The Zishen Yutai Pill increased the live birth rate after fresh embryo transfer when compared with placebo.
Abstract
OBJECTIVE:
To assess the efficacy of the Zishen Yutai Pill compared with placebo on live birth rates among women after fresh embryo transfer cycles.
METHODS:
We conducted a double-blind, multicenter, placebo-controlled, randomized trial to investigate whether administration of the Zishen Yutai Pill would improve pregnancy outcomes among women undergoing fresh embryo transfer after in vitro fertilization or intracytoplasmic sperm injection. The primary outcome was live birth rate. Secondary outcomes were rates of implantation, biochemical pregnancy, clinical pregnancy, pregnancy loss, cycle cancellation, and maternal, fetal, and neonatal complications. A total sample size of 2,265 women (1:1 in two groups) was used to detect a live birth rate difference between the Zishen Yutai Pill and placebo. Participants were enrolled and randomized to receive 5 g of the Zishen Yutai Pill or placebo orally, three times per day during the study.
RESULTS:
Recruitment was completed between April 2014 and June 2017, with 2,580 patients screened. Two thousand two hundred sixty-five patients were randomized: 1,131 to the Zishen Yutai Pill and 1,134 to placebo. Characteristics were similar between groups. In intention-to-treat analysis, the rates of live birth in the Zishen Yutai Pill (ZYP) group and placebo group were 26.8% and 23.0% (rate ratio [RR], 1.16; 95% CI 1.01–1.34; P=.038), respectively. The implantation rates were 36.8% and 32.6% in the ZYP and placebo groups, respectively (RR 1.13; 95% CI 1.01–1.25; P=.027). The biochemical pregnancy rate for the ZYP group was 35.5% compared with 31.1% in the placebo group (RR 1.14; 95% CI 1.02–1.28; P=.026). The rates of clinical pregnancy in the ZYP and placebo groups were 31.2% compared with 27.3%, respectively (RR 1.14; 95% CI 1.00–1.30; P=.043). There were no significant between-group differences in the rates of pregnancy loss, maternal, or neonatal complications (all P>.05).
CONCLUSION:
The Zishen Yutai Pill increased the rate of live birth after fresh embryo transfer compared with placebo.
CLINICAL TRIAL REGISTRATION:
Chictr.org.cn, Chictr-TRC-14004494.
In vitro fertilization (IVF) is widely performed as infertility treatment and has resulted in more than 5 million births worldwide.1–3 In recent years, traditional Chinese medicine (TCM) therapies are frequently used by women undergoing IVF.4–7 Data from a prospective cohort study in the United States showed that 17% of couples used TCM therapies for infertility.4 Another cross-sectional study found that 46% of Irish patients undergoing IVF used TCM, with 38% having taken TCM in the 3 months before presenting for infertility treatment.8 Previous studies suggest a possible benefit from TCM in improving IVF outcomes.9,10
In Asia, the Zishen Yutai Pill is one of the representative TCM preparations used in IVF. In 2018, the Zishen Yutai Pill was included in the National Essential Medicine List of China.11 The Zishen Yutai Pill contains 15 Chinese traditional medicinal herbs.12 It ameliorates advanced endometrial maturation through the upregulation of HOXA10,13 and a high-dose Zishen Yutai Pill may up-regulate levels of transforming growth factor-β and improve oocyte function.14 A metabonomic study suggested that metabolites changed after administration of the Zishen Yutai Pill were highly enriched in sphingolipids, alanine, aspartic acid, glutamic acid, taurine and hypotaurine metabolites, and aminoacyl tRNA biosynthesis. These metabolites may be involved in proliferation of endometrium, regulation of oxidative stress and lipid metabolism, and thus lead to improvements in endometrial receptivity and oocyte quality.15 Clinically, a previous report demonstrates that using the Zishen Yutai Pill in luteal phase could improve pregnancy outcomes among patients undergoing in vitro fertilization-embryo transfer (IVF-ET).16 However, the efficacy and safety of the Zishen Yutai Pill have not been demonstrated in any multicenter randomized controlled trial (RCT) among patients with infertility undergoing IVF or intracytoplasmic sperm injection (ICSI).
We conducted a double-blind, multicenter, placebo-controlled, randomized trial to investigate whether administration of the Zishen Yutai Pill would increase the live birth rates among women undergoing IVF or ICSI.
