Abstract
Introduction
Multicenter studies involving patients with acute kidney injury (AKI) associated with the disease caused by the new coronavirus (COVID-19) and treated with renal replacement therapy (RRT) in developing countries are scarce. The objectives of this study were to evaluate the demographic profile, clinical picture, risk factors for mortality, and outcomes of critically ill patients with AKI requiring dialysis (AKI-RRT) and with COVID-19 in the megalopolis of São Paulo, Brazil.
Methods
This multicenter, retrospective, observational study was conducted in the intensive care units of 13 public and private hospitals in the metropolitan region of the municipality of São Paulo. Patients hospitalized in an intensive care unit, aged ≥ 18 years, and treated with RRT due to COVID-19-associated AKI were included.
Results
The study group consisted of 375 patients (age 64.1 years, 68.8% male). Most (62.1%) had two or more comorbidities: 68.8%, arterial hypertension; 45.3%, diabetes; 36.3%, anemia; 30.9%, obesity; 18.7%, chronic kidney disease; 15.7%, coronary artery disease; 10.4%, heart failure; and 8.5%, chronic obstructive pulmonary disease. Death occurred in 72.5% of the study population (272 patients). Among the 103 survivors, 22.3% (23 patients) were discharged on RRT. In a multiple regression analysis, the independent factors associated with death were the number of organ dysfunctions at admission and RRT efficiency.
Conclusion
AKI-RRT associated with COVID-19 occurred in patients with an elevated burden of comorbidities and was associated with high mortality (72.5%). The number of organ dysfunctions during hospitalization and RRT efficiency were independent factors associated with mortality. A meaningful portion of survivors was discharged while dependent on RRT (22.3%).
Introduction
Approximately 20 to 25% of patients with acute kidney injury (AKI) in intensive care units (ICUs) require renal replacement therapy (RRT) [1,2]. The mortality rate for patients with AKI requiring dialysis (AKI-RRT) in the ICU is greater than 50% [3–6], which can be explained by the increase in age and the burden of comorbidities in critically ill patients in recent years. The COVID-19 pandemic caused by the new coronavirus SARS-CoV-2 significantly affected nephrological practice, increasing the demand for nephrologists, nephrology-specialized nurses, RRT equipment and supplies [7]. Knowing the incidence, characteristics, and outcomes of AKI associated with COVID-19 is essential for health planning.
The frequencies of AKI and AKI-RRT reported in patients with COVID-19 range from 10 to 17% and 2.4 to 4.3%, respectively [8,9]. Subgroup analyses showed a higher odds ratio (OR) for AKI in the elderly population (OR = 3.53, CI 2.92–4.25; p <0.001) and in males (OR = 1.36, CI 0.84–2.20; p = 0.21) [9]. Patients with AKI associated with COVID-19 are 15 times more likely to die [10,11], and this increase is proportional to the stage of AKI (4.5, 8.0, 19.9, and 30.2 times more likely in stages 1, 2, 3—no dialysis, and 3—requiring dialysis, respectively, p <0.001) [12].
The available information on the mortality and recovery of patients with AKI-RRT associated with COVID-19 is mostly from cohorts in high-income countries located in the Northern Hemisphere, which show a mortality rate of 63.3% to 79.3% and dependence on RRT after discharge in 22.0% to 38% of survivors [13–16]. Data on the epidemiological profile and outcomes of patients with AKI-RRT associated with SARS-CoV-2 are scarce in Latin American countries.
The objectives of this study were to evaluate the demographic profile, clinical picture, risk factors for mortality, and outcomes of critically ill patients with AKI-RRT-associated COVID-19 in the megalopolis of São Paulo, Brazil.
Patients and methods
Study design, location, and population
This was a multicenter, retrospective, observational study conducted in 13 hospitals (public and private) in the metropolitan region of the city of São Paulo. The choice of participating centers was made by convenience.
The inclusion criteria were as follows: patients ≥ 18 years of age admitted to the ICU due to COVID-19 between 1 April 2020 and 31 August 2020 who required RRT due to AKI. The exclusion criteria were patients with chronic kidney disease (CKD) dependent on RRT before hospitalization and those in exclusive palliative care. The study was approved by the research ethics committees of the participating centers under CAAE (certificate of presentation of ethical appreciation) number n31693820.8.1001.5485.
Variables of interest
Demographic data (age, sex, and ethnicity), profiles of comorbidities, and hospital admission parameters (symptoms and time of symptom onset related to COVID-19, vital signs, and laboratory tests) were obtained. A patient was considered to have hypertension (HTN) if the diagnosis was recorded in the medical record, or the patient used antihypertensive drugs. A patient was considered to have diabetes mellitus (DM) if the diagnosis was recorded in the medical record, or the patient reported the previous use of oral antidiabetics drugs or insulin. A patient was considered to have CKD, heart failure (HF), chronic liver disease, and chronic obstructive pulmonary disease (COPD) if such diagnoses were reported in the medical record. A patient was considered to have coronary artery disease if she or he had a history of acute myocardial infarction, stent placement, or myocardial revascularization. A patient was considered obese with a body mass index > 30 kg/m2 or if this diagnosis was reported in the medical record. A patient was considered to have anemia if the serum hemoglobin (Hb) concentration at admission was < 13.0 g/dl in men or Hb < 12.0 g/dl in women. In addition, the following variables were of interest: the severity of pulmonary involvement on chest tomography (mild, < 25%; moderate, between 25 and 50%; and severe, > 50%), Simplified Acute Physiology Score (SAPS) at ICU admission, the incidence of organ dysfunction other than kidney dysfunction during hospitalization (pulmonary, circulatory, hepatic, or coagulation) and the use of the following medications during hospitalization: azithromycin, hydroxychloroquine, corticosteroids, antibiotics (in addition to azithromycin), and heparin by continuous infusion. Pulmonary, circulatory, hepatic, and coagulation dysfunction was defined as a PaO2/FiO2 ratio < 400 or the need for mechanical ventilation, the use of vasopressors, serum levels of total bilirubin ≥ 1.2 mg/dl, and platelets < 150,000/mm3, respectively [17].
