WHAT HAPPENED?
After more than 50 years of human spaceflight with a perpetual focus on altered blood flow in microgravity, the recent and unforeseen in-flight internal jugular venous thrombosis (VT) in an astronaut has caused serious concern in the aerospace medicine community (1).
Auñón-Chancellor et al. (1) identified a potentially occlusive internal jugular VT in an asymptomatic astronaut approximately 2 mo into a flight based on secondary analysis of a scientific evaluation of jugular venous flow (2). The diagnosis of VT was subsequently made based on real-time remote-guided ultrasound self-examination by the astronaut and ground-based clinical evaluation of the images. Treatment with enoxaparin was initiated within 24 h and the suspected VT receded over the following weeks. Treatment was arrested 4 days prior to re-entry due to the associated risk of injury and hemorrhage. No blood clot was detected on ultrasound postflight and no follow-up treatment was deemed necessary (1).
This case was resolved with no adverse clinical consequences but still, cast a long shadow on future human deep-space exploration missions. On Earth, the suspicion of VT is based on symptoms. Diagnostic tools such as blood biomarkers, advanced ultrasounds, and expert sonographers, and effective treatments are readily available. In the spaceflight environment, some symptoms may be masked or present differently, which, in combination with the limited medical capabilities and general operational challenges in weightlessness lead to an elevated medical risk (3). The recent suspected VT underlines the need for a better understanding of risk factors (4), screening procedures (5), countermeasures (6, 7), and treatment options (1) for VT in space to avoid future medical emergencies.
WHAT MIGHT THE UNDERPINNING MECHANISMS BE?
On Earth, the lifetime risk of developing a venous thrombosis is 1:12, with lifestyle/hereditary factors significantly elevating this risk (8). However, internal jugular VT is exceedingly rare and commonly a result of catheterization or underlying malignancy (9). Although astronaut selection ensures an overall low-medical-risk population, headward fluid shift, inactivity, and associated venous stasis may increase the risk of blood clot formation in weightlessness (5). Virchow’s triad summarizes the three conventional VT risk factors: altered blood flow/stasis, vascular wall injury, and hypercoagulability (10). Venous stasis in the internal jugular vein (IJV), combined with vessel-wall distention could arguably constitute spaceflight-associated risk factors for cephalad thrombosis (5, 11, 12).
Marshall-Goebel et al. (2) performed a retrospective analysis of IJV flow on 11 astronauts including the VT case described in the first section. They reported an alarming observation of stagnation and even reversal of the cerebral venous outflow in six of 11 astronauts, suggesting that spaceflight may cause venous blood to flow from the central veins through the internal jugular vein into the brain (2). Such venous retrograde flow patterns have never been reported in ground-based analogs nor in actual weightlessness (10), and warrant further consideration in terms of physiological consequences and the methodology used to quantify venous flow.
Cerebral blood flow is governed by cerebral autoregulation through intricate myogenic, neurogenic, and metabolic mechanisms (13, 14). Due to the rigid skull, overall cerebral arterial inflow must be perfectly matched beat-to-beat by venous outflow to maintain intracranial pressures (ICP) within the life-sustaining range (15–17). Cerebral arterial flow is narrowly maintained relative to changes in posture, blood pressures, and ICP (18). In supine postures on Earth, IJVs are fully open and constitute the primary draining route from the brain (19). In weightlessness, the IJVs remain patently open (2, 20–22). Conversely, on Earth, gravitational collapse of these large neck veins in more upright postures (21) shifts part of the drainage to the much smaller posterior vertebral veins (18, 19, 21). As such, the cerebral drainage pattern is gravity-dependent, but in no instance does the IJV flow reverse. Venous blood obligatorily flows from high to low pressure. Given the fact that central venous pressure is lower in space than during supine on Earth (23), cerebral resistance (ICP) would have to decrease relatively more to accommodate this reversal of flow. Current data does not indicate significant decreases in ICP during spaceflight. In fact, ocular and cerebral findings in astronauts are compatible with an increase in ICP to above values in upright and likely even a slight but persistent elevation in 24-h average ICP compared with the 24-h average ICP on Earth (24). Taken together, there seems to be no clear hemodynamic underpinning mechanism suggesting that flow in open IVJs would reverse (2).