METHODS
This double-blind, multicenter RCT was conducted at 19 IVF centers encompassing all China regions (nine in Southern China, four in Eastern China, one in Western China, and five in Northern China). The protocol (Appendix 1, http://links.lww.com/AOG/C554), with two amendments (Appendix 2, available online at http://links.lww.com/AOG/C555), was approved by the Ethics Review Committee of each study center. A data-safety and monitoring board was established to oversee the study. All participants provided written informed consent to participate in this study. Registration was made on April 13, 2014 (Chictr.org.cn, Chictr-TRC-14004494).
Patients with infertility undergoing an IVF cycle or ICSI were recruited from 19 reproductive medical centers throughout China. The inclusion criteria were 1) women with infertility aged 43 years or younger, 2) patients who planned to undergo IVF or ICSI-ET (long protocol and antagonist protocol), 3) body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) 30 or lower, and 4) both ovaries present. The exclusion criteria were 1) repeated implantation failures (three or more IVF or ICSI-ET failure cycles); 2) severe endometriosis, including adenomyosis of the uterus and ovarian “chocolate” cysts; 3) untreated bilateral hydrosalpinx; 4) untreated endometrial diseases (eg, endometritis, endometrial polyp, intrauterine adhesion); or 5) diseases that were not suitable for assisted reproductive technology (ART) or pregnancy.
If a potential participant expressed interest in the study, a face-to-face interview regarding the entire trial process was conducted by clinical staff. In total, 236 patients did not express interest. Patients who met inclusion criteria were enrolled after providing written informed consent.
The participants were randomized 1:1 to receive double-blind and single-dummy monotherapy with placebo or the Zishen Yutai Pill. Sequence generation was done by independent statisticians, with a block size of 4. The randomization was stratified by study site.
The packaging and pills were the same in appearance for placebo and the Zishen Yutai Pill. Patients, investigators and clinical staff performing this trial were masked to treatment allocation. Allocation information was sealed in an opaque envelope and kept by independent statisticians. Blinding was maintained until the completion of the analysis. Unblinding was allowed in case of a medical emergency. The cause and timing of unblinding were recorded in detail and signed by the treating physicians when deemed necessary.
All data entry, management, and analyses were coordinated or performed at Sun Yat-Sen University, where the data coordinating center for this study resided.
In patients receiving the gonadotropin-releasing hormone analogue long protocol, both the Zishen Yutai Pill and placebo were administered orally three times per day in a dose of 5 g from the day of downregulation. In patients receiving GnRH antagonist protocol, both the Zishen Yutai Pill and placebo were administered orally three times per day in a dose of 5 g from day 19–23 in a previous cycle until the day of the pregnancy test (2 weeks after ET). For patients in both protocols, study intervention was stopped if the serum pregnancy test was β-hCG–negative. For those with positive serum β-hCG results, study intervention continued until an embryo with cardiac activity was visualized by ultrasonography (see Appendix 2, http://links.lww.com/AOG/C555, Schematic diagram for study design, intervention and visit settings).
The Zishen Yutai Pill contains 15 Chinese traditional medicinal herbs. It is produced under Good Manufacturing Practice, which can therefore ensure the uniformity among different batches. The detailed formula and dosage of the Zishen Yutai Pill are presented in the protocol (Appendix 1, http://links.lww.com/AOG/C554). The placebo was made up of pregelatinized starch, black iron oxide, refined honey, and dextrin. Both the Zishen Yutai Pill and placebo were obtained from Baiyunshan Zhongyi Co. Ltd.
The participants' ovarian stimulation protocol in this study was determined by the clinician. In the GnRHa long protocol, triptorelin (Decapeptyl, 1.25 mg, once) was administered on day 21 of the preceding menstrual cycle, subcutaneously. After 14 days of downregulation, follicle-stimulating hormone (FSH) was started and was continued until the day of human chorionic gonadotropin (hCG) administration. In the GnRH antagonist protocol, FSH was administered daily beginning on day 2 of the cycle. The GnRH antagonist (Cetrotide, Serono, Geneva, Switzerland) was given at a daily dose of 0.25 mg subcutaneously when the leading follicle diameter was 12–14 mm. In both cases, when the diameter of the leading follicle reached 18 mm or greater, ovulation was triggered with 10,000 international units hCG intramuscularly. Serum progesterone, luteinizing hormone (LH), and estradiol (E2) were analyzed on the day of hCG administration.