The RRT parameters evaluated were serum creatinine, urea, sodium, potassium, and bicarbonate up to 24 hours before the first RRT session, simple mean values of serum creatinine, urea, sodium, potassium, and bicarbonate during the period on RRT and the RRT method used: peritoneal dialysis (PD), intermittent hemodialysis (IHD), sustained low-efficiency dialysis (SLED), or continuous renal replacement therapy (CRRT). Efficient RRT was defined as the presence of two or more of the following criteria, which were evaluated during the period in which the patient underwent RRT: simple mean values of urea < 100 mg/dl, potassium < 5.0 mEq/l, and bicarbonate > 22 mEq/l. The number of RRT sessions, the total hours of CRRT, and the indications for the RRT method could not be obtained.
The outcomes of interest were the length of hospital stay, death, and discharge (with or without RRT dependence). The patient follow-up time was up to 90 days of hospitalization.
The diagnosis of COVID-19 was defined as a positive RT–PCR (real-time polymerase chain reaction) result or as a combination of respiratory symptoms and chest tomography with typical changes (peripheral and bilateral ground glass opacities, multifocal ground glass opacities of rounded morphology, and/or an inverted halo sign) [18].
Statistical analysis
Categorical variables are presented as frequencies. Quantitative variables with a normal distribution are presented as the mean and standard deviation, and those with a no normal distribution are presented as the median and interquartile range. Frequencies were compared using the χ2 or Fischer’s test as appropriate. Intergroup comparisons for quantitative variables were performed using Student’s t test and the Mann–Whitney U test for normally distributed and nonnormally distributed data, respectively.
The analysis of independent risk factors for mortality was performed using logistic regression. Due to the occurrence of missing data and their no monotonic pattern, multiple inputs were performed using the Monte Carlo method via Markov chains, which generated 15 complete datasets. All variables included in the multiple logistic regression were used for the input of missing data, for which logistic regression was performed for categorical variables and linear regression was performed for continuous variables. Multiple logistic regression analysis was performed for each of the 15 complete datasets using a backward strategy and maintaining in the model the variables that, when removed, led to a change greater than 10% in the estimate of the betas of the variables present. The presence of multicollinearity was verified by the variance inflation factor.
The initial logistic regression model included the following variables: type of hospital (public or private), age, sex, smoking, one or more comorbidities (no reference), lower tercile of platelets, upper tercile of creatinine at admission, number of organ dysfunctions during hospitalization (circulatory, pulmonary, coagulopathic, and hepatic), use of vasoactive drugs, mechanical ventilation, urea > 150 mg/dl, potassium > 5 mEq/l, bicarbonate < 22 mEq/l, upper tercile of serum creatinine levels measured on the day of the first indication for RRT, and efficient RRT. The quality of the model fit was evaluated using the Hosmer–Lemeshow test, and the significance of the variables was evaluated using the Wald test. The results obtained with the analysis of the 15 datasets were averaged [19]. Statistical analysis was performed using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA). The significance level adopted was < 0.05.
Results
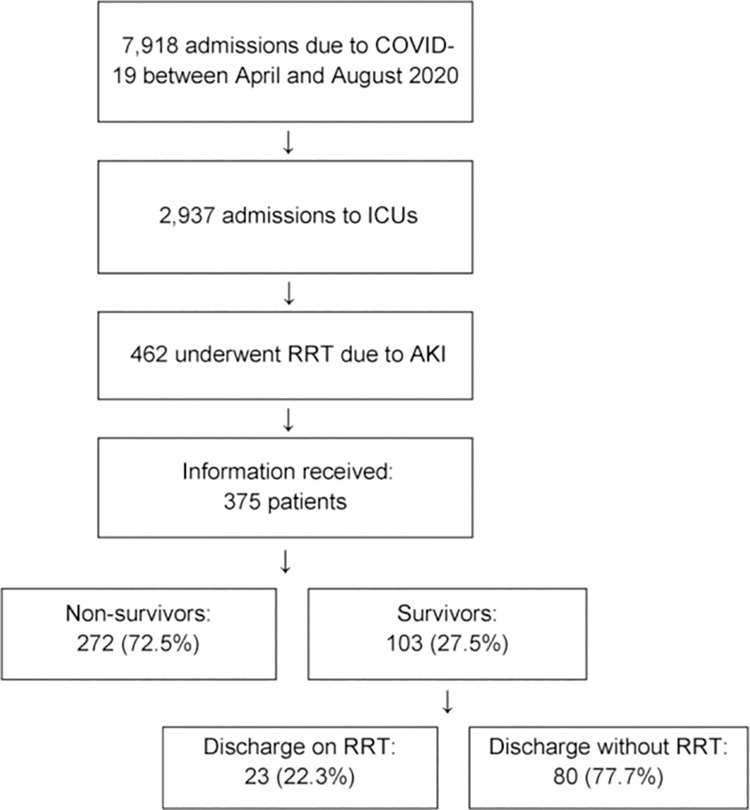
Between April and August 2020, the numbers of patients—total and ICU—admitted for COVID-19 in the participating hospitals were 7,918 and 2,937, respectively. In the same period, 462 of these 2,937 ICU patients (15.7%) underwent RRT due to AKI. The data for 375 (81.1%) of these patients (39.5% admitted to public hospitals and 60.5% admitted to private hospitals) were obtained (Fig 1). Patients with positive RT–PCR results for COVID-19 accounted for 97.1% of the population (364 patients). Individuals with unavailable RT–PCR results but with clinical and radiological findings highly compatible with COVID-19 accounted for 2.9% of the population (11 patients).
