Abstract
The evolutionary conserved Polycomb Group (PcG) repressive system comprises two central protein complexes, PcG repressive complex 1 (PRC1) and PRC2. These complexes, through the incorporation of histone modifications on chromatin, have an essential role in the normal development of eukaryotes. In recent years, a significant effort has been made to characterize these complexes in the different kingdoms, and despite there being remarkable functional and mechanistic conservation, some key molecular principles have diverged. In this review, we discuss current views on the function of plant PcG complexes. We compare the composition of PcG complexes between animals and plants, highlight the role of recently identified plant PcG accessory proteins, and discuss newly revealed roles of known PcG partners. We also examine the mechanisms by which the repression is achieved and how these complexes are recruited to target genes. Finally, we consider the possible role of some plant PcG proteins in mediating local and long-range chromatin interactions and, thus, shaping chromatin 3D architecture.
Keywords: Polycomb Group, PRC1, PRC2, histone modifications, gene repression, chromatin organization
The current picture of how the Polycomb Group repressive system acts at multiple levels to regulate gene expression in plants is examined and discussed in this review.
Introduction
The development of multicellular organisms relies on cells and tissues that establish unique gene expression programs. These programs depend on transcription factors (TFs) that guide the binding of the transcriptional machinery to specific gene promoters and control its subsequent activity. It has become evident that nucleosome organization within chromatin can either expose or hide functionally important regulatory DNA sequences that are required for the binding of TFs and the transcriptional machinery, thus strongly influencing gene expression. Furthermore, nucleosome organization can determine the formation of higher-order structures that maintain transcriptional states through cell division.
The organization of nucleosomes within chromatin can be altered in several ways (Rosa and Shaw, 2013), including the incorporation of posttranslational modifications on nucleosomal histone tails (Bannister and Kouzarides, 2011). The importance of histone modifications in the control of developmental processes was revealed a long time ago during investigations of the role of Polycomb Group (PcG) proteins. PcG genes were initially discovered in Drosophila melanogaster (Drosophila), where they are necessary for the repression of homeotic genes and therefore for the specification of the body plan (for review, see Kassis et al., 2017). Subsequently, Drosophila PcG genes were shown to have homologs in vertebrates and plants, where, among other processes, they also regulate development (reviewed in Whitcomb et al., 2007; Mozgova and Hennig, 2015; Xiao and Wagner, 2015).
Biochemical analyses have shown that PcG proteins normally assemble into two large multiprotein complexes with different histone-modifying activities. Polycomb Repressive Complex 1 (PRC1) has H2A E3 ubiquitin ligase activity toward lysine 119, 120, and 121 in Drosophila, vertebrates, and Arabidopsis, respectively (Wang et al., 2004; Cao et al., 2005; Bratzel et al., 2010; Yang et al., 2013), and PRC2 has H3 lysine 27 (H3K27) trimethyltransferase activity (Müller et al., 2002; Makarevich et al., 2006; Mozgova and Hennig, 2015). Detailed studies over the last decade have revealed that, despite remarkable functional and mechanistic conservation among the PcG systems of different kingdoms, some key molecular principles have diverged.
In this review, we discuss the current view of how the PcG system regulates gene expression in plants. We first present the composition and activities of PcG complexes as a prerequisite to understanding how PRC1 and PRC2 perform their functions. Excellent reviews have recently examined the composition of the core components of these complexes and their roles in the regulation of different developmental processes (Wang and Shen, 2018; Bieluszewski et al., 2021; Hinsch et al., 2021). We therefore focus our survey on the role of recently identified accessory proteins and on the new functions that have been attributed to previously known components. We then examine the mechanisms by which PRC1 and PRC2 regulate gene expression and how they identify their target sites. Finally, we discuss their possible role in the formation of local and long-range chromatin interactions and, thus, their contribution to shaping chromatin 3D structure.
Placing plant PRC1 within the context of animal PRC1 complexes
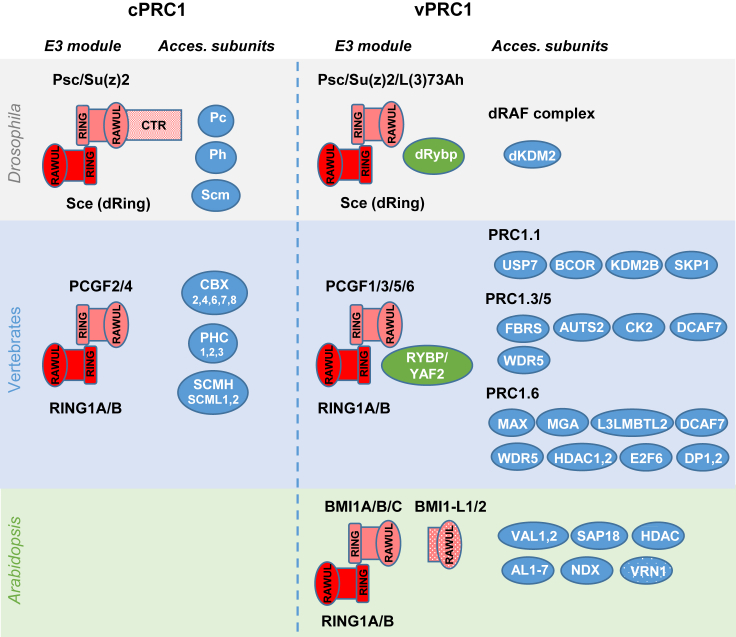
PRC1 complexes incorporate H2A monoubiquitin marks into chromatin. In Drosophila, the H2A E3 monoubiquitin ligase module (the E3 module hereafter) consists of Sex Comb Extra (Sce, also known as dRing) and Posterior Sex Combs (Psc) or Su(z)2 (Cao et al., 2005) (Figure 1). The E3 module is more diverse in vertebrates than in Drosophila. It comprises RING1A or RING1B and one of the six Polycomb Group RING finger (PCGF1–6) proteins (Barbour et al., 2020; Geng and Gao, 2020; Blackledge and Klose, 2021) (Figure 1).
Figure 1.
A diverse repertoire of PRC1s.
Schematic representation of PRC1 E3 modules in Drosophila, vertebrates, and Arabidopsis and accessory proteins found in canonical PRC1s (cPRC1s) and variant PRC1s (vPRC1s) in the different cases. Possible accessory proteins that have not been verified by AP–MS are indicated in a dotted background. The complete names of vertebrate accessory proteins that are not mentioned in the text are: AUTS2, autism susceptibility protein 2; BCOR, BCL-6 co-repressor; CK2, casein kinase 2; FBRS, fibrosin; L3MBTL2, lethal(3)malignant brain tumor-like protein 2; SKP1, S-phase kinase-associated protein 1; DCAF7, DDB1 and CUL4-Associated Factor 7; and WDR5, WD40-repeat protein 5.
The RING1 and PCGF proteins have similar domain architectures, including an N-terminal RING finger domain, a C-terminal RING finger, and a WD40-associated ubiquitin-like (RAWUL) domain (Sanchez-Pulido et al., 2008; Merini and Calonje, 2015) (Figure 1). The RING1 proteins provide the catalytic activity, and the PCGF proteins enhance their enzymatic activity (Buchwald et al., 2006; Li et al., 2006). RING1 and PCGF proteins dimerize through their RING domains, and this dimerization facilitates their interaction with an E2-conjugating enzyme to enable histone ubiquitination (Bentley et al., 2011). The RAWUL domain binds to a range of accessory subunits to give rise to different PRC1s (for review see Geng and Gao, 2020; Barbour et al., 2020; Blackledge and Klose, 2021). Drosophila Sce, Psc, and Su(z)2 also contain these domains; however, Psc and Su(z)2 have, in addition, a long, intrinsically disordered and positively charged C-terminal region (CTR) (Lo et al., 2009; Beh et al., 2012) (Figure 1). This CTR is able to bind chromatin and DNA in vitro (King et al., 2005; Emmons et al., 2009; Beh et al., 2012; Kang et al., 2020) and to mediate chromatin compaction (Francis et al., 2004), nucleosome bridging (Lo et al., 2012), transcription inhibition (King et al., 2005), and chromatin remodeling (King et al., 2005; Lo and Francis, 2010).
The first characterized PRC1 in animals was the one currently known as canonical PRC1 (cPRC1). In Drosophila, this complex contains the E3 module (Sce and Psc/Su(z)2) and the accessory proteins Polycomb (Pc), Polyhomeotic (Ph), and Sex Comb on Midleg (Scm) (Shao et al., 1999; Saurin et al., 2001) (Figure 1). Similarly, vertebrate cPRC1 contains RING1A/B, PCGF2/4, Chromobox (CBX)2/4/6/7/8, Polyhomeotic homolog (PHC)1/2/3, and Scm homolog (SCMH)1, Scm-like 1 (SCML1), or SCML2 (Gao et al., 2012). Both Pc and the CBXs have a CHROMO domain that binds H3K27me3; however, the different CBXs also exhibit unique functions (Bernstein et al., 2006; Kaustov et al., 2011). For instance, CBX2, but not other CBXs, has an intrinsically disordered region (IDR) enriched in basic amino acids that displays functions similar to those of the Psc-CTR (Grau et al., 2011; Plys et al., 2019; Kent et al., 2020). On the other hand, Ph and PHC1/2/3 have a sterile alpha motif (SAM) domain that mediates oligomerization (Kim et al., 2002; Isono et al., 2013). Finally, the SCM proteins also contain an SAM domain whose architecture is similar to that of the PHCs, but the exact role of the SCM proteins is not clear (Kim et al., 2005) (Figure 1).
Several variant PRC1s (vPRC1s) have subsequently been identified. In Drosophila, the dRing-associated factor (dRAF) complex contains Sce, Psc/Su(z)2, and the H3K36-specific demethylase dKDM2 (Lagarou et al., 2008). The Drosophila homolog of the vertebrate Ring1 and Ying Yang (YY)1 binding protein (dRybp) has also been connected to this complex (Fereres et al., 2014) (Figure 1). The dRAF complex promotes both monoubiquitination of H2A and demethylation of H3K36me2 (Lagarou et al., 2008), and most of the H2A monoubiquitination activity in Drosophila has been attributed to this complex. However, loss of Psc/Su(z)2 function decreases total H2A monoubiquitination by 30%–40%, whereas the knockdown of L(3)73Ah, a less-characterized PCGF homolog in Drosophila (Irminger-Finger and Nöthiger, 1995), decreases H2A monoubiquitination by 70%. This result suggests the existence of an alternative L(3)73Ah-dRing/Sce module that is responsible for incorporating most of this modification (Lee et al., 2015). In vertebrates, vPRC1s contain an E3 module composed of RING1A/B, one of the six PCGFs, and RYBP or its paralog YY1-associated factor (YAF) 2, which regulates the catalytic activity of the E3 module (Figure 1) (reviewed in Geng and Gao, 2020; Barbour et al., 2020; Blackledge and Klose, 2021). The associations of CBX proteins and RYBP/YAF2 with the E3 module are mutually exclusive. Whereas RYBP/YAF2 strongly stimulates the activity of the module, CBX proteins have a less stimulatory effect. Accordingly, cPRC1 displays much lower E3 ligase activity than the vPRC1s (Buchwald et al., 2006). In addition, each vPRC1 presents different accessory proteins depending on the PCGF that it contains (Figure 1). Although we are not going to describe the vast array of vPRC1 accessory proteins identified in vertebrates (for review see Barbour et al., 2020; Chetverina et al., 2020; Geng and Gao, 2020; Blackledge and Klose, 2021), we would like to highlight the diversity of their biochemical properties. For instance, these proteins include DNA binding activities that can target PRC1 to specific sites (e.g. KDM2B, the dimer MYC-associated factor X [MAX] and MAX gene-associated protein [MGA], and the dimer E2F6 and dimerization partner [DP]1/2), histone-modifying activities directed to remove counteracting marks (e.g. the histone deacetylases HDAC1 and HDAC2 and the H3K36me2 demethylase KDM2B), and proteins that regulate PRC1 activity (e.g. the ubiquitin C-terminal hydrolase ubiquitin-specific protease 7 [USP7] that prevents self-ubiquitination of RING1 and PCGF to stabilize the complex and thus regulates H2A monoubiquitination levels [de Bie et al., 2010; Maertens et al., 2010; Hu et al., 2014; Maat et al., 2021]) (Figure 1).
In Arabidopsis, there are two RING1-like proteins, RING1A and RING1B, and three PCGF-like proteins named BMI1A, BMI1B, and BMI1C after the PCGF4/B lymphoma Mo-MLV insertion region 1 homolog (BMI1) (Merini and Calonje, 2015; Wang and Shen, 2018) (Figure 1). In animals, the E3 module is formed by a RING1/PCGF heterodimer, but in Arabidopsis, RING1A and RING1B can self-interact, cross-interact, and also interact with each of the BMI1s (Xu and Shen, 2008; Bratzel et al., 2010; Chen et al., 2010). Moreover, the five proteins alone have H2A E3 ligase activity in vitro (Bratzel et al., 2010, 2012), and loss of either RING1A/B or BMI1A/B/C activity causes a reduction in H2AK121ub (Bratzel et al., 2010; Yang et al., 2013; Li et al., 2017a). Therefore, it remains unclear whether or not heterodimerization takes place between the RING1s and the BMI1s and whether this association enhances the in vivo H2A E3-ligase activity.
Interestingly, a recent work has revealed the existence of BMI1-like truncated genes (BMI1-L) restricted to embryophytes (Figure 1). These genes encode a RAWUL domain but lack the N-terminal RING finger domain, which is the catalytic part of BMI1. They share part of the BMI1 exon–intron structure, suggesting a common evolutionary history. A BLASTP search identified 138 homologs. Whereas Marchantia polymorpha and Selaginella moellendorffii contain a single gene, Physcomitrella patens, Amborella trichopoda, Pinus pinaster, and most dicots contain two genes, and monocots usually contain three (López et al., 2021). The tomato, rice, and maize homologs are involved in shoot apical meristem and axillary meristem development (Tabuchi et al., 2011; Yao et al., 2019; López et al., 2021). One of the tomato homologs, Super determinant1A (Sde1A), and the Marchatia polymorpha homolog, MpBMI1-L, have been shown to genetically interact with the BMI1 proteins (Liu et al., 2021; López et al., 2021). These findings raise the possibility that these genes may be involved in the regulation of PRC1 activity by, for instance, competing with BMI1 for interaction partners or, alternatively, in the formation of non-enzymatically active vPRC1s.