All participants underwent fresh ET. Oocyte retrieval was performed 35–36 hours after hCG administration, and ET was performed 3 days later. The maximum number of embryos transferred was three. Intramuscular progesterone at a daily dose of 40 mg was given as luteal phase support. Quantitative β-hCG estimation was performed 14 days after ET. The oocytes were inseminated approximately 4–6 hours after follicular aspiration by a conventional method or ICSI, according to sperm quality. Morphologic criteria were used for embryo scoring. On day 3, one to three good-quality embryos were picked for fresh ET. The good-quality embryos were defined as the cleavage stage embryo at level I. The cleavage-stage embryo at level I was defined as uniform cells in size and cytoplasm, with 0–10% cellular debris and no multinucleation.17
All baseline demographics, medical history, maternal, and neonatal outcomes were obtained through review of medical records. Baseline demographics included age and BMI. Medical history included etiology of infertility, duration of the attempt to conceive, infertility factors, previous IVF cycles, miscarriage history, smoking history, comorbidities such as hypertension and diabetes, antral follicle count at baseline and basic sex hormone levels including E2, FSH, and LH. The ovulation induction protocol, days of ovarian stimulation, total gonadotropin dose, E2 level, progesterone level, and endometrial thickness on hCG trigger day, the number of cleavage, fertilization, 2 PN fertilization, oocytes retrieved, available embryos, good-quality embryos, and embryos transferred were also collected.
The primary outcome of the study was the live birth rate. Secondary outcomes included rates of implantation, biochemical pregnancy, clinical pregnancy, pregnancy loss, cycle cancellation, and incidences of maternal, fetal and neonatal complications. Clinical pregnancy was defined as the observation of fetal cardiac activity by vaginal ultrasonography performed 2–3 weeks after positive serum β-hCG results. Live birth was defined as delivering any viable neonate at 28 weeks of gestation or more.18,19 Biochemical pregnancy was defined as serum β-hCG–positive (greater than 10 milli-international units/mL), as measured 14 days after ET. Cycle cancellation included cases with no embryos transferred due to either failed oocyte retrieval or failed fertilization, high risk of ovarian hyperstimulation syndrome, and higher progesterone level on hCG day. Detailed reasons for cycle cancellation are included in Appendix 2 (http://links.lww.com/AOG/C555, Cancellation of embryo transfer in the intention-to-treat [ITT] population). Clinical pregnancy rate was defined as number of clinical pregnancies per total embryo transfer cycles. Biochemical pregnancy rate was defined as number of patients with positive serum β-hCG pregnancy test results per total embryo transfer cycles. The live birth rate was defined as number of live births per total embryo transfer cycles. Pregnancy loss rate was defined as number of abortions per biochemical pregnancies or clinical pregnancies. Implantation rate was defined as number of gestational sacs per number of embryos transferred. Incidences of maternal, fetal and neonatal complications were assessed, including moderate or severe ovarian hyperstimulation syndrome, gestational diabetes mellitus, gestational hypertension, postpartum hemorrhage, preterm delivery, congenital anomalies, puerperal infection, stillbirth, neonatal jaundice, neonatal infection, neonatal death, ectopic pregnancy, and low birth weight neonate. The definitions of these secondary outcomes are included in Appendix 2 (http://links.lww.com/AOG/C555, Definitions of secondary outcomes).
Each center was assigned a trained fertility doctor to follow the participants, perform outpatient follow-up on the ovulation induction day, embryo transfer day, 2 weeks after transfer, and 5 weeks after transfer, as well as telephone follow-up after delivery to ascertain the live birth outcome for participants who delivered at another hospital.
We conducted a pilot study to collect preliminary data on the clinical pregnancy rate of with the Zishen Yutai Pill (38.3%) compared with placebo (31%) to guide our sample size estimation. Because the live birth rate (our primary outcome) is generally 10% lower than the clinical pregnancy rate,20 the estimated live birth rate was 34% for the Zishen Yutai Pill and 28% for the placebo. With 960 patients per study group, we had greater than 80% power to detect an absolute difference of 6% assuming a baseline rate of live birth of 28% in the placebo group, with a significance level of .05. We increased the sample size from 960 to 1,130 to allow for a dropout rate of 15%.
We employed the ITT strategy for our primary data analysis. Per-protocol analyses were performed by excluding the participants who dropped out of the study, failed to comply with the study protocol, or canceled ET. An interim analysis was planned when 25% of the participants were recruited; however, live birth outcome data were not available at that point, and the interim analysis was therefore not performed.
For the comparison of outcomes, categorical data were presented as frequency and percentage. Between-group differences were assessed using the χ2 test or Fisher exact test for expected frequencies of less than five, and rate ratio (RR) with 95% CIs were presented. Continuous data were expressed as mean±SD and were analyzed using t test or the Wilcoxon rank-sum test as appropriate. Characteristics that are counts of events and variables that were not normally distributed were expressed as median with interquartile ranges. All statistical calculations were done using SPSS 19 for Microsoft Windows. P<.05 was considered statistically significant.
