Abstract
Prospective population studies suggest that psychotic syndromes may be an emergent phenomenon—a function of severity and complexity of more common mental health presentations and their nonpsychotic symptoms. Examining the relationship between nonpsychotic and subthreshold psychotic symptoms in individuals who later developed the ultimate outcome of interest, a first episode of psychosis (FEP), could provide valuable data to support or refute this conceptualization of how psychosis develops. We therefore conducted a detailed follow-back study consisting of semistructured interviews with 430 patients and families supplemented by chart reviews in a catchment-based sample of affective and nonaffective FEP. The onset and sequence of 27 pre-onset nonpsychotic (NPS) or subthreshold psychotic (STPS) symptoms was systematically characterized. Differences in proportions were analyzed with z-tests, and correlations were assessed with negative binomial regressions. Both the first psychiatric symptom (86.24% NPS) and the first prodromal symptom (66.51% NPS) were more likely to be NPS than STPS. Patients reporting pre-onset STPS had proportionally more of each NPS than did those without pre-onset STPS. Finally, there was a strong positive correlation between NPS counts (reflecting complexity) and STPS counts (β = 0.34, 95% CI [0.31, 0.38], P < 2 e-16). Prior to a FEP, NPS precede STPS, and greater complexity of NPS is associated with the presence and frequency of STPS. These findings complement recent arguments that the emergence of psychotic illness is better conceptualized as part of a continuum—with implications for understanding pluripotential developmental trajectories and strengthening early intervention paradigms.
Keywords: psychosis, first-episode psychosis, subthreshold psychotic symptoms, at-risk mental state, psychopathology, trajectory
Introduction
The use of the term schizophrenia, rooted in Kraepelin’s description of dementia praecox in the early 20th century, has become increasingly controversial.1 Some have called for abolishing what is seen as an imprecise and even harmful diagnosis.2 Others, however, have continued to advocate that schizophrenia and related psychoses should remain a category distinct from nonpsychotic disorders,3–5 as seen in both DSM-IV6 and DSM-5.7,8 Even some recent attempts to advance the construct by adding dimensions of severity and functional impairment frame the earliest stages of psychosis as “light” or attenuated versions of the prototypical condition,9,10 thereby inadvertently propagating the notion that the range of psychotic phenomena—from transient experiences to threshold-level disorders—together represents a discrete entity.
Yet there is also accumulating evidence that schizophrenia and related psychoses occur not in a distinct silo, but as part of a continuum with admixtures of nonpsychotic phenomena.11–16 First, there are surprisingly common subclinical psychotic experiences and subthreshold psychotic symptoms in the general population, most of whom do not go on to develop a psychotic disorder.17–21 Second, psychotic experiences are associated with overall distress, poor functioning,19,22 and suicidality,23,24 and are predictive of both psychotic as well as nonpsychotic disorders.25–27 Third, clinical samples with subthreshold psychotic syndromes followed prospectively demonstrate that a threshold-level psychosis is one of a range of possible outcomes; few individuals at risk develop a first episode of psychosis (FEP), while a large proportion develop nonpsychotic disorders (primarily mood, anxiety, or substance use).28–30 In this view, then, psychotic syndromes likely represent one end of an overall continuum:31 they are not a categorically distinct entity, but instead an emergent phenomenon2,13,16,32–34 indicating the most complex and severe forms of common mental disorders and conditions.
Furthermore, just as nonpsychotic disorders can emerge in individuals with previous subthreshold psychotic syndromes, those with nonpsychotic syndromes can develop a subsequent threshold-level psychosis.11,35 This means that psychotic disorders may be the culmination of either homotypic (from early psychotic experiences or the attenuated psychotic syndrome) and/or heterotypic (from nonpsychotic syndromes or common mental disorders) illness progression.36,37 Data supporting the notion that trajectories to psychosis emerge from a variety of diagnostic silos comes from a range of studies. Diverse clinical evidence suggests that psychotic syndromes are often preceded by common mental disorders, such as depression or anxiety.12,38–40 Epidemiological cohorts also demonstrate co-occurring and reciprocal relationships between affective/anxious and psychotic psychopathology.13,33,41,42 And these shifts across diagnostic boundaries are meaningful: while the operationalized at-risk mental state for psychosis has an increased risk for development of threshold-level psychosis (hazard ratio [HR] 7.86; population attributable fraction [PAF] 36.9), the fraction of psychosis attributable to prior mood states is substantially higher still (HR 10.67, PAF 66.2).43
Proposals of trajectories to psychosis have often drawn on hierarchical models of symptoms.44 Reflecting their end-point of interest and the belief that psychotic syndromes are among the most complex and persistent of mental illnesses, primacy in these models has historically been given to the most apparent (positive) psychotic symptoms. Thus, nonpsychotic symptoms (NPS) such as anxiety, depression, or sleep disturbances are considered to precede subthreshold psychotic symptoms (STPS), which in turn occur before the threshold-level psychotic symptoms FEP; 45–49 negative symptoms and cognitive impairments (which too play a central role in psychotic disorders) are seen as less specific ones that also accompany nonpsychotic presentations.
Altogether, the idea that psychotic disorders represent not a categorically distinct illness type, but rather the most complex and severe forms of common mental disorders, has to date been primarily investigated in prospective studies.2,41,50 Yet critical support for (or against) this theory could also come from examining when and in what order NPS and subthreshold STPS occur prior to the ultimate outcome of interest, a FEP. Because the at-risk mental state (with its constitutive STPS7) was conceptualized after pioneering follow-back studies of FEP,51–54 systematically cataloguing that pre-onset psychopathology in a representative FEP sample can now provide additional texture regarding how psychotic syndromes develop from across a range of early stage mental phenomena.
To that end, we examined the symptom profiles experienced by a retrospective follow-back cohort of FEP patients entering a catchment-based early intervention program, focusing on the timing, distribution, and relationship among NPS and STPS. We theorized that individuals experiencing a FEP should represent those with the most complex nonpsychotic syndromes, indexed as the number of pre-onset symptoms identified. We hypothesized that the initial STPS would follow NPS chronologically, that NPS would be more frequent and complex in those with STPS, and that greater numbers of NPS would be predictive of more STPS.
