Summary
Background
We report on the safety and immunogenicity of V591, a measles vector-based SARS-CoV-2 vaccine candidate.
Methods
In this multicentre, randomised, placebo-controlled, double-blind, phase 1/2 trial, healthy adults with no history of COVID-19 disease were assigned to intramuscular injection of V591 or placebo (4:1 ratio). In part 1, younger adults (18-55 years) received V591 median tissue culture infectious dose (TCID50)-levels of 1×105 or 1×106 or placebo, 56 days apart. In part 2, younger and older (>55 years) adults received a single dose of one of four (104/105/106/107) or one of two (105/106) V591 TCID50 levels, respectively, or placebo. Primary outcome: safety/tolerability. Secondary outcome: humoral immunogenicity. ClinicalTrials.gov: NCT04498247.
Findings
From August–December 2020, 444 participants were screened and 263 randomised (210 V591; 53 placebo); 262 received at least one and 10 received two doses of V591 or placebo. Adverse events were experienced by 140/209 (67.0%) V591 dose-group participants and 37/53 (69.8%) placebo-group participants following injection 1; most frequent were fatigue (57 [27.3%] vs 20 [37.7%]), headache (57 [27.3%] vs 19 [35.8%]), myalgia (35 [16.7%] vs 10 [18.9%]), and injection-site pain (35 [16.7%] vs 4 [7.5%]). No deaths nor vaccine-related serious adverse events occurred. At Day 29, no anti-SARS-CoV-2 spike serum neutralising antibody and IgG-responses were identified in placebo or the three lower V591 dose-groups; responses were detected with V591 1×107 TCID50, although titres were lower than convalescent serum.
Interpretation
V591 was generally well tolerated, but immunogenicity was insufficient to warrant continued development.
Funding
Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Keywords: COVID-19, SARS-CoV-2, Vaccine
Research in context.
Evidence before this study
The COVID-19 pandemic has accelerated vaccine development against SARS-CoV-2. According to the World Health Organisation, 104 COVID-19 vaccines were in clinical development as of June 25, 2021, using a wide range of platforms, including RNA or DNA, protein subunits, replicating and non-replicating viral vectors, inactivated viruses, live attenuated viruses, virus-like particles, and viral vectors plus antigen presenting cells. The Schwarz measles virus strain, which elicits a strong and persistent humoral immune response, is being explored as a vaccine platform against several viral pathogens. In a randomised phase 2 trial, the measles vector-based vaccine candidate against chikungunya virus (MV-CHIK) demonstrated good immunogenicity and favourable safety and tolerability. Experience with other measles vector-based vaccine candidates was used to develop V591, a Schwarz measles vector-based SARS-CoV-2 vaccine candidate.
Added value of this study
We present the phase 1 results of the COVID-19 vaccine candidate V591, which, while generally well tolerated, demonstrated low immunogenicity following intramuscular administration in healthy adult participants. Given the strong immunogenicity observed with the chikungunya virus candidate vaccine (MV-CHIK) using the same platform, the low immunogenicity observed with V591 was unexpected.
Implications of all the available evidence
Development of V591 as a candidate vaccine for COVID-19 will not continue due to results from this study. The low immunogenicity observed in this study warrants further investigation to inform the development of future measles vector-based vaccines.
Alt-text: Unlabelled box
Introduction
The rapidly evolving coronavirus disease 2019 (COVID-19) global pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is associated with an unprecedented impact on individuals, healthcare systems and the global economy.1 Safe and effective vaccines against SARS-CoV-2 have a critical role in preventing morbidity and mortality, and although several vaccines have received conditional regulatory authorisation, multiple vaccines using different platforms may be required to control and end the pandemic.2
Viral vector vaccines are created by inserting selected gene fragments encoding antigens into attenuated viral vectors.3 Non-replicating recombinant adenovirus-based vector vaccines that deliver the SARS-CoV-2 spike protein are among the COVID-19 vaccines conditionally authorised for use in certain countries, including AZD1222 (AstraZeneca/Oxford University), Ad5-nCoV (CanSino), Ad26.COV2.S (Johnson & Johnson), and Sputnik V/Gam-COVID-Vac (Gamaleya Research Institute of Epidemiology and Microbiology).4 With only one exception, all of these vaccines require two injections to achieve the desired immune response. By comparison, replicating viral vector vaccine candidates for SARS-CoV-2 are in earlier stages of clinical development,5 and have the potential to be effective with a single injection. V591 is a live, attenuated, recombinant measles vector-based vaccine encoding a stabilised SARS-CoV-2 spike protein. The vector used in V591 is the Schwarz vaccine strain of the measles virus, which elicits a strong and persistent humoral and cellular immune response6 and is being explored for a wide variety of experimental vaccines against viral pathogens, including chikungunya virus, West Nile virus, dengue virus, human immunodeficiency virus, Lassa virus, Zika virus, Middle East Respiratory Syndrome coronavirus and SARS-CoV-1.6, 7, 8, 9, 10, 11, 12, 13, 14, 15
This phase 1/2, randomised, double-blind, placebo-controlled, dose-ranging trial was designed to evaluate the safety and immunogenicity of multiple regimens of V591 in healthy adults.
