Abstract
Mucus is a slimy hydrogel that lines the mucosal surfaces in our body, including the intestines, stomach, eyes, lungs and urogenital tract. This glycoprotein-rich network is truly the jack of all trades. As a barrier, it lubricates surfaces, protects our cells from physical stress, and selectively allows the passage of nutrients while clearing out pathogens and debris. As a home to our microbiota, it supports a level of microbial diversity that is unattainable with most culture methods. As a reservoir of complex carbohydrate structures called glycans, it plays critical roles in controlling cell adhesion and signaling, and it alters the behavior and spatial distribution of microbes. On top of all this, mucus regulates the passage of sperm during fertilization, heals wounds, helps us smell, and prevents the stomach from digesting itself, to name just a few of its functions. Given these impressive features, it is no wonder that mucus crosses boundaries of species and kingdoms — mucus gels are made by organisms ranging from the simplest metazoans to corals, snails, fish, and frogs. It is also no surprise that mucus is exploited in everyday applications, including foods, cosmetics, and other products relevant to medicine and industry.
From its physical properties to its chemical composition, mucus is a highly effective interface between our epithelia and the world. Yet, the properties most important for mucus’ exquisite qualities are also the most mystifying, particularly with regard to the extent and diversity of glycan structures present on its glycoprotein-rich network. This gap leaves us with many structure–function relationships to uncover and engineering applications to invent. In this Primer, we will take a look at how mucins, the building blocks of mucus, are synthesized, how their expression is regulated and achieved, and how they make crucial contributions to health and disease.
Mucins are a family of polymers with complex glycan modifications that underpin the mucus gel
Although mucus is typically envisioned as the slimy gel lining our noses and digestive tracts, mucin proteins, the components of mucus that are encoded by MUC genes, actually take on a diversity of forms that are expressed in different areas of the body (Figure 1A). In humans, mucins can be membrane-bound, or they can be secreted. Secreted mucins can be gel-forming polymers or can remain as small, non-polymeric glycoproteins. In this section, we will focus on secreted, gel-forming mucins — the kind that are 80% carbohydrate by mass and cover an impressive 185 m2 of surface area in the intestines.
Figure 1. Locations of secreted mucins in the body and their structural diversity.
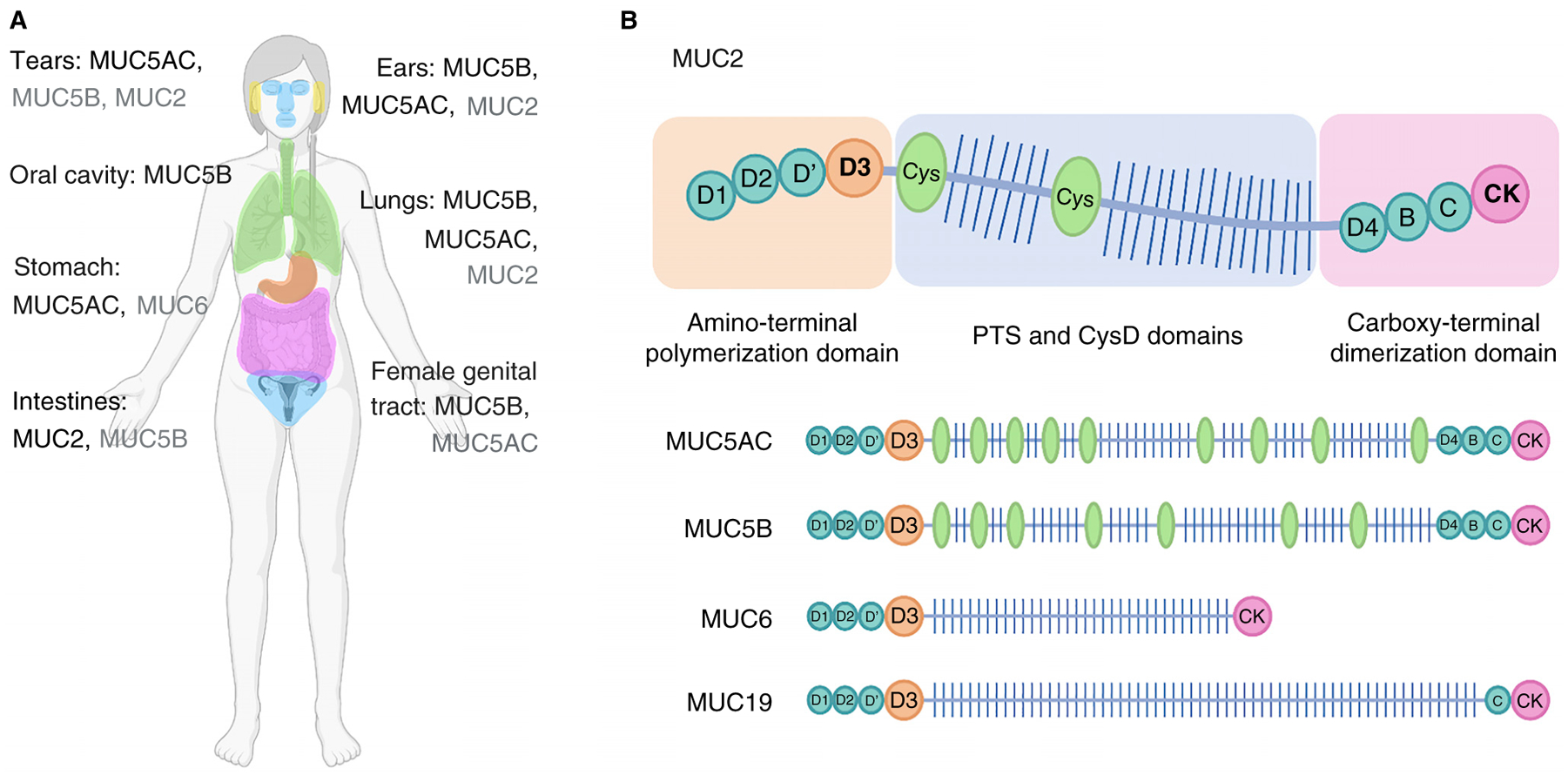
(A) Mucus coats all non-keratinized wet epithelial surfaces. Various MUC genes are expressed throughout the body to form mucin gels that are adapted to various physiological environments. For tissues expressing multiple mucin types, the dominant mucins are listed in black, with non-dominant mucin types in grey. (B) Summary of the features of the gel-forming mucin, MUC2. The cartoon illustrates the domain organization of MUC2 (not drawn to scale), highlighting the vWF-like cysteine-rich domains at the amino and carboxy termini (B, C, and D domains; teal circles), the centrally located, highly O-glycosylated PTS domains (glycans shown as lines), and the internal cysteine-rich domains (CysD; green ovals). The amino- and carboxy-terminal domains are essential for polymer formation. Mucin polymers are stabilized by tail-to-tail disulfide linkages between carboxy-terminal CK domains (pink) and head-to-head disulfide linkages between amino-terminal vWF-like D3 domains (orange circles). (All figures created with BioRender.com.)