RESULTS
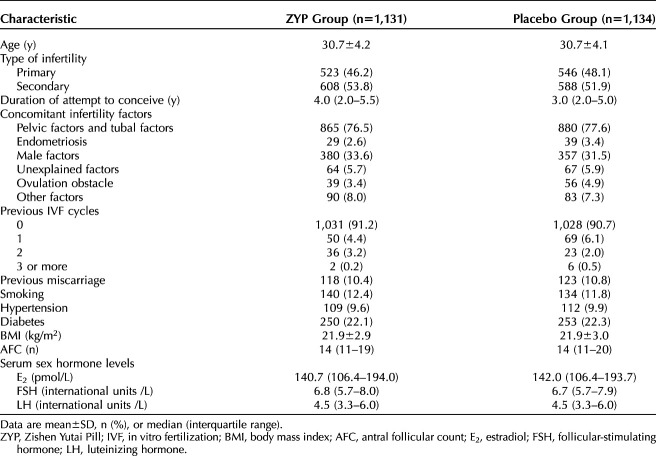
Overall, 2,580 patients were initially screened, and 2,265 were randomized. Recruitment was performed between April 2014 and June 2017. Follow-up was complete in June 2018. A total of 297 and 84 participants dropped out of the study or deviated from the protocol before and after fresh ET, respectively, including 146 of 1,131 (12.9%) in the ZYP group and 151 of 1,134 (13.3%) in the placebo group before fresh ET and 42 of 1,131 (3.7%) in the ZYP group and 42 of 1,134 (3.7%) in the placebo group. A total of 656 participants cancelled the cycle, including 314 of 1,131 (27.8%) in the ZYP group and 342 of 1,134 (30.2%) in the placebo group (Fig. 1). Appendix 2 (http://links.lww.com/AOG/C555, Cancellation of embryo transfer in the ITT population), presents the reasons for cycle cancellation. Characteristic of participants were shown in Table 1, and characteristics of cycle response details for study population were presented as in Table 2.
Fig. 1. Participant flow chart. PGD, preimplementation genetic diagnosis; ZYP, Zishen Yutai Pill; ET, embryo transfer; hCG, human chorionic gonadotropin.
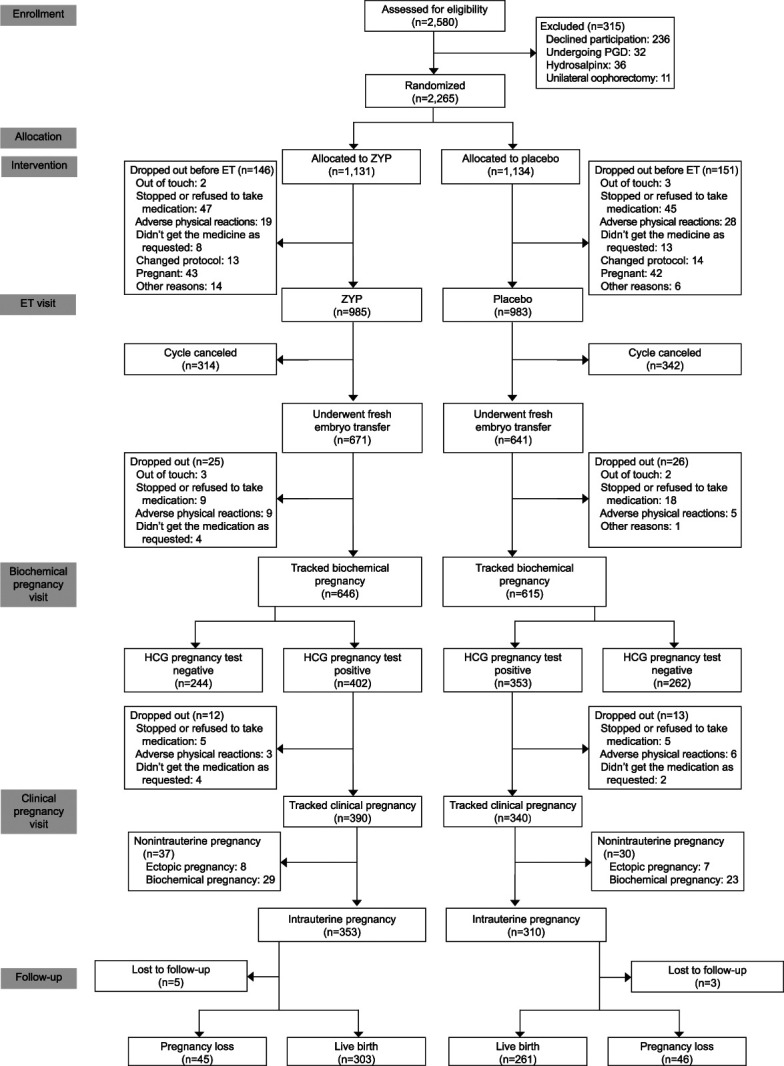
Chen. Zishen Yutai Pill for Live Birth in ART. Obstet Gynecol 2022.
