Abstract
OBJECTIVES
Robotic lung resections (RLRs) are conventionally performed using look-up views of the thorax from the caudal side. To conduct RLR with views similar to those in open thoracotomy, we adopted a vertical port placement and confronting upside-down monitor setting, which we called robotic ‘open-thoracotomy-view approach’. We herein present our experience of this procedure.
METHODS
We retrospectively reviewed 58 patients who underwent RLR (43 with lobectomy; 15 with segmentectomy) with 3-arm open-thoracotomy-view approach using the da Vinci Surgical System between February 2019 and October 2020. The patient cart was rolled in from the left cranial side of the patient regardless of the side to be operated on. Robotic ports were vertically placed along the axillary line, and 2 confronting monitors and 2 assistants were positioned on each side of the patient. The right-side monitor, which was set up for the left-side assistant to view, projected the upside-down image of the console surgeon’s view.
RESULTS
All procedures were safely performed. The median duration of surgery and console operation was 215 and 164 min, respectively. Emergency conversion into thoracotomy and severe morbidities did not occur, and the median postoperative hospitalization duration was 3 days. In all procedures, the console surgeon and 2 assistants had direct ‘bird-eye’ views of the cranially located intrathoracic structures and instrument tips, which are sometimes undetectable with the conventional look-up view.
CONCLUSIONS
The open-thoracotomy-view approach setting is a possible option for RLR. It offers natural thoracotomy views and can circumvent some of the known limitations of the conventional procedure.
Keywords: Robotic lung resection, Open-thoracotomy-view approach, Vertical port placement, Confronting monitors
INTRODUCTION
Robotic lung resection (RLR) is a robot-assisted thoracoscopic surgery (RATS). Conventionally, RLR is performed using look-up views of the thorax from the caudal side; this viewing technique is currently the most commonly used RLR approach worldwide [1–5] including Japan [6–8].
In our practice, open thoracotomy surgery (OTS) is routinely performed using the vertical muscle sparing/splitting thoracotomy (VMST) [9], with the operating surgeon standing on the right side of the patient (i.e. patient’s dorsal side during right-lung surgery and ventral side during left-lung surgery) regardless of the side to be operated on. During a video-assisted thoracoscopic surgery (VATS), the operating surgeon also stands on the patient’s right side and uses the confronting upside-down (CUD) monitor setting [10]. This enables the operating and assisting surgeons to have the same surgical view in OTS and VATS, and also, the procedural flow and the placements of the instrumentations always remain the same for both OTS and VATS. Upon introducing RATS to our practice, however, we encountered an issue concerning how to perform RLR consistently with OTS and VATS. In Japan, the insurance coverage for RATS started in April 2018, and we began to perform RATS at a full scale in February 2019. To obtain the same surgical views as in OTS and VATS during RLR, we adopted a vertical placement of robotic ports and the CUD monitor setting for RLR; we called this approach as the ‘open-thoracotomy-view approach (OTVA)’. In this report, we present our initial experience of RLR using a 3-arm OTVA and discuss the technical aspects of the procedure.
PATIENTS AND METHODS
Patients
The institutional review board of Aichi Cancer Center Hospital approved the study (#2020-1-232). Each patient provided informed consent for the use of clinical data.
Of the 76 patients who underwent RATS in our department from February 2019 to October 2020, we retrospectively reviewed 58 patients who underwent major RLR (lobectomy, 43 patients; segmentectomy, 15 patients) using a 3-arm OTVA with the CUD monitor setting (Table 1). Among those who were excluded from this study were 3 patients who underwent RLR using the conventional look-up approach, 11 who underwent robotic mediastinal tumour resection in supine position, one who underwent RLR with 1 monitor and 4-arm OTVA without CUD monitor and 3 who underwent only partial lung resection using the presented method. The procedures were performed for clinical stage I primary lung cancer and lesions strongly suspected to be early-stage lung cancer based on the eighth tumour–node–metastasis classification system or resectable metastatic lung tumours. Preoperatively, the lesion and the lung including pulmonary vessels, bronchi and fissures were thoroughly assessed using the axial, sagittal and coronal images of the high-resolution computed tomography and its 3D reconstruction. Considering that small lesions with an almost ground-glass appearance have a small pathological invasive size based on preoperative high-resolution computed tomography [11], mediastinal lymph node dissection was omitted for such less invasive lesions. Segmentectomy was considered for such less invasive lesions, multiple lesions or metastatic lung tumours.