In any case, a cPRC1 is not conserved in plants, as there are no plant homologs of the Pc/CBXs, Ph/PHCs, or SCM proteins. Nevertheless, it was proposed that plants have a cPRC1-like complex that contains RING1A/B, BMI1A/B/C, and the plant-specific proteins LIKE-HETEROCHROMATIN PROTEIN 1 (LHP1), also known as TERMINAL FLOWER 2 (TFL2), and EMBRYONIC FLOWER 1 (EMF1). LHP1 was considered to be the functional equivalent of the Pc/CBX proteins because, although LHP1 contains a CHROMO domain and a CHROMO SHADOW domain like animal Heterochromatin Protein 1 (HP1) (Gaudin et al., 2001; Kotake et al., 2003), it localizes in euchromatin and binds H3K27me3 marks (Zhang et al., 2007b; Turck et al., 2007). In support of this notion, LHP1 was shown to interact with the RING1 and BMI1 proteins in yeast two-hybrid assays (Xu and Shen, 2008; Chen et al., 2010). However, recent affinity purification followed by mass spectrometry (AP–MS) experiments have revealed that LHP1 co-purifies with PRC2 (Derkacheva et al., 2013; Liang et al., 2015; Bloomer et al., 2020). Similarly, EMF1 was proposed to be a PRC1 component because of its structural and functional similarities to Drosophila Psc-CTR (Calonje et al., 2008; Beh et al., 2012). However, EMF1 is required for H3K27me3 marking at some PcG target genes (Calonje et al., 2008; Kim et al., 2012; Yin et al., 2021), and it also co-purifies with PRC2 components (Liang et al., 2015; Bloomer et al., 2020). Therefore, LHP1 and EMF1 are now considered PRC2-associated proteins and, as such, they are included in the next section.
Plants appear to contain only vPRC1s, although few accessory proteins have been identified to date (Figure 1). The recruitment of an Arabidopsis E3 module has been associated with VIVIPAROUS1/ABI3-LIKE (VAL)1/2 TFs (Yang et al., 2013; Qüesta et al., 2016; Baile et al., 2021; Mikulski et al., 2021). VAL factors contain a B3 DNA binding domain that specifically recognizes RY elements (CATGCA) (Suzuki et al., 2007). Several lines of evidence indicate that the VAL factors are bona fide PRC1 accessory proteins. First, the val1/2 mutant phenocopies the bmi1abc phenotype (Yang et al., 2013). Second, VAL1/2 are required for the incorporation of H2AK121ub marks (Yang et al., 2013; Baile et al., 2021). Third, VAL1 interacts with BMI1 proteins in vitro (Yang et al., 2013); and fourth, BMI1 and RING1 proteins co-purify with VAL1 in AP–MS experiments (Qüesta et al., 2016; Mikulski et al., 2021). Interestingly, VAL1 also co-purifies with SIN3-associated protein 18 (SAP18), SR45, and ACINUS (Qüesta et al., 2016). SAP18 is a component of both the SIN3-HISTONE DEACETYLASE COMPLEXES (HDACs) (Zhang et al., 1997) and, together with SR45 and ACINUS, the APOPTOSIS AND SPLICING-ASSOCIATED PROTEIN (ASAP) complex (Deka and Singh, 2017). In addition, several reports have shown that VAL factors directly or indirectly interact with different HDAs associated with SIN3-HDACs (Zhou et al., 2013; Zeng et al., 2020), suggesting a crosstalk between histone deacetylation and PcG repression. However, it remains to be shown how the interaction of VAL1 with ASAP, SIN3-HDACs, and PRC1 is coordinated spatially and temporally.
In addition, it has been shown that ALFIN1-like 6 (AL6) interacts with RING1 and BMI1 proteins (Molitor et al., 2014). AL1–7 form a plant-specific protein family whose members contain a PAL domain at the N terminus and a plant homeodomain (PHD) at the C terminus (Molitor et al., 2014). The PHD domain of AL proteins was reported to bind H3K4me3 (Zhao et al., 2018), and the PAL domain has been involved in the interaction with RING1 and BMI1 (Peng et al., 2018). Based on several results, the ALs have been proposed to target PRC1 to H3K4me3-marked active chromatin in order to promote the transition from the active to the repressed stage (Molitor et al., 2014; Peng et al., 2018).
Interestingly, two recent reports have shown that Nodulin Homeobox Factor (NDX) interacts with the RING1s but not with the BMI1 proteins and that it co-purifies with VAL1, BMI1, and RING1 in AP–MS experiments (Zhu et al., 2020; Mikulski et al., 2021). Furthermore, the levels of H2AK121ub are affected in ndx mutants (Zhu et al., 2020; Mikulski et al., 2021), pointing to NDX as a new PRC1 accessory protein. NDX is an atypical PHD-containing protein that has been previously implicated in single-stranded DNA recognition and stabilization of the R-loop formed at the 3′ end of FLOWERING LOCUS C (FLC) (Sun et al., 2013), as well as the regulation of abscisic acid signaling (Zhu et al., 2020); however, its exact role in PRC1 function remains to be investigated.
VERNALIZATION 1 (VRN1), a B3 domain-containing plant-specific protein involved in the vernalization response (Levy et al., 2002), has also been proposed to be a PRC1 component (Mylne et al., 2006; Holec and Berger, 2012); however, there is not yet biochemical evidence to support this association. Finally, the H3K4me3 demethylase JMJ14 was shown to interact with BMI1A/B and EMF1 (Wang et al., 2014); however, it has also recently been found to co-purify with PRC2 (see next section).
In summary, there are not many confirmed PRC1 accessory proteins in plants. In addition, it is not known whether these or other unknown accessory proteins associate with different E3 modules constituting different plant vPRC1s. It is likely that, as in vertebrates, different specialized complexes act in different tissues or developmental stages.
The number of plant PRC2-associated proteins has recently significantly increased
PRC2 core components are broadly conserved (Figure 2). These core components are essential for the H3K27 trimethyltransferase activity of the complex (reviewed in Blackledge et al., 2015; Chetverina et al., 2020; Bieluszewski et al., 2021; Blackledge and Klose, 2021).
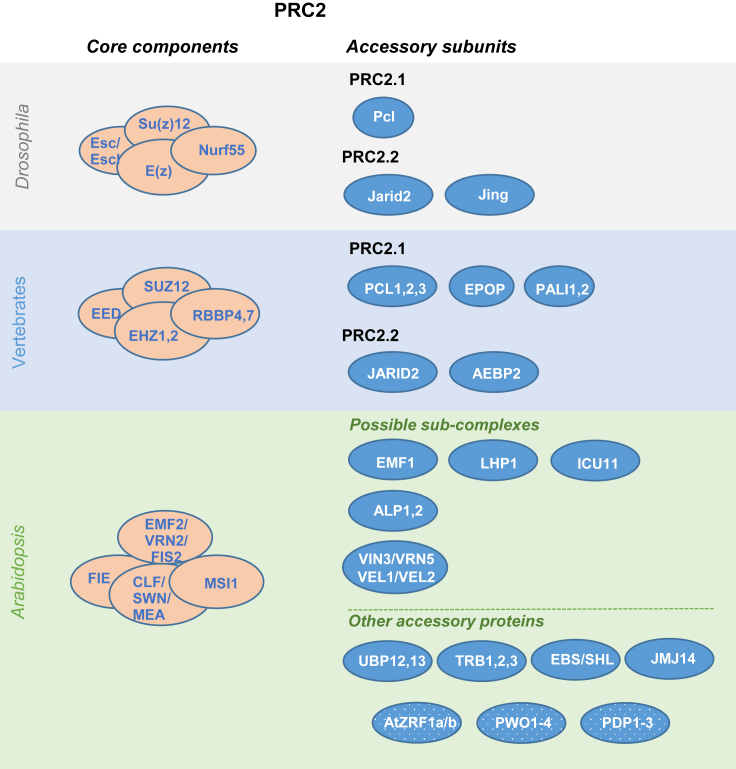
Figure 2.
PRC2 cores and accessory proteins.
Schematic representation of PRC2 core components in Drosophila, vertebrates, and Arabidopsis and accessory proteins found in the different PRC2 sub-complexes. Possible accessory proteins that have not been verified by AP–MS are indicated in a dotted background.
The Drosophila PRC2 core is composed of the SET-domain-containing methyltransferase Enhancer of zeste (E(z)), the scaffold protein Suppressor of zeste 12 (Su(z)12), the H3K27me3 binding protein Extra sex combs (Esc) or Esc-like (Escl), and the nucleosome-remodeling factor Nurf55 (reviewed in Chetverina et al., 2020; Blackledge and Klose, 2021). Similarly, the vertebrate core components are EHZ1 or EHZ2 (of which EZH2 displays higher methyltransferase activity [Margueron et al., 2008]), SUZ12, EED, and RBBP4 or RBBP7 (Chetverina et al., 2020; Blackledge and Klose, 2021) (Figure 2).
In Arabidopsis, homologs of all four PRC2 core subunits have been identified, but some of them show multiplication (Huang et al., 2017). CURLY LEAF (CLF), MEDEA (MEA), and SWINGER (SWN) are homologs of E(z) (Goodrich et al., 1997; Grossniklaus et al., 1998; Chanvivattana et al., 2004). FERTILIZATION INDEPENDENT SEED 2 (FIS2), EMBRYONIC FLOWER 2 (EMF2), and VERNALIZATION 2 (VRN2) are homologs of Su(z)12 (Luo et al., 1999; Yoshida et al., 2001; Gendall et al., 2001). FERTILIZATION INDEPENDENT ENDOSPERM (FIE) is the unique Esc homolog (Ohad et al., 1999), and MULTIPLE SUPPRESSOR OF IRA 1 (MSI1) is the homolog of Nurf55 (Köhler et al., 2003). Molecular and biochemical characterizations have revealed that Arabidopsis has at least three different PRC2 cores, named VRN2–PRC2 (VRN2, CLF/SWN, FIE, MSI1), EMF2–PRC2 (EMF2, CLF/SWN, FIE, MSI1), and FIS2–PRC2 (FIS2, MEA, FIE, MSI1) (Figure 2). These PRC2 cores display distinct and overlapping functions in the regulation of gene expression throughout plant development (Mozgova and Hennig, 2015; Xiao and Wagner, 2015; Hinsch et al., 2021).
In Drosophila and vertebrates, PRC2 diversity is achieved through the association of accessory proteins that can modulate its enzymatic activity or chromatin target sites. There are two PRC2s, PRC2.1 and PRC2.2. PRC2.1 includes Polycomb-like (Pcl) in Drosophila and the mutually exclusive Pcl homologs PCL1, PCL2, and PCL3 in vertebrates. These proteins stimulate the methyltransferase activity of E(z)/EZH (Nekrasov et al., 2007; Sarma et al., 2008). Vertebrate PRC2.1 also contains Elongin BC and PRC2-associated protein (EPOP) and PRC2-associated LCOR isoform 1 (PALI1) or PALI2 (Beringer et al., 2016; Liefke et al., 2016; Conway et al., 2018), which also support methyltransferase activity through an undefined mechanism. These proteins have not been found in Drosophila PRC2.1 (Figure 2). PRC2.2 is defined by the presence of Jardin2 in Drosophila or JARID2 in vertebrates, which bind to H2A monoubiquitination marks through their N-terminal ubiquitin interaction motif, and Jing in Drosophila or AEBP2 in vertebrates, which enhance the enzymatic activity and regulate the chromatin binding of the complex (Figure 2) (Chetverina et al., 2020; Blackledge and Klose, 2021).
In Arabidopsis, the number of identified PRC2 accessory proteins has significantly increased in recent years. These proteins co-purify with PRC2 core components and affect H3K27me3 levels (Figure 2). However, with several exceptions, it is not clear whether they preferentially associate with a specific PRC2 core or whether they give rise to sub-complexes through their mutually exclusive association with the same PRC2 core.
As mentioned in the previous section, despite the fact that LHP1 and EMF1 were considered PRC1 components, they co-purify with PRC2 core components and affect H3K27me3 levels (Derkacheva et al., 2013; Liang et al., 2015; Zhou et al., 2017; Bloomer et al., 2020; Yin et al., 2021). Thus, although in animals these two activities are associated with PRC1, in plants they are included in the list of PRC2-associated proteins (Figure 2). Similarly, in the yeast Cryptococcus neoformans, the PRC2 components co-purify with a CHROMO domain-containing subunit, Ccc1, that recognizes H3K27me3 (Dumesic et al., 2015) as LHP1 does, suggesting that this could be a more common event than expected based on the information available from Drosophila and vertebrates.
The best characterized PRC2 accessory proteins in Arabidopsis are VERNALIZATION INSENSITIVE 3 (VIN3), VIN3-LIKE 1/VERNALIZATION 5 (VIL1/VRN5), VIL2/VEL1, and VIL3/VEL2 (Sung et al., 2006; Greb et al., 2007) (Figure 2), which participate in the vernalization response by enhancing the activity of the VRN2-PRC2 core (Sung et al., 2006; Greb et al., 2007; Costa and Dean, 2019). These proteins share the PHD domain and winged helix DNA contact domain with Pcl proteins (Costa and Dean, 2019), which in vertebrates constitute different PRC2.1s. It has been shown that the VRN2-PRC2 core components VIN3 and VRN5 co-purify with SAP18, SR45, ACINUS, and HDA19 (Qüesta et al., 2016). Interestingly, these proteins also co-purify with the PRC1-associated protein VAL1, suggesting a connection between PRC1 and PRC2 established through VAL proteins.
More recently, telomere repeat-binding factors (TRB1–3), which contribute to PRC2 recruitment (Zhou et al., 2018a), and the harbinger transposase-derived ANTAGONISTIC OF LHP1 1 (ALP1) and ALP2 proteins, which antagonize PRC2 activity, have also been shown to co-purify with PRC2 core components (Liang et al., 2015; Velanis et al., 2020) (Figure 2). Interestingly, it seems that LHP1 and EMF1 do not associate with PRC2 when the ALP proteins are present, and vice versa (Velanis et al., 2020), suggesting that they are accessory components of separate sub-complexes (Figure 2).