Structurally, mucins share several key characteristics. All of them have a protein backbone (or ‘apomucin’ core) with a similar composition of domains. In particular, this backbone is enriched with variable-length, tandemly repeating regions of proline-threonine-serine (PTS) domains, where exposed hydroxyl groups on threonine and serine act as potential sites of O-glycosylation (Figure 1B). PTS domains are responsible for the bottlebrush-like structure of mucins, where densely grafted glycans are displayed radially outward from the protein core. Within these PTS domains, glycan structures are not only abundant but incredibly diverse; individual mucins can harbor > 200 unique glycan structures, leading to vast — and largely mysterious — complexity. As we will discuss later, mucin glycans are responsible for essential mechanical properties but can also act as decoys for pathogens, serve as signals to regulate microbial virulence pathways, modulate immune cell function, divert pathogens and toxins away from cell surfaces, and agglutinate materials for clearance.
Mucins contain cysteine-rich amino- and carboxy-terminal regions flanking their PTS domains that create linear polymers via end-to-end disulfide bonds, enabling mucins to form gels (for example, Figure 1B; although some studies suggest that specific mucins can form branched polymers). Additional small cysteine-rich segments are interspersed among PTS domains with variable numbers of segments among mucin isoforms. After formation, these long polymer chains are secreted from the apical faces of mucosal epithelial cells and, upon hydration, expand dramatically in size to produce the hydrogel barrier.
The identities and orders of the domains in gel-forming mucins have been conserved over hundreds of millions of years. This conservation is especially evident in the amino- and carboxy-terminal cysteine-rich domains involved in covalent polymer formation and assembly. Given that these domains serve as the basis of barrier formation, the physical protective function of mucus has likely been an important contributor to fitness in a diverse range of organisms throughout evolution. By contrast, the PTS regions, which become decorated with glycans, are poorly conserved among species, with respect to both sequence identity and sequence length. There seems to be a general evolutionary trend of increasing the length of PTS domains by introducing more repeats. The length and sequence identity of PTS domains are integral to mucus physical properties as well as its biological activities, but the exact selection mechanisms governing these evolutionary changes are unknown. Nonetheless, these mechanisms may be driven by factors such as fluctuations in the microbial and chemical composition of an organism’s native environment and appear to be coupled to the expansion and proliferation of enzymes involved in O-glycosylation.
Mucin biosynthesis is a complex, energy-intensive process
The biosynthesis of a mucin polymer is carried out in specialized secretory cells that must tackle a variety of challenges such as assembling the polymer, controlling glycosylation, and managing molecules whose fully hydrated dimensions surpass those of some of the intracellular structures in which they are trafficked and stored. Although the general stages and intracellular locations of the polymeric mucin biosynthetic pathway have been described — based mainly on studies of MUC2, MUC5AC, MUC5B, and pig submaxillary mucin (MUC19) — full mechanistic details have yet to be elucidated for any mucin.
Mucins are translated in the rough endoplasmic reticulum (rER) of secretory cells, where membrane-bound ribosomes synthesize peptides that are co-translationally transported into the rER lumen. The rER harbors an abundance of modifying enzymes involved in folding, N-glycosylation, C-mannosylation, and disulfide-bond formation along the nascent protein. Next, individual mucin proteins dimerize through non-covalent twisting of domains downstream of the PTS, followed by formation of disulfide bonds between ‘cysteine knot’ domains at the distal carboxy termini. Finally, the newly formed dimers are exported to the Golgi apparatus.
O-glycosylation occurs in the Golgi. Here, mucins obtain the bottlebrush-like structure that renders them slimy upon hydration. Creation of the glycan chains is a step-wise process where various glycosyltransferases coordinate their initiation, extension, and termination. Initiation of the glycan chains occurs via the addition of N-acetylgalactosamine (GalNAc) to serine or threonine residues by polypeptide GalNAc (ppGalNAc) transferases. As the O-glycosylated mucin dimer moves from the cis-Golgi to later compartments, glycan chains are extended via addition of galactose and N-acetylglucosamine (GlcNAc) residues and then terminated via addition of additional sugars, such as fucose or highly charged sialic acid groups. Terminal sialic acid residues confer a net negative charge, which is important for the rod-like structure and hydration capacity of mucins, among other signature properties. Importantly, these glycosylation steps happen rapidly — mucin dimers contain 4,000 potential glycosylation sites, but they pass through the Golgi in just 2–4 hours (Figure 2A).
Figure 2. Mucins are synthesized, packaged and secreted from specialized cells to form the mucus gel.
