Abstract
Stenotrophomonas maltophilia has recently emerged as an important nosocomial pathogen in immunocompromised patients, in transplant recipients, and in persons with cystic fibrosis (CF). While this organism is nonpathogenic in healthy individuals, it is increasingly associated with morbidity and mortality in susceptible populations. Recent studies have indicated that for approximately 10% of CF patients with moderate lung disease, S. maltophilia can be cultured from respiratory tract secretions. Identification of S. maltophilia can be problematic, and analysis of isolates from the Burkholderia cepacia Research Laboratory and Repository showed that several isolates presumptively identified as B. cepacia by clinical microbiology laboratories were in fact S. maltophilia. To overcome the problems associated with definitive identification, we developed species-specific PCR (SS-PCR) primers, designated SM1 and SM4, directed to the 23S rRNA gene, and tested their utility to accurately identify S. maltophilia directly from sputum. The SS-PCR was developed and tested against a panel of 112 S. maltophilia isolates collected from diverse geographic locations. To test for specificity, 43 isolates from 17 different species were analyzed. PCR with the SM1-SM4 primer pair and isolated genomic DNA as a template resulted in amplification of a band from all S. maltophilia isolates and was uniformly negative for all other species tested, yielding a sensitivity and a specificity of 100% for the SS-PCR. The utility of the SS-PCR to directly identify S. maltophilia in sputum was examined. Thirteen expectorated sputum samples from CF patients were analyzed by SS-PCR. Three samples were PCR positive, in complete concordance with the conventional laboratory culture. Thus, we have developed an SS-PCR protocol that can rapidly and accurately identify S. maltophilia isolates and which can be used for the direct detection of this organism in CF patient sputum.
Stenotrophomonas maltophilia (9, 24) is a free living, non-glucose-fermenting, gram-negative bacillus widely distributed in a variety of environmental habitats. While predominantly isolated from the rhizosphere of diverse crops such as chicory and wheat (8), sugar beets (19), sunflowers (10), and orchids (35), this bacterium has also been isolated from well and river water, raw milk, frozen fish, raw sewage, and rabbit and human feces (14, 16). Originally considered to be a harmless commensal, S. maltophilia is emerging as an important nosocomial pathogen in the immunocompromised, in cancer patients, in transplant recipients, and in patients undergoing peritoneal dialysis (23, 27, 28, 29). S. maltophilia has been associated with infections of the eyes (25) and of the urinary and respiratory tracts (31, 36). Due to endogenous β-lactamase production and low outer membrane permeability, S. maltophilia is resistant to many broad-spectrum antibiotics including penicillins, carbapenems, and aminoglycosides (12) and is increasingly isolated from respiratory samples in patients with cystic fibrosis (CF) (36). These patients are routinely managed with aggressive antimicrobial therapy, and multiple antibiotic resistance (21) has made S. maltophilia a serious concern for the CF community. With the increase in prevalence, problems relating to accurate identification have become more evident (4). In a recent review of isolates forwarded to the Burkholderia cepacia Reference Laboratory and Repository (BcRLR), isolates identified by the referring laboratory as B. cepacia were later identified as S. maltophilia (22). Thus, a simple, reliable, and accurate test would enhance the identification of this organism. Previously, we have examined the efficacy of species-specific PCR (SS-PCR) to identify other pulmonary pathogens, including B. cepacia genomovar I, B. vietnamiensis, B. stabilis, B. multivorans, and B. gladioli (20, 32–34). Here we report the development of an SS-PCR for the identification of S. maltophilia and its application to identification of this organism directly from sputum samples.
MATERIALS AND METHODS
Bacterial strains.