PRC2 core components also co-purify with the ubiquitin C-terminal hydrolases UBIQUITIN-SPECIFIC PROTEASE 12 (UBP12) and UBP13 (Liang et al., 2015; Derkacheva et al., 2016; Bloomer et al., 2020) (Figure 2). Phylogenetic analysis of these proteins showed that they share a similar protein sequence with the vertebrate vPRC1 accessory protein USP7 (March and Farrona, 2017; Derkacheva et al., 2016). USP7 regulates PRC1 activity by maintaining the integrity of the complex. Consequently, USP7 inhibition results in decreased levels of H2A monoubiquitination at PRC1 target loci (de Bie et al., 2010; Maertens et al., 2010; Hu et al., 2014; Maat et al., 2021). A recent report showed that the ubp12/13 mutant displays a higher number of upregulated genes than downregulated genes, suggesting a role for UBP12/13 in repression. Also, a larger number of genes lose H2AK121ub than gain H2AK121ub in the ubp12/13 mutant, and it therefore shows a global reduction in H2AK121ub levels (Kralemann et al., 2020). These results suggest a role of UBP12/13 in regulating PRC1 integrity/activity, similar to that of USP7. However, based on analyses of the less-abundant loci that gained H2AK121ub in the ubp12/13 mutant, it has been proposed that UBP12/13 may directly regulate H2A monoubiquitination levels (Kralemann et al., 2020).
EARLY BOLTING IN SHORT DAYS (EBS) and its homolog SHORT LIFE (SHL), which contain an N-terminal Bromo-Adjacent Homology (BAH) domain followed by a PHD domain with a C-terminal extension (Yang et al., 2018), also co-purify with PRC2 core components (Li et al., 2018) (Figure 2). These proteins bind to H3K4me3 and H3K27me3 marks, interact with EMF1, and are required for the maintenance of H3K27me3 levels. ebs/shl/lhp1 triple mutants show a significant global reduction in H3K27me3 levels (Li et al., 2018), leading to propose that, together with LHP1, they are the H3K27me3 readers in Arabidopsis (Krause and Turck, 2018). In line with this proposal, EPR-1, a BAH protein of the filamentous fungus Neurospora crassa, has been show to bind H3K27me2/3 and to be implicated in gene repression. Although PRC1 core components are absent in N. crassa, EPR-1 acts as an H3K27me2/3 reader and is required for the formation of nuclear foci reminiscent of Polycomb bodies. epr-1 mutants do not exhibit appreciably altered H3K27 methylation levels, indicating that EPR-1 acts downstream of PRC2 (Wiles et al., 2020). Moreover, vertebrate BAH and coiled-coil domain-containing protein 1 (BAHCC1) and BAH domain-containing protein 1 (BAHD1) also act as H3K27me3 readers and interact with HDACs, resulting in optimal repression of PcG target genes (Fan et al., 2020, 2021). Phylogenetic analysis has shown that EPR-1 orthologs are widely distributed across the eukaryotes, suggesting an ancient role of EPR-1 homologs in PcG repression (Wiles et al., 2020).
Interestingly, a member of a novel family of putative Jumonji-type 2-oxoglutarate/Fe(II)-dependent dioxygenases, INCURVATA 11 (ICU11), robustly co-purified with PRC2 core components and accessory proteins (Bloomer et al., 2020) (Figure 2). Neither ICU11 nor EMF1 where found to interact with VIN3 or its homologs VRN5, VEL1, and VEL2, reinforcing the view that distinct PcG complexes operate over different spatial and temporal scales. Several lines of evidence support a role for ICU11 in mediating H3K36me3 demethylation, suggesting that ICU11 may enable the transition from an H3K36me3-marked active to an H3K27me3-marked inactive chromatin state (Bloomer et al., 2020). The H3K4me3 demethylase JMJ14 was detected in ICU11-enriched peptides and also interacts with EMF1 (Wang et al., 2014), supporting its association with PRC2 (Figure 2). Therefore, a combination of activities in the whole complex would link demethylation of active histone modifications and the incorporation of repressive modifications.
Unlike in animals (Blackledge and Klose, 2021), a PRC2-associated protein that can recognize and bind H2A monoubiquitination marks has not yet been identified in plants. Nevertheless, the Arabidopsis ZUOTIN RELATED FACTOR 1 (ZRF1) homologs AtZRF1a and AtZRF1b have been proposed to perform PcG-related and PcG-independent functions (Feng et al., 2016). All homologs of ZRF1 have a zuotin domain at their N terminus, which is composed of a DnaJ domain and a potential ubiquitin-binding domain, and tandem repeats of the SANT domain at their C terminus (Feng et al., 2021). Vertebrate ZRF1 can specifically recognize and bind to monoubiquitinated H2A (Richly et al., 2010; Barbour et al., 2020). Likewise, AtZRF1b can bind ubiquitin in vitro and pull down monoubiquitinated H2A from plant protein extracts, acting as a possible H2AK121ub reader (Feng et al., 2016, 2021). However, in contrast to vertebrate ZRF1, which competes with PRC1 to activate many PcG-repressed target genes, AtZRF1a/b seem to promote H3K27me3 deposition for gene repression (Feng et al., 2016), which may provide a mechanism for PRC2 reading of H2AK121ub after PRC1 deposition. Nevertheless, it remains to be investigated whether ZRF1 proteins associate with PRC2 in Arabidopsis.
Finally, although they have not been found to co-purify with PRC2 core components, the PWWP domain-containing proteins PWWP DOMAIN INTERACTOR OF POLYCOMBS 1–4 (PWO1–4), which are H3 readers via their PWWP domain, have been shown to interact with the three H3K27 trimethyltransferases in Arabidopsis and to recruit PcG proteins to subnuclear domains (Hohenstatt et al., 2018). In addition, another subgroup of PWWP domain proteins (PDP1–3) has been proposed to regulate PcG function (Zhou et al., 2018b), suggesting a role for PWWP domain-containing proteins in sustaining PcG function.
In summary, the existence of this long list of PRC2 accessory proteins indicates that the regulation of PRC2 activity may be critical for specifying cell identity during development and differentiation.
Role of PcG complexes and their hallmarks in plant transcriptional regulation
PcG complexes play a crucial role in the regulation of gene expression; however, the exact mechanism by which they exert this regulation is not yet clear. Previous studies in Drosophila and vertebrates proposed a PRC2-initiated hierarchical model in which PRC2 establishes H3K27me3, which is recognized by the PRC1 subunit Pc/CBXs. PRC1 in turn incorporates H2A monoubiquitination to maintain stable repression (Wang et al., 2004). However, later studies in vertebrates found that the activity of vPRC1s can recruit PRC2 for H3K27me3 marking, revealing a reverse alternative model for the incorporation of PcG marks (Blackledge et al., 2014; Kalb et al., 2014). In support of this notion, several results indicate that the recognition of H2A monoubiquitination is crucial for PRC2 nucleation at target sites. Moreover, the PRC2 accessory proteins Jarid2/JARID2 are able to bind H2A monoubiquitination marks (Barbour et al., 2020). The incorporation of H3K27me3 is then necessary to reinforce the binding of PRC2 and the spread of H3K27me3, in which EED and possibly other H3K27me3 readers play an important role (Blackledge and Klose, 2021). Nevertheless, because H3K27me3 is also recognized by the Pc/CBXs, PRC2 can in turn recruit cPRC1, indicating that both models, the reverse and the classical, take place in animals. Interestingly, cPRC1 contributes only minimally to H2A monoubiquitination levels in vivo, whereas it seems to be required for the formation of Polycomb bodies (Blackledge and Klose, 2021), as will be discussed below. Despite all these results, the participation of H2A monoubiquitination in gene repression has remained controversial. Some reports have provided evidence that it is indispensable (Kundu et al., 2017), whereas others have shown that it is not (Illingworth et al., 2015; Pengelly et al., 2015). Therefore, additional work is needed to determine which chromatin context acts in concert with this modification to regulate gene expression.
Genome-wide distribution analyses of H2AK121ub and H3K27me3 marks in Arabidopsis showed that most H2AK121ub peaks overlap with regions immediately downstream of transcriptional start sites (Zhou et al., 2017; Kralemann et al., 2020). Conversely, H3K27me3 peaks occupy complete gene bodies and thus partially overlap with H2AK121ub (Zhang et al., 2007a; Turck et al., 2007; Lafos et al., 2011; Zhou et al., 2017; Kralemann et al., 2020), unlike their extended co-localization in animals. In addition, H2AK121ub marks in Arabidopsis are widespread, often co-localizing with H3K27me3 but also occupying a set of genes devoid of H3K27me3 (Zhou et al., 2017; Kralemann et al., 2020), revealing three different subsets of PcG targets based on the presence of H2AK121ub, H3K27me3, or both.
Transcriptional analyses of these PcG target subsets showed that most H3K27me3-marked genes are not expressed or display low expression levels in WT seedlings, consistent with previous reports (Zhang et al., 2007a; Turck et al., 2007; Lafos et al., 2011). Conversely, a considerable percentage of genes with only H2AK121ub marks are transcriptionally active (Zhou et al., 2017; Kralemann et al., 2020), although their average expression levels are still lower than those of active genes that lack H2AK121ub marks (Yin et al., 2021). These results indicate that the two modifications may have different roles in transcriptional repression. In addition, profiling of H2AK121ub and H3K27me3 marks in the clf28swn7 mutant showed that PRC2 activity was not required for H2AK121ub marking (Zhou et al., 2017), ruling out the classical hierarchical model for the incorporation of PcG marks proposed in animals. By contrast, loss of BMI1 function affects the incorporation of H3K27me3 at the genes in which H2AK121ub and H3K27me3 marks co-localize (Zhou et al., 2017; Kralemann et al., 2020), consistent with the reverse alternative model (Blackledge et al., 2014; Calonje, 2014; Kalb et al., 2014; Merini and Calonje, 2015).
PRC1 components have been shown to interact with PRC2-associated proteins in Arabidopsis. For instance, RING1 and BMI1 interact with LHP1 and EMF1 (Xu and Shen, 2008; Bratzel et al., 2010), which may suggest that these interactions, rather than H2AK121ub, promote PRC2 recruitment and H3K27me3 marking. However, recent results in Marchantia polymorpha, which shares many signaling pathways with Arabidopsis but displays low genetic redundancy, revealed that H2A monoubiquitin marks are essential for PRC2 recruitment (Liu et al., 2021). Through mutation of the single gene encoding canonical H2A in M. polymorpha, H2A monoubiquitination was shown to be required for H3K27me3 incorporation (Liu et al., 2021). Consistent with this result, recent works showed that loss of vertebrate RING1B catalytic activity largely phenocopies the complete removal of the RING1B protein (Blackledge et al., 2020; Tamburri et al., 2020). Furthermore, the animal PRC2 accessory protein Jarid2/JARID2 binds H2A monoubiquitination marks, supporting the participation of this modification in PRC2 recruitment (Barbour et al., 2020). Unfortunately, an Arabidopsis PRC2 accessory protein that can bind H2AK121ub marks has not been identified; however, the fact that AtZRF1a/b proteins bind H2AK121ub and promote H3K27me3 marking makes them potential candidates to carry out this function. Alternatively, another unknown H2AK121ub reader associated with PRC2 may perform this function. In any case, recent results indicate that a combination of both H2AK121ub binding and direct interaction between PRC1 and PRC2 components may participate in PRC2 recruitment (Zhou et al., 2017; Baile et al., 2021; Liu et al., 2021).
Despite the implication of H2K121ub in PRC2 recruitment, the fact that average transcription levels of genes marked with H2AK121ub only are higher than those of genes marked with H2AK121ub/H3K27me3—and those of H2AK121ub/H3K27me3-marked genes are higher than those of H3K27me3-marked genes—led us to propose that H2AK121ub is not a repressive mark per se (Kralemann et al., 2020).
However, in support of a repressive role for this modification, it has recently been shown that BMI1 proteins also mediate monoubiquitination of the H2A variant H2A.Z. H2A.Z can be monoubiquitinated at lysine 129 (H2A.ZK129ub). The incorporation of this modification is required for H2A.Z-mediated transcriptional repression (Gómez-Zambrano et al., 2019). Transcriptomic comparison among the H2A.Z mutant hta9hta11, hta9hta11 complemented with a native form of H2A.Z, and hta9hta11 complemented with a mutated H2A.Z that cannot be monoubiquitinated showed that most genes upregulated in hta9hta11 recovered WT-like expression levels in the presence of native H2A.Z, but not in the presence of mutated H2A.Z (Gómez-Zambrano et al., 2019). Interestingly, a considerable number of the genes upregulated in hta9hta11 are already active in the WT, but their expression levels are further increased in hta9hta11, suggesting that H2A.ZK129ub marks modulate the expression of target genes.
Furthermore, a recent report showed that H2AK121ub marks are associated with a less accessible chromatin state at transcriptional regulation hotspots that are enriched for the binding of TFs (Yin et al., 2021), and this presumably interferes with transcription. This report showed that decreased levels of H2AK121ub at both only-H2AK121ub and H2AK121ub/H3K27me3-marked chromatin led to an increase in chromatin accessibility. Nonetheless, the fact that average expression levels of only-H2AK121ub genes are higher than those of H2AK121ub/H3K27me3 genes indicates that chromatin marked only with H2AK121ub is transcriptionally permissive, suggesting a role for this modification in modulating rather than switching off gene expression (Figure 3). Chromatin accessibility is further reduced when H3K27me3 is present. However, although H2AK121ub/H3K27me3-mediated inaccessible chromatin is still transcriptionally responsive, as it can be reactivated when the levels of these modifications are reduced, only-H3K27me3 marked chromatin is less responsive. Interestingly, only-H3K27me3 chromatin is not usually associated with transcriptional regulation hotspots (Yin et al., 2021), indicating that these sites are required for gene responsiveness (Yin et al., 2021) and supporting a role for H2K121ub in the modulation of accessibility at these sites (Figure 3). Accordingly, the presence of H2AK121ub marks has been positively linked to gene responsiveness, whereas the presence of H3K27me3 marks showed a weak negative association (Kralemann et al., 2020).