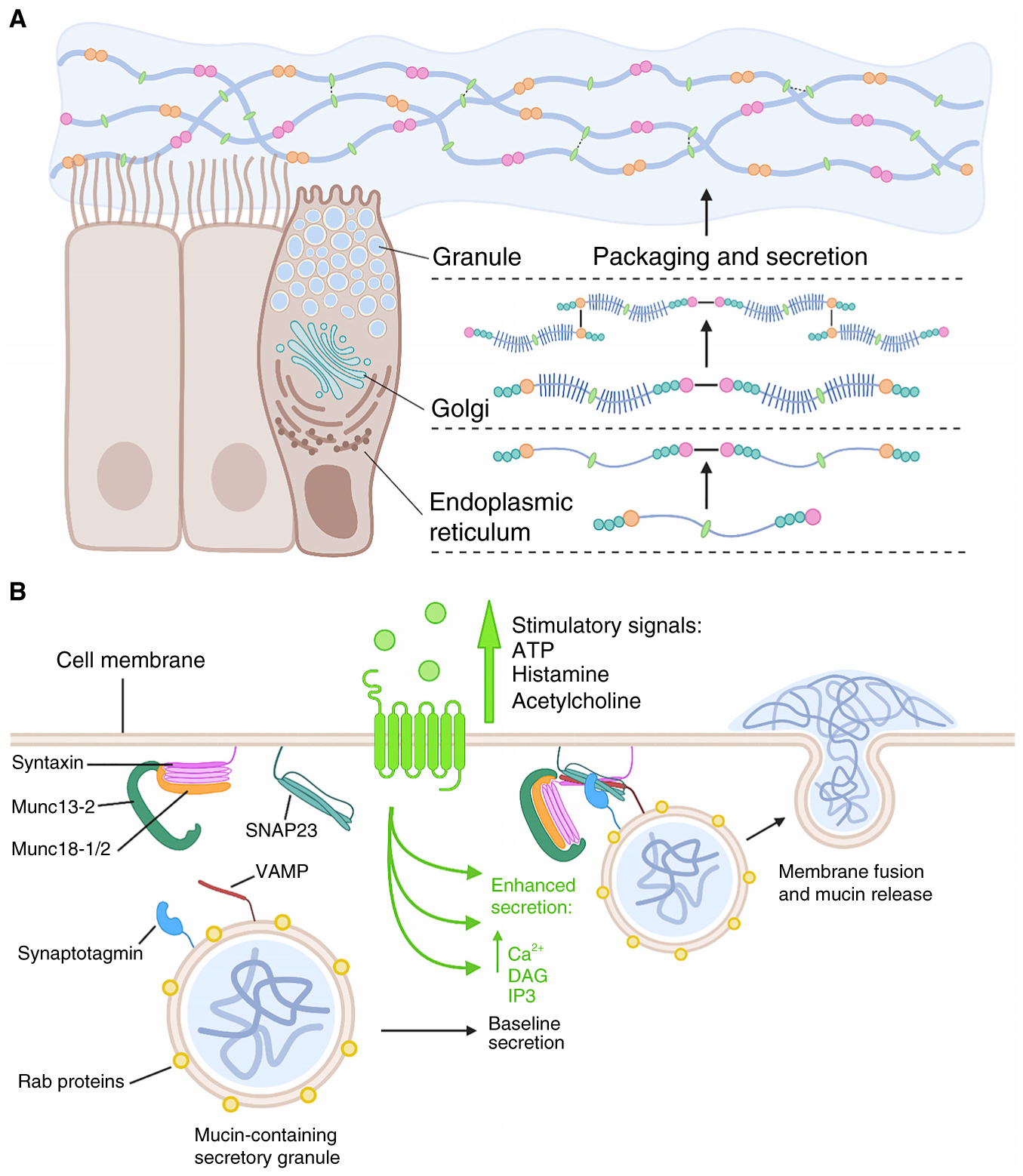
(A) In brief, CK-domain-mediated dimerization of the mucin polypeptide occurs in the endoplasmic reticulum followed by O-glycan addition in the Golgi and then D3-domain-mediated multimerization. Assembled polymers are then compacted for storage in secretory granules via calcium- and pH-dependent mechanisms. After secretion, the polymers hydrate and expand to form a dynamic mucin network that is stabilized by a combination of non-covalent and covalent interactions; the relative contributions of these types of interactions are tissue-dependent, with intestinal mucus containing the highest level of covalent interactions stabilizing its structure. Colors and symbols are as defined in Figure 1. (B) Exocytosis of mucin granules is highly regulated. In the resting state (left), mucin-containing granules decorated with Rab proteins become tethered to the plasma membrane by interacting with Munc13-2 and other tethering proteins. Stimulatory signals (light green) binding to heptahelical receptors lead to the generation of second messengers diacylglycerol (DAG) and inositol triphosphate (IP3). DAG activates Munc13-2 and, together with Munc18, opens syntaxin, which then interacts with the other SNARE proteins SNAP23 and VAMP to form a four-helix-bundle SNARE complex (right). IP3 induces the release of Ca2+ from the ER that in turn activates the calcium sensor synaptotagmin, which promotes further coiling of the SNARE complex. This action drives the fusion of the mucin-containing granule with the plasma membrane and the subsequent release of mucins into the extracellular space. Both baseline and stimulated mucin-granule exocytosis are regulated, as they are dependent on extracellular signals and second messengers.
With most types of protein glycosylation, initiation is controlled by just 1–2 gene products. However, in the case of mucin-type O-glycosylation, initiation can be accomplished by an entire family of at least 15 homologous ppGalNAc transferases. After initiation, the O-GalNAc residues can be lengthened by 30 additional glycosyltransferases, thus extending the glycan structure (Figure 3). Given that these glycosyltransferases have unique recognition motifs and distinct donor and acceptor sugars, differential expression of these enzymes in various cells and tissues means that the same mucin gene product can possess site-specific glycosylation patterns. Beyond being helpful for generating diversity in glycan composition, such a large number of glycosyltransferases may hint at the important biological role of O-glycans within the body. What appears as redundancy may actually provide protection against deleterious mutations that compromise this important protein modification. Although O-glycosylation is not exclusive to mucins, the sheer number of O-glycans present on a single mucin gene product suggests that this process is particularly demanding in mucus-producing cells.
Figure 3. Many glycosyltransferases contribute to the generation of mucin-type O-glycan diversity.