Methods
Setting
Participants were recruited between 2003 and 2019 from the Prevention and Early Intervention Program for Psychosis (PEPP-Montreal) within the Douglas Mental Health University Institute, the only early intervention service serving a catchment of over 300 000 individuals in the southwest of Montreal, Canada.55 Prior to intake, help-seeking patients were assessed first by a screening clinician and psychiatrist, then by a research assistant with the Structured Clinical Interview for DSM‐IV (SCID‐IV)56 which was later reviewed in a consensus meeting, to confirm a diagnosis of FEP. Inclusion criteria into both the service and the study were (1) meeting diagnostic criteria for a nonaffective or affective psychotic disorder not attributable to substance use alone, based on the SCID-IV; (2) having received less than 30 days of antipsychotic medication; (3) IQ ≥ 70; (4) having no organic mental disorder, such as epilepsy; and (5) being between 14 and 35 years of age. Once accepted to the program, participants were provided with up to 2 years of multicomponent FEP care.57 The present study was part of a larger, long-term study of outcome and early intervention in FEP approved by the Douglas Hospital Research Centre’s research ethics board. Participants provided written informed consent, or assent with written parental consent if younger than 18 years.
Instruments
Within the first 3 months of entry to PEPP-Montréal, semi-structured interviews with patients and family members were conducted along with a review of all available health and social records. This information was then used to inform the Circumstances of Onset and Relapse Schedule (CORS) and the Topography of Psychotic Episode (TOPE),58 to determine when and which of 27 early signs or symptoms were experienced by each subject before the onset of psychosis. Despite understandable concerns regarding the reliability of information acquired in this manner and the potential for recall bias,59–61 retrospective and follow-back studies have been employed with some success to examine a range of issues affecting those experiencing mental illnesses, including psychosis.35,52,60–65
Given variation in available collateral information (chart review, interviews with family members, and accessible records from external assessments) and written records among patients, research assistants synthesized the information acquired into a narrative in advance of regular meetings chaired by a research psychiatrist (AKM, RJ, or JLS), during which consensus was derived on early signs/symptoms and associated dates. Research assistants received orientations, training on rating videotapes, role-playing, conducting interviews under supervision, and identifying early signs/symptoms as well as key dates related to illness course. In addition, to ensure accuracy in timing, anchor points were employed during the interview (eg, “your 16th birthday” or “during 8th grade”). To assess inter-rater reliability, cases were randomly selected and independently evaluated by 3–8 raters for variables such as length of treatment delays and number of help-seeking contacts (intraclass correlation coefficient, 0.82–0.98).66
Of the 27 early signs and symptoms, nine had been previously identified by experts as constituting “attenuated positive symptoms/subthreshold psychotic symptoms (STPS), if they appeared at a time when an individual would not have met criteria for a syndromal level psychotic episode”:11 suspiciousness, odd ideas of reference, odd behavior, unusual perceptual experiences, disorganized speech, inappropriate affect, hallucinations, delusions, and passivity (in decreasing order of frequency).11 The remaining 18 represent a wide range of nonpsychotic signs and symptoms, including (in decreasing frequency) depression, anxiety, impaired role functioning, social withdrawal, impaired concentration, sleep disturbance, decreased energy, irritability, change in weight, restlessness, blunted affect, memory problems, mood elation, poor grooming, self-harm, obsessive compulsive, and motor symptoms (extrapyramidal or catatonia).
Course of Illness
In addition to early signs and symptoms, we examined several aspects of illness course that were reported as occurring before the index FEP. During the pre-onset period, the majority of individuals experience a prodrome, defined retrospectively as a period of one or more continuous symptoms up to the onset of a threshold-level psychosis.67 Syndromes that occurred but subsequently resolved before onset of the prodrome or FEP are known as outposts.68 Prior to a FEP, individuals could experience either no identifiable early signs/symptoms whatsoever, an outpost syndrome, and/or a prodrome.
Exclusion
Between 2003 and 2019, of 992 individuals receiving services, 626 participants consented to be included in the study. Of the initial 626, full data (including early signs/symptoms and illness course) were available for 430 participants included in the following analyses.
Statistical Analyses
Statistical analysis was conducted from March 2019 to March 2020. All visualizations and analyses were performed in R, version 3.6.3. Of the participants assessed with TOPE, 272 (63%) had one or more identifiable STPS (STPSp group), while 158 (37%) had no identifiable STPS (STPSa group). Distributions of symptoms were visually examined as histograms, radial arm plots, and bar charts. Differences of proportion were tested with the two proportion z-test. Investigations of the correlations between counts of NPS and STPS prior to the FEP were analyzed with negative binomial regressions to appropriately model overdispersed count data.
Results
Sample Characteristics
There were no differences in sociodemographic or clinical variables between the 430 individuals excluded in the analysis and the 196 excluded due to missing data (Table 1).
Table 1.
Participant demographics
| Characteristic | Participants (N = 430)* | Excluded sample (N = 196) | Comparing excluded and included (P value) | STPSp (N = 271) | STPSa (N = 158) | Comparing NPS and STPS (P value) |
|---|---|---|---|---|---|---|
| Age at psychosis onset, mean (SD) | 22.65 (4.61) | 22.81 (5.07) | 0.7 | 22.47 (4.48) | 22.96 (4.84) | 0.29 |
| Age of first psychiatric symptom, mean (SD) | 17.36 (5.31) | 17.78 (6.21) | 0.42 | 16.92 (5.28) | 18.13 (5.29) | 0.03* |
| Age of onset of prodrome, mean (SD) | 20.73 (5.18) | 21.01 (5.91) | 0.58 | 20.33 (5.06) | 21.41 (5.31) | 0.04* |
| Sex (Male; Female; other), No. (%) | 291 (67.83); 136 (31.70); 2 (0.47) | 144;53 | 0.22 | 187 (69.00); 83 (30.63); 1 (0.37) | 104 (65.82%); 53 (33.54%); 1 (0.63%) | 0.57 |
| Visible minority status (Caucasian; Other), No. (%) | 260 (63.88);147 (36.12) | 106;71 | 0.41 | 161 (61.92); 99 (38.08) | 99 (65.13%); 53 (34.87%) | 0.59 |
| Education level (>high school; high school or less), No. (%) | 213 (52.59); 192 (47.41) | 102;76 | 0.34 | 135 (50.75); 131 (49.25) | 83 (56.46%); 64 (43.54%) | 0.31 |
| Secondary diagnosis of substance use disorder (yes; no), No. (%) | 219 (53.16); 193 (46.84) | 75;82 | 0.29 | 145 (55.13); 118 (44.87) | 74 (49.66%); 75 (50.34%) | 0.33 |
| Baseline diagnosis (schizophrenia spectrum disorder; affective), No. (%) | 307 (72.41); 117 (27.59) | 122;60 | 0.22 | 203 (75.47); 66 (24.54) | 104 (67.10%); 51 (32.90%) | 0.07 |
Note: Total participant counts differ per row based on available demographic data. Data presented in the table represent means (standard deviation) for continuous variables, count (percentages) for categorical variables, or P values where indicated in the column heading.