The strains of S. maltophilia used to determine the 23S rRNA gene sequence were American Type Culture Collection (ATCC) strain 13637 and three clinical isolates (AU760, AU680, and AU789). Other organisms used in development and testing of S. maltophilia species-specific PCR primers included 112 clinical CF isolates of S. maltophilia, comprised of 16 isolates from this laboratory, 46 isolates characterized by the BcRLR, and 50 isolates from the clinical trials of inhaled tobramycin (6), kindly provided by the PathoGenesis Corporation, Seattle, Wash. The latter organisms were obtained from a total of 33 different patients at 24 geographically unrelated CF centers across the United States. A subset of these isolates was evaluated by RAPD [random(ly) amplified polymorphic DNA]-PCR for genetic relatedness, and strains from distinct patients were all found to have unique profiles (J. L. Burns, unpublished data). In addition to isolates of S. maltophilia, the following microbial strains were tested: Pseudomonas fluorescens ATCC 13525; Pseudomonas stutzeri ATCC 17588; Klebsiella pneumoniae ATCC 13883; Proteus mirabilis ATCC 29906; Moraxella catarrhalis ATCC 25238; B. gladioli strains ATCC 10854, 19302, and 10248; Ralstonia solanacearum ATCC 11696; and B. caryophylli ATCC 11441. Clinical isolates tested included one each of B. multivorans, B. stabilis, B. vietnamiensis, B. cepacia genomovar I, B. cepacia genomovar III, P. aeruginosa, Haemophilus influenzae, R. pickettii, Alcaligenes xylosoxidans, 16 isolates of B. gladioli, and 8 isolates of the B. cepacia complex (genomovar unknown).
Isolation of genomic DNA.
Genomic DNA was purified from cultures of each individual strain using the QIAamp Tissue Kit (Qiagen, Valencia, Calif.). DNA was resuspended in sterile high-pressure liquid chromatography-grade water at a final concentration of 1 μg/ml.
Cloning of the 23S ribosomal RNA and determination of the nucleotide sequence.
PCR primers were designed based upon sequences available in the National Center for Biotechnology Information databases. Target regions were chosen based on sequence conservation across a wide range of species. PCR with two primers, 23SRNA3 and SMRNA5 (Table), amplified a segment of the S. maltophilia ATCC 13637 23S rRNA gene comprising approximately the first 1,400 bp. These primers were used in separate PCRs to amplify an identical-sized product from genomic DNA purified from AU760, AU680, and AU789. The products were cloned into the pCR2.1 TOPO vector (TopoTA Cloning Kit; Invitrogen, Carlsbad, Calif.) to produce p13637A, p760A, p680A, and p789A. The sequence of the insert of each clone was determined using an ABI 373 automated sequencer (Perkin-Elmer Applied Biosystems, Foster City, Calif.). DNA sequences were assembled and analyzed using the GCG Wisconsin Package (Genetics Computer Group Inc., Madison, Wis.).
Development of S. maltophilia specific PCR.
The consensus nucleotide sequence of the amplified 1,381-bp fragment from the four S. maltophilia 23S rRNA genes was determined from the inserts of p13637A, p760A, p680A, and p789A. Differences between the consensus and the published B. cepacia 23S rRNA (15) were identified. These regions were further analyzed using the BLASTN nucleotide sequence alignment algorithm (1) to determine if the putatively specific S. maltophilia regions shared homology with other 23S rRNA genes. Two regions putatively specific for S. maltophilia were selected. Oligonucleotide PCR primers, designated SM1 and SM4 (Table 1), were designed to incorporate the S. maltophilia specific nucleotides at the 3′ end.
TABLE 1.