Fig 1. Patient inclusion flowchart and outcomes.
COVID-19, a disease caused by the new coronavirus; ICUs: Intensive care units; RRT, renal replacement therapy; AKI, acute kidney injury.
The demographic data, comorbidities, and hospital admission parameters are summarized in Table 1. The age was 64.1 (55.0–74.2) years, and a predominance of males (68.8%) and white ethnicity (38.9%) was noted. The main comorbidities were HTN (68.8%), DM (45.3%), anemia (36.3%), obesity (30.9%), CKD (18.7%), coronary artery disease (15.7%), and HF (10.4%). Among the patients, 62.1% had two or more comorbidities.
Table 1. General characteristics of the study population (N = 375).
| Demographics | |
| Age, years | 64.1 (55.0–74.2) |
| Male sex, % (n) | 68.8 (258) |
| Ethnicity | |
| White, % (n) | 38.9 (146) |
| Afro-descendants, % (n) | 19.0 (71) |
| Asian, % (n) | 3.7 (14) |
| Missing data, % (n) | 38.1 (143) |
| Smoking, % (n) | 18.1 (68) |
| Comorbidities | |
| Hypertension, % (n) | 68.0 (255) |
| Diabetes mellitus, % (n) | 45.3 (170) |
| Obesity | |
| Yes, % (n) | 30.9 (116) |
| No, % (n) | 46.9 (176) |
| Unknown, % (n) | 22.1 (83) |
| Chronic kidney disease, % (n) | 18.7 (70) |
| Coronary insufficiency, % (n) | 15.7 (59) |
| Heart failure, % (n) | 10.4 (39) |
| Chronic obstructive pulmonary disease, % (n) | 8.5 (32) |
| Neoplasia, % (n) | 3.5 (13) |
| Chronic liver disease, % (n) | 2.1 (8) |
| Participating institution | |
| Public hospital, % (n) | 39.5 (148) |
| Private hospital, % (n) | 60.5 (227) |
| Parameters at hospital admission | |
| Symptoms | |
| Dyspnea, % (n) | 74.4 (279) |
| Cough, % (n) | 74.1 (278) |
| Fever, % (n) | 52.3 (196) |
| Diarrhea, % (n) | 10.9 (41) |
| Coryza, % (n) | 9.3 (35) |
| Odynophagia, % (n) | 6.9 (26) |
| Expectoration, % (n) | 6.7 (25) |
| Anosmia, % (n) | 6.7 (25) |
| Ageusia, % (n) | 4.8 (18) |
| Mean arterial pressure (mmHg) | 91.3 ± 18.5 |
| Oxygen saturation (%) | 92 (88–95) |
| Time since symptom onset, days | 5 (3–7) |
Data are presented as the mean ± SD, the median and interquartile range (p25-p75), or a percentage.
Subsidiary examinations, clinical characteristics, organ dysfunction, treatments, and outcomes of the patients are shown in Table 2. Severe pulmonary involvement occurred in 39.7% of the patients (chest tomography was not performed or not reported for 15.2%). Organ dysfunction, in addition to kidney dysfunction, included circulatory (85.3%), pulmonary (78.7%), coagulopathic (30.4%), and hepatic dysfunction (13.3%). The isolated use of IHD, SLED, and CRRT was 56.5% (212), 8.3% (31), and 18.7% (70), respectively. A total of 16.5% (62) of patients received any combination of RRT methods. No patient underwent PD. Death occurred in 72.5% of the population (272/375 patients). Among the survivors, 22.3% (23/103 patients) were discharged while dependent on RRT.
Table 2. Subsidiary test results, clinical characteristics, and outcomes of the patients included in the study (N = 375).
