallowing function including swallowing manoeuvres, surgical and pharmacological interventions. We considered any comparison, for example other interventions, placebo, no intervention, or standard care.
Types of outcome measures
We applied prespecified outcome measures from a previous version of the review (Hill 2004).
Primary outcomes
The stabilisation of previously documented progressive weight loss not attributable to any other cause, weight gain of at least 5 kg in adults or increase in weight to at least the 10th centile in children, maintained for at least six months following the intervention.
Secondary outcomes
Reduction in laryngeal penetration of bolus and/or aspiration observed on videofluoroscopy (modified barium swallow), assessed between three and six months after the intervention.
Reduction in chest infections attributable to aspiration over a six‐month period.
Reduction in the number of episodes of pharyngonasal or pharyngo‐oral aspirations maintained for at least six months.
Improvement in quality of life using a validated rating scale maintained for at least six months.
Proportion of participants who refuse the intervention.
Proportion of carers who refuse the intervention.
Serious adverse events related to the intervention within the first 12 months after intervention, namely events that are life‐threatening, result in hospitalisation or prolongation of stay in hospital, result in a temporary or permanent worsening of swallow, or result in increased disability.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Specialised Register (11 January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL in the Cochrane Register of Studies Online), MEDLINE (January 1966 to November 2015), EMBASE (January 1980 to January 2016), AMED (January 1985 to December 2015), CINAHL Plus (January 1937 to January 2016) and LILACS (January 1982 to December 2015).
The detailed search strategies are in the appendices: Appendix 1 (Cochrane Neuromuscular Specialised Register), Appendix 2 (CENTRAL), Appendix 3 (MEDLINE), Appendix 4 (EMBASE), Appendix 5 (AMED), Appendix 6 (CINAHL), and Appendix 7 (LILACS).
Searching other resources
We checked references in the identified trials for any additional randomised and quasi‐randomised controlled trials. We also searched ClinicalTrials.gov (www.clinicaltrials.gov) on 12 January 2016 and the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/) on 12 January 2016 for ongoing or completed but unpublished clinical trials. The search strategies are in Appendix 8. We applied no language limitations.
Data collection and analysis
Selection of studies
Three review authors (TH, KJ, and RP) independently identified RCTs eligible for inclusion from the search results. In the absence of sufficient RCTs for meta‐analysis, we also identified non‐randomised studies through our searches. Review authors (including TH, KJ, and RP) discussed the selection of studies for inclusion in this review and resolved any differences in opinion by discussion.
Data extraction and management
Four review authors (UB, MH, KJ, and SM) independently extracted RCT and non‐randomised trial data using a standardised data extraction form. Two review authors assessed each identified study and resolved any discrepancies in data extraction by discussion. The review authors would have attempted to obtain missing data from the trial authors if necessary, but were already aware that no additional data were available for the study included at this update. One review author (KJ) entered data, which another review author (from among UB, MH, and SM) checked. The review authors resolved any disagreements in data entry by discussion.
Assessment of risk of bias in included studies
We assessed risk of bias in the included studies, using the standard criteria of random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Two review authors (KJ and MR) assessed the risk of bias in included studies as 'high', 'low', or 'unclear' according to criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The review authors resolved any disagreements by discussion.
Measures of treatment effect
When considering the primary outcome measure, we planned to calculate the risk ratio and its 95% confidence interval (CI) of achieving sustained improvement in swallowing or nutrition with a given intervention. For continuous outcomes, we planned to report the mean difference with 95% CI for studies using the same measurement scale, or standardised mean difference with 95% CI for studies using different measurement scales for an outcome.
Unit of analysis issues
We planned to include randomised cross‐over trials with a suitable wash‐out period, as this design can be appropriate in slowly progressive conditions. We would have included suitable data from a paired analysis using the generic inverse variance facility in Review Manager (RevMan) 5.3 (RevMan 2014). If we suspected carry‐over effects from the first period, we may have decided to report first‐period data only, while acknowledging the drawbacks of this approach (Higgins 2011). We included only first‐period data from the included cross‐over study as it was not truly cross‐over in design because participants chose the second‐period intervention.
Assessment of heterogeneity
We would have assessed heterogeneity through visual assessment of forest plots. We would have used the I2 statistic calculated by RevMan as a measure of statistical heterogeneity, as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data synthesis
We anticipated that different studies would use different criteria to analyse the secondary outcome measure of a reduction in laryngeal penetration or aspiration as assessed by videofluoroscopy. Where possible, we planned to pool results from these studies to calculate a standardised mean difference with 95% CI. We planned to calculate change in weight and number of chest infections as mean differences with 95% CI. Where different studies used the same validated quality of life rating scale, we planned to calculate a mean difference with 95% CI, and where different scales were used, a standardised mean difference with 95% CI.
We planned to review all studies, randomised and non‐randomised, to identify possible adverse events arising from an intervention and the cost‐effectiveness of that intervention. If the studies were of sufficient quality, we would have calculated a risk ratio of an adverse event arising from a procedure. Where it was not possible to perform a statistical analysis, we planned to comment on adverse events and cost‐effectiveness from the available non‐randomised evidence.
We planned to pool results using RevMan software with a fixed‐effect model, and to assess the results for heterogeneity. If we had found heterogeneity, we would have attempted to explain the heterogeneity by omitting the trials at high risk of bias. If there still appeared to be significant heterogeneity that we could not explain, we planned to repeat the meta‐analysis using a random‐effects model.
'Summary of findings' table
If future updates include more than one eligible study for any comparison, we will include a 'Summary of findings' table presenting the quality of evidence for key outcomes, namely:
The stabilisation of previously documented progressive weight loss not attributable to any other cause; weight gain of at least 5 kg in adults, or increase in weight to at least the 10th centile in children, maintained for at least six months following the intervention.
Serious adverse events related to the intervention within the first 12 months after intervention, namely events that are life‐threatening, result in hospitalisation or prolongation of stay in hospital, result in a temporary or permanent worsening of swallow, or result in increased disability.
In the absence of a formal 'Summary of findings' table, we discussed the included study with reference to Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria in Quality of the evidence.
We will upgrade or downgrade the evidence based on the five GRADE considerations: study limitations, consistency of effect, imprecision, indirectness, and publication bias (Higgins 2011). We will provide footnotes to explain our decisions.
Subgroup analysis and investigation of heterogeneity
We planned to analyse adults and children (aged less than 18 years) with chronic muscle disease separately. For studies that examined an intervention in a specific condition such as OPMD, we planned to present the results for the specific condition, as well as for chronic muscle disease as a whole. Due to the rarity of many muscle conditions, we anticipated that most studies would look at an intervention in a range of progressive muscle conditions.