Table 1:
Baseline characteristics of the 58 patients who underwent major lung resection using the robotic open-thoracotomy-view approach
| Variables | Dataa |
|---|---|
| Age (median, range; years) | 70 (36–86) |
| Sex | |
| Male/female | 24 (41)/34 (59) |
| Smoking status | |
| Never/former or current | 33 (57)/25 (43) |
| Brinkman index (median, range) | 0 (0–1920) |
| Body condition | |
| Height (mean ± SD, range; cm) | 160 ± 9 (143–181) |
| Weight (mean ± SD, range; kg) | 59 ± 12 (37–91) |
| Body mass index (mean ± SD, range; kg/m2) | 23 ± 3 (16–34) |
| Respiratory function | |
| %VC (mean ± SD, range; % predicted) | 100 ± 14 (62–152) |
| %FEV1 (mean ± SD, range; % predicted) | 97 ± 20 (40–172) |
| %DLCO (mean ± SD, range; % predicted) | 110 ± 22 (71–181) |
| HRCT findings and size | |
| Pure GGO/partly solid/solid | 6 (10)/33 (57)/19 (33) |
| LDb (mean ± SD, range; cm) | 2.0 ± 1.0 (0.7–5.7) |
| CDb (mean ± SD, range; cm) | 1.2 ± 0.9 (0–3.7) |
| MDb (mean ± SD, range; cm) | 0.7 ± 0.7 (0–3.5) |
| Preoperative diagnosis | |
| Lung cancer (c-stage 0/IA1/IA2/IA3/IB) | 56 (5/24/17/8/2) |
| Metastatic lung tumour | 2 |
| Surgical procedure | |
| Lobectomy | 43 (74) |
| Segmentectomy | 15 (26) |
Data are presented as indicated or as the number of patients.
LD, CD, MD were previously reported [11].
CD: consolidation dimension in HRCT lung window; DLCO: diffusing capacity of the lung for carbon monoxid; FEV1: forced expiratory volume in 1 s; GGO: ground-glass opacity; HRCT: high-resolution computed tomography; LD: whole tumour dimension in the HRCT lung window; MD: tumour dimension in HRCT mediastinal window; SD: standard deviation; VC: vital capacity.
Surgical management
Patients were placed in conventional right or left lateral decubitus position and managed by general anaesthesia and double-lumen intubation. Before the start of the surgery, the anaesthesiologist introduced a paravertebral block or retrolaminar block by percutaneously injecting 0.25% ropivacaine hydrochloride hydrate for intraoperative analgesia.
System setting
We used the da Vinci Xi® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA). Regardless of the side to be operated on, the patient cart was always rolled in at 45°–60° angle from the patient’s left cranial side. In the 3-robotic-arm setting, arm 1 was not used but was pushed off towards the cranial side of the patient, while arm 2 was positioned at the cranial side of the patient for the console surgeon’s left hand, arm 3 was used for 30°-robotic endoscopy and arm 4 was positioned at the patient’s caudal side for the console surgeon’s right hand.
Assistants and confronting upside-down monitor setting
Figure 1 and Video 1 display the positions of the 2 assistants and the CUD monitor setting. The assistant standing on the right side of the patient (i.e. the patient’s dorsal side for the right-lung surgery or ventral side for the left-lung surgery; assistant A) was mainly responsible for the docking procedure and the exchange of Endowrist® instruments. The assistant standing on the left side of the patient (i.e. the patient’s ventral side for the right-lung surgery or dorsal side for the left-lung surgery; assistant B) assisted directly in retracting lungs and other intrathoracic structures, suctioning blood and firing non-robotic stapling devices.