*Totals <430 due to small amounts of missing data.
Of the included patients, there were no significant differences between the STPSa and STPSp groups in sociodemographics, proportion with affective versus nonaffective psychosis, or comorbid substance use; the STPSp group was younger at the age of first psychiatric symptom and age of onset of the prodrome (Table 1).
Timing of Symptoms
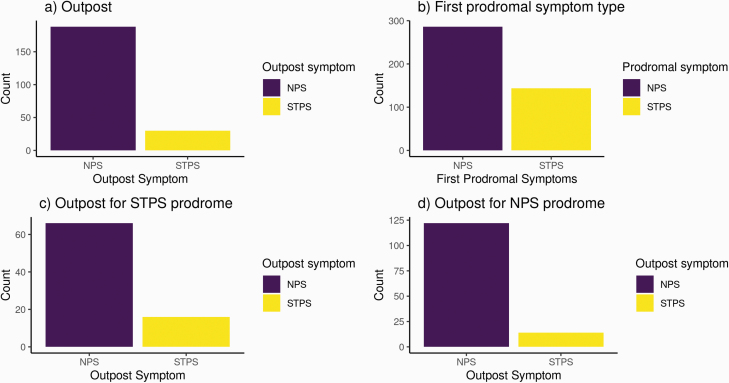
A total of 218 participants had an outpost distinct from the prodrome. Of this group, 188 (86.24%) experienced an NPS as the outpost symptom, and 30 (14%) experienced an STPS as the outpost symptom (Figure 1a).
Figure 1.
Timing of symptom onset. Histograms of distribution for first symptom type for a) outpost syndrome b) prodrome c) outpost if first prodromal symptom was STPS d) outpost if first prodromal symptom was NPS.
The prodromal symptom was the first recorded symptom for 211 out of the total 430 participants, and was NPS for 66.51% of the sample (N = 286) and STPS for 33% of the sample (N = 144; Figure 1b). The prodrome was significantly more likely to start with a STPS than was the outpost syndrome (z = 5.35, P = 1.44 e-07).
Of those with both a distinct outpost and a prodrome, a majority of individuals whose prodrome began with a STPS had an outpost syndrome with NPS (N = 66/82, 80%, compared with STPS outpost, N = 16/82, 20%; Figure 1c). Those whose prodrome began with an NPS were also more likely to have an NPS outpost syndrome (N = 122/136, 90% versus STPS outpost, N = 14/136, 10%; Figure 1d).
Distribution of Symptoms
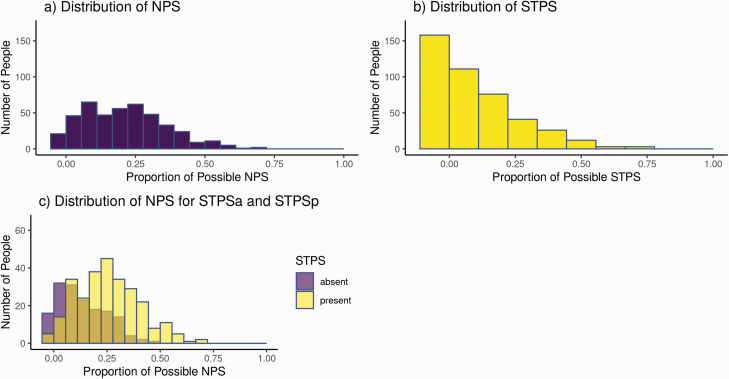
Overall, NPS were far more common than STPS in the entire sample (Figure 2a). With a median of 4 and mode of 2 NPS per subject, the histogram is moderately right-skewed (0.52), roughly following a normal distribution. In contrast, the STPS distribution is highly right-skewed (1.18), with a median of 1 and mode of 0 (Figure 2b).
Figure 2.
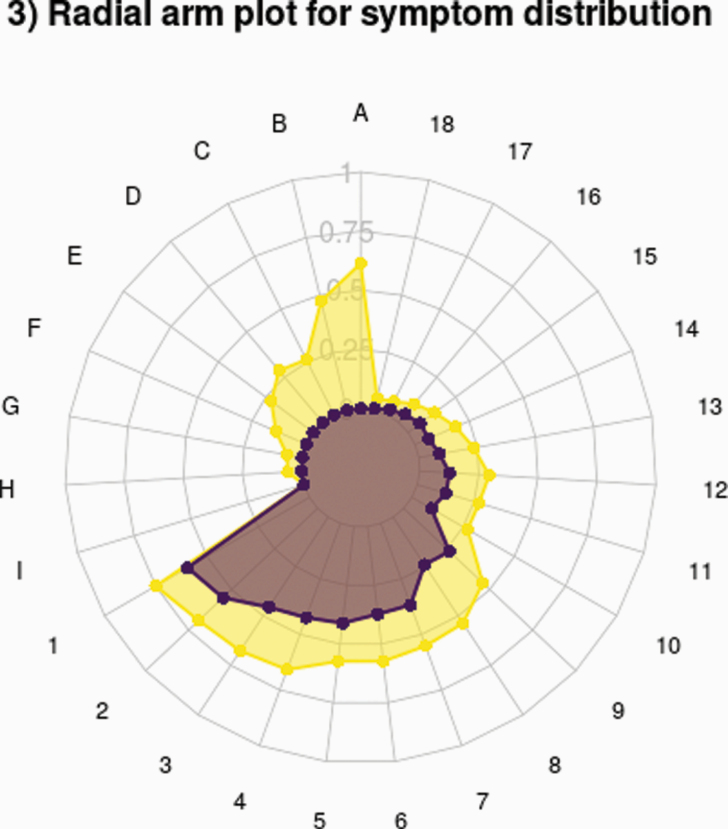
Radial arm plot for symptom distribution. Radial arm plot for STPSa group and STPSp group for STPS (A = suspiciousness, B = odd ideas of reference, C = odd behavior, D = Unusual perceptual experienced, E = disorganized speech, F = inappropriate affect, G = hallucinations, H = delusions, I = passivity) and NPS (1 = depression, 2 = anxiety, 3 = impaired role functioning, 4 = social withdrawal, 5 = impaired concentration, 6 = sleep disturbance, 7 = decreased energy, 8 = irritability, 9 = change in weight, 10 = restlessness, 11 = blunted affect, 12 = memory problems, 13 = elated mood, 14 = poor grooming, 15 = self harm, 16 = obsessive compulsive, 17 = extrapyramidal symptoms, 18 = catatonia).
