Abstract
Xanthomonas oryzae pv. oryzae (Xoo), the causal agent of bacterial leaf blight in rice, delivers transcription activator-like effector (TALE) proteins into host cells to activate susceptibility or resistance (R) genes that promote disease or immunity, respectively. Nonhost plants serve as potential reservoirs of R genes; consequently, nonhost R genes may trap TALEs to trigger an immune response. In this study, we screened 17 Xoo TALEs for their ability to induce a hypersensitive response (HR) in the nonhost plant Nicotiana benthamiana (Nb); only AvrXa10 elicited an HR when transiently expressed in Nb. The HR generated by AvrXa10 required both the central repeat region and the activation domain, suggesting a specific interaction between AvrXa10 and a potential R-like gene in nonhost plants. Evans blue staining and ion leakage measurements confirmed that the AvrXa10-triggered HR was a form of cell death, and the transient expression of AvrXa10 in Nb induced immune responses. Genes targeted by AvrXa10 in the Nb genome were identified by transcriptome profiling and prediction of effector binding sites. Using several approaches (in vivo reporter assays, electrophoretic mobility-shift assays, targeted designer TALEs, and on-spot gene silencing), we confirmed that AvrXa10 targets NbZnFP1, a C2H2-type zinc finger protein that resides in the nucleus. Functional analysis indicated that overexpression of NbZnFP1 and its rice orthologs triggered cell death in rice protoplasts. An NbZnFP1 ortholog was also identified in tomato and was specifically activated by AvrXa10. These results demonstrate that NbZnFP1 is a nonhost R gene that traps AvrXa10 to promote plant immunity in Nb.
Key words: Xanthomonas oryzae pv. oryzae, AvrXa10, hypersensitive response, nonhost plant, zinc finger protein
Seventeen Xoo TALEs were screened for their ability to cause a hypersensitive response (HR) in N. benthamiana (Nb), and only AvrXa10 elicited a stable HR in Nb. AvrXa10 was shown to activate NbZnFP1, a C2H2-type zinc finger protein, which led to HR in Nb. These results demonstrate that TALE proteins can be trapped by R-like genes in nonhost plants to promote immunity.
Introduction
Plants are constantly under attack by microbial pathogens in nature and cope by deploying an innate immune system to resist infection (Jones et al., 2016). The first layer of immunity is triggered by recognition of pathogen-associated molecular patterns via pattern recognition receptors; the latter are often receptor-like proteins or receptor-like kinases (Tang et al., 2017). At the cellular level, pathogen-associated molecular pattern-triggered immunity (PTI) includes the generation of reactive oxygen species (ROS), mitogen-activated protein kinase cascades, induction of pathogenesis-related (PR) genes, and deposition of phenolic compounds (Schwessinger and Ronald, 2012). To suppress PTI, pathogens have evolved virulence “effectors” that interfere with PTI and inhibit basal defense, resulting in effector-triggered susceptibility (Jones and Dangl, 2006; Dou and Zhou, 2012). As a countermeasure, plants have developed additional receptors that recognize effectors, resulting in a second layer of immunity known as effector-triggered immunity (ETI). ETI occurs more rapidly than PTI and is often accompanied by a hypersensitive response (HR) at the invasion site that inhibits pathogen multiplication (Jones and Dangl, 2006; Jones et al., 2016).
Bacteria in the genus Xanthomonas infect many important crops, including rice, wheat, cassava, soybean, and cotton. The pathogenicity of Xanthomonas spp. depends on the type III secretion system (T3SS) (Yang and White, 2004), which delivers effector proteins into plant cells. Effectors include Xanthomonas outer proteins or transcription activator-like effector proteins (TALEs); the latter are highly conserved in Xanthomonas spp. (Boch and Bonas, 2010). TALEs share a conserved modular structure that comprises a type III secretion signal at the N terminus, nuclear localization signals, an acidic activation domain (AD) at the C terminus, and a central repeat region (CRR). The nuclear localization signals guide the TALEs into the plant nucleus, where they utilize the CRR to bind specific promoter sequences known as effector-binding elements (EBE); the AD then triggers expression of the target gene (Moscou and Bogdanove, 2009; Boch and Bonas, 2010). The CRR of TALEs comprises 1.5–33.5 copies of nearly identical, tandemly arranged repeats that are mostly 33 or 34 amino acids long. These highly conserved repeats are polymorphic at positions 12 and 13, and the amino acids at these locations are known as repeat variable di-residues (RVDs). The RVDs recognize specific nucleotides in the EBE of the host gene promoter and can be used to help identify TALE targets (Boch et al., 2009; Grau et al., 2013; Cernadas et al., 2014).
Some TALEs function as virulence factors by inducing host susceptibility genes that promote plant diseases. For example, the TALE AvrBs3 from Xanthomonas euvesicatoria targets the pepper transcription factor UPA20 and contributes to disease by inducing hypertrophy (Kay et al., 2007). Tal2g in Xanthomonas oryzae pv. oryzicola (Xoc) contributes to lesion development in rice by targeting the sulfate transporter OsSULTR3;6 (Cernadas et al., 2014). In citrus, CsLOB1 is targeted by multiple TAL effectors and promotes bacterial growth and pustule formation (Hu et al., 2014; Li et al., 2014). The SWEET genes in host plants encode sugar transporters and are the most important virulence targets of TALEs (Yang et al., 2006; Antony et al., 2010; Cohn et al., 2014; Cox et al., 2017; Xu et al., 2019). Tal2 in Xanthomonas citri pv. malvacearum contributes to bacterial blight of cotton by targeting a yet-unknown susceptibility gene in cotton (Haq et al., 2020).
As a countermeasure, plants have evolved resistance (R) genes that “trap” TALEs and confer immunity. Some R genes, including Xa10, Xa23, Xa27, and Xa7 in rice and Bs3 and Bs4C-R in solanaceous plants, trap TALEs and exhibit TALE-dependent transcription to promote an HR (Bogdanove et al., 2010; Xue et al., 2020; Chen et al., 2021; Luo et al., 2021). The resistance spectra of R genes are diverse and depend on the cognate or “trapped” TALEs in the Xanthomonas population. For example, Xa10-mediated resistance is very limited owing to the absence of AvrXa10 in most Xanthomonas oryzae pv. oryzae (Xoo) races (Wang et al., 2017). Several rice R genes originated from the wild species Oryza rufipogon (Zhang et al., 2001; Wang et al., 2015); thus it seems possible that Xanthomonas TALEs may occasionally be trapped in nonhost plants.