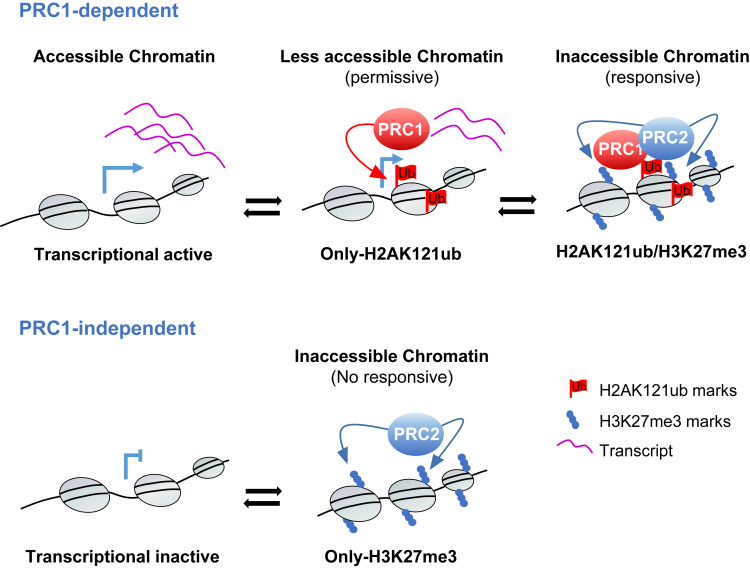
Figure 3.
Model for the two proposed PcG-mediated repressive mechanisms in plants.
Before the recruitment of PcG complexes, PRC1-dependent genes are active, whereas PRC1-independent genes are repressed. Once PRC1 is targeted to active genes, chromatin becomes less accessible, and the transcription is downregulated. PRC2 then recognizes H2AK121ub marks and PRC1 components and mediates the transcriptional repression of these genes by promoting an inaccessible chromatin state, which is responsive to reactivation. Repressed genes are targeted only by PRC2, which maintains an inaccessible chromatin state.
Interestingly, the histone demethylase RELATIVE OF EARLY FLOWERING 6 (REF6) (Lu et al., 2011) binds to and removes H3K27me3 preferentially from genes marked with H2AK121ub (Kralemann et al., 2020), further supporting the possibility that H2A monoubiquitination creates a partially repressed state that enables a quick response to stimuli. All together, these data also indicate that PcG regulation of only-H3K27me3 marked genes may involve a different mechanism. Accordingly, PRC1-independent H3K27me3-marked genes are already repressed before PRC2 recruitment, unlike PRC1-dependent genes that require PRC2 for repression (Kralemann et al., 2020) (Figure 3).
On the other hand, it has been proposed that H2AK121ub should be removed from H2AK121ub/H3K27me3-marked genes after recruitment of PRC2 to maintain a stable repression, placing UBP12/13 as key factors to remove these marks (Kralemann et al., 2020; Hinsch et al., 2021). To verify this possibility, it would be necessary to identify direct targets of UBP12/13 and to determine whether these proteins affect PRC1 integrity and activity or whether they directly deubiquitinate H2A.
Targeting PcG complexes in plants
Despite the fact that PcG activities are essential for controlling the transcriptional state of genes at a particular stage, time, or condition, none of the PcG core components are able to bind specific DNA sequences; therefore, this regulation may require different intermediaries that attract PcG complexes to specific target genes.
In Drosophila, multiple TFs acting in combination have been shown to recruit PcG complexes to cis-elements in small genomic regions called Polycomb response elements (PREs) (Kassis and Brown, 2013; Steffen and Ringrose, 2014). In vertebrates, the recruitment of PcG complexes is broadly related to unmethylated CpG-rich DNA regions that are proximal to promoters and are known as CpG islands (Deaton and Bird, 2011). These regions are recognized and bound by the zinc-finger-CXXC domain of KDM2B and by the winged helix domain of PCL1/2/3 (Blackledge et al., 2014; Li et al., 2017b). Other TFs and histone-modification binding associated proteins also collaborate in the recruitment of PcG complexes (Blackledge and Klose, 2021). Moreover, certain PcG complexes transiently interact with TFs for target site recognition in certain contexts (Blackledge and Klose, 2021). On the other hand, as an alternative to TFs, long non-coding RNAs (lncRNAs) have been involved in targeting of PcG complexes and, more recently, RNA–DNA hybrid structures, which are known as R-loops (Chetverina et al., 2020; Blackledge and Klose, 2021), suggesting that different mechanisms can contribute to the specific tethering of PcG complexes.
In Arabidopsis, the recruitment of PRC1 has been related to VAL1/2 factors. The B3 domain of VAL factors specifically recognizes RY elements (CATGCA) in the regulatory regions of several target genes (Suzuki et al., 2007; Qüesta et al., 2016; Wu et al., 2018a; Sasnauskas et al., 2018). In fact, as mentioned previously, several lines of evidence suggest that VAL factors are PRC1-associated proteins (Yang et al., 2013; Qüesta et al., 2016; Mikulski et al., 2021). In addition to the B3 domain, VAL1/2 factors also contain a plant homeodomain-like (PHD-L) domain, a cysteine- and tryptophan-rich zinc finger domain (CW), and an ethylene-responsive element binding factor-associated amphiphilic repression (EAR) domain (Suzuki et al., 2007). The PHD-L has been shown to participate in the homo- or heterodimerization of VAL1 and VAL2 (Chen et al., 2020) and has also been proposed to act as a reader of H3 methylation states, like the CW domain (Hoppmann et al., 2011; Yuan et al., 2016). The EAR domain is involved in the interaction with TOPLESS (TPL)/TPL-RELATED (TPR) 1–4 corepressors or SAP18, which in turn recruit HDA activities (Kagale and Rozwadowski, 2011). Accordingly, VAL1/2 have been reported to interact with HDA activities (Zhou et al., 2013; Zeng et al., 2020). Together, these data indicate that VAL1/2 binding to chromatin involves, in addition to cis-elements, interaction with histone modifications. Furthermore, VAL factors are able to recruit other histone-modifying activities (Zhou et al., 2013; Zeng et al., 2020; Baile et al., 2021), suggesting that they can act as platforms for the simultaneous assembly of different epigenetic mechanisms.
Apart from the VAL factors, the AL proteins have been proposed to bind H3K4me3 through their PHD domain and to recruit PRC1 (Molitor et al., 2014; Peng et al., 2018). Interestingly, most AL proteins can also bind to the conserved cis-element GNGGTG/GTGGNG (ALFIN1 elements; Bastola et al., 1998; Wei et al., 2015), raising the possibility that PRC1 could be tethered to specific sites by the AL proteins independently of VAL1/2. In support of this possibility, the promoters of genes upregulated in the bmi1abc mutant are enriched in ALFIN1 elements (Merini et al., 2017).
Whereas few factors have been implicated in PRC1 recruitment to date, the recruitment of PRC2 for H3K27me3 marking, in addition to depending on PRC1 activity (Zhou et al., 2017; Kralemann et al., 2020; Liu et al., 2021), has been linked to a wide diversity of TFs. These include the MYB TF ASYMMETRIC LEAVES 1 (AS1) (Lodha et al., 2013), the MADS-box TFs FLC and SHORT VEGETATIVE PHASE (SVP) (Wang et al., 2014; Richter et al., 2019), the GAGA motif binding proteins BASIC PENTACYSTEINE (BPC) 1–6 (Hecker et al., 2015; Xiao et al., 2017), the TELOBOX motif binding proteins ARABIDOPSIS ZINC FINGER 1 (AZF1) and ZINC FINGER OF ARABIDOPSIS THALIANA 6 (ZAT6) (Xiao et al., 2017), the C2H2 TFs SUPERMAN (SUP) (Xu et al., 2018) and KNUCKLES (KNU) (Sun et al., 2019), and VAL1/2 factors (Yuan et al., 2016, 2021; Chen et al., 2020). Nevertheless, although some of these TFs have been shown to interact with one or more PRC2 components, they may be engaged in more transient interactions than accessory proteins, as they have not been detected in AP–MS experiments.
The existence of Arabidopsis PRE-like sequences containing cis-elements for the binding of TFs has been proposed in several independent studies (Berger et al., 2011; Lodha et al., 2013; Mu et al., 2017; Xiao et al., 2017). Moreover, two key families of TFs, the class I BPC TFs and the ZINC FINGER TFs, co-localize with PRC2 at thousands of loci, and loss or reduction of their function causes a loss of PRC2 binding (Xiao et al., 2017).
A recent report described the in vivo mediation of TF binding to a synthetic locus whose promoter lacked any of the known cis-elements involved in PcG recruitment. Interestingly, the results implicated some of these factors in recruiting one or the other PcG complex in Arabidopsis (Baile et al., 2021). These findings showed that the binding of VAL1 leads to the incorporation of H2AK121ub and H3K27me3 and the removal of H3 acetylation marks (Figure 4A). Whereas BMI1 proteins directly interact with VAL1, PRC2 marking requires both PRC1 activity and VAL1, indicating that different interactions collaborate in PRC2 recruitment. Interestingly, SAP18 and HDA activities co-purify with VAL1 and the PRC2-associated proteins VIN3 and VRN5 (Qüesta et al., 2016), suggesting a connection between HDACs and PRC2 via some of these proteins. Furthermore, this report showed that PRC2 activity could be recruited independently of PRC1 by the binding of TFs from different families that, like the VALs, contain an EAR domain (Figure 4A). The EAR domains have been shown to bind SAP18 and in turn to recruit HDA activities. The binding of these EAR factors also leads to the removal of H3 acetylation marks (Baile et al., 2021). These results are consistent with the existence of PcG targets in which the PRC2 recruitment is dependent or independent of PRC1 activity. Moreover, they suggest that the EAR–SAP18 interaction acts as a link between PRC2 and HDACs to mediate gene repression.
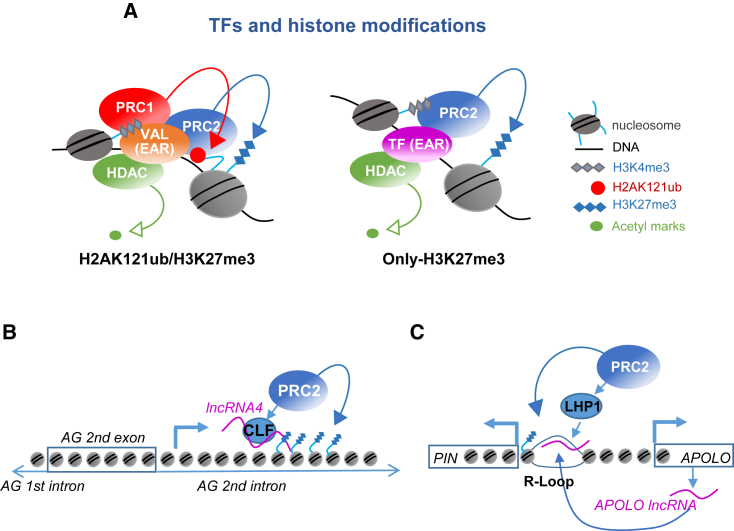
Figure 4.
Factors implicated in target site identification in plants.
(A) TFs recognize their DNA binding motifs at PRE-like sequences of target sites. TFs that contain an EAR domain as a common feature participate in PRC2 and HDAC complex recruitment. While VAL factors act as a platform for the assembly of PRC1, PRC2, and HDAC (left panel), other TFs lead to the recruitment of PRC2 and HDAC independently of PRC1 activity (right panel). The ability of VAL factors and other PRC1 and PRC2 accessory proteins to bind histone modifications, such as H3K4me3, may stabilize the recruitment of the complexes to target genes.
(B) An intronic lncRNA from the floral homeotic AGAMOUS (AG) gene, lncRNA-4, is expressed in leaves and interacts with CLF to deposit H3K27me3 histone marks into the AG locus.
(C) The lncRNA APOLO mediates the formation of R-loops by sequence complementarity with its targets, which attract LHP1 and PRC2 core components.
Finally, lncRNAs have been associated with PcG repression in plants. For instance, several lncRNAs generated from the FLC locus have been implicated in the recruitment of PRC2 and FLC repression (Costa and Dean, 2019). COLDAIR sense lncRNA interacts with CLF (Heo and Sung, 2011), COLDWRAP is an FLC promoter-associated lncRNA that also interacts with CLF to form a repressive intragenic chromatin loop (Kim and Sung, 2017), and COOLAIR antisense lncRNAs recruit CLF indirectly via an RNA binding protein called FLOWERING CONTROL LOCUS A (FCA) (Tian et al., 2019). In addition, lncRNA-4, an intronic lncRNA from the floral homeotic AGAMOUS (AG) gene, is expressed in leaves and interacts with CLF to deposit H3K27me3 histone marks into the AG locus (Wu et al., 2018b) (Figure 4B). In addition to lncRNAs, recent studies have indicated that R-loops may also support PcG target site recognition. For example, the lncRNA APOLO mediates the formation of R-loops by sequence complementarity with its targets, which attract LHP1 (Ariel et al., 2020) (Figure 4C). Interestingly, through the analysis of a COOLAIR-induced R-loop at the 3′ end of FLC, a recent report characterized the mechanism by which resolution of a nascent-transcript-induced R-loop promotes chromatin silencing (Xu et al., 2021). Stabilization of a COOLAIR-induced R-loop at the 3′ end of FLC is mediated by NDX, which inhibits further antisense transcription (Sun et al., 2013). This enables the RNA binding protein FCA and other 3′-end processing factors to polyadenylate the nascent antisense transcript, which clears the R-loop and recruits chromatin modifiers to remove H3K4me1 and H3K36me3 and promote H3K27me3 accumulation (Xu et al., 2021). Also, NDX has been recently shown to co-purify with PRC1 components and to be required for H2AK121ub accumulation and H3K27me3 incorporation at the FLC nucleation region (Mikulski et al., 2021). Therefore, it will be important to establish whether this NDX-PRC1 association points to an additional NDX/R-loop interaction in the FLC nucleation region or is the result of the gene loop.