The distribution of NPS for participants with one or more STPS (STPSp) is right-shifted compared with those with no STPS (STPSa), indicating that the former group experiences overall relatively higher numbers of NPS (Figure 2c).
A radial arm plot shows the distribution of specific symptoms as proportions for the STPSp (N = 271) and STPSa (N = 149) groups (Figure 3). In both, depression suspiciousness, anxiety, and impaired role functioning were the most common symptoms. Notably, all NPS were more frequent in the STPSp group: in other words, STPSp subjects were distinguished not just by the presence of at least one STPS but also by having greater numbers of all NPS.
Figure 3.
Radial arm plot for symptom distribution. Radial arm plot for STPSa group and STPSp group for STPS (A = suspiciousness, B = odd ideas of reference, C = odd behavior, D = Unusual perceptual experienced, E = disorganized speech, F = inappropriate affect, G = hallucinations, H = delusions, I = passivity) and NPS (1 = depression, 2 = anxiety, 3 = impaired role functioning, 4 = social withdrawal, 5 = impaired concentration, 6 = sleep disturbance, 7 = decreased energy, 8 = irritability, 9 = change in weight, 10 = restlessness, 11 = blunted affect, 12 = memory problems, 13 = elated mood, 14 = poor grooming, 15 = self harm, 16 = obsessive compulsive, 17 = extrapyramidal symptoms, 18 = catatonia).
Relationship Between NPS and STPS
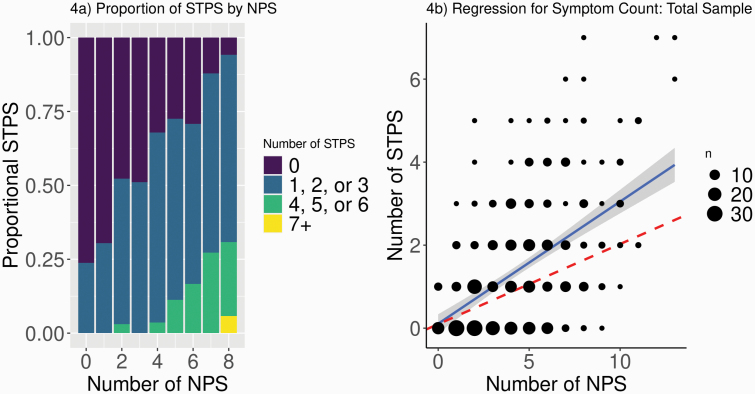
Examining NPS and STPS symptom counts (Figure 4a), those with large numbers of NPS almost always had four or more STPS, while those with few NPS also had few STPS. Notably, while more than a third of the sample was STPSa, only 21 participants (4.8%) experienced no NPS. Of these NPS-absent individuals, 16 had no STPS either, meaning their FEP arose with no pre-onset symptoms whatsoever. The other 5 all experienced only one STPS, demonstrating the rarity of STPS without NPS.
Figure 4.
Relationship among symptoms. a) Binned histogram with count of NPS on x-axis (8+ combined to protect anonymity of participants) and proportion of sample reporting each NPS on the y-axis. Total sample for each NPS count indicated at the top of each bar. Colors represent binned counts of STPS. b) Graph representing NBR for the total sample. Dashed line indicates standardized slope for STPSp sample for comparison.
For the STPSp group alone, the negative binomial regression revealed a relationship between NPS and STPS symptom counts (β = 0.19, 95% CI [0.17, 0.22], P = 8.55 e-11), such that for every unit increase in NPS there was a 0.19 unit increase in STPS (Figure 4b). When combining the STPSp and STPSa groups, the strength of this relationship increased, with every unit increase in NPS associated with a 0.34 unit increase in STPS (β = 0.34, 95% CI [0.31, 0.38], P < 2 e-16). Including covariates of age, sex, and education level in the latter equation did not affect the relationship between counts of NPS and STPS (β = 0.34, 95% CI [0.32, 0.38], P < 2 e-16).
NPS and STPS in Affective and Nonaffective FEP
Nearly 30% of the sample (27.6%) experienced an affective psychosis, with the remaining majority diagnosed with a nonaffective (schizophrenia spectrum) disorder (Table 1). There was a trend-level difference in age of onset of psychosis between these two subgroups (P = 0.06), but no difference in age of prodromal onset or first psychiatric change.
Sub-analyses of these two groups revealed that compared with the affective group, the nonaffective group was more likely to have an STPS as the first outpost (P = 0.008, z = 2.76) and first prodromal symptoms (P = 0.004, z = 2.99; Supplementary Figure S1). However, for both it was more common to experience an NPS before an STPS.
There were no differences in the distributions of NPS and STPS for the two groups between either NPS (Supplementary Figure S2a; affective: skewness = 0.32, median = 5; nonaffective: skewness = 0.59, median = 4) or STPS (Supplementary Figure S2b; affective: skewness = 1.23, median = 1; nonaffective: skewness = 1.13, median = 1). Additionally, negative binomial regressions revealed that the positive relationship between count of NPS and count of STPS was preserved for both affective (β = 0.40, 95% CI [0.34, 0.47], P = 2.18 e-9) and nonaffective (β = 0.33, 95% CI [0.30, 0.37], P = 2.00 e-16) subgroups (Supplementary Figure S3).