Nonhost plants exhibit durable, broad-spectrum resistance to a wide range of phytopathogens. The mechanistic basis of nonhost resistance (NHR) is complex and involves both preformed and induced defense responses that may result in symptomless reactions or the HR (Uma et al., 2011; Senthil-Kumar and Mysore, 2013). Nonhost plants can also recognize effectors from pathogens, and this recognition can trigger ETI. For example, the effector AvrRxo1 from the rice pathogen Xoc was recognized by the maize R gene product Rxo1, and this recognition induced a nonhost defense in maize (Zhao et al., 2004). Furthermore, rice lines expressing Rxo1 exhibited a high level of resistance to Xoc containing avrRxo1 (Zhao et al., 2005), thus demonstrating that R genes from nonhost plants can be used in host resistance. Similarly, the effectors XopQ from Xanthomonas and HopQ1 from Pseudomonas are recognized by Roq1 in the nonhosts Nicotiana benthamiana (Nb) and Nicotiana tabacum, resulting in ETI (Wei et al., 2007; Schwartz et al., 2015; Schultink et al., 2017). Recently, our lab reported that the effector XopL from Xoo triggered an HR in Nb by interacting with ferredoxin (Ma et al., 2020). Collectively, these studies indicate that nonhost plants possess a repository of R genes that could be deployed in plant disease resistance breeding.
Xoo elicits an HR in the nonhost Nb (Li et al., 2012); however, the role of TALEs in NHR has not previously been investigated. In this study, 17 TALEs from Xoo were analyzed for their ability to elicit an HR-like response in Nb by Agrobacterium-mediated transient expression. The TALE AvrXa10 was shown to elicit an HR in Nb, and AvrXa10-mediated cell death was dependent on the induced expression of NbZnFP1, which encodes a C2H2-type zinc finger protein (ZnFP). AvrXa10 was also shown to trigger HR in tomato, likely by activating an NbZnFP1 ortholog. These findings suggest that NbZnFP1 functions in a manner analogous to that of an R gene in Nb in response to the TALE AvrXa10.
Results
AvrXa10 induces HR and immune responses in N. benthamiana
Xoo was previously shown to induce the HR in the nonhost Nb in a T3SS-dependent manner (Li et al., 2012). In this study, the role of Xoo TAL effectors in mediating NHR was investigated by cloning 17 tal genes into the binary vector pHB, which contains an N-terminal FLAG epitope tag driven by the 35S promoter (Supplemental Figure 1A and 1B). Genes encoding the TALEs were transiently expressed in Nb via Agrobacterium-mediated transformation to evaluate their ability to induce HR-like cell death (Supplemental Methods 1 and 2). The TALE AvrXa10 induced cell death at 3 days post inoculation (dpi), whereas the other 16 effectors (Supplemental Figure 1C) failed to induce an HR when expressed in Nb.
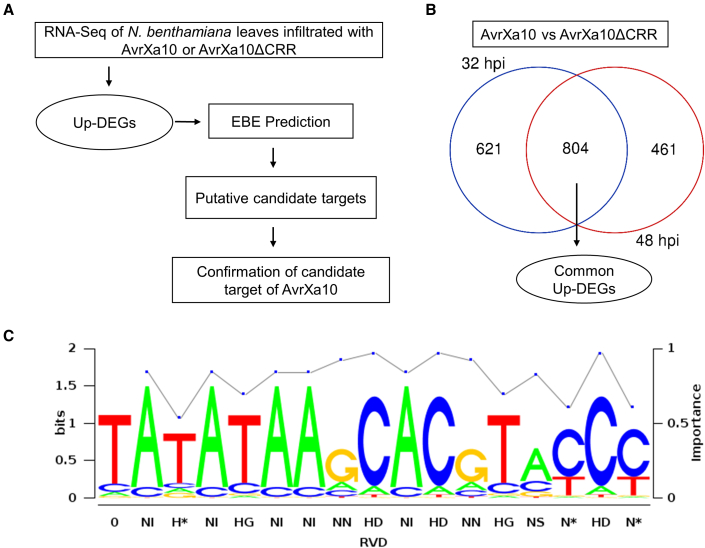
Figure 2.
Experimental design for identification of candidate AvrXa10 target genes in N. benthamiana.
(A) Schematic flowchart of experimental design. RNA was sequenced from Nb leaves transiently expressing AvrXa10 or AvrXa10ΔCRR at 32 and 48 hpi. Differentially regulated genes were identified at the two time points, and upregulated DEGs common to both time points were identified. The TALgetter computational tool was then used to predict AvrXa10 EBEs in the promoter regions of the upregulated genes.
(B) Venn diagram of DEGs upregulated by AvrXa10 but not by AvrXa10ΔCRR at 32 and 48 hpi.
(C) The predicted theoretical EBE of AvrXa10 RVDs. The AvrXa10 RVDs and their associated nucleotides are shown. The logo was produced using TALgetter (Galaxy v.1.1 http://galaxy.informatik.uni-halle.de/).
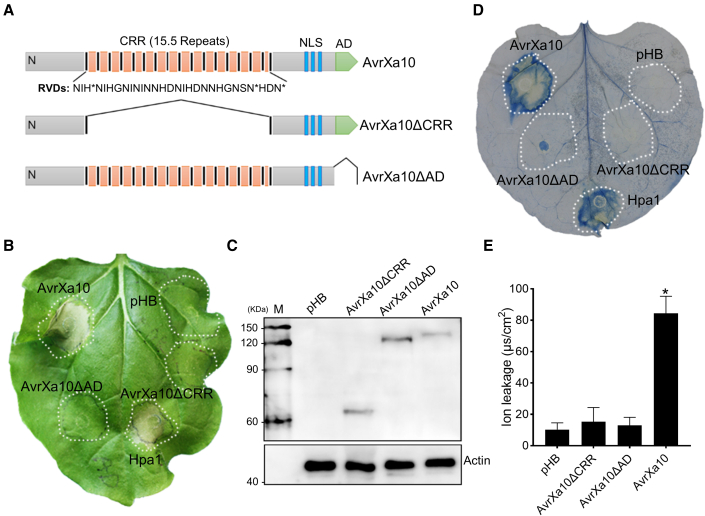
The truncated proteins AvrXa10ΔCRR and AvrXa10ΔAD, which lack the CRR and AD, respectively (Figure 1A), were constructed as described in Supplemental Methods 1. When the mutant constructs pHB-AvrXa10ΔCRR and pHB-AvrXa10ΔAD were transiently expressed in Nb, the HR was absent, suggesting that the cell death triggered by AvrXa10 occurs when the CRR and AD target an unknown Nb gene (Figure 1B). The HR-inducing ability of AvrXa10 was further tested by infiltrating Nb with Agrobacterium strains carrying pHB-AvrXa10 at OD600 0.5, 0.2, 0.1, 0.01, and 0.001. AvrXa10 triggered an HR at OD600 0.5, 0.2, and 0.1, but not at 0.01 or 0.001 (Supplemental Figure 1D). Immunoblotting confirmed that AvrXa10 was clearly detectable in leaves infiltrated with OD600 0.5, 0.2, and 0.1, but not with lower values (OD600 0.01 and 0.001) (Supplemental Figure 1E). Evans blue staining and ion leakage measurements confirmed that AvrXa10 elicited cell death when transiently expressed in Nb, whereas AvrXa10ΔAD and AvrXa10ΔCRR did not (Figure 1D and 1E).