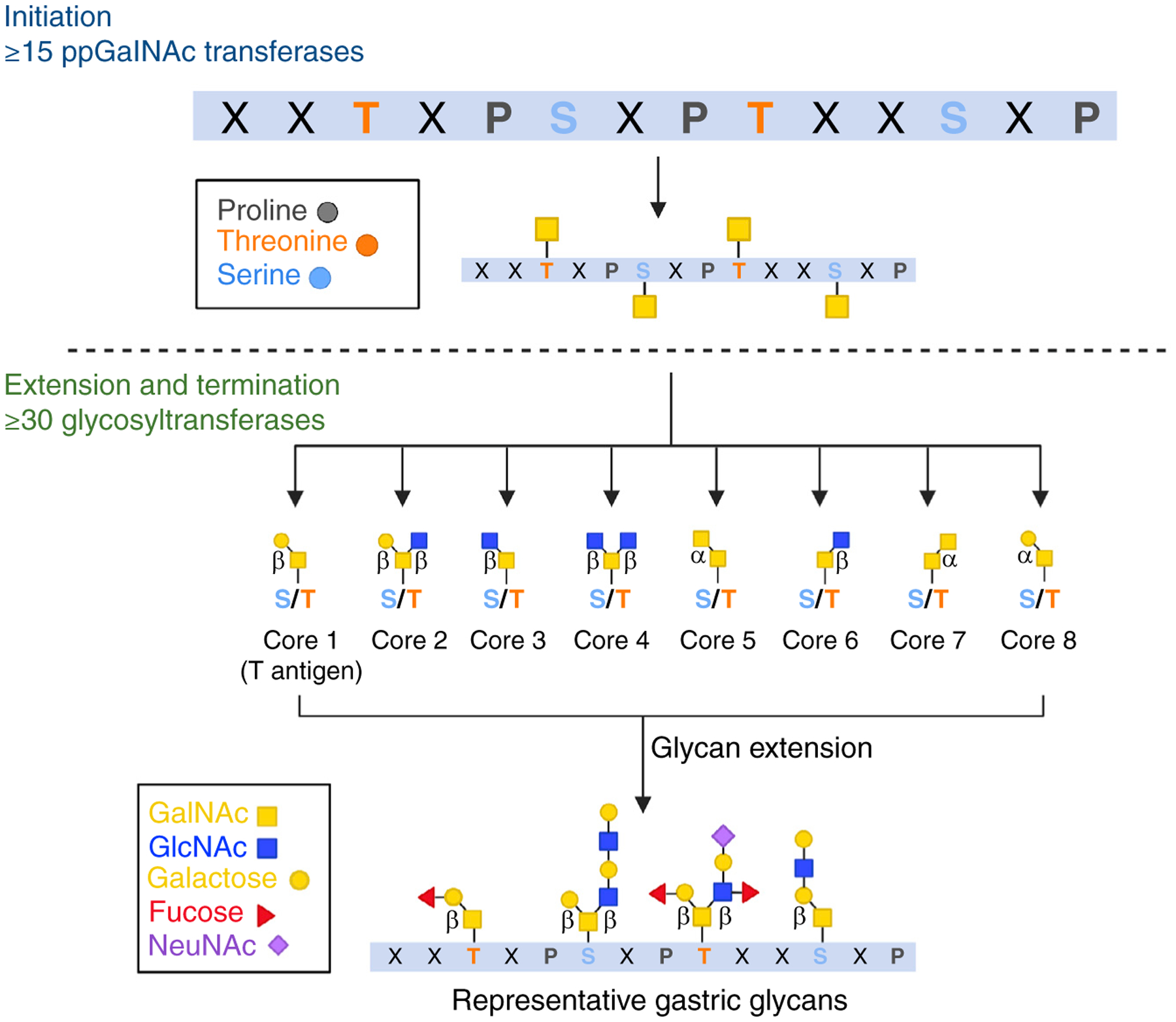
Serine and threonine residues act as potential sites of O-glycosylation within PTS domains. At least 15 ppGalNAc transferases can accomplish the first step, initiation, in which a GalNAc is transferred to a serine or threonine residue. As the O-glycosylated mucin moves through later compartments of the Golgi apparatus, glycan residues are further extended by at least 30 different additional glycosyltransferases with distinct donor and acceptor sugars using both α and β linkages. This combinatorial approach generates a plethora of diverse glycan structures based on eight core scaffolds, with cores 1–4 being the most common. Representative glycan structures present on human gastric mucin are shown.
It has yet to be explored how the glycosylation machinery in mucin-secreting cells differs from that in other cell types, how glycosyltransferases interact with the mucin backbone, and whether distinct enzymes act together in complexes. Further, it is unclear whether polymer size is controlled stochastically or whether it is limited by the spatial constraints of intracellular structures involved in storage and trafficking and/or by temporal constraints related to continuous exocytosis versus stimulated secretory bursts. What is clear is that mucin biosynthesis, particularly O-glycosylation, requires a huge expenditure of energy and resources, including entire families of glycosyltransferases and countless sugars that are used to make thousands of glycan chains per mucin. Thus, the complexity and diversity of glycan structures must be functionally important.
Dedicated signaling pathways regulate mucin expression
Although many mucins seem to have related roles in the body, their production is differentially regulated and their glycosylation signatures are distinct. This points to important functional differences. Unique glycosylation patterns on mucus gels likely mediate the interplay between epithelial cells, the immune system, and microbes (both commensal and pathogenic) in a manner specific to the challenges faced by each mucosal surface. Accordingly, mucin production and secretion are tightly controlled both spatially and temporally.
For example, MUC2 is expressed by surface goblet cells throughout the intestines. In the stomach, MUC5AC is widely expressed by surface and glandular mucous cells, whereas MUC6 is restricted to pyloric gland mucous cells. In nasal and tracheobronchial airways, MUC5B is abundant in submucosal glands but largely absent from surface goblet cells. In healthy airways, MUC5AC is predominantly expressed by surface mucosal cells, and its expression can increase > 50-fold during inflammation.
Epigenetic control has been proposed as one way of regulating the location of mucin expression, presumably by restricting transcription factor accessibility. Emerging evidence in the developing mouse respiratory tract shows that dedicated signaling pathways, namely Notch- and bHLH-dependent signals, coordinate secretory cell fate determination, affecting homeostatic mucus defenses by controlling the production of Muc5b, the murine counterpart of human MUC5B.
In health, there is very little deviance from these distribution patterns. However, the rapid and sometimes dramatic changes in mucin expression and localization in disease have motivated biomedical investigations into mechanisms regulating homeostatic and inducible mucin expression. After injury or inflammation, mucosal surfaces may undergo profound remodeling that is characterized by induction of mucin expression within existing mucous cells or by differentiation of additional epithelial precursors into mucous cells. In acute and chronic airway disease, increased expression of MUC5B and the induction of MUC5AC are driven by these differentiation cues along with inflammatory mediator signals, such as NFκB and STAT6, and growth-factor-response signals such as EGFR, FOXA2/3, and SPEDF.
As mentioned above, the spatial distribution of mucins and patterns of mucin glycosylation are also regulated by the immune system. The recent discovery that infection at one mucosal barrier site — the gut — initiated mucin-mediated host protection to pathogen challenge at a distal mucosal site — the lung — reveals an important innate defense mechanism in which mucins play a key role in protecting against potential infectious challenges encountered at multiple mucosal surfaces. These effects reinforce the need to understand how mucous cell differentiation and mucin biosynthesis are driven by the coordinated efforts of genetic, environmental, and immunological cues.
The heterogeneity of these signaling mechanisms highlights the importance of adapting — and integrating — the responses of mucin-expressing cells to diverse stimuli, as well as the potential for things to go awry and lead to disease. Accordingly, these variable yet tightly controlled expression patterns are also coordinated with expression of genes involved in polymer assembly, glycosylation, and protein homeostasis.
Balance of mucin expression and secretion is determined by location and external signals
Mucus secretion is an explosive event — upon hydration, mucins expand several hundred-fold in volume almost instantaneously, as if they are being released from a pressurized container. It is clear that secretory cells must expertly handle dehydrated mucins on the pathway to secretion. This goal is achieved via a process of regulated exocytosis.