Sensitivity analysis
In the presence of heterogeneity, we would have examined the sensitivity of meta‐analyses to studies at high risk of bias as described above.
Results
Description of studies
Results of the search
The search strategies in the appendices produced the following results: Cochrane Neuromuscular Specialised Register 19 records, CENTRAL 206 records, MEDLINE 359 records, EMBASE 272 records, AMED 16 records, CINAHL Plus 256 records, and LILACS 29. We found 202 records of ongoing studies in ClinicalTrials.gov and 42 records of ongoing studies in the World Health Organization International Clinical Trials Registry Platform (Appendix 8). We have included a flow chart to illustrate the study selection process (see Figure 1) and a separate flow chart to illustrate the identification of potentially relevant ongoing studies (see Figure 2).
1.
Study flow diagram.
2.

Ongoing study flow diagram.
Included studies
We identified one study fulfilling the selection criteria (Dalakas 1997). This RCT investigated the effect of intravenous immunoglobulin (IVIg) on swallowing function in inclusion body myositis (IBM); see Characteristics of included studies.
In Dalakas 1997, 22 adults (19 analysed) with IBM, diagnosed according to Griggs 1995 diagnostic criteria, undertook a cross‐over study of three months' IVIg and three months of placebo, separated by a wash‐out period of at least one month. The placebo consisted of dextrose in half normal saline, and IVIg dosage was 2 g/kg body weight. However, three participants also received concomitant treatment with prednisone. Both trial interventions were administered once every three months by blinded assessors. The participants were also blinded to treatment intervention and were block randomised at the pharmacy to intervention groups, although they were subsequently given the option of crossing over to the alternative treatment. The primary outcome for this trial of IVIg therapy was muscle strength sum scores, as assessed in another Cochrane review (Rose 2015). However, trial authors also examined the change in bulbar muscle strength using ultrasound swallowing and videofluoroscopy, as well as changes in swallowing function using a self assessment questionnaire. Age and muscle strength were reported to be comparable between IVIg and placebo groups at baseline, although there were differences in mean swallowing duration in ultrasound studies. Funding sources were not disclosed.
Excluded studies
We excluded one ongoing trial, NCT00773227, and three completed trials that did not meet our inclusion criteria because they were not randomised (Dobloug 2012; Horowitz 1987; Sjögreen 2010). Dobloug 2012 investigated the effects of long‐term IVIg therapy on swallowing dysfunction in people with IBM. Horowitz 1987 also only included a one‐off administration of metoclopramide for delayed gastric emptying rather than an intervention for dysphagia per se. Sjögreen 2010 studied the effects of lip‐strengthening exercises in people with myotonic dystrophy type 1, assessing change in strength and functional capacities.
Ongoing studies
We identified a potentially relevant ongoing RCT of IV trehalose (Cabaletta) drug treatment in oculopharyngeal muscular dystrophy, NCT02328482, and an RCT comparing different treatment methods for cricopharyngeal dysfunction, ISRCTN84905610. See Characteristics of ongoing studies.
Risk of bias in included studies
One controlled trial fulfilled the inclusion criterion for attempting to randomise participants to two or more different interventions (Dalakas 1997). Despite adequate initial randomisation and allocation concealment, participants were allowed to choose whether to change their intervention during the second cross‐over period, breaking randomisation. We also identified a high risk of reporting bias across the results of both cross‐over periods, and possible attrition bias associated with dropouts following the first period of the trial (see Risk of bias in included studies and Figure 3). We therefore considered the trial to have a high overall risk of bias.
3.
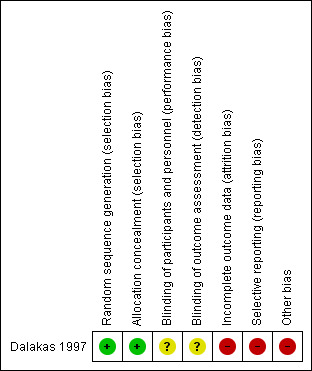
Risk of bias summary: review authors' judgements about each risk of bias item for the included study. Green (+) = low risk of bias; yellow (?) = unclear risk of bias; red (‐) = high risk of bias.
Effects of interventions
IVIg versus placebo in IBM
Efficacy
Eighteen participants chose to cross over intervention, and one participant refused to cross over from IVIg to placebo. Two other participants also dropped out during the first period of the trial, although it is unclear if they were allocated to IVIg or placebo. As the second period of this cross‐over trial was not randomised, we reviewed only the first‐period results. The trial presented no data for the primary outcome measure of this review, weight gain or maintenance.
No efficacy data were available for the review's secondary outcome measures, although the trial investigators undertook videofluoroscopy.
The trial authors found some supporting evidence for IVIg treatment effect in ultrasound swallowing studies. Specifically, a reduction in mean swallowing duration (seconds) was used as an indicator of improved swallowing function. Baseline mean swallowing duration was consistently lower for participants randomised to IVIg compared with placebo; this effect was reported as statistically significant (P < 0.05) for the second of three dry swallows and the final third wet swallow. However, the reporting of outcome data was incomplete because the study did not provide standard deviation values alongside the mean changes in swallowing duration. Furthermore, no corrections were made for multiple comparisons, which could produce statistically significant results by chance. The trial authors reported a significant improvement in the duration of swallows following three months' IVIg and worsening following placebo, but did not include a statistical between‐group comparison for the first‐period results. Instead, the trial authors analysed the within‐group before‐after data, which may be influenced by period effects. The trialists measured but did not report swallowing function using videofluoroscopy and a self assessment questionnaire, although they included a subjective evaluation of overall treatment effect, whereby 13 of the 19 participants who completed the trial experienced some improvement with IVIg. Sixteen participants were able to correctly identify their intervention allocation, suggesting possible unblinding and an increased risk of performance bias.
Data were insufficient to generate a 'Summary of findings' table. However, in accordance with the GRADE considerations, we double‐downgraded the quality of the available efficacy evidence to 'low' due to limitations in the study design and implementation (incomplete outcome reporting), and indirectness, as the presence of dysphagia at the start of the study was not quantified.
Adverse events
Trial authors did not identify any serious adverse events as defined in this review. Although some participants retrospectively reported headaches, it was unclear whether these adverse events occurred in the treatment or placebo group. The trial did not report an overall incidence of adverse events. Three unexplained dropouts (14% attrition) occurred during the study, including one participant who refused to continue after completing the IVIg period. It is unclear whether the other two participants dropped out of the IVIg or placebo period.