Materials and methods
Trial design and participants
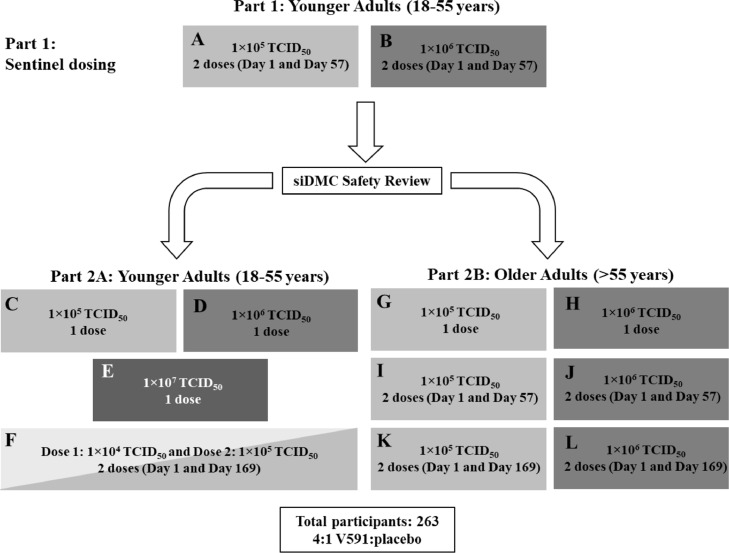
This randomised, double-blind, placebo-controlled, dose-ranging trial was conducted at nine centres across the United States, Austria, and Belgium [Protocol number: 001-03]. The trial was conducted in two parts (Figure 1). In part 1 (sentinel dosing), 10 adults (18–55 years of age) were randomly assigned to receive two intramuscular (IM) injections of V591 at different dosing levels (1×105 [panel A] or 1×106 [panel B] of the median tissue culture infectious dose [TCID50]) or placebo on Days 1 and 57 in a 4:1 ratio. A standing internal Data Monitoring Committee reviewed at least 7 days of safety and tolerability data following the first injection for all 10 participants in part 1 before initiating part 2 of the trial. In part 2, adults aged 18–55 years (part 2A) and >55 years (part 2B) were randomly assigned to receive V591 (either a single dose or two doses) or placebo in a 4:1 ratio at different dosing regimens (panels C–L), as outlined in Figure 1. All participants in part 2A were assigned to receive 1×104, 1×105, 1×106 or 1×107 TCID50 of V591 or placebo and all participants in part 2B were assigned to receive 1×105 or 1×106 TCID50 of V591 or placebo. Dose levels were selected based on previous experience with measles vector-based vaccine candidates.7,16 Due to the decision to terminate the trial early, participants were requested to complete activities up to at least Day 56, including all protocol-specified safety laboratory and routine adverse event (AE) assessments. Participants were not actively followed beyond end-of-trial activities (to occur on or after Day 56); spontaneous reporting of serious AEs (SAEs) and other protocol-specified events were instructed to continue through the protocol-specified durations.
Figure 1.
Trial design. A siDMC safety review of 7 days of safety/tolerability data after first dose in the sentinel dosing cohort (Panels A and B) was performed to trigger parts 2A and 2B (Panels C–L). A siDMC safety review of 7 days of safety/tolerability after second dose in the sentinel dosing cohort (Panel A and B) was performed to trigger second dose for those assigned to two doses in Parts 2A and 2B (Panels F, I–L). Shading of boxes corresponds to assigned V591 concentrations ranging from 1×104 TCID50 in very light grey, 1×105 TCID50 in light grey, 1×106 TCID50 in medium grey, and 1×107 TCID50 in dark grey. siDMC, standing internal Data Monitoring Committee; TCID50, median tissue culture infectious dose.
Eligible adults were 18 years of age or older with a body mass index <30 kg/m2 and in overall good health based on medical history, physical examination, electrocardiogram, vital sign measurements and safety laboratory tests performed prior to randomisation. Participants were required to practice social distancing for at least 2 weeks prior to planned injection on Day 1, with no close contacts with confirmed case(s) of active SARS-CoV-2 infection during that time period. Adherence to these rules was self-reported by participants. All participants in part 1 and the first five participants assigned to receive a single dose of 1×107 TCID50 of V591 or placebo in part 2A were required to be seronegative for SARS-CoV-2 prior to randomisation.
Ethics
The trial was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate institutional review boards or independent ethics committees and regulatory agencies in conformance with applicable country or local requirements. For Belgium, the FAMHP (Federal Agency for Medicines and Health Products) and the Ethics Committee Research UZ/KU Leuven approved the trial. For Austria, the BASG (Austrian Federal Office for Safety in Health Care), the AGES (Austrian Agency for Health & Food Safety) and the Ethics Committee of the Medical University of Vienna approved the trial. For US, the U.S. Food and Drug Administration and the central ethics committee Advarra IRB, Columbia, MD, USA approved the trial. Written informed consent was obtained from each participant prior to any study procedure.
Randomisation and masking
Eligible participants were randomly assigned to receive either V591 or placebo in a 4:1 ratio with a blocking factor of 5 within each panel separately (Figure 1) by means of a computer-generated randomisation schedule. Randomisation numbers were manually assigned by the Sponsor to each clinical site by e-mail correspondence just prior to dosing. It was ensured that once a randomisation number was assigned to a participant, it could not be re-assigned to another participant.
Participants, investigators, and the Sponsor or delegates involved in administration of the trial intervention or clinical evaluation of the participants were blinded to the intervention assignments. Vaccine dose preparation and administration were performed by an unblinded pharmacist or medically qualified trial personnel (e.g., physician, nurse, physician's assistant), as allowed by local/state, country, and institutional guidance.
Intervention
Based on experience with a preclinical measles vector-based SARS-CoV-1 vaccine candidate,15 a human codon-optimised gene encoding a modified full-length spike protein of SARS-CoV-2, described in the companion paper by Launay et al.,17 was inserted as an additional transcription unit into the Schwarz measles vector.6 Detailed information on the sequence of the spike protein gene insert and preclinical data for V591 will be reported separately by Institut Pasteur.
The V591 vaccine was manufactured in Vero 10-87 cells and vialed by ABL Europe S.A.S. (France) or Biofabri (Spain) according to Good Manufacturing Practices and placebo (0.9% sodium chloride, USP or BP sterile saline) was sourced locally by each centre. Vials containing the V591 product were stored frozen (≤-65°C) before preparation and injection. The vaccine was thawed for up to 30 minutes and administered within 60 minutes after removal from the freezer. IM injections were administered into the left or right deltoid muscle.
Primary and secondary endpoints
The primary objective of the trial was to assess the safety and tolerability of V591 compared with placebo at each dose regimen examined. Primary safety endpoints included solicited injection-site AEs (pain/tenderness, swelling, redness) from Day 1 through Day 5 after trial intervention, solicited systemic AEs (fever [oral temperature], muscle pain, joint pain, headache, fatigue, rash, nausea) from Day 1 through Day 14, unsolicited AEs from Day 1 through Day 28, and SAEs and medically attended AEs (MAAEs) collected from Day 1 throughout the duration of trial. AEs were graded by the trial investigators according to the guidance document Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials of the Food and Drug Administration (September 2007).18
The secondary objective of the trial was to evaluate the humoral immunogenicity of V591 at all time points (initially planned to include Days 15, 29, 57, 85, 115, 211, and 365 prior to early termination of the trial) compared with placebo, with a focus on Day 29 for all panels, Day 85 for the two-dose panels A, B, I, and J, and Day 197 for the two-dose panels K and L. Due to early termination of the trial, immunogenicity results are reported at Days 15 and 29 for those who received injection 1.