Oligonucleotide primers used for PCR
| Primer | Oligonucleotide sequencea (5′-3′) | Targetb (nucleotide positions, type) |
|---|---|---|
| 23SRNA3 | GAATATTGACCTGCTTCCC | 1363–1381, 23S |
| SM23RNA5 | GGTCAAGCGAATAAGCGC | 1–18, 23S |
| SM1 | CAGCCTGCGAAAAGTA | 62–77, 23S |
| SM4 | TTAAGCTTGCCACGAACAG | 593–575, 23S |
| PSL | AGGATTAGATACCCTGGTAGTCCA | 780–803, 16S |
| PSR | ACTTAACCCAACATCTCACGACAC | 1068–1092, 16S |
Individual PCRs were optimized for the primer pair SM1-SM4 using genomic DNA derived from S. maltophilia ATCC 13637. PCRs were performed using the Rapid Cycler thermocycler (Idaho Technologies, Idaho Falls, Idaho). All PCRs had an initial denaturation of 95°C for 5 min with a subsequent 30 cycle amplification and contained a 1 μM concentration of each primer, 10 ng of genomic DNA, a 200 μM concentration of each deoxynucleotide triphosphate, and 1.25 U of Taq DNA polymerase (Boehringer Mannheim, Indianapolis, Ind.) in a 3 mM MgCl2 PCR buffer (Idaho Technologies), in a total volume of 50 μl. The cycle parameters consisted of annealing at 58°C for 10 s, extension at 72°C for 60 s, and denaturation at 95°C for 10 s. For the last cycle the extension step was 2 min. After amplification, 20 μl of each reaction mixture was subjected to electrophoresis in a 0.8% agarose gel in 0.5× Tris-borate-EDTA (TBE) buffer (pH 8.0) alongside a 100-bp ladder. The PCR products were visualized and photographed after ethidium bromide staining. Positive results were assessed by the amplification of a 531-bp product. Any samples that failed to amplify a product using these primer pairs were further analyzed with the PSL-PSR primer pair (7) as a control.
PCR directly from sputum.
A portion of sputum samples from CF patients submitted for routine culture was liquefied according to the method of Reischl et al. (26). Approximately 1 ml of sputum was mixed with an equal volume of suspension buffer (50 mM trisodium citrate, 1% N-acetyl-l-cysteine, 2% NaOH), incubated at room temperature for 15 min, and centrifuged at 15,000 × g for 5 min. The resulting pellet was suspended in 100 μl of extraction buffer (1% Triton X-100, 0.5% Tween 20, 1 mM EDTA, and 10 mM Tris-HCl [pH 8.0]) and centrifuged at 15,000 × g for 5 min, and the pellet was resuspended in 50 μl of TBE buffer (pH 8.0). The bacterial suspension was lysed by five cycles of freezing in liquid nitrogen for 3 min and heating for 3 min in boiling water. After a final centrifugation step of 15,000 × g for 5 min the supernatant, containing total DNA, was used as template (5 μl) in the PCR.
Pulsed-field gel electrophoresis (PFGE).
Three milliliters of an overnight culture of each S. maltophilia isolate was centrifuged, and the bacterial pellet was resuspended in SE buffer (75 mM NaCl, 25 mM EDTA; pH 7.4) to an optical density at 620 nm of 0.8 to 0.9. Plugs were prepared by mixing 200 μl of the S. maltophilia suspension with 200 μl of 1.0% Pulse Field Certified Agarose (BioRad Laboratories, Richmond, Calif.) in 0.5% TBE cooled to 45°C and poured into the CHEF Plug mold. Following chilling at 4°C for 15 min, the plugs were extruded into sterile PEN buffer (0.5 mM EDTA, 1.0% N-lauroyl sarcosine; pH 9.6) containing 1 mg of Pronase (Roche Molecular Biochemicals, Indianapolis, Ind.) per ml and incubated at 37°C for 24 h. Plugs were washed twice in TE buffer at 4°C for 24 h and three times at room temperature for 1 h with gentle rocking. A 5-mm slice of each plug was digested with 40 U of SpeI (New England Biolabs, Inc., Beverly, Mass.) in 150 μl of reaction buffer at 37°C for 24 h prior to loading in to a 1.0% Pulse Field Certified Agarose (Bio-Rad Laboratories) gel in 0.5× TBE. Electrophoresis was performed in a CHEF-DRIII system (Bio-Rad Laboratories) at 14°C. Initial and final switch times were 25 and 45 s, respectively, with a linear ramping factor and a run time of 20 h at 6.0 V/cm. Bacteriophage lambda concatamers (New England Biolabs, Inc.) were used as molecular markers. DNA was visualized with UV light after staining with ethidium bromide (1.0 μg/ml).
RESULTS
Cloning and sequencing of the 23S rRNA gene.
