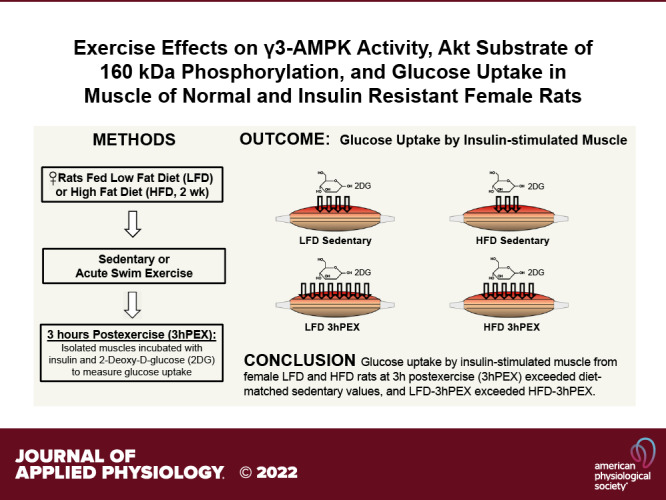
Keywords: acute exercise, AMP-activated protein kinase, glucose uptake, insulin resistance, TBC1D4
Abstract
Previous studies demonstrated that acute exercise can enhance glucose uptake (GU), γ3-AMP-activated protein kinase (AMPK) activity, and Akt substrate of 160 kDa (AS160) phosphorylation in skeletal muscles from low-fat diet (LFD)- and high-fat diet (HFD)-fed male rats. Because little is known about exercise effects on these outcomes in females, we assessed postexercise GU by muscles incubated ± insulin, delta-insulin GU (GU of muscles incubated with insulin minus GU uptake of paired muscles incubated without insulin), and muscle signaling proteins from female rats fed a LFD or a brief HFD (2 wk). Rats were sedentary (LFD-SED, HFD-SED) or swim exercised. Immediately postexercise (IPEX) or 3 h postexercise (3hPEX), epitrochlearis muscles were incubated (no insulin IPEX; ±insulin 3hPEX) to determine GU. Muscle γ3-AMPK activity (IPEX, 3hPEX) and phosphorylated AS160 (pAS160; 3hPEX) were also assessed. γ3-AMPK activity and insulin-independent GU of IPEX rats exceeded sedentary rats without diet-related differences in either outcome. At 3hPEX, both GU by insulin-stimulated muscles and delta-insulin GU exceeded their respective diet-matched sedentary controls. GU by insulin-stimulated muscles, but not delta-insulin GU for LFD-3hPEX, exceeded HFD-3hPEX. LFD-3hPEX versus LFD-SED had greater γ3-AMPK activity and greater pAS160. HFD-3hPEX exceeded HFD-SED for pAS160 but not for γ3-AMPK activity. pAS160 and γ3-AMPK at 3hPEX did not differ between diet groups. These results revealed that increased γ3-AMPK activity at 3hPEX was not essential for greater GU in insulin-stimulated muscle or greater delta-insulin GU in HFD female rats. Similarly elevated γ3-AMPK activity in LFD-IPEX versus HFD-IPEX and pAS160 in LFD-3hPEX versus HFD-3hPEX may contribute to the comparable delta-insulin GU at 3hPEX in both diet groups.
NEW & NOTEWORTHY Glucose uptake (GU) and phosphorylated AS160 (pAS160) by insulin-stimulated muscles at 3 h postexercise (3hPEX) exceeded diet-matched controls in female low-fat diet-fed (LFD) or high-fat diet-fed (HFD) rats. GU with insulin for LFD-3hPEX exceeded HFD-3hPEX, whereas pAS160 was similar between these groups. γ3-AMPK immediately postexercise (IPEX) was similarly elevated in LFD and HFD, but only LFD-3hPEX had increased γ3-AMPK. These results suggest that greater γ3-AMPK at IPEX and pAS160 at 3hPEX may contribute to elevated GU with insulin, but greater γ3-AMPK at 3hPEX was dispensable for female HFD rats.
INTRODUCTION
It is well documented that a single exercise session can result in subsequently elevated insulin-stimulated glucose uptake by skeletal muscle from healthy humans and rodents (1–8). The vast majority of this research has been on males, but a few studies have also reported elevated insulin sensitivity after acute exercise by healthy female humans and rodents (9–11). It has also been demonstrated that one exercise session can enhance insulin-stimulated glucose uptake by skeletal muscle from insulin-resistant male humans and rodents (12–16). Published information is extremely limited about the effects of acute exercise on insulin-stimulated glucose uptake by skeletal muscle from insulin-resistant females.
Insulin resistance was detectable after only 1–3 wk of a high-fat diet (HFD) in male mice, before major increases in body mass or body fat, and muscle insulin resistance did not deteriorate further when the HFD was continued for 16 wk (17). Kraegen et al. (18) reported that 3 wk of HFD resulted in insulin resistance in the soleus, red gastrocnemius, and white gastrocnemius (WG) but not the extensor digitorum longus (EDL) of male rats. Insulin resistance has also been reported in the epitrochlearis of male rats eating a HFD for 2 wk (16, 19). The fiber type profile is quite similar for the WG, EDL, and epitrochlearis of rats (20), so the differing results for the EDL are not attributable to fiber type. Castorena et al. (12) studied the effects of acute exercise on insulin-stimulated glucose uptake by skeletal muscle from male rats after either a low-fat diet (LFD) or a HFD (2 wk) to assess the ability of exercise to oppose insulin resistance with a brief HFD. Insulin-stimulated glucose uptake by skeletal muscle from both LFD and HFD rats was elevated after exercise compared with diet-matched, sedentary control rats. Furthermore, insulin-stimulated glucose uptake after exercise was greater in muscles from the LFD versus the HFD male rats. Subsequent studies in male rats assessed exercise effects on glucose uptake and changes in potential regulatory processes in skeletal muscle with the same 2-wk HFD protocol (16, 21, 22). In the context of these foundational studies focused exclusively on male rats, we used the same 2-wk HFD protocol in female rats in the present study. The first aim of the present study was to determine the effects of one exercise session on subsequent insulin-stimulated glucose uptake by skeletal muscles from female LFD and HFD rats. We hypothesized that the insulin-stimulated glucose uptake would be greatest for the postexercise LFD group and that the postexercise HFD values would exceed the values for the sedentary HFD group.
It has been widely reported that the improved insulin sensitivity after acute exercise can occur without elevation in proximal insulin signaling events, including Akt phosphorylation (6, 7, 12, 23, 24). Phosphorylation of the Rab-GTPase activating protein known as Akt substrate of 160 kDa (also called AS160 or TBC1D4) is important for insulin-stimulated glucose uptake (25–28). A number of studies have found that prior exercise can result in elevated phosphorylation of AS160 in insulin-stimulated skeletal muscle (23, 24, 29–32). However, virtually all of the published research on insulin signaling after acute exercise has focused on skeletal muscles from male humans or rodents. Therefore, the second aim of the present study was to evaluate the influence of acute exercise on the phosphorylation of Akt and AS160 in skeletal muscles from female LFD and HFD rats. We hypothesized that insulin-stimulated Akt phosphorylation would not differ among the groups and that insulin-stimulated AS160 phosphorylation would be greatest for the postexercise LFD group, with the values for the postexercise HFD group greater than the values for sedentary HFD group.
AMP-activated protein kinase (AMPK), a heterotrimeric protein that includes a catalytic subunit (α1 or α2 isoform) and two regulatory subunits (β1 or β2 isoform and γ1, γ2, or γ3 isoform), has been implicated in the regulation of muscle glucose uptake after exercise (33). The γ3 regulatory isoform is selectively expressed in skeletal muscle, and γ3-AMPK activity has been reported to be elevated both immediately postexercise (IPEX) and several hours after exercise that leads to enhanced insulin-stimulated glucose uptake in male LFD and HFD rats (16, 24). Furthermore, prior treatment of skeletal muscle with the AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) leads to subsequently enhanced insulin-stimulated glucose uptake by skeletal muscle from wild-type mice but not γ3-null mice (34). In addition, prior electrically stimulated muscle contractions lead to elevation of both γ3-AMPK activity and insulin-stimulated glucose uptake in mouse skeletal muscles 3 h after contraction (35). These observations support the idea that γ3-AMPK may have a role in enhanced insulin sensitivity after various interventions, including muscle contractions, AICAR treatment, and exercise (36). The postexercise increase in γ3-AMPK activity has not been assessed in skeletal muscle from female rats. Accordingly, the third aim of this study was to determine the effect of exercise [IPEX and 3 h postexercise (3hPEX)] on γ3-AMPK in muscles from female LFD and HFD rats. We hypothesized that γ3-AMPK from the postexercise LFD rats would be greater than all other groups and that the postexercise HFD values would be greater than both of the sedentary groups.
MATERIALS AND METHODS
Materials
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Hanover Park, IL) unless otherwise noted. The reagents and apparatus for SDS-PAGE and nonfat dry milk (no. 170-6404) were from Bio-Rad (Hercules, CA). Pierce MemCode Reversible Protein Stain Kit (#24585), bicinchoninic acid protein assay (#23225), Tissue Protein Extraction Reagent (T-PER; #78510), and protein G-magnetic beads (#10004 D) and DynaMag-2 magnet (#12321 D) were obtained from Thermo Fisher Scientific (Waltham, MA). Anti-phospho Akt Ser473 (pAktSer473; #9271), anti-phospho Akt Thr308 (pAktThr308; #13038), anti-Akt (#4691), anti-phospho AS160 Thr642 (pAS160Thr642; #8881), anti-phospho AS160 Ser588 (pAS160Ser588; #8730), anti-phospho AMPKα Thr172 (pAMPKαThr172; #2531), anti-AMPK-α (AMPKα; #5831), anti-acetyl CoA carboxylase (ACC; #3676), anti-phospho ACC Ser79 (pACCSer79; #3661), anti-hexokinase II (HKII; #2867), and anti-rabbit IgG horseradish peroxidase (HRP) conjugate (#7074) were from Cell Signaling Technology (Danvers, MA). Anti-phospho AS160 Ser704 (pAS160Ser704) was custom made by Capra Science (Angelholm, Sweden) and provided by J.T.T. Anti-AMP-activated protein kinase γ3 (γ3-AMPK) was provided by Dr. David Thomson (Brigham Young University, Provo, UT) (37). Anti-Akt substrate of 160 kDa (AS160; #ABS54), anti-glucose transporter type 4 (GLUT4; #CBL243), P81 Phosphocellulose Squares (#20–134), and enhanced chemiluminescence Luminata Forte Western HRP Substrate (#WBLUF0100) were from EMD Millipore (Billerica, MA). 2-Deoxy-d-[3H]-glucose ([3H]-2-DG) and [14C]mannitol were from PerkinElmer (Boston, MA). [γ-33P]ATP was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Liquid scintillation cocktail (#111195-CS) was obtained from Research Products International (Mount Prospect, IL).
Table 1 provides the source, dilution, and validation for each of the antibodies used (24, 38–40).
Table 1.
Names, sources, dilutions, and validation of antibodies used
| Antibody Name (abbreviation) | Source (catalog no.) | Dilution | Validation |
|---|---|---|---|
| Anti-Akt | Cell Signaling Technology (#4691) | 1:1,000 | • Validated using cells transfected with siRNA (38) • Widely cited (∼2,600 citations) |
| Anti-AS160 | MilliporeSigma (#ABS54) | 1:1,000 | Validated using muscles from wild-type and AS160-KO rats (39, 40) |
| Anti-γ3-AMPK | Dr. David Thomson, Brigham Young Univ., Provo, UT | 1:1,000 | Validated using muscles from wild-type and γ3-AMPK-KO mice (24) |
| Anti-GLUT4 | MilliporeSigma (#CBL243) | 1:100 | Validated using muscles from rats injected with AAV-GLUT4 vs. muscles from rats with sham injection (unpublished data from G.D.C.) |
| Anti-phospho Akt Ser473 (pAktSer473) | Cell Signaling Technology (#4060) | 1:1,000 | • Vendor validated antibody using cells stimulated by PDGF ± specific inhibitors (LY294002/wortmannin) • Widely cited (∼5,600 citations) |
| Anti-phospho Akt Thr308 (pAktThr308) | Cell Signaling Technology (#13038) | 1:1,000 | • Vendor verified antibody using cells stimulated with PDGF • Widely cited (∼500 citations) |
| Anti-phospho AMPKα Thr172 (pAMPKThr172) | Cell Signaling Technology (#2535) | 1:1,000 | Vendor validated antibody using cells treated with oligomycin |
| Anti-phospho AS160 Ser588 (pAS160Ser588) | Cell Signaling Technology (#8730) | 1:1,000 | • Vendor validated antibody phosphospecificity by λ phosphatase treatment. • Validated using AS160-KO rat muscles injected with AAV-AS160 with Ser588 mutated to Ala588 vs. muscles from wild-type rats with sham injection (unpublished data from G.D.C.) |
| Anti-phospho AS160 Thr642 (pAS160Thr642) | Cell Signaling Technology (#4288) | 1:1,000 | • Vendor validated antibody phosphospecificity by λ phosphatase treatment • Validated using AS160-KO rat muscles injected with AAV-AS160 with Thr642 mutated to Ala642 vs. muscles from wild-type rats with sham injection (unpublished data from G.D.C.) |
| Anti-phospho AS160 Ser704 (pAS160Ser7104) | J.T.T. | 1:1,250 | • Validated using mouse muscles with gene electrotransfer of flag-tagged AS160 with Ser704 mutated to Ala704 vs. mouse muscles with electrotransferred flag-tagged wild-type AS160 (unpublished data from J.T.T.) • Validated using AS160-KO rat muscles injected with AAV-AS160 with Ser704 mutated to Ala704 vs. muscles from wild-type rats with sham injection (unpublished data from G.D.C.) |
| Anti-rabbit IgG, HRP-linked | Cell Signaling Technology (#7074) | 1:20,000 | Widely cited (∼7,600 citations) |
AAV, adeno-associated virus; AMPK, AMP-activated protein kinase; AS160, Akt substrate of 160 kDa; GLUT4, glucose transporter type 4; HRP, horseradish peroxidase; KO, knockout; PDGF, platelet-derived growth factor.
Animal Treatment
Animal care procedures were approved by the University of Michigan Committee on Use and Care of Animals and performed in accordance with the guidelines from the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Female Wistar rats (8–9 wk old when diet intervention was initiated) were individually housed on a 12:12-h light-dark cycle (lights out at 1800) and provided with standard rodent chow (LFD: 14% kcal fat, 57% kcal carbohydrate, and 29% kcal protein; Laboratory Diet no. 5L0D; LabDiet, St. Louis, MO) or high-fat chow (HFD: 60% kcal fat, 20% kcal carbohydrate, and 20% kcal protein; Laboratory Diet no. D12492; Research Diets, New Brunswick, NJ) and water ad libitum for 2 wk.
Rats were fasted the night before the experiment day at ∼1900. The following morning at ∼0830, rats either remained sedentary or swam in a barrel filled with water (35°C, ∼45-cm depth, 6 rats swimming at a time) for 4 × 30-min bouts, with 5-min rest between the bouts. Then rats were anesthetized with an intraperitoneal injection of ketamine-xylazine cocktail (50 mg/kg ketamine and 5 mg/kg xylazine) either immediately postexercise (IPEX) or 3 h postexercise (3hPEX) along with time-matched sedentary (SED) rats. Both epitrochlearis muscles from each rat were dissected out before euthanasia while the rat was deeply anesthetized. After muscle dissections, the retroperitoneal fat pads were dissected and weighed.
Muscle Incubation
For the IPEX experiment, one epitrochlearis muscle was trimmed, freeze-clamped with aluminum clamps cooled to the temperature of liquid N2, and stored at −80°C until being processed for further analysis. The contralateral epitrochlearis muscle was placed in a vial containing 2 mL of Krebs–Henseleit buffer (KHB) supplemented with 0.1% bovine serum albumin (BSA), 2 mM sodium pyruvate, and 6 mM mannitol (solution 1) for 10 min. Muscles were then transferred to another vial containing 2 mL of KHB-BSA, 1 mM 2-DG (with final specific activity of 2.25 mCi/mmol [3H]-2-DG), and 9 mM mannitol (with final specific activity of 0.022 mCi/mmol [14C]mannitol) (solution 2) for 15 min. For the 3hPEX experiment, paired epitrochlearis muscles were incubated for 30 min in vials containing 2 mL of solution 1 with or without insulin (0.6 nM). After this initial incubation, the muscles were incubated for 20 min in another vial containing 2 mL of solution 2 with the same insulin concentration as in the previous incubation step. For all incubation steps, the vials were shaken at 45 oscillations/min and continuously gassed (95% O2-5% CO2) in a heated (35°C) water bath. After the final incubation step, muscles were blotted, freeze-clamped, and stored at −80°C until later processing and analysis.
Muscle Lysate Preparation
Frozen muscles were weighed and then homogenized with 1 mL of ice-cold lysis buffer with a glass pestle attached to a motorized homogenizer (Caframo, Georgian Bluffs, ON, Canada). For muscle lysates used for determination of γ3-AMPK activity, the lysis buffer included 10% glycerol, 20 mmol/L sodium pyrophosphate (NaPP), 1% NP-40, 2 mmol/L phenylmethylsulfonyl fluoride (PMSF), 150 mmol/L sodium chloride (NaCl), 50 mmol/L HEPES (pH 7.5), 20 mmol/L β-glycerophosphate, 10 mmol/L sodium fluoride (NaF), 1 mmol/L EDTA, 1 mmol/L EGTA, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 2 mmol/L sodium vanadate (Na3VO4). For muscle lysates used for 2-deoxy-d-glucose (2-DG) uptake and immunoblotting, the lysis buffer contained T-PER supplemented with 1 mM EDTA, 1 mM EGTA, 2.5 mM NaPP, 1 mM Na3VO4, 1 mM β-glycerophosphate, 1 µg/mL leupeptin, and 1 mM PMSF. Homogenates were rotated for 1 h at 4°C before centrifugation (15,000 g for 15 min at 4°C). The supernatants were transferred to microfuge tubes and stored at −80°C until subsequent analyses. Protein concentration was measured with the bicinchoninic acid procedure.
Muscle 2-DG Uptake
Aliquots of the supernatants (200 μL) from muscle lysates were pipetted into a vial together with 8 mL of scintillation cocktail. A scintillation counter (PerkinElmer) was used to determine the 3H and 14C disintegrations per minute. 2-DG uptake was calculated as previously described (41, 42). Delta-insulin 2-DG uptake of muscles was calculated (2-DG uptake of muscles incubated with insulin minus 2-DG uptake of paired muscles incubated without insulin).
γ3-AMPK Activity Assay
The specificity of the γ3-AMPK antibody used for immunoprecipitation (IP) has been previously confirmed (24). AMPK activity was determined as previously described (34). Briefly, muscle lysates (300 µg protein) were rotated at 4°C overnight with γ3-AMPK antibody (1:500) and IP buffer [50 mmol/L NaCl, 1% Triton X-100, 50 mmol/L NaF, 5 mmol/L NaPP, 20 mmol/L Tris-base (pH 7.5), 500 μmol/L PMSF, 2 mmol/L dithiothreitol (DTT), 5 μg/mL leupeptin, 50 μg/mL soybean trypsin inhibitor, 6 mM benzamidine, and 250 mmol/L sucrose]. Then 50 μL of protein G-magnetic beads were added to the muscle lysate/antibody mixture and rotated for 2 h at 4°C. A DynaMag-2 magnet was used to pellet the protein G-immunocomplex. Each immunopellet was washed once in IP buffer, once in 6× assay buffer (240 mmol/L HEPES, 480 mmol/L NaCl, pH 7.0), and twice in 3× assay buffer (1:1). Then the activity assay was performed in 30 μL of kinase mix buffer (40 mmol/L HEPES, pH 7.5, 80 mmol/L NaCl, 800 μmol/L DTT, 200 μmol/L AMP, 100 μmol/L AMARA peptide, 5 mmol/L magnesium chloride, 200 μmol/L ATP, and 2 µCi of [γ-33P]ATP) for 30 min at 30°C. The reaction was stopped by the addition of 10 μL of 1% phosphoric acid. Next, 30 µL of supernatant was spotted on P81 phosphocellulose paper. After 4 × 15-min washing with 1% phosphoric acid and 1 × 5-min washing with acetone, the phosphocellulose paper was dried and placed in the vials containing 8 mL of scintillation cocktail for scintillation counting. Results are expressed relative to the normalized mean of all the samples from each experiment.
Muscle Glycogen Measurement
Muscle glycogen was determined with a Glycogen Assay Kit (#MAK016, Sigma-Aldrich, St. Louis, MO). Briefly, epitrochlearis muscles were homogenized in ice-cold water. The homogenates were boiled for 5 min at 95°C to inactivate enzymes. Then samples were centrifuged at 13,000 g for 5 min, and supernatant was saved. Colorimetric measurements were performed according to the manufacturer’s protocol. The absorbance was measured at 570 nm with a microplate reader.
Immunoblotting
An equal amount of protein from each muscle lysate was mixed with 6× Laemmli buffer, boiled at 95°C for 5 min, subjected to SDS-PAGE, and transferred to polyvinylidene difluoride membranes. Equal loading was confirmed with the MemCode protein stain (43). Membranes were blocked with TBST (Tris-buffered saline pH 7.5 plus 0.1% Tween 20) that was supplemented with either 5% BSA or 5% nonfat milk for 1 h at room temperature, incubated with appropriate concentrations of primary and secondary antibodies, subjected to enhanced chemiluminescence, and quantified by densitometry (AlphaView; ProteinSimple, San Jose, CA). Each gel used for immunoblotting included equal numbers of samples from each treatment condition in the respective experiments (IPEX or 3hPEX). For IPEX experiments there were four treatment conditions (LFD-SED, LFD-IPEX, HFD-SED, HFD-IPEX), and n = 2 samples from each treatment group were included on each immunoblot. For 3hPEX experiments, there were eight treatment conditions (LFD-SED without insulin, LFD-SED with insulin, HFD-SED without insulin, HFD-SED with insulin, LFD-3hPEX without insulin, LFD-3hPEX with insulin, HFD-3hPEX without insulin, HFD-3hPEX with insulin), and n = 1 sample from each treatment group was included on each immunoblot. Results for each sample (densitometric units) were expressed relative to the normalized average of all the samples on the blot. These normalized values were divided by the corresponding MemCode loading control value for each sample (using individual sample MemCode values that were normalized by dividing the mean MemCode values for all samples on each blot). Values for phosphorylated proteins are expressed as the ratio of phosphorylated protein to total protein (determined for each sample with a separate immunoblot with a primary antibody against the appropriate total protein). Delta-insulin for phosphorylated insulin signaling proteins (Akt and AS160) was calculated (phosphoprotein ratio value of muscles incubated with insulin minus phosphoprotein ratio value of paired muscles incubated without insulin).
Statistical Analysis
Student’s t test was used for comparisons between two groups. Two-way analysis of variance (ANOVA) was used to assess the main and interaction effects of diet (LFD or HFD) and exercise (SED or 3hPEX) within each insulin level (minus or plus insulin). Post hoc analysis was performed with the Tukey test (SigmaPlot version 14.0; Systat Software, San Jose, CA). Data lacking normal distribution and/or equal variance were mathematically transformed to achieve normality and equal variance before statistical analysis.
RESULTS
Body Mass, Retroperitoneal Fat Mass, and Retroperitoneal-to-Body Mass Ratio
After the 2-wk diet intervention, the HFD rats versus LFD rats had greater body mass (261 ± 9 vs. 233 ± 8 g; P < 0.0001), retroperitoneal fat mass (1,502 ± 179 vs. 556 ± 64 mg; P < 0.001), and retroperitoneal-to-body mass ratio (5.7 ± 0.6 vs. 2.4 ± 0.2; P < 0.001).
IPEX: 2-DG Uptake, Muscle Glycogen, and γ3-AMPK Activity
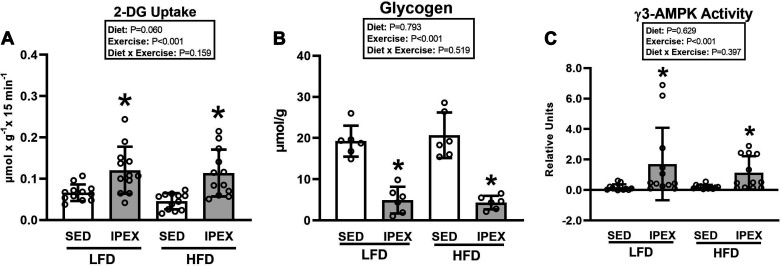
For insulin-independent 2-DG uptake in muscles, there was a significant main effect of exercise (IPEX exceeded SED; P < 0.001; Fig. 1A). Post hoc analysis indicated that 2-DG uptake was greater for muscles from IPEX versus SED in each diet group (P < 0.001 for LFD; P < 0.01 for HFD).
Figure 1.
A: 2-deoxy-d-glucose (2-DG) uptake (insulin independent) in epitrochlearis muscles immediately postexercise (IPEX). *IPEX vs. sedentary (SED) within each diet [P < 0.001 for low-fat diet (LFD) group; P < 0.01 for high-fat diet (HFD) group]. B: glycogen in epitrochlearis muscles at IPEX. *IPEX vs SED within each diet (P < 0.001). C: γ3-AMP-activated protein kinase (AMPK) activity in epitrochlearis muscles at IPEX. *IPEX vs. SED within each diet (P < 0.05). Data were analyzed by 2-way analysis of variance. Tukey post hoc analysis was performed to identify significant differences. Values are means ± SD; n = 6–12 rats per treatment group.
For muscle glycogen, there was a significant main effect of exercise (IPEX exceeded SED; P < 0.001; Fig. 1B). Post hoc analysis indicated that glycogen was lower for IPEX versus SED in both LFD (P < 0.001) and HFD (P < 0.001) groups.
For γ3-AMPK activity, there was a significant main effect of exercise (IPEX exceeded SED; P < 0.001; Fig. 1C). Post hoc analysis indicated that γ3-AMPK activity was significantly increased for IPEX versus SED within each diet group (P < 0.05). γ3-AMPK activity did not differ between diet groups for either SED or IPEX.
IPEX: Immunoblotting
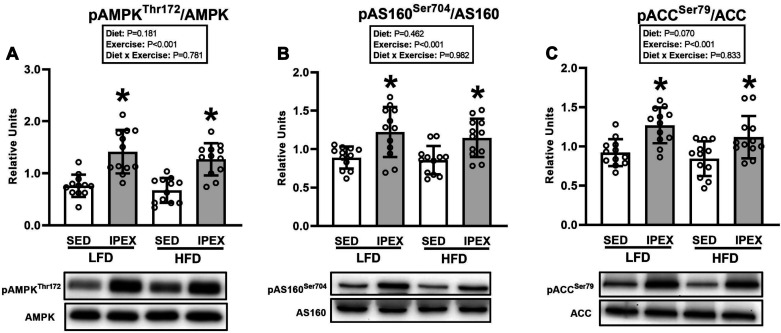
There were no significant effects of diet or exercise on AMPK or AS160 total abundance (not shown). For ACC total abundance analyzed by two-way ANOVA, there was a significant main effect of diet (HFD exceeded LFD; P < 0.05, not shown). Post hoc analysis revealed that the HFD-SED group was slightly greater than the LFD-SED group (∼13%, P < 0.01).
For all of the phosphorylated proteins, the data are expressed as a ratio of the phosphorylated to total protein values. For AMPKα Thr172 phosphorylation, there was a significant main effect of exercise (IPEX exceeded SED; P < 0.001; Fig. 2A). Post hoc analysis revealed that pAMPKαThr172/AMPKα was greater for muscles from IPEX versus SED within each diet group (P < 0.001). For AS160 Ser704 phosphorylation, there was a significant main effect of exercise (IPEX exceeded SED, P < 0.001, Fig. 2B). Post hoc analysis indicated that pAS160Ser704/AS160 was significantly increased for muscles from IPEX versus SED in both LFD (P < 0.001) and HFD (P < 0.01) groups. For ACC Ser79 phosphorylation, there was a significant main effect of exercise (IPEX exceeded SED; P < 0.001; Fig. 2C). Post hoc analysis indicated greater pACCSer79/ACC in the IPEX group compared with SED controls with each diet (P < 0.01).
Figure 2.
A: phosphorylated (p) AMP-activated protein kinase (AMPK)αThr172/AMPKα in epitrochlearis muscles immediately postexercise (IPEX). *IPEX vs. sedentary (SED) within each diet (P < 0.001). HFD, high-fat diet; LFD, low-fat diet. B: phosphorylated Akt substrate of 160 kDa (AS160)Ser704/AS160 in epitrochlearis muscles at IPEX. *IPEX vs. SED within each diet (P < 0.001 for LFD group; P < 0.01 for HFD group). C: phosphorylated acetyl CoA carboxylase (ACC)Ser79/ACC in epitrochlearis muscles at IPEX. *IPEX vs. SED within each diet (P < 0.01). Data were analyzed by 2-way analysis of variance. Tukey post hoc analysis was performed to identify significant differences. Values are means ± SD; n = 12 rats per treatment group. The representative blots are aligned with the group identification in the bar graph.
3hPEX: 2-DG Uptake
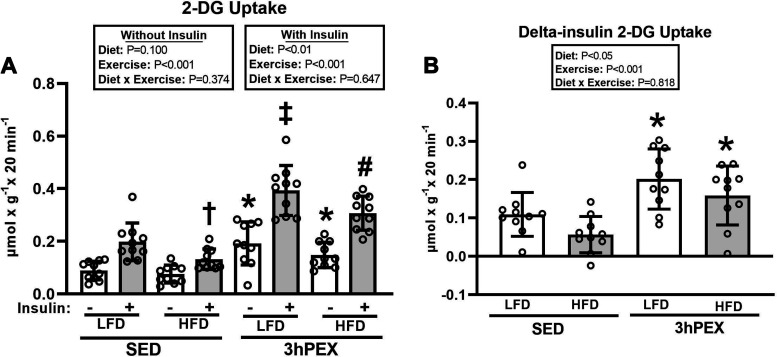
For 2-DG uptake in muscles incubated without insulin (Fig. 3A), there was a significant main effect of exercise (3hPEX exceeded SED; P < 0.001), and post hoc analysis indicated that 2-DG uptake was greater for muscles from 3hPEX versus SED in both LFD (P < 0.001) and HFD (P < 0.05) groups. There was no significant difference (P = 0.079) for 2-DG uptake without insulin between the LFD-3hPEX and HFD-3hPEX groups. For 2-DG uptake in muscles incubated with insulin, there were significant main effects of diet (LFD exceeded HFD; P < 0.01) and exercise (3hPEX exceeded SED; P < 0.001; Fig. 3A). Post hoc analysis revealed that 2-DG uptake values in HFD-SED rats were lower than values in LFD-SED rats. The LFD-3hPEX group had greater 2-DG uptake than both LFD-SED (P < 0.001) and HFD-3hPEX (P < 0.01) groups. In the HFD rats, 2-DG uptake was greater (P < 0.001) for muscles from 3hPEX versus SED.
Figure 3.
A: 2-deoxy-D-glucose (2-DG) uptake in paired epitrochlearis muscles incubated ± 100 µU/mL insulin at 3 h postexercise (3hPEX). With no insulin: *3hPEX vs. sedentary (SED) within each diet [P < 0.001 for low-fat diet (LFD) group; P < 0.05 for high-fat diet (HFD) group]. With insulin: †HFD-SED < LFD-SED (P < 0.05); ‡LFD-3hPEX > LFD-SED (P < 0.001) and HFD-3hPEX (P < 0.01); #HFD-3hPEX > HFD-SED (P < 0.001). B: delta-insulin 2-DG uptake values (delta = value with insulin − value without insulin from paired muscles). *3hPEX vs. SED within each diet (P < 0.01). Data were analyzed by 2-way analysis of variance within each insulin level (without or with insulin) or for delta-insulin values. Tukey post hoc analysis was performed to identify significant differences. Values are means ± SD; n = 10 rats per treatment group.
Delta-insulin 2-DG uptake provides insights about insulin’s ability to increase 2-DG uptake above the 2-DG uptake value found in paired muscles in the absence of insulin. For delta-insulin 2-DG uptake, there were significant main effects of diet (LFD exceeded HFD; P < 0.05) and exercise (3hPEX exceeded SED; P < 0.001; Fig. 3B). Post hoc analysis indicated that delta-insulin 2-DG uptake was not significantly different for LFD-SED versus HFD-SED (P = 0.084). Post hoc analysis revealed that delta-insulin 2-DG uptake for both LFD-3hPEX and HFD-3hPEX exceeded their respective, diet-matched sedentary controls (P < 0.01). There was no significant diet-related difference in delta-insulin glucose uptake at 3hPEX.
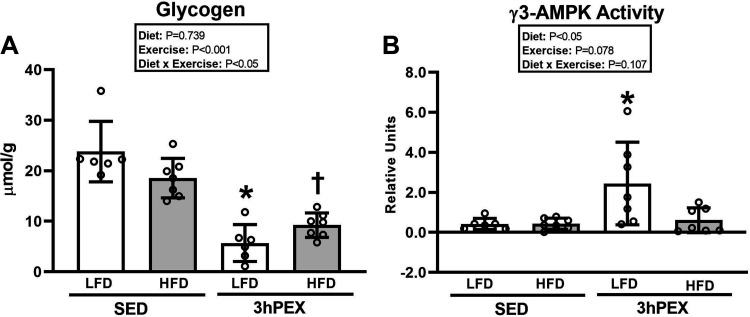
3hPEX: Muscle Glycogen
There was a significant diet × exercise interaction (P < 0.01; Fig. 4A) for muscle glycogen. Post hoc analysis revealed that muscle glycogen was lower (P < 0.001) for 3hPEX versus SED within each diet group. The muscle glycogen concentration from LFD-3hPEX rats was lower than that from HFD-3hPEX rats (P < 0.05).
Figure 4.
A: glycogen in epitrochlearis muscles at 3 h postexercise (3hPEX). HFD, high-fat diet; LFD, low-fat diet; SED, sedentary. *LFD-3hPEX < LFD-SED (P < 0.001) and HFD-3hPEX (P < 0.05); †HFD-3hPEX < HFD-SED (P < 0.001). B: γ3-AMP-activated protein kinase (AMPK) activity in epitrochlearis muscles at 3hPEX. Data were analyzed by 2-way analysis of variance. Tukey post hoc analysis was performed to identify significant differences. Comparisons between SED and 3hPEX rats within each diet group that were made with Student’s t test revealed significantly greater γ3-AMPK activity in muscles from LFD-3hPEX vs. LFD-SED rats (*P< 0.05), but no significant difference was detected between HFD-3hPEX and HFD-SED rats. Values are means ± SD; n = 6 or 7 rats per treatment group.
3hPEX: γ3-AMPK Activity
There was a significant main effect of diet (LFD exceeded HFD, P < 0.05; Fig. 4B), no significant main effect of exercise (P = 0.078), and no significant diet × exercise interaction (P = 0.107) for γ3-AMPK activity. We used a Student’s t test for comparisons between SED and 3hPEX groups within each diet. The results revealed significantly greater (P < 0.05) γ3-AMPK activity in muscles from LFD-3hPEX versus LFD-SED rats. In contrast, no significant difference was detected between HFD-3hPEX and HFD-SED groups. The γ3-AMPK activity assays for IPEX and 3hPEX experiments were run separately, with each experiment using different samples for SED controls.
3hPEX: Immunoblotting
Total abundance of signaling proteins.
Total abundance of signaling proteins was analyzed by two-way ANOVA. For total Akt abundance, there was a significant diet × exercise interaction (P < 0.001, not shown). Post hoc analysis revealed that the HFD-SED group was slightly less than the LFD-SED group (∼12%, P < 0.001). For total AS160 abundance, there was a significant diet × exercise interaction (P < 0.01, not shown). Post hoc analysis indicated that the LFD-SED group was slightly lower than the HFD-SED group (∼14%, P < 0.05) and the HFD-3hPEX group was lower than the HFD-SED group (∼23%, P < 0.001). For total AMPKα abundance, there was a significant main effect of exercise (SED exceeded 3hPEX, P < 0.05, not shown). Post hoc analysis demonstrated that the LFD-SED group was slightly greater than the LFD-3hPEX group (∼7%, P < 0.01). For total ACC abundance, there were no significant effects of diet or exercise.
Akt phosphorylation.
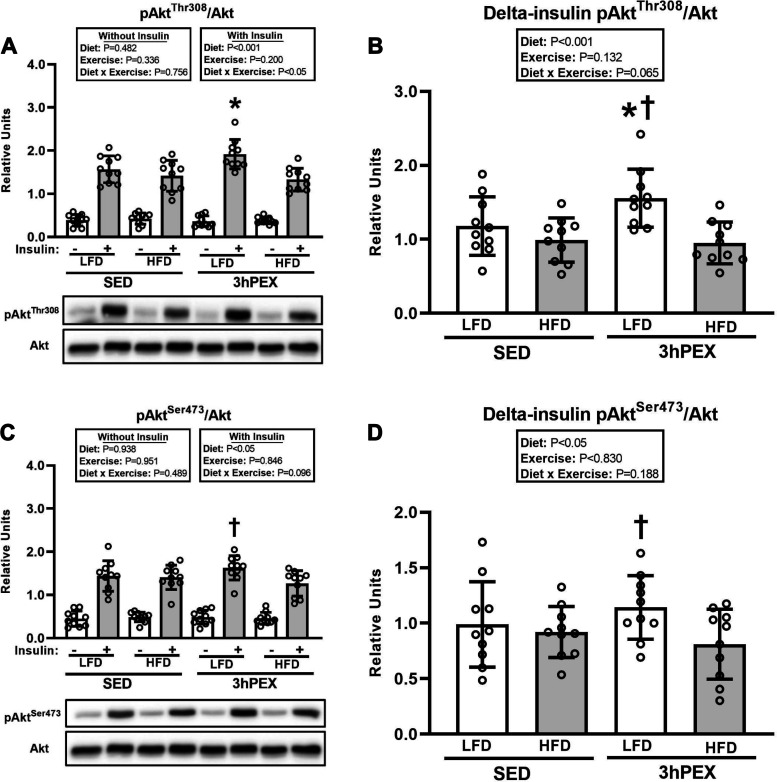
For pAktThr308/Akt in muscles incubated without insulin, there were no significant effects of diet or exercise (Fig. 5A). For pAktThr308/Akt in muscles incubated with insulin, there was a significant diet × exercise interaction (P < 0.05; Fig. 5A). Post hoc analysis revealed that the LFD-3hPEX group was greater than both LFD-SED (P < 0.001) and HFD-3hPEX (P < 0.05) groups. For delta-insulin for pAktThr308/Akt, there was a significant main effect of diet (LFD exceeded HFD, P < 0.001; Fig. 5B). Post hoc analysis detected that LFD-3hPEX exceeded both LFD-SED (P < 0.05) and HFD-3hPEX (P < 0.001). For pAktSer473/Akt in muscles incubated without insulin, there were no significant effects of diet or exercise (Fig. 5C). For pAktSer473/Akt in muscles incubated with insulin, there was a significant main effect of diet (LFD exceeded HFD, P < 0.05; Fig. 5C). Post hoc analysis indicated that the LFD-3hPEX values exceeded the HFD-3hPEX values (P < 0.05). For delta-insulin for pAktSer473/Akt, there was a significant main effect of diet (LFD exceeded HFD, P < 0.05; Fig. 5D). Post hoc analysis detected that LFD-3hPEX exceeded HFD-3hPEX (P < 0.05).
Figure 5.
A: phosphorylated (p)AktThr308/Akt in paired epitrochlearis muscles incubated ± 100 µU/mL insulin at 3 h postexercise (3hPEX). HFD, high-fat diet; LFD, low-fat diet; SED, sedentary. With insulin: *LFD-3hPEX > LFD-SED (P < 0.05) and HFD-3hPEX (P < 0.001). B: delta-insulin pAktThr308/Akt. *LFD-3hPEX > LFD-SED (P < 0.05); †LFD-3hPEX > HFD-3hPEX (P < 0.001). C: pAktSer473/Akt in paired epitrochlearis muscles incubated ± 100 µU/mL insulin at 3hPEX. With insulin: †LFD-3hPEX > HFD-3hPEX (P < 0.05). D: delta-insulin pAktSer473/Akt. †LFD-3hPEX > HFD-3hPEX (P < 0.05). Data were analyzed by 2-way analysis of variance within each insulin level (without or with insulin) or for delta-insulin values. Delta-insulin values = value with insulin − value without insulin from paired muscles. Tukey post hoc analysis was performed to identify significant differences. Values are means ± SD; n = 10 rats per treatment group. The representative blots are aligned with the group identification in the bar graph.
AS160 phosphorylation.
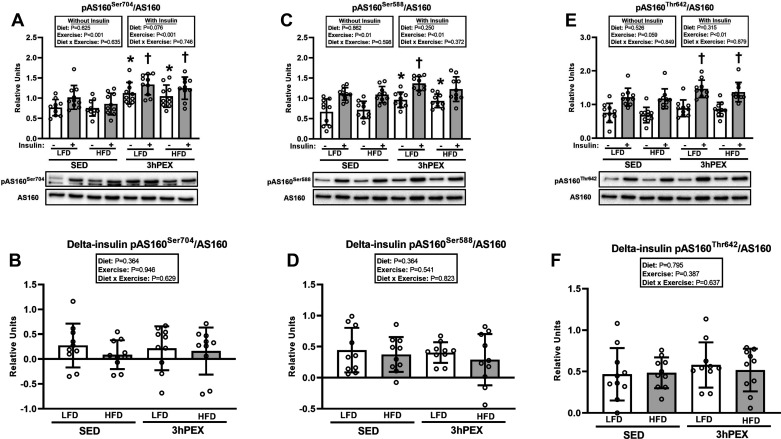
For pAS160Ser704/AS160 in muscles incubated without or with insulin, there was a significant main effect of exercise (3hPEX exceeded SED, P < 0.001; Fig. 6A). Post hoc analysis revealed that pAS160Ser704/AS160 was greater (P < 0.05) for muscles from 3hPEX versus SED in both LFD and HFD groups, regardless of insulin concentration. No significant exercise or diet effects or interactions were detected for delta-insulin for pAS160Ser704/AS160 (Fig. 6B).
Figure 6.
A: phosphorylated (p) Akt substrate of 160 kDa (AS160)Ser704/AS160 in paired epitrochlearis muscles incubated ± 100 µU/mL insulin at 3 h postexercise (3hPEX). HFD, high-fat diet; LFD, low-fat diet; SED, sedentary. *3hPEX vs. SED within each diet with no insulin (P < 0.01 for LFD group; P < 0.05 for HFD group); †3hPEX vs. SED within each diet with insulin (P < 0.05 for LFD group; P < 0.01 for HFD group). B: delta-insulin pAS160Ser704/AS160. C: pAS160Ser588/AS160 in paired epitrochlearis muscles incubated ± 100 µU/mL insulin at 3hPEX. *3hPEX vs. SED within each diet with no insulin (P < 0.01 for LFD group; P < 0.05 for HFD group); †3hPEX vs. SED within LFD group with insulin (P < 0.05). D: delta-insulin pAS160Ser588/AS160. E: pAS160Thr642/AS160 in paired epitrochlearis muscles incubated ± 100 µU/mL insulin at 3hPEX. †3hPEX vs. SED within each diet with insulin (P < 0.05). F: delta-insulin pAS160Thr642/AS160. Data were analyzed by 2-way analysis of variance within each insulin level (without or with insulin) or for delta-insulin values. Delta-insulin values= value with insulin − value without insulin from paired muscles. Tukey post hoc analysis was performed to identify significant differences. Values are means ± SD; n = 10 rats per treatment group. The representative blots are aligned with the group identification in the bar graph.
For pAS160Ser588/AS160 in muscles incubated without insulin, there was a significant main effect of exercise (3hPEX exceeded SED, P < 0.01; Fig. 6C). Post hoc analysis demonstrated that pAS160Ser588/AS160 values were greater for 3hPEX versus SED within each diet group (P < 0.01 for LFD, P < 0.05 for HFD). For pAS160Ser588/AS160 in muscles incubated with insulin, there was a significant main effect of exercise (3hPEX exceeded SED, P < 0.01; Fig. 6C). Post hoc analysis indicated that the LFD-3hPEX group was greater than the LFD-SED group (P < 0.05). No significant exercise or diet effects or interactions were detected for delta-insulin for pAS160Ser588/AS160 (Fig. 6D).
For pAS160Thr642/AS160 in muscles incubated without insulin, there were no significant effects of diet or exercise (Fig. 6E). For pAS160Thr642/AS160 in muscles incubated with insulin, there was a significant main effect of exercise (3hPEX exceeded SED, P < 0.01; Fig. 6E), and post hoc analysis indicated that pAS160Thr642/AS160 was greater (P < 0.05) for muscles from 3hPEX versus SED in both LFD and HFD groups. No significant exercise or diet effects or interactions were detected for delta-insulin for pAS160Thr642/AS160 (Fig. 6F).
AMPKα and ACC phosphorylation.
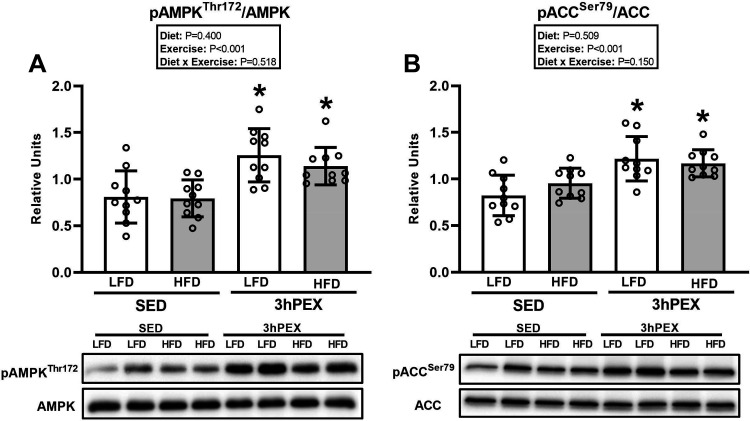
For pAMPKαThr172/AMPKα, there was a significant main effect of exercise (3hPEX exceeded SED, P < 0.001; Fig. 7A). Post hoc analysis revealed that pAMPKαThr172/AMPKα was greater for muscles from 3hPEX versus SED within each diet (P < 0.001 for LFD group; P < 0.01 for HFD group).
Figure 7.
A: phosphorylated (p) AMP-activated protein kinase (AMPK)αThr172/AMPKα in epitrochlearis muscles at 3 h postexercise (3hPEX). HFD, high-fat diet; LFD, low-fat diet; SED, sedentary. *3hPEX vs. SED within each diet (P < 0.001 for LFD group; P < 0.01 for HFD group). B: phosphorylated acetyl CoA carboxylase (ACC)Ser79/ACC in epitrochlearis muscles at 3hPEX. *3hPEX vs. SED within each diet (P < 0.001 for LFD group; P < 0.05 for HFD group). Data were analyzed by 2-way analysis of variance. Tukey post hoc analysis was performed to identify significant differences. Values are means ± SD; n = 10 rats per treatment group.
For pACCSer79/ACC, there was a significant main effect of exercise (3hPEX exceeded SED, P < 0.001; Fig. 7B). Post hoc analysis indicated that 3hPEX values were greater than SED values for each diet (P < 0.001 for LFD group; P < 0.05 for HFD group).
GLUT4 and HKII Abundance
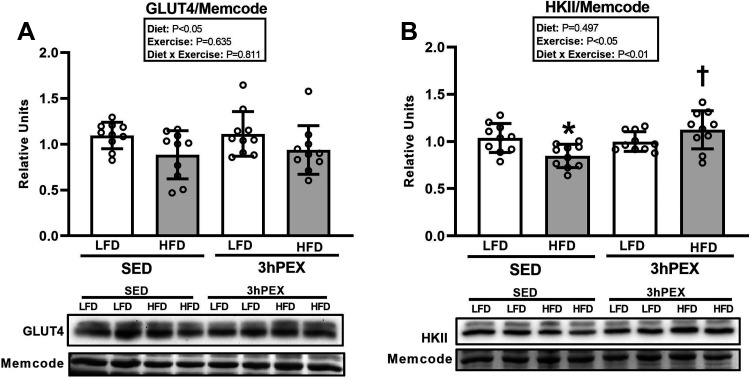
For GLUT4 abundance, there was a significant main effect of diet (LFD exceeded HFD, P < 0.05; Fig. 8A). Post hoc analysis indicated that GLUT4 abundance was not significantly lower for HFD-SED versus LFD-SED (P = 0.052).
Figure 8.
A: glucose transporter type 4 (GLUT4) abundance in epitrochlearis muscles at 3 h postexercise (3hPEX). HFD, high-fat diet; LFD, low-fat diet; SED, sedentary. B: hexokinase II (HKII) abundance in epitrochlearis muscles at 3hPEX. *HFD-SED < LFD-SED (P < 0.05); †HFD-3hPEX > HFD-SED (P < 0.05). Data were analyzed by 2-way analysis of variance. Tukey post hoc analysis was performed to identify significant differences. Values are means ± SD; n = 10 rats per treatment group.
For HKII abundance, there was a significant diet × exercise interaction (P < 0.01; Fig. 8B). Post hoc analysis revealed that HFD values were lower than LFD values for SED rats (P < 0.05). In the HFD group, HKII abundance was greater (P < 0.01) for 3hPEX versus SED rats (P < 0.01).
DISCUSSION
Many previous studies evaluated potential mechanisms for increased insulin-stimulated glucose uptake after exercise in the skeletal muscle of male rodents and humans. The present research filled a major gap in the literature as the first study to assess the effect of exercise on glucose uptake and key potential mechanisms for enhanced insulin sensitivity in both normal and insulin-resistant skeletal muscle of females. The effect of prior exercise on glucose uptake in muscles stimulated by insulin was evaluated by two commonly used approaches: 1) glucose uptake was determined for muscles that were incubated with insulin, and 2) delta-insulin glucose uptake was calculated based on the difference in glucose uptake of muscles incubated with insulin and paired muscles incubated without insulin. The first value is important because the total glucose disposal by muscle under normal, physiological conditions comprises the sum of insulin-independent and insulin-dependent glucose uptake. Delta-insulin glucose uptake is a mathematically derived value that provides valuable insights that are specific to insulin-mediated glucose uptake. The results indicated that prior exercise can enhance glucose uptake as assessed by each of these approaches in both normal and insulin-resistant skeletal muscle from female rats. Interestingly, glucose uptake determined in insulin-stimulated muscles was greater for LFD-3hPEX versus HFD-3hPEX rats, whereas delta-insulin glucose uptake was not significantly different between the diet groups. Prior exercise led to greater AS160 phosphorylation at 3hPEX and elevated γ3-AMPK activity at IPEX in both diet groups, and no diet-related differences were detected in either outcome at these time points after exercise. These results suggest that AS160 phosphorylation at 3hPEX and γ3-AMPK at IPEX may be important for improving muscle insulin sensitivity after exercise in both diet groups of female rats. However, only LFD rats had greater γ3-AMPK activity at 3hPEX. The diet-related difference in 3hPEX γ3-AMPK may be relevant for the glucose uptake in insulin-stimulated muscles from LFD-3hPEX versus HFD-3hPEX rats, but it was not essential for elevated delta-insulin glucose uptake in HFD-3hPEX rats.
Both diet groups had similar IPEX effects on each of the metabolic end points measured in skeletal muscle (insulin-independent glucose uptake, glycogen, γ3-AMPK activity, and phosphorylation of AMPK Thr172, ACC Ser79, and AS160 Ser704). Earlier research in male rats undergoing the same diet and exercise protocols also reported similar IPEX effects between the diet groups for insulin-independent glucose uptake, glycogen, and phosphorylation of AMPK Thr172 (12). Other research using male rats with the same exercise and diet protocols as the present study demonstrated IPEX-related increases in γ3-AMPK activity and phosphorylation of AS160 Ser704 in both LFD (24) and HFD (16) rats, but these studies also did not directly compare exercise effects on male LFD versus HFD rats. The present results indicate that the diet-related difference in glucose uptake by insulin-stimulated muscle observed at 3hPEX in female rats, similar to previous research in male rats, was not attributable to differences between LFD-IPEX and HFD-IPEX groups in these markers of skeletal muscle energetic stress.
Consistent with earlier studies in female humans and rodents with normal insulin sensitivity (9–11), glucose uptake by insulin-stimulated muscle was elevated after acute exercise in the present study. Prior exercise elevated pAktThr308 and delta-insulin pAktThr308, but did not alter pAktSer473, in LFD-3hPEX versus LFD-SED muscles. Earlier research in LFD-fed male rats undergoing the same exercise protocol did not detect postexercise effects on pAkt or delta-insulin pAkt on either phosphosite (12). The present study was the first to test in female rats the influence of acute exercise on Ser588, Thr642, and Ser704 phosphorylation of AS160. Both Thr642 and Ser588 phosphorylation were greater in insulin-stimulated muscles from the LFD-3hPEX group compared with sedentary, diet-matched control animals. AS160 Ser704 (corresponds to mouse Ser711) has been identified as an AMPK-phosphomotif (44). Previous studies provided compelling evidence for a link between γ3-AMPK and increased insulin-stimulated glucose uptake induced by the AMPK activator AICAR (34). Additionally, expression of the AS160 Ser704A (Ser704 to Ala704) mutant to prevent the phosphorylation of Ser704 in mouse skeletal muscle resulted in decreased insulin-stimulated phosphorylation of AS160 Thr642 (34). The present results showed that exercise increased AS160 Ser704 phosphorylation concomitant with greater γ3-AMPK activity and AMPK phosphorylation on Thr172. Increased abundance of HKII or GLUT4 can result in greater insulin-stimulated glucose uptake by muscle (35), but there was no effect of exercise on GLUT4 or HKII abundance in LFD-3hPEX versus diet-matched controls. The relative magnitude of the postexercise increases in both glucose uptake by insulin-stimulated muscle and delta-insulin glucose uptake was roughly similar in female LFD-3hPEX rats (99% and 83%, respectively) compared with earlier studies in normal male LFD-3hPEX rats (averaging ∼65% and ∼90%, respectively) after the same exercise protocol (12, 16, 24, 45). These earlier studies of LFD-fed male rats also found that improved insulin-stimulated glucose uptake was accompanied by greater AS160 phosphorylation and γ3-AMPK activity, without altered GLUT4 expression, but HKII abundance was not reported in these studies. Taken together, the present results in normal female rats and earlier results in LFD male rats support the idea that AS160 and γ3-AMPK may be linked to enhanced insulin-stimulated glucose uptake after exercise.
The present study performed a dietary protocol that has been shown to induce skeletal muscle insulin resistance in male rats (12, 16, 19). In these earlier studies using male HFD-SED rats, glucose uptake by isolated muscles incubated with insulin was ∼30% lower than values in LFD-SED rats. Similarly, skeletal muscles that were incubated with insulin after being isolated from HFD-SED rats had 33% lower glucose uptake compared with LFD-SED muscles that were incubated with insulin. In the earlier studies with male rats (12, 16, 19), delta-insulin glucose uptake was ∼50% lower in HFD-SED versus LFD-SED groups. In the present study, there was a main effect of diet on the delta-insulin glucose uptake value in HFD-fed versus LFD-fed female rats. However, post hoc analysis did not detect a significant difference for delta-insulin glucose uptake between HFD-SED versus LFD-SED female rats. There was a significant main effect of diet for GLUT4, with values for LFD rats exceeding HFD rats, which would be expected to favor lower glucose uptake by muscles with insulin from HFD versus LFD rats. In contrast, earlier research using male rats undergoing the same diet protocol did not find LFD versus HFD differences in muscle GLUT4 abundance (12, 16). HFD-SED versus LFD-SED female rats in the present study had lower HKII abundance. Earlier research in males with the same HFD protocol did not measure HKII abundance. AS160 site-selective phosphorylation (Thr642, Ser588, or Ser704) did not differ between the female LFD-SED and HFD-SED rats. In male rats, AS160 phosphorylation was greater for insulin-stimulated muscles from LFD-SED versus HFD-SED rats (12, 16). The results suggested that impaired glucose uptake in muscles from female rats incubated with insulin induced by HFD may be attributable, in part, to reduced GLUT4 and HKII abundance but not to attenuated phosphorylation of AS160 on several key regulatory sites. The mechanisms whereby the HFD protocol resulted in lower muscle glucose uptake may not be identical between the sexes.
To the best of our knowledge, the present study is the first to investigate the effect of acute exercise on insulin signaling by insulin-resistant skeletal muscle from female HFD rats. Earlier research in male rats undergoing the same HFD and exercise protocols also reported elevated glucose uptake by muscles stimulated by insulin and delta-insulin glucose uptake (12, 16). The observation that delta-insulin glucose uptake is increased in skeletal muscle of both male and female rats consuming a HFD has important implications with regard to the efficacy of exercise for improving insulin sensitivity, regardless of sex, under conditions of lipid overload. The relative magnitude of the postexercise increases in glucose uptake by insulin-stimulated muscles (133%) and delta-insulin glucose uptake (179%) versus HFD sedentary control animals was greater for the female rats in the present study compared with male rats in earlier studies (∼40% for glucose uptake by insulin-stimulated muscles and ∼90% for delta-insulin glucose uptake). These are intriguing results that should be addressed in future experiments to pursue mechanistic explanations for the apparently greater relative postexercise increases in glucose uptake in insulin-stimulated muscles and delta-insulin glucose uptake in muscles from female HFD rats compared with male HFD rats.
The present results indicated that there was no exercise effect on Akt phosphorylation on either Thr308 or Ser473 in insulin-stimulated muscles from HFD rats. These results are similar to previously reported results in male HFD-fed rats (12). For HFD-3hPEX compared with HFD-SED control animals, insulin-stimulated AS160 Thr642 phosphorylation was significantly increased. Prior exercise by HFD-fed rats also induced increases in phosphorylation of AS160 on Ser704 and AMPK on Thr172, regardless of insulin concentration. However, there was no exercise effect on γ3-AMPK activity in muscles from HFD-3hPEX rats. These results suggest that attaining greater AS160 phosphorylation at 3hPEX may be involved in the mechanism for the exercise benefit on insulin-resistant muscles from female HFD rats. Increased γ3-AMPK activity in muscles from female HFD rats at IPEX may play a role in triggering the subsequent increase in delta-insulin glucose uptake at 3hPEX. However, enhanced γ3-AMPK activity in female HFD-3hPEX rats was not essential for improved insulin sensitivity compared with HFD-SED rats. Previously published research in male rats undergoing the same HFD and exercise protocols has reported greater AS160 phosphorylation at 3hPEX and greater γ3-AMPK at both IPEX and 3hPEX (12, 16). Skeletal muscles from female LFD-3hPEX versus HFD-3hPEX rats had lower glycogen concentration. Earlier studies using the same diet and exercise protocols in male rats did not report muscle glycogen at 3hPEX.
Previous research in male rats undergoing the same diet and exercise protocols reported that both glucose uptake by insulin-stimulated muscles and delta-insulin glucose uptake were lower in HFD-3hPEX compared to LFD-3hPEX rats (12). Similar to male rats, female rats also had significantly lower glucose uptake by insulin-stimulated muscles in HFD-3hPEX versus LFD-3hPEX rats. The relative magnitude of this diet-related decrement was similar for female (22%) compared with male (28%) rats. Taken together, these results suggest that although HFD-fed rats of both sexes have a substantial postexercise enhancement of glucose uptake by insulin-stimulated muscle, they do not attain the same absolute glucose uptake as LFD rats after exercise. In contrast, delta-insulin glucose uptake was significantly lower (34%) for HFD-3hPEX versus LFD-3hPEX male rats but not significantly different between the 3hPEX female diet groups.
What might be the biological explanation for the similar values for delta-insulin glucose uptake in LFD-3hPEX compared with HFD-3hPEX female rats? In female rats, AS160 Thr642, Ser588, and Ser704 phosphorylation, as well as delta-insulin for these phosphosites, were not different between diet groups at 3hPEX. The lack of diet-related differences in AS160 phosphorylation may be relevant for the lack of diet-related differences in delta-insulin glucose uptake in female rats. In male rats, AS160 Thr642 and Ser588 phosphorylation, as well as delta-insulin for both phosphosites, of LFD-3hPEX rats exceeded HFD-3hPEX values, suggesting that these differences might be related to the greater delta-insulin for male LFD rats versus male HFD rats at this time point (12). AS160 Ser704 phosphorylation has not been directly compared in male LFD-3hPEX rats versus HFD-3hPEX rats. Interestingly, LFD-3hPEX female rats, LFD-3hPEX male rats, and HFD-3hPEX male rats, but not HFD-3hPEX female rats, had elevated γ3-AMPK activity compared with sedentary control rats. Thus, a sustained increase in γ3-AMPK was not essential for the elevated delta-insulin glucose uptake found in HFD-3hPEX female rats. Future mechanistic experiments will be required to elucidate the underlying causes for sex- and diet-related differences in delta-insulin glucose uptake.
The exercise effects in the present study were reminiscent of earlier results after one-legged exercise by humans (31) or after treadmill running exercise by rats (2). Research in humans with the one-legged exercise model demonstrated that insulin-stimulated glucose uptake was greater in the exercised versus the nonexercised muscle, providing support for the idea that exercise effects on insulin-stimulated glucose uptake are found in the previously contracting skeletal muscle. In an earlier study using the same exercise protocol as the present study, we reported greater glucose uptake by the insulin-stimulated rat epitrochlearis after exercise without any exercise effect on insulin-stimulated glucose uptake by another rat forelimb muscle with similar mass and fiber type composition as the epitrochlearis (extensor digiti quinti proprius) (24). Taking all of these results together, it seems reasonable to suspect that the swim exercise protocol leads to greater glucose uptake that is attributable to muscle contractile activity rather than a nonspecific, systemic stress response.
There was a modest but statistically significant increase in pACCSer79 in both diet groups of female rats at 3hPEX, whereas no increase was detected for male rats in either diet group (16). The increased pACCSer79 in females but not males is not attributable to a lack of postexercise elevation in pAMPKThr172 or γ3-AMPK activity in male rats. However, γ3-AMPK activity is measured with a muscle lysate under conditions that do not capture possible effects on enzyme colocalization with substrate or concentrations of allosteric regulators. It is also possible that the effects are related to γ1-AMPK activity or the activity of the phosphatases that dephosphorylate pACCSer79. Discernment about these various possibilities will require future research.
The present study focused on the effects of brief HFD. Would acute exercise have similar effects on individuals with greater insulin resistance and metabolic dysfunction? Several studies have evaluated acute exercise effects on individuals with severe insulin resistance, profound obesity, or longer-duration HFD. In each study, acute exercise improved insulin-stimulated glucose uptake. The relative exercise-induced increase in glucose disposal during a hyperinsulinemic clamp in women and men was similar for people with normal insulin sensitivity compared with people who were extremely insulin resistant but not diabetic or obese (47). However, the glucose disposal of the severely insulin-resistant people remained well below the values of the people with normal insulin sensitivity, and the results were not separately reported for women and men. Improved glucose uptake by insulin-stimulated muscles has also been reported after exercise by male genetically obese Zucker rats (14) and male rats eating a HFD for either 4 wk (15) or 3 mo (48). However, none of these animal studies included a group of rats with normal insulin sensitivity that were exercised for comparison.
A limitation of the present study was that male rats were not included to enable a direct statistical comparison between the sexes. An earlier study using electrical stimulation to elicit contractions by isolated mouse skeletal muscles reported that contraction-stimulated glucose uptake was greater for female compared with male mice (49). However, this previous study did not assess the effects of prior exercise on subsequent insulin-stimulated glucose uptake or include a HFD-fed group. It would be valuable for future research to make a direct comparison between the sexes, including both normal and insulin-resistant animals. Elucidating the underlying biological mechanisms responsible for both the differences and the similarities between the sexes with regard to insulin resistance is a complex and crucial topic for ongoing investigation (50–53).
In conclusion, the present study revealed that both glucose uptake by insulin-stimulated muscle and delta-insulin glucose uptake can be increased after one session of exercise in both normal and insulin-resistant skeletal muscles from female rats. Many aspects of the present results in female rats were generally similar to earlier results in male rats undergoing the same diet and exercise protocols. The present results suggest that greater phosphorylation of AS160 may potentially play a role in these exercise benefits for each diet group in female rats. Neither the delta-insulin glucose uptake, an indicator of insulin-stimulated glucose uptake, nor AS160 phosphorylation was significantly different at 3hPEX in LFD compared with HFD rats. However, glucose uptake by insulin-stimulated muscle, a physiologically important outcome with regard to muscle’s ability to dispose of extracellular glucose that comprises both insulin-dependent and insulin-independent glucose uptake, was greater for LFD-3hPEX versus HFD-3hPEX rats, and only the LFD-3hPEX rats had elevated γ3-AMPK activity at 3hPEX. Future research should investigate 1) whether greater AS160 phosphorylation is essential for exercise effect on insulin-stimulated glucose uptake in muscles from female LFD and/or HFD rats; 2) the mechanism responsible for the lack of increased γ3-AMPK activity in female 3hPEX rats; 3) the extent to which the diet-related difference in γ3-AMPK activity at 3hPEX contributes to greater glucose uptake by insulin-stimulated muscle in the LFD-3hPEX versus HFD-3hPEX rats; and 4) the mechanisms underlying potential differences between the sexes with regard to exercise effects on muscle glucose uptake.
GRANTS
These experiments were supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK-71771). J.T.T. was supported by the Novo Nordisk Foundation Center for Basic Metabolic Research (CBMR). CBMR is an independent Research Center at the University of Copenhagen and partially funded by an unrestricted donation from the Novo Nordisk Foundation (NNF18CC0034900).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.W. and G.D.C. conceived and designed research; H.W. performed experiments; H.W. analyzed data; H.W. and G.D.C. interpreted results of experiments; H.W. prepared figures; H.W. and G.D.C. drafted manuscript; H.W., E.B.A., J.T.T., and G.D.C. edited and revised manuscript; H.W., E.B.A., J.T.T., and G.D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Xiaohua Zheng, Seongeun Kwak, Jiahui Zhao, and Gengfu Dong for technical assistance and Dr. David Thomson for generously supplying the γ3-AMPK antibody.
REFERENCES
- 1.Cartee GD. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol Endocrinol Metab 309: E949–E959, 2015. doi: 10.1152/ajpendo.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest 69: 785–793, 1982. doi: 10.1172/jci110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab 256: E494–E499, 1989. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- 4.Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle—interaction between exercise and insulin. J Appl Physiol (1985) 65: 909–913, 1988. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]
- 5.Hamada T, Arias EB, Cartee GD. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. J Appl Physiol (1985) 101: 1368–1376, 2006. doi: 10.1152/japplphysiol.00416.2006. [DOI] [PubMed] [Google Scholar]
- 6.Wojtaszewski JF, Hansen BF, Gade, Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49: 325–331, 2000. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- 7.Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes 46: 1775–1781, 1997. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- 8.Wojtaszewski JF, Richter EA. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem 42: 31–46, 2006. doi: 10.1042/bse0420031. [DOI] [PubMed] [Google Scholar]
- 9.Davis TA, Klahr S, Tegtmeyer ED, Osborne DF, Howard TL, Karl IE. Glucose metabolism in epitrochlearis muscle of acutely exercised and trained rats. Am J Physiol Endocrinol Metab 250: E137–E143, 1986. doi: 10.1152/ajpendo.1986.250.2.E137. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Patterson BW, Smith GI, Kampelman J, Reeds DN, Sullivan SA, Mittendorfer B. A ∼60-min brisk walk increases insulin-stimulated glucose disposal but has no effect on hepatic and adipose tissue insulin sensitivity in older women. J Appl Physiol (1985) 114: 1563–1568, 2013. doi: 10.1152/japplphysiol.01364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Baracos VE, Quinney HA, Clandinin MT. Dietary fat modifies exercise-dependent glucose transport in skeletal muscle. J Appl Physiol (1985) 80: 1219–1224, 1996. doi: 10.1152/jappl.1996.80.4.1219. [DOI] [PubMed] [Google Scholar]
- 12.Castorena CM, Arias EB, Sharma N, Cartee GD. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes 63: 2297–2308, 2014. doi: 10.2337/db13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devlin JT, Horton ES. Effects of prior high-intensity exercise on glucose metabolism in normal and insulin-resistant men. Diabetes 34: 973–979, 1985. doi: 10.2337/diab.34.10.973. [DOI] [PubMed] [Google Scholar]
- 14.Betts JJ, Sherman WM, Reed MJ, Gao JP. Duration of improved muscle glucose uptake after acute exercise in obese Zucker rats. Obes Res 1: 295–302, 1993. doi: 10.1002/j.1550-8528.1993.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka S, Hayashi T, Toyoda T, Hamada T, Shimizu Y, Hirata M, Ebihara K, Masuzaki H, Hosoda K, Fushiki T, Nakao K. High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metabolism 56: 1719–1728, 2007. doi: 10.1016/j.metabol.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Pataky MW, Arias EB, Wang H, Zheng X, Cartee GD. Exercise effects on γ3-AMPK activity, phosphorylation of Akt2 and AS160, and insulin-stimulated glucose uptake in insulin-resistant rat skeletal muscle. J Appl Physiol (1985) 128: 410–421, 2020. doi: 10.1152/japplphysiol.00428.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 18.Kraegen EW, James DE, Storlien LH, Burleigh KM, Chisholm DJ. In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment by euglycaemic clamp plus deoxyglucose administration. Diabetologia 29: 192–198, 1986. doi: 10.1007/BF02427092. [DOI] [PubMed] [Google Scholar]
- 19.Pataky MW, Wang H, Yu CS, Arias EB, Ploutz-Snyder RJ, Zheng X, Cartee GD. High-fat diet-induced insulin resistance in single skeletal muscle fibers is fiber type selective. Sci Rep 7: 13642, 2017. doi: 10.1038/s41598-017-12682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castorena CM, Mackrell JG, Bogan JS, Kanzaki M, Cartee GD. Clustering of GLUT4, TUG, and RUVBL2 protein levels correlate with myosin heavy chain isoform pattern in skeletal muscles, but AS160 and TBC1D1 levels do not. J Appl Physiol (1985) 111: 1106–1117, 2011. doi: 10.1152/japplphysiol.00631.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pataky MW, Van Acker SL, Dhingra R, Freeburg MM, Arias EB, Oki K, Wang H, Treebak JT, Cartee GD. Fiber type-specific effects of acute exercise on insulin-stimulated AS160 phosphorylation in insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab 317: E984–E998, 2019. doi: 10.1152/ajpendo.00304.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pataky MW, Yu CS, Nie Y, Arias EB, Singh M, Mendias CL, Ploutz-Snyder RJ, Cartee GD. Skeletal muscle fiber type-selective effects of acute exercise on insulin-stimulated glucose uptake in insulin-resistant, high-fat-fed rats. Am J Physiol Endocrinol Metab 316: E695–E706, 2019. doi: 10.1152/ajpendo.00482.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297: E242–E251, 2009. doi: 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Arias EB, Pataky MW, Goodyear LJ, Cartee GD. Postexercise improvement in glucose uptake occurs concomitant with greater γ3-AMPK activation and AS160 phosphorylation in rat skeletal muscle. Am J Physiol Endocrinol Metab 315: E859–E871, 2018. doi: 10.1152/ajpendo.00020.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cartee GD. Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia 58: 19–30, 2015. doi: 10.1007/s00125-014-3395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartee GD, Funai K. Exercise and insulin: convergence or divergence at AS160 and TBC1D1? Exerc Sport Sci Rev 37: 188–195, 2009. doi: 10.1097/JES.0b013e3181b7b7c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 28.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 29.Schweitzer GG, Arias EB, Cartee GD. Sustained postexercise increases in AS160 Thr642 and Ser588 phosphorylation in skeletal muscle without sustained increases in kinase phosphorylation. J Appl Physiol (1985) 113: 1852–1861, 2012. doi: 10.1152/japplphysiol.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treebak JT, Frøsig C, Pehmøller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath JR, Hardie DG, Kiens B, Richter EA, Pilegaard H, Wojtaszewski JF. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia 52: 891–900, 2009. doi: 10.1007/s00125-009-1294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pehmøller C, Brandt N, Birk JB, Høeg LD, Sjøberg KA, Goodyear LJ, Kiens B, Richter EA, Wojtaszewski JF. Exercise alleviates lipid-induced insulin resistance in human skeletal muscle-signaling interaction at the level of TBC1 domain family member 4. Diabetes 61: 2743–2752, 2012. doi: 10.2337/db11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- 33.Kjøbsted R, Hingst JR, Fentz J, Foretz M, Sanz MN, Pehmøller C, Shum M, Marette A, Mounier R, Treebak JT, Wojtaszewski JF, Viollet B, Lantier L. AMPK in skeletal muscle function and metabolism. FASEB J 32: 1741–1777, 2018. doi: 10.1096/fj.201700442R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjøbsted R, Treebak JT, Fentz J, Lantier L, Viollet B, Birk JB, Schjerling P, Björnholm M, Zierath JR, Wojtaszewski JF. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes 64: 2042–2055, 2015. doi: 10.2337/db14-1402. [DOI] [PubMed] [Google Scholar]
- 35.Kjøbsted R, Munk-Hansen N, Birk JB, Foretz M, Viollet B, Björnholm M, Zierath JR, Treebak JT, Wojtaszewski JF. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes 66: 598–612, 2017. doi: 10.2337/db16-0530. [DOI] [PubMed] [Google Scholar]
- 36.Kjøbsted R, Wojtaszewski JF, Treebak JT. Role of AMP-activated protein kinase for regulating post-exercise insulin sensitivity. Exp Suppl 107: 81–126, 2016. doi: 10.1007/978-3-319-43589-3_5. [DOI] [PubMed] [Google Scholar]
- 37.Hardman SE, Hall DE, Cabrera AJ, Hancock CR, Thomson DM. The effects of age and muscle contraction on AMPK activity and heterotrimer composition. Exp Gerontol 55: 120–128, 2014. doi: 10.1016/j.exger.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNamara CR, Ahuja R, Osafo-Addo AD, Barrows D, Kettenbach A, Skidan I, Teng X, Cuny GD, Gerber S, Degterev A. Akt Regulates TNFalpha synthesis downstream of RIP1 kinase activation during necroptosis. PLoS One 8: e56576, 2013. doi: 10.1371/journal.pone.0056576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng X, Arias EB, Qi NR, Saunders TL, Cartee GD. In vivo glucoregulation and tissue-specific glucose uptake in female Akt substrate 160 kDa knockout rats. PLoS One 15: e0223340, 2020. doi: 10.1371/journal.pone.0223340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arias EB, Zheng X, Agrawal S, Cartee GD. Whole body glucoregulation and tissue-specific glucose uptake in a novel Akt substrate of 160 kDa knockout rat model. PLoS One 14: e0216236, 2019. doi: 10.1371/journal.pone.0216236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol Endocrinol Metab 268: E902–E909, 1995. doi: 10.1152/ajpendo.1995.268.5.E902. [DOI] [PubMed] [Google Scholar]
- 42.Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol (1985) 76: 979–985, 1994. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- 43.Antharavally BS, Carter B, Bell PA, Krishna Mallia A. A high-affinity reversible protein stain for Western blots. Anal Biochem 329: 276–280, 2004. doi: 10.1016/j.ab.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 44.Treebak JT, Taylor EB, Witczak CA, An D, Toyoda T, Koh HJ, Xie J, Feener EP, Wojtaszewski JF, Hirshman MF, Goodyear LJ. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am J Physiol Cell Physiol 298: C377–C385, 2010. doi: 10.1152/ajpcell.00297.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Arias EB, Cartee GD. Reduced membrane cholesterol content in skeletal muscle is not essential for greater insulin-stimulated glucose uptake after acute exercise by rats. Appl Physiol Nutr Metab 46: 685–689, 2021. doi: 10.1139/apnm-2021-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335: 1357–1362, 1996. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 48.Ropelle ER, Pauli JR, Prada PO, de Souza CT, Picardi PK, Faria MC, Cintra DE, Fernandes MF, Flores MB, Velloso LA, Saad MJ, Carvalheira JB. Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol 577: 997–1007, 2006. doi: 10.1113/jphysiol.2006.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang JH, Park JE, Dagoon J, Masson SW, Merry TL, Bremner SN, Dent JR, Schenk S. Sirtuin 1 is not required for contraction-stimulated glucose uptake in mouse skeletal muscle. J Appl Physiol (1985) 130: 1893–1902, 2021. doi: 10.1152/japplphysiol.00065.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hevener AL, Zhou Z, Moore TM, Drew BG, Ribas V. The impact of ERalpha action on muscle metabolism and insulin sensitivity—strong enough for a man, made for a woman. Mol Metab 15: 20–34, 2018. doi: 10.1016/j.molmet.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Paoli M, Zakharia A, Werstuck GH. The role of estrogen in insulin resistance: a review of clinical and preclinical data. Am J Pathol 191: 1490–1498, 2021. doi: 10.1016/j.ajpath.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Goossens GH, Jocken JW, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol 17: 47–66, 2021. doi: 10.1038/s41574-020-00431-8. [DOI] [PubMed] [Google Scholar]
- 53.Tramunt B, Smati S, Grandgeorge N, Lenfant F, Arnal JF, Montagner A, Gourdy P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 63: 453–461, 2020. doi: 10.1007/s00125-019-05040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]