Using GRADE considerations, we double‐downgraded the quality of the available evidence for adverse events to 'low' due to limitations in the study design and implementation (incomplete outcome reporting) and indirectness.
Discussion
Summary of main results
One RCT of 22 adults with inclusion body myositis (IBM) fulfilled the inclusion criteria for our review (Dalakas 1997). The study assessed change in swallowing function without defining the swallowing impairment. Dalakas 1997 produced some findings that supported an improvement in swallowing function with IVIg treatment, but the study was associated with a high risk of bias, and the clinical relevance of these findings remains unclear. Applying GRADE criteria, we assessed the quality of the evidence from the trial as low. We downgraded the evidence twice, for study design and implementation limitations, and indirectness.
In the absence of sufficient RCTs for meta‐analysis, we also considered the evidence from non‐randomised studies assessing the effects of intervention for managing dysphagia in long‐term, progressive muscle disease. As part of this discussion, we considered outcome measures for eating and drinking difficulties along with the assessment of swallowing indices, as it was possible that prespecified review outcome measures were inappropriate. For example, our primary outcome referred to a generic weight gain with intervention, which could be considered too prescriptive.
Evidence from non‐RCTs
Long‐term IVIg in IBM
Dobloug 2012 included 16 adults with IBM according to Griggs 1995 diagnostic criteria, treated with IVIg over a mean period of 23 months. The trial used a standard treatment protocol in which participants received a total IVIg dose of 2 g/kg body weight, infused over three to five days. Trialists administered this treatment monthly or once every third month and "tailored according to the perceived need of each individual patient", including dose adjustment in response to side effects. The IVIg‐treated cohort received a total of 10 infusions on average. Seven participants in the cohort received concomitant drug therapy (corticosteroids or corticosteroids combined with methotrexate); one of these participants received infusions of methylprednisone before IVIg therapy. Retrospective assessment included comparison of the cohort with an internal control group composed of six adults with IBM who were treated with other drugs (prednisolone, methotrexate, and azathioprine). Dysphagia was assessed subjectively by participants and using barium X‐rays of the oesophagus to detect and quantify the presence of dysmotility. The study made no comparison between the baseline characteristics of the control and intervention groups.
Efficacy
In addition to blood tests and manual muscle strength testing, Dobloug 2012 reviewed self reported dysphagia and dynamic studies of the oesophagus by barium X‐ray. The study authors did not complete group‐level analyses to assess the effect of long‐term IVIg use across the cohort. Three participants treated with IVIg indicated subjective improvement in swallowing function during their follow‐up compared to none in the control group. However, study authors did not specify the timing of subjective assessment, which could have varied between 11 and 48 months across intervention and control groups. Radiological dynamic studies of the oesophagus found that only 2 of 14 people treated with IVIg who were assessed had normal oesophageal motility; however, the study authors did not assess change in dysmotility over time as part of this study.
Adverse events
There were no serious adverse events. Mild‐to‐moderate headaches during and after IVIg infusions were reported as side effects. Two participants received a dose reduction because of side effects, but the study paper did not report overall incidence of side effects associated with treatment.
Lip‐strengthening exercises in myotonic dystrophy type 1
In Sjögreen 2010, eight school‐aged children diagnosed with either congenital or childhood‐onset myotonic dystrophy type 1 undertook a cross‐over study of 16 weeks' training with an oral screen and 16 weeks without intervention to assess the effect of lip‐strengthening exercises. The training involved 16 minutes of active and passive exercises with an oral screen performed five days per week at home or in school. The exercises could be completed independently by the child or with support from a teacher or parent. The trial authors did not directly assess dysphagia but used a range of outcome measures to assess eating and drinking difficulties, including: maximal lip force measured using a force meter; endurance of lip muscles measured at 50% maximal lip force; 3D‐analysis of lip mobility; lip articulation assessment using the Swedish Articulation and Nasality Test (SVANTE); and parental assessment of eating and saliva control by questionnaire. The study authors made no comparison between the children's baseline characteristics immediately prior to the intervention and non‐intervention periods. The study authors also did not describe any wash‐out period between intervention and non‐intervention periods.
Efficacy
There was no significant difference between the change in maximal lip force following intervention and non‐intervention periods (z = ‐0.911; P = 0.362), although half of the participants showed significant improvement in maximal lip force and endurance (P value unspecified). Trial authors reported "wide intra‐individual variation for speech and eating ability within and between assessments". They observed that changes in lip strength and endurance did not necessarily result in improved function. This study did not provide group‐level results for speech and eating function.
Adverse events
Trial authors reported no adverse events, but highlighted that recurrent infections affected routine training in some children.
Overall completeness and applicability of evidence
Since publication of the original review (Hill 2004), there remains a lack of supporting evidence for interventions to manage dysphagia in long‐term, progressive muscle disease. However, the existing trials highlight a fundamental issue: that we still have no standardised approach for assessing swallowing function and its change over time. The value of intervention trials in dysphagia is therefore unclear while outcome measures are inadequate to assess the effect of treatment. There was also a lack of evidence from the studies discussed in this review on the cost‐effectiveness of different interventions for dysphagia.
The sensitivity of weight change as a primary outcome measure in dysphagia is uncertain. The severity of dysphagia is expected to influence the absolute amount of weight change with intervention. Establishing a stable baseline or pre‐morbid weight could also be difficult in some progressive muscle diseases. Manometry and videofluoroscopy are established clinical methods for investigating swallowing function. Videofluoroscopy is often considered to be the gold standard for assessing dysphagia, but its visual interpretation has poor inter‐rater reliability, particularly when dysfunction is less obvious (Scott 1998). Philpot 1999 included abnormal findings on videofluoroscopy as a criterion for offering enteral feeding to participants, but the normal and abnormal findings corresponded inconsistently with weight gain. A later study of young people with juvenile dermatomyositis, a form of inflammatory myopathy, also found that the normality of videofluoroscopic swallow studies was not always consistent with symptom assessment (McCann 2007). Similarly, upper oesophageal manometry does not appear to reliably discriminate between those people with clinically relevant dysphagia and an apparently healthy population (Pandolfino 2005). Although intervention with either cricopharyngeal myotomy or upper oesophageal sphincter dilatation may affect upper oesophageal pressures, Pandolfino 2005 clarified that no meaningful relationship has been established between manometric abnormality and functional abnormality of either bolus transit or particular symptoms.