Dehydrated mucins are compacted by shielding the negative charges on the glycans with calcium and by establishing calcium- and pH-dependent, reversible, non-covalent interactions between the cysteine-rich domains of the mucin polymer. After compaction, mucin polymer-containing vesicles are trafficked along the secretory pathway in vesicles decorated with Rab proteins. At the plasma membrane, Rabs interact with tethering proteins on the inner leaflet of the plasma membrane, where they mediate granule docking. In response to specific biological cues, docked granules associate more tightly to and fuse with the plasma membrane, releasing mucins to the epithelial surface, where they instantly become hydrated and balloon in size. This fusion process uses highly conserved molecular machinery comprising a four-helix bundle (the SNARE complex), a Munc18 scaffolding protein, and other regulatory proteins that respond to diverse secondary messengers (Figure 2B).
To protect and prevent the drying of epithelial surfaces, mucins are constantly secreted at low rates. In the lungs, low levels of mucin exocytosis occur in equilibrium with mucin biosynthesis in order to maintain mucociliary clearance and innate defense. In tissues where mucin biosynthesis is robust, exocytosis results in an accumulation of mucus on epithelial surfaces. In the stomach, colon, or endocervix, this accumulation may be protective. In contrast, in the lungs, excessive mucin release can obstruct airflow and negatively impact defensive mucus clearance.
Beyond a baseline level of secretion, mucin exocytosis can be triggered in a controlled manner by various signals, including increased levels of Ca2+, diacylglycerol, ATP, histamine, and acetylcholine. For instance, when stomach epithelial cells experience disruption of their plasma membrane, Ca2+ is released rapidly into the local environment. This signal triggers the rapid release of mucus by stomach mucous cells, which is thought to promote the survival of cells with damaged plasma membranes and confer extra protection and lubrication to sites that endure high amounts of mechanical stress.
The triggers and machinery that regulate mucin exocytosis appear to be similar across tissues, but variations in exocytic machinery isoforms may help tailor secretion rates and magnitudes to local needs. In the lung, for example, some components are shared between the low baseline and the rapidly stimulated secretory pathways (such as SNAP23 and Munc13-2). However, others are distinct (for example, Munc18-1 versus 18-2). Whether these exocytic components are linked selectively with secretion of specific mucin isoforms is still an open question. Overall, the presence of distinct machineries in the airway epithelium enables strategies for selective manipulation of the stimulated secretory machinery to reduce obstruction in diseases such as asthma, cystic fibrosis, and chronic obstructive pulmonary disease, while preserving the baseline secretory machinery to maintain particle clearance.
Mucins assemble into mucus to perform important biological functions
After their secretion, hydrated mucins interact with each other and scores of other proteins and macromolecules. The resulting supramolecular organization is still not completely understood. One key technical limitation is that it is hard to ‘visualize’ native mucus without perturbing its structure, but here are a few high-level insights that we currently understand.
In aqueous solution, high molecular-weight mucin molecules form a network via a complex series of reversible bonds that are sensitive to variations in pH as well as to concentrations of ions and small molecules. Mucus is a viscoelastic material, meaning that its mechanical response to an imposed deformation lies somewhere between that of a pure solid (Hooke’s law of elasticity) and that of a pure liquid (Newton’s law of viscosity). The spinnability of mucus, or its propensity to form filaments when stretched, is an important determinant of physiological functions such as mucociliary clearance, food bolus formation, and swallowing. The extent of cross-linking in the mucin network, the length of the mucin molecules, mucin concentration, and the composition of mucin glycans all impact spinnability.
Crucially, mucus layers possess distinct mechanical and biochemical properties that depend on their location and intended physiological function. Consider that the eye has a thin, watery mucin solution on its surface for hydration and lubrication, while the mucus lining the stomach is a stiffer gel that protects the epithelium against acidic (pH ≈ 1–2) gastric juices. Mechanical and biochemical properties can be altered by changing the degree of physical and chemical cross-linking in the mucin network, modifying mucin conformations through variations in pH and salt, manipulating the types and densities of the mucin glycans to impact gel swelling, and changing mucin concentration. Mucus stiffness, or its ability to resist deformation, varies throughout the body; mucus can rapidly self-heal to recover its initial stiffness after mechanical deformations associated with processes such as coughing.
Mucus barriers are selectively permeable
The cross-linked network of mucin polymers establishes a selectively sticky, web-like filter that — together with other mucosal components such as water, salts, lipids, nucleic acids, and proteins — enables mucus to regulate the diffusion of microorganisms, particulates, and small molecules. Large particles are filtered on the basis of size by the cross-linked mucus mesh, whereas passage of molecules smaller than the pore size are carefully controlled through physical binding interactions by charged and hydrophobic residues on mucin polymers.
There are multiple molecular features that play a role in mucus binding and hence diffusive penetration through mucus barriers, but it is difficult to predict how a molecule will behave in mucosal environments based only on its structure. In PTS regions, mucins have a net negative charge due to the abundance of carboxyl and sulfate groups present on O-glycans. Other anionic compounds such as DNA, glycosaminoglycans, and shed epithelial cells are also present in mucus and play a role in binding interactions. Thus, positive charge on a molecule is generally associated with tighter binding to mucus and decreased mucopenetration. However, net charge does not necessarily predict binding. Molecules with the same net positive charge have been shown to interact differently with mucus, suggesting that the magnitude of charge as well as the density and spatial arrangement of charged moieties are important contributors to this interaction. Likewise, there are also hydrophobic domains present in mucus that mediate self-assembly of the polymer network and can bind to hydrophobic moieties on molecules. Similarly, the spatial location of double bonds, aromatic groups, and proximal charges on a molecule all likely mediate interactions with hydrophobic components of mucus.
Improved understanding of the mechanisms that determine adhesion to the mucus barrier will be critical for the design of particles with specific transport rates through mucus, including for drug delivery. In general, there is a tradeoff between enrichment and longevity in the mucus layer, as molecules that bind and become enriched in mucus layers will also be shed more rapidly along with the outer mucus layer. By contrast, mucus-inert particles can penetrate deeper into the mucus barrier and will clear more slowly, but will not become enriched (Figure 4). Although most drug-delivery applications (particularly in sites of rapid mucus turnover) have sought to discourage mucus binding, interactions with mucin and other mucus components may be beneficial if temporary enrichment within the mucus layer is desired. For instance, polymer scaffolds, films, or patches that are mucoadhesive can be used to direct drug release to the desired site of absorption in the body. This strategy has been used to treat conditions such as corneal inflammation of the eye, stomach ulcers, vaginitis, and periodontal disease.