The 1.4-kb 23S rRNA gene fragment was amplified from four separate isolates of S. maltophilia using the primer pair 23SRNA3-SMRNA5. Each amplified product was cloned into the TopoTA vector (Invitrogen) to yield p13637A, p760A, p680A, and p789A. Following screening to confirm the presence of the correct insert, each clone was sequenced in both directions. The resulting nucleotide sequences were aligned using the GCG PILEUP algorithm, yielding a consensus sequence for the 1.4-kb S. maltophilia 23S rRNA gene. The EMBL accession numbers for the 23S rRNA of S. maltophilia strains ATCC 13637, AU760, AU680, and AU789 are AF273255, AF175765, AF175763, and AF175764, respectively.
Development of an S. maltophilia-specific PCR.
The consensus sequence for the S. maltophilia 23S rRNA gene fragment was compared with the published sequence for the B. cepacia 23S rRNA gene described by Hopfl et al. (15). Regions that displayed variability were noted and analyzed against all other 23S rRNA genes contained in the GenBank database using the BLAST algorithm. Two regions, 531 bp apart, were determined to be putatively specific to S. maltophilia and were selected for further study. Opposing oligonucleotides were designed targeting each site and to encompass the variable nucleotide(s) at the 3′ end for use in SS-PCR. These primers were designated SM1 and SM4 (Table 1).
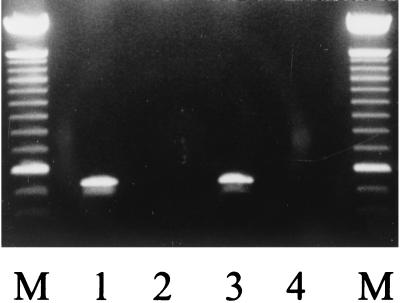
The specificities of primer pair SM1-SM4 were tested by performing individual PCRs with genomic DNA purified from S. maltophilia isolates ATCC 13637, PC760, AU680, and AU789 and one isolate each of B. multivorans, B. stabilis, B. vietnamiensis, and genomovars I and III of the B. cepacia complex. As a control, a second PCR with each genomic DNA sample was performed with primers that amplify a band from all bacteria tested. The results demonstrated that PCR with primer pair SM1-SM4 amplified a band of the correct size from each S. maltophilia isolates; however, these primers did not yield a band from the other DNA tested (Fig. 1). All genomic DNA was amplified in PCRs with the PSL-PSR primer pair. To assess the sensitivity and specificity of the SS-PCR protocol, a panel comprised of 112 S. maltophilia and 43 isolates of other bacteria representing 17 species was examined. The results demonstrated a positive result for 116 S. maltophilia, while all other isolates were uniformly negative.
FIG. 1.
SS-PCR of S. maltophilia. Lane 1, genomic DNA derived from S. maltophilia ATCC 13637; lane 2, negative control lacking genomic DNA; lane 3, SS-PCR of culture-positive sputum; lane 4, culture-negative sputum. The molecular marker (M) is a 100-bp ladder; the most distinct band is at 600 bp.
SS-PCR directly from sputum.
To assess the potential of the SS-PCR protocol to directly identify S. maltophilia, a series of sputum samples from CF patients were analyzed. Expectorated sputum was forwarded to the clinical microbiology laboratory, where the sample was split in two. One portion was analyzed utilizing the conventional diagnostic techniques employed by the clinical laboratory, while the remainder was frozen, pending analysis by PCR. Thirteen different sputa were blindly examined. The results demonstrated three PCR positive samples, in complete concordance with the laboratory culture. The three positive samples were submitted by two patients. In addition to S. maltophilia, the 13 sputum samples were variously culture positive for the following species: Staphylococcus aureus, P. aeruginosa, Aspergillus fumigatus, Alcaligenes xylosoxidans, Nocardia asteroides, Aspergillus flavus, and isolates of a Haemophilus species, alpha-hemolytic Streptococcus sp., and yeast (species unidentified).