Figure 1:

Two assistants and the confronting monitor setting. The patient’s head is at the far side of the operating room in this photograph. Assistant A stands on the right side of the patient and has the same view as the console surgeon, whereas assistant B stands on the left side of the patient and views the upside-down monitor. The dorsal or ventral side indicated on the monitors shows the dorsal side of the thorax during the right-lung surgery or ventral side of the thorax during the left-lung surgery.
Two confronting monitors were placed on each side of the patient. The left-side monitor, which was set up for assistant A, showed the same image as on the surgeon console. Conversely, the right-side monitor, which was set up for assistant B, projected the upside-down image of the surgeon console view. These settings enabled the console surgeon and the 2 assistants to naturally acquire the same views as in our OTS and VATS procedures.
Port placements
Figure 2 illustrates the vertical port placements. For right-side surgeries, 3 robotic ports were placed along the posterior axillary line, while 1 assist port was placed on the ventral surface. For left-side surgeries, 3 robotic ports were placed along the anterior axillary line, while the assist port was placed on the dorsal surface. For the right upper lobe, an 8 mm robotic port, another 8 mm robotic port, and a 12 mm robotic port were placed at the third, fifth and seventh intercostal spaces along the posterior axillary line, respectively, and an accessory port (Alnote Lapsingle, AL-LS-51–1318, Alfresa Pharma Corporation, Osaka, Japan) was placed on the ventral surface of the fifth or sixth intercostal space. This set-up is described as ‘3/5/7/A5 or A6’ (Fig. 2A). Similarly, set-ups such as ‘3/5/8/A6 or A7’ or ‘4/6/8/A6 or A7’ for the right middle lobe, ‘4/6/8/A6 or A7’ for the right lower lobe, ‘3/5/7/A8 or A9’ for the left upper lobe (Fig. 2B), and ‘3/5/8/A9 or A10’ or ‘4/6/8/A9 or A10’ for the left lower lobe were also used. In these set-ups, 3 robotic ports were placed around the VMST incision line [9].
Figure 2:
Vertical port placements for right-side (A) and left-side (B) surgeries. The lines and numbers drawn on the patient’s body indicate the location of the ribs, and the circles indicate the incision size and the intercostal space where each port is placed. Arrows show the roll-in direction of the patient cart. These figures show the settings for the upper lobes. For middle and lower lobes, the port locations are caudally moved, as described in the text. ICS: intercostal space.
Insufflation system
To maintain a stable pneumothorax environment, smoke evacuation and CO2 recirculation, we used an insufflation system (AirSeal® System, ConMed Corporation, Utica, NY, USA) and maintained a positive intrathoracic pressure of 5–10 mmHg.
Other surgical affairs
The target structures were around the azygos vein for the right upper lobe, around the superior pulmonary vein for the right middle and lower lobes, at the area distal to the lesser curvature of the aortic arch for the left upper lobe, and around the ventral side of the descending aorta for the left lower lobe.
In selecting Endowrist® instruments, we preferred the combination of fenestrated bipolar forceps (arm 2) and monopolar curved scissors (arm 4) for most dissecting manoeuvres. For lymph node dissection, we frequently used Vessel Sealer Extend and medium-large clip appliers. Robotic staplers were standardized for resecting each pulmonary lobe and blood vessel and were used through the caudal 12 mm port. Regarding nonrobotic staplers, we used the 30 mm types for the vessels and the 45 mm types, which are easier to handle than the 60 mm types, for pulmonary parenchyma and bronchus.
During segmentectomies, after resecting the segment-specific pulmonary arteries, veins, and bronchi, we diluted 25 mg of indocyanine green with 10 ml of sterile water and intravenously administered 5 mg (2 ml), which was one-fifth of the usual amount used in a hepatic function test, of the solution; the segmental boundaries were then identified using a fluorescence observation mode (Firefly®).