Figure 4. Molecular properties influence enrichment and longevity within mucus layers.
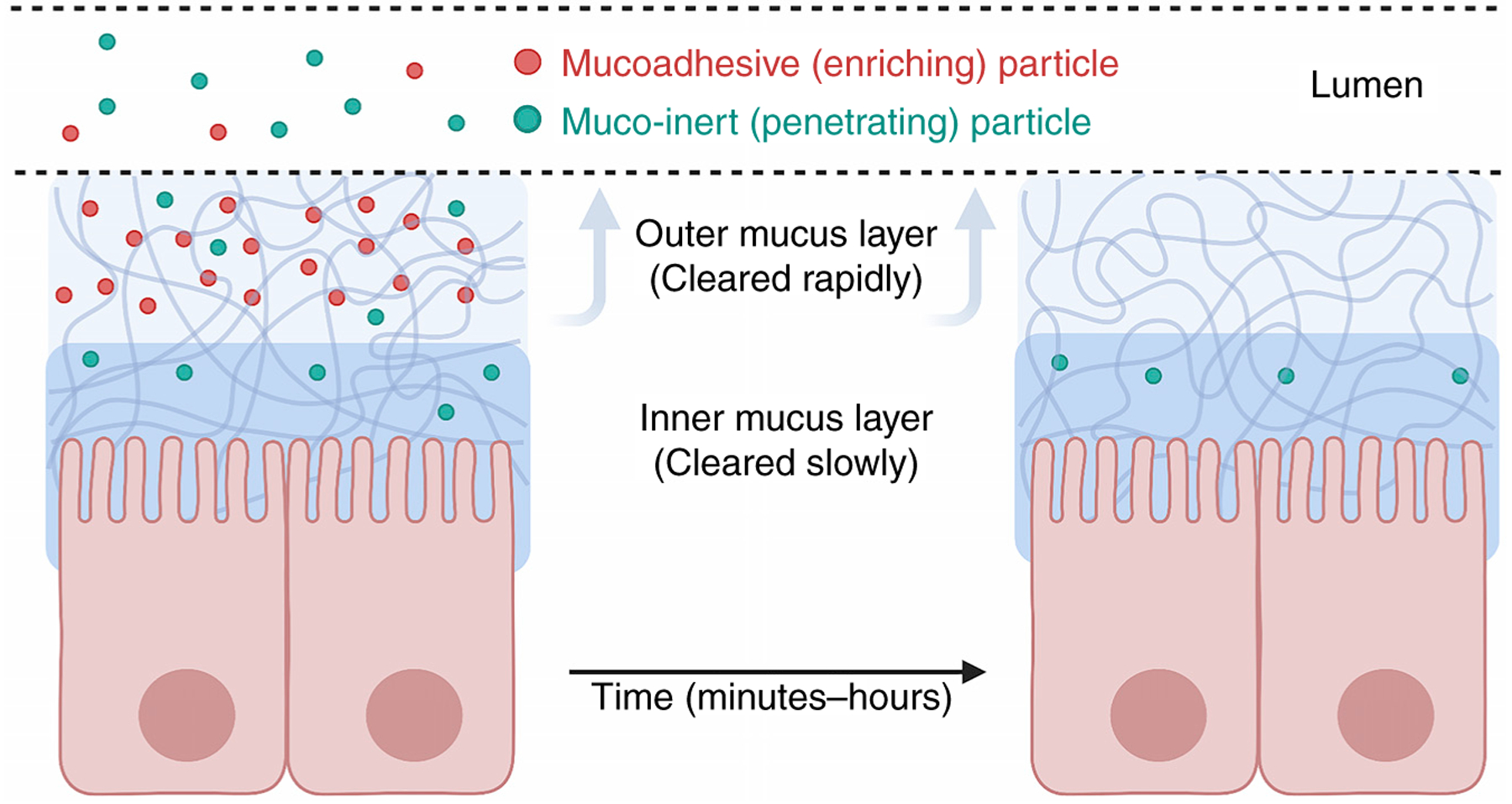
Particles with concentrated regions of positive charge or hydrophobicity can bind to and become enriched in mucus layers (left, red dots). However, binding to mucus leads to faster clearance from the body due to sloughing off of the outer mucus layer. Particles that do not bind to mucus do not become enriched in mucus layers but are able to penetrate deeper and are not cleared as quickly from the body (right, blue dots). Both mucoadhesive and muco-inert particles present advantages and tradeoffs in terms of drug design and delivery. (This figure was adapted from Lai et al. (2009).)
Mucus selects for and houses the microbiota
Mucus serves as a major ecological niche, a function that is conserved throughout evolution. Surface mucus layers first arose in some of the simplest animals in existence, including corals, anemones, and jellyfish (Cnidarians and Ctenophores). Homologs of gel-forming mucins have been found in comb jellies, one of the most distant animal relatives of humans, indicating that gel-forming mucins have existed for nearly as long as multicellular animals.
In aquatic environments, marine organisms are bombarded with environmental bacteria. As a result, sponges (Porifera), which are not considered to have a functional surface mucosa, can attribute up to 40–50% of their wet mass to bacterial cells. By contrast, mucus-producing Cnidarians and Ctenophores have lower bacterial loads than sponges, suggesting that mucus helps restrict bacterial colonization.
Beyond simply preventing bacterial overgrowth, mucus seems to be an important factor in selecting for a core microbiota. Corals, which can dedicate up to 40% of their daily carbon fixation to mucus production, have an ability to house a microbiota within mucus such that the microbial community is unique to each coral species and distinct from the local environment. Remarkably, mucus from the squid Euprymna scolopes helps specifically select for the bioluminescent symbiont Vibrio fischeri within an environmental context that contains ~106 non-symbiotic bacteria.
It is no surprise that mucus also plays a role in shaping microbiota composition in humans, where it accommodates an incredible 100 trillion microbes at extreme densities approaching 1011–1012 cells/mL. Similar to what is observed in aquatic organisms, mucus in humans plays a dual role by protecting epithelia from dangerous microbes while accommodating beneficial ones. In addition to acting as a physical barrier that can aggregate and clear away bacteria, it is becoming increasingly clear that mucus employs several mechanisms to actively regulate the microbiota.