There are several barriers to the development of robust RCTs even with the application of a standard method of assessing dysphagia. Blinding procedures may be difficult to incorporate into interventional studies due to the nature of the interventions for dysphagia, such as surgery. An active comparator may be considered in place of a non‐intervention comparator, as in the ongoing study of balloon dilation versus surgery for cricopharyngeal dysfunction (ISRCTN84905610); however, this does not remove the risk of bias associated with lack of blinding. Longitudinal data on how patients perceive the effort of swallowing, as well as the frequency, severity, and rate of progression of dysphagia across different progressive muscle conditions are insufficient. The heterogeneity in muscle disease is an additional complication, although studies of a single, well‐defined patient group should be feasible. Indeed, a 12‐year follow‐up study of people with IBM identified mixed obstruction‐ and aspiration‐related symptoms affecting the majority of a surveyed cohort, suggesting that a complex of symptoms or symptom descriptions may be required to monitor dysphagia. Although the rate of symptom progression was not determined, cachexia was identified as a leading cause of death in this study, illustrating the potential impact of dysphagia symptoms at the end stage of disease (Cox 2011). We also postulate that existing clinical trials of interventions for dysphagia may not adequately account for prevalent, concurrent breathing‐related impairments. Factors such as reduced forced vital capacity and an ineffective cough may have critical implications for airway clearance in people with swallowing difficulties.
The two non‐randomised studies discussed in this review presented very low‐quality evidence (Dobloug 2012; Sjögreen 2010). As Dobloug 2012 was a retrospective rather than prospective study, the long‐term follow‐up of an IVIg‐treated cohort was subject to a high risk of selection and reporting bias. In Sjögreen 2010, the response to lip‐strengthening exercises appeared to be highly individual and variable among a small number of children with myotonic dystrophy type 1. Together, the RCT and non‐RCTs applied a range of outcome measures, which are expected to assess different and potentially unrelated indicators of treatment effect. In relation to dysphagia, the studies included subjective assessment and objective examination that failed to demonstrate baseline similarity, or depended on within‐study estimates of apparently normal swallowing duration and oesophageal motility. Trialists also indirectly assessed dysphagia through subjective assessment of eating and drinking function and objective examination of lip strength and endurance. Where reported, within‐participant variations in objective measurements made it difficult to interpret any change as evidence of treatment effect. As small, non‐randomised studies, these were underpowered to determine group‐level treatment effect from any change in the reported outcomes.
We propose that our search for answers on managing dysphagia may benefit from a more holistic approach by instead considering interventions for oral feeding difficulties. This approach could help to identify potential determinants of whether a person is able to feed by mouth, with or without detectable changes in swallowing indices. Findings from Sjögreen 2010 appear to support this need to refocus our attention on patient experiences of difficulties associated with eating and drinking; these difficulties may include, for example, external factors such as food consistency and the availability of carers. In terms of defining a normal range for swallowing function at the level of disability rather than impairment, Hughes 1996 previously combined quantitative indices for drinking a fixed volume of water with qualitative observation of this bedside test. The trial authors established that age, sex, and height were major determinants of swallowing function in apparently healthy adults, based on timed swallowing indices. Results from this study were validated by the association between reduced swallowing capacity and the number of symptoms and signs indicative of a swallowing problem in people with motor neuron disease (Hughes 1996). However, the predictive value of such swallowing indices remains to be determined across different muscle diseases.
Quality of the evidence
In this review, the included study and one of the non‐randomised studies reviewed in the Discussion assessed the same intervention in the same patient population, IBM (Dalakas 1997 and Dobloug 2012). Only one of the identified trials was an RCT (Dalakas 1997), and we assessed this as at a high risk of bias (see Risk of bias in included studies). Similarly, there was a high risk of bias in the two non‐randomised trials identified in this review. In addition to such limitations of study design and implementation, none of the trials included data for the prespecified primary outcome of weight maintenance or change. The trials reported the occurrence of adverse events but not according to predefined review criteria. The indirectness of outcome measures for dysphagia also lowered the quality of the evidence. We could only consider overall quality of the evidence low or very low for the identified trials.
Potential biases in the review process
No review author was an investigator or author in any of the identified trials. To help ensure that relevant expertise was represented in the review process, our review authors included specialist medical and allied health professionals to evaluate the effects of various pharmacological, surgical, and supportive interventions for managing dysphagia in muscle disease.
One potential bias in the review process was that RCTs could be inadequate to identify adverse events over a 12‐month period after intervention, as specified in the review's secondary outcomes.
Another potential bias in the review process was that we identified non‐randomised trials through the search for randomised trials; we did not perform a separate search for non‐randomised trials, which might have identified relevant studies for discussion.
Agreements and disagreements with other studies or reviews
In this review, eating and drinking ability were assessed as part of a controlled trial of lip‐strengthening exercises (Sjögreen 2010). The trialists identified a high degree of variability in lip force measurements and concluded that improvement in such indices does not automatically correspond with improvement in eating and drinking. Elsewhere, lingual training with an air‐filled bulb has been associated with improved strength in a single case study in IBM (Malandraki 2012); controlled studies would be required to investigate the possible treatment effect further. The therapeutic potential of the Mendelsohn manoeuvre, defined as voluntary prolongation of hyolaryngeal elevation at the peak of the swallow (McCullough 2012), has also been studied in cases with IBM and dysphagia; Oh 2008 found that participants' weight was maintained over a review period of one to five years, and there were no reported incidences of aspiration. It is important to highlight, however, that adherence can be problematic in the application of exercise and swallowing techniques for dysphagia. For example, Sjögreen 2010 acknowledged that training was interrupted by recurrent infections for some participants. Another study reported that 21% of participants never complied with the advice given on safe swallowing (Low 2001). In a systematic review of children with swallowing disorders, there was no clear evidence to support treatment with oral motor exercises (Arvedson 2010).
Despite potential issues with intervention adherence, the previous review highlighted that trialists considered participants' subjective evaluation of swallowing to be a reliable method of detecting improvement following interventions (Hill 2004). All three studies appraised in this review included subjective evaluation, although none detailed the validity and reliability of selected outcome measures. There is a clear need for validated, reliable and standardised assessment of dysphagia that can be applied and reported in clinical trials.