Fibrin glue and polyglycolic acid sheet were used according to the degree of air leak, and a 20 Fr chest tube was placed at the camera port.
Postoperative management
Water intake was allowed 2 h postoperatively, and food intake was started on the evening of the same day; if no air leak or chyle was detected, the chest tube was removed 2 h after food intake. For pain management, we orally administered 60 mg of loxoprofen sodium thrice a day. Chest X-ray was performed the day after tube removal; when no abnormalities were detected, the patients were discharged.
RESULTS
Table 2 summarizes the surgical outcomes. The median duration of surgery and console operation was 215 (147–311) and 164 (93–271) min, respectively. No emergency conversion into OTS occurred. One patient underwent unplanned conversion into regular VATS for safety because of moderate haemorrhage from a pulmonary artery branch during left upper lobectomy. The median postoperative time of chest tube removal was 0 (0–7) days, and the duration of postoperative hospitalization was 3 (1–9) days. No serious postoperative complication was observed. One patient experienced a prolonged air leak (>5 postoperative days), and another had a worsening subcutaneous emphysema. Slight malaise on postoperative day 1 was observed in 3 patients, seemingly associated with CO2 insufflation.
Table 2:
Surgical outcomesa
| Variables | All procedures (n = 58) | Lobectomy (n = 43) | Segmentectomyb (n = 15) |
|---|---|---|---|
| Operating time (median, range; min) | |||
| Total time | 215 (147–311) | 215 (149–311) | 200 (147–262) |
| Console time | 164 (93–271) | 167 (94–271) | 151 (93–210) |
| Time from surgery start to roll-in | 16 (10–31) | 16 (10–31) | 16 (12–27) |
| Time from surgery start to console start | 23 (16–43) | 22 (16–43) | 23 (19–36) |
| Time from roll-out to end of surgery | 26 (14–106) | 25 (16–106) | 32 (14–51) |
| Surgical procedure | |||
| RU/RM/RL/LU/LL | 20/9/9/14/6 | 18/9/5/6/5 | 2/0/4/8/1 |
| Right S1 + 3/S3/S6/S8 | 1/1/3/1 | ||
| Left S1 + 2+S3/S1 + 2/S3/S4+S5/S6 | 4/3/1/0/1 | ||
| Node dissection | |||
| ND1/ND2a-1/ND2a-2 | 31 (53)/23 (40)/4 (7) | 18 (42)/21 (49)/4 (9) | 13 (87)/2 (13)/0 (0) |
| Bleeding (median, range; g) | |||
| <5 (<5–290) | <5 (<5–290) | <5 (<5–20) | |
| Number of stapling devicesc (median, range) | |||
| 7 (3–12) | 6 (3–12) | 8 (5–12) | |
| Fibrin glue and polyglycolic acid sheet | |||
| –/+ | 24 (41)/34 (59) | 17 (40)/26 (60) | 7 (47)/8 (53) |
| Conversion | |||
| To VATSd/to open | 1/0 | 1/0 | 0/0 |
| Morbidity | |||
| Prolonged air leak (>5 postoperative days) | 1 (2) | 1 (2) | 0 |
| Subcutaneous emphysema | 1 (2) | 0 | 1 (7) |
| Slight malaise | 3 (5) | 2 (5) | 1 (7) |
| Postoperative course (median, range; days) | |||
| Chest tube removal | 0 (0–7) | 0 (0–7) | 0 (0–5) |
| Hospital stay | 3 (1–9) | 3 (1–9) | 3 (2–7) |
| Resection | |||
| R0/R1–2 | 58/0 | 43/0 | 15/0 |
| Histology | |||
| Primary lung cancer | 55 | 41 | 14 |
| Adenocarcinoma | 51 | 39 | 12 |
| Squamous-cell carcinoma | 2 | 1 | 1 |
| Small-cell carcinoma | 1 | 1 | 0 |
| Carcinoid | 1 | 0 | 1 |
| pT status | |||
| Tis/T1a/T1b/T1c/T2a/T2b/T3 | 2/20/24/5/3/0/1 | 2/10/20/5/3/0/1 | 0/10/4/0/0/0/0 |
| pN status | |||
| N0/N1/N2 | 54/1/0 | 40/1/0 | 14/0/0 |
| p-Stage | |||
| 0/IA1/IA2/IA3/IB/IIA/IIB | 2/20/24/5/2/0/2 | 2/10/20/5/2/0/2 | 0/10/4/0/0/0/0 |
| Metastatic lung tumour | 2 | 1 | 1 |
| Other | 1 | 1 | 0 |
| Adjuvant chemotherapy | |||
| –/+ | 57/1 | 42/1 | 15/0 |
| Postoperative observation time (median, range; months) | |||
| 11.0 (1.0–20.3) | 12.2 (1.0–20.3) | 10.0 (2.0–15.8) |
Data are presented as indicated or as the number of patients.