Immunogenicity
Humoral immunogenicity was quantified through measurement of anti-SARS-CoV-2 spike serum neutralising antibody (nAb) responses using the pseudo-virus neutralisation assay,19 and anti-SARS-CoV-2 spike immunoglobulin G (IgG) using an enzyme-linked immunosorbent assay (ELISA).20 The ELISA used a recombinant, purified, stabilised extracellular domain of the spike protein, which was trimerised using a fold-on motif (ELISA tri-S).20 Immunogenicity measurements were conducted by the contract research organisation, Nexelis (Laval, Quebec).
Human convalescent sera from donors who had recovered from COVID-19 were tested to compare with the immunogenicity obtained after treatment with V591. These sera were residual biological samples obtained from a study sponsored by Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, investigating the humoral response in patients with COVID-19 (MK-0000 P411). A total of 28 serum samples were tested from donors 23 to 70 years of age (median age, 47.5 years); samples were obtained at least 4 weeks after a confirmed diagnosis of symptomatic COVID-19 and after symptom resolution. Donors did not require mechanical ventilation, and were not immunosuppressed based on clinical history or recent/current immunosuppressive therapy.
Statistical analysis
Safety analyses were conducted in the All Participants as Treated (APaT) population, which included all randomised participants who received at least one dose of trial intervention. Participants were included in the treatment group corresponding to the trial intervention they received for the analysis of safety data using the APaT population. Immunogenicity analyses were conducted in the per-protocol population, which included all randomised participants without deviations from the protocol that may have substantially affected the results of the immunogenicity endpoints. Potential deviations that may have resulted in the exclusion of a participant from the per-protocol population for all immunogenicity analyses included failure to receive any trial vaccine at Day 1, failure to receive the correct dose of trial vaccine at Day 1, or receipt of prohibited medication or prohibited vaccine prior to the Day 1 blood sample collection. For immunogenicity analyses, the observed nAb geometric mean titres (GMTs) at each timepoint with serum collection and geometric mean fold-rises (GMFRs) from prevaccination to postvaccination were provided within each vaccination group separately. Descriptive statistics with point estimates and within-group 95% confidence intervals (CIs) were provided. The point estimates were calculated by exponentiating the estimates of the mean of the natural log values and the within-group CIs were derived by exponentiating the bounds of CIs of the mean of the natural log values based on the t-distribution. A similar statistical approach was used to evaluate the anti-SARS-CoV-2 spike serum IgG responses. For responses smaller than the lower limit of quantitation (LLOQ), half of the LLOQ were used for analysis when calculating the nAb GMTs and IgG geometric mean concentrations (GMCs). Safety results were reported as the number and percentage of participants who experienced one or more event(s). This is a phase 1 dose finding study and no formal hypothesis was tested. The sample size provided appropriate assessments for both safety endpoints and the variability of each dose level for immunogenicity endpoints. SAS software version 9.4 was used for all immunogenicity and safety analyses described above.
Role of the funding source
Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, participated in trial design, data collection, data analysis, data interpretation and writing of the report. The funders reviewed a draft of this manuscript. All authors had access to the trial results and approved the decision to submit for publication.
Results
Participants
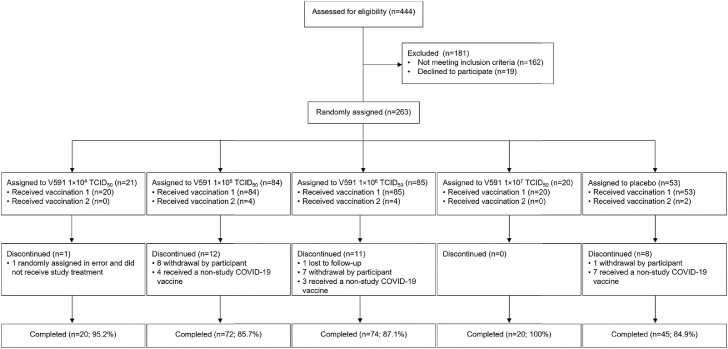
From 27 August 2020 to 1 December 2020, 444 participants underwent screening for enrolment, of whom 263 participants were randomised and 231 completed the trial (Figure 2). All randomised participants except one, who was enrolled in error and did not receive study intervention, received at least one injection of V591 (n=209) or placebo (n=53), and 10 participants received a second injection of V591 (n=8) or placebo (n=2). The trial was terminated early based on interim immunogenicity results. At trial termination, all 10 participants in part 1 had received a second injection, but no participants in part 2 had received a second injection. The interim results reported herein are considered the final results for this study.
Figure 2.
Participant disposition (all randomised participants). TCID50, median tissue culture infectious dose.
Baseline demographics for all randomised participants are shown in Table 1. All participants who received V591 dose levels of 1×104 or 1×107 TCID50 were 18–55 years of age (part 2A), in accordance with the trial design, and those who received the V591 dose of 1×105 or 1×106 TCID50 were 18–55 years of age (28%) and >55 years of age (72%).
Table 1.