In this review, we found that IVIg therapy was the only pharmacological treatment for which a change in swallowing function was assessed as part of a controlled trial (Dalakas 1997; Dobloug 2012). The results were subject to a high risk of bias and did not specifically target participants with swallowing impairment. We propose that findings of an improvement in swallowing function in both the short‐ and longer‐term studies of IVIg are attributable to large inter‐ and intra‐participant variation in individual responses, but the RCT did not report standard deviation values to confirm or refute this interpretation. These IVIg studies targeted people with IBM, a condition that may have a primary inflammatory rather than degenerative aetiology, and is not always associated with dysphagia. Any evidence of an IVIg treatment effect in these trials might therefore not be applicable to either long‐term, progressive primary muscle disease or dysphagia. Furthermore, a Cochrane review of treatment for IBM could not determine whether IVIg improved or stabilised overall muscle strength because the relevant RCT data were not available for meta‐analysis (Rose 2015).
Authors' conclusions
Implications for practice.
There is currently a lack of evidence from randomised controlled studies on interventions for dysphagia in muscle disease. Clinically relevant effects of intravenous immunoglobulin for dysphagia in inclusion body myositis can neither be confirmed nor excluded using the evidence presented in this review.
Implications for research.
Universal, validated, and reliable outcome measures are needed for assessing dysphagia. Clinically meaningful outcomes for dysphagia may require a shift in focus from measures of impairment to disability associated with oral feeding difficulties. In the absence of a definitive measurement for dysphagia, there is still a need to map proxy outcomes over time as part of a prospective longitudinal study; large‐scale data collection will be essential for evaluating treatment effects in future clinical trials.
What's new
| Date | Event | Description |
|---|---|---|
| 22 September 2015 | New citation required and conclusions have changed | We also considered trials in inclusion body myositis in this review update. Katherine Jones, Robert DS Pitceathly, Michael R Rose, Susan McGowan, and Umesh A Badrising joined the review at this update; Chris Milford withdrew. |
| 22 September 2015 | New search has been performed | This is an update of a Cochrane review in which authors consider the latest randomised controlled trials of interventions for dysphagia in long‐term, progressive muscle disease and the continuing challenges in study design. New searches incorporated. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 30 July 2008 | Amended | Converted to new review format. |
| 6 May 2008 | New citation required and conclusions have changed | Substantive amendment |
| 30 September 2007 | New search has been performed | Searches updated to August 2007. Description of two more non‐randomised studies and two case reports added. |
Acknowledgements
The Cochrane Neuromuscular Trials Search Co‐ordinator completed the search strategies for this review update.
The Methods section has been updated to current standards using in part standard text provided by Cochrane Neuromuscular.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, National Institute for Health Research, National Health Service, or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register (CRS) search strategy
#1 MeSH DESCRIPTOR Deglutition Disorders Explode All [REFERENCE] [STANDARD] #2 Dysphagi* or swallow* or deglutition* [REFERENCE] [STANDARD] #3 #1 or #2 [REFERENCE] [STANDARD] #4 MeSH DESCRIPTOR Muscular Diseases Explode All [REFERENCE] [STANDARD] #5 "inclusion body" NEAR2 myositis [REFERENCE] [STANDARD] #6 muscl* or muscul* [REFERENCE] [STANDARD] #7 disease* or disorder* or weaknes* [REFERENCE] [STANDARD] #8 #6 and #7 [REFERENCE] [STANDARD] #9 #4 or #5 or #8 [REFERENCE] [STANDARD] #10 #3 and #9 [REFERENCE] [STANDARD] #11 (#3 and #9) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL (CRSO) search strategy
Search run on Mon Jan 11 2016
#1 MESH DESCRIPTOR Deglutition Disorders EXPLODE ALL TREES1759 #2 (Dysphagi* or swallow* or deglutition*):TI,AB,KY3203 #3 #1 OR #2 4363 #4 MESH DESCRIPTOR Neuromuscular Diseases EXPLODE ALL TREES 4567 #5 "inclusion body" NEAR2 myositis 39 #6 (muscl* or muscul*):TI,AB,KY 36204 #7 (disease* or disorder* or weaknes*):TI,AB,KY 231720 #8 #6 AND #71 1750 #9 #4 OR #5 OR #8 15556 #10 #3 AND #9 206
Appendix 3. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to November Week 3 2015> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (417624) 2 controlled clinical trial.pt. (92270) 3 randomized.ab. (309508) 4 placebo.ab. (159698) 5 drug therapy.fs. (1862631) 6 randomly.ab. (219030) 7 trial.ab. (322047) 8 groups.ab. (1378466) 9 or/1‐8 (3517639) 10 exp animals/ not humans.sh. (4156219) 11 9 not 10 (2999126) 12 exp Deglutition Disorders/ (44220) 13 (Dysphagi$ or (swallow$ or deglutition$)).mp. (43420) 14 12 or 13 (68551) 15 exp Muscular Diseases/ (143257) 16 ((muscle$ or muscul$) adj5 (disease$ or disorder$ or weaknes$)).mp. (68927) 17 inclusion body myositis.mp. (1204) 18 or/15‐17 (176206) 19 11 and 14 and 18 (368) 20 remove duplicates from 19 (359)
Appendix 4. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2016 Week 02> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure/ (45414) 2 double‐blind procedure/ (124985) 3 randomized controlled trial/ (388894) 4 single‐blind procedure/ (21252) 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (1533722) 6 or/1‐5 (1616325) 7 exp animals/ (20527856) 8 exp humans/ (16476972) 9 7 not (7 and 8) (4050884) 10 6 not 9 (1460070) 11 limit 10 to embase (1200272) 12 exp Dysphagia/ (47908) 13 (Dysphagi$ or swallow$ or deglutition$).mp. (76279) 14 12 or 13 (78165) 15 exp Neuromuscular Disease/ (146270) 16 ((muscle$ or muscul$) adj5 (disease$ or disorder$ or weaknes$)).mp. (109756) 17 inclusion body myositis.mp. (2185) 18 or/15‐17 (232644) 19 11 and 14 and 18 (272)
Appendix 5. AMED (OvidSP) search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to December 2015> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Randomized controlled trials/ (1752) 2 Random allocation/ (313) 3 Double blind method/ (571) 4 Single‐Blind Method/ (67) 5 exp Clinical Trials/ (3501) 6 (clin$ adj25 trial$).tw. (6280) 7 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. (2602) 8 placebos/ (569) 9 placebo$.tw. (2823) 10 random$.tw. (15486) 11 research design/ (1828) 12 Prospective Studies/ (858) 13 meta analysis/ (156) 14 (meta?analys$ or systematic review$).tw. (2704) 15 control$.tw. (32082) 16 (multicenter or multicentre).tw. (897) 17 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. (11549) 18 or/1‐17 (49684) 19 (Dysphagi$ or (swallow$ or deglutition$)).mp. (881) 20 exp Muscular disease/ (7728) 21 ((muscle$ or muscul$) adj5 (disease$ or disorder$ or weaknes$)).mp. (5170) 22 inclusion body myositis.mp. (16) 23 or/20‐22 (11376) 24 18 and 19 and 23 (16) 25 remove duplicates from 24 (16)