One patient who underwent right S6 segmentectomy and middle lobectomy for double primary lesions was included in the segmentectomy group because the primary lesion was located in the S6.
Including robotic and nonrobotic devices.
Unplanned conversion for moderate bleeding from a pulmonary artery branch.
LL: left lower; LU: left upper; RL: right lower; RM: right middle; RU: right upper; VATS: video-assisted thoracoscopic surgery.
No 30- or 90-day mortalities transpired. The median duration of postoperative observation was 11.0 (1.0–20.3) months. Five patients had a follow-up time of <3 months. None of the patients experienced recurrence. Additionally, the operating and console durations were slightly shorter, stapling devices were used more often and the procedure was performed on earlier stage lesions in the segmentectomy group than in the lobectomy group.
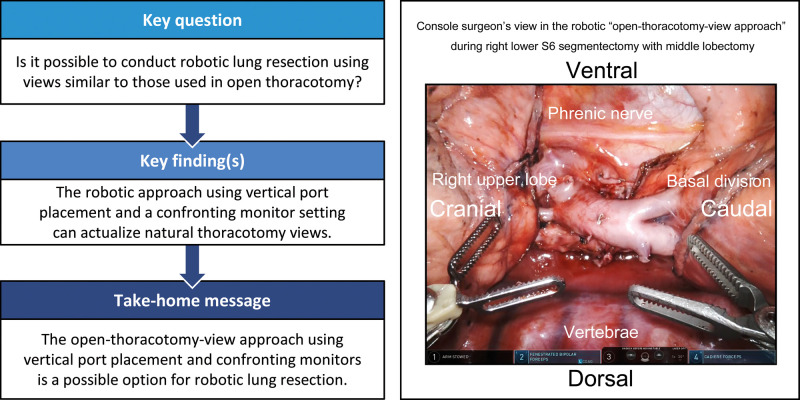
Figures 3 and 4 and Video 2 show the surgical views displayed on the surgeon console for the following procedures: right upper lobectomy (Fig. 3A), left upper lobectomy (Fig. 3B), right lower S6 segmentectomy with middle lobectomy for double primary lesions (Fig. 3C), right upper mediastinal lymph node dissection (Fig. 4A), left upper mediastinal lymph node dissection (Fig. 4B) and subcarinal mediastinal lymph node dissection during left lower lobectomy (Fig. 4C). In all procedures, ‘bird-eye’ views, which were different from those in the conventional look-up procedure, were achieved as in OTS. Cranially located intrathoracic structures or instrument tips, which are sometimes hidden and difficult to confirm in the look-up view, were visually confirmed in the front.