| Hospital admission exams | Normal range | |
|---|---|---|
| Hemoglobin (g/dl) | 12.0–16.0 | 13.0 ± 2.1 |
| Total leukocytes (n/mm3) | 4,000–8,000 | 8,117 (5,500–11,200) |
| Total lymphocytes (n/mm3) | 1,000–3,900 | 974 (699–1489) |
| Platelets (n/mm3) | 150,000–400,000 | 179,000 (141,000–231,750) |
| Serum creatinine (mg/dl) | 0.6–1.3 | 1.16 (0.90–1.78) |
| D-dimer (ng/ml) | < 0.5 | 1.5 (0.7–9.1) |
| C-reactive protein (mg/dl) | < 0.1 | 15.5 (7.6–25.1) |
| Pulmonary involvement on tomography | ||
| Mild, % (n) | 13.1 (49) | |
| Moderate, % (n) | 32.0 (120) | |
| Severe, % (n) | 39.7 (149) | |
| Unknown, % (n) | 15.2 (57) | |
| Severity score at ICU admission | ||
| SAPS 3 | 54 (46–66) | |
| Unknown, % (n) | 42.9 (161) | |
| Mechanical ventilation, % (n) | 88.5 (332) | |
| Organ dysfunctions during hospitalization | ||
| Circulatory, % (n) | 85.3 (320) | |
| Pulmonary, % (n) | 78.7 (295) | |
| Coagulopathy, % (n) | 30.4 (114) | |
| Hepatic | ||
| Yes, % (n) | 13.3 (50) | |
| No, % (n) | 73.3 (275) | |
| Unknown, % (n) | 13.3 (50) | |
| Kidney, % (n) | 100.0 (375) | |
| Medications | ||
| Azithromycin + hydroxychloroquine, % (n) | 37.6 (141) | |
| Other antibiotics, not azithromycin | ||
| Yes, % (n) | 85.9 (322) | |
| No, % (n) | 2.7 (10) | |
| Unknown, % (n) | 11.5 (43) | |
| Corticosteroids, % (n) | 60.3 (226) | |
| Continuous heparin infusion | ||
| Yes, % (n) | 23.7 (89) | |
| No, % (n) | 75.9 (286) | |
| Laboratory exams at first RRT indication | ||
| Creatinine (mg/dl) | 0.6–1.3 | 4.05 (2.90–5.60) |
| Urea (mg/dl) | 16–40 | 152.8 (98.5–211.0) |
| Potassium (mEq/l) | 3.5–5.1 | 4.8 (4.2–5.5) |
| Bicarbonate (mEq/l) | 22–27 | 22.1 (19.0–25.9) |
| Exams during the RRT period | ||
| Creatinine (mg/dl) | 0.6–1.3 | 3.63 (2.12–5.10) |
| Urea (mg/dl) | 16–40 | 126.5 (88.0–174.1) |
| Potassium (mEq/l) | 3.5–5.1 | 4.7 (4.1–5.4) |
| Bicarbonate (mEq/l) | 22–27 | 23.0 (19.6–26.0) |
| RRT method | ||
| Intermittent, % (n) | 56.5 (212) | |
| SLED, % (n) | 8.3 (31) | |
| Continuous, % (n) | 18.7 (70) | |
| Combination of methods, % (n) | 16.5 (62) | |
| Time on RRT (days) | 6 (2–15) | |
| Outcomes | ||
| Total length of stay (days) | 19 (11–30) | |
| Death, % (n) | 72.5 (272) | |
| Discharge without RRT, % (n) | 77.7 (80/103) | |
| Discharge dependent on RRT, % (n) | 22.3 (23/103) |
Data are presented as the mean ± SD, the median and interquartile range (p25-p75), or a percentage. SLED, sustained low-efficiency dialysis.
In the univariate analysis, nonsurvivors exhibited a shorter hospital stay than survivors (15 [9–23] vs. 36 [22–47] days, p < 0.001); a higher frequency of circulatory dysfunction (89.7% vs. 73.8%, p < 0.001), pulmonary dysfunction (82.0% vs. 69.9%, p = 0.01), and liver dysfunction (19.1% vs. 6.3%, p = 0.004); and a higher frequency of corticosteroid use (65.7% vs. 46.6%, p = 0.001).
Nonsurvivors had higher serum potassium levels (4.9 [4.3–5.6] vs. 4.5 [3.9–5.2] mEq/l, p = 0.003) on the day of the first RRT indication. In addition, nonsurvivors had higher simple medians of creatinine (3.72 [2.30–5.25] vs. 3.20 [1.80–4, 82] mg/dl, p = 0.04), urea (131.5 [91.0–180.4] vs. 108.4 [79.5–159.2] mg/dl, p = 0.03), and potassium (4.8 [4.2–5.5] vs. 4.3 [3.8–4.9] mEq/l, p < 0.001), and lower serum bicarbonate (22.5 [19, 1–25.6] vs. 23.8 [20.6–27.4] mEq/l, p = 0.006) during the RRT period (Table 3).
Table 3. Comparison between surviving and nonsurviving patients.
| Survivors (N = 103) | No survivors (N = 272) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 62.5 (54.0–74.3) | 64.9 (55.4–74.2) | 0.38 |
| Male sex, % (n) | 68.9 (71) | 68.8 (187) | 1.00 |
| White ethnicity, % (n) | 56.3 (40) | 65.8 (106) | 0.16 |
| Smoking, % (n) | 18.4 (19) | 18.1 (49) | 0.95 |
| Comorbidities | |||
| Hypertension, % (n) | 69.9 (72) | 67.3 (183) | 0.63 |
| Diabetes mellitus, % (n) | 49.5 (51) | 43.8 (119) | 0.32 |