In summary, TFs and specific combinations of their binding sites play an important role in the recruitment of PcG complexes to chromatin in plants, and epigenetic modifications of histones and RNA-protein interactions can stabilize interactions between the complexes and chromatin.
The role of PcG proteins in shaping chromatin structure in plants
PcG proteins are also related to a higher-order level of chromatin organization. PcG proteins form nuclear bodies (Polycomb bodies), suggesting that parts of the genome bound by PcG proteins gather together in the nucleus to interact, to share common machinery, and to create local concentrations of specific factors (Pirrotta and Li, 2012). Accordingly, PcG proteins are able to fold chromatin at multiple scales by establishing local loops, compacting chromatin domains, and mediating long-range interactions among H3K27me3-associated domains (Figure 5) (Cheutin and Cavalli, 2019). The formation of chromatin domains covered by H3K27me3 and the interaction among these domains shapes a chromatin network that seems to be crucial for PcG function in animals. Recent reports have shown that, in animals, cPRC1 plays a crucial role in creating these domains and in establishing the interaction among them (for a review, see Guo et al., 2021).
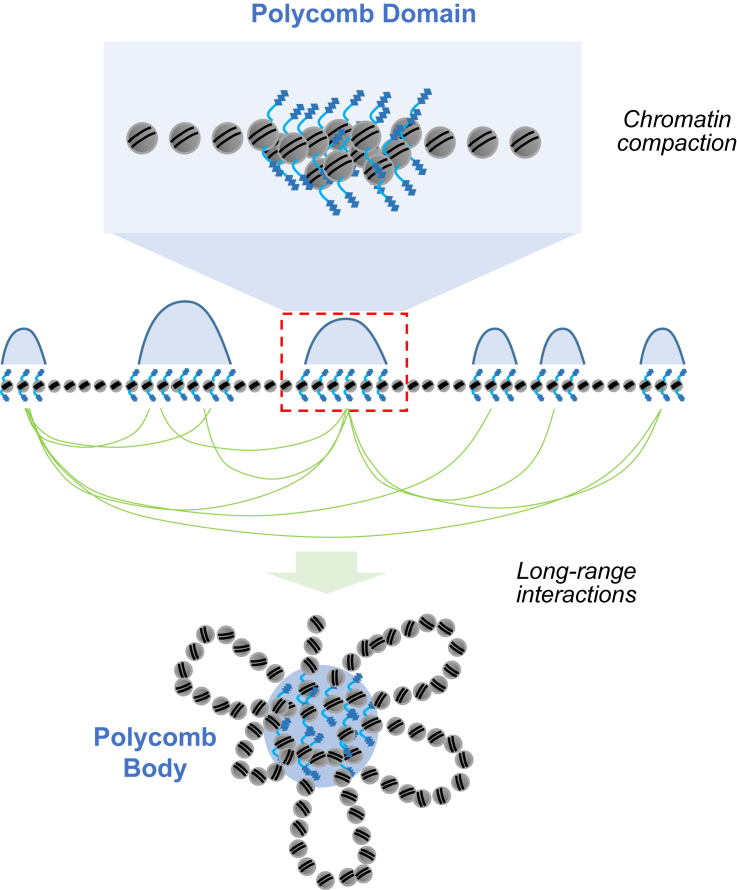
Figure 5.
PcG proteins and chromatin structure in plants.
Different PcG proteins, such as EMF1, LHP1, or BAH proteins, may promote the formation of H3K27me3-marked domains, chromatin compaction, long-range chromatin loops, and phase-separated Polycomb bodies, which contribute to the maintenance of genes in a stable repressed state.
This cPRC1 ability relies mainly on Drosophila Psc and Ph and vertebrate CBX2 and PHC (Grau et al., 2011; Isono et al., 2013; Wani et al., 2016), but it seems to be independent of cPRC1 monoubiquitination activity, as it persists when RING1B catalytic activity is impaired (Boyle et al., 2020). The IDR of CBX2, which shares biochemical and functional properties with Drosophila Psc-CTR (Grau et al., 2011), and the SAM domain of Ph/PHC, which mediates head-to-tail oligomerization of cPRC1 (Isono et al., 2013), confer this ability. Accordingly, loss of these cPRC1 proteins leads to the dissolution of Polycomb bodies (Isono et al., 2013; Wani et al., 2016; Tatavosian et al., 2019; Plys et al., 2019) (Figure 5). Furthermore, CBX2 and PHC are able to mediate liquid–liquid phase separation (LLPS) that underlies the formation of condensates (Grau et al., 2011; Seif et al., 2020) and has been proposed to induce chromatin compaction and segregate repressed chromatin from transcriptional machinery. Interestingly, data have suggested that H3K27me3 may not be the seeding site for CBX2-mediated LLPS, as H3K27me3 does not prevent the formation of CBX2 condensates in live cells. However, removal of H3K27me3 greatly reduces the bound level of other CBXs, such as CBX7 or CBX8, to chromatin, indicating that they play a different role (Tatavosian et al., 2019). By integrating these and other results, a model has recently been proposed to explain the function of PcG complexes in the mediation of higher order chromatin organization. The model is known as the scaffold-adaptor-client phase separation: CBX2-PRC1 is the scaffold, CBX7-PRC1 is the adaptor, and H3K27me3-marked chromatin is the client. In this model, CBX7-PRC1 recruits H3K27me3-marked chromatin into established CBX2-PRC1 condensates through interactions between CBX7 and H3K27me3 and polymerization of PHC between CBX2-PRC1 and CBX7-PRC1 (Kent et al., 2020). In addition, the ability of BAH-containing proteins to bind H3K27me3 (for instance, BAHCC1; Fan et al., 2020) suggests that these proteins contribute to establishing bridges between surrounding chromatin domains covered with H3K27me3. However, the interplay between these and other factors remains to be dissected.
In plants, several studies have suggested that H3K27me3 is a key contributor to chromatin topology. Local interaction of H3K27me3 domains is reduced in the Arabidopsis clfswn double mutant background (Feng et al., 2014). In addition, H3K27me3 is enriched at long-distance interacting loci across the Arabidopsis genome (Liu et al., 2016; Huang et al., 2021). However, it remains unknown whether any plant PcG proteins help to shape these interactions. EMF1 has been reported to show structural and functional similarities to Drosophila Psc-CTR and the IDR of CXB2 in vitro (Grau et al., 2011; Beh et al., 2012). Moreover, a region in the center of EMF1 is required for the formation of Polycomb bodies (Calonje et al., 2008). Therefore, it may participate in mediating phase-separated condensates, which may help to compact chromatin, promote H3K27me3 marking, and establish interactions between H3K27me3-marked domains. In addition, LHP1 contains a disordered hinge region that, when perturbed, causes the Polycomb bodies to be disrupted (Berry et al., 2017), suggesting that LHP1 participates in PcG condensate formation in plants. Moreover, as in animals, the ability of the CHROMO domain of LHP1 and the BAH domain proteins EBS and SHL to bind H3K27me3 could mediate interactions among H3K27me3 domains in plants. In addition, PWO1–4 proteins are proposed to recruit PcG proteins to subnuclear domains and to participate in chromatin compaction (Hohenstatt et al., 2018). In summary, although further work is required to explore the potential roles of these and other proteins in the mediation of plant 3D chromatin structures, the fact that Pc/CBX2 and Psc-CTR activities are linked to PRC2 instead of PRC1 in plants suggest differences in the way these interactions are established and maintained.
Concluding remarks and perspectives
Although our understanding of the PcG repressive mechanism in plants has increased significantly in recent years, many gaps remain. It is becoming clear that PcG complexes regulate gene expression at multiple levels; however, to understand how these different levels of regulation are achieved in plants, we need to identify all the players involved in the system. On the one hand, it is not known whether different plant PRC1 E3 modules exist and whether the BMI1-L truncated proteins can associate in a PRC1. Because the BMI1-Ls do not contain the RING domain, their role in PcG-mediated repression may be independent of H2A monoubiquitination. An intriguing possibility is that they play a role in creating Polycomb domains and establishing interactions among them, similar to animal cPRC1, which displays this function independently of E3 monoubiquitin ligase activity. In addition, compared with animals, the number of identified PRC1 accessory proteins in plants is still not very large; moreover, it is not known whether these or other unknown accessory proteins associate with different E3 modules constituting different plant vPRC1s. On the other hand, even though many more PRC2 accessory proteins are known now than some years ago, in most cases we still do not know whether they associate with different PRC2 cores constituting different PRC2 sub-complexes and whether these sub-complexes are involved in different levels of regulation. Addressing all these questions would require performing AP–MS experiments in different cell types and conditions.
Interestingly, although PRC1 and PRC2 enzymatic activities are conserved between animals and plants, several animal PRC1-associated activities have been linked to PRC2 in Arabidopsis. These activities participate in reading and propagating H3K27me3 marks or in mediating chromatin compaction, such as the activities displayed by LHP1 and EMF1. A possible explanation for this finding could lie in the different genomic distributions of H2AK121ub and H3K27me3 marks between animals and plants. In Arabidopsis, H2AK121ub marks are enriched at what could be considered the PcG nucleation region of target genes, whereas H3K27me3 marks co-localize with H2AK121ub at these regions but also extend over gene bodies. By contrast, these two marks co-localize over large regions in animals. Therefore, the spreading of H3K27me3 and chromatin compaction abilities may well be associated with PRC1 in animals, as the two complexes follow the same pattern and maintain a feedforward loop. However, these activities should be linked to PRC2 in plants, as PRC1 and PRC2 markings do not follow the same pace. Interestingly, a considerable number of PRC1 and PRC2 accessory proteins are plant specific, indicating that in plants the PcG system has been re-invented, incorporating a different array of proteins. Nevertheless, similar activities have been recruited, demonstrating the effectiveness of the combination for the repressive mechanism.
Finally, it will be important to extend our understanding of the Arabidopsis PcG system to other plants species with larger genomes to determine whether or not the recruitment of the complexes and their roles in shaping chromatin 3D structure follow the same rules.
Funding
This work was supported by a PID2019-106664GB-I00 grant from the Spanish Ministry of Science and Innovation.
Author contributions
F.B., A.G.-Z., and M.C. contributed equally to this work.
Acknowledgments
We apologize to those authors whose work could not be discussed due to space limitations. No conflict of interest declared.