Figure 3:

Views of the surgeon console during lung resections. The left and right sides of all the images are the cranial and caudal sides of the intrathorax, respectively. The images are of different patients. (A) Resection of the superior pulmonary vein during right upper lobectomy. (B) Dissection of the pulmonary artery in an interlobar part during left upper lobectomy. (C) Segmental boundaries between right S6 and basal segments fluorescently enhanced in Firefly after intravenous indocyanine green injection. AZ: azygos vein; LLL: left lower lobe; LUL: left upper lobe; RML: right middle lobe; RUL: right upper lobe; SVC: superior vena cava.
Figure 4:

Lymph node (LN) dissections. (A) Right upper mediastinal zone. The brachiocephalic artery (BCA) was identified in the front. (B) Left upper mediastinal zone. The LNs around the left recurrent laryngeal nerve were dissected. (C) Completion of the subcarinal zone dissection following left lower lobectomy. The left main bronchus (LMB) and the contralateral right main bronchus (RMB) as well as the contralateral right vagus nerve were confirmed. AZ: azygos vein; BCA: brachiocephalic artery; LMB: left main bronchus; LN: lymph node; RMB: right main bronchus; SVC: superior vena cava.
DISCUSSION
This robotic OTVA can be explained as a modified conversion of the 3-arm robotic surgery for mediastinal neoplasms in the supine position to lung resection in lateral decubitus position. By combining the CUD monitor with this setting, the console surgeon and 2 assistants can obtain natural bird-eye views and perform RLR as though they were performing OTS or confronting VATS. During the look-up VATS from the caudal side of the thorax, cranially located intrathoracic structures or instrument tips can occasionally be obscured from view. Similar situation can occur during the conventional robotic look-up view approach. This situation can sufficiently be circumvented by the surgeon’s own techniques; however, there can certainly be some occasions where surgeons would have such difficulties, although demonstrating this characteristic quantitatively is difficult.
In this approach, especially in small female patients, the robotic ports and the target structures are often in close proximity, occasionally resulting in the limited manoeuvrability of the camera and the Endowrist® instruments. Nonetheless, this approach is made sufficiently feasible by expanding the thoracic cavity using an insufflation system (AirSeal®). Given that this approach could be successfully implemented in Japanese elderly small female patients, it could be implemented more easily in European and American patients who tend to be larger. Regarding camera manoeuvrability, the ventral or dorsal side of the pulmonary hilum can clearly become visible by switching the 30°-robotic camera up or down accordingly, as demonstrated in Video 2. Furthermore, the locations of the 3 robotic ports placed along the incision line for VMST [9] indicate that the OTVA views are similar to those in VMST. Surgeons may feel that the insertion of a robotic stapler via the caudally placed port is similar to the insertion of a stapler via the port placed in the seventh or eighth intercostal space that can be used for thoracoscope or chest tube placement in OTS.
Mun et al. [10] reported on VATS using the CUD monitor setting in detail, and we adopted this method for routine VATS because cranially located intrathoracic structures can be visualized and confirmed easily. They also explored an approach involving confronting monitors used for robotic surgeries, and we received extensive advice from them regarding robotic OTVA.
To conduct RLR using OTVA with CUD monitors, the operating console surgeon and assistants need to be well trained in VATS using the CUD monitor setting. Performing RLR using this set-up without the necessary proficiency is challenging. Considering that only 3 (not 4) robotic arms are used, assistant B needs to perform the important task of delicate and careful counter-retraction manoeuvres. In this setting, the space in which assistant B stands to perform tasks tends to be small; therefore, the boom rotation during targeting should be finely adjusted to secure the space. When the boom is positioned such that the caudal 12 mm port, the assist port and arm 4 are lined up perpendicularly to the patient, a sufficient space is secured for assistant B.
Yamazaki et al. [12] reported another approach to RLR different from conventional methods; this approach always provides a view of the intrathorax from the ventral side. In their approach, the left intrathoracic view is similar to that in our OTVA, and the right intrathoracic view is opposite to ours; in addition, they perform RLR using a 4-arm setting. In our first case of OTVA, we performed right lower lobectomy using the 4-arm setting with 1 monitor and 1 assistant; however, the procedure was made difficult by interference from the arms. Our next challenge is to develop our current 3-arm OTVA into a stable 4-arm procedure. By adopting the 4-arm method, only the assistant A would provide all the assistance.