| Obesity, % (n) | 31.0 (26) | 43.3 (90) | 0.05 |
| Heart failure, % (n) | 8.7 (9) | 11.0 (30) | 0.52 |
| Coronary insufficiency, % (n) | 13.6 (14) | 16.5 (45) | 0.48 |
| CKD, % (n) | 15.5 (16) | 19.9 (54) | 0.33 |
| COPD, % (n) | 6.8 (7) | 9.2 (25) | 0.46 |
| Chronic liver disease, % (n) | 2.9 (3) | 1.8 (5) | 0.54 |
| Neoplasia, % (n) | 4.9 (5) | 3.0 (8) | 0.38 |
| Participating institution, public hospital, % (n) | 59.2 (61) | 61.0 (166) | 0.75 |
| Mean arterial pressure at hospital admission (mmHg) | 94 ± 19 | 90 ± 18 | 0.07 |
| Oxygen saturation at hospital admission (%) | 91 (88–95) | 92 (88–95) | 0.86 |
| Time to symptom onset (days) | 5 (3–7) | 5 (3–8) | 0.28 |
| Hospital admission exams | |||
| Hemoglobin (g/dl) (N.R. 12.0–16.0) | 13.3 (11.9–14.3) | 13.2 (11.8–14.5) | 0.51 |
| Total leukocytes (n/mm3) (N.R. 4,000–8,000) | 8.425 (5.658–11.600) | 8020 (5475–11040) | 0.26 |
| Total lymphocytes (n/mm3) (N.R. 1,000–3,900) | 909 (699–1348) | 995 (697–1516) | 0.37 |
| Platelets (103/mm3) (N.R. 150,000–400,000) | 186 (143.5–260.1) | 177 (141–225) | 0.09 |
| Serum creatinine (mg/dl) (N.R. 0.6–1.3)) | 1.20 (0.94–1.75) | 1.14 (0.89–1.80) | 0.21 |
| D-dimer (ng/ml) (N.R. < 0.5) | 1.30 (0.64–8.94) | 1.83 (0.78–9.2) | 0.25 |
| C-reactive protein (mg/dl) (N.R. < 0.1) | 16.8 (10–5–28.2) | 14.9 (7.0–22.9) | 0.07 |
| SAPS 3 | 52 (44–61) | 54 (47–67) | 0.13 |
| Severe involvement on tomography, % (n) | 40.8 (42) | 39.3 (107) | 0.46 |
| Mechanical ventilation | 80.6 (83) | 91.5 (249) | 0.003 |
| Organ dysfunctions | |||
| Circulatory, % (n) | 73.8 (76) | 89.7 (244) | < 0.001 |
| Pulmonary, % (n) | 69.9 (72) | 82.0 (223) | 0.011 |
| Coagulopathy, % (n) | 23.3 (24) | 33.3 (90) | 0.06 |
| Liver, % (n) | 6.3 (6) | 19.1 (44) | 0.004 |
| Medications | |||
| Vasoactive drug, % (n) | 73.8 (76) | 89.7 (244) | < 0.001 |
| Vasopressin, % (n) | 14.6 (13) | 52.1 (124) | < 0.001 |
| Noradrenaline, % (n) | 72.8 (75) | 88.6 (241) | < 0.001 |
| Dobutamine, % (n) | 7.9 (6) | 18.7 (38) | 0.03 |
| Azithromycin + hydroxychloroquine, % (n) | 41.7 (43) | 36.8 (99) | 0.47 |
| Corticosteroids, % (n) | 46.6 (48) | 65.7 (178) | 0.001 |
| Continuous heparin infusion, % (n) | 24.5 (25) | 24.0 (64) | 0.91 |
| Laboratory exams at first RRT indication | |||
| Creatinine (mg/dl) (N.R. 0.6–1.3) | 4.00 (2.90–5.52) | 4.10 (2.90–5.60) | 0.78 |
| Urea (mg/dl) (N.R. 16–40) | 140.0 (86.5–212.5) | 157.5 (100.2–211.0) | 0.18 |
| Potassium (mEq/l) (N.R. 3.5–5.1) | 4.5 (3.9–5.2) | 4.9 (4.3–5.6) | 0.003 |
| Bicarbonate (mEq/l) (N.R. 22–27) | 23.0 (18.9–26.0) | 22.0 (19.0–25.3) | 0.31 |
| Laboratory values during the RRT period | |||
| Creatinine (mg/dl) (N.R. 0.6–1.3) | 3.20 (1.80–4.82) | 3.72 (2.30–5.25) | 0.04 |
| Urea (mg/dl) (N.R. 16–40) | 108.4 (79.5–159.2) | 131.5 (91.0–180.4) | 0.03 |
| Potassium (mEq/l) (N.R. 3.5–5.1) | 4.3 (3.8–4.9) | 4.8 (4.2–5.5) | < 0.001 |
| Bicarbonate (mEq/l) (N.R. 22–27) | 23.8 (20.6–27.4) | 22.5 (19.1–25.6) | 0.006 |
| RRT method | |||
| Intermittent, % (n) | 53.4 (55) | 57.7 (157) | 0.45 |
| SLED, % (n) | 9.7 (10) | 7.7 (21) | 0.53 |
| Continuous, % (n) | 18.4 (19) | 18.8 (51) | 0.94 |
| Combination of methods, % (n) | 18.4 (19) | 15.8 943) | 0.54 |
| Time on RRT (days) | 15 (7–24) | 4 (2–11) | < 0.001 |
| Length of hospital stay (days) | 36 (22–47) | 15 (9–23) | < 0.001 |
Data are presented as the mean ± SD, the median and interquartile range (p25-p75), or a percentage. SLED, sustained low-efficiency dialysis, N.R., normal range.
Among the survivors, those discharged while dependent on RRT exhibited a higher frequency of obesity (52.9% vs. 25.4%, p = 0.03) and higher use of isolated IHD (82.6% vs. 57.5%, p = 0.03) based on the univariate analysis. They showed higher serum creatinine levels (2.95 [1.32–6.97] vs. 1.07 [0.90–1.53] mg/dl, p < 0.001) and lower total leukocyte counts (7,275 [5,25–9,185] vs. 13,050 [6,105–13,242]/mm3, p = 0.04) at admission. (Table 4).