Discussion
Prospective evidence has long suggested that psychotic symptoms appear not de novo, but as emergent phenomena signifying the most complex and severe of psychiatric syndromes. Here we attempted to determine whether this conceptualization could be supported by a detailed follow-back examination of the developmental trajectories of nonpsychotic and subthreshold psychotic symptoms leading up to the ultimate outcome of interest, a FEP. We found that the timing, progression, distribution, and relationship between these symptoms were each consistent with the hypothesis that psychotic syndromes tend to emerge in the context of previous nonpsychotic symptoms—with implications for our understanding of the onset and evolution of the early phases of mental disorders.
Although the co-occurrence of NPS and STPS in early psychosis populations is well documented, there are few detailed descriptions of their close intermingling prior to the onset of FEP. In addition to being consistent with the continuum model articulated by van Os and colleagues, the sequences and relationships seen in this dataset echo long-recognized patterns of psychotic relapse intimately preceded by NPS,69–71 and of hierarchical models of the development of mental illness.44,49,72 Where differences are seen, they exist in timing alone—with NPS less common but still the most likely outpost or first prodromal symptom in nonaffective compared with affective psychosis subsamples, and no differences in the distribution or relationship between NPS and STPS in these subgroups. Together, this suggests that the observed sequential relationships between initial nonpsychotic and subsequent psychotic symptoms may exist prior to both FEP and later stages of psychosis, and raises the possibility that similar patterns may also be seen in other severe mental illnesses. Other reports reveal comparable observations: for example, individual presentations shift across diagnostic silos in studies of life course epidemiology,73,74 and heterotypy is a core feature of both theoretical and empirical models of clinical staging in youth mental health.36,75
Our results may also have relevance for models of etiopathogenesis (such as vulnerability-stress frameworks)76–80 that are consistent with the objectives of early intervention paradigms. For example, those with pre-existing vulnerabilities such as family history or childhood trauma may have the greatest risk for earlier onset of psychosis, especially in the event of multiple or accumulating stressors. A corollary is that individuals who manifest psychotic (as compared with, say, anxiodepressive) symptoms in the context of stressful experiences might have particularly high levels of vulnerability and/or stressors that should call for preventative or more intensive clinical interventions. In combination with prospective studies demonstrating that NPS occur before STPS,13 our conclusions strengthen the importance of designing and developing youth mental health services that embrace the pluripotent, fluid nature of such presentations, including across critical periods81 and early stages.36,66,67
Given that the focus of our analysis was on the interplay between NPS and STPS, we paid specific attention to individuals who experienced both of these symptom sets (the STPSp subgroup). However, other subdivisions of the overall sample likewise illustrate that STPS emerge in the context of NPS: there were relatively few individuals with no identifiable NPS, of whom all had either zero or one STPS, and even nonaffective psychosis was generally characterized by NPS before STPS. Additionally, the 37% of the study population without STPS (STPSa) still emerged as part of a continuum of nonpsychotic symptoms beginning with prior NPS (albeit fewer NPS on average than for the STPSp group; Figures 2c and 3), but with the first identifiable psychotic symptom being the onset of FEP. Along with the delayed first psychiatric symptom and prodrome onset experienced by the STPSa group (Table 1), this suggests a more acute onset of threshold-level psychosis that might in turn be due to a different balance of vulnerabilities and stressors compared with the STPSp group.
While our data satisfy and support assumptions regarding the sequence, distribution and relationship between NPS and STPS in continuum models, we cannot definitively exclude the possibility that psychosis and common mental disorders have qualitatively, rather than quantitatively, different origins and presentations. However, the data would have more strongly supported maintaining categorical distinctions between psychosis and other mental disorders if STPS had largely preceded NPS, were as or more frequent than NPS, or if many individuals had STPS without NPS. Thus, our findings are at minimum consistent with—and represent novel complementary evidence in favor of—the continuum hypothesis.
This detailed follow-back study has a number of strengths. First, it builds upon previous work demonstrating the predictive validity of STPS status55 in which individuals with pre-onset STPS (STPSp) were similar to those without (STPSa), but exhibited poorer psychotic symptom and functional outcomes over time. Second, the trajectories to psychosis in this large sample were consistently and systematically collected and well-characterized from 2003 to 2019, thereby reinforcing the generalizability of our findings. Third, while a subset (31.3%) of potential participants were excluded due to incomplete CORS/TOPE data, this excluded group did not differ in demographics from the included subjects, suggesting that our analyses are not subject to a major sampling bias. Fourth, the fact that participants were only minimally exposed to antipsychotic medications or FEP interventions reduces the likelihood of treatment-related confounds. Finally, the presenting population is derived from a catchment-based organization of health services with no competing public or private services in the same geographic area, representing a near treated incidence sample.
Nonetheless, follow-back approaches and data collection are subject to limitations of recall (and potential associated bias), particularly in reconstructing the course of the prodrome with its multiple (often overlapping) symptoms. Depending on the direction of any such bias, this could have skewed reports of pre-FEP symptoms to be either underreported or overreported, if individuals do not recall their symptoms or preferentially recall psychotic symptoms respectively. Attempts to examine this issue, particularly in the context of an intense illness experience and cognitive impairment, have on the one hand noted reasonable levels of convergent validity and agreement between self-report and either clinical interviews or data provided by key informants, while on the other finding variable consistency in endorsement of pre-FEP symptoms along with relatively small sample sizes.59–61 To the extent possible, we attempted to address this not just with our large sample but by defining detailed timestamps within the CORS/TOPE, along with integrating information provided by family members, a review of all available health and social/educational records, and the standardized use of probes and anchor-points (such as birthdays, milestones, and major events). A further limitation is that we indexed syndrome complexity as a function of numbers of symptoms, without regard to the distress experienced at corresponding timepoints; in fact, small numbers of severe symptoms, whether NPS82–84 or STPS,85 might confer an equivalent or even greater burden of illness compared with large numbers of mild symptoms. And because our focus was on the developmental trajectories of NPS and STPS prior to a FEP, we did not consider additional variables that might have been contributors to the emergence of STPS,86–88 such as early life trauma, genetic predisposition, and cannabis use.