Given that the ports were placed across multiple intercostal spaces, we need to consider that pain may be enhanced in OTVA. Although the pain was not quantitatively assessed and some patients slightly complained of intercostal neuralgia-like pain, we did not observe any clinical differences with our 4-port VATS. Considering that 5 ports (or 6 ports in some cases) are generally placed in conventional RLR, the lower number (4) of ports in our OTVA may not be inferior to the conventional approach in terms of pain. The assist port and its location may also affect pain. For future studies, quantitative investigations for pain must be required.
From an educational perspective, considering the benefits of consistency, we standardized the procedural flow for lung lobectomy regardless of whether OTS, VATS or RATS is performed. Even when RLR is occasionally performed among many other routine surgeries, the staff and residents sufficiently understand the procedural flow of lung resection; therefore, they can focus on the unique technical aspects of RLR. This way, robotic surgeries are not completely separated from our other routine surgeries in terms of skill development.
Currently, we perform OTS, VATS or RATS for patients with lung cancer according to the following considerations: OTS is indicated for advanced diseases, cases that may require manual manoeuvres such as a vessel or bronchial plasty, cases of severe lymph node calcification, cases requiring induction therapies, rethoracotomies or unusual highly invasive procedures; VATS and RATS are indicated for patients with up to clinical stage I disease; and RATS is indicated for lesions with a low likelihood of lymph node metastasis on preoperative radiological examination [11] and cases that are well lobulated, because it is still in the introductory phase. More recently, we have expanded the indications for VATS and RATS.
Finally, the emergency roll-out in this setting can be more straightforward and quicker than in the 4-arm setting. Considering the port location, the roll-out procedure and direction are consistently the same regardless of the side to be operated on. The procedure is as follows: (i) the console surgeon and assistants confirm that the instruments of arms 2 and 4 are free, and assistant A removes these instruments; (ii) assistant A undocks arms 2 and 4 from the respective ports; (iii) assistant A removes the camera from arm 3 and promptly inserts it manually into the caudal port and observes the intrathorax; and (iv) arm 3 is undocked, and the patient cart is rolled out. This procedure can be completed in ∼20 s. Meanwhile, assistant B concentrates on haemostasis. Alternatively, the system can easily be converted into routine VATS using CUD monitors by adding a scope port. Contrary to the trend towards future robotic surgeries performed by a single surgeon without assistants, our approach still requires 2 assistants. Nevertheless, having 2 assistants in close proximity to the patient helps to ensure safety in cases of emergency roll-out or conversion into thoracotomy.
Limitations
Our robotic OTVA presented herein has several limitations as described above. Our experiences are still in the introduction phase, and further acquisition of experience is necessary. A retrospective analysis of data from a single institution also limits generalization of the findings.
CONCLUSION
Because our robotic OTVA is different from well-established worldwide conventional approach, our method may be controversial and may present supporting and detracting perspectives, and it would be natural that many experts in conventional RATS may have some critical insights. This report does not emphasize the possible limitations of the conventional method; rather, it suggests that various approaches can be considered for RLR, similarly as VATS has several approaches including the look-up method and the CUD monitor method. We undoubtedly consider the widely used look-up view method as the current mainstream approach to RLR.
Robotic surgery is exponentially evolving and will change over a considerably shorter time span compared with conventional OTS and VATS. While we are attempting to solve the many issues associated with current RATS, new robots will likely be developed, giving rise to another set of unique challenges. Nevertheless, OTVA using the vertical port placement and CUD monitor setting is a possible option for RLR. It can actualize RLR with natural thoracotomy views and can, therefore, circumvent some of the known limitations of conventional look-up procedure.
