Abstract
Background and Objectives
Despite detection of autoantibodies, anti-IgLON5 disease was historically considered a tau-associated neurodegenerative disease, with limited treatment options and detrimental consequences for the patients. Observations in increasing case numbers hint toward underlying inflammatory mechanisms that, early detection provided, open a valuable window of opportunity for therapeutic intervention. We aimed to further substantiate this view by studying the CSF of patients with anti-IgLON5.
Methods
We identified 11 patients with anti-IgLON5 from our database and compared clinical, MRI, and CSF findings with a cohort of 20 patients with progressive supranuclear palsy (PSP) (as a noninflammatory tauopathy) and 22 patients with functional neurologic disorder.
Results
Patients with anti-IgLON5 show inflammatory changes in routine CSF analysis, an increase in B-lymphocyte frequency, and the presence of plasma cells in comparison to the PSP-control group and functional neurologic disease controls. Patients with intrathecal plasma cells showed a clinical response to rituximab.
Discussion
Our findings indicate the importance of inflammatory mechanisms, in particular in early and acute anti-IgLON5 cases, which may support the use of immune-suppressive treatments in these cases. The main limitation of the study is the small number of cases due to the rarity of the disease.
Anti-IgLON5 disease is a multifaceted and heterogeneous disease presenting with sleep disorder, bulbar dysfunction, ocular symptoms, movement disorder, and cognitive dysfunction, defined by the presence of antibodies against the neuronal cell adhesion protein IgLON5.1 Postmortem studies in 6 cases showed evidence of neuronal accumulation of hyperphosphorylated tau but no inflammatory changes.2 Thus, it shares histopathologic features with neurodegenerative forms of tau pathology, including progressive supranuclear palsy (PSP).
Despite the absence of inflammatory changes in pathologic specimens, there is evidence of protein elevation with no signs of oligoclonal bands (OCBs) in patients with anti-IgLON5 disease3; however, detailed cellular CSF analyses are lacking. We characterized 11 patients with anti-IgLON5 disease combining clinical parameters and routine CSF analysis including detailed CSF flow cytometry and compare them with patients having PSP and a control group with functional neurologic disorders.
Methods
We retrospectively screened our clinical database for patients with anti-IgLON5 disease (eFigure 1, links.lww.com/NXI/A690) and age matched them with patients diagnosed with PSP, in whom standard and flow cytometric CSF data were collected during routine clinical differential diagnostic processes following standardized procedures (eMethods, http://links.lww.com/NXI/A690). IgLON5 antibodies in serum or CSF were detected by EUROIMMUN commercial kit. CSF and blood samples were analyzed as described previously.4 Flow cytometric data of patients with anti-IgLON5 disease and PSP were compared with an age-matched control group of functional neurologic disorders without any signs of inflammatory or epileptic CNS disorder.
Standard Protocol Approvals, Registrations, and Patient Consents
Patients gave written informed consent for the use of the clinical data as part of research projects. Ethics approval was given by the ethics committee of the Medical Faculty of the University of Münster, Germany (AZ 2013 350-f-S).
Data Availability
Data are available from the corresponding author on reasonable request.