Appendix 6. CINAHL (EBSCOhost) search strategy
Monday, January 11, 2016 8:32:02 AM S26 S24 AND S25 36 S25 EM 20141110‐ 476,011 S24 S18 and S23 256 S23 S19 and S22 794 S22 S20 or S21 59,299 S21 ( muscle* or muscul* ) and ( disease* or disorder* or weaknes* ) 36,180 S20 (MH "Muscular Diseases+") 32,523 S19 Dysphagi* or ( swallow* or deglutition* ) 10,190 S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 801,400 S17 ABAB design* 91 S16 TI random* or AB random* 163,434 S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 325,199 S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 115,928 S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 42,524 S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 25,172 S11 PT ("clinical trial" or "systematic review") 131,254 S10 (MH "Factorial Design") 964 S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 277,109 S8 (MH "Meta Analysis") 23,911 S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 49 S6 (MH "Quasi‐Experimental Studies") 7,671 S5 (MH "Placebos") 9,596 S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 32,950 S3 (MH "Clinical Trials+") 195,146 S2 (MH "Crossover Design") 13,507 S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 71,518
Appendix 7. LILACS (IAHx) search strategy
("deglutition disorders" or MH:C06.405.117.119$ or "trastornos de deglucion" or "transtornos de degluticao" or dysphagia or swallow$ or deglutition) and ("muscular diseases" or MH:C05.651$ "enfermedades musculares" or "doencas musculares" or ((muscle$ or muscular) and (disease$ or disorder$ or weakness))) and ((PT:"Randomized Controlled Trial" or "Randomized Controlled trial" or "Ensayo Clínico Controlado Aleatorio" or "Ensaio Clínico Controlado Aleatório" or PT:"Controlled Clinical Trial" or "Ensayo Clínico Controlado" or "Ensaio Clínico Controlado" or "Random allocation" or "Distribución Aleatoria" or "Distribuição Aleatória" or randon$ or Randomized or randomly or "double blind" or "duplo‐cego" or "duplo‐cego" or "single blind" or "simples‐cego" or "simples cego" or placebo$ or trial or groups) AND NOT (B01.050$ AND NOT (humans or humanos or humanos)))
Appendix 8. Clinical trials registries search strategies
deglutition AND muscle
dysphagia AND muscle
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dalakas 1997.
| Methods | Double‐blind, placebo‐controlled, cross‐over study; 3 months' IVIg and 3 months of placebo, separated by a wash‐out period of at least 1 month | |
| Participants | 22 randomised (gender not specified) IVIg group mean age: 61.2 (42 to 74 years); mean disease duration 5.6 (3 to 10 years) Placebo group mean age: 66.1 (35 to 76 years); mean disease duration 7.4 (4 to 16 years) Inclusion criteria: diagnostic criteria of s‐IBM; active disease characterised by progressive muscle weakness; impaired ability to perform fully the activities of daily living; absence of another systemic illness Exclusion criteria: coronary artery disease; IgA deficiency; kidney dysfunction; bedridden patients |
|
| Interventions | 3 months IVIg versus placebo with a wash‐out period of at least 1 month. The placebo consisted of dextrose in half normal saline, and IVIg dosage was 2 g/kg body weight. 3 participants also received concomitant treatment with prednisone | |
| Outcomes |
|
|
| Funding | Not reported | |
| Conflicts of interest | Not reported | |
| Notes | "Patients were referred...for therapeutic studies during the period 1992‐1994" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random component in the sequence generation process described: "The patients were assigned to receive IVIg or placebo by a block‐randomization procedure" |
| Allocation concealment (selection bias) | Low risk | Central allocation described: "Randomization was performed at the pharmacy" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement: "The principal investigator, the physicians, nurses, physical therapists, and statisticians were unaware of which treatment was administered" but "Sixteen of the 19 patients correctly identified the period during which they received placebo or IVIg" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement: blinding following allocation concealment not described fully |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 3 dropouts after the first period of the trial (14% attrition); it was unclear whether 2 of the 3 dropouts received treatment or placebo |
| Selective reporting (reporting bias) | High risk | Outcomes for swallowing function incompletely reported (videofluoroscopy and self assessment questionnaire). No standard deviation values reported. No published protocol available |
| Other bias | High risk | Randomisation was broken by giving participants the option to cross over intervention. There was a minimum wash‐out period of 1 month, which may not be long enough to exclude a carry‐over effect. There was also a possible carry‐over effect from drug treatment taken prior to the trial |
IgA: immunoglobulin A IVIg: intravenous immunoglobulin s‐IBM: sporadic inclusion body myositis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dobloug 2012 | Non‐randomised controlled trial |
| Horowitz 1987 | Non‐randomised controlled trial |
| NCT00773227 | Treatment of dysphagia in oculopharyngeal muscular dystrophy by autologous transplantation of myoblasts. Single group assignment. Non‐randomised controlled trial |
| Sjögreen 2010 | Non‐randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN84905610.
| Trial name or title | Comparative study on treatment methods in cricopharyngeal dysfunction |
| Methods | RCT (pilot) |
| Participants | Oropharyngeal dysphagia caused by cricopharyngeal dysfunction |
| Interventions | Balloon dilation versus laser myotomy |
| Outcomes | Videomanometry Sydney Swallow Questionnaire |
| Starting date | January 2008 |
| Contact information | ÖNH Kliniken Skanes Universitetssjukhuset Jan Waldenström gata 18 Malmö 205 02 Sweden |
| Notes |
NCT02328482.
| Trial name or title | Continuation protocol to protocol BBCO‐001 |
| Methods | RCT |
| Participants | Oculopharyngeal muscular dystrophy |
| Interventions | Drug: IV trehalose (Cabaletta) 30 g |
| Outcomes | Changes in disease markers Changes in swallowing quality of life Safety and tolerability evaluation including adverse events, vital signs, safety labs, and physical examination |
| Starting date | January 2015 |
| Contact information | BioBlast Pharma Ltd. |
| Notes |
RCT: randomised controlled trial
Differences between protocol and review
In the updated review we changed the title of the review from 'Treatment for swallowing difficulties (dysphagia) in chronic muscle disease' to 'Interventions for dysphagia in long‐term, progressive muscle disease' to clarify that we would include any type of intervention.