On one hand, mucosal environments are thought to support diverse microbial communities through promoting the coexistence of metabolically distinct species. Mucins provide nutrition for some mucus-dwelling microbes, perhaps contributing to the selection of commensal microbes and supporting a beneficial and diverse microbiota. Given that microbes contain unique metabolic capabilities, modulating mucin glycosylation levels and profiles could establish niche-specific communities.
On the other hand, mucus may harbor signaling molecules that trigger changes in microbial gene expression and phenotypes. Mucosal environments seem to discourage competitive behaviors among microbes. For example, MUC5B appears to promote cooperation of the normally competing species Streptococcus sanguinis and Streptococcus mutans by promoting a harmless planktonic state rather than the more insidious biofilm mode of growth. Further, mucins, and in some cases mucin glycans, have been shown to reduce harmful behaviors in opportunistic pathogens, such as in Candida albicans and Pseudomonas aeruginosa, thus enabling them to coexist as part of a stable microbiota. Therefore, mucosal environments seem to regulate the microbiota by reducing competition, both among microbes and between microbes and their host.
Interestingly, mucus also spatially organizes populations of microbes; various theoretical and experimental models have demonstrated that a heterogeneous spatial structure supports diverse bacterial populations. Some microbes bind mucins, which could allow for their positive selection and resistance to displacement. Mucin could also bind secreted bacterial products and alter the diffusion of metabolism by-products or small molecules involved in cell–cell communication. Further characterizing the spatial structure of bacterial communities within mucus may reveal fascinating insights into how the 3D mesh-like network of mucus controls microbiota composition.
Mucus and disease
Together, our emerging insights into the diverse properties, structures, and functions of mucus underscore this hydrogel’s multipronged roles in promoting health and preventing disease. Changes in the permeability, glycosylation profiles, and charge distributions of mucus barriers have been linked to altered physiology, resulting in devastating pulmonary, gastrointestinal, and urogenital diseases, among other ailments (Figure 5).
Figure 5. Healthy mucus protects against barrier dysfunction and disease.
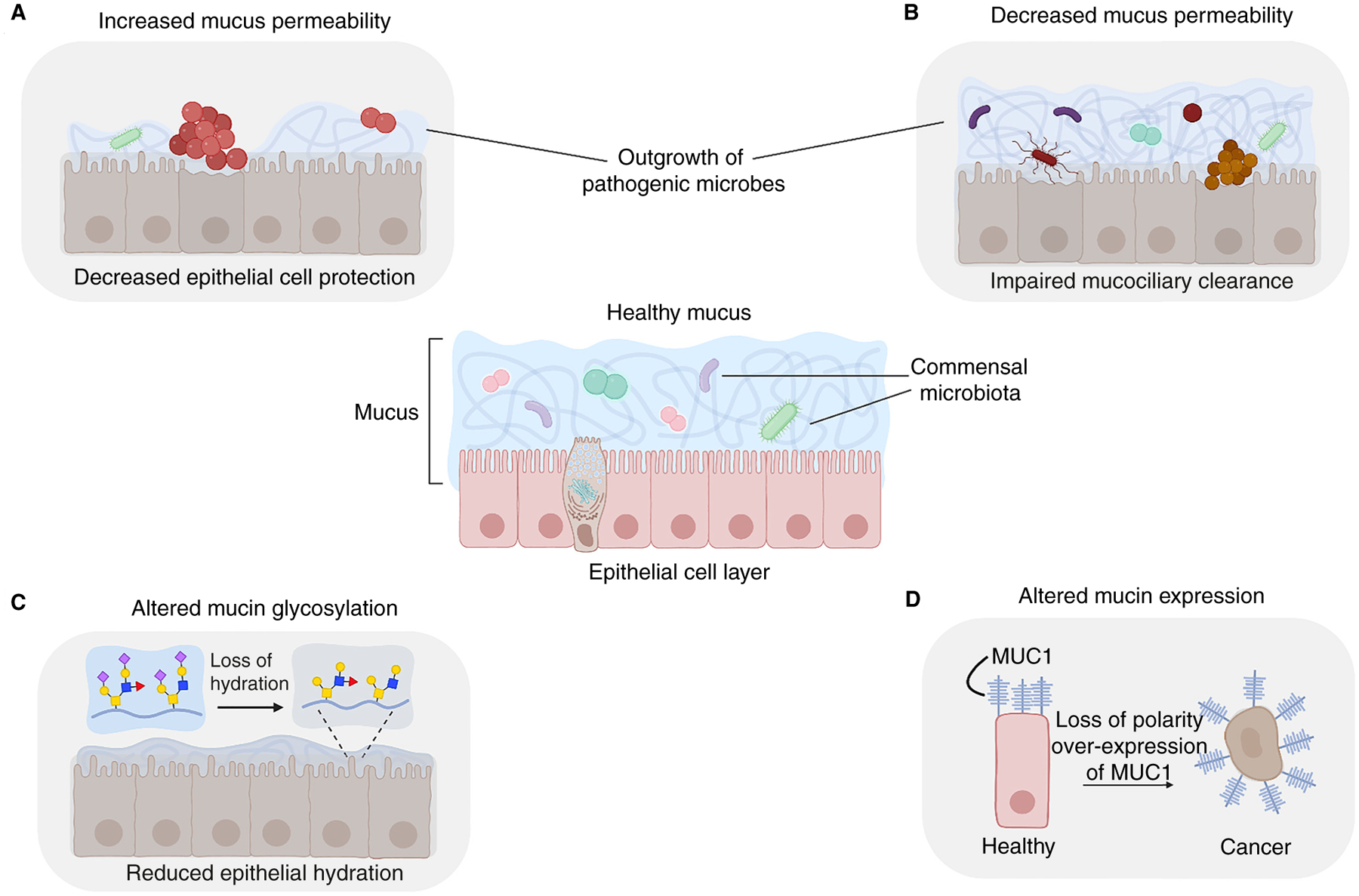
Healthy mucus (center) protects epithelia by acting as a dynamic barrier that provides hydration, regulates diffusion and accommodates commensal microbes. Changes in mucin expression and identity are associated with disease. (A) Increased mucus permeability in various tissues potentially allows the outgrowth of pathogenic microbes leading to intra-amniotic infections and stomach ulcers. (B) Decreased mucus permeability impairs mucociliary clearance, leading to increased microbial colonization, as observed in cystic fibrosis and chronic obstructive pulmonary disease. (C) Altered mucin glycosylation is associated with various diseases, including Sjögren’s syndrome, cancer, and cystic fibrosis. Loss of sialylated glycans in Sjögren’s syndrome reduces the hydrophilicity of mucin glycans, causing dry mouth. (D) Altered mucin expression can lead to cancer. Membrane-associated MUC1 promotes the survival of epithelial cells, but MUC1 overexpression can lead to aberrant regulation of growth factor signaling.