Baseline demographics (all randomised participants).
| V591 1×104 TCID50 (n=21) | V591 1×105 TCID50 (n=84) | V591 1×106 TCID50 (n=85) | V591 1×107 TCID50 (n=20) | Placebo (n=53) | Total (n=263) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||||||
| Male | 12 | (57.1) | 35 | (41.7) | 38 | (44.7) | 6 | (30.0) | 24 | (45.3) | 115 | (43.7) |
| Female | 9 | (42.9) | 49 | (58.3) | 47 | (55.3) | 14 | (70.0) | 29 | (54.7) | 148 | (56.3) |
| Age, years | ||||||||||||
| 18–55 years | 21 | (100.0) | 24 | (28.6) | 24 | (28.2) | 20 | (100.0) | 22 | (41.5) | 111 | (42.2) |
| >55 years | 0 | (0.0) | 60 | (71.4) | 61 | (71.8) | 0 | (0.0) | 31 | (58.5) | 152 | (57.8) |
| Mean (SD) | 39.5 | (10.3) | 58.9 | (14.8) | 57.6 | (15.5) | 26.6 | (6.8) | 55.3 | (18.0) | 53.7 | (17.6) |
| Race | ||||||||||||
| White | 19 | (90.5) | 81 | (96.4) | 82 | (96.5) | 20 | (100.0) | 52 | (98.1) | 254 | (96.6) |
| Black or African American | 0 | (0.0) | 2 | (2.4) | 3 | (3.5) | 0 | (0.0) | 0 | (0.0) | 5 | (1.9) |
| Asian | 0 | (0.0) | 1 | (1.2) | 0 | (0.0) | 0 | (0.0) | 1 | (1.9) | 2 | (0.8) |
| Multiple | 2 | (9.5) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 2 | (0.8) |
| Ethnicity | ||||||||||||
| Not Hispanic or Latino | 20 | (95.2) | 64 | (76.2) | 72 | (84.7) | 20 | (100.0) | 50 | (94.3) | 226 | (85.9) |
| Hispanic or Latino | 1 | (4.8) | 20 | (23.8) | 13 | (15.3) | 0 | (0.0) | 3 | (5.7) | 37 | (14.1) |
Data are n (%) or mean (SD).
SD, standard deviation; TCID50, median tissue culture infectious dose.
Vaccine safety
V591 was generally well tolerated (Table 2). Across V591 and placebo groups, one or more AEs were experienced by 177 (67.6%) of 262 participants following injection 1 (140 [67.0%] of 209 in the V591 dose groups and 37 [69.8%] of 53 in the placebo group) and by 5 (50.0%) of 10 participants following injection 2 (4 [50.0%] of 8 in the V591 dose groups and 1 [50.0%] of 2 in the placebo group). Three participants reported a total of four SAEs, none of which were considered vaccine-related by the investigator. SAEs included grade 4 COVID-19, which occurred 23 days after receiving injection 1 of V591 1×105 TCID50 and resolved after 2.1 months; coronary artery disease and myocardial infarction (both grade 3 and in the same participant), which occurred 41 days after receiving injection 1 of V591 1×106 TCID50 and resolved after 29 days; and grade 3 migraine, which occurred 24 days after receiving injection 1 of placebo and resolved after 4 days.
Table 2.
Adverse event summary (following injection 1 & 2) (all participants as treated).
| Following injection 1 |
Following injection 2 |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V591 1×104 TCID50 |
V591 1×105 TCID50 |
V591 1×106 TCID50 |
V591 1×107 TCID50 |
Placebo |
TOTAL |
V591 1×105 TCID50 |
V591 1×106 TCID50 |
Placebo |
TOTAL |
|||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Participants in population with follow-up | 20 | 84 | 85 | 20 | 53 | 262 | 4 | 4 | 2 | 10 | ||||||||||
| With one or more AEs* | 15 | (75.0) | 52 | (61.9) | 53 | (62.4) | 20 | (100.0) | 37 | (69.8) | 177 | (67.6) | 1 | (25.0) | 3 | (75.0) | 1 | (50.0) | 5 | (50.0) |
| Injection site | 5 | (25.0) | 10 | (11.9) | 15 | (17.6) | 14 | (70.0) | 4 | (7.5) | 48 | (18.3) | 1 | (25.0) | 1 | (25.0) | 0 | (0.0) | 2 | (20.0) |
| Non-injection site | 14 | (70.0) | 50 | (59.5) | 49 | (57.6) | 15 | (75.0) | 35 | (66.0) | 163 | (62.2) | 1 | (25.0) | 3 | (75.0) | 1 | (50.0) | 5 | (50.0) |
| With vaccine-related† AEs | 11 | (55.0) | 27 | (32.1) | 27 | (31.8) | 17 | (85.0) | 20 | (37.7) | 102 | (38.9) | 1 | (25.0) | 3 | (75.0) | 1 | (50.0) | 5 | (50.0) |
| Injection site | 5 | (25.0) | 10 | (11.9) | 15 | (17.6) | 14 | (70.0) | 4 | (7.5) | 48 | (18.3) | 1 | (25.0) | 1 | (25.0) | 0 | (0.0) | 2 | (20.0) |
| Non-injection site | 10 | (50.0) | 22 | (26.2) | 19 | (22.4) | 10 | (50.0) | 17 | (32.1) | 78 | (29.8) | 1 | (25.0) | 3 | (75.0) | 1 | (50.0) | 5 | (50.0) |
| With SAEs | 0 | (0.0) | 0 | (0.0) | 2 | (2.4) | 0 | (0.0) | 1 | (1.9) | 3 | (1.1) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| With events of clinical interest‡ | 2 | (10.0) | 11 | (13.1) | 10 | (11.8) | 3 | (15.0) | 7 | (13.2) | 33 | (12.6) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| With MAAEs | 6 | (30.0) | 9 | (10.7) | 10 | (11.8) | 8 | (40.0) | 5 | (9.4) | 38 | (14.5) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Discontinued vaccine due to an AE | 1 | (5.0) | 5 | (6.0) | 4 | (4.7) | 0 | (0.0) | 3 | (5.7) | 13 | (5.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Discontinued vaccine due to a vaccine-related AE | 1 | (5.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (0.4) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
AE, adverse event; MAAE, medically attended adverse event; PCR, polymerase chain reaction; SAE, serious adverse event; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TCID50, median tissue culture infectious dose.
There were no deaths, serious vaccine-related AEs, or vaccine discontinuations due to a SAE.
Determined by the investigator to be related to the vaccine. SAEs, MAAEs and events of clinical interest were collected throughout the duration of the trial. Other non-SAEs were collected from Day 1 to Day 28 following each injection.