We did not specify the diagnostic criteria for dysphagia or muscle disease in the updated review on the basis that dysphagia is described as a syndrome and there are no standard criteria for diagnosis of dysphagia or muscle disease.
We included participants with inclusion body myositis in the updated review as explained in the main text.
An original review author, Chris Milford, did not co‐author this review update. Katherine Jones, Robert DS Pitceathly, Michael R Rose, Susan McGowan, and Umesh A Badrising joined the review at this update.
The protocol did not describe methods for assessment of heterogeneity; we added these at this update to comply with current standards. We used the current Cochrane 'Risk of bias' tool (Higgins 2011), rather than the Jadad scale, for methodological quality assessment.
Contributions of authors
All review authors were involved in data collection or critical appraisal of the included studies, or both.
All review authors reviewed drafts and agreed on the final text.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Association Franҫaise contre les Myopathies, France.
Grant: Katherine Jones
-
Myositis UK, UK.
Grant: Katherine Jones
Declarations of interest
KJ: Her research contribution to this review has been paid for by grants from Myositis UK and the Association Française contre les Myopathies. KJ was Assistant Managing Editor of Cochrane Neuromuscular at the time of publication. Her work on the review largely predated this appointment.
RP: None known.
MR: None known.
SM: None known.
MH: None known for current review update. She works primarily as a consultant neurologist and Associate Professor at Swansea University. She also has an established private medical practice, and as part of this work could review patients with dysphagia secondary to muscle disease.
UB: None known for current review update. His institution received a consulting fee for trial design and a fee for an ongoing clinical trial of bimagrumab in inclusion body myositis from Novartis.
TH: None known.
KJ and TH are joint first authors
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Dalakas 1997 {published data only}
- Dalakas MC, Sonies B, Dambrosia J, Sekul E, Cupler E, Sivakumar K. Treatment of inclusion‐body myositis with IVIg: a double‐blind, placebo‐controlled study. Neurology 1997;48(3):712‐6. [PUBMED: 9065553] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dobloug 2012 {published data only}
- Dobloug C, Walle‐Hansen R, Gran JT, Molberg Ø. Long‐term follow‐up of sporadic inclusion body myositis treated with intravenous immunoglobulin: a retrospective study of 16 patients. Clinical and Experimental Rheumatology 2012;30(6):838‐42. [PUBMED: 22935197] [PubMed] [Google Scholar]
Horowitz 1987 {published data only}
- Horowitz M, Maddox A, Maddern GJ, Wishart J, Collins PJ, Shearman DJ. Gastric and esophageal emptying in dystrophia myotonica. Effect of metoclopramide. Gastroenterology 1987;92(3):570‐7. [PUBMED: 3817383] [DOI] [PubMed] [Google Scholar]
NCT00773227 {published data only}
- NCT00773227. Treatment of dysphagia in oculopharyngeal muscular dystrophy by autologous transplantation of myoblasts. http://clinicaltrials.gov/show/NCT00773227 (accessed 12 January 2016).
Sjögreen 2010 {published data only}
- Sjögreen L, Tulinius M, Kiliaridis S, Lohmander A. The effect of lip strengthening exercises in children and adolescents with myotonic dystrophy type 1. International Journal of Pediatric Otorhinolaryngology 2010;74(10):1126‐34. [PUBMED: 20638139] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN84905610 {published data only}
- ISRCTN84905610. Comparative study on treatment methods in cricopharyngeal dysfunction. http://apps.who.int/trialsearch/trial2.aspx?trialid=isrctn84905610 (accessed 12 January 2016).
NCT02328482 {published data only}
- NCT02328482. Continuation protocol to protocol BBCO‐001. http://clinicaltrials.gov/show/NCT02328482 (accessed 12 January 2016).
Additional references
Abu‐Baker 2007
- Abu‐Baker A, Rouleau GA. Oculopharyngeal muscular dystrophy: Recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies. Biochimica et Biophysica Acta 2007;1772:173‐85. [DOI] [PubMed] [Google Scholar]
Archer 2013
- Archer SK, Garrod R, Hart N, Miller S. Dysphagia in Duchenne muscular dystrophy assessed by validated questionnaire. International Journal of Language & Communication Disorders 2013;48(2):240‐6. [DOI] [PubMed] [Google Scholar]
Arvedson 2010
- Arvedson J, Clark H, Lazarus C, Schooling T, Frymark T. The effects of oral‐motor exercises on swallowing in children: an evidence‐based systematic review. Developmental Medicine and Child Neurology 2010;52(11):1000‐13. [DOI] [PubMed] [Google Scholar]
Bülow 2001
- Bülow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in patients with pharyngeal dysfunction. Dysphagia 2001;16(3):190‐5. [DOI] [PubMed] [Google Scholar]
Cox 2009
- Cox FM, Verschuuren JJ, Verbist BM, Niks EH, Wintzen AR, Badrising UA. Detecting dysphagia in inclusion body myositis. Neurology 2009;256(12):2009‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2011
- Cox FM, Titulaer MJ, Sont JK, Wintzen AR, Verschuuren JJ, Badrising UA. A 12‐year follow‐up in sporadic inclusion body myositis: an end stage with major disabilities. Brain 2011;134(Pt 11):3167‐75. [DOI] [PubMed] [Google Scholar]
Darrow 1992
- Darrow DH, Hoffman HT, Barnes GJ, Wiley CA. Management of dysphagia in inclusion body myositis. Archives of Otolaryngology ‐ Head and Neck Surgery 1992;118(3):313‐7. [DOI] [PubMed] [Google Scholar]
Drake 1997
- Drake W, O'Donoghue S, Bartram C, Lindsay J, Greenwood R. Eating in side‐lying facilitates rehabilitation in neurogenic dysphagia. Brain Injury 1997;11(2):137‐42. [DOI] [PubMed] [Google Scholar]
Durmus 2011
- Durmus H, Laval SH, Deymeer F, Parman Y, Kiyan E, Gokyigiti M, et al. Oculopharyngodistal myopathy is a distinct entity: clinical and genetic features of 47 patients. Neurology 2011;76(3):227‐35. [DOI] [PubMed] [Google Scholar]
