Abstract
Background:
Recent studies have reported that air pollution exposure may have neurotoxic properties.
Objective:
To examine longitudinal associations between prenatal particles less than 2.5 micrometers in diameter (PM2.5) exposure and neurodevelopment during the first two years of children’s life..
Methods:
Analysis was conducted in PROGRESS, a longitudinal birth cohort between 2007–2013 in Mexico City. We used satellite data to predict daily PM2.5 concentrations at high spatial resolution. Multivariate mixed-effect regression models were adjusted to examine cognitive, language and motor scores in children up to 24 months of age (n=740) and each trimester-specific and whole pregnancy exposure to PM2.5. Results: Models adjusted by child sex, gestational age, birth weight, smoking and mother’s IQ, showed that each increase of 1μg/m3 of PM2.5 was associated with a decreased language function of −0.38 points (95% CI: −0.77, −0.01). PM2.5 exposure at third trimester of pregnancy contributed most to the observed association.
Conclusion:
Our findings suggest that language development up to 24 months of age may be particularly sensitive to PM2.5 exposure during pregnancy.
Keywords: neurodevelopment, PM2.5, particulate matter, prenatal exposure
INTRODUCTION
The impact of particulate air pollution has been the subject of a growing body of epidemiological research around the world. In Mexico City (MC), the largest city of Mexico and one of the most-populated city in North America. Exposure to particulate air pollution has been associated with respiratory (Téllez-Rojo 2000, Rojas-Martínez 2007, Barraza-Villarreal 2008), cardiovascular (Riojas-Rodríguez 2006) and cardiorespiratory events (Carbajal-Arroyo 2010), and recently with neurodevelopment (Calderon-Garcidueñas 2012). A possible pathogenic mechanism that associate prenatal exposure to particulate matter and fetal brain development is an increase in reactive oxygen species (ROS). An alteration in the systemic balance of ROS may induce pro-inflammatory reactions (Block 2009), particularly if the particles enter the blood stream,translocate the placental barrier (Oberdoster 2004; Elder 2006) and cross the blood brain barrier (BBB), a border of endothelial cells that prevents substances crossing into the extracellular fluid of the brain, where neurons reside (PMID: 25147109). Local neuroinflammation in prenatal life may inhibit neuronal differentiation, myelination and synaptogenesis (Johanson 2007).
Studies with mice exposed to particulate matter have demostrated alterations in proinflammatory markers such as interleukin IL-6 and IL-1β (Guerra 2013). These inflammatory mediators may induce the production of nitric oxide (NO) synthase, wich lead to opening the BBB (Calderon-Garcidueñas 2008). Evidence from epidemiological research indicates that early life exposure to particles less than 2.5 micrometers in diameter (PM2.5) may affect children development, including reduced intelligence (Chiu et al., 2016), decreased cognitive and psychomotor development (Lertxundi 2015), behavioral development (McGuinn 2020) and patterns of hemispheric brain development (Cserbik 2020). . Studies focusing on PM2.5 prenatal exposure and developmental assessment at different ages, have found a negative association with behavior scores in newborn (Chen et al., 2000); behavioral and cognition development, particularly adaptive skills (McGuinn 2000) as well as memory, motor and verbal cognition (Lertxundi 2019) in children at 4–6 years of age; intelligence among boys and memory function in girls in children age 6.5 ± 0.98 years (Chiu et al., 2016) and child cognitive development in school children (average age 8.5 years) (Basagaña et al., 2016). However, the language component in early childhood, such as 0–2 years, has not been studied. To address these issues, we examined the association between prenatal PM2.5 exposure and motor, language and cognitive development among children up to 24 months of age in a birth cohort in Mexico City.
MATERIALS AND METHODS
Study population
This study was conducted in the ongoing Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) pregnancy-birth cohort in Mexico City. A complete description of the cohort and study design is presented elsewhere (Braun 2014). Briefly, women were enrolled between 2007 and 2011 during their 2nd trimester of pregnancy in their prenatal care visits at 4 clinics belonging to the Mexican Social Security System. The target population was restricted to women attending prenatal care less than 20 weeks pregnant, at least 18 years old, had planned to live in Mexico City during the next 3 years, did not consumpt alcohol daily, had no medical history of heart or kidney disease, and had access to a telephone.
Of the 1054 mothers originally recruited, 106 were lost to follow-up because they did not provide information of delivery. From the remaining 948 participants 760 mother-children pairs returned for follow-up visits up to 24 months of chldren’s age, and among these, seven did not complete development assessment due to concurrent illness or other factors that could affect performance. We first restricted our analyses to the 753 mother-child pairs whose children had at least one neurodevelopmental assessment between 6 and 24 months of age, however 13 children were excluded: five for problems of development, three did not have address information to assign air pollution exposure, two were very premature (<32 weeks of gestation), two were infants with very low birth weight (<1500 g) and one children was both very premature and very low birth weight. The final analytical sample included 740 mother-child pairs .
All women received information about the aims of the study and research procedures, data and sample collection. All participants gave written informed consent. Study methods were reviewed and approved by the Research, Ethics and Biosafety Committees at the National Institute of Public Health, the Mexican Social Security System, and the National Institute of Perinatology, in Mexico; the Harvard School of Public Health and the Icahn School of Medicine at Mount Sinai in the United States.
Neurodevelopmental assessment
We used the Bayley Scales of Infant Development, Third edition (BSID-III) adapted to Mexican population, to assess cognitive, language (expressive and receptive communication) and motor (fine and gross) development for children at ages 6, 12, 18 and 24 months (Bayley-III: Bayley, 2006, 2009). BSID-III test were administered to each children by psychologists specifically trained for these examinations. To analyze the heterogeneity between the different examiners, we applied a test of difference of variances and we did not find any significant differences.
The BSID-III scales yield raw scores based on chronological age, to generate cognitive, language and motor subscores based on US norms (with a range from 40 to 160 points). We standardized raw scores for each domain using conversion tables provided in the manual with an expected mean of 100 points and a standard deviation of 15 points (Cromwell 2014).
Exposure assessment
PM2.5 exposure data were generated through a satellite based hybrid model. The methods used to obtain these estimates have been described previously (Just 2015) and are briefly summarized here. The exposure model is based on Optical Depth (AOD) satellite data calibrated with daily ground-level PM2.5 measurements using mixed models with random slopes for day. The AOD data was combined with classic land use regression (LUR) techniques and meteorological data for temperature, planetary boundary layer height, relative humidity and wind speed to estimate daily PM2.5 in a 1-km × 1-km grid. After model calibration, we used the coefficients of the calibrated model to predict daily values of PM2.5 in each grid cell with available AOD data. Finally, to predict the daily PM2.5 concentrations in the cells without AOD measurements, the average PM2.5 daily concentrations were smoothed by season to impute predictions at these missing locations.
Exposure metrics
For each children, we calculated the average level of PM2.5 during the entire gestation and each trimester of pregnancy. Based on PM2.5 previous estimates, each mother’s residence was linked to 1-km × 1-km grid. We aggregated weekly averages of daily PM2.5 starting with the self-reported first day of the last menstrual period (LMP) as day 1.
The entire pregnancy exposure was calculated by averaging daily PM2.5 concentrations since day 1 up to the delivery date. Then we estimated exposure of the first, second, and third trimester of pregnancy averaging the PM2.5 concentrations over weeks 1 to 13, 14 to 26, and 27 to delivery date, respectively.
Covariates
We obtained the following maternal information through standardized questionnaires: age at delivery (years), education (years of schooling), smoking during pregnancy, parity, gestational age (weeks of pregnancy) and breastfeeding (never, exclusive at 1 month ever, ever breastfeeding at 12 months). Mother’s intellectual coefficient was assessed at one month postpartum using the Spanish version of Wechsler Adult Intelligence Scale (Wechsler 1981). The perceived stress of the mother was measured at third trimester of pregnancy with the Perceived Stress Scale (Cohen 1994), which measures how stressful life events are appraised.
When the children were 24 months of age, a Spanish version of the Home Observation for Measurement of the Environment (HOME) Inventory score was administered during home visits. HOME is an instrument that measures the stimulation and emotional support provided to the children at home (Caldwell and Bradley 1984) and it is predictive of developmental scores in early childhood (Torres-Sánchez, 2007).
Since epidemiologic studies have well documented that prenatal exposure to lead has an adverse effect on neurodevelopment (Hu et al, 2006; Schnaas 2006, Sanders et al., 2009). , prenatal lead (Pb) exposure was assessed using maternal blood samples during the second and third trimesters of pregnancy. Lead concentration was measured using a dynamic reaction cell inductively coupled plasma mass spectrometer (Elan 6100; PerkinElmer, Norwalk, CT) in the trace metals laboratory at the Icahn School of Medicine at Mount Sinai. The limit of detection is 0.02 μg/dL and the details of sampling process have been explained elsewhere (y Ortiz 2017). We also collected infant characteristics such as sex and birth weight.
Statistical analysis
To assess the effect of prenatal PM2.5 exposure and cognitive, language and motor subtest scaled scores by age, we used a mixed-effect regression model for a multivariate outcome variable, wich allowed us to adjust for potential correlation between Bayley scores within individuals over age and covariance among the three subscales. We performed separate analyses for PM2.5 at each trimester of pregnancy as well as for the entire pregnancy evaluating exposures as continuous variable associated with the three subtest scores simultaneously. These scores were longitudinally assessed from 6 to 24 months of age (McLean et al. 1991). The general structure of the models was:
Where Yijk corresponds to the Bayley’s score (k=cognitive, language and motor) in the ith participant at the j= 6, 12, 18 and 24 months of age; PM2.5i is the average exposure during the different study time periods for participant i; Xi are the fixed effects associated with individual potential confounders that do not vary over time. Zijγi are random effects variables in participant i for assessment j.
For all models, we considered a random intercept (β0) term representing overall performance at each period of assessment and a random linear slope term representing age at evaluation. We considered unstructured covariance because we were not imposing any constraints on the values. Since cognitive, language and motor scores were considered joint measures we used independent covariance structure for the joint subscale measures.
Covariates with fixed effects (Xi) included maternal age (years), maternal stress perception during pregnancy, maternal education (years), parity (first pregnancy/more than one pregnancy), maternal smoking (never smoking, ex-smoker, current smoker), maternal IQ, gestational age at birth (weeks of pregnancy), child’s sex (female/male), birth weight (kilograms), breastfeeding history (never, 1 month exclusive, ever breastfeeding at 12 months), HOME scale scores at 24 months of age, and maternal blood lead (μg/dL). Factors with random effect (Zijkγi) were age at evaluation j and Bayley subscales nested within age.
Since gestational age and birthweight may be on the causal pathway between PM2.5 pregnancy exposures and development (Harris 2015), we restricted the analysis for those children who were not very premature and/or very low birth weight.
In a first step, we ran fully adjusted models retaining all covariates in the multivariate model for control of confounding regardless of their association with cognitive, language and motor scales. In a second step, we adjusted models with stimulation at home
Finally, we excluded covariates such as lead exposure, breastfeeding and perceived stress from the models to increase sample size since none of them significantly contributed to the response. Regression models adjusted with a minimal set of covariates included sex of the child, gestational age, birth weight, smoking and mother’s IQ.
Sensitivity analysis was performed assessing the effect of prenatal PM2.5 exposure on development functions without the stimulation at HOME variable and then with this variable included in the model. Although home observation for measurement of the environment scores were only collected at 24 months, that is 18 months after the initial assessment, it has been observed stability of home environment with a median change score of zero for most subscales, suggesting that measuring home environments every 2 years at early childhood would be sufficient in longitudinal studies (Mitchell and Gray, 1981; Bradley et al, 1989; Rousey et al., 2002).
We determined the goodness of fit of each model using residual diagnosis. We set statistical significance at a p-value < 0.05. We used STATA 13© (Stata Corp., College Station, TX, USA) for data processing and mixed-effect regression analysis.
RESULTS
Descriptive characteristics of the 740 mother-child pairs included in this analysis, and participants excluded (noneligible) and lost to follow-up, are reported in Table 1.
Table 1.
Selected mother-child pairs characteristics of the study sample (n=740) a
| Characteristics | Included (n=740) b | Lost to follow up and excluded (n=208) |
|---|---|---|
| Mother | ||
| Maternal age (years) | 27.1 ± 5.5 | 27.4 ± 5.5 |
| Maternal education (years) | 11.6 ± 2.9 | 11.9 ± 3.3 |
| Parity | ||
| 1 | 267 (35.8) | 71(35) |
| ≥2 | 478 (64.2) | 132(65) |
| Smoking | ||
| Never | (45.8) | (38.5) |
| Ex-smoker | (19.4) | (26) |
| Smoker | (34.8) | (35.5) |
| Maternal IQ c | 84.9 ± 12.7 | 83.8 ± 16.6 |
| Blood lead (μg/dL)d | ||
| 2nd trimester | 3.4 ± 2.9 | 3.4 ± 3.0 |
| 3rd trimester | 3.6 ± 3.2 | 4.3 ± 3.5 |
| Children | ||
| Sex (% boys) | 52.2 | 53.9 |
| Gestational age (week) | 38.9 ± 1.4 | 34.7 ± 4.3 |
| Birth weight (Kg) | 3.1 ± 0.4 | 2.9 ± 0.7 |
| Breastfeeding | ||
| Never | 45 (6.4) | 6 (9.2) |
| No exclusive | 471 (66.9) | 45 (69.2) |
| Exclusive | 188 (26.7) | 14 (21.6) |
| HOME score | 31.7 ± 5.5 | 31.8 ± 5.1 |
Values are percentages for categorical variables and mean ± SD for continuous variables.
Differences tested using chi-square for categorical variables and t-test for continuous variables. Differences were not statistically significant between participants included in the analysis and those who were lost to follow-up, except for maternal education.
Sample sizes for covariates: maternal education n= 740; parity n= 452; prenatal smoking n= 464; mother’s IQ n= 714; blood lead levels (3T) n= 738; blood lead levels (3T) n= 653; breastfeeding n= 697; HOME n= 449.
Wechsler Adult Intelligence Scale
Geometric mean for blood lead (μg/dL)
The 740 children included in the analyses had a total of 2,233 BSDI-III evaluations (10.3% had 1 evaluation, 13.3% had 2 evaluations, 26.9% had 3 evaluations and 49.5% had 4 evaluations). Cognitive, language and motor scores by age of assessment are reported in Table 2. Girls showed significantly (p<0.05) higher performance than boys on cognitive (96.7 vs. 94.6), language (87.4 vs. 85.1) and motor (94.0 vs 91.7) component (Table S1).
Table 2.
Mean scores (± SD) a of Bayley Scales of Infant Development (BSID-III) components by age at evaluation (n=740)
| Scale | 6 months of age (n=623) |
12 months of age (n=552) |
18 months of age (n=520) |
24 months of age (n=542) |
|---|---|---|---|---|
| Cognitive | 97.7 ± 11.2 | 96.0 ± 9.6 | 96.6 ± 9.9 | 92.6 ± 8.4 |
| Language | 86.1 ± 11.1 | 81.4 ± 9.7 | 88.7 ± 10.4 | 89.8 ± 8.9 |
| Motor | 94.4 ± 11.3 | 88.6 ± 9.1 | 95.0 ± 9.0 | 94.1 ± 9.4 |
Score standardized by age at evaluation.
The exposure levels of PM2.5 across the three trimester and during the entire pregnancy are shown in Table 3. Mean levels of this pollutant ranged from 22.2 μg/m3 to 23.0 μg/m3.
Table 3.
Maternal mean PM2.5 (μg/m3) exposure among study participants throughout pregnancy (n=740) a
| Minimum | Median | Maximum | ||
|---|---|---|---|---|
| 1st trimester | 22.6 ± 4.3 | 12.3 | 22.0 | 33.5 |
| 2nd trimester | 22.2 ± 4.1 | 13.0 | 21.1 | 31.8 |
| 3rd trimester | 22.9 ± 5.0 | 12.5 | 22.4 | 35.3 |
| All pregnancy | 23.0 ± 2.6 | 16.4 | 22.8 | 29.3 |
Values calculated by the Satellite Hybrid Model with AOD data.
In models adjusted by sex of the child, gestational age, birth weight, smoking and mother’s IQ (minimally adjusted models), increased PM2.5 predicted lower cognitive and language scores. The most consistent results were in language assessment.
Table 4 shows the marginal effects and 95% confidence intervals of 1 unit increase in PM2.5 on cognitive, language and motor composite scale scores. During the 6 months assessment, cognitive and language scores significantly decrease with all-time windows exposure. The marginal effects were larger for the entire pregnancy exposure. We estimate an adjusted cognitive score reduction of −0.93 (95% confidence interval [CI] : −1.27, −0.58) and an adjusted language score reduction of −0.95 (95% CI: −1.29, −0.60) at 6 months assessment.Language scores also showed a reduction of −0.58 (95% CI: −88 and −0.28) and −0.38 (95% CI: −0.77, - 0.01) associate to total pregnancy exposures at 12 and 18 month follow up respectively. However, no changes were observed in the motor component.
Table 4.
Cognitive, language and motor composite scale scores during the first 24 months of age associated with 1 μg/m3 increase in the mean PM2.5 prenatal levela
| Scale | Ageb | PM2.5 window of exposure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st trimester |
2nd trimester |
3rd trimester |
All pregnancy |
||||||||||
| β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | β | p | 95% CI | ||
| Cognitive | −0.26 | 0.04 | −0.51, −0.02 | −0.50 | 0.00 | −0.75, −0.25 | −0.21 | 0.04 | −0.42, −0.01 | −0.93 | 0.00 | −1.27, −0.58 | |
| Language | 6 | −0.31 | 0.02 | −0.56, −0.06 | −0.39 | 0.00 | −0.65, −0.13 | −0.30 | 0.01 | −0.52, −0.09 | −0.95 | 0.00 | −1.29, −0.60 |
| Motor | −0.13 | 0.31 | −0.37, 0.12 | −0.11 | 0.40 | −0.36, 0.14 | 0.10 | 0.33 | −0.10, 0.31 | −0.14 | 0.42 | −0.49, 0.20 | |
|
| |||||||||||||
| Cognitive | 0.05 | 0.70 | −0.19. 0.28 | 0.03 | 0.80 | −0.21, 0.27 | −0.26 | 0.01 | −0.45, −0.07 | −0.14 | 0.35 | −0.43, 0.15 | |
| Language | 12 | −0.18 | 0.14 | −0.41. 0.06 | −0.05 | 0.69 | −0.29, 0.19 | −0.27 | 0.01 | −0.47, −0.08 | −0.58 | 0.00 | −0.88, −0.28 |
| Motor | 0.07 | 0.55 | −0.16, 0.30 | 0.14 | 0.26 | −0.10, 0.38 | −0.17 | 0.09 | −0.36, 0.02 | −0.01 | 0.93 | −0.31, 0.28 | |
|
| |||||||||||||
| Cognitive | 0.17 | 0.16 | −0.07, 0.41 | −0.02 | 0.86 | −0.27, 0.23 | −0.20 | 0.04 | −0.39, −0.01 | −0.13 | 0.40 | −0.44, 0.18 | |
| Language | 18 | 0.00 | 0.99 | −0.25, 0.24 | 0.01 | 0.96 | −0.25, 0.26 | −0.08 | 0.43 | −0.27, 0.12 | −0.08 | 0.63 | −0.38, 0.23 |
| Motor | 0.14 | 0.26 | −0.11, 0.39 | 0.08 | 0.52 | −0.17, 0.34 | −0.04 | 0.71 | −0.23, 0.16 | 0.13 | 0.41 | −0.18, 0.44 | |
|
| |||||||||||||
| Cognitive | 0.26 | 0.05 | 0.00, 0.51 | 0.14 | 0.34 | −0.14, 0.42 | −0.14 | 0.20 | −0.35, 0.07 | 0.07 | 0.73 | −0.31, 0.45 | |
| Language | 24 | −0.11 | 0.41 | −0.36, 0.15 | −0.12 | 0.40 | −0.40, 0.16 | −0.12 | 0.28 | −0.33, 0.09 | −0.38 | 0.05 | −0.77, −0.01 |
| Motor | 0.24 | 0.07 | −0.02, 0.50 | 0.13 | 0.35 | −0.15, 0.41 | −0.02 | 0.88 | −0.23, 0.20 | 0.19 | 0.33 | −0.19, 0.57 | |
95% CI: 95% confidence interval; P: p value
The number of mother–child pairs included in the analysis n=445
Models adjusted by child’s sex, gestational age, birthweight, prenatal smoking and maternal IQ.
Age at evaluation in months
Effects of trimester-specific exposure yielded a similar effect for third trimester average exposure on cognitive domain at 12 (−0.26 points; 95% CI: −0.45, −0.07) and 18 (−0.20 points; 95% CI: −0.39, −0.01) months assessment and language domain at 12-month follow-up assessment (−0.27 points; 95% CI: −0.47, −0.08).
Figure 2 illustrates the mean changes scores of the minimally adjusted model for the three domains of development (cognitive, language and motor) associated to changes in PM2.5 mean exposure of the entire pregnancy over the follow-up period. Children at 6 months of age have lower cognitive scores expected for this age, but in the follow-up no changes were observed.
Fig. 2.
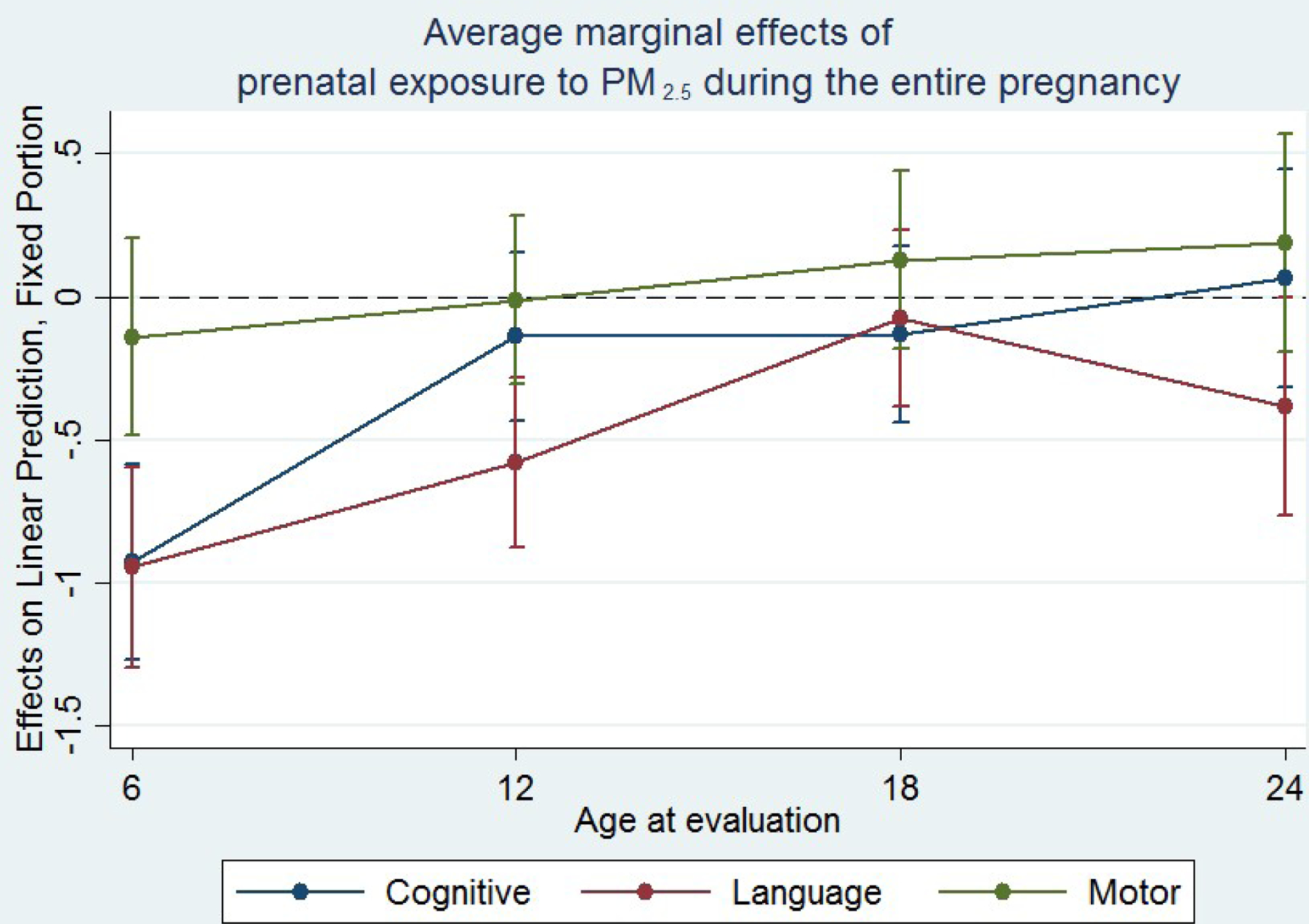
Changes in cognitive, language and motor functions associated with each 1 μg/m3 increase in PM2.5 mean exposure of the entire pregnancy.
In fully adjusted models, all pregnancy, second and third trimester exposures were associated with a decrease in cognitive and language scores at 6 months of age. Nevertheless, third trimester and all pregnancy exposures associated with cognitive and language function at 6, 18 and 24 months were most of the times attenuated and motor function significantly increased at 18 and 24 months follow-up (Supplemental Material, Table S3).
DISCUSSION
We found negative associations between prenatal PM2.5 exposure during all pregnancy and language function at 6 months, 12 months and up to 24 months of age. Our findings suggest that PM2.5 exposure during pregnancy modifies the performance of language function in early childhood. These results were strongest in the 6 month old assessment and the magnitude of the association remained stable after including stimulation at HOME as a potential confounding variable.
The mechanisms by which air pollution could affect fetal neurodevelopment include intrauterine inflammation, which contributes to cell loss within the central nervous system (Brookmeyer 2016). Gestational exposure to PM2.5 may increase maternal oxidative stress that leads to an increase in pro-inflammatory cytokine production (Xu 2012) with subsequent placental dysfunction. Similarly, increased fetal oxidative stress produces cytokines that modulate various neurodevelopmental processes, including cell differentiation, maturation and survival (Fest 2007, Meyer 2007). In addition, it has been proposed that the finest particles can reach endocrine glands and cause alterations in the endocrine system (Predieri 2020). Epidemiological evidence suggested that first trimester hypothyroxinemia, when neuronal migratory starts, increases the risk of poor neuropsychological development of the fetus due to a decreased availability of maternal thyroxine (T4) to the developing brain (Morreale de Escobar 2000). Furthermore, it has been suggested that gestational exposure to PM2.5 increases levels of apoptotic proteins during an important stage of brain development (Zheng 2019).
How PM2.5 in utero exposure might influence language development in early childhood is not well understood, however, the hypothesis is that early language development may be affected through cell loss that inhibits processing of available linguistic information (Brockmeyer 2016, Bates 1992). It is known that communicative interaction among parents and children starts in utero, as early as the third trimester of pregnancy, when human fetus brain is ready to learn and it is capable of learning some aspects of speech, including speech sounds. Therefore, infants are capable of perceiving the speech at birth and/or within the first months of life. (Bates 1992). Accordingly, we found negative associations up to the first year of life with exposure to PM2.5 during the whole pregnancy and the third trimester of pregnancy. Previous studies that assessed language or verbal domain showed inconsistent results. In children ages 4 through 6 years, a negative verbal domain was associated with exposure to PM2.5 during the whole pregnancy (β = −0.30; CI 95%: −0.78, 0.17), though it was not statistically significant. However, after stratifying by sex, the associations become negative and statistically significant for boys (β = −0.68; CI 95%: −1.34, −0.02), but not for girls (β = 0.05; CI 95%: −0.76, 0.85). Another study observed similar results in children at 15 months of age, an age group included in our study. PM2.5 exposure during pregnancy was not significantly associated with a decrease of language development (β = −0.64; CI 95%: −1.64, 0.36) (Guxens 2014),
We also found an adverse association of PM2.5 exposure during the whole pregnancy with cognitive development through the first 18 months of life, but the association does not persist up to 24 months. This is consistent with previous research that observed that an increase of 1μg/m3 of exposure to PM2.5 over the whole pregnancy was associated with decreased in the cognitive (β = −0.70; 90% CI: −1.17; −0.23) development scales at 15 months of age (Lertxundi 2015).
In addition, cognitive and motor results in our study showed increasing scores up to 24 months with all the exposure metrics except with the third trimester exposure. However, these higher scores were not statistically significant.
Estimated associations of PM2.5 during all pregnancy and language development in fully adjusted models were similar to those obtained by minimally adjusted models, but with no statistical significance (p=0.073 up to 12 months and p = 0.064 up to 24 months). However; it may be due to the low levels of statistical power when covariates were included in the model. In fully adjusted models, predictors of function development such as maternal stress and prenatal lead exposure, indicated that both parameters had not significant associations with the outcomes. In addition, it is important to note that we considered potential confounders like socioeconomic status and important determinants of a childrens such as parity and breastfeeding, but including these variables in the model reduced the sample size and did not contribute substantially to the estimates.
Consistent with previous research, boys lag slightly behind girls in most measures of cognitive, language and motor development (Chiu 2016). One explanation is that boys may be more vulnerable to prenatal oxidant damage and may have greater response to air pollution exposure during early development (Minghetti 2013). Since there was an indication of a larger effect on males, we examined the interaction between sex and PM2.5 but found the coefficients were not significant. We did find stronger negative effects on cognitive scores in boys and language scores in girls (FS2 supplemental material).
Our study has some important strengths. These include a novel high-resolution satellite-based model in our PM2.5 exposure estimates, which allowed us to overcome many of the spatial coverage and interpolation problems that occur when using only data from monitoring stations. We also adjusted for important confounders and covariates that enabled us to take into account the effects of these factors. However, some limitations should be noted. We used each mother’s residential location at time of recruitment to assign PM2.5 exposures, and it is possible that movements throughout the city could change the level of exposure. However, studies with prenatal residential address history suggest minimal exposure misclassification when using address at conception, since it may not significantly differ from address at delivery, even amongst movers (Hodgson 2015). We also were unable to control for postnatal PM2.5 exposure. Even so, studies have reported that results do not change when postnatal PM2.5 exposure is included (Chiu 2016). Moreover, we hypothesize that if we have great variability in exposure, children with very high or low PM2.5 estimates, it may attenuate associations towards the null due to exposure misclassification. Finally, although we assessed a highly susceptible period to environmental factors, further research will be required to understand the overall association between PM2.5 and children’s neurodevelopment. Another limitation is that only 740 mother–child pairs were included for the study among the 948 mothers with delivery information. Nevertheless, there were no significant differences in maternal characteristics between the two groups. .
CONCLUSIONS
In our study, exposure to PM2.5 during pregnancy showed a consistent pattern of language deficits in children up to 2 years old, which may have important consequences in later academic success and social adaptation (Bashir 1992). Because language development is fundamental for reading and writing skills (Donahue 1986), health benefits might be achieved through regulatory interventions that reduce such exposure during pregnancy.
Supplementary Material
Fig. 1.
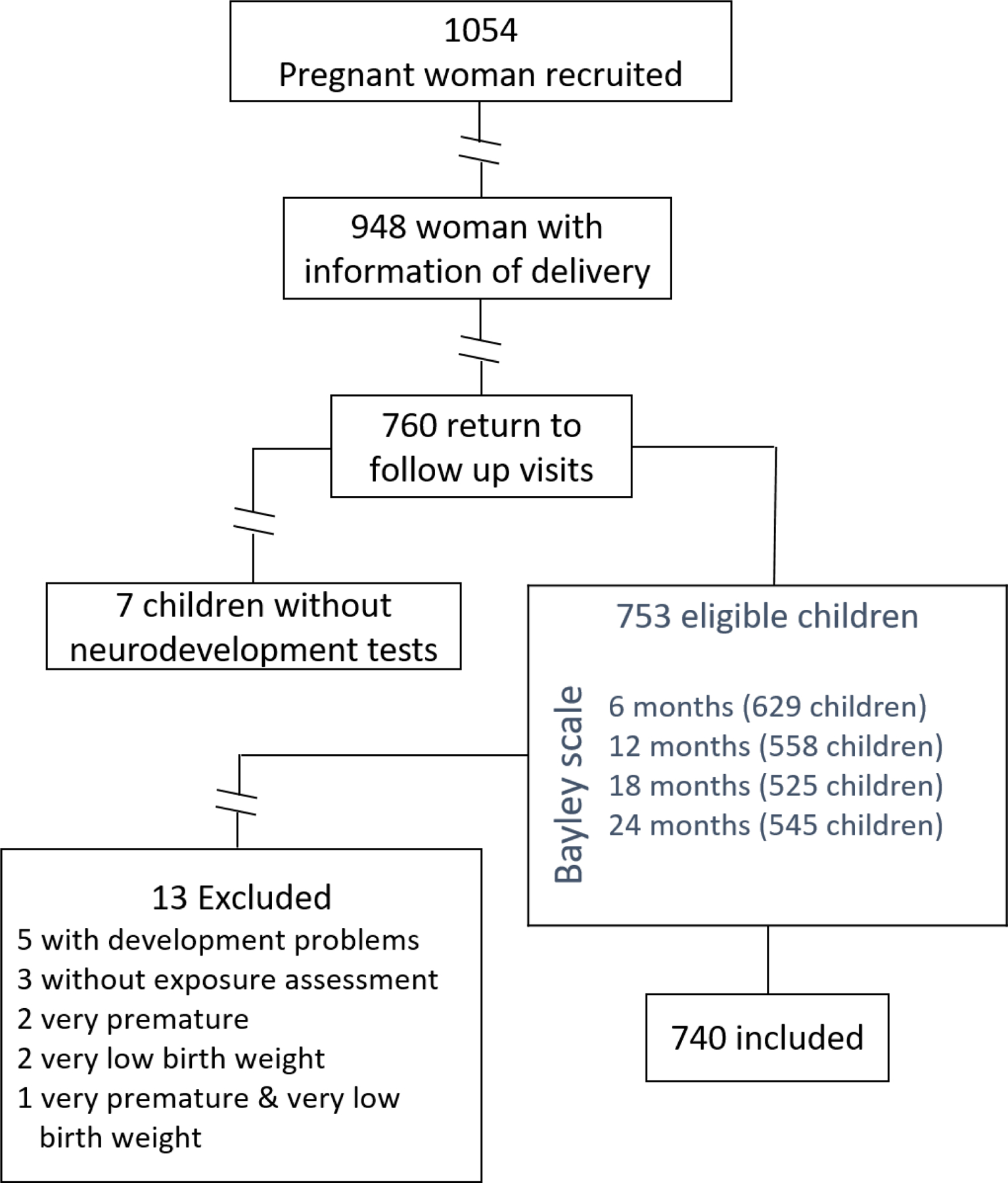
PRGORESS cohort study, Mother-child pairs selected for the analysis.
Acknowledgments
This work was supported by the U.S. Government National Institute of Environmental and Health Sciences (NIEHS) with financing numbers R00 ES023450, R01 ES013744, R01 ES014930, R01 ES021357 and P30 ES023515. The study was partially financed by the Instituto Nacional de Salud Pública de México (INSP). The authors would like to thank the Instituto Nacional de Perinatología and the Centro Médico ABC for the support during this research.
Abbreviations
- %
percentage
- μg/m3
microgram/cubic meter
- 95% CI
95% Confidence Interval
- BBB
Blood-brain barrier
- CI
Confidence Interval
- DALYs
Disability-adjusted life years
- Min
Minimal value
- Max
Maximal value
- p25
25th percentile
- NO
Nitric oxide
- p75
75th percentile
- ROS
Reactive oxygen species
- SD
Standard Deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
Conflicts of Interest
The authors declare no conflicts of interest
REFERENCES
- Barraza-Villarreal AS,J; Hernandez-Cadena L; Escamilla-Nunez MC; Sienra-Monge JJ; Ramirez-Aguilar M; Cortez-Lugo M; Holguin F; Diaz-Sanchez D; Olin AC; Romieu I, Air pollution, airway inflammation, and lung function in a cohort study of Mexico City schoolchildren. Environ Health Perspect, 2008. 116(6): p. 832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basagaña X, Esnaola M, Rivas I, Amato F, Alvarez-Pedrerol M, Forns J, ... & Sunyer J (2016). Neurodevelopmental deceleration by urban fine particles from different emission sources: a longitudinal observational study. Environmental Health Perspectives, 124(10), 1630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir Anthony S., and Scavuzzo Annebelle. “Children with language disorders: Natural history and academic success.” Journal of learning disabilities 25.1 (1992): 53–65. [DOI] [PubMed] [Google Scholar]
- Bates E, Thal D, & Janowsky JS (1992). Early language development and its neural correlates. Handbook of neuropsychology, 7, 69–69. [Google Scholar]
- Bayley N Bayley Scales of Infant Development 2nd ed. San Antonio, TX: Psychological [Google Scholar]
- Beyer HL (2004). Hawth’s analysis tools for ArcGIS.Corporation; 1993. [Google Scholar]
- Block ML, & Calderón-Garcidueñas L (2009). Air pollution: mechanisms of neuroinflammation and CNS disease. Trends in neurosciences, 32(9), 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM, Rock SL, Ramey CT, Barnard KE, Gray C, ... & Johnson DL (1989). Home environment and cognitive development in the first 3 years of life: A collaborative study involving six sites and three ethnic groups in North America. Developmental psychology, 25(2), 217. [Google Scholar]
- Braun JM, Wright RJ, Just AC, Power MC, Tamayo Y, Ortiz M, Schnaas L, et al. , 2014. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ. Health 13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer S, & D’Angiulli A (2016). How air pollution alters brain development: the role of neuroinflammation. Translational Neuroscience, 7(1), 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, Gómez-Garza G, Barragán-Mejía G, Broadway, and Engle RW (2008). Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain and cognition, 68(2), 117–127. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Styner M, Gómez-garza G, Zhu H, Torres-Jardón R, .. & D’Angiulli A (2012). White matter hyperintensities, systemic inflammation, brain growth, and cognitive functions in children exposed to air pollution. Journal of Alzheimer’s Disease, 31(1), 183–191. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. 1984. Home Observation for Measurement of the Environment Little Rock, AR:University of Arkansas. [Google Scholar]
- Carbajal-Arroyo L, et al. , Effect of PM10 and O3 on infant mortality among residents in the Mexico City Metropolitan Area: a case-crossover analysis, 1997–2005. J Epidemiol Community Health, 2011. 65(8): p. 715–21. [DOI] [PubMed] [Google Scholar]
- Chen B, Huang S, He J, He Q, Chen S, Liu X, ... & Duan Y (2020). Sex-specific influence of prenatal air pollutant exposure on neonatal neurobehavioral development and the sensitive window. Chemosphere, 126824. [DOI] [PubMed] [Google Scholar]
- Chiu YHM, Hsu HHL, Coull BA, Bellinger DC, Kloog I, Schwartz J, ... & Wright RJ (2016). Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environment international, 87, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, ... & Feigin V (2017). Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1994). Perceived stress scale. Measuring stress: A guide for health and social scientists [Google Scholar]
- Cromwell EA, Dube Q, Cole SR, Chirambo C, Dow AE, Heyderman RS, & Van Rie A (2014). Validity of US norms for the Bayley Scales of Infant Development-III in Malawian children. european journal of pediatric neurology, 18(2), 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserbik D, Chen JC, McConnell R, Berhane K, Sowell ER, Schwartz J, ... & Herting MM (2020). Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environment International, 143, 105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LW (2017). Saturday Driving Restrictions Fail to Improve Air Quality in Mexico City. Scientific Reports, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue M. Linguistic and communicative development in learning-disabled children. In: Ceci SJ, editor. Handbook of Cognitive, Social, and Neuropsychological Aspects of Learning Disabilities Lawrence Erlbaum Associates; Hillsdale, NJ: 1986. pp. 263–289. [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect 2006;114(8):1172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, Chen R, ... & Mor G (2007). Trophoblast–macrophage interactions: a regulatory network for the protection of pregnancy. American journal of reproductive immunology, 57(1), 55–66. [DOI] [PubMed] [Google Scholar]
- Guerra R, Vera-Aguilar E, Uribe-Ramirez M, Gookin G, Camacho J, Osornio-Vargas AR et al. (2013) Exposure to inhaled particulate matter activates early markers of oxidative stress, inflammation and unfolded protein response in rat striatum. Toxicol Lett 222:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Garcia-Esteban R, Giorgis-Allemand L, Forns J, Badaloni C, Ballester F, ... & De Nazelle A (2014). Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology, 636–647. [DOI] [PubMed] [Google Scholar]
- Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, ... & Bellinger DC (2015). Prenatal and childhood traffic-related pollution exposure and childhood cognition in the project viva cohort (Massachusetts, USA). Environmental health perspectives, 123(10), 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson S, Lurz PW, Shirley MD, Bythell M, & Rankin J (2015). Exposure misclassification due to residential mobility during pregnancy. International journal of hygiene and environmental health, 218(4), 414–421. [DOI] [PubMed] [Google Scholar]
- Hu H, Téllez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, ... & Hernández-Avila M (2006). Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environmental health perspectives, 1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME). Global burden of disease 2010. In: http://ghdx.healthmetricsandevaluation.org/record/global-burden-disease-study-2010-gbd-2010-ambient-air-pollution-risk-model-1990-2010. Consulted el 30 de enero de 2014. [Google Scholar]
- Johansson C, Castoldi AF, Onishchenko N, Manzo L, Vahter M, Ceccatelli S. Neurobehavioural and molecular changes induced by methylmercury exposure during development. Neurotox Res 2007; 11: 241–260. [DOI] [PubMed] [Google Scholar]
- Just AC, Wright RO, Schwartz J, Coull BA, Baccarelli AA, Téllez-R MM, ... & Kloog I (2015). Using high-resolution satellite aerosol optical depth to estimate daily PM2. 5 geographical distribution in Mexico City. Environmental Science & Technology, 49(14), 8576–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FJ, & Fussell JC (2015). Air pollution and public health: emerging hazards and improved understanding of risk. Environmental geochemistry and health, 37(4), 631–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Park H, Hong YC, Ha M, Kim Y, Kim BN, ... & Ha EH (2014). Prenatal exposure to PM10 and NO2 and children’s neurodevelopment from birth to 24months of age: Mothers and Children’s Environmental Health (MOCEH) study. Science of The Total Environment, 481, 439–445. [DOI] [PubMed] [Google Scholar]
- Lertxundi A, Baccini M, Lertxundi N, Fano E, Aranbarri A, Martínez MD, ... & Ibarluzea J (2015). Exposure to fine particle matter, nitrogen dioxide and benzene during pregnancy and cognitive and psychomotor developments in children at 15 months of age. Environment international, 80, 33–40. [DOI] [PubMed] [Google Scholar]
- Lertxundi A, Andiarena A, Martínez MD, Ayerdi M, Murcia M, Estarlich M, ... & Ibarluzea J (2019). Prenatal exposure to PM2. 5 and NO2 and sex-dependent infant cognitive and motor development. Environmental research, 174, 114–121. [DOI] [PubMed] [Google Scholar]
- McGuinn LA, Bellinger DC, Colicino E, Coull BA, Just AC, Kloog I, ... & Wright RO (2020). Prenatal PM2. 5 Exposure and Behavioral Development in Children from Mexico City. NeuroToxicology [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R, Sanders WL, Stroup WW0. A Unified Approach to Mixed Linear Models. The American Statistician0 1991;45:54–640. [Google Scholar]
- Meyer U, Yee BK, & Feldon J (2007). The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse?. The Neuroscientist, 13(3), 241–256. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Zanardo V, Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern Fetal Neonatal Med 2013;26:259–262 [DOI] [PubMed] [Google Scholar]
- Mitchell SK, & Gray CA (1981). Developmental generalizability of the HOME inventory. Educational and Psychological Measurement, 41(4), 1001–1010. [Google Scholar]
- Morreale de Escobar G, Jesús Obregón M, & Escobar del Rey F (2000). Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia?. The Journal of Clinical Endocrinology & Metabolism, 85(11), 3975–3987 [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, & Cox C (2004). Translocation of inhaled ultrafine particles to the brain. Inhalation toxicology, 16(6–7), 437–445. [DOI] [PubMed] [Google Scholar]
- Predieri B, Bruzzi P, Bigi E, Ciancia S, Madeo SF, Lucaccioni L, & Iughetti L (2020). Endocrine Disrupting Chemicals and Type 1 Diabetes. International Journal of Molecular Sciences, 21(8), 2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, et al. , Personal PM2.5 and CO exposures and heart rate variability in subjects with known ischemic heart disease in Mexico City. J Expos Sci Environ Epidemiol, 2006. 16(2): p. 131–137. [DOI] [PubMed] [Google Scholar]
- Rojas-Martinez R, et al. , Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med, 2007. 176(4): p. 377–84. [DOI] [PubMed] [Google Scholar]
- Rousey AM, Wild M, & Blacher J (2002). Stability of measures of the home environment for families of children with severe disabilities. Research in Developmental Disabilities, 23(1), 17–35. [DOI] [PubMed] [Google Scholar]
- Sanders T, Liu Y, Buchner V, & Tchounwou PB (2009). Neurotoxic effects and biomarkers of lead exposure: a review. Reviews on environmental health, 24(1), 15–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaas L, Rothenberg SJ, Flores MF, Martinez S, Hernandez C, Osorio E, ... & Perroni E (2006). Reduced intellectual development in children with prenatal lead exposure. Environmental health perspectives, 114(5), 791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott EO, Arena VC, Rager JR, Clougherty JE, Michanowicz DR, Sharma RK, & Stacy SL (2015). Fine particulate matter and the risk of autism spectrum disorder. Environmental Research, 140, 414–420. [DOI] [PubMed] [Google Scholar]
- Tamayo y Ortiz MT, Téllez-Rojo MM, Trejo-Valdivia B, Schnaas L, Osorio-Valencia E, Coull B, ... & Wright RO (2017). Maternal stress modifies the effect of exposure to lead during pregnancy and 24-month old children’s neurodevelopment. Environment International, 98, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Rojo MMR,I; Ruiz-Velasco S; Lezana MA; Hernandez-Avila MM, Daily respiratory mortality and PM10 pollution in Mexico City: importance of considering place of death. Eur Respir J, 2000. 16(3): p. 391–6. [DOI] [PubMed] [Google Scholar]
- Torres-Sánchez L, Rothenberg SJ, Schnaas L, Cebrián ME, Osorio E, del Carmen Hernández M, ... & López-Carrillo L (2007). In utero p, p’-DDE exposure and infant neurodevelopment: a perinatal cohort in Mexico. Environmental Health Perspectives, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 1981. WAIS-Español. Escala de Inteligencia para Adultos. Manual México, DF:El Manual Moderno, S.A. [Google Scholar]
- World Health Organization. (2016). Ambient air pollution: a global assessment of exposure and burden of disease. In Ambient air pollution: a global assessment of exposure and burden of disease [Google Scholar]
- Xu X, Deng F, Guo X, Lv P, Zhong M, Liu C, ... & Rajagopalan S (2012). Association of systemic inflammation with marked changes in particulate air pollution in Beijing in 2008. Toxicology letters, 212(2), 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang X, Wang T, Zhang H, Wu H, Zhang C, ... & Guan Y (2019). Gestational exposure to particulate matter 2.5 (PM2. 5) leads to spatial memory dysfunction and neurodevelopmental impairment in hippocampus of mice offspring. Frontiers in neuroscience, 12, 1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