Conclusion
A major debate in psychosis studies is whether schizophrenia and related psychoses can be considered a discrete illness type qualitatively different from other disorders, or whether their development should be more appropriately viewed as an emergent phenomenon reflecting complex forms of common mental illnesses. Utilizing detailed clinical data on the trajectories of nonpsychotic and sub-threshold psychotic symptoms in a FEP sample, our results are consistent with the notion that psychosis emerges from within a broad continuum of nonpsychotic syndromes rather than as a qualitatively distinct disorder. This evidence should now be incorporated into continuum models that better recognize the role of pluripotentiality and dynamic shifts in the risk and onset of this and other severe mental illnesses.
Supplementary Material
Acknowledgments
The authors would like Dr M. Mallar Chakravarty for his suggestions for data visualizations, and Nicole Pawliuk for her assistance with organizing and maintaining the dataset.
Funding
This work was supported by grants 68961 and MCT-94189 from the Canadian Institutes of Health Research (Dr Iyer), salary awards from the Fonds de Recherche du Quebec-Sante (Drs Joober and Shah) and the Canada Research Chairs Program (Dr Malla), the Sackler Foundation, and the Lobeer Foundation.
Conflict of Interest statement
AKM served as a research consultant to and gave lectures at conferences supported by Lundbeck and Otsuka and was on an advisory board meeting for those two companies, for which he received honoraria.
SNI reports grant and salary awards from the Canadian Institutes for Health Research and Fonds de recherche du Québec–Santé, outside the submitted work.
RJ served as speaker and member of advisory board committees for Pfizer, Janssen, BMS, Sunovian, Myelin, Otsuka, Lundbeck, Shire and Perdue; he also received grants from Janssen, BMS, Otsuka, Lundbeck, Astra Zeneca and HLS, within the past 3 years, unrelated to the submitted work.
JLS reports grants and salary awards from the Canadian Institutes of Health Research and Fonds de recherche du Québec–Santé, outside the submitted work.
References
- 1. van Os J. “ Schizophrenia” does not exist. Br Med J. 2016;352. http://search.proquest.com/openview/40c8d7375972dede6e1655927f8baa7e/1?pq-origsite=gscholar&cbl=2043523. [DOI] [PubMed]
- 2. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229–244. [DOI] [PubMed] [Google Scholar]
- 3. Jablensky A. The diagnostic concept of schizophrenia: its history, evolution, and future prospects. Dialogues Clin Neurosci. 2010;12(3):271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoghbi AW, Lieberman JA. Alive but not well: the limited validity but continued utility of the concept of schizophrenia. Psychol Med. 2018;48(2):245–246. [DOI] [PubMed] [Google Scholar]
- 5. Lawrie SM, Hall J, McIntosh AM, Owens DG, Johnstone EC. The ‘continuum of psychosis’: scientifically unproven and clinically impractical. Br J Psychiatry. 2010;197(6):423–425. [DOI] [PubMed] [Google Scholar]
- 6. American Psychiatric Association Staff. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text Revision (DSM-IV-TR®). Washington, DC: American Psychiatric Association Publishing; 2010. [Google Scholar]
- 7. Tandon R, Gaebel W, Barch DM, et al. . Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3–10. [DOI] [PubMed] [Google Scholar]
- 8. Association D-5 AP, Others. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Publishing. 2013. [Google Scholar]
- 9. Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry. 2017;16(3):251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Os J, Murray RM. Can we identify and treat “schizophrenia light” to prevent true psychotic illness? BMJ. 2013;346:f304. [DOI] [PubMed] [Google Scholar]
- 11. Shah JL, Crawford A, Mustafa SS, Iyer SN, Joober R, Malla AK. Is the clinical high-risk state a valid concept? retrospective examination in a first-episode psychosis sample. Psychiatr Serv. 2017;68(10):1046–1052. [DOI] [PubMed] [Google Scholar]
- 12. Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull. 2014;40(1):120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wigman JT, van Nierop M, Vollebergh WA, et al. . Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity–implications for diagnosis and ultra-high risk research. Schizophr Bull. 2012;38(2):247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamminga CA, Ivleva EI, Keshavan MS, et al. . Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170(11):1263–1274. [DOI] [PubMed] [Google Scholar]
- 15. Reininghaus U, Böhnke JR, Chavez-Baldini U, et al. . Transdiagnostic dimensions of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). World Psychiatry. 2019;18(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Os J, Linscott RJ. Introduction: The extended psychosis phenotype–relationship with schizophrenia and with ultrahigh risk status for psychosis. Schizophr Bull. 2012;38(2):227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tien AY. Distributions of hallucinations in the population. Soc Psychiatry Psychiatr Epidemiol. 1991;26(6):287–292. [DOI] [PubMed] [Google Scholar]
- 18. van Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: a psychosis continuum in the general population? Schizophr Res. 2000;45(1–2):11–20. [DOI] [PubMed] [Google Scholar]
- 19. Rössler W, Riecher-Rössler A, Angst J, et al. . Psychotic experiences in the general population: a twenty-year prospective community study. Schizophr Res. 2007;92(1–3):1–14. [DOI] [PubMed] [Google Scholar]
- 20. Verdoux H, van Os J. Psychotic symptoms in non-clinical populations and the continuum of psychosis. Schizophr Res. 2002;54(1–2):59–65. [DOI] [PubMed] [Google Scholar]
- 21. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43(6):1133–1149. [DOI] [PubMed] [Google Scholar]
- 22. Oh H, Koyanagi A, Kelleher I, DeVylder J. Psychotic experiences and disability: Findings from the Collaborative Psychiatric Epidemiology Surveys. Schizophr Res. 2018;193:343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bromet EJ, Nock MK, Saha S, et al. . Association between psychotic experiences and subsequent suicidal thoughts and behaviors: a cross-national analysis from the World Health Organization World Mental Health Surveys. JAMA Psychiatry. 2017;74(11):1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yates K, Lång U, Cederlöf M, et al. . Association of psychotic experiences with subsequent risk of suicidal ideation, suicide attempts, and suicide deaths: a systematic review and meta-analysis of longitudinal population studies. JAMA Psychiatry. 2019;76(2):180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGrath JJ, Saha S, Al-Hamzawi A, et al. . The bidirectional associations between psychotic experiences and DSM-IV mental disorders. Am J Psychiatry. 2016;173(10):997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kırlı U, Binbay T, Drukker M, et al. . DSM outcomes of psychotic experiences and associated risk factors: 6-year follow-up study in a community-based sample. Psychol Med. 2019;49(8):1346–1356. [DOI] [PubMed] [Google Scholar]