A significant limitation of these investigations (2) is the lack of a robust method to measure cerebral venous flow patterns. Conventional pulsed-wave Doppler ultrasound is strictly angle-dependent and based on the, often erroneous, assumption of laminar blood flow in plane with the imaging. Pulsed-wave Doppler relies on a sample gate to interrogate velocity; when the gate is widened sufficiently to encompass the entire vessel, a spectrum of flow velocities is captured and displayed as a temporal waveform (25). The blood flow pattern of IJV, even at rest on Earth, is distinctly nonuniform with asymmetrical walls oscillating with both respiratory and cardiac cycles resulting in complex recirculatory patterns and nonlinear streams (26). In addition, the presence of a vascular intrusion such as carotid stenosis or partial VT that obstructs or redirects flow would cause even more complex patterns such as eddy pools and potentially the appearance of retrograde flow on a traditional pulsed-wave Doppler device.
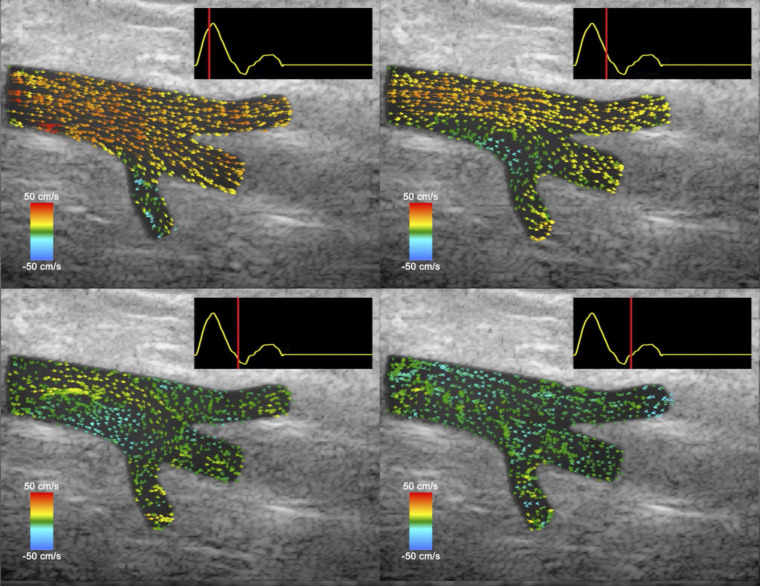
Newer technologies, such as vector flow imaging, employ high-frame-rate ultrasound, which provides a more sensitive, angle-independent tool to quantify complex circulatory flows and could significantly improve both in-flight diagnostic and research specificity (27), as shown in Fig. 1.
Figure 1.
This figure demonstrates the ability of vector flow imaging to characterize turbulent flow throughout the cardiac cycle. Beginning at the top left, one can see that much of the flow in the vessel is away from the heart (positive, red/yellow color). As the cardiac cycle progresses (as shown by the red line in the top right corner of each image), the flow in the vessel appears to stagnate (green color) or move backward (blue color) due to the eddies caused by the trifurcation of the vessel, much like the retrograde flow noted by Marshall-Goebel et al. (2). These images were reproduced from Supplemental Video S1: “Ultrasound vector flow imaging of a femoral trifurcation” of Au et al. (28) and are used with permission. The full video is available at https://doi.org/10.6084/m9.figshare.8344004.v1 under Creative Commons Attribution CC-BY.
WHAT ARE THE NEXT STEPS?
Clarification of cerebral drainage patterns relative to gravitational stress and weightlessness is critical. Further study into venous blood flow in space with appropriate tools is necessary to characterize the contribution of altered blood flow to blood clot formation. Limitations of currently available ultrasound technology and operator expertise in-flight introduce uncertainties in interpretation of venous flow data. These limitations are easier to overcome in terrestrial analogs or clinical trials and may explain why retrograde IJV flow has not been seen before (10).
Since VT was not previously considered a major risk, contributing risk factors in astronauts are currently insufficiently characterized (5). Aspects such as endothelial dysfunction (11), the influence of mission durations (5), and sex (4) should be investigated and quantified as potential contributing factors for thrombus formation. Screening for thrombophilia and other predisposing clotting factors and correlating these to clinical events or asymptomatic VT in-flight is critical (5). Finally, gene expression analysis and biochemical markers of coagulation in blood would provide insight into the molecular changes associated with hemodynamic changes. Taken together, we recommend defining a standardized set of screening measurements to be completed in-flight, pre-flight, post-flight, and during bed rest and dry immersion studies that include the above-mentioned parameters, as well as high rate flow ultrasound imaging of larger veins.