Table 4. Comparison between patients discharged while dependent and not dependent on kidney replacement therapy.
| Discharged without dependence on RRT (N = 80) | Discharged while dependent on RRT (N = 23) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 62.5 (54.1–74.2) | 62.3 (53.4–74.9) | 0.94 |
| Male sex, % (n) | 70.0 (56) | 65.2 (15) | 0.66 |
| Comorbidities | |||
| Hypertension, % (n) | 71.3 (57) | 65.2 (15) | 0.58 |
| Diabetes mellitus, % (n) | 46.3 (37) | 60.9 (14) | 0.22 |
| Obesity, % (n) | 25.4 (17) | 52.9 (9) | 0.03 |
| Heart failure, % (n) | 10.0 (8) | 4.3 (1) | 0.40 |
| Coronary insufficiency, % (n) | 12.5 (10) | 17.4 (4) | 0.57 |
| CKD, % (n) | 17.5 (14) | 8.7 (2) | 0.28 |
| Hospital admission exams | |||
| Hemoglobin (g/dl) (N.R. 12.0–16.0) | 13.0 (11.9–14.3) | 13.8 (11.9–15.3) | 0.25 |
| Total leukocytes (n/mm3) (N.R. 4,000–8,000) | 13.050 (6.105–13.242) | 7.275 (5.025–9.185) | 0.04 |
| Total lymphocytes (n/mm3) (N.R. 1,000–3,900) | 931 (699–1305) | 852 (699–1495) | 0.79 |
| Platelets (103/mm3) (N.R. 150,000–400,000) | 193 (151–272) | 158 (120–245) | 0.11 |
| Serum creatinine (mg/dl) (N.R. 0.6–1.3) | 1.07 (0.9–1.53) | 2.95 (1.32–6.97) | < 0.001 |
| SAPS 3 | 51 (44–61) | 56 (42–69) | 0.70 |
| Severe pulmonary involvement on tomography, % (n) | 45.2 (33) | 42.9 (9) | 0.81 |
| Number of organ dysfunctions | 2 (1–2) | 2 (1–2) | 0.36 |
| Laboratory exams at first RRT indication | |||
| Creatinine (mg/dl) (N.R. 0.6–1.3) | 4.00 (2.85–5.44) | 4.6 (4.2–5.32) | 0.28 |
| Urea (mg/dl) (N.R. 16–40) | 130 (83–198) | 173 (120–243) | 0.05 |
| Potassium (mEq/l) (N.R. 3.5–5.1) | 4.5 (3.9–5.2) | 4.8 (4.2–5.3) | 0.09 |
| Bicarbonate (mEq/l) (N.R. 22–27) | 23.0 (19.7–26.6) | 21.2 (18.8–25.1) | 0.14 |
| Laboratory values during the RRT period | |||
| Creatinine (mg/dl) (N.R. 0.6–1.3) | 2.73 (1.80–4.59) | 3.85 (2.47–5.17) | 0.10 |
| Urea (mg/dl) (N.R. 16–40) | 110 (79–154) | 99 (77–165) | 0.76 |
| Potassium (mEq/l) (N.R. 3.5–5.1) | 4.3 (3.8–4.8) | 4.7 (3.9–5.2) | 0.13 |
| Bicarbonate (mEq/l) (N.R. 22–27) | 24.0 (20.8–27.6) | 23.4 (19.3–26.3) | 0.27 |
| RRT method | |||
| Intermittent, % (n) | 57.5 (46) | 82.6 (19) | 0.03 |
| SLED, % (n) | 7.5 (6) | 0.0 (0) | 0.33 |
| Continuous, % (n) | 11.3 (9) | 4.3 (1) | 0.45 |
| Combination of methods, % (n) | 23.8 (19) | 13.0 (3) | 0.39 |
| Time on RRT (days) | 15 (6–23) | 15 (7–36) | 0.23 |
| Length of hospital stay (days) | 37 (24–47) | 26 (16–45) | 0.06 |
Data are presented as the medians and interquartile ranges (p25-p75) or as percentages; RRT: Kidney replacement therapy; CKD: Chronic kidney disease. SLED, sustained low-efficiency dialysis; N.R., normal range.
The independent factors associated with death in the multiple logistic regression analysis were the number of organ dysfunctions during hospitalization and efficient RRT (Table 5).
Table 5. Multivariate analysis of factors related to death.
| Variable | Adjusted OR (CI 95%) | P value |
|---|---|---|
| Number of organ dysfunctions | 1.52 (1.11–2.09) | 0.009 |
| Efficient RRT | 0.41 (0.25–0.69) | < 0.001 |
Multiple logistic regression with variables adjusted for type of hospital, age, sex, smoking, number of comorbidities, lower tercile of platelets and higher creatinine at admission, use of vasoactive drugs, mechanical ventilation, and serum measurements (potassium < 5 mEq/ml, bicarbonate > 22 mEq/ml, urea > 150 mg/dl, and upper tercile of creatinine). OR: Odds ratio; RRT: Kidney replacement therapy.
Discussion
The results of this multicenter study in the metropolitan region of the megalopolis of São Paulo indicated that AKI-RRT associated with COVID-19 has high lethality and occurs mainly in patients with a high burden of comorbidities and other organ dysfunctions related to the critical state. A higher number of organ dysfunctions increased and efficient RRT decreased the OR for death in the multiple regression analysis. More than one-fifth of the survivors were discharged from the hospital while dependent on RRT, an outcome associated with obesity and a higher creatinine level at hospital admission. To date, this is the largest report related to the epidemiological profile and outcomes of critically ill patients with AKI-RRT and COVID-19 in the Southern Hemisphere.
In our study, the lethality of critically ill patients with COVID-19 and AKI-RRT was similar to that reported in other multicenter international studies (63–79%) [12–15,20]. Unfortunately, few studies have reported the SAPS score (severity), impeding opportunities to compare populations. Nevertheless, the percentages of patients who required mechanical ventilation and vasoactive drugs reported in these studies were similar to those found in the present study (79–97% and 51–92%, respectively). The lower lethality (63%) found in the cohort in the study by Gupta et al. may be partly explained by the lower severity of disease in the patients (79% and 51% required mechanical ventilation and vasopressor use, respectively) [13]. The independent risk factors for death reported in other studies were age, obesity, COPD, coronary artery disease, neoplasia, oliguria on the day of the first indication for RRT, and the need for two or more vasopressors [13,15,16]. In our study, the presence of pulmonary, circulatory, and liver dysfunctions alone and in combination was associated with death.