- 27. Kelleher I, Keeley H, Corcoran P, et al. . Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Br J Psychiatry. 2012;201(1):26–32. [DOI] [PubMed] [Google Scholar]
- 28. Addington J, Cornblatt BA, Cadenhead KS, et al. . At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168(8):800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simon AE, Borgwardt S, Riecher-Rössler A, Velthorst E, de Haan L, Fusar-Poli P. Moving beyond transition outcomes: meta-analysis of remission rates in individuals at high clinical risk for psychosis. Psychiatry Res. 2013;209(3):266–272. [DOI] [PubMed] [Google Scholar]
- 30. McGorry PD, Nelson B, Phillips LJ, et al. . Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. J Clin Psychiatry. 2013;74(4):349–356. [DOI] [PubMed] [Google Scholar]
- 31. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35(3):549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johns LC, van Os J. The continuity of psychotic experiences in the general population. Clin Psychol Rev. 2001;21(8):1125–1141. [DOI] [PubMed] [Google Scholar]
- 33. Krabbendam L, Myin-Germeys I, Hanssen M, et al. . Development of depressed mood predicts onset of psychotic disorder in individuals who report hallucinatory experiences. Br J Clin Psychol. 2005;44(Pt 1):113–125. [DOI] [PubMed] [Google Scholar]
- 34. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468(7321):203–212. [DOI] [PubMed] [Google Scholar]
- 35. Schultze-Lutter F, Rahman J, Ruhrmann S, et al. . Duration of unspecific prodromal and clinical high risk states, and early help-seeking in first-admission psychosis patients. Soc Psychiatry Psychiatr Epidemiol. 2015;50(12):1831–1841. [DOI] [PubMed] [Google Scholar]
- 36. Shah JL, Scott J, McGorry PD, et al. . Transdiagnostic clinical staging in youth mental health: a first international consensus statement. World Psychiatry. 2020;19(2):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scott J, Henry C. Clinical staging models: From general medicine to mental disorders. BJPsych Advances. 2017;23(5):292–299. [Google Scholar]
- 38. Sündermann O, Onwumere J, Kane F, Morgan C, Kuipers E. Social networks and support in first-episode psychosis: exploring the role of loneliness and anxiety. Soc Psychiatry Psychiatr Epidemiol. 2014;49(3):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanssen M, Peeters F, Krabbendam L, Radstake S, Verdoux H, van Os J. How psychotic are individuals with non-psychotic disorders? Soc Psychiatry Psychiatr Epidemiol. 2003;38(3):149–154. [DOI] [PubMed] [Google Scholar]
- 40. Schultze-Lutter F, Ruhrmann S, Picker H, von Reventlow HG, Brockhaus-Dumke A, Klosterkötter J. Basic symptoms in early psychotic and depressive disorders. Br J Psychiatry Suppl. 2007;51:s31–s37. [DOI] [PubMed] [Google Scholar]
- 41. van Rossum I, Dominguez MD, Lieb R, Wittchen HU, van Os J. Affective dysregulation and reality distortion: a 10-year prospective study of their association and clinical relevance. Schizophr Bull. 2011;37(3):561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Varghese D, Scott J, Welham J, et al. . Psychotic-like experiences in major depression and anxiety disorders: a population-based survey in young adults. Schizophr Bull. 2011;37(2):389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guloksuz S, Pries LK, Ten Have M, et al. . Association of preceding psychosis risk states and non-psychotic mental disorders with incidence of clinical psychosis in the general population: a prospective study in the NEMESIS-2 cohort. World Psychiatry. 2020;19(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Jong A, Giel R, Lindeboom EG, Slooff CJ, Wiersma D. Foulds’ hierarchical model of psychiatric illness in a Dutch cohort: a re-evaluation. Psychol Med. 1984;14(3):647–654. [DOI] [PubMed] [Google Scholar]
- 45. Tsuang MT, Van Os J, Tandon R, et al. . Attenuated psychosis syndrome in DSM-5. Schizophr Res. 2013;150(1):31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tandon N, Shah J, Keshavan MS, Tandon R. Attenuated psychosis and the schizophrenia prodrome: current status of risk identification and psychosis prevention. Neuropsychiatry (London). 2012;2(4):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. . The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353–370. [DOI] [PubMed] [Google Scholar]
- 49. Docherty JP, Van Kammen DP, Siris SG, Marder SR. Stages of onset of schizophrenic psychosis. Am J Psychiatry. 1978;135(4):420–426. [DOI] [PubMed] [Google Scholar]
- 50. Binbay T, Drukker M, Elbi H, et al. . Testing the psychosis continuum: differential impact of genetic and nongenetic risk factors and comorbid psychopathology across the entire spectrum of psychosis. Schizophr Bull. 2012;38(5):992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Häfner H, Maurer K, an der Heiden W. ABC Schizophrenia study: an overview of results since 1996. Soc Psychiatry Psychiatr Epidemiol. 2013;48(7):1021–1031. [DOI] [PubMed] [Google Scholar]
- 52. Häfner H, Maurer K, Löffler W, an der Heiden W, Hambrecht M, Schultze-Lutter F. Modeling the early course of schizophrenia. Schizophr Bull. 2003;29(2):325–340. [DOI] [PubMed] [Google Scholar]
- 53. Häfner H, Maurer K, Löffler W, et al. . The ABC Schizophrenia Study: a preliminary overview of the results. Soc Psychiatry Psychiatr Epidemiol. 1998;33(8):380–386. [DOI] [PubMed] [Google Scholar]
- 54. Häfner H. From onset and prodromal stage to a life-long course of schizophrenia and its symptom dimensions: how sex, age, and other risk factors influence incidence and course of illness. Psychiatry J. 2019;2019:9804836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosengard RJ, Malla A, Mustafa S, et al. . Association of pre-onset subthreshold psychotic symptoms with longitudinal outcomes during treatment of a first episode of psychosis. JAMA Psychiatry. 2019;76(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. First MB, Spitzer RL, Gibbon M, et al. . Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York, NY: SCID-I/P; 2002. [Google Scholar]