The modus operandi of space medicine is prevention rather than evaluation and management of medical issues. Prophylactic blood thinners greatly elevate the risk during any trauma, and considering the low incidence of VT, cannot be recommended. Lower-body negative pressure (LBNP) at low, and demonstrated safe, levels (i.e., <30 mmHg) may be a useful countermeasure to reintroduce caudal venous blood volume distribution thereby simulating the effects of gravitational stress including cerebral drainage patterns (6, 7, 20, 27, 29), but may in itself elevate the risk of VT in the legs (30). A better understanding of risk factors, underpinning mechanisms, and actual prevalence of VT both in the upper and lower part of the vasculature in space is needed to evaluate and determine the best possible countermeasures including LBNP.
GRANTS
This study was supported by the National Aeronautics and Space Administration (NASA) Grant 10NSSC19K0020 to L. G. Petersen.
DISCLOSURES
The funder KBR GmbH provided support in the form of salaries for the authors David Green and Tobias Weber but did not have any role in the research, analysis, decision to publish, or preparation of this manuscript. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
K.M.H., T.W., and L.G.P. conceived and designed research; D.G. and L.G.P. interpreted results of experiments; K.M.H., T.W., D.G., and L.G.P. drafted manuscript; K.M.H., T.W., D.G., D.A.G., N.G., and L.G.P. edited and revised manuscript; K.M.H., T.W., D.G., D.A.G., N.G., and L.G.P. approved final version of manuscript.
REFERENCES
- 1.Auñón-Chancellor SM, Pattarini JM, Moll S, Sargsyan A. Venous thrombosis during spaceflight. N Engl J Med 382: 89–90, 2020. doi: 10.1056/NEJMc1905875. [DOI] [PubMed] [Google Scholar]
- 2.Marshall-Goebel K, Laurie SS, Alferova IV, Arbeille P, Auñón-Chancellor SM, Ebert DJ, Lee SMC, Macias BR, Martin DS, Pattarini JM, Ploutz-Snyder R, Ribeiro LC, Tarver WJ, Dulchavsky SA, Hargens AR, Stenger MB. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open 2: e1915011, 2019. doi: 10.1001/jamanetworkopen.2019.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blue RS, Bayuse TM, Daniels VR, Wotring VE, Suresh R, Mulcahy RA, Antonsen EL. Supplying a pharmacy for NASA exploration spaceflight: challenges and current understanding. NPJ Microgravity 5: 14, 2019. doi: 10.1038/s41526-019-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain V, Ploutz-Snyder R, Young M, Charvat JM, Wotring VE. Potential venous thromboembolism Risk in female astronauts. Aerosp Med Hum Perform 91: 432–439, 2020. doi: 10.3357/AMHP.5458.2020. [DOI] [PubMed] [Google Scholar]
- 5.Limper U, Tank J, Ahnert T, Maegele M, Grottke O, Hein M, Jordan J. The thrombotic risk of spaceflight: has a serious problem been overlooked for more than half of a century? Eur Heart J 42: 97–100, 2021. doi: 10.1093/eurheartj/ehaa359. [DOI] [PubMed] [Google Scholar]
- 6.Harris KM, Petersen LG, Weber T. Reviving lower body negative pressure as a countermeasure to prevent pathological vascular and ocular changes in microgravity. NPJ Microgravity 6: 38, 2020. doi: 10.1038/s41526-020-00127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen LG, Hargens A, Bird EM, Ashari N, Saalfeld J, Petersen JCG. Mobile lower body negative pressure suit as an integrative countermeasure for spaceflight. Aerosp Med Hum Perform 90: 993–999, 2019. doi: 10.3357/AMHP.5408.2019. [DOI] [PubMed] [Google Scholar]
- 8.Bell EJ, Lutsey PL, Basu S, Cushman M, Heckbert SR, Lloyd-Jones DM, Folsom AR. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med 129: 339.e19–339.e26, 2016. doi: 10.1016/j.amjmed.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gbaguidi X, Janvresse A, Benichou J, Cailleux N, Levesque H, Marie I. Internal jugular vein thrombosis: outcome and risk factors. QJM 104: 209–219, 2011. doi: 10.1093/qjmed/hcq179. [DOI] [PubMed] [Google Scholar]
- 10.Kim DS, Vaquer S, Mazzolai L, Roberts LN, Pavela J, Watanabe M, Weerts G, Green DA. The effect of microgravity on the human venous system and blood coagulation: a systematic review. Exp Physiol 106: 1149–1158, 2021. doi: 10.1113/EP089409. [DOI] [PubMed] [Google Scholar]