Figure 1.
HR-like cell death induced by Xoo TALE AvrXa10 in N. benthamiana.
(A) Diagram showing RVDs in AvrXa10 and the construction of mutants AvrXa10ΔCRR and AvrXa10ΔAD, which lack the central repeat region (CRR) and activation domain (AD), respectively.
(B) Phenotypes of Nb leaves expressing AvrXa10, AvrXa10ΔCRR, and AvrXa10ΔAD. Agrobacterium strains containing constructs pHB-AvrXa10, pHB-AvrXa10ΔCRR, pHB-AvrXa10ΔAD, pHB-Hpa1 (positive control), and empty pHB were used to transform Nb leaves at OD600 1.0. Photographs were taken at 3 dpi.
(C) Detection of AvrXa10, AvrXa10ΔCRR, and AvrXa10ΔAD expression in Nb leaves by western blotting. Actin was used as a loading control.
(D) Detection of cell death in Nb leaves infiltrated with Agrobacterium containing AvrXa10, AvrXa10ΔCRR, AvrXa10ΔAD, and empty pHB by Evans blue staining.
(E) Measurement of ion leakage in Nb leaves expressing AvrXa10, AvrXa10ΔCRR, AvrXa10ΔAD, or empty pHB vector. Error bars show means ± SD (n = 3), and columns labeled with an asterisk are significantly different (∗P < 0.01) from the pHB control. The experiment was repeated at least three times with similar results.
The cell death reaction in plants is generally preceded by other immune responses, such as the generation of ROS and the expression of defense-related genes (Asai and Yoshioka, 2009; Deb et al., 2018). Therefore, we tested whether AvrXa10 and its derivatives could induce ROS accumulation in Nb. Agrobacterium strains carrying pHB-AvrXa10, pHB-AvrXa10ΔAD, pHB-AvrXa10ΔCRR, or empty pHB were infiltrated into Nb leaves, and ROS accumulation was measured. AvrXa10, but not AvrXa10ΔAD or AvrXa10ΔCRR, induced ROS accumulation at 2 and 3 dpi (Supplemental Figure 2A). Furthermore, we monitored the expression of eight defense-related genes after agroinfiltration of Nb leaves with pHB-AvrXa10 and pHB at 0, 32, and 46 h post inoculation (hpi) (Supplemental Figure 2B). Compared with the pHB control, genes highly induced by AvrXa10 included the PR genes PR1, PR2, PR4, PR5, and PR10; the HR marker gene EDS1; the master immune regulatory gene NPR1; and the pattern-triggered immunity gene PTI5. Taken together, these results indicated that AvrXa10 induced HR-like cell death and defense responses when transiently expressed in Nb.
Candidate targets of AvrXa10 in N. benthamiana
Our results indicated that the AvrXa10-mediated HR in Nb is dependent on the CRR and AD, implying that the AvrXa10 RVDs bind to an unknown R gene promoter. Two complementary approaches were used to identify putative targets of AvrXa10 (Figure 2A). In one approach, RNA was sequenced from Nb leaves transiently expressing AvrXa10 or AvrXa10ΔCRR at 32 and 48 hpi (Figure 2A). A two-fold change in expression and P ≤ 0.05 were used as cutoff values, and the Nb genome (v.0.4.4) was used as a reference (ftp://ftp.solgenomics.net/genomes/Nicotiana_benthamiana). Using this approach, 1425 and 1265 genes were differentially upregulated in the presence of AvrXa10 at 32 and 48 hpi, respectively, compared with AvrXa10 ΔCRR (Figure 2B and Supplemental Dataset 2). We then compared the upregulated genes at the two different time points and identified 804 genes that were induced at 32 and 48 hpi (Figure 2B). The TALgetter computational tool (Grau et al., 2013) was then used to predict AvrXa10 EBEs in the promoter regions of these upregulated genes. A script for promoter extraction (Supplemental Dataset 1) and the Nb genome were used to identify promoter regions ∼2.0 kb upstream of the ATG site in the upregulated genes. EBEs potentially recognized by AvrXa10 were identified in the promoter regions of upregulated differentially expressed genes (DEGs) using the default parameters of TALgetter. Among the 804 DEGs, 16 had promoter regions that contained predicted EBEs for AvrXa10 (Supplemental Dataset 2). These 16 genes were ranked based on their EBE prediction scores, and the proximity of the EBE to the ATG and TATA box was noted; 6 genes were deleted as candidate targets because the AvrXa10 EBE was located too far away from the ATG and TATA box. The 10 genes listed in Supplemental Table 1 were designated as the putative targets of AvrXa10.