Published: November 26, 2021
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
References
- Ariel F., Lucero L., Christ A., Mammarella M.F., Jegu T., Veluchamy A., Mariappan K., Latrasse D., Blein T., Liu C., et al. R-loop mediated trans action of the APOLO long noncoding RNA. Mol. Cell. 2020;77:1055–1065.e4. doi: 10.1016/j.molcel.2019.12.015. [DOI] [PubMed] [Google Scholar]
- Baile F., Merini W., Hidalgo I., Calonje M. EAR domain-containing transcription factors trigger PRC2-mediated chromatin marking in Arabidopsis. Plant Cell. 2021;33:2701–2715. doi: 10.1093/plcell/koab139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour H., Daou S., Hendzel M., Affar E.B. Polycomb group-mediated histone H2A monoubiquitination in epigenome regulation and nuclear processes. Nat. Commun. 2020;11:5947. doi: 10.1038/s41467-020-19722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastola D.R., Pethe V.V., Winicov I. Alfin1, a novel zinc-finger protein in alfalfa roots that binds to promoter elements in the salt-inducible MsPRP2 gene. Plant Mol. Biol. 1998;38:1123–1135. doi: 10.1023/a:1006081926699. [DOI] [PubMed] [Google Scholar]
- Beh L.Y., Colwell L.J., Francis N.J. A core subunit of Polycomb repressive complex 1 is broadly conserved in function but not primary sequence. Proc. Natl. Acad. Sci. U S A. 2012;109:E1063–E1071. doi: 10.1073/pnas.1118678109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley M.L., Corn J.E., Dong K.C., Phung Q., Cheung T.K., Cochran A.G. Recognition of UbcH5c and the nucleosome by the Bmi1/Ring1b ubiquitin ligase complex. EMBO J. 2011;30:3285–3297. doi: 10.1038/emboj.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N., Dubreucq B., Roudier F., Dubos C., Lepiniec L. Transcriptional regulation of Arabidopsis LEAFY COTYLEDON2 involves RLE, a cis-element that regulates trimethylation of histone H3 at lysine-27. Plant Cell. 2011;23:4065–4078. doi: 10.1105/tpc.111.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer M., Pisano P., Di Carlo V., Blanco E., Chammas P., Vizán P., Gutiérrez A., Aranda S., Payer B., Wierer M., et al. EPOP functionally links Elongin and polycomb in pluripotent stem cells. Mol. Cell. 2016;64:645–658. doi: 10.1016/j.molcel.2016.10.018. [DOI] [PubMed] [Google Scholar]
- Bernstein E., Duncan E.M., Masui O., Gil J., Heard E., Allis C.D. Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol. Cell Biol. 2006;26:2560–2569. doi: 10.1128/MCB.26.7.2560-2569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry S., Rosa S., Howard M., Bühler M., Dean C. Disruption of an RNA-binding hinge region abolishes LHP1-mediated epigenetic repression. Genes Dev. 2017;31:2115–2120. doi: 10.1101/gad.305227.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieluszewski T., Xiao J., Yang Y., Wagner D. PRC2 activity, recruitment, and silencing: a comparative perspective. Trends Plant Sci. Adv. 2021;26:1186–1198. doi: 10.1016/j.tplants.2021.06.006. [DOI] [PubMed] [Google Scholar]
- Blackledge N.P., Klose R.J. Getting under the skin of polycomb-dependent gene regulation. Genes Dev. 2021;35:301–303. doi: 10.1101/gad.348257.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge N.P., Farcas A.M., Kondo T., King H.W., McGouran J.F., Hanssen L.L.P., Ito S., Cooper S., Kondo K., Koseki Y., et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014;157:1445–1459. doi: 10.1016/j.cell.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge N.P., Rose N.R., Klose R.J. Targeting Polycomb systems to regulate gene expression: modifications to a complex story. Nat. Rev. Mol. Cell Biol. 2015;16:643–649. doi: 10.1038/nrm4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge N.P., Fursova N.A., Kelley J.R., Huseyin M.K., Feldmann A., Klose R.J. PRC1 catalytic activity is central to polycomb system function. Mol. Cell. 2020;77:857–874.e9. doi: 10.1016/j.molcel.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer R.H., Hutchison C.E., Bäurle I., Walker J., Fang X., Perera P., Velanis C.N., Gümüs S., Spanos C., Rappsilber J., et al. The Arabidopsis epigenetic regulator ICU11 as an accessory protein of polycomb repressive complex 2. Proc. Natl. Acad. Sci. U S A. 2020;117:16660–16666. doi: 10.1073/pnas.1920621117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S., Flyamer I.M., Williamson I., Sengupta D., Bickmore W.A., Illingworth R.S. A central role for canonical PRC1 in shaping the 3D nuclear landscape. Genes Dev. 2020;34:931–949. doi: 10.1101/gad.336487.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratzel F., López-Torrejón G., Koch M., Del Pozo J.C., Calonje M. Keeping cell identity in Arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr. Biol. 2010;20:1853–1859. doi: 10.1016/j.cub.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Bratzel F., Yang C., Angelova A., López-Torrejón G., Koch M., del Pozo J.C., Calonje M. Regulation of the new Arabidopsis imprinted gene AtBMI1C requires the interplay of different epigenetic mechanisms. Mol. Plant. 2012;5:260–269. doi: 10.1093/mp/ssr078. [DOI] [PubMed] [Google Scholar]
- Buchwald G., van der Stoop P., Weichenrieder O., Perrakis A., van Lohuizen M., Sixma T.K. Structure and E3-ligase activity of the ring–ring complex of polycomb proteins Bmi1 and Ring1b. EMBO J. 2006;25:2465–2474. doi: 10.1038/sj.emboj.7601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calonje M. PRC1 marks the difference in plant PcG repression. Mol. Plant. 2014;7:459–471. doi: 10.1093/mp/sst150. [DOI] [PubMed] [Google Scholar]
- Calonje M., Sanchez R., Chen L., Sung Z.R. EMBRYONIC FLOWER1 participates in polycomb group-mediated AG gene silencing in Arabidopsis. Plant Cell. 2008;20:277–291. doi: 10.1105/tpc.106.049957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R., Tsukada Y.-I., Zhang Y. Role of bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell. 2005;20:845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Chanvivattana Y., Bishopp A., Schubert D., Stock C., Moon Y.-H., Sung Z.R., Goodrich J. Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development. 2004;131:5263–5276. doi: 10.1242/dev.01400. [DOI] [PubMed] [Google Scholar]
- Chen D., Molitor A., Liu C., Shen W.-H. The Arabidopsis PRC1-like ring-finger proteins are necessary for repression of embryonic traits during vegetative growth. Cell Res. 2010;20:1332–1344. doi: 10.1038/cr.2010.151. [DOI] [PubMed] [Google Scholar]
- Chen N., Wang H., Abdelmageed H., Veerappan V., Tadege M., Allen R.D. HSI2/VAL1 and HSL1/VAL2 function redundantly to repress DOG1 expression in Arabidopsis seeds and seedlings. New Phytol. 2020;227:840–856. doi: 10.1111/nph.16559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetverina D.A., Lomaev D.V., Erokhin M.M. Polycomb and Trithorax group proteins: the long road from mutations in Drosophila to use in medicine. Acta Naturae. 2020;12:66–85. doi: 10.32607/actanaturae.11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheutin T., Cavalli G. The multiscale effects of polycomb mechanisms on 3D chromatin folding. Crit. Rev. Biochem. Mol. Biol. 2019;54:399–417. doi: 10.1080/10409238.2019.1679082. [DOI] [PubMed] [Google Scholar]
- Conway E., Jerman E., Healy E., Ito S., Holoch D., Oliviero G., Deevy O., Glancy E., Fitzpatrick D.J., Mucha M., et al. A family of vertebrate-specific polycombs encoded by the LCOR/LCORL genes balance PRC2 subtype activities. Mol. Cell. 2018;70:408–421.e8. doi: 10.1016/j.molcel.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Costa S., Dean C. Storing memories: the distinct phases of Polycomb-mediated silencing of Arabidopsis FLC. Biochem. Soc. Trans. 2019;47:1187–1196. doi: 10.1042/BST20190255. [DOI] [PubMed] [Google Scholar]
- de Bie P., Zaaroor-Regev D., Ciechanover A. Regulation of the Polycomb protein RING1B ubiquitination by USP7. Biochem. Biophys. Res. Commun. 2010;400:389–395. doi: 10.1016/j.bbrc.2010.08.082. [DOI] [PubMed] [Google Scholar]
- Deaton A.M., Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka B., Singh K.K. Multifaceted regulation of gene expression by the apoptosis- and splicing-associated protein complex and its components. Int. J. Biol. Sci. 2017;13:545–560. doi: 10.7150/ijbs.18649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkacheva M., Steinbach Y., Wildhaber T., Mozgová I., Mahrez W., Nanni P., Bischof S., Gruissem W., Hennig L. Arabidopsis MSI1 connects LHP1 to PRC2 complexes. EMBO J. 2013;32:2073–2085. doi: 10.1038/emboj.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkacheva M., Liu S., Figueiredo D.D., Gentry M., Mozgova I., Nanni P., Tang M., Mannervik M., Köhler C., Hennig L. H2A deubiquitinases UBP12/13 are part of the Arabidopsis polycomb group protein system. Nat. Plants. 2016;2:16126. doi: 10.1038/nplants.2016.126. [DOI] [PubMed] [Google Scholar]
- Dumesic P.A., Homer C.M., Moresco J.J., Pack L.R., Shanle E.K., Coyle S.M., Strahl B.D., Fujimori D.G., Yates J.R., Madhani H.D. Product binding enforces the genomic specificity of a yeast polycomb repressive complex. Cell. 2015;160:204–218. doi: 10.1016/j.cell.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons R.B., Genetti H., Filandrinos S., Lokere J., Wu C. Molecular genetic analysis of suppressor 2 of zeste identifies key functional domains. Genetics. 2009;182:999–1013. doi: 10.1534/genetics.108.097360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Lu J., Guo Y., Li D., Zhang Z.-M., Tsai Y.-H., Pi W.-C., Ahn J.H., Gong W., Xiang Y., et al. BAHCC1 binds H3K27me3 via a conserved BAH module to mediate gene silencing and oncogenesis. Nat. Genet. 2020;52:1384–1396. doi: 10.1038/s41588-020-00729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Guo Y., Tsai Y.-H., Storey A.J., Kim A., Gong W., Edmondson R.D., Mackintosh S.G., Li H., Byrum S.D., et al. A conserved BAH module within mammalian BAHD1 connects H3K27me3 to Polycomb gene silencing. Nucleic Acids Res. 2021;49:4441–4455. doi: 10.1093/nar/gkab210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Cokus S.J., Schubert V., Zhai J., Pellegrini M., Jacobsen S.E. Genome-wide Hi-C analyses in wild-type and mutants reveal high-resolution chromatin interactions in Arabidopsis. Mol. Cell. 2014;55:694–707. doi: 10.1016/j.molcel.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Chen D., Berr A., Shen W.-H. ZRF1 chromatin regulators have polycomb silencing and independent roles in development. Plant Physiol. 2016;172:1746–1759. doi: 10.1104/pp.16.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Gao Y., Wang K., Jiang M. A novel epigenetic regulator ZRF1: insight into its functions in plants. Genes. 2021;12:1245. doi: 10.3390/genes12081245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereres S., Simón R., Mohd-Sarip A., Verrijzer C.P., Busturia A. dRYBP counteracts chromatin-dependent activation and repression of transcription. PLoS One. 2014;9:e113255. doi: 10.1371/journal.pone.0113255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis N.J., Kingston R.E., Woodcock C.L. Chromatin compaction by a polycomb group protein complex. Science. 2004;306:1574–1577. doi: 10.1126/science.1100576. [DOI] [PubMed] [Google Scholar]
- Gao Z., Zhang J., Bonasio R., Strino F., Sawai A., Parisi F., Kluger Y., Reinberg D. PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol. Cell. 2012;45:344–356. doi: 10.1016/j.molcel.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin V., Libault M., Pouteau S., Juul T., Zhao G., Lefebvre D., Grandjean O. Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development. 2001;128:4847–4858. doi: 10.1242/dev.128.23.4847. [DOI] [PubMed] [Google Scholar]
- Gendall A.R., Levy Y.Y., Wilson A., Dean C. The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell. 2001;107:525–535. doi: 10.1016/s0092-8674(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Geng Z., Gao Z. Mammalian PRC1 complexes: compositional complexity and diverse molecular mechanisms. Int. J. Mol. Sci. 2020;21:E8594. doi: 10.3390/ijms21228594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Zambrano Á., Merini W., Calonje M. The repressive role of Arabidopsis H2A.Z in transcriptional regulation depends on AtBMI1 activity. Nat. Commun. 2019;10:2828. doi: 10.1038/s41467-019-10773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J., Puangsomlee P., Martin M., Long D., Meyerowitz E.M., Coupland G. A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature. 1997;386:44–51. doi: 10.1038/386044a0. [DOI] [PubMed] [Google Scholar]
- Grau D.J., Chapman B.A., Garlick J.D., Borowsky M., Francis N.J., Kingston R.E. Compaction of chromatin by diverse Polycomb group proteins requires localized regions of high charge. Genes Dev. 2011;25:2210–2221. doi: 10.1101/gad.17288211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greb T., Mylne J.S., Crevillen P., Geraldo N., An H., Gendall A.R., Dean C. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr. Biol. 2007;17:73–78. doi: 10.1016/j.cub.2006.11.052. [DOI] [PubMed] [Google Scholar]
- Grossniklaus U., Vielle-Calzada J.P., Hoeppner M.A., Gagliano W.B. Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science. 1998;280:446–450. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- Guo Y., Zhao S., Wang G.G. Polycomb gene silencing mechanisms: PRC2 chromatin targeting, H3K27me3 “Readout”, phase separation-based compaction. Trends Genet. 2021;37:547–565. doi: 10.1016/j.tig.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker A., Brand L.H., Peter S., Simoncello N., Kilian J., Harter K., Gaudin V., Wanke D. The Arabidopsis GAGA-binding factor BASIC PENTACYSTEINE6 recruits the POLYCOMB-REPRESSIVE COMPLEX1 component LIKE HETEROCHROMATIN PROTEIN1 to GAGA DNA motifs. Plant Physiol. 2015;168:1013–1024. doi: 10.1104/pp.15.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.B., Sung S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science. 2011;331:76–79. doi: 10.1126/science.1197349. [DOI] [PubMed] [Google Scholar]
- Hinsch V., Adkins S., Manuela D., Xu M. Post-embryonic phase transitions mediated by polycomb repressive complexes in plants. Int. J. Mol. Sci. 2021;22:7533. doi: 10.3390/ijms22147533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenstatt M.L., Mikulski P., Komarynets O., Klose C., Kycia I., Jeltsch A., Farrona S., Schubert D. PWWP-DOMAIN INTERACTOR OF POLYCOMBS1 interacts with polycomb-group proteins and histones and regulates Arabidopsis flowering and development. Plant Cell. 2018;30:117–133. doi: 10.1105/tpc.17.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holec S., Berger F. Polycomb group complexes mediate developmental transitions in plants. Plant Physiol. 2012;158:35–43. doi: 10.1104/pp.111.186445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppmann V., Thorstensen T., Kristiansen P.E., Veiseth S.V., Rahman M.A., Finne K., Aalen R.B., Aasland R. The CW domain, a new histone recognition module in chromatin proteins. EMBO J. 2011;30:1939–1952. doi: 10.1038/emboj.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Li S., Zhang X., Zheng X. HSCARG, a novel regulator of H2A ubiquitination by downregulating PRC1 ubiquitin E3 ligase activity, is essential for cell proliferation. Nucleic Acids Res. 2014;42:5582–5593. doi: 10.1093/nar/gku230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Chen D.-H., Liu B.-Y., Shen W.-H., Ruan Y. Conservation and diversification of polycomb repressive complex 2 (PRC2) proteins in the green lineage. Brief. Funct. Genomics. 2017;16:106–119. doi: 10.1093/bfgp/elw007. [DOI] [PubMed] [Google Scholar]