Table 1.
Characteristics of the Study Population for the Intention-To-Treat Analyses
Table 2.
Cycle Response Details for Study Population (Intention-To-Treat Analyses)
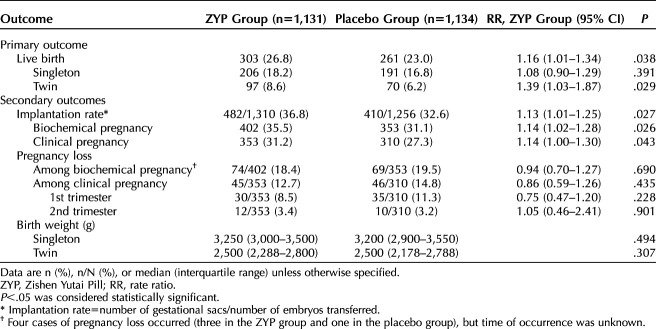
Table 3 presents the efficacy assessment for the primary and secondary outcomes in the ITT analysis. The rate of live birth was 26.8% in the ZYP group and 23.0% in the placebo group (RR of 1.16, 95% CI 1.01–1.34). Regarding the secondary outcomes, compared with the placebo group, the ZYP group had higher implantation rates (36.8% vs 32.6%; RR 1.13; 95% CI 1.01–1.25) and biochemical pregnancy rates (35.5% vs 31.1%; RR 1.14; 95% CI 1.02–1.28). The rates of clinical pregnancy in the ZYP and placebo groups were 31.2% compared with 27.3%, respectively (RR 1.14, 95% CI 1.00–1.30). There were no significant differences in the rates of pregnancy loss (all P>.05) (Table 3).
Table 3.
Treatment Outcomes in the Study Population for Intention-To-Treat Analyses
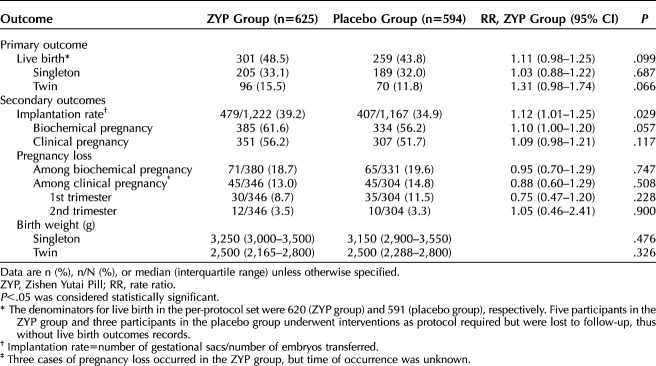
Table 4 presents the results of per-protocol analyses for the primary and secondary outcomes. The rates of live birth were 48.5% in the ZYP group compared with 43.8% in placebo (RR 1.11; 95% CI 0.98–1.25). Biochemical pregnancy rate was 61.6% in the ZYP group and 56.2% in placebo group (RR 1.10; 95% CI 1.00–1.20). For clinical pregnancy, the rates were 56.2% compared with 51.7% for the ZYP and placebo groups, respectively (RR 1.09; 95% CI 0.98–1.21). But the differences were not statistically significant, due to smaller sample sizes in the per-protocol analyses than that in the ITT analysis. The implantation rate was 39.2% in the ZYP group and 34.9% in the placebo group (RR 1.12; 95% CI 1.01–1.25).
Table 4.
Treatment Outcomes of Study Population for Per-Protocol Analyses
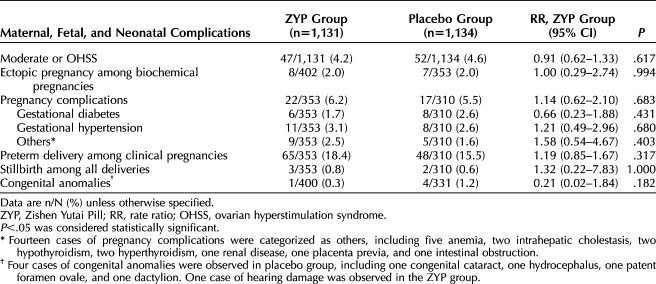
There were no significant between-group differences in the rates of ovarian hyperstimulation syndrome, ectopic pregnancy, other pregnancy complications, neonatal complications, or congenital anomalies (Table 5).