Analysis of candidate AvrXa10 target genes in N. benthamiana
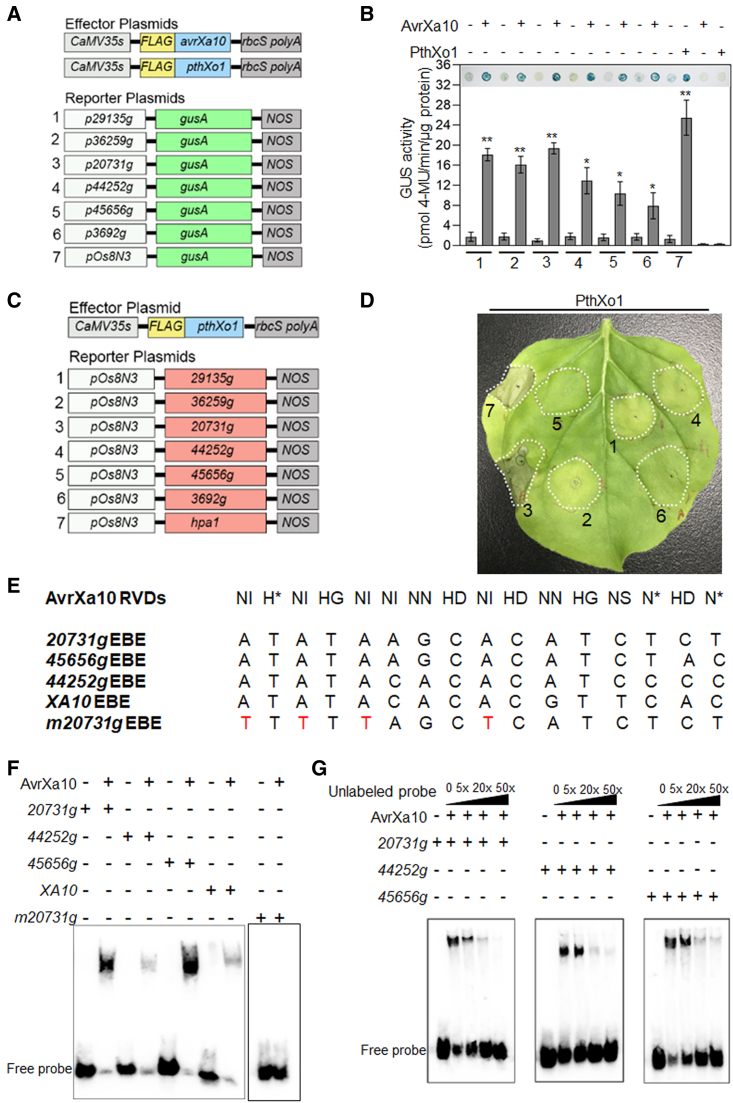
Our working hypothesis was that one of the genes in Supplemental Table 1 functions as an R gene targeted by AvrXa10. To identify the direct target of AvrXa10, expression of the candidate genes listed in Supplemental Table 1 was monitored in Nb leaves transiently expressing AvrXa10 or AvrX10ΔCRR (control). We found that genes 29135g, 36259g, 20731g, 44252g, 45656g, and 3269g were significantly induced by AvrXa10 compared with AvrXa10ΔCRR at both 32 and 48 hpi (Supplemental Figure 3), which agreed with the RNA-sequencing data. To investigate whether the presence of AvrXa10 induces the expression of the candidate genes, we cloned ∼1 kb promoter regions of the six highly induced genes upstream of a gusA reporter gene in pCAMBIA1381. The six promoter::GUS fusions (p29135g∷GUS, p32659g∷GUS, p20731g∷GUS, p44252g∷GUS, p45656g∷GUS, and p3692g∷GUS) were individually co-expressed in Nb with pHB-AvrXa10 by Agrobacterium-mediated transformation (Figure 3A and Supplemental Table 2). As a control, the Os8N3 promoter region, which has an EBE bound by the TALE PthXo1 (Yang et al., 2006), was inserted upstream of gusA in pCAMBIA1381 to form the pOs8N3∷GUS construct; this was co-expressed in Nb with pHB-pthXo1 (Figure 3A and Supplemental Table 2). There was a significant level of GUS expression when pHB-AvrXa10 was co-expressed with p29135g∷GUS, p32659g∷GUS, p20731g∷GUS, p44252g∷GUS, p45656g∷GUS, or p3692g∷GUS, but not with pOs8N3::GUS (Figure 3B). Furthermore, GUS expression was higher with the p29135g, p32659g, and p20731g promoters (P ≤ 0.01) compared with those of p44252g, p45656g, and p3692g (P ≤ 0.05); this may be caused by weaker binding between effector and promoter, or it may reflect reduced activation ability. These results indicate that AvrXa10 can induce the expression of these six genes in Nb by potentially targeting an EBE site in their promoter regions.
Figure 3.
AvrXa10 activates the expression of potential target genes by binding EBEs in promoter regions.
(A) Functional maps of effector and reporter plasmid constructs. The effector constructs contained FLAG-tag fused AvrXa10 or PthXo1 in vector pHB under the control of the CaMV 35S promoter. Reporter constructs contained gusA reporter cassettes that were driven by the candidate gene promoters (∼1 kb in length); these were cloned in pCAMBIA1381. Abbreviations: rbcS, ribulose-1,5-bisphosphate carboxylase, small subunit; polyA, polyadenylation site; NOS, NOS terminator site.
(B)Nb promoters from six genes direct the AvrXa10-dependent, transient expression of GUS in N. benthamiana. The photographs of qualitative GUS assays are shown above the bars. The TALE PthXo1 and the Os8N3 promoter were used as a control. Samples were collected at 36 hpi, and GUS activity was calculated. Error bars indicate means ± SD (n = 3), and asterisks indicate significant differences (∗P ≤ 0.05; ∗∗P ≤ 0.01). The experiment was performed at least three times with similar results.
(C) Schematic map of effector and reporter constructs used to identify target genes that promote an HR in Nb. The effector constructs contained FLAG-tag fused pthXo1 under the control of the CaMV 35S promoter. The reporter constructs contained the coding sequences of candidate target genes fused with the Os8N3 promoter, and the Os8N3-hpa1 construct served as a positive control.
(D) HR assay in N. benthamiana. Agrobacterium strains containing the effector construct (pHB-pthXo1) and one of the six reporter constructs were infiltrated into fully expanded Nb leaves, which were evaluated for the HR at 4–7 dpi. Representative results were chosen from five independent experiments. Legend: 1, PthXo1 + pOs8N3::29135g; 2, PthXo1 + pOs8N3::36259g; 3, PthXo1 + pOs8N3::20731g; 4, PthXo1 + pOs8N3::44252g; 5, PthXo1 + pOs8N3::45656g; 6, PthXo1 + pOs8N3::3692g; and 7, PthXo1 + pOs8N3::Hpa1.
(E) Oligonucleotide sequences of the EBE probes used in electrophoretic mobility-shift assays. Xa10 EBE was used as a positive control, and the mutated 20731g EBE was used as a negative control. The AvrXa10 RVDs corresponding to the EBE sequences are shown above.
(F) Electromobility shift assays using biotin-labeled putative EBE fragments derived from the promoter regions of 20731g, 44252g, and 45656g. The XA10 EBE probe was used as a positive control.
(G) Binding specificity of AvrXa10 to the target EBEs. Competition of biotinylated probes with unlabeled probes that were used at increasing concentrations (0, 5, 20, and 50×). The experiments were repeated three times.