- Huang Y., Sicar S., Ramirez-Prado J.S., Manza-Mianza D., Antunez-Sanchez J., Brik-Chaouche R., Rodriguez-Granados N.Y., An J., Bergounioux C., Mahfouz M.M., et al. Polycomb-dependent differential chromatin compartmentalization determines gene coregulation in Arabidopsis. Genome Res. Adv. 2021;31:1230–1244. doi: 10.1101/gr.273771.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth R.S., Moffat M., Mann A.R., Read D., Hunter C.J., Pradeepa M.M., Adams I.R., Bickmore W.A. The E3 ubiquitin ligase activity of RING1B is not essential for early mouse development. Genes Dev. 2015;29:1897–1902. doi: 10.1101/gad.268151.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irminger-Finger I., Nöthiger R. The Drosophila melanogaster gene lethal(3)73Ah encodes a ring finger protein homologous to the oncoproteins MEL-18 and BMI-1. Gene. 1995;163:203–208. doi: 10.1016/0378-1119(95)00326-2. [DOI] [PubMed] [Google Scholar]
- Isono K., Endo T.A., Ku M., Yamada D., Suzuki R., Sharif J., Ishikura T., Toyoda T., Bernstein B.E., Koseki H. SAM domain polymerization links subnuclear clustering of PRC1 to gene silencing. Dev. Cell. 2013;26:565–577. doi: 10.1016/j.devcel.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Kagale S., Rozwadowski K. EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics. 2011;6:141–146. doi: 10.4161/epi.6.2.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb R., Latwiel S., Baymaz H.I., Jansen P.W.T.C., Müller C.W., Vermeulen M., Müller J. Histone H2A monoubiquitination promotes histone H3 methylation in Polycomb repression. Nat. Struct. Mol. Biol. 2014;21:569–571. doi: 10.1038/nsmb.2833. [DOI] [PubMed] [Google Scholar]
- Kang J.J., Faubert D., Boulais J., Francis N.J. DNA binding reorganizes the intrinsically disordered C-terminal region of PSC in Drosophila PRC1. J. Mol. Biol. 2020;432:4856–4871. doi: 10.1016/j.jmb.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J.A., Brown J.L. Polycomb group response elements in Drosophila and vertebrates. Adv. Genet. 2013;81:83–118. doi: 10.1016/B978-0-12-407677-8.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J.A., Kennison J.A., Tamkun J.W. Polycomb and Trithorax group genes in Drosophila. Genetics. 2017;206:1699–1725. doi: 10.1534/genetics.115.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaustov L., Ouyang H., Amaya M., Lemak A., Nady N., Duan S., Wasney G.A., Li Z., Vedadi M., Schapira M., et al. Recognition and specificity determinants of the human cbx chromodomains. J. Biol. Chem. 2011;286:521–529. doi: 10.1074/jbc.M110.191411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S., Brown K., Yang C.-H., Alsaihati N., Tian C., Wang H., Ren X. Phase-separated transcriptional condensates accelerate target-search process revealed by live-cell single-molecule imaging. Cell Rep. 2020;33:108248. doi: 10.1016/j.celrep.2020.108248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-H., Sung S. Vernalization-triggered intragenic chromatin loop formation by long noncoding RNAs. Dev. Cell. 2017;40:302–312.e4. doi: 10.1016/j.devcel.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.A., Gingery M., Pilpa R.M., Bowie J.U. The SAM domain of polyhomeotic forms a helical polymer. Nat. Struct. Biol. 2002;9:453–457. doi: 10.1038/nsb802. [DOI] [PubMed] [Google Scholar]
- Kim C.A., Sawaya M.R., Cascio D., Kim W., Bowie J.U. Structural organization of a Sex-comb-on-midleg/polyhomeotic copolymer. J. Biol. Chem. 2005;280:27769–27775. doi: 10.1074/jbc.M503055200. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Lee J., Eshed-Williams L., Zilberman D., Sung Z.R. EMF1 and PRC2 cooperate to repress key regulators of Arabidopsis development. PLoS Genet. 2012;8:e1002512. doi: 10.1371/journal.pgen.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I.F.G., Emmons R.B., Francis N.J., Wild B., Müller J., Kingston R.E., Wu C.-T. Analysis of a polycomb group protein defines regions that link repressive activity on nucleosomal templates to in vivo function. Mol. Cell. Biol. 2005;25:6578–6591. doi: 10.1128/MCB.25.15.6578-6591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C., Hennig L., Bouveret R., Gheyselinck J., Grossniklaus U., Gruissem W. Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. EMBO J. 2003;22:4804–4814. doi: 10.1093/emboj/cdg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake T., Takada S., Nakahigashi K., Ohto M., Goto K. Arabidopsis TERMINAL FLOWER 2 gene encodes a heterochromatin protein 1 homolog and represses both FLOWERING LOCUS T to regulate flowering time and several floral homeotic genes. Plant Cell Physiol. 2003;44:555–564. doi: 10.1093/pcp/pcg091. [DOI] [PubMed] [Google Scholar]
- Kralemann L.E.M., Liu S., Trejo-Arellano M.S., Muñoz-Viana R., Köhler C., Hennig L. Removal of H2Aub1 by ubiquitin-specific proteases 12 and 13 is required for stable Polycomb-mediated gene repression in Arabidopsis. Genome Biol. 2020;21:144. doi: 10.1186/s13059-020-02062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K., Turck F. Plant H3K27me3 has finally found its readers. Nat. Genet. 2018;50:1206–1208. doi: 10.1038/s41588-018-0201-1. [DOI] [PubMed] [Google Scholar]
- Kundu S., Ji F., Sunwoo H., Jain G., Lee J.T., Sadreyev R.I., Dekker J., Kingston R.E. Polycomb repressive complex 1 generates discrete compacted domains that change during differentiation. Mol. Cell. 2017;65:432–446.e5. doi: 10.1016/j.molcel.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafos M., Kroll P., Hohenstatt M.L., Thorpe F.L., Clarenz O., Schubert D. Dynamic regulation of H3K27 trimethylation during Arabidopsis differentiation. PLoS Genet. 2011;7:e1002040. doi: 10.1371/journal.pgen.1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarou A., Mohd-Sarip A., Moshkin Y.M., Chalkley G.E., Bezstarosti K., Demmers J.A.A., Verrijzer C.P. dKDM2 couples histone H2A ubiquitylation to histone H3 demethylation during Polycomb group silencing. Genes Dev. 2008;22:2799–2810. doi: 10.1101/gad.484208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-G., Kahn T.G., Simcox A., Schwartz Y.B., Pirrotta V. Genome-wide activities of Polycomb complexes control pervasive transcription. Genome Res. 2015;25:1170–1181. doi: 10.1101/gr.188920.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y.Y., Mesnage S., Mylne J.S., Gendall A.R., Dean C. Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science. 2002;297:243–246. doi: 10.1126/science.1072147. [DOI] [PubMed] [Google Scholar]
- Li Z., Cao R., Wang M., Myers M.P., Zhang Y., Xu R.-M. Structure of a Bmi-1-Ring1B polycomb group ubiquitin ligase complex. J. Biol. Chem. 2006;281:20643–20649. doi: 10.1074/jbc.M602461200. [DOI] [PubMed] [Google Scholar]
- Li J., Wang Z., Hu Y., Cao Y., Ma L. Polycomb group proteins RING1A and RING1B regulate the vegetative phase transition in Arabidopsis. Front. Plant Sci. 2017;8:867. doi: 10.3389/fpls.2017.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liefke R., Jiang J., Kurland J.V., Tian W., Deng P., Zhang W., He Q., Patel D.J., Bulyk M.L., et al. Polycomb-like proteins link the PRC2 complex to CpG islands. Nature. 2017;549:287–291. doi: 10.1038/nature23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Fu X., Wang Y., Liu R., He Y. Polycomb-mediated gene silencing by the BAH-EMF1 complex in plants. Nat. Genet. 2018;50:1254–1261. doi: 10.1038/s41588-018-0190-0. [DOI] [PubMed] [Google Scholar]
- Liang S.C., Hartwig B., Perera P., Mora-García S., de Leau E., Thornton H., de Alves F.L., Rapsilber J., Yang S., James G.V., et al. Kicking against the PRCs—a domesticated transposase antagonises silencing mediated by polycomb group proteins and is an accessory component of polycomb repressive complex 2. PLoS Genet. 2015;11:e1005660. doi: 10.1371/journal.pgen.1005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liefke R., Karwacki-Neisius V., Shi Y. EPOP interacts with Elongin BC and USP7 to modulate the chromatin landscape. Mol. Cell. 2016;64:659–672. doi: 10.1016/j.molcel.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Wang C., Wang G., Becker C., Zaidem M., Weigel D. Genome-wide analysis of chromatin packing in Arabidopsis thaliana at single-gene resolution. Genome Res. 2016;26:1057–1068. doi: 10.1101/gr.204032.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Trejo-Arellano M.S., Qiu Y., Eklund D.M., Köhler C., Hennig L. H2A ubiquitination is essential for Polycomb Repressive Complex 1-mediated gene regulation in Marchantia polymorpha. Genome Biol. 2021;22:253. doi: 10.1186/s13059-021-02476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S.M., Francis N.J. Inhibition of chromatin remodeling by polycomb group protein posterior sex combs is mechanistically distinct from nucleosome binding. Biochemistry. 2010;49:9438–9448. doi: 10.1021/bi100532a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S.M., Ahuja N.K., Francis N.J. Polycomb group protein Suppressor 2 of zeste is a functional homolog of Posterior Sex Combs. Mol. Cell. Biol. 2009;29:515–525. doi: 10.1128/MCB.01044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S.M., Follmer N.E., Lengsfeld B.M., Madamba E.V., Seong S., Grau D.J., Francis N.J. A bridging model for persistence of a polycomb group protein complex through DNA replication in vitro. Mol. Cell. 2012;46:784–796. doi: 10.1016/j.molcel.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodha M., Marco C.F., Timmermans M.C.P. The ASYMMETRIC LEAVES complex maintains repression of KNOX homeobox genes via direct recruitment of Polycomb-repressive complex2. Genes Dev. 2013;27:596–601. doi: 10.1101/gad.211425.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López H., Schmitz G., Thoma R., Theres K. Super determinant1A, a RAWULdomain-containing protein, modulates axillary meristem formation and compound leaf development in tomato. Plant Cell. 2021;33:2412–2430. doi: 10.1093/plcell/koab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F., Cui X., Zhang S., Jenuwein T., Cao X. Arabidopsis REF6 is a histone H3 lysine 27 demethylase. Nat. Genet. 2011;43:715–719. doi: 10.1038/ng.854. [DOI] [PubMed] [Google Scholar]
- Luo M., Bilodeau P., Koltunow A., Dennis E.S., Peacock W.J., Chaudhury A.M. Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U S A. 1999;96:296–301. doi: 10.1073/pnas.96.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maat H., Atsma T.J., Hogeling S.M., Rodríguez López A., Jaques J., Olthuis M., de Vries M.P., Gravesteijn C., Brouwers-Vos A.Z., van der Meer N., et al. The USP7-TRIM27 axis mediates non-canonical PRC1.1 function and is a druggable target in leukemia. iScience. 2021;24:102435. doi: 10.1016/j.isci.2021.102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens G.N., El Messaoudi-Aubert S., Elderkin S., Hiom K., Peters G. Ubiquitin-specific proteases 7 and 11 modulate Polycomb regulation of the INK4a tumour suppressor. EMBO J. 2010;29:2553–2565. doi: 10.1038/emboj.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarevich G., Leroy O., Akinci U., Schubert D., Clarenz O., Goodrich J., Grossniklaus U., Köhler C. Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep. 2006;7:947–952. doi: 10.1038/sj.embor.7400760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March E., Farrona S. Plant deubiquitinases and their role in the control of gene expression through modification of histones. Front. Plant Sci. 2017;8:2274. doi: 10.3389/fpls.2017.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R., Li G., Sarma K., Blais A., Zavadil J., Woodcock C.L., Dynlacht B.D., Reinberg D. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol. Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merini W., Calonje M. PRC1 is taking the lead in PcG repression. Plant J. Adv. 2015;83:110–120. doi: 10.1111/tpj.12818. [DOI] [PubMed] [Google Scholar]
- Merini W., Romero-Campero F.J., Gomez-Zambrano A., Zhou Y., Turck F., Calonje M. The Arabidopsis polycomb repressive complex 1 (PRC1) components AtBMI1A, B, and C impact gene networks throughout all stages of plant development. Plant Physiol. 2017;173:627–641. doi: 10.1104/pp.16.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulski P., Wolff P., Lu T., Zhu D., Dean C. VAL1 as an assembly platform co-ordinating co-transcriptional repression and chromatin regulation at Arabidopsis FLC. bioRxiv. 2021 doi: 10.1101/2021.07.21.453204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor A.M., Bu Z., Yu Y., Shen W.-H. Arabidopsis AL PHD-PRC1 complexes promote seed germination through H3K4me3-to-H3K27me3 chromatin state switch in repression of seed developmental genes. PLoS Genet. 2014;10:e1004091. doi: 10.1371/journal.pgen.1004091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozgova I., Hennig L. The polycomb group protein regulatory network. Annu. Rev. Plant Biol. 2015;66:269–296. doi: 10.1146/annurev-arplant-043014-115627. [DOI] [PubMed] [Google Scholar]
- Mu Y., Zou M., Sun X., He B., Xu X., Liu Y., Zhang L., Chi W. BASIC PENTACYSTEINE proteins repress ABSCISIC ACID INSENSITIVE4 expression via direct recruitment of the polycomb-repressive complex 2 in Arabidopsis root development. Plant Cell Physiol. 2017;58:607–621. doi: 10.1093/pcp/pcx006. [DOI] [PubMed] [Google Scholar]