Table 5.
Maternal, Fetal, and Neonatal Complications in the Study Population for Intention-To-Treat Analyses
DISCUSSION
In this randomized, double-blind, controlled clinical trial involving 2,265 women undergoing IVF or ICSI, the administration of the Zishen Yutai Pill compared with placebo around the time of ovarian stimulation and ET resulted in increased live birth rates.
This RCT showed higher implantation, biochemical and clinical pregnancy rates in fresh ART cycles. However, the exact mechanisms of the Zishen Yutai Pill remain unknown. Controlled ovarian hyperstimulation drugs could stimulate a dramatic change of sex hormones and disrupt physiologic hormonal balance.21 The dyssynchrony between the endometrial implantation window and embryonic development after controlled ovarian hyperstimulation drug administration could decrease endometrial receptivity and pregnancy rates.22,23 The Zishen Yutai Pill could alleviate the downregulation of HOXA10 caused by controlled ovarian hyperstimulation as shown in a mouse model study.13 Metabonomic study also indicated the potential of the Zishen Yutai Pill in improving endometrial receptivity and oocytes quality.15 It can be hypothesized that the Zishen Yutai Pill can maintain proper endometrial differentiation, improve uterine receptivity and oocytes quality, thus resulting in an elevation of implantation rates.
Previously, various TCM decoction or preparations have been shown to improve pregnancy outcomes, highlighting the feasibility and possible effectiveness of TCM to improve pregnancy outcomes in RCTs, including after IVF.16,24–27 However, trials using decoction may be inconvenient for patients. Some trials also used different TCM formula during treatment, which makes it difficult to compare efficacy.25,26 In addition, most studies evaluating TCM in patients with infertility use clinical pregnancy as the primary outcome rather than live birth.25,27 This RCT addresses the limitations of previous studies providing information on efficacy and safety of a frequently-used TCM preparation during ART.
We found no significant differences in the rates of pregnancy-related or neonatal complications between groups. The safety of the Zishen Yutai Pill had been previously assessed regarding menstruation, endocrine indices, body temperature, endometrium, pregnancy loss rate, abdominal pain, and vaginal bleeding.28 Nonetheless, the available evidence remains insufficient to confirm safety for all women.
The main strength of this study is that it is a randomized, double-blind, multicenter clinical trial with a large sample size, including fertility units across the China mainland to explore the efficacy and safety of the Zishen Yutai Pill during ART. In addition, the trial had long follow-up duration, enabling the collection of live birth information and neonatal outcomes. The treatment protocol was based on best practice and was developed through consensus from expert clinicians. Blinding was intact in both groups during the whole study.
The study has several limitations. First, about 29% of the patients in each group had their cycles cancelled. Because this study aimed to compare live birth rates after a fresh cycle, the per-protocol population had a large reduction in available sample size for analysis. Second, about 18% of the patients in each group were canceled because of the risk of ovarian hyperstimulation syndrome in high-response patients or higher progesterone levels on hCG day. Previous studies have found that freeze-thaw ET cycles have higher pregnancy rates than fresh ET in high responders (with 15 or more oocytes retrieved) who undergo their first IVF transfer. The median number of oocytes in our study was 12 (interquartile range 8–17), and almost one third of the patients were high responders, which might result in high cancellation rates. In this RCT, only fresh ETs were performed, and further studies of frozen ET cycles for the high-response patients should be performed to determine the efficacy of the Zishen Yutai Pill on pregnancy outcomes. Third, this study performed only day 3 ET in all patients. Therefore, these findings might not be generalizable to all women undergoing ET. Fourth, the current sample size was insufficient to determine whether different subgroups (eg, those of more advanced maternal age) would experience larger benefits of taking the Zishen Yutai Pill when undergoing ART (see the post hoc analysis in Appendix 2, http://links.lww.com/AOG/C555). The pregnancy outcomes in women aged 35 years or older will be examined in our next clinical trial (Clinical Trial No. NCT03703700).
In summary, we found that the Zishen Yutai Pill improved the live birth rate after fresh embryo IVF cycles. There were no significant differences in the rates of maternal, fetal and neonatal complications between the ZYP and placebo groups. The mechanism by which the Zishen Yutai Pill functions to improve outcomes warrants further study.