A novel in vivo reporter system was developed to determine which of the six candidate target genes could trigger an HR once activated in planta. In this experiment, gusA was replaced with the coding sequence (CDS) of a target gene, and expression was driven by the Os8N3 promoter (Figure 3C and Supplemental Table 2); a pOs8N3-hpa1 construct served as a positive control. The reporter constructs were transiently co-expressed in Nb with the effector pthXo1 (Figure 3C and 3D). In this screening assay, an HR would develop if a target gene were expressed under the Os8N3 promoter, which depends on PthXo1 binding in this assay (Yang et al., 2006). The construct pOs8N3-hpa1 served as a positive HR-inducing control; hpa1 encodes a harpin protein that elicits an HR in Nb (Zou et al., 2006). pOs8N3-EV was co-infiltrated with pHB-pthXo1 as a negative control (Supplemental Figure 4). When PthXo1 was co-expressed with pOs8N3∷20731g, HR-like cell death was apparent starting at 4 dpi (Figure 3D, infiltration site 3). The co-expression of PthXo1 with pOs8N3∷44252 and pOs8N3∷45656 induced a partial HR starting at 7 dpi, but the HR was not produced consistently (Supplemental Figure 4). The other three candidate genes, 29135g, 32659g, and p3692g, did not produce an HR in this assay. Based on these results, 20731g, 44252g, and 45656g were subjected to further analyses.
The potential binding of AvrXa10 to the promoter region of the three candidate target genes 20731g, 44252g, and 45656g was investigated in electrophoretic mobility-shift assays (EMSAs); the Xa10 EBE (Tian et al., 2014) was used as a positive AvrXa10-interacting control. AvrXa10 was purified from pET30a-AvrXa10 as a C-terminal histidine fusion protein and incubated with the biotin-labeled putative EBE fragments present in the promoter regions of 20731g, 44252g, and 45656g (Figure 3E and 3F). EMSA indicated that all three candidate genes contained potential EBE sites that were recognized and bound by AvrXa10 (Figure 3F). AvrXa10 was unable to bind a mutated EBE fragment of 20731g (Figure 3F), confirming its binding specificity to the targeted EBE. The specificity of AvrXa10 binding was further confirmed by performing competition assays with labeled and unlabeled EBEs (Figure 3G). The intensity of the AvrXa10-bound putative EBE fragments from 20731g, 44252g, and 45656g was reduced by increasing the concentration of unlabeled EBE probes (Figure 3G). Collectively, these results suggest that AvrXa10 binds to all three promoters via effector binding sites.
Overexpression of NbZnFP1 elicits HR in N. benthamiana
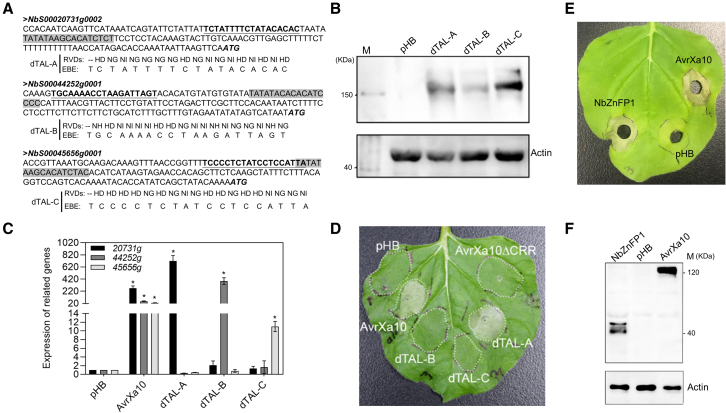
Designer TALEs (dTALEs) designated dTAL-A, dTAL-B, and dTAL-C were generated to target other sites upstream of the AvrXa10-EBEs in the promoter regions of 20731g, 44252g, and 45656g, respectively (Supplemental Figure 5A and Figure 4A), and were used to identify genes responsible for AvrXa10-triggered HR in Nb. The dTALEs were cloned into a binary pHB vector with an N-terminal FLAG-tag epitope and were transiently expressed in Nb via Agrobacterium; western blot analysis confirmed that the dTALEs were expressed in Nb leaves (Figure 4B and Supplemental Methods 3). qRT-PCR assays showed that 20731g, 44252g, and 45656g were specifically and significantly induced by dTAL-A, dTAL-B, and dTAL-C, respectively (Figure 4C). Functional analysis indicated that transient expression of dTAL-A targeting 20731g elicited an HR in Nb at 3 dpi; HR-mediated cell death was not observed with dTAL-B or dTAL-C targeting 44252g or 45656g, respectively (Figure 4D). Collectively, these data demonstrate that the activation of Nb target 20731g, which encodes a C2H2-type ZnFP 1 (hereafter NbZnFP1; Supplemental Table 1), elicits the HR in Nb.
Figure 4.
Overexpression of 20731g (NbZnFP1) results in the HR in N. benthamiana.
(A) Designer TALE assays. Promoter sequences of Nb20731g, Nb44252g, and Nb45656g; bold, underscored bases indicate sites for dTALE insertion. The gray highlighted region shows the putative AvrXa10 EBE, and the repeat variable di-residues of dTAL-A, dTAL-B, and dTAL-C and target EBEs are shown.
(B) Detection of dTAL-A, dTAL-B, and dTAL-C by western blot analysis. Fully expanded Nb leaves were infiltrated with pHB-TAL constructs, and FLAG-tagged dTALs were detected at 48 hpi. The pHB vector and actin protein were used as negative and loading controls, respectively.
(C) Expression analysis of 20731g, 44252g, and 45656g in Nb leaves infiltrated with Agrobacterium carrying the pHB constructs dTAL-A, dTAL-B, and dTAL-C; pHB and pHB-AvrXa10 served as negative and positive controls, respectively. Four-week-old Nb leaves were infiltrated and collected at 36 hpi for qRT-PCR. Error bars indicate means ± SD (n = 3), and asterisks indicate significant differences (∗P ≤ 0.01). The results shown are representative of three independent replicates.
(D) dTAL-A induces the HR in N. benthamiana. Constructs were transiently expressed in fully expanded Nb leaves via infiltration with Agrobacterium (OD600 0.8). Constructs included pHB-dTAL-A, pHB-dTAL-B, pHB-dTAL-C, pHB-AvrXa10 (positive control), pHB-AvrXa10ΔCRR, and pHB. Nb leaves were photographed at 3 dpi. All experiments were repeated three times with similar results.
(E) Transient overexpression of NbZnFP1 in Nb leaves. The Agrobacterium strains containing pHB-AvrXa10, pHB-NbZnFP1, or empty pHB vector were infiltrated into Nb leaves with a needleless syringe. The phenotype was photographed at 3 dpi.
(F) For the western blotting assay, the center region of infiltrated leaf samples was collected at 2 dpi.