Events of clinical interest included: an overdose of V591 (>1 dose of study vaccine in a 24-hour period or >2 doses of study vaccine throughout the study); laboratory-confirmed diagnosis of SARS-CoV-2 infection (virologically confirmed by PCR) and any resulting sequelae; known exposure to a confirmed case of active SARS-CoV-2; AEs of grade 3 or above reported as a solicited AE on the vaccine report card; acute respiratory distress syndrome; pneumonitis; acute cardiac injury; arrhythmias; septic shock-like syndrome; acute kidney injury; vasculitis; new-onset autoimmune disease; meningitis; atypical measles; anosmia; dysgeusia; encephalitis; encephalopathy; clinically significant arthralgias.
Solicited injection-site pain was reported by 38 participants (14.5%) after injection 1, including 5 (25.0%) of 20 who received V591 1×104 TCID50, 5 (6.0%) of 84 who received V591 1×105 TCID50, 13 (15.3%) of 85 who received V591 1×106 TCID50, 11 (55.0%) of 20 who received V591 1×107 TCID50, and 4 (7.5%) of 53 who received placebo (Table 3). Solicited injection-site erythema was reported by 3 participants (1.1%) after injection 1, including 1 (1.2%) of 84 who received V591 1×105 TCID50 and 2 (2.4%) of 85 who received V591 1×106 TCID50. Solicited injection-site swelling was reported by 3 participants (1.1%) after injection 1; all 3 received V591 1×105 TCID50. Among the 10 participants who received a second injection, one participant in the V591 1×106 TCID50 group reported solicited injection-site pain and one participant in the V591 1×106 TCID50 group reported injection-site erythema.
Table 3.
Solicited adverse events (following injection 1 & 2) (all participants as treated).
| Following injection 1 |
Following injection 2 |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V591 1×104 TCID50 |
V591 1×105 TCID50 |
V591 1×106 TCID50 |
V591 1×107 TCID50 |
Placebo |
TOTAL |
V591 1×105 TCID50 |
V591 1×106 TCID50 |
Placebo |
TOTAL |
|||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Participants in population | 20 | 84 | 85 | 20 | 53 | 262 | 4 | 4 | 2 | 10 | ||||||||||
| Solicited injection-site AEs* | ||||||||||||||||||||
| With ≥1 solicited injection-site AE | 5 | 25.0 | 5 | 6.0 | 13 | 15.3 | 11 | 55.0 | 4 | 7.5 | 38 | 14.5 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 1 | 10.0 |
| Injection-site pain | 5 | 25.0 | 5 | 6.0 | 13 | 15.3 | 11 | 55.0 | 4 | 7.5 | 38 | 14.5 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 1 | 10.0 |
| Solicited injection-site erythema or swelling† | ||||||||||||||||||||
| Injection-site erythema | 0 | 0.0 | 1 | 1.2 | 2 | 2.4 | 0 | 0.0 | 0 | 0.0 | 3 | 1.1 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 | 1 | 10.0 |
| Injection-site swelling | 0 | 0.0 | 3 | 3.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Solicited systemic AEs* | ||||||||||||||||||||
| With ≥1 solicited systemic AE | 10 | 50.0 | 38 | 45.2 | 32 | 37.6 | 13 | 65.0 | 30 | 56.6 | 123 | 46.9 | 1 | 25.0 | 3 | 75.0 | 1 | 50.0 | 5 | 50.0 |
| Arthralgia | 2 | 10.0 | 8 | 9.5 | 7 | 8.2 | 1 | 5.0 | 7 | 13.2 | 25 | 9.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Fatigue | 8 | 40.0 | 22 | 26.2 | 19 | 22.4 | 7 | 35.0 | 20 | 37.7 | 76 | 29.0 | 1 | 25.0 | 2 | 50.0 | 0 | 0.0 | 3 | 30.0 |
| Headache | 4 | 20.0 | 22 | 26.2 | 20 | 23.5 | 7 | 35.0 | 19 | 35.8 | 72 | 27.5 | 0 | 0.0 | 2 | 50.0 | 1 | 50.0 | 3 | 30.0 |
| Myalgia | 3 | 15.0 | 14 | 16.7 | 14 | 16.5 | 3 | 15.0 | 10 | 18.9 | 44 | 16.8 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 1 | 10.0 |
| Nausea | 3 | 15.0 | 6 | 7.1 | 1 | 1.2 | 3 | 15.0 | 7 | 13.2 | 20 | 7.6 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 1 | 10.0 |
| Pyrexia | 1 | 5.0 | 0 | 0.0 | 1 | 1.2 | 0 | 0.0 | 0 | 0.0 | 2 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Rash | 2 | 10.0 | 0 | 0.0 | 2 | 2.4 | 1 | 5.0 | 0 | 0.0 | 5 | 1.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
AE, adverse event; TCID50, median tissue culture infectious dose.
Solicited from Day 1 to Day 5 following each injection. All cases of injection-site erythema and injection-site swelling were ≤2.4 cm.
Solicited from Day 1 to Day 14 following each injection.
One or more solicited systemic AEs were reported by 123 participants (46.9%) after injection 1, including 10 (50.0%) of 20 who received V591 1×104 TCID50, 38 (45.2%) of 84 who received V591 1×105 TCID50, 32 (37.6%) of 85 who received V591 1×106 TCID50, 13 (65.0%) of 20 who received V591 1×107 TCID50, and 30 (56.6%) of 53 who received placebo (Table 3). The frequency of participants reporting solicited systemic AEs after injection 1 across the four V591 dose groups (n=209) versus the placebo group (n=53), respectively, were fatigue (56 [26.8%] vs 20 [37.7%]), headache (53 [25.4%] vs 19 [35.8%]), myalgia (34 [16.3%] vs 10 [18.9%]), arthralgia (18 [8.6%] vs 7 [13.2%]), nausea (13 [6.2%] vs 7 [13.2%]), rash (5 [2.4%] vs 0 [0.0%]), and pyrexia (2 [1.0%] vs 0 [0.0%]).