AUTHORS’ DATA SHARING STATEMENT
Will individual participant data be available (including data dictionaries)? Yes.
What data in particular will be shared? Individual participant data that underlie the results reported in this article, after deidentification.
What other documents will be available? Study protocol and statistical analysis plan.
When will data be available (start and end dates)? 24 months after publication.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? Researchers who gain Ethic Approval from Sun Yat-sen Memorial Hospital of Sun Yat-sen University and provide study proposal.
Footnotes
This study was supported by the Guangdong Provincial Bureau of Traditional Chinese Medicine, Guangdong Provincial Secondary Development Project of Famous and Excellent Chinese Patent Medicine, Secondary Development of Famous and Excellent Chinese Patent Medicine Zishen Yutai Pill (Project No.: 20174002), and the Research on Livelihood Science and Technology of Guangzhou Science and Technology Plan Project, Clinical and Experimental Study on the Application of Kidney Tonifying Chinese Patent Medicine in in Vitro Fertilization and Embryo Transplantation for Elderly Infertile Women (Project No.: 201704020046).
Financial Disclosure Dongzi Yang disclosed that the Zishen Yutai Pill is not approved by the U.S. Food and Drug Administration, but in China, the Zishen Yutai Pill is widely used in assisted reproductive technologies. The production process of Zishen Yutai Pill complies with the relevant requirements of law of China's Drug Administration and GMP, with approval from the China National Medical Products Administration (Permit No.Z44020008). The other authors did not report any potential conflicts of interest.
The authors thank all members of the Study Group for their discussion in the protocol development and approval process; the IVF patients who enrolled in this study and all staff at the participating sites, without whom this research could not have been conducted; and the clinical affairs team for site recruitment, coordination, and monitoring.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews are available at http://links.lww.com/AOG/C556.
REFERENCES
- 1.Kissin DM, Jamieson DJ, Barfield WD. Monitoring health outcomes of assisted reproductive technology. N Engl J Med 2014;371:91–3. doi: 10.1056/NEJMc1404371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Child T. Optimising the management of patients with infertility. Practitioner 2013;257, 19–22, 2–3. [PubMed] [Google Scholar]
- 3.Lindsay TJ, Vitrikas KR. Evaluation and treatment of infertility. Am Fam Physician 2015;91:308–14. [PubMed] [Google Scholar]
- 4.Smith JF, Eisenberg ML, Millstein SG, Nachtigall RD, Shindel AW, Wing H, et al. The use of complementary and alternative fertility treatment in couples seeking fertility care: data from a prospective cohort in the United States. Fertil Steril 2010;93:2169–74. doi: 10.1016/j.fertnstert.2010.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulson C, Jenkins J. Complementary and alternative medicine utilization in NHS and private clinic settings: a United Kingdom survey of 400 infertility patients. J Exp Clin Assist Reprod 2005;2:5. doi: 10.1186/1743-1050-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rayner JA, Willis K, Burgess R. Women’s use of complementary and alternative medicine for fertility enhancement: a review of the literature. J Altern Complement Med 2011;17:685–90. doi: 10.1089/acm.2010.0435 [DOI] [PubMed] [Google Scholar]
- 7.Stankiewicz M, Smith C, Alvino H, Norman R. The use of complementary medicine and therapies by patients attending a reproductive medicine unit in South Australia: a prospective survey. Aust N Z J Obstet Gynaecol 2007;47:145–9. doi: 10.1111/j.1479-828X.2007.00702.x [DOI] [PubMed] [Google Scholar]
- 8.Shannon J, El Saigh I, Tadrous R, Mocanu E, Loughrey J. Usage of herbal medications in patients undergoing IVF treatment in an Irish infertility treatment unit. Ir J Med Sci 2010;179:63–5. doi: 10.1007/s11845-009-0378-5 [DOI] [PubMed] [Google Scholar]