Ertekin 2001
- Ertekin C, Keskin A, Kiylioglu N, Kirazli Y, On AY, Tarlaci S, et al. The effect of head and neck positions on oropharyngeal swallowing: a clinical and electrophysiologic study. Archives of Physical Medicine and Rehabilitation 2001;82(9):1255‐60. [DOI] [PubMed] [Google Scholar]
Ganger 1990
- Ganger D, Craig RM. Swallowing disorders and nutritional support. Dysphagia 1990;4(4):213‐9. [DOI] [PubMed] [Google Scholar]
Griggs 1995
- Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, et al. Inclusion body myositis and myopathies. Annals of Neurology 1995;38(5):705‐13. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 2002b
- Hill M, Hughes T. Workshop: Management of adults and children with feeding difficulties secondary to chronic muscle disease, 22nd March 2002, Sheffield, UK. Neuromuscular Disorders 2002;12(10):970–4. [DOI] [PubMed] [Google Scholar]
Hughes 1996
- Hughes TA, Wiles CM. Clinical measurement of swallowing in health and in neurogenic dysphagia. Quarterly Journal of Medicine 1996;89(2):109‐16. [DOI] [PubMed] [Google Scholar]
Jaradeh 2006
- Jaradeh S. Muscle disorders affecting oral and pharyngeal swallowing. GI Motility Online 2006. [DOI: 10.1038/gimo35] [DOI] [Google Scholar]
Logemann 1994
- Logemann JA, Rademaker AW, Pauloski BR, Kahrilas PJ. Effects of postural change on aspiration in head and neck surgical patients. Otolaryngology ‐ Head and Neck Surgery 1994;110(2):222‐7. [DOI] [PubMed] [Google Scholar]
Low 2001
- Low J, Wyles C, Wilkinson T, Sainsbury R. The effect of compliance on clinical outcomes for patients with dysphagia on videofluoroscopy. Dysphagia 2001;16(2):123‐7. [DOI] [PubMed] [Google Scholar]
Malandraki 2012
- Malandraki GA, Kaufman A, Hind J, Ennis S, Gangnon R, Waclawik A, et al. The effects of lingual intervention in a patient with inclusion body myositis and Sjögren's syndrome: a longitudinal case study. Archives of Physical Medicine and Rehabilitation 2012;93(8):1469‐75. [DOI] [PubMed] [Google Scholar]
Martin 1991
- Martin AW. Dietary management of swallowing disorders. Dysphagia 1991;6(3):129‐34. [DOI] [PubMed] [Google Scholar]
McCann 2007
- McCann LJ, Garay SM, Ryan MM, Harris R, Riley P, Pilkington CA. Oropharyngeal dysphagia in juvenile dermatomyositis (JDM): an evaluation of videofluoroscopy swallow study (VFSS) changes in relation to clinical symptoms and objective muscle scores. Rheumatology 2007;46(8):1363‐6. [DOI] [PubMed] [Google Scholar]
McCullough 2012
- McCullough GH, Kamarunas E, Mann GC, Schmidley JW, Robbins JA, Crary MA. Effects of Mendelsohn maneuver on measures of swallowing duration post stroke. Top Stroke Rehabilitation 2012;19(3):234‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norton 1996
- Norton B, Homer‐Ward M, Donnelly MT, Long RG, Holmes GK. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ 1996;312(7022):13‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Gara 1990
- O'Gara JA. Dietary adjustments and nutritional therapy during treatment for oral‐pharyngeal dysphagia. Dysphagia 1990;4(4):209‐12. [DOI] [PubMed] [Google Scholar]
Oh 2008
- Oh TH, Brumfield KA, Hoskin TL, Kasperbauer JL, Basford JR. Dysphagia in inclusion body myositis: clinical features, management, and clinical outcome. American Journal of Physical Medicine and Rehabilitation 2008;87(11):883‐9. [DOI] [PubMed] [Google Scholar]
Palmer 2010
- Palmer PM, Neel AT, Sprouls G, Morrison L. Swallow characteristics in patients with oculopharyngeal muscular dystrophy. Journal of Speech, Language & Hearing Research 2010;53(6):1567‐78. [DOI] [PubMed] [Google Scholar]
Pandolfino 2005
- Pandolfino JE, Kahrilas PJ, American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005;128(1):209‐24. [DOI] [PubMed] [Google Scholar]
Philpot 1999
- Philpot J, Bagnall A, King C, Dubowitz V, Muntoni F. Feeding problems in merosin deficient congenital muscular dystrophy. Archives of Disease in Childhood 1999;80(6):542‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2015
- Rose MR, Jones, K, Leong K, Walter MC, Miller J, Dalakas MC, et al. Treatment for inclusion body myositis. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD001555.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 1998
- Scott A, Perry A, Bench J. A study of interrater reliability when using videofluoroscopy as an assessment of swallowing. Dysphagia 1998;13(4):223‐7. [DOI] [PubMed] [Google Scholar]
Seguy 2002
- Seguy D, Michaud L, Guimber D, Cuisset JM, Devos P, Turck D, et al. Efficacy and tolerance of gastrostomy feeding in pediatric forms of neuromuscular diseases. Journal of Parenteral and Enteral Nutrition 2002;26(5):298‐304. [DOI] [PubMed] [Google Scholar]
Straathof 2014
- Straathof CS, Doorenweerd N, Wokke BH, Dumas EM, Bergen JC, Buchem MA, et al. Temporalis muscle hypertrophy and reduced skull eccentricity in Duchenne muscular dystrophy. Journal of Child Neurology 2014;29(10):1344‐8. [DOI] [PubMed] [Google Scholar]
Tieleman 2009
- Tieleman AA, Knuijt S, Vliet J, Swart BJ, Ensink R, Engelen BG. Dysphagia is present but mild in myotonic dystrophy type 2. Neuromuscular Disorders 2009;19:196‐8. [DOI] [PubMed] [Google Scholar]
Willig 1994
- Willig TN, Paulus J, Lacau Saint Guily J, Béon C, Navarro J. Swallowing problems in neuromuscular disorders. Archives of Physical Medicine & Rehabilitation 1994;75(11):1175‐81. [DOI] [PubMed] [Google Scholar]
Wintzen 1988
- Wintzen AR, Bots GT, Bakker HM, Hulshof JH, Padberg GW. Dysphagia in inclusion body myositis. Journal of Neurology, Neurosurgery and Psychiatry 1988;51(12):1542‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hill 2002a
- Hill M, Hughes T, Milford C. Treatment for dysphagia in chronic muscle disease. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD004303] [DOI] [PubMed] [Google Scholar]
Hill 2004
- Hill M, Hughes T, Milford C. Treatment for swallowing difficulties (dysphagia) in chronic muscle disease. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004303.pub2] [DOI] [PubMed] [Google Scholar]