To test whether transient expression of NbZnFP1 in Nb leaves causes HR, the full-length CDS of NbZNFP1 was amplified from Nb cDNA using the primers NbZnFP1-F/Nb-ZnFP1-R listed in Supplemental Table 3 and was cloned into the pHB vector, which is driven by the CaMV35S promoter. The plasmid pHB-NbZnFP1 was transformed into Agrobacterium strain EHA105, and the strain was infiltrated into Nb leaves. The Agrobacterium strains containing pHB-AvrXa10 and empty pHB vector were used as controls. The results showed that transient overexpression of NbZnFP1 in Nb leaves can also cause HR (Figure 4E). Western blot results indicated that the NbZnFP1 protein was detected in high amounts in the leaf sample showing visible HR (Figure 4F). Overall, these results confirmed that overexpression of NbZnFP1, either by activation of NbZnFP1 using dTALE or by transient expression, can cause HR in Nb leaves. Thus, NbZnFP1 is the biologically relevant target of AvrXa10 and functions as an R gene in Nb.
AvrXa10 activates NbZnFP1 and causes cell death when delivered into plant cells by Xanthomonas
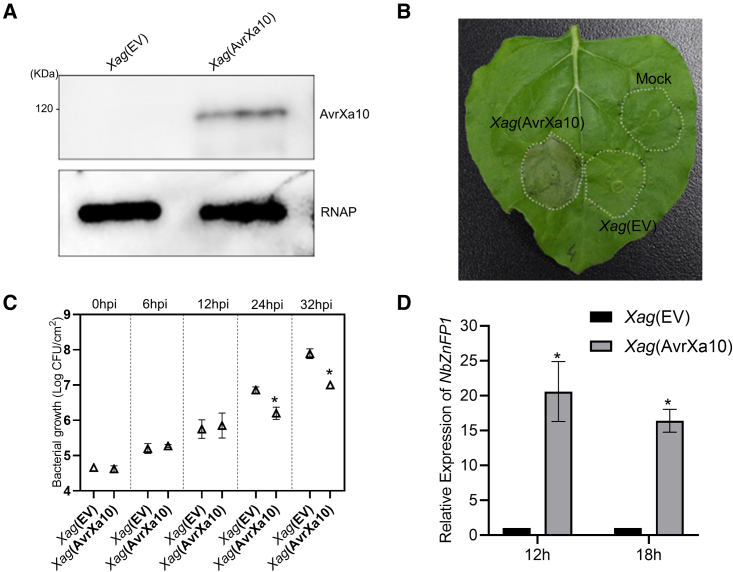
Expression of AvrXa10 in Nb leaves via Agrobacterium showed that AvrXa10 triggers HR by activating NbZnFP1. We further evaluated this phenomenon by delivering AvrXa10 into plant cells using the Xanthomonas axonopodis pv. glycines (Xag) strain ATCC43911, which possesses a functional T3SS but does not cause HR in Nb (Liu et al., 2016). The pHZW-AvrXa10 construct and the pHM1 empty vector (EV) were transformed into Xag ATCC43911 (Supplemental Methods 4). The expression of AvrXa10 in Xag ATCC43911 was confirmed by western blotting (Figure 5A). The leaves of Nb were infiltrated with Xag strain ATCC43911 carrying AvrXa10 or an EV and mock control. AvrXa10 caused HR at 24 hpi in Nb leaves when delivered by Xag (Figure 5B) and also significantly reduced bacterial growth at 24 and 32 hpi (Figure 5C). The qRT-PCR result showed that AvrXa10 activated NbZnFP1 in Nb leaves (Figure 5D). Overall, these data further confirmed that AvrXa10 activates NbZnFP1 to trigger immunity in Nb when delivered by Xanthomonas (Xag).
Figure 5.
AvrXa10 causes cell death when delivered by X. axonopodis pv. glycines (Xag) in N. benthamiana.
(A) Detection of AvrXa10 production in Xag by western blotting using an anti-FLAG primary antibody (see methods). RNA polymerase subunit alpha (RNAP) from E. coli was used as a loading control.
(B) HR phenotype at 24 hpi. The Xag strains containing AvrXa10 or empty vector (EV) were inoculated into Nb leaves. The simple buffer MgCl2 was used as a mock control. The experiment was repeated three times with similar results.
(C) Quantification of bacterial growth at different time points. The Xag strains containing AvrXa10 or empty vector (EV) were inoculated into Nb leaves. The infiltrated leaf samples were collected with a 1-cm diameter cork borer at different time points (0, 12, 24, and 32 hpi). Three leaf discs from three different plants were used as a single replicate, and three replicates were used for this experiment. Error bars indicate means ± SD (n = 3), and asterisks indicate significant differences (∗P ≤ 0.01).
(D) qRT-PCR analysis of NbZnFP1 in Nb leaves upon inoculation with derivatives of the Xag strain. Fully expanded leaves were infiltrated with Xag strains carrying AvrXa10 or pHM1 (EV), then collected at 24 hpi for RNA isolation. NbEF1α was used as an internal control. Error bars represent means ± SD (n = 3), and asterisks indicate significant differences (∗P ≤ 0.05). The results shown are representative of three independent replicates.
On-spot silencing of NbZnFP1 inhibits AvrXa10-mediated HR
We used the on-spot silencing approach (Johansen and Carrington, 2001) to further confirm that NbZnFP1 activation by AvrXa10 mediates the HR in Nb. A partial CDS (285 bp) of NbZnFP1 was amplified from Nb and cloned into pYL156, resulting in pYL156-NbZnFP1 (Supplemental Table 2). Nb leaves were infiltrated with Agrobacterium suspensions containing the helper plasmid pTRV-RNA1, the virus-induced gene silencing (VIGS) construct pYL156-NbZnFP1 or pYL156 (EV), and pHB-AvrXa10 (see methods). Leaves inoculated with AvrXa10 + VIGS-NbZnFP1 showed no obvious HR in comparison to leaves inoculated with AvrXa10 + VIGS-EV at 3 dpi (Figure 6A), suggesting that NbZnFP1 expression was silenced in the VIGS-NbZnFP1 treatment. RT-PCR analysis of inoculated leaves in Figure 6A indicated that the expression of NbZnFP1 was reduced in the VIGS-NbZnFP1 treatment compared with the VIGS-EV-inoculated leaves (Figure 6B). These results suggested that on-spot silencing of NbZnFP1 impaired the HR caused by AvrXa10, providing further evidence that NbZnFP1 is the R gene activated by AvrXa10 for HR induction in Nb.
Figure 6.
NbZnFP1 is the biologically relevant target of AvrXa10 in N. benthamiana.