DISCUSSION
S. maltophilia is becoming increasingly associated with infection in humans (12). This increase in colonization is of particular concern in patients with CF. The aberrant lung physiology makes these patients particularly susceptible to pulmonary infection. In two independent studies, the incidences of S. maltophilia in German and British CF centers were 6.8 and 10%, respectively (3, 13), while in France up to 13% of patients attending a regional center were colonized (21). Results from a comprehensive study in Spain indicated that 30.7% of the CF patients had submitted at least one culture-positive sample for S. maltophilia; of these, 9.6% were chronically colonized over a period of at least 6 months (2). Studies of therapeutic agents administered to these groups indicated that 90% had received aerosol therapy with ceftazidime or aztreonam. This study also revealed that all patients receiving aminoglycoside therapy were chronically colonized with S. maltophilia. Thus, the use of such antimicrobials may be a predisposing factor for colonization by S. maltophilia (2). The American experience of S. maltophilia colonization within the CF population has indicated an increase in prevalence. Saiman et al. reported a prevalence of 1.9% in 1994 (L. Saiman et al., North American Cystic Fibrosis conference, 1994), whereas Burns et al. found that 10.3% of patients included in their study had sputum cultures positive for S. maltophilia (5), a level similar to that observed in Europe. Interestingly, the levels of prevalence reported by Burns et al. are higher than the figures in the Cystic Fibrosis Patient Registry of 1995 and 1996, which records, incidences of S. maltophilia as 2.9 and 3.9%, respectively. This study was based on a selected population of patients colonized with P. aeruginosa. However, since in excess of 70% of CF patients are P. aeruginosa culture positive (11), the current prevalence of S. maltophilia may exceed the level recorded in the Patient Registry. The increase in rate of colonization presents numerous problems to the microbiology laboratory, notably the accurate identification of lung bacteria. This is complicated by a high degree of phenotypic similarity between S. maltophilia and certain members of the B. cepacia complex. This may potentially lead to the misdiagnosis of patients as B. cepacia positive (4), a diagnosis that carries severe psychosocial consequences for the patient. In addition, such a misdiagnosis may lead to a patient joining a cohort of other “B. cepacia-positive” patients, exposing that patient to potential acquisition of B. cepacia. In a recent study examining four commercial identification systems in the United States, the discriminatory power of the tests for S. maltophilia ranged from 66 to 100%; however, only three isolates were analyzed. The same systems correctly identified 25 to 90% of the B. cepacia isolates analyzed (30). Similar studies on the identification of S. maltophilia have demonstrated 87 to 100% correct identification; however, the identification of B. cepacia by the same kits ranges from 50 to 86% (18). Molecular methods for the detection of S. maltophilia have been previously reported (17); however, the PCR protocol employed displayed low sensitivity (17). Previously, we have reported molecular methods with high sensitivity and specificity for B. cepacia complex and B. gladioli isolates (20, 32–34). Here we demonstrate the use of SS-PCR to identify S. maltophilia. To design PCR assays to identify all isolates of S. maltophilia, we sought species-level signature sequences in the 23S rRNA. To overcome the possibility of selecting isolate specific sequences, we derived a consensus sequence from four distinct isolates: one type strain and three clinical isolates. This strategy allowed easier identification of species-level target sites. From the putatively species-specific sequences two primers were developed: SM1 and SM4. The SS-PCR protocol correctly identified 100% of the S. maltophilia strains and was routinely negative for all other isolates.
Utilizing the same methodology, CF sputum samples were analyzed. While this test panel was limited in size and bacterial diversity, the results clearly demonstrate the potential utility of the SS-PCR for the direct detection of S. maltophilia directly from sputum.