There were 38 participants (14.5%) with 1 or more MAAE, including 6 (30.0%) of 20 who received V591 1×104 TCID50, 9 (10.7%) of 84 who received V591 1×105 TCID50, 10 (11.8%) of 85 who received V591 1×106 TCID50, 8 (40.0%) of 20 who received V591 1×107 TCID50, and 5 (9.4%) of 53 who received placebo. The most frequent MAAEs, experienced by 3 (1.1%) or more participants overall, were COVID-19 (1 [1.2%] of 84 participants who received V591 1×105 TCID50, 4 [4.7%] of 85 who received V591 1×106 TCID50, and 1 [1.9%] of 53 who received placebo) and nasopharyngitis (1 [5.0%] of 20 who received V591 1×104 TCID50, 1 [1.2%] of 84 who received V591 1×105 TCID50, and 1 [1.2%] of 85 who received V591 1×106 TCID50).
Immunogenicity
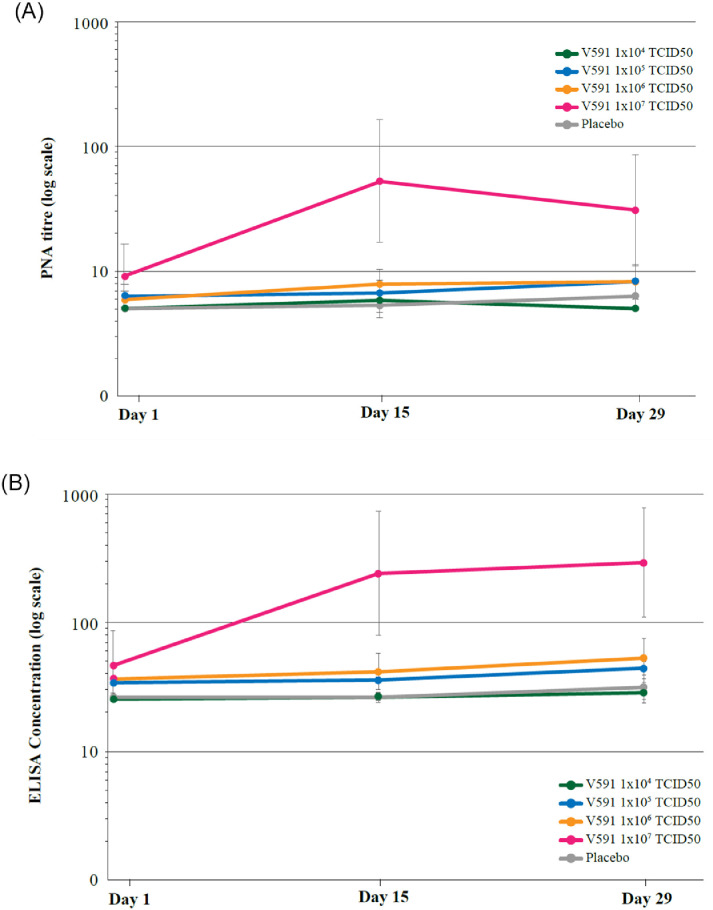
The GMTs of anti-SARS-CoV-2 spike serum nAbs in participants who received a single V591 dose of 1×104, 1×105, or 1×106 TCID50 were comparable at all post-injection time points tested (Day 15 and Day 29) to levels in participants who received placebo; higher titres were observed in participants who received a single V591 dose of 1×107 TCID50 (Figure 3). The GMFR (95% CI) in anti-SARS-CoV-2 spike serum nAbs from Day 1 to Day 29 was 1.0 (1.0–1.0), 1.2 (1.0–1.5), 1.3 (1.1–1.6), and 2.6 (1.2–5.5) for V591 doses of 1×104, 1×105, 1×106 and 1×107 TCID50, respectively, and 1.2 (1.0–1.5) for placebo.
Figure 3.
(A) Longitudinal plot of anti-SARS-CoV-2 spike serum neutralising antibody geometric mean titres (pseudo-virus neutralisation assay) from Day 1 through Day 29 (per-protocol population, 1×104 TCID50 n=20; 1×105 TCID50 n=84, 1×106 TCID50 n=85; 1×107 TCID50 n=20). Error bars represent the geometric standard deviation. (B) Longitudinal plot of anti-SARS-CoV-2 spike immunoglobulin geometric mean concentrations from Day 1 through Day 29 ELISA (per-protocol population, 1×104 TCID50 n=20; 1×105 TCID50 n=84, 1×106 TCID50 n=85; 1×107 TCID50 n=20). Error bars represent the geometric standard deviation. ELISA, enzyme-linked immunosorbent assay; PNA, pseudo-virus neutralisation assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The GMCs of anti-SARS-CoV-2 spike IgG in participants who received a single V591 dose of 1×104, 1×105, or 1×106 TCID50 were comparable at all post-injection time points tested (Day 15 and Day 29) to levels in participants who received placebo; higher titres were observed in participants who received a single V591 dose of 1×107 TCID50 (Figure 3). The GMFR (95% CI) in anti-SARS-CoV-2 spike IgGs from Day 1 to Day 29 was 1.1 (1.0–1.2), 1.2 (1.0–1.4), 1.3 (1.1–1.6), and 3.9 (1.8–8.6) for V591 doses of 1×104, 1×105, 1×106, and 1×107, respectively, and 1.2 (1.0–1.4) for placebo.
Anti-SARS-CoV-2 spike serum nAbs or spike IgG responses in participants who received a single V591 dose, including the highest dose level tested of 1×107 TCID50 (Figure 3), were generally lower than those observed in a panel of sera obtained from patients outside of this study that had recovered from COVID-19 (MK-0000 P411, Table 4). Specifically, in participants who received V591 1×107 TCID50, GMTs (95% CI) of anti-SARS-CoV-2 spike serum nAb at Days 1, 15 and 29 were: 9.1 (5.0, 16.7), 53.1 (17.2, 164.6) and 31.2 (95% CI: 11.2, 86.7), respectively, and GMCs (95% CI) of anti-SARS-CoV-2 spike IgG at Day 1, 15 and 29 were: 46.3 (24.7, 86.8), 239.2 (78.8, 725.9) and 294.8 (110.3, 788.2), respectively (Figure 3). In contrast, in the panel of sera obtained from patients outside of this study that had recovered from COVID-19, GMT (95% CI) of anti-SARS-CoV-2 spike serum nAbs was 164.9 (92.0, 295.5) and GMC (95% CI) of anti-SARS-CoV-2 spike IgG was 1449.9 (833.0, 2523.5) (Table 4).
Table 4.
Summary of responses and GMTs in convalescent sera comparators.
| Assay | n | Observed response (m/n)* | GMT (95% CI)† |
|---|---|---|---|
| ELISA | 28 | 100% (28/28) | 1449.86 (833.00, 2523.52) |
| PNA | 28 | 96.4% (27/28) | 164.89 (92.01, 295.48) |
CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; GMT, geometric mean titre; PNA, pseudo-virus neutralisation assay.
n is the number of patients contributing to the analysis; m is the number of participants with results greater than or equal to the cut-off value that corresponds to an antibody response. For ELISA, the cut-off value is 50.3; for PNA, the cut-off value is 10.
GMTs were calculated based on all participants contributing to the analysis. For assay results below the cut-off value, half of the cut-off value was used, i.e., for ELISA, if x<50.3, then x=25; for PNA, if x<10, then x=5.
Because the trial was terminated early, only 8 participants received two doses of V591 (Day 1 and Day 57): 4 received two doses of 1×105 TCID50 and 4 received two doses of 1×106 TCID50 and 2 received two doses of placebo. Although the sample size in these two groups is too small to yield meaningful results, the GMFR (95% CI) in anti-SARS-CoV-2 spike serum nAbs from Day 1 to Day 85, was 3.5 (0.3–41.3) and 5.4 (3.6–8.1) for V591 doses of 1×105 and 1×106 TCID50, respectively, and 1.0 (1.0–1.0) for placebo.
Discussion
This was one of the first clinical trials of a measles vector-based SARS-CoV-2 vaccine candidate. Although V591 was generally well tolerated, interim immunogenicity results indicated immune responses to a single dose of V591 were inferior to those reported either after natural infection by SARS-CoV-2 or after vaccination with other SARS-CoV-2/COVID-19 vaccines,21, 22, 23 and were predicted24,25 to provide suboptimal protection from disease. There was evidence that neutralizing antibody titres against SARS-COV-2 were predictive of clinical protection against COVID-19. The COVID-19 vaccines approved for emergency use in the US or EU, at the time, all had immunogenicity greater than that developed with natural immunity.23,24 Therefore, the Sponsor decided to terminate the study early, allowing participants to seek COVID-19 vaccinations outside of the study and the development of V591 was discontinued.
It is unclear why V591 resulted in limited immunogenicity, although further analyses of archived samples collected from this study may provide greater understanding. An inherently low immunogenicity potential of the delivered spike antigen is possible, but considered unlikely given that the chosen antigen design17 was similar to that used in other, immunogenic, conditionally authorised SAR2-CoV-2 vaccines.23,26 Another hypothesis is the existence of pre-existing measles (i.e., anti-vector) immunity in participants prior to V591 dosing.27 Pre-existing anti-vector immunity has been a concern with other SARS-CoV-2 vaccines, such as the Ad5-nCOV vaccine (CanSino), which demonstrated approximately two-fold lower SARS-CoV-2 nAb levels in those with high pre-existing anti-vector immunity compared with those with low pre-existing anti-vector immunity.28 In support of this hypothesis, another early-phase trial of V591 in healthy adults (COVID-19-101; NCT04497298, V591 was named TMV-083 in the study protocol) that was conducted at the same time as this trial also found that V591 injection elicited insufficient immunogenicity, and the immune response to V591 appeared to be impacted by pre-existing anti-measles immunity.17 This reduced immunogenicity in measles-experienced subjects was not seen in previous trials with the Schwarz measles vector platform, which demonstrated a strong and persistent cellular and humoral immune response.7,16 Also, the vaccine candidate against chikungunya virus (MV-CHIK), using the same measles vector, demonstrated good immunogenicity and favourable safety and tolerability in randomised phase 1 and 2 trials, despite the presence of pre-existing anti-vector immunity.7,16 This suggests that the measles vector platform is not the sole explanation for the results with V591, but does not rule out the possibility that pre-existing anti-measles immunity may reduce immunogenicity at the vaccine doses tested in this study. It is also possible that not all inserts used with this vector will be expressed similarly, resulting in a variable relationship between pre-existing immunity to the vector and immunogenicity to the target antigen.
It is also possible that multiple doses of V591 could result in a more favourable immunogenicity profile. While this was intended to be tested in this trial, early termination resulted in only a few participants from part 1 with available data post-dose 2. Given the small sample size, no meaningful conclusions can be drawn from these data. A two-dose regimen was tested, however, in the other early-phase trial of V591 referenced above, and while the second immunisation elicited nAbs in the majority of subjects receiving the 1×105 dose, the frequency and magnitude of the response was still insufficient to warrant further development.
Various specific hypotheses are being explored to explain the reasons for the limited immunogenicity of the V591 vaccine candidate and findings will be reported separately.
Declaration of interests
WL, RTW, LH, XC, MD, JH, JL, MMcG, KR, YT, RT, DDB, WX, KR, SAS and EL are employees of Merck Sharpe & Dohme, Corp., and division of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock or hold stock options in the Company. JRS is an employee of Merck Sharpe & Dohme, Corp. and division of Merck & Co., Inc., Kenilworth, NJ, USA, holding stocks alongside stocks in various pharmaceutical and biotechnology companies and service providers, has participated on various editorial boards and committees for Society for Industrial and Applied Mathematics and is a member and participant in committees for the International Society of Pharmacometrics. KB was an employee of Merck Sharpe & Dohme, Corp., and division of Merck & Co., Inc., Kenilworth, NJ, USA until February 2021. AA was an employee of Merck Sharpe & Dohme, Corp., and division of Merck & Co., Inc., Kenilworth, NJ, USA, until September 2021. All other authors report no conflicts of interest.
Acknowledgments
Contributors
FV contributed to the trial conduct, data collection and data interpretation. AA and RT contributed to the trial conceptualisation, trial design, data interpretation and the writing of the report. WL, RTW, XC and DDB contributed to the trial design, data analysis and interpretation. LH contributed to the trial conceptualisation, trial design, trial implementation and monitoring, and data collection. KB contributed to the trial conceptualisation, trial design, data collection and interpretation. MD contributed to the management of the trial. JH and MMcG contributed to the data interpretation and the writing of the report. JL contributed to the trial design, data collection, data analysis and interpretation. KR contributed to the trial design, trial supervision and data interpretation. YT contributed to the trial design, data interpretation and the writing of the report. WX contributed to the data collection, analysis, and interpretation. JRS contributed to the conceptualisation of the trial, trial design, data analysis and interpretation and the writing of the report. KR contributed to the trial design, trial implementation and monitoring, and data interpretation. SAS contributed to the trial conceptualisation, trial design, data collection, data analysis and interpretation. EL contributed to the trial conceptualisation, supervision of trial conduct and writing of the report. The underlying data were verified by EL, WL, RTW, XC and JRS and all authors had access to the study data. The final version of this paper was reviewed and approved by all authors.
Acknowledgments
Professional medical writing and editorial assistance was provided by Megan Perkins of ApotheCom (London, UK) and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. We are grateful to Christiane Gerke and Nicolas Escriou (Institut Pasteur, Paris, France) for critical review of the manuscript. The trial was funded by Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Data sharing statement
The data sharing policy, including restrictions, of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the Engage Zone site or via email to dataaccess@merck.com.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103811.
Appendix. Supplementary materials
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 17 May 2021 2021. https://covid19.who.int/.
- 2.Brisse M, Vrba SM, Kirk N, Liang Y, Ly H. Emerging concepts and technologies in vaccine development. Front Immunol. 2020;11:2578. doi: 10.3389/fimmu.2020.583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shang Z, Chan SY, Liu WJ, Li P, Huang W. Recent insights into emerging coronavirus: SARS-CoV-2. ACS Infectious Dis. 2020;7(6):1369–1388. doi: 10.1021/acsinfecdis.0c00646. [DOI] [PubMed] [Google Scholar]
- 4.Nagy A, Alhatlani B. An overview of current COVID-19 vaccine platforms. Comput Struct Biotechnol J. 2021;19:2508–2517. doi: 10.1016/j.csbj.2021.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarabel L, Guardascione M, Dal Bo M, Toffoli G. Pharmacological strategies to prevent SARS-CoV-2 infection and to treat the early phases of COVID-19 disease. Int J Infect Dis. 2021;104:441–451. doi: 10.1016/j.ijid.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Combredet C, Labrousse V, Mollet L, et al. A molecularly cloned Schwarz strain of measles virus vaccine induces strong immune responses in macaques and transgenic mice. J Virol. 2003;77(21):11546–11554. doi: 10.1128/JVI.77.21.11546-11554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reisinger EC, Tschismarov R, Beubler E, et al. Immunogenicity, safety, and tolerability of the measles-vectored chikungunya virus vaccine MV-CHIK: a double-blind, randomised, placebo-controlled and active-controlled phase 2 trial. Lancet North Am Ed. 2018;392(10165):2718–2727. doi: 10.1016/S0140-6736(18)32488-7. [DOI] [PubMed] [Google Scholar]
- 8.Brandler S, Marianneau P, Loth P, et al. Measles vaccine expressing the secreted form of West Nile virus envelope glycoprotein induces protective immunity in squirrel monkeys, a new model of West Nile virus infection. J Infect Dis. 2012;206(2):212–219. doi: 10.1093/infdis/jis328. [DOI] [PubMed] [Google Scholar]
- 9.Brandler S, Ruffié C, Combredet C, et al. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine. 2013;31(36):3718–3725. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- 10.Lorin C, Mollet L, Delebecque F, et al. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J Virol. 2004;78(1):146–157. doi: 10.1128/JVI.78.1.146-157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodmer BS, Fiedler AH, Hanauer JRH, Prüfer S, Mühlebach MD. Live-attenuated bivalent measles virus-derived vaccines targeting Middle East respiratory syndrome coronavirus induce robust and multifunctional T cell responses against both viruses in an appropriate mouse model. Virology. 2018;521:99–107. doi: 10.1016/j.virol.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stebbings R, Février M, Li B, et al. Immunogenicity of a recombinant measles-HIV-1 clade B candidate vaccine. PLoS One. 2012;7(11):e50397. doi: 10.1371/journal.pone.0050397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateo M, Reynard S, Carnec X, et al. Vaccines inducing immunity to Lassa virus glycoprotein and nucleoprotein protect macaques after a single shot. Sci Transl Med. 2019;11(512) doi: 10.1126/scitranslmed.aaw3163. [DOI] [PubMed] [Google Scholar]
- 14.Nürnberger C, Bodmer BS, Fiedler AH, Gabriel G, Mühlebach MD. A measles virus-based vaccine candidate mediates protection against Zika virus in an allogeneic mouse pregnancy model. J Virol. 2019;93(3) doi: 10.1128/JVI.01485-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escriou N, Callendret B, Lorin V, et al. Protection from SARS coronavirus conferred by live measles vaccine expressing the spike glycoprotein. Virology. 2014;452:32–41. doi: 10.1016/j.virol.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsauer K, Schwameis M, Firbas C, et al. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect Dis. 2015;15(5):519–527. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- 17.Launay O, Artaud C, Lachâtre M, et al. Safety and immunogenicity of a measles-vectored SARS-CoV-2 vaccine candidate, V591 /TMV-083, in healthy adults: results of a randomized, placebo-controlled Phase I study. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2021.103810. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Food & Drug Administration. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. 2007. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical (accessed 25 May 2021).
- 19.Bewley KR, Coombes NS, Gagnon L, et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat Protoc. 2021;16:3114–3140. doi: 10.1038/s41596-021-00536-y. [DOI] [PubMed] [Google Scholar]
- 20.Grzelak L, Temmam S, Planchais C, et al. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci Transl Med. 2020;12(559) doi: 10.1126/scitranslmed.abc3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet North Am Ed. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet North Am Ed. 2020;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a Trial of Ad26. COV2. S Covid-19 Vaccine. N Engl J Med. 2021;384(19):1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Largajolli A, Plock N, Kandala B, et al. Cross-species translation of correlates of protection for COVID-19 vaccine candidates using quantitative tools. ID Week. Virtual. 2021 [Google Scholar]
- 25.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021:1–7. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 26.Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu F-C, Guan X-H, Li Y-H, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet North Am Ed. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.