- Müller J., Hart C.M., Francis N.J., Vargas M.L., Sengupta A., Wild B., Miller E.L., O’Connor M.B., Kingston R.E., Simon J.A. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell. 2002;111:197–208. doi: 10.1016/s0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Mylne J.S., Barrett L., Tessadori F., Mesnage S., Johnson L., Bernatavichute Y.V., Jacobsen S.E., Fransz P., Dean C. LHP1, the Arabidopsis homologue of HETEROCHROMATIN PROTEIN1, is required for epigenetic silencing of FLC. Proc. Natl. Acad. Sci. U S A. 2006;103:5012–5017. doi: 10.1073/pnas.0507427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasov M., Klymenko T., Fraterman S., Papp B., Oktaba K., Köcher T., Cohen A., Stunnenberg H.G., Wilm M., Müller J. Pcl-PRC2 is needed to generate high levels of H3-K27 trimethylation at Polycomb target genes. EMBO J. 2007;26:4078–4088. doi: 10.1038/sj.emboj.7601837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad N., Yadegari R., Margossian L., Hannon M., Michaeli D., Harada J.J., Goldberg R.B., Fischer R.L. Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell. 1999;11:407–416. doi: 10.1105/tpc.11.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Wang L., Zhang Y., Dong A., Shen W.-H., Huang Y. Structural analysis of the Arabidopsis AL2-PAL and PRC1 complex provides mechanistic insight into active-to-repressive chromatin state switch. J. Mol. Biol. 2018;430:4245–4259. doi: 10.1016/j.jmb.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Pengelly A.R., Kalb R., Finkl K., Müller J. Transcriptional repression by PRC1 in the absence of H2A monoubiquitylation. Genes Dev. 2015;29:1487–1492. doi: 10.1101/gad.265439.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Li H.-B. A view of nuclear Polycomb bodies. Curr. Opin. Genet. Dev. 2012;22:101–109. doi: 10.1016/j.gde.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plys A.J., Davis C.P., Kim J., Rizki G., Keenen M.M., Marr S.K., Kingston R.E. Phase separation of Polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 2019;33:799–813. doi: 10.1101/gad.326488.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qüesta J.I., Song J., Geraldo N., An H., Dean C. Arabidopsis transcriptional repressor VAL1 triggers Polycomb silencing at FLC during vernalization. Science. 2016;353:485–488. doi: 10.1126/science.aaf7354. [DOI] [PubMed] [Google Scholar]
- Richly H., Rocha-Viegas L., Ribeiro J.D., Demajo S., Gundem G., Lopez-Bigas N., Nakagawa T., Rospert S., Ito T., Di Croce L. Transcriptional activation of polycomb-repressed genes by ZRF1. Nature. 2010;468:1124–1128. doi: 10.1038/nature09574. [DOI] [PubMed] [Google Scholar]
- Richter R., Kinoshita A., Vincent C., Martinez-Gallegos R., Gao H., van Driel A.D., Hyun Y., Mateos J.L., Coupland G. Floral regulators FLC and SOC1 directly regulate expression of the B3-type transcription factor TARGET OF FLC AND SVP 1 at the Arabidopsis shoot apex via antagonistic chromatin modifications. PLoS Genet. 2019;15:e1008065. doi: 10.1371/journal.pgen.1008065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa S., Shaw P. Insights into chromatin structure and dynamics in plants. Biology. 2013;2:1378–1410. doi: 10.3390/biology2041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pulido L., Devos D., Sung Z.R., Calonje M. RAWUL: a new ubiquitin-like domain in PRC1 ring finger proteins that unveils putative plant and worm PRC1 orthologs. BMC Genomics. 2008;9:308. doi: 10.1186/1471-2164-9-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma K., Margueron R., Ivanov A., Pirrotta V., Reinberg D. Ezh2 requires PHF1 to efficiently catalyze H3 lysine 27 trimethylation in vivo. Mol. Cell. Biol. 2008;28:2718–2731. doi: 10.1128/MCB.02017-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasnauskas G., Kauneckaite K., Siksnys V. Structural basis of DNA target recognition by the B3 domain of Arabidopsis epigenome reader VAL1. Nucleic Acids Res. 2018;46:4316–4324. doi: 10.1093/nar/gky256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin A.J., Shao Z., Erdjument-Bromage H., Tempst P., Kingston R.E. A Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature. 2001;412:655–660. doi: 10.1038/35088096. [DOI] [PubMed] [Google Scholar]
- Seif E., Kang J.J., Sasseville C., Senkovich O., Kaltashov A., Boulier E.L., Kapur I., Kim C.A., Francis N.J. Phase separation by the polyhomeotic sterile alpha motif compartmentalizes Polycomb group proteins and enhances their activity. Nat. Commun. 2020;11:5609. doi: 10.1038/s41467-020-19435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z., Raible F., Mollaaghababa R., Guyon J.R., Wu C.T., Bender W., Kingston R.E. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell. 1999;98:37–46. doi: 10.1016/S0092-8674(00)80604-2. [DOI] [PubMed] [Google Scholar]
- Steffen P.A., Ringrose L. What are memories made of? How Polycomb and Trithorax proteins mediate epigenetic memory. Nat. Rev. Mol. Cell Biol. 2014;15:340–356. doi: 10.1038/nrm3789. [DOI] [PubMed] [Google Scholar]
- Sun Q., Csorba T., Skourti-Stathaki K., Proudfoot N.J., Dean C. R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science. 2013;340:619–621. doi: 10.1126/science.1234848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Zhou Y., Cai J., Shang E., Yamaguchi N., Xiao J., Looi L.-S., Wee W.-Y., Gao X., Wagner D., et al. Integration of transcriptional repression and polycomb-mediated silencing of WUSCHEL in floral meristems. Plant Cell. 2019;31:1488–1505. doi: 10.1105/tpc.18.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S., Schmitz R.J., Amasino R.M. A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev. 2006;20:3244–3248. doi: 10.1101/gad.1493306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Wang H.H.-Y., McCarty D.R. Repression of the LEAFY COTYLEDON 1/B3 regulatory network in plant embryo development by VP1/ABSCISIC ACID INSENSITIVE 3-LIKE B3 genes. Plant Physiol. 2007;143:902–911. doi: 10.1104/pp.106.092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi H., Zhang Y., Hattori S., Omae M., Shimizu-Sato S., Oikawa T., Qian Q., Nishimura M., Kitano H., Xie H., et al. LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems. Plant Cell. 2011;23:3276–3287. doi: 10.1105/tpc.111.088765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburri S., Lavarone E., Fernández-Pérez D., Conway E., Zanotti M., Manganaro D., Pasini D. Histone H2AK119 mono-ubiquitination is essential for polycomb-mediated transcriptional repression. Mol. Cell. 2020;77:840–856.e5. doi: 10.1016/j.molcel.2019.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatavosian R., Kent S., Brown K., Yao T., Duc H.N., Huynh T.N., Zhen C.Y., Ma B., Wang H., Ren X. Nuclear condensates of the Polycomb protein chromobox 2 (CBX2) assemble through phase separation. J. Biol. Chem. 2019;294:1451–1463. doi: 10.1074/jbc.RA118.006620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Zheng H., Zhang F., Wang S., Ji X., Xu C., He Y., Ding Y. PRC2 recruitment and H3K27me3 deposition at FLC require FCA binding of COOLAIR. Sci. Adv. 2019;5:eaau7246. doi: 10.1126/sciadv.aau7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck F., Roudier F., Farrona S., Martin-Magniette M.-L., Guillaume E., Buisine N., Gagnot S., Martienssen R.A., Coupland G., Colot V. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007;3:e86. doi: 10.1371/journal.pgen.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanis C.N., Perera P., Thomson B., de Leau E., Liang S.C., Hartwig B., Förderer A., Thornton H., Arede P., Chen J., et al. The domesticated transposase ALP2 mediates formation of a novel Polycomb protein complex by direct interaction with MSI1, a core subunit of Polycomb Repressive Complex 2 (PRC2) PLoS Genet. 2020;16:e1008681. doi: 10.1371/journal.pgen.1008681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Shen W.-H. Chromatin modulation and gene regulation in plants: insight about PRC1 function. Biochem. Soc. Trans. 2018;46:957–966. doi: 10.1042/BST20170576. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang L., Erdjument-Bromage H., Vidal M., Tempst P., Jones R.S., Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Wang Y., Gu X., Yuan W., Schmitz R.J., He Y. Photoperiodic control of the floral transition through a distinct polycomb repressive complex. Dev. Cell. 2014;28:727–736. doi: 10.1016/j.devcel.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Wani A.H., Boettiger A.N., Schorderet P., Ergun A., Münger C., Sadreyev R.I., Zhuang X., Kingston R.E., Francis N.J. Chromatin topology is coupled to Polycomb group protein subnuclear organization. Nat. Commun. 2016;7:10291. doi: 10.1038/ncomms10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Zhang Y.-Q., Tao J.-J., Chen H.-W., Li Q.-T., Zhang W.-K., Ma B., Lin Q., Zhang J.-S., Chen S.-Y. The Alfin-like homeodomain finger protein AL5 suppresses multiple negative factors to confer abiotic stress tolerance in Arabidopsis. Plant J. 2015;81:871–883. doi: 10.1111/tpj.12773. [DOI] [PubMed] [Google Scholar]
- Whitcomb S.J., Basu A., Allis C.D., Bernstein E. Polycomb Group proteins: an evolutionary perspective. Trends Genet. 2007;23:494–502. doi: 10.1016/j.tig.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Wiles E.T., McNaught K.J., Kaur G., Selker J.M.L., Ormsby T., Aravind L., Selker E.U. Evolutionarily ancient BAH-PHD protein mediates Polycomb silencing. Proc. Natl. Acad. Sci. U S A. 2020;117:11614–11623. doi: 10.1073/pnas.1918776117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Zhang M., Su S., Liu H., Gan J., Ma J. Structural insight into the role of VAL1 B3 domain for targeting to FLC locus in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2018;501:415–422. doi: 10.1016/j.bbrc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Wu H.-W., Deng S., Xu H., Mao H.-Z., Liu J., Niu Q.-W., Wang H., Chua N.-H. A noncoding RNA transcribed from the AGAMOUS (AG) second intron binds to CURLY LEAF and represses AG expression in leaves. New Phytol. 2018;219:1480–1491. doi: 10.1111/nph.15231. [DOI] [PubMed] [Google Scholar]
- Xiao J., Wagner D. Polycomb repression in the regulation of growth and development in Arabidopsis. Curr. Opin. Plant Biol. 2015;23:15–24. doi: 10.1016/j.pbi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Xiao J., Jin R., Yu X., Shen M., Wagner J.D., Pai A., Song C., Zhuang M., Klasfeld S., He C., et al. Cis and trans determinants of epigenetic silencing by Polycomb repressive complex 2 in Arabidopsis. Nat. Genet. 2017;49:1546–1552. doi: 10.1038/ng.3937. [DOI] [PubMed] [Google Scholar]
- Xu L., Shen W.-H. Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr. Biol. 2008;18:1966–1971. doi: 10.1016/j.cub.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Xu Y., Prunet N., Gan E.-S., Wang Y., Stewart D., Wellmer F., Huang J., Yamaguchi N., Tatsumi Y., Kojima M., et al. SUPERMAN regulates floral whorl boundaries through control of auxin biosynthesis. EMBO J. 2018;37:e97499. doi: 10.15252/embj.201797499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Wu Z., Duan H.-C., Fang X., Jia G., Dean C. R-Loop resolution promotes co-transcriptional chromatin silencing. Nat. Commun. 2021;12:1790. doi: 10.1038/s41467-021-22083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Bratzel F., Hohmann N., Koch M., Turck F., Calonje M. VAL- and AtBMI1-mediated H2Aub initiate the switch from embryonic to postgerminative growth in Arabidopsis. Curr. Biol. 2013;23:1324–1329. doi: 10.1016/j.cub.2013.05.050. [DOI] [PubMed] [Google Scholar]
- Yang Z., Qian S., Scheid R.N., Lu L., Chen X., Liu R., Du X., Lv X., Boersma M.D., Scalf M., et al. EBS is a bivalent histone reader that regulates floral phase transition in Arabidopsis. Nat. Genet. 2018;50:1247–1253. doi: 10.1038/s41588-018-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Skirpan A., Wardell B., Matthes M.S., Best N.B., McCubbin T., Durbak A., Smith T., Malcomber S., McSteen P. The barren stalk2 gene is required for axillary meristem development in maize. Mol. Plant. 2019;12:374–389. doi: 10.1016/j.molp.2018.12.024. [DOI] [PubMed] [Google Scholar]
- Yin X., Romero-Campero F.J., de Los Reyes P., Yan P., Yang J., Tian G., Yang X., Mo X., Zhao S., Calonje M., et al. H2AK121ub in Arabidopsis associates with a less accessible chromatin state at transcriptional regulation hotspots. Nat. Commun. 2021;12:315. doi: 10.1038/s41467-020-20614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N., Yanai Y., Chen L., Kato Y., Hiratsuka J., Miwa T., Sung Z.R., Takahashi S. EMBRYONIC FLOWER2, a novel polycomb group protein homolog, mediates shoot development and flowering in Arabidopsis. Plant Cell. 2001;13:2471–2481. doi: 10.1105/tpc.010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Luo X., Li Z., Yang W., Wang Y., Liu R., Du J., He Y. A cis cold memory element and a trans epigenome reader mediate Polycomb silencing of FLC by vernalization in Arabidopsis. Nat. Genet. 2016;48:1527–1534. doi: 10.1038/ng.3712. [DOI] [PubMed] [Google Scholar]
- Yuan L., Song X., Zhang L., Yu Y., Liang Z., Lei Y., Ruan J., Tan B., Liu J., Li C. The transcriptional repressors VAL1 and VAL2 recruit PRC2 for genome-wide Polycomb silencing in Arabidopsis. Nucleic Acids Res. 2021;49:98–113. doi: 10.1093/nar/gkaa1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Gao Z., Jiang C., Yang Y., Liu R., He Y. HISTONE DEACETYLASE 9 functions with polycomb silencing to repress FLOWERING LOCUS C expression. Plant Physiol. 2020;182:555–565. doi: 10.1104/pp.19.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Iratni R., Erdjument-Bromage H., Tempst P., Reinberg D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Zhang X., Clarenz O., Cokus S., Bernatavichute Y.V., Pellegrini M., Goodrich J., Jacobsen S.E. Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 2007;5:e129. doi: 10.1371/journal.pbio.0050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Germann S., Blus B.J., Khorasanizadeh S., Gaudin V., Jacobsen S.E. The Arabidopsis LHP1 protein colocalizes with histone H3 Lys27 trimethylation. Nat. Struct. Mol. Biol. 2007;14:869–871. doi: 10.1038/nsmb1283. [DOI] [PubMed] [Google Scholar]
- Zhao S., Zhang B., Yang M., Zhu J., Li H. Systematic profiling of histone readers in Arabidopsis thaliana. Cell Rep. 2018;22:1090–1102. doi: 10.1016/j.celrep.2017.12.099. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Tan B., Luo M., Li Y., Liu C., Chen C., Yu C.-W., Yang S., Dong S., Ruan J., et al. HISTONE DEACETYLASE19 interacts with HSL1 and participates in the repression of seed maturation genes in Arabidopsis seedlings. Plant Cell. 2013;25:134–148. doi: 10.1105/tpc.112.096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Romero-Campero F.J., Gómez-Zambrano Á., Turck F., Calonje M. H2A monoubiquitination in Arabidopsis thaliana is generally independent of LHP1 and PRC2 activity. Genome Biol. 2017;18:69. doi: 10.1186/s13059-017-1197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang Y., Krause K., Yang T., Dongus J.A., Zhang Y., Turck F. Telobox motifs recruit CLF/SWN-PRC2 for H3K27me3 deposition via TRB factors in Arabidopsis. Nat. Genet. 2018;50:638–644. doi: 10.1038/s41588-018-0109-9. [DOI] [PubMed] [Google Scholar]
- Zhou J.-X., Liu Z.-W., Li Y.-Q., Li L., Wang B., Chen S., He X.-J. Arabidopsis PWWP domain proteins mediate H3K27 trimethylation on FLC and regulate flowering time. J. Integr. Plant Biol. 2018;60:362–368. doi: 10.1111/jipb.12630. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Hu X., Duan Y., Li S., Wang Y., Rehman A.U., He J., Zhang J., Hua D., Yang L., et al. The Arabidopsis Nodulin homeobox factor AtNDX interacts with AtRING1A/B and negatively regulates abscisic acid signaling. Plant Cell. 2020;32:703–721. doi: 10.1105/tpc.19.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]