ACKNOWLEDGEMENTS
We thank Dr Takashi Suda, Dr Koji Kawaguchi and Dr Mingyon Mun who gave us instruction and advice regarding our RATS.
Conflict of interest: All authors have no conflicts of interest to declare.
ABBREVIATIONS
- CUD
Confronting upside-down
- OTS
Open thoracotomy surgery
- OTVA
Open-thoracotomy-view approach
- RATS
Robot-assisted thoracoscopic surgery
- RLR
Robotic lung resection
- VATS
Video-assisted thoracoscopic surgery
- VMST
Vertical muscle sparing/splitting thoracotomy
Author contributions
Noriaki Sakakura: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing—original draft; Writing—review & editing. Takeo Nakada: Data curation; Methodology; Writing—review & editing. Suguru Shirai: Data curation; Methodology. Hirotomo Takahara: Data curation; Methodology. Keita Nakanishi: Data curation; Methodology. Takuya Matsui: Data curation; Methodology. Harushi Ueno: Data curation; Methodology. Yusuke Takahashi: Data curation; Methodology; Writing—review & editing. Hiroaki Kuroda: Conceptualization; Data curation; Methodology; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Frank A. Baciewicz Jr., Alper Toker and Alessandro Pardolesi for their contribution to the peer review process of this article.
REFERENCES
- 1.Cerfolio RJ, Ghanim AF, Dylewski M, Veronesi G, Spaggiari L, Park BJ.. The long-term survival of robotic lobectomy for non-small cell lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerfolio RJ, Watson C, Minnich DJ, Calloway S, Wei B.. One hundred planned robotic segmentectomies: early results, technical details, and preferred port placement. Ann Thorac Surg 2016;101:1089–95. [DOI] [PubMed] [Google Scholar]
- 3.Jiao W, Zhao Y, Qiu T, Xuan Y, Sun X, Qin Y. et al. Robotic bronchial sleeve lobectomy for central lung tumors: technique and outcome. Ann Thorac Surg 2019;108:211–8. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han Y, Zhang Y, Li C, Yang S, Li H.. Robotic lung cancer surgery: from simple to complex, from surgery to clinical study. J Thorac Dis 2020;12:51–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haruki T, Kubouchi Y, Takagi Y, Kidokoro Y, Matsui S, Nakanishi A. et al. Comparison of medium-term survival outcomes between robot-assisted thoracoscopic surgery and video-assisted thoracoscopic surgery in treating primary lung cancer. Gen Thorac Cardiovasc Surg 2020;68:984–92. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi Y, Nakamura H, Miwa K, Haruki T, Araki K, Takagi Y. et al. Initial results of robotic surgery for primary lung cancer: feasibility, safety and learning curve. Yonago Acta Med 2017;60:162–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura H, Suda T, Ikeda N, Okada M, Date H, Oda M. et al. Initial results of robot-assisted thoracoscopic surgery in Japan. Gen Thorac Cardiovasc Surg 2014;62:720–5. [DOI] [PubMed] [Google Scholar]
- 9.Sakakura N, Mizuno T, Arimura T, Kuroda H, Sakao Y.. Design variations in vertical muscle-sparing thoracotomy. J Thorac Dis 2018;10:5115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mun M, Ichinose J, Matsuura Y, Nakao M, Okumura S.. Video-assisted thoracoscopic surgery lobectomy via confronting upside-down monitor setting. J Vis Surg 2017;3:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakakura N, Inaba Y, Yatabe Y, Mizuno T, Kuroda H, Yoshimura K. et al. Estimation of the pathological invasive size of pulmonary adenocarcinoma using high-resolution computed tomography of the chest: a consideration based on lung and mediastinal window settings. Lung Cancer 2016;95:51–56. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki K, Toyokawa G, Shoji F, Takeo S.. A novel technique for robotic-assisted lobectomy for lung cancer: the anterior approach. Interact CardioVasc Thorac Surg 2020;30:328–328. [DOI] [PubMed] [Google Scholar]