- 9.Ried K, Stuart K. Efficacy of Traditional Chinese Herbal Medicine in the management of female infertility: a systematic review. Complement Ther Med 2011;19:319–31. doi: 10.1016/j.ctim.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 10.Ried K. Chinese herbal medicine for female infertility: an updated meta-analysis. Complement Ther Med 2015;23:116–28. doi: 10.1016/j.ctim.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 11.Zuo W, Mei D, Sun W, Tang X, Niu Z, Gao D, et al. The interpretation of China national essential medicines list 2018. Expert Rev Clin Pharmacol 2020;13:191–200. doi: 10.1080/17512433.2020.1713749 [DOI] [PubMed] [Google Scholar]
- 12.Maharajan K, Xia Q, Duan X, Tu P, Zhang Y, Liu K. Therapeutic importance of Zishen Yutai Pill on the female reproductive health: a review. J Ethnopharmacology 2021;281:114523. doi: 10.1016/j.jep.2021.114523 [DOI] [PubMed] [Google Scholar]
- 13.Gao Q, Han L, Li X, Cai X. Traditional Chinese medicine, the Zishen Yutai pill, ameliorates precocious endometrial maturation induced by controlled ovarian hyperstimulation and improves uterine receptivity via upregulation of HOXA10. Evid Based Complement Alternat Med 2015;2015:317586. doi: 10.1155/2015/317586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Yan W, Ge PF, Li Y, Ye Q. Study on prevention effect of Zishen Yutai pill combined with progesterone for threatened abortion in rats. Asian Pac J Trop Med 2016;9:577–81. doi: 10.1016/j.apjtm.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 15.Li L, Ning N, Wei J-A, Huang Q-L, Lu Y, Pang X-F, et al. Metabonomics study on the infertility treated with zishen yutai pills combined with fertilization-embryo transfer. Front Pharmacol 2021;12:686133. doi: 10.3389/fphar.2021.686133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L, Han H, Hu M, Luan H, Liu Y. The effect of Zishen Yutai pill on embryo implantation and pregnancy outcome in luteal phase of cycles undergoing in vitro fertilization and embryo transfer. Clin J Tradit Chin Med 2016;28:4. doi: 10.16448/j.cjtcm.2016.0079 [DOI] [Google Scholar]
- 17.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011;26:1270–83. doi: 10.1093/humrep/der037 [DOI] [PubMed] [Google Scholar]
- 18.Xie X, Gou W. Obstetrics and gynecology 8th ed. People’s Medical Publishing House; 2013. p. 168. [Google Scholar]
- 19.Chen Z-J, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016;375:523–33. doi: 10.1056/NEJMoa1513873 [DOI] [PubMed] [Google Scholar]
- 20.Olivius K, Friden B, Lundin K, Bergh C. Cumulative probability of live birth after three in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril 2002;77:505–10. doi: 10.1016/s0015-0282(01)03217-4 [DOI] [PubMed] [Google Scholar]
- 21.Slater CC, Chang L, Stanczyk FZ, Paulson RJ. Altered balance between the 5 alpha-reductase and aromatase pathways of androgen metabolism during controlled ovarian hyperstimulation with human menopausal gonadotropins. J Assist Reprod Genet 2001;18:527–33. doi: 10.1023/a:1011914218410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update 2003;9:515–22. doi: 10.1093/humupd/dmg045 [DOI] [PubMed] [Google Scholar]
- 23.Bentin-Ley U. Relevance of endometrial pinopodes for human blastocyst implantation. Hum Reprod 2000;15(suppl 6):67–73. [PubMed] [Google Scholar]
- 24.Hullender Rubin LE, Opsahl MS, Wiemer KE, Mist SD, Caughey AB. Impact of whole systems traditional Chinese medicine on in-vitro fertilization outcomes. Reprod Biomed Online 2015;30:602–12. doi: 10.1016/j.rbmo.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J, Li D, Liu C, Ji X, Li R, Du X. Effects of Chinese herbs combined with in vitro fertilization and embryo transplantation on infertility: a clinical randomized controlled trial. J Tradit Chin Med 2014;34:267–73. doi: 10.1016/s0254-6272(14)60089-3 [DOI] [PubMed] [Google Scholar]
- 26.Xia W, Huang J, Zhou H, Zhong Y, Xue H, Wang Zhen Department of Gynecology, et al. Curative effect of assisted reproduction technology by Traditional Chinese Medicine multi-channel interventional therapy on 95 cases of in vitro fertilization and embryo transfer failure. J Tradit Chin Med 2017;37:681–7. [PubMed] [Google Scholar]
- 27.Fang L, Rui-Xia W, Feng-Mei M, Zhen-Gao S, Li-Hong W, Lei S. Effects of Chinese medicines for tonifying the kidney on DNMT1 protein expression in endometrium of infertile women during implantation period. J Altern Complement Med 2013;19:353–9. doi: 10.1089/acm.2011.0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Q, Zhao R, Mao Y. Zishenyutaiwan in the treatment of threatened abortion 231 cases. Chin J Perinat Med 2001;4:85–7. [Google Scholar]