- 57. Iyer S, Jordan G, MacDonald K, Joober R, Malla A. Early intervention for psychosis: a Canadian perspective. J Nerv Ment Dis. 2015;203(5):356–364. [DOI] [PubMed] [Google Scholar]
- 58. Norman RMG, Malla AK.. Prevention and Early Intervention for Psychosis Program (PEPP): Course of Onset and Relapse Schedule: Interview and Coding Instruction Guide. Ontario, Canada: London Health Sciences Centre. 2002. [Google Scholar]
- 59. Hambrecht M, Häfner H, Löffler W. Beginning schizophrenia observed by significant others. Soc Psychiatry Psychiatr Epidemiol. 1994;29(2):53–60. [DOI] [PubMed] [Google Scholar]
- 60. Fisher HL, Craig TK, Fearon P, et al. . Reliability and comparability of psychosis patients’ retrospective reports of childhood abuse. Schizophr Bull. 2011;37(3):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gayer-Anderson C, Reininghaus U, Paetzold I, et al. . A comparison between self-report and interviewer-rated retrospective reports of childhood abuse among individuals with first-episode psychosis and population-based controls. J Psychiatr Res. 2020;123:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Conus P, Ward J, Lucas N, et al. . Characterisation of the prodrome to a first episode of psychotic mania: results of a retrospective study. J Affect Disord. 2010;124(3):341–345. [DOI] [PubMed] [Google Scholar]
- 63. Maurer K, Zink M, Rausch F, Häfner H. The early recognition inventory ERIraos assesses the entire spectrum of symptoms through the course of an at-risk mental state. Early Interv Psychiatry. 2018;12(2):217–228. [DOI] [PubMed] [Google Scholar]
- 64. Register-Brown K, Hong LE. Reliability and validity of methods for measuring the duration of untreated psychosis: a quantitative review and meta-analysis. Schizophr Res. 2014;160(1–3):20–26. [DOI] [PubMed] [Google Scholar]
- 65. Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances–systematic review and meta-analysis. Addict Behav. 2012;37(3):225–233. [DOI] [PubMed] [Google Scholar]
- 66. Iyer S, Mustafa S, Gariépy G, et al. . A NEET distinction: youths not in employment, education or training follow different pathways to illness and care in psychosis. Soc Psychiatry Psychiatr Epidemiol. 2018;53(12):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Iyer SN, Boekestyn L, Cassidy CM, King S, Joober R, Malla AK. Signs and symptoms in the pre-psychotic phase: description and implications for diagnostic trajectories. Psychol Med. 2008;38(8):1147–1156. [DOI] [PubMed] [Google Scholar]
- 68. Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A. Monitoring and care of young people at incipient risk of psychosis. Schizophr Bull. 1996;22(2):283–303. [DOI] [PubMed] [Google Scholar]
- 69. Herz MI, Melville C. Relapse in schizophrenia. Am J Psychiatry. 1980;137(7):801–805. [DOI] [PubMed] [Google Scholar]
- 70. Norman RM, Malla AK. Prodromal symptoms of relapse in schizophrenia: a review. Schizophr Bull. 1995;21(4):527–539. [DOI] [PubMed] [Google Scholar]
- 71. Malla AK, Norman RM. Prodromal symptoms in schizophrenia. Br J Psychiatry. 1994;164(4):487–493. [DOI] [PubMed] [Google Scholar]
- 72. Conrad K. Die beginnende schizophrenie [Beginning schizophrenia]. Stuttgart, Germany: Thieme Verlag; 1958. [Google Scholar]
- 73. Plana-Ripoll O, Pedersen CB, Holtz Y, et al. . Exploring comorbidity within mental disorders among a Danish national population. JAMA Psychiatry. 2019;76(3):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Caspi A, Houts RM, Ambler A, et al. . Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin birth cohort study. JAMA Netw Open. 2020;3(4):e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Iorfino F, Scott EM, Carpenter JS, et al. . Clinical stage transitions in persons aged 12 to 25 years presenting to early intervention mental health services with anxiety, mood, and psychotic disorders. JAMA Psychiatry. 2019;76(11):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev. 2017;73:191–218. [DOI] [PubMed] [Google Scholar]
- 77. Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. [DOI] [PubMed] [Google Scholar]
- 78. Corcoran C, Walker E, Huot R, et al. . The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29(4):671–692. [DOI] [PubMed] [Google Scholar]
- 79. Zubin J, Spring B. Vulnerability–a new view of schizophrenia. J Abnorm Psychol. 1977;86(2):103–126. [DOI] [PubMed] [Google Scholar]
- 80. Shah JL, Malla AK. Much ado about much: stress, dynamic biomarkers and HPA axis dysregulation along the trajectory to psychosis. Schizophr Res. 2015;162(1–3):253–260. [DOI] [PubMed] [Google Scholar]
- 81. Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl. 1998;172(33):53–59. [PubMed] [Google Scholar]
- 82. Angst J, Gamma A, Clarke D, Ajdacic-Gross V, Rössler W, Regier D. Subjective distress predicts treatment seeking for depression, bipolar, anxiety, panic, neurasthenia and insomnia severity spectra. Acta Psychiatr Scand. 2010;122(6):488–498. [DOI] [PubMed] [Google Scholar]
- 83. Fervaha G, Zakzanis KK, Foussias G, Agid O, Remington G. Distress related to subclinical negative symptoms in a non-clinical sample: Role of dysfunctional attitudes. Psychiatry Res. 2015;230(2):249–254. [DOI] [PubMed] [Google Scholar]
- 84. Pfeifer S, van Os J, Hanssen M, Delespaul P, Krabbendam L. Subjective experience of cognitive failures as possible risk factor for negative symptoms of psychosis in the general population. Schizophr Bull. 2009;35(4):766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pruessner M, Iyer SN, Faridi K, Joober R, Malla AK. Stress and protective factors in individuals at ultra-high risk for psychosis, first episode psychosis and healthy controls. Schizophr Res. 2011;129(1):29–35. [DOI] [PubMed] [Google Scholar]
- 86. Padmanabhan JL, Shah JL, Tandon N, Keshavan MS. The “polyenviromic risk score”: Aggregating environmental risk factors predicts conversion to psychosis in familial high-risk subjects. Schizophr Res. 201```7;181:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Radua J, Ramella-Cravaro V, Ioannidis JPA, et al. . What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry. 2018;17(1):49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fusar-Poli P, Tantardini M, De Simone S, et al. . Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur Psychiatry. 2017;40:65–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.