(A) On-spot VIGS-mediated silencing of NbZnFP1 partially inhibits AvrXa10-induced HR. Nb leaves were co-infiltrated with Agrobacterium carrying pHB-AvrXa10 + pTRV-RNA1 + pYL156-NbZnFP1 or pHB-AvrXa10 + pTRV-RNA1 + pYL156 (EV); these two combinations are labeled AvrXa10 + VIGS-NbZnFP1 and AvrXa10 + VIGS-EV, respectively. Nb leaves were photographed at 3 dpi.
(B) RT-PCR analysis of NbZnFP1 expression in Nb after on-spot VIGS. Infiltrated leaves were collected at 3 dpi and analyzed for NbZnFP1 expression by RT-PCR; NbEF1α served as a reference gene.
(C) Transient expression of NbZnFP1 induces cell death in rice protoplasts. Constructs pRTVcHA-NbZnFP1 (NbZnFP1) and pRTVcHA (EV, empty vector) were co-expressed with the LUC reporter construct pRTVcVC-LUC in rice protoplasts. Co-transfection of pRTVcHA-Xa10 (XA10) with the LUC construct was used as a positive, cell-death-inducing control. LUC activity was measured after 24 h of transfection using the Promega LUC assay system. The images on the left side of the graph show microtiter plates containing protoplasts expressing the constructs. The image of LUC fluorescence was taken with a CCD imaging system (IVIS Spectrum, PerkinElmer, USA). The graph on the right shows the relative LUC activity measured with a luminometer (Tecan, M200). Cell death in protoplasts was monitored by the reduction in luciferase activity. Error bars represent means ± SD (n = 3), and asterisks indicate significant differences (∗P ≤ 0.01). The results are representative of three replicates.
Transient overexpression of NbZnFP1 causes cell death in rice protoplasts
To investigate the potential use of NbZnFP1 in bacterial leaf blight resistance, we attempted to generate rice transgenic lines expressing NbZnFP1 with its native promoter, which contains the AvrXa10 EBE; however, stable transgenic plantlets were not obtained. We therefore used a transient expression system to investigate the functionality of NbZnFP1 in rice protoplasts. NbZnFP1 was co-expressed with a luciferase reporter construct in rice protoplasts, and cell death was evaluated by monitoring changes in LUC activity (Figure 6C). Transient co-expression of the EV (pRTVcHA) or Xa10 (pRTVcHA-Xa10) with the LUC construct served as negative and positive controls, respectively. Transient expression of NbZnFP1 significantly reduced LUC activity compared with the EV (Figure 6C), indicating that the expression of the candidate target NbZnFP1 causes cell death in rice protoplasts.
Phylogenetic analysis of NbZnFP1 and subcellular localization
Phylogenetic analysis revealed that NbZnFP1 is closely related to AtZFP1 (Supplemental Figure 6A). NbZnFP1 and orthologs from different plant species contained a conserved C2H2 domain and an ethylene-responsive transcription factor-associated amphiphilic repressor (EAR) motif (Supplemental Figure 6B). Because some ZnFPs function as transcription factors in the plant nucleus (Koguchi et al., 2017; Zhang et al., 2019; Han et al., 2020), the subcellular localization of NbZnFP1 was investigated. The full-length CDS of NbZnFP1 was cloned into pYFP and transiently expressed in Nb leaves. At 2 dpi, confocal microscopy indicated that NbZnFP1 was localized in the nucleus (Supplemental Figure 6C).
Rice has nine orthologs of NbZnFP1 that contain the conserved C2H2 domain and EAR motif at the C terminus (Supplemental Figure 7A). However, the promoter regions of these nine genes lack EBE sites that might be recognized by AvrXa10. Rice leaves were infiltrated with Xoo strain PH (tal-free strain, Supplemental Table 2) carrying AvrX10, AvrXa10ΔCRR, or an EV control. There were no consistent differences in gene expression among the nine homologs in response to AvrXa10 or AvrXa10ΔCRR as measured by qRT-PCR (Supplemental Figure 7B), implying that the increased expression of these nine genes may cause cell death in rice. To test this possibility, four rice orthologs of NbZnFP1 (Os41110, Os26210, Os44190, and Os13600) were randomly selected and individually co-expressed with the luciferase reporter construct in rice protoplasts, and cell death was evaluated by monitoring changes in LUC activity (Supplemental Figure 7C). Indeed, the overexpression of these four rice genes, like NbZnFP1, significantly reduced LUC activity compared with the EV (Supplemental Figure 7C), indicating that transient overexpression of NbZnFP1 family genes causes cell death in rice protoplasts.
AvrXa10 triggers the HR in tomato by activating an NbZnFP1 ortholog
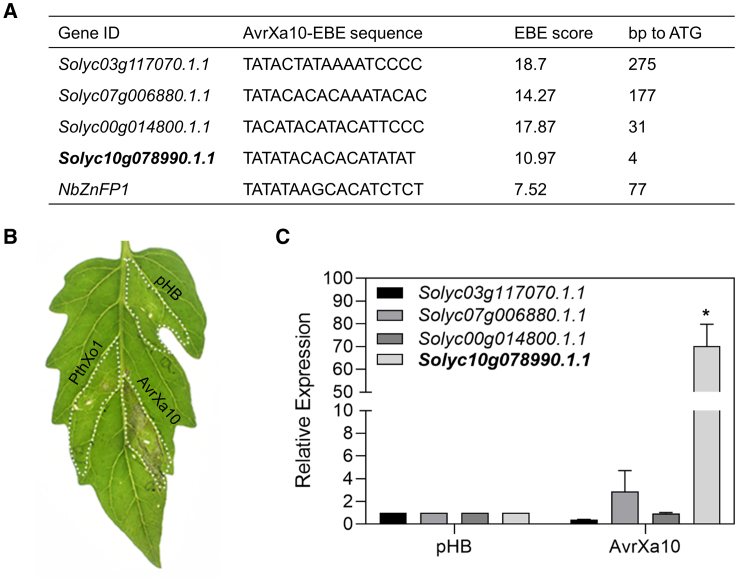
Tomato, like Nb, is a member of the Solanaceae. Interestingly, four orthologs of NbZnFP1 that contained a putative EBE site for AvrXa10 in their promoter regions were identified in tomato (Figure 7A). When 4-week-old tomato (cv. Ailsa Craig) leaves were infiltrated with Agrobacterium strains containing pHB-AvrXa10, pHB-PthXo1, or pHB (EV), a prominent HR induced by AvrXa10 was observed (Figure 7B). We then investigated whether AvrXa10 could activate any of the four NbZnFP1 homologs in tomato (Figure 7B). qRT-PCR analysis indicated that one homolog, Solyc10g078990.1.1, was significantly induced by AvrXa10 at 32 hpi (Figure 7C). This suggested that AvrXa10 specifically activates the expression of Solyc10g078990.1.1 for HR cell death, similar to that in Nb.