Results
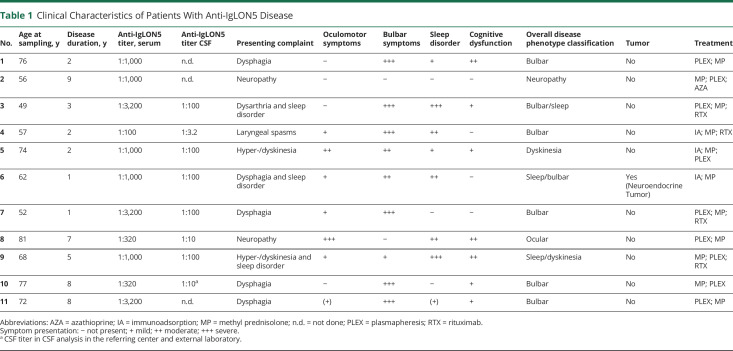
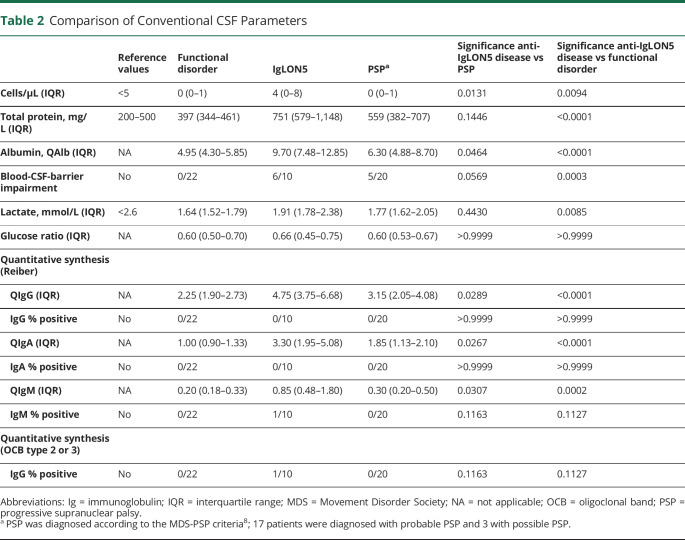
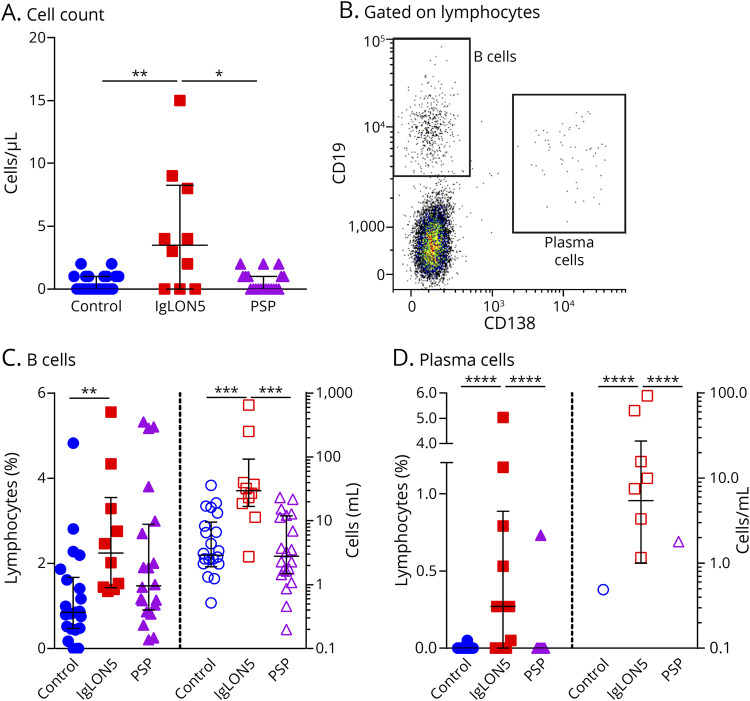
Eleven patients with anti-IgLON5 disease were identified (Table 1), CSF analyses including immune profiling by flow cytometry, were available in 10. The clinical phenotype was in line with the previous literature1 with a predominance of a bulbar and sleep disorder–related phenotype. In only 1 case, we found a metastasis of a neuroendocrine tumor with low-grade of differentiation and unknown primary 2 years after diagnosis of anti-IgLON5 disease. All cases were therapy naive at the time of CSF analysis—except for 1 treated with azathioprine and 1 with immunoadsorption and steroid treatment 6 weeks before CSF sampling. Four patients later on received second-line treatment with rituximab (RTX) resulting in stabilization of symptoms. Compared with 20 patients with PSP, who were matched in age at presentation and onset of disease, as well as clinical severity measured with the mRS, brain atrophy was seen in both groups, but specific midbrain atrophy was restricted to PSP cases (eTable 1, links.lww.com/NXI/A690). In comparison to patients with functional disorders and PSP cases, patients with IgLON5 exhibited increased total protein levels compared with patients with a functional disorder (Table 2). Six of 10 patients displayed blood-CSF-barrier dysfunction indicated by CSF/serum albumin quotient, with 1 patient showing an intrathecal immunoglobulin G and another one an increased immunoglobulin M synthesis. Three of 10 patients with anti-IgLON5 showed a mild pleocytosis (Table 2; Figure 1A). Immune profiling of CSF cells4 revealed increased frequencies of B lymphocytes and occurrence of plasma cells (Figure 1, B–D) suggesting a B cell–related pathology, whereas other immune cell subtypes were not specifically affected (eFigures 2–4, CSF, 5–7 blood). Four patients with increased CSF plasma cells at initial presentation received treatment with RTX later on in the disease course, which resulted in clinical stabilization of the disease.