In several diseases, compromised selectivity of the mucosal layer results in serious physiological consequences. The cervical mucus of women at high risk for preterm birth is more permeable to peptide probes than samples from low-risk pregnant counterparts. This finding presents a possible connection between increased rates of intra-amniotic infection observed in cases of preterm birth and increased permeability of the cervical mucus plug to microbes. In general, healthy mucus layers serve as protective barriers against harmful microbes. However, certain pathogens are able to degrade or alter the rheological properties of the mucus barrier to facilitate penetration. Helicobacter pylori, a causative agent of peptic ulcers and gastric cancer, modifies the properties of mucus in the stomach by locally increasing pH, decreasing the viscoelastic properties of mucus and enabling the pathogen to swim more easily through it.
On the other hand, mucus barriers that are too impermeable present challenges as well. In addition to causing poorly cleared mucus to act as an incubator for colonized microbes, dense mucus has lower permeability, which impairs drug delivery to the lung in cystic fibrosis. Important causes of these defects include hyper-concentration of mucus components, increased mucin cross-linking, and calcium-mediated compaction due to impaired bicarbonate secretion. Patients with chronic obstructive pulmonary disease also hypersecrete mucus, causing a decline in lung performance due to decreased airflow, chronic infection, and poor gas exchange.
Other diseases are linked to mucus with altered glycosylation profiles. For example, Sjögren’s syndrome is an autoimmune disorder leading to chronic dry mouth and eyes that is associated with problems swallowing and tasting, as well as increased rates of tooth decay and yeast infections. Patients with this disorder have decreased residual saliva production and flow rate. Although the overall salivary concentrations of MUC5B and MUC7 (a non-polymeric secreted mucin) did not differ between healthy controls and Sjögren’s patients, MUC7 O-glycans in Sjögren’s patients have a significant reduction in hydrophilic sialic acid groups, resulting in dryness.
Over the past 20 years, research has revealed that changes in secreted and membrane-bound mucins are linked to cancer progression and carcinogenesis. Compromised MUC2 barriers in the intestine, as observed with ulcerative colitis, are associated with genotoxic stress and chronic-inflammation-induced cancer progression. Further, MUC1, a transmembrane mucin, evolved as an anti-inflammatory protective mechanism for epithelial cells; however, its role in promoting the proliferation and survival of epithelial cells is exploited in cancer. Mucins are typically only present on the apical surface, but when epithelial cells lose polarity during carcinogenesis, mucins can become expressed all over the cell surface, meaning that they become much more available to interact with various growth factor receptors involved in cancer signaling. Consequently, 65% of tumors diagnosed in the United States each year aberrantly express MUC1. Another membrane-bound mucin that is upregulated in certain cancers, MUC4, is correlated with resistance to antibody treatment and evasion of the immune system, and also appears to contribute to growth, motility, and invasiveness of carcinoma cells. Further, ovarian, lung, breast, and pancreatic cancers are associated with aberrant expression of the membrane-bound mucin MUC16. Together, these examples point to mucins as promising anticancer drug targets.
Mucosal defects and aberrant expression of mucin genes (both membrane-bound and secreted mucins) have been linked to many other ailments, including but not limited to infertility, endometriosis, kidney disease, and Chrohn’s disease. This wide variety of conditions underscores the potential of non-invasive, mucin-based diagnostics for disease. It also suggests that beyond harboring biomarkers of disease states, mucus and its component mucins can be directly involved in disease pathology.
Outlook
The ability of mucus to achieve such a wide variety of functions is due to the complexity that underpins mucin structure and the abilities of mucus gels to adapt their properties to diverse environments and needs. The more we learn about the importance of mucus, the more new and exciting areas of research emerge. For example, the identity and arrangement of mucin glycans have been shown to be essential for activity, but it is unknown how these properties are regulated. Similarly, the ability of mucin to signal to pathogens and alter physiology suggests that there must be other relationships between mucin and mucus-resident cells, such as immune cells and commensal bacteria, yet to be discovered. Expanding our understanding of how mucin functions in health will enable us to better understand and treat disease states associated with mucus dysfunction. Moreover, research on mucin structure will uncover its properties with potential applications in biomedical engineering, such as wound healing and infection prevention. These exciting discoveries will shift the textbook model of mucus from one in which mucus serves solely as a barrier to one that encompasses its additional, multifaceted and vital biochemical functions.
FURTHER READING
- Bakshani CR, Morales-Garcia AL, Althaus M, Wilcox MD, Pearson JP, Bythell JC, and Burgess JG (2018). Evolutionary conservation of the antimicrobial function of mucus: a first defence against infection. NPJ Biofilms Microbiomes 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansil R, and Turner BS (2018). The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev 124, 3–15. [DOI] [PubMed] [Google Scholar]
- Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, and Tabak LA (2012). Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC (2019). Muco-obstructive lung diseases. N. Engl. J. Med 380, 1941–1953. [DOI] [PubMed] [Google Scholar]
- Campbell L, Hepworth MR, Whittingham-Dowd J, Thompson S, Bancroft AJ, Hayes KS, Shaw TN, Dickey BF, Flamar A-L, Artiset E, et al. (2019). ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. J. Exp. Med 216, 2714–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AM, Azzegagh Z, Tuvim MJ, and Dickey BF (2018). Airway mucin secretion. Ann. Am. Thorac. Soc 15, S164–S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe DW (2010). Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer 9, 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang Y-Y, and Hanes J (2009). Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev 61, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Hansson GC, and Samuelsson T (2007). Gel-forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. USA 104, 16209–16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Tanaka T, and McNeil PL (2006). Disruption-induced mucus secretion: repair and protection. PLoS Biol. 4, e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, et al. (2014). Muc5b is required for airway defence. Nature 505, 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P (2012). Supramolecular dynamics of mucus. Cold Spring Harb. Perspect. Med 2, a009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CE, Wheeler KM, and Ribbeck K (2018). Mucins and their role in shaping the functions of mucus barriers. Annu. Rev. Cell Dev. Biol 34, 189–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler KM, Cárcamo-Oyarce G, Turner BS, Dellos-Nolan S, Co JY, Lehoux S, Cummings RD, Wozniak DJ, and Ribbeck K (2019). Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol 4, 2146–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten J, Samad T, and Ribbeck K (2018). Selective permeability of mucus barriers. Curr. Opin. Biotechnol 52, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]