Figure 7.
AvrXa10 triggers HR in tomato, probably by activating a zinc finger-like protein gene (ZnFP)
(A) Putative AvrXa10 EBEs in the promoters of ZnFP homologs in tomato. The AvrXa10-EBE in the promoter of NbZnFP is also shown.
(B) HR phenotypes at 4 dpi.
(C) qRT-PCR analysis of the four ZnFP homologs. Leaves of 4-week-old tomato cv. Ailsa Craig were infiltrated with Agrobacterium strains containing pHB-AvrXa10, pHB-AvrXa10ΔCRR, pHB-AvrXa10ΔAD, or pHB (empty vector). Samples were collected at 32 hpi for RNA isolation and qRT-PCR analysis. The SolyEF1α gene was used to normalize the data. Error bars represent means ± SD (n = 3), and asterisks indicate significant differences (∗P ≤ 0.01). The results shown are representative of three independent replicates.
Discussion
Xoo elicits a T3SS-dependent HR in Nb (Li et al., 2012). Known Xoo factors that elicit HR in Nb include Hpa1, Ssb, XopL, XopP1, and XopY (Li et al., 2013; Ma et al., 2020; Zou et al., 2006). In this study, the role of 17 Xoo TALE proteins in mediating the HR in Nb was systematically evaluated, and only AvrXa10 elicited a stable HR when transiently expressed in Nb (Supplemental Figure 1). AvrXa10 elicited a strong HR in Nb at relatively low OD values, suggesting that the interaction was specific. AvrXa10 has previously been used in Nb with promoter–GUS reporter assays, and no cell death was reported (Boch et al., 2009; Tian et al., 2014), perhaps because of timing issues. In GUS reporter assays, samples are typically collected at 36 hpi, and the AvrXa10-triggered HR in Nb leaves starts at 3 dpi. This can also be observed in our GUS results, in which AvrXa10 did not show visible HR when the samples were collected early (Figure 3B). To meet the criteria for NHR, the target R gene must be dependent on a cognate TALE for transcription (Zhang et al., 2015). Our results show that the CRR and AD of AvrXa10 are specifically required to induce the HR in Nb, suggesting that the target gene is dependent on AvrXa10 for activation (Figure 1). AvrXa10 was previously shown to activate the expression of the R gene Xa10 in rice, and this activation resulted in the HR (Tian et al., 2014). However, Xa10 homologs are absent in Nb, indicating that AvrXa10 activates a different target gene for HR induction in Nb.
Transcriptome analysis and EBE prediction are valid approaches for the identification of TALE-activated target genes. For example, the R gene Bs4c was identified in pepper by sequencing host RNA in the presence or absence of AvrBs4 (Strauß et al., 2012). Similarly, GhSWEET10 in cotton and TaNCED-5BS in wheat were identified based on transcriptome profiling and EBE prediction of TALE-binding sites (Cox et al., 2017; Peng et al., 2019). In the current study, RNA sequencing and EBE prediction were used to identify 16 putative AvrXa10 targets in Nb (Figure 2). Analysis by qRT-PCR, EMSA, and a novel reporter assay indicated that 20731g, 44252g, and 45656g were probable AvrXa10 targets (Supplemental Figures 3 and 4 and Figure 3). The 44252g gene encodes a GDSL-esterase/lipase; interestingly, a similar enzyme was involved in the NHR of Arabidopsis thaliana (Langenbach et al., 2016). The 45656g gene encodes an ethylene-responsive transcription factor, which is relevant because the overexpression of a similar tobacco gene, NtERF3, triggered an HR in tobacco (Ogata et al., 2012). The target 20731g encodes a C2H2-type ZnFP, and ZnFPs are known to function in the plant stress response (Kim et al., 2004; Shi et al., 2014; Yu et al., 2016; Han et al., 2020). It is noteworthy that many R gene products and defense proteins contain zinc finger domains (Gupta et al., 2012).
Although qRT-PCR, in vivo reporter assays, and EMSA indicated that 20731g, 44252g, and 45656g were potential targets of AvrXa10, it was unclear which gene was the biologically relevant target of AvrXa10 or whether the HR caused by AvrXa10 was due to the collective activity of all three genes. This question was addressed by utilizing dTALEs, which have been used to identify potential TALE gene targets (Cernadas et al., 2014; Cohn et al., 2014; Cox et al., 2017; Peng et al., 2019). We constructed dTALEs that specifically induced each candidate gene by targeting a unique sequence in its promoter region. Only dTAL-A targeting 20731g triggered the HR in Nb when transiently expressed (Figure 4). qRT-PCR showed that dTAL-A specifically induced the expression of target 20731g and not the expression of the other two candidate genes. dTAL-B and dTAL-C specifically activated 44252g and 45656g, but did not cause the HR in Nb. On-spot VIGS was used to further validate that 20731g is targeted by AvrXa10 in Nb. The inoculation site where 20731g was silenced showed a reduced HR in response to AvrXa10, thus confirming that 20731g (NbZnFP1) was the biologically significant target of AvrXa10 in Nb (Figure 6). It is important to mention that transient expression of NbZnFP1 elicited the HR in Nb leaves when it was expressed in very high amounts (Figure 4E and 4F). Also, the silencing of NbZnFP1 did not fully reduce the transcript level, but the HR was no longer completely visible (Figure 6). These results suggest that the AvrXa10-NbZnFP1 mediated HR is somehow dose dependent.
R genes for race-specific resistance to Xanthomonas TALEs have been cloned from rice (Xa10, Xa23, Xa27, and Xa7) and solanaceous plants (Bs3 and Bs4C-R) (Xue et al., 2020; Chen et al., 2021; Luo et al., 2021). These R genes are not homologous to classical NLR genes and are subdivided into two groups (Zhang et al., 2015). Group 1 probably functions in plant development or physiology and includes the Bs3-encoded flavin monooxygenases (Römer et al., 2007). Group 2 consists of transmembrane proteins that possess multiple hydrophobic domains (Zhang et al., 2015). This study shows that nonhost plants may encode a third type of resistance protein, namely the C2H2-type ZnFPs.
Phylogenetic analysis of ZnFPs from different plant species indicated that NbZnFP1 is closely related to ZnFPs in Arabidopsis, tomato (Solanum lycopersicum), and eggplant (Solanum melongena) (Supplemental Figure 6). Although homologs of NbZnFP1 were identified in rice, their promoter regions did not encode the EBE recognized by AvrXa10 and were not induced by AvrXa10 in rice (