Table 1.
Clinical Characteristics of Patients With Anti-IgLON5 Disease
Table 2.
Comparison of Conventional CSF Parameters
Figure 1. CSF Findings in Anti-IgLON5 Disease.
(A) Cell count in CSF samples obtained from 22 controls without any sign of inflammatory CNS disorders (blue circles), 10 IgLON5 patients (red squares) and 20 PSP patients (violet triangles) as determined by Fuchs-Rosenthal chamber. (B) Representative dot plot from flow-cytometric analysis of CSF used to quantify CD19highCD138neg B cells and CD19lowCD138high plasma cells. (C, D) Relative (left, closed symbols) and total (right, open symbols) levels of B cells (C) and plasma cells (D) in the CSF. Error bars indicate the median and interquartile range. Statistical analysis was done by Kruskal-Wallis test with Dunn's post-test, *p < 0.05. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
Our cohort of patients with anti-IgLON5 showed signs of inflammatory processes indicated by molecular markers and significant changes in the B-cell compartment with detection of plasma cells in some patients. These changes were not observed in patients having PSP. This observation suggests that the presence of an antibody is crucial in the disease process as suggested in in vitro studies.5,6 Comparable CSF studies in anti-IgLON5 disease are limited. A recent meta-analysis of routine CSF findings suggested that in anti-IgON5 disease, high levels of protein were observed in the absence of pleocytosis and OCBs.3 We were able to corroborate high protein levels and absence of OCBs in most patients. In our cohort, significant CSF pleocytosis was found in a subgroup of 3 patients. Second-line treatment with RTX was effective in halting disease progression as has been suggested in a previous review.7
Overall, our study illustrates that patients with anti-IgLON5 disease have inflammatory changes in the CSF. Therefore, the use of immune-modulatory therapies might be considered in these cases, but further studies are required to issue a general recommendation.
Appendix. Authors
Contributor Information
Christine Strippel, Email: christine.strippel@ukmuenster.de.
Anna Heidbreder, Email: anna.heidbreder@i-med.ac.at.
Andreas Schulte-Mecklenbeck, Email: andreas.schulte-mecklenbeck@ukmuenster.de.
Lisanne Korn, Email: lisanne.korn@ukmuenster.de.
Tobias Warnecke, Email: tobias.warnecke@ukmuenster.de.
Nico Melzer, Email: nico.melzer@med.uni-duesseldorf.de.
Heinz Wiendl, Email: heinz.wiendl@ukmuenster.de.
Matthias Pawlowski, Email: matthias.pawlowski@ukmuenster.de.
Catharina C. Gross, Email: catharina.gross@ukmuenster.de.
Study Funding
No targeted funding reported.
Disclosure
The authors declare no conflict of interests. Go to Neurology.org/NN for full disclosures.
References
- 1.Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13(6):575-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelpi E, Höftberger R, Graus F, et al. Neuropathological criteria of anti-IgLON5-related tauopathy. Acta Neuropathol. 2016;132(4):531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blinder T, Lewerenz J. Cerebrospinal fluid findings in patients with autoimmune encephalitis—a systematic analysis. Front Neurol. 2019;10:804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross CC, Schulte-Mecklenbeck A, Madireddy L, et al. Classification of neurological diseases using multi-dimensional cerebrospinal fluid analysis. Brain J Neurol. 2021;144(9):2625-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landa J, Gaig C, Plagumà J, et al. Effects of IgLON5 antibodies on neuronal cytoskeleton: a link between autoimmunity and neurodegeneration. Ann Neurol. 2020;88(5):1023-1027. [DOI] [PubMed] [Google Scholar]
- 6.Ryding M, Gamre M, Nissen MS, et al. Neurodegeneration induced by anti-IgLON5 antibodies studied in induced pluripotent stem cell-derived human neurons. Cells. 2021;10(4):837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabezudo-García P, Mena-Vázquez N, Estivill Torrús G, Serrano-Castro P. Response to immunotherapy in anti-IgLON5 disease: a systematic review. Acta Neurol Scand. 2020;141(4):263-270. [DOI] [PubMed] [Google Scholar]
- 8.Hoglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the Movement Disorder Society Criteria. Mov Disord. 2017;32(6):853-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author on reasonable request.