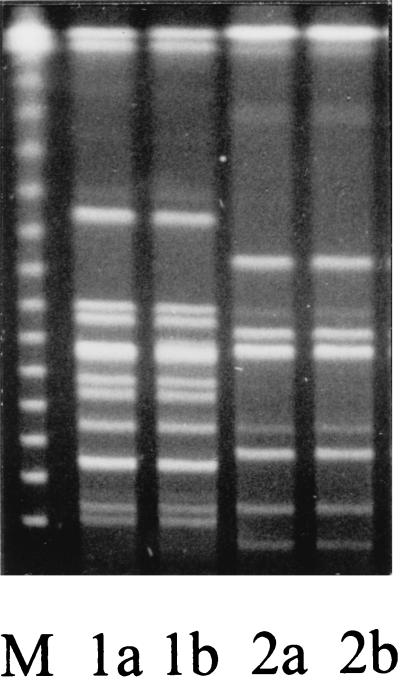
Upon examination of the culture results for the sputum test panel, it was apparent that two of the three PCR-positive sputum samples, from separate patients, were each culture positive for two phenotypically distinct S. maltophilia isolates. In both cases the two isolates varied from each other in antibiotic profile, with one isolate having a lower level of resistance to ceftazidime, piperacillin, and ticarcillin. To determine if the SS-PCR amplified a product from each phenotypically distinct isolate, the isolates were examined using the above protocol. In addition, they were subjected to PFGE to determine genotypic similarity. The results showed that all isolates yielded a product with the SS-PCR and that both isolates from a single patient were genotypically identical and yet different from the restriction fragment pattern of the other patient (Fig. 2). The difference in the antibiotic resistance profiles between isolates probably reflects a different level of expression of endogenous β-lactamase.
FIG. 2.
SpeI-digested PFGE of S. maltophilia clinical isolates. Lanes 1a and 1b show two phenotypically distinct isolates derived from a single sputum samples of one patient, and lanes 2a and 2b show phenotypically distinct isolates derived from a single sputum sample of a separate patient. The molecular markers (M) are bacteriophage lambda concatamers; the lowest band is 48.5 kb, and each successive band represents an increase of 48.5 kb.
In summary, we have developed an SS-PCR protocol for the detection of S. maltophilia. Use of this technique in combination with other phenotypic analysis will facilitate the accurate and rapid identification of S. maltophilia.
ACKNOWLEDGMENTS
This work was supported by grants to T.L.S., P.W.W., and J.J.L awarded from the Cystic Fibrosis Foundation. P.W.W. and T.L.S. acknowledge the financial support of the Children's Medical Research Institute (Oklahoma City, Okla.).
We thank Denise Robison, Debbie Berry, Theresa Zaccone, and Kenneth Hatter for technical support.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballestero S, Virseda I, Escobar H, Suarez L, Baquero F. Stenotrophomonas maltophilia in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 1995;14:728–729. doi: 10.1007/BF01690887. [DOI] [PubMed] [Google Scholar]
- 3.Bauernfeind A, Bertele R M, Harms K, Horl G, Jungwirth R, Petermuller C, Przyklenk B, Weisslein-Pfister C. Qualitative and quantitative microbiological analysis of sputa of 102 patients with cystic fibrosis. Infection. 1987;15:270–277. doi: 10.1007/BF01644137. [DOI] [PubMed] [Google Scholar]
- 4.Burdge D R, Noble M A, Campbell M E, Krell V L, Speert D P. Xanthomonas maltophilia misidentified as Pseudomonas cepacia in cultures of sputum from patients with cystic fibrosis: a diagnostic pitfall with major clinical implications. Clin Infect Dis. 1995;20:445–448. doi: 10.1093/clinids/20.2.445. [DOI] [PubMed] [Google Scholar]
- 5.Burns J L, Emerson J, Stapp J R, Yim D L, Krzewinski J, Louden L, Ramsey B W, Clausen C R. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 6.Burns J L, Van Delfsen J M, Shawer R M, Otto K L, Garber R L, Quan J M, Montgomery A B, Albers G B, Ramsey B W, Smith A L. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis. 1999;179:1190–1196. doi: 10.1086/314727. [DOI] [PubMed] [Google Scholar]
- 7.Campbell P W I, Phillips III J A, Heidecker G J, Krishnamani M R S, Zahorchak R, Stull T L. Detection of Pseudomonas (Burkholderia) cepacia using PCR. Pediatr Pulmonol. 1995;20:44–49. doi: 10.1002/ppul.1950200109. [DOI] [PubMed] [Google Scholar]
- 8.Debette J, Blondeau R. Présence de Pseudomonas maltophilia dans la rhizosfère de quelques plantes cultivées. Can J Microbiol. 1980;26:460–463. [PubMed] [Google Scholar]
- 9.Drancourt M, Bollet C, Raoult D. Stenotrophomonas africana sp. nov., an opportunistic human pathogen in Africa. Int J Syst Bacteriol. 1997;47:160–163. doi: 10.1099/00207713-47-1-160. [DOI] [PubMed] [Google Scholar]
- 10.Fages J, Arsac J F. Sunflower inoculation with Azospirillum and other plant growth promoting rhizobacteria. Plant Soil. 1991;137:87–90. [Google Scholar]
- 11.Gilligan P H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilligan P H, Whittier S. Burkholderia, Stenotrophomonas, Ralstonia, Brevundimonas, Comamonas, and Acidovorax. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology Press; 1999. pp. 526–538. [Google Scholar]
- 13.Gladman G, Connor P J, Williams R F, David T J. Controlled study of Pseudomonas cepacia and Pseudomonas maltophilia in cystic fibrosis. Arch Dis Child. 1992;27:192–195. doi: 10.1136/adc.67.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauben L, Vauterin L, Moore E R B, Hoste B, Swings J. Genomic diversity of the genus Stenotrophomonas. Int J Syst Bacteriol. 1999;49:1749–1760. doi: 10.1099/00207713-49-4-1749. [DOI] [PubMed] [Google Scholar]
- 15.Hopfl P, Ludwig W, Schleifer K H, Larsen N. The 23S ribosomal RNA higher-order structure of Pseudomonas cepacia and other prokaryotes. Eur J Biochem. 1989;185:355–364. doi: 10.1111/j.1432-1033.1989.tb15123.x. [DOI] [PubMed] [Google Scholar]
- 16.Hugh R, Ryschenkow E. Pseudomonas maltophilia and Alcaligenes-like species. J Gen Microbiol. 1961;26:123–132. doi: 10.1099/00221287-26-1-123. [DOI] [PubMed] [Google Scholar]
- 17.Karpati F, Jonasson J. Polymerase chain reaction for the detection of Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Burkholderia cepacia in sputum of patients with cystic fibrosis. Mol Cell Probes. 1996;10:397–403. doi: 10.1006/mcpr.1996.0055. [DOI] [PubMed] [Google Scholar]
- 18.Kiska D L, Kerr A, Jones M C, Caracciolo J A, Eskridge B, Jordan M, Miller S, King N, Gilligan P H. Accuracy of four commercial systems for identification of Burkholderia cepacia and other gram-negative nonfermenting bacilli recovered from patients with cystic fibrosis. J Clin Microbiol. 1996;34:886–891. doi: 10.1128/jcm.34.4.886-891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert B, Meire P, Joos H, Lens P, Swings J. Fast-growing, aerobic, heterotrophic bacteria from the rhizosphere of young sugar beet plants. Appl Environ Microbiol. 1990;56:3375–3381. doi: 10.1128/aem.56.11.3375-3381.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LiPuma J J, Dulaney B J, McMenamin J D, Whitby P W, Stull T L, Coenye T, Vandamme P. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J Clin Microbiol. 1999;37:3167–3170. doi: 10.1128/jcm.37.10.3167-3170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marty N. Epidemiological typing of Stenotrophomonas maltophilia. J Hosp Infect. 1997;36:261–266. doi: 10.1016/s0195-6701(97)90052-9. [DOI] [PubMed] [Google Scholar]
- 22.McMenamin J D, Zaccone T M, Coenye T, Vandamme P, LiPuma J J. Misidentification of Burkholderia cepacia in U.S. cystic fibrosis treatment centers: an analysis of 1051 recent sputum samples. Chest. 2000;117:1661–1665. doi: 10.1378/chest.117.6.1661. [DOI] [PubMed] [Google Scholar]
- 23.Nagai T. Association of Pseudomonas maltophilia with malignant lesions. J Clin Microbiol. 1984;20:1003–1005. doi: 10.1128/jcm.20.5.1003-1005.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palleroni N J, Bradbury J F. Stenotrophomonas, a new bacterial genus for Xanthomonas maltophilia (Hugh 1980) Swings et al. 1983. Int J Syst Bacteriol. 1993;43:606–609. doi: 10.1099/00207713-43-3-606. [DOI] [PubMed] [Google Scholar]
- 25.Penland R L, Wilhelmus K R. Stenotrophomonas maltophilia ocular infections. Arch Ophthalmol. 1996;114:433–436. doi: 10.1001/archopht.1996.01100130429013. [DOI] [PubMed] [Google Scholar]
- 26.Reischl U, Pulz M, Ehret W, Wolf H. PCR-based detection of mycobacteria in sputum samples using a simple and reliable DNA extraction protocol. BioTechniques. 1994;17:844–845. [PubMed] [Google Scholar]
- 27.Roilides E, Buttler K M, Husson R N, Mueller B U, Lewis L L, Pizzo P A. Pseudomonas infections in children with human immunodeficiency virus infection. Pediatr Infect Dis J. 1992;11:547–553. doi: 10.1097/00006454-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Spencer R C. The emergence of epidemic, multiple-antibiotic-resistant Stenotrophomonas (Xanthomonas) maltophilia and Burkholderia (Pseudomonas) cepacia. J Hosp Infect. 1995;30:453–464. doi: 10.1016/0195-6701(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 29.Taylor G, McKenzie M, Buchanan-Chell M, Perry D, Chui L, Dasgupta M. Peritonitis due to Stenotrophomonas maltophilia in patients undergoing chronic peritoneal dialysis. Peritoneal Dialysis Int. 1999;19:259–262. [PubMed] [Google Scholar]
- 30.van Pelt C, Verduin C M, Goessens W H F, Vos M C, Tümmler B, Segonds C, Reubsaet F, Verbrugh H, van Belkum A. Identification of Burkholderia spp. in the clinical microbiology laboratory: comparison of conventional and molecular methods. J Clin Microbiol. 1999;37:2158–2164. doi: 10.1128/jcm.37.7.2158-2164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vartivarian S E, Papadakis K A, Anaissie E J. Stenotrophomonas maltophilia urinary tract infection. A disease that is usually severe and complicated. Arch Intern Med. 1996;156:433–435. [PubMed] [Google Scholar]
- 32.Whitby P W, Carter K B, Hatter K L, LiPuma J J, Stull T L. Identification of members of the Burkholderia cepacia complex by species-specific PCR. J Clin Microbiol. 2000;38:2962–2965. doi: 10.1128/jcm.38.8.2962-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitby P W, Dick H L, Campbell P W, Tullis D E, Matlow A, Stull T L. Comparison of culture and PCR for detection of Burkholderia cepacia in sputum samples of patients with cystic fibrosis. J Clin Microbiol. 1998;36:1642–1645. doi: 10.1128/jcm.36.6.1642-1645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitby P W, Pope L C, Carter K B, LiPuma J, Stull T L. Species-specific PCR as a tool for the identification of Burkholderia gladioli. J Clin Microbiol. 2000;38:282–285. doi: 10.1128/jcm.38.1.282-285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson K G, Dixon K W, Sivasithamparam K, Ghisalberti E L. Effect of IAA on symbiotic germination of an Australian orchid and its production by orchid-associated bacteria. Plant Soil. 1994;159:291–295. [Google Scholar]
- 36.Wust J, Frei R, Gunthard H, Altwegg M. Analysis of restriction fragment length polymorphism and ribotyping of multiresistant Stenotrophomonas maltophilia isolated from persisting lung infection in a cystic fibrosis patient. Scand J Infect Dis. 1995;27:499–502. doi: 10.3109/00365549509047053. [DOI] [PubMed] [Google Scholar]