- 11.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord 15: 130, 2015. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 6: 16–26, 2009. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lassen NA. Autoregulation of cerebral blood flow. Circ Res 15: 201–204, 1964. [PubMed] [Google Scholar]
- 14.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Bothwell SW, Janigro D, Patabendige A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 16: 9, 2019. doi: 10.1186/s12987-019-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen LG, Ogoh S. Gravity, intracranial pressure, and cerebral autoregulation. Physiol Rep 7: e14039, 2019. doi: 10.14814/phy2.14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogoh S, Sato K, de Abreu S, Denise P, Normand H. Arterial and venous cerebral blood flow responses to long-term head-down bed rest in male volunteers. Exp Physiol 105: 44–52, 2020. doi: 10.1113/EP088057. [DOI] [PubMed] [Google Scholar]
- 18.Ogoh S, Washio T, Sasaki H, Petersen LG, Secher NH, Sato K. Coupling between arterial and venous cerebral blood flow during postural change. Am J Physiol Regul Integr Comp Physiol 311: R1255–R1261, 2016. doi: 10.1152/ajpregu.00325.2016. [DOI] [PubMed] [Google Scholar]
- 19.Gisolf J, Van Lieshout JJ, Van Heusden K, Pott F, Stok WJ, Karemaker JM. Human cerebral venous outflow pathway depends on posture and central venous pressure. J Physiol 560: 317–327, 2004. doi: 10.1113/jphysiol.2004.070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen LG, Lawley JS, Lilja-Cyron A, Petersen JCG, Howden EJ, Sarma S, Cornwell WK, Zhang R, Whitworth LA, Williams MA, Juhler M, Levine BD. Lower body negative pressure to safely reduce intracranial pressure. J Physiol 597: 237–248, 2019. doi: 10.1113/JP276557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen LG, Petersen JCG, Andresen M, Secher NH, Juhler M. Postural influence on intracranial and cerebral perfusion pressure in ambulatory neurosurgical patients. Am J Physiol Regul Integr Comp Physiol 310: R100–R104, 2016. doi: 10.1152/ajpregu.00302.2015. [DOI] [PubMed] [Google Scholar]
- 22.Lan M, Phillips SD, Archambault‐Leger V, Chepko AB, Lu R, Anderson AP, Masterova KS, Fellows AM, Halter RJ, Buckey JC. Proposed mechanism for reduced jugular vein flow in microgravity. Physiol Rep 9: e14782, 2021. doi: 10.14814/phy2.14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckey JC Jr, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG. Orthostatic intolerance after spaceflight. J Appl Physiol (1985) 81: 7–18, 1996. doi: 10.1152/jappl.1996.81.1.7. [DOI] [PubMed] [Google Scholar]
- 24.Lawley JS, Petersen LG, Howden EJ, Sarma S, Cornwell WK, Zhang R, Whitworth LA, Williams MA, Levine BD. Effect of gravity and microgravity on intracranial pressure. J Physiol 595: 2115–2127, 2017. doi: 10.1113/JP273557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pozniak MA, Zagzebski JA, Scanlan KA. Spectral and color Doppler artifacts. Radiographics 12: 35–44, 1992. doi: 10.1148/radiographics.12.1.1734480. [DOI] [PubMed] [Google Scholar]
- 26.Simka M, Latacz P, Redelbach W. Blood flow in the internal jugular veins during the spaceflight – is it actually bidirectional? Life Sci Space Res (Amst) 25: 103–106, 2020. doi: 10.1016/j.lssr.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Au JS, Yiu BYS, So H, Chee AJY, Greaves DK, Hughson RL, Yu ACH. Ultrasound vector projectile imaging for detection of altered carotid bifurcation hemodynamics during reductions in cardiac output. Med Phys 47: 431–440, 2020. doi: 10.1002/mp.13905. [DOI] [PubMed] [Google Scholar]
- 28.Au J, Yiu B, Yu A. Case Studies in Physiology: Visualization of blood recirculation in a femoral artery “trifurcation” using ultrasound vector flow imaging. J Appl Physiol (1985) 127: 1809–1813, 2019. doi: 10.1152/japplphysiol.00451.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goswami N, Blaber AP, Hinghofer-Szalkay H, Convertino VA. Lower body negative pressure: physiological effects, applications, and implementation. Physiol Rev 99: 807–851, 2019. doi: 10.1152/physrev.00006.2018. [DOI] [PubMed] [Google Scholar]
- 30.Cvirn G, Waha JE, Brix B, Rössler A, Jantscher A, Schlagenhauf A, Koestenberger M, Wonisch W, Wagner T, Goswami N. Coagulation changes induced by lower-body negative pressure in men and women. J Appl Physiol (1985) 126: 1214–1222, 2019. doi: 10.1152/japplphysiol.00940.2018. [DOI] [PubMed] [Google Scholar]