The present study showed that patients with higher potassium and urea and lower bicarbonate levels during the RRT period had higher mortality rates. These findings may suggest that an insufficient RRT dose affected patient outcomes. Another possible explanation is that more severe disease and hemodynamic instability hindered adequate metabolic control. In fact, in the present study, patients who died received fewer days of RRT and had shorter hospital stays. In previous studies, information on laboratory tests at the beginning of RRT and metabolic control for patients with AKI-RRT and COVID-19 has been scarce or absent. Oliguria at RRT initiation was reported as an independent risk factor for death in patients with these conditions, potentially indicating more severe AKI as well as a delay in the indication for RRT [13]. Unfortunately, the present study was unable to obtain information on the participants’ urine output and fluid balance.
The RRT method was likely not directly related to mortality in the present study or in other studies involving AKI in patients with COVID-19 [15,19,20]. The percentage of patients who underwent continuous RRT in this study was lower than those in other multicenter studies (46–98%) [13,15,16,20], reinforcing the hypothesis that factors other than the RRT method determine the outcomes of critically ill patients with AKI-RRT. In Brazil, continuous RRT is the modality of choice in a few private hospitals and university centers [21,22]. No patient participating in this study was treated with PD, which is consistent with previous reports on RRT methods used more frequently in Latin America. However, studies have shown that PD can be successfully used in adults with AKI, including COVID-19-associated AKI [23–26].
As in our study, other authors reported that COVID-19-associated AKI-RRT occurred more frequently in men (68–83%) and in patients with a high burden of comorbidities (62–70 years of age; HTN, 62–71%; diabetes, 40–53%; obesity, 41–45%; CKD, 26–35%; and coronary artery disease, 13–16%) [12–15,20]. Although the Kidney Disease Improving Global Outcomes guideline [27] includes female sex on the list of susceptibilities for AKI, a higher prevalence of males with severe forms of AKI has been described for patients with and without COVID-19 [2,28–31]. The present study included only patients affected by the first wave of COVID-19 and showed a high burden of comorbidities in this cohort of critically ill patients treated with RRT. During the second wave, although the mean age of affected patients was lower, high percentages of HTN, diabetes, CKD, and HF continued to be reported in individuals with severe forms of the disease [32,33].
In previous studies, among survivors of AKI-RRT associated with COVID-19, the percentage discharged from the hospital while dependent on RRT varied between 30–38% [12–15]. The relatively lower rate of requiring RRT at discharge observed in our study (22%) may be associated with the lower proportion of patients with CKD (19%). In fact, the presence of CKD in patients with and without COVID-19 is the main risk factor for RRT dependence after an AKI-RRT event [14,31].
The limitations of this study are the retrospective design, the lack of data from all patients who met the inclusion criteria, and the lack of information on kidney function recovery after hospital discharge. In addition, considering the regional differences in Brazil and the fact that the global mortality of critically ill patients has a strong association with national income [34], the results presented here may not represent Brazilian ICUs or those in the Southern Hemisphere. In fact, a recent Brazilian study conducted in public hospitals with limited resources showed a mortality rate of 93% for patients with COVID-19 and severe AKI [35].
Conclusion
COVID-19-associated AKI-RRT occurred in patients with an elevated burden of comorbidities and showed high lethality. The number of organ dysfunctions during hospitalization and the efficiency of RRT were independent factors associated with mortality. A significant portion of the survivors were discharged without kidney function recovery.
Supporting information
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Grupo NotreDame Intermédica, Brazil, has provided the financial support for the English review of the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212. doi: 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annigeri RA, Ostermann M, Tolwani A, Vasquez-Rangel A, Ponce D, Bagga A, et al. Renal Support for Acute Kidney Injury in the Developing World. Kidney Int Rep. 2017; 2: 559–578. doi: 10.1016/j.ekir.2017.04.006 [DOI] [Google Scholar]
- 3.Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW. National trends in acute kidney injury requiring dialysis in England between 1998 and 2013. Kidney Int. 2015; 88(5):1161–9. doi: 10.1038/ki.2015.234 [DOI] [PubMed] [Google Scholar]
- 4.Wald R, McArthur E, Adhikari NK, Bagshaw SM, Burns KE, Garg AX, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015; 65(6):870–7. doi: 10.1053/j.ajkd.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 5.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010; 376(9749):1339–46. doi: 10.1016/S0140-6736(10)60446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008; 359:7–20. doi: 10.1056/NEJMoa0802639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anger MS, Mullon C, Ficociello LH, Thompson D, Kraus MA, Newcomb P, et al. Meeting the Demand for Renal Replacement Therapy during the COVID-19 Pandemic: A Manufacturer’s Perspective. KIDNEY360 2021; 2: 350–354. doi: 10.34067/KID.0006192020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Tang Y, Huang Q, Fu S, Li X, Lin B, et al. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrology 2021; 22:52. doi: 10.1186/s12882-021-02244-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin L, Wang X, Ren J, Sun Y, Yu R, Li K, et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open 2020; 10:e042573. doi: 10.1136/bmjopen-2020-042573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabrizi F, Alfieri CM, Cerutti R, Lungui G and Messa P. COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Pathogens 2020; 9 (12): 1052. doi: 10.3390/pathogens9121052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J, et al. Outcomes for Patients With COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Kidney Int Rep 2020. 5:1149–1160; doi: 10.1016/j.ekir.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. Mount Sinai COVID Informatics Center (MSCIC). AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 2021; 32(1):151–160. doi: 10.1681/ASN.2020050615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, et al. AKI Treated with Renal Replacement Therapy in Critically Ill Patients with COVID-19. J Am Soc Nephrol. 2021; 32(1):161–176. doi: 10.1681/ASN.2020060897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, et al. Outcomes Among Patients Hospitalized With COVID-19 and Acute Kidney Injury. Am J Kidney Dis. 2021; 77(2):204–215.e1. doi: 10.1053/j.ajkd.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charytan DM, Parnia S, Khatri M, Petrilli CM, Jones S, Benstein J, et al. Decreasing Incidence of Acute Kidney Injury in Patients with COVID-19 Critical Illness in New York City. Kidney Int Rep. 2021; 6(4):916–927. doi: 10.1016/j.ekir.2021.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens JS, King KL, Robbins-Juarez SY, Khairallah P, Toma K, Alvarado Verduzco H, et al. High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLoS ONE 2020; 15(12):e0244131. doi: 10.1371/journal.pone.0244131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016; 315(8):775–87. doi: 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological Society of North America Expert Consensus Document on Reporting Chest CT Findings Related to COVID-19: Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol Cardiothorac Imaging. 2020; 2(2):e200152. doi: 10.1148/ryct.2020200152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin D and Schenker N. Multiple Imputation for Interval Estimation from Simple Random Samples with Ignorable Nonresponse. Journal of the American Statistical Association, 1986; 81(394): 366–374. doi: 10.2307/2289225 [DOI] [Google Scholar]
- 20.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020; 98(1):209–218. doi: 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash DM, Przech S, Wald R, O’Reilly D. Systematic review and meta-analysis of renal replacement therapy modalities for acute kidney injury in the intensive care unit. J Crit Care. 2017; 41:138–144. doi: 10.1016/j.jcrc.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Lombardi R, Ferreiro A, Claure-Del Granado R, Burdmann EA, Rosa-Diez G, Yu L, et al. EPILAT-IRA Study: A contribution to the understanding of the epidemiology of acute kidney injury in Latin America. PLoS One. 2019;14(11):e0224655. doi: 10.1371/journal.pone.0224655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowes E, Joslin J, Braide-Azikiwe DCB, Tulley C, Bramham K, Saha S, et al. Acute Peritoneal Dialysis with Percutaneous Catheter Insertion for COVID-19-Associated Acute Kidney Injury in Intensive Care: Experience from a UK Tertiary Center. Kidney Int Rep. 2021;6(2):265–271. doi: 10.1016/j.ekir.2020.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sourial MY, Sourial MH, Dalsan R, Graham J, Ross M, Chen W, et al. Urgent Peritoneal Dialysis in Patients With COVID-19 and Acute Kidney Injury: A Single-Center Experience in a Time of Crisis in the United States. Am J Kidney Dis. 2020;76(3):401–406. doi: 10.1053/j.ajkd.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parapiboon W, Ponce D, Cullis B. Acute peritoneal dialysis in COVID-19. Perit Dial Int. 2020;40(4):359–362. doi: 10.1177/0896860820931235 [DOI] [PubMed] [Google Scholar]
- 26.Cullis B, Abdelraheem M, Abrahams G, Balbi A, Cruz DN, Frishberg Y, et al. Peritoneal dialysis for acute kidney injury. Perit Dial Int. 2014;34(5):494–517. doi: 10.3747/pdi.2013.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi: 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 28.Neugarten J, Golestaneh L, Kolhe NV. Sex differences in acute kidney injury requiring dialysis. BMC Nephrol. 2018;19(1):131. doi: 10.1186/s12882-018-0937-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vahidy FS, Pan AP, Ahnstedt H, Munshi Y, Choi HA, Tiruneh Y, et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: Cross-sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16(1):e0245556. doi: 10.1371/journal.pone.0245556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8. doi: 10.1001/jama.294.7.813 [DOI] [PubMed] [Google Scholar]
- 31.De Corte W, Dhondt A, Vanholder R, De Waele J, Decruyenaere J, Sergoyne V, et al. Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: a prospective cohort study. Crit Care. 2016;20(1):256. doi: 10.1186/s13054-016-1409-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iftimie S, López-Azcona AF, Vallverdú I, Hernández-Flix S, de Febrer G, Parra S, et al. First and second waves of coronavirus disease-19: A comparative study in hospitalized patients in Reus, Spain. PLoS One. 2021;16(3):e0248029. doi: 10.1371/journal.pone.0248029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharif N, Opu RR, Ahmed SN, Sarkar MK, Jaheen R, Daullah MU, et al. Prevalence and impact of comorbidities on disease prognosis among patients with COVID-19 in Bangladesh: A nationwide study amid the second wave [published online ahead of print, 2021 Jun 26]. Diabetes Metab Syndr. 2021;15(4):102148. doi: 10.1016/j.dsx.2021.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–6. doi: 10.1016/S2213-2600(14)70061-X [DOI] [PubMed] [Google Scholar]
- 35.Bezerra R, Teles F, Mendonca PB, Damte T, Likaka A, Ferrer-Miranda E, et al. Outcomes of critically ill patients with acute kidney injury in COVID-19 infection: an observational study. Ren Fail. 2021;43(1):911–918. doi: 10.1080/0886022X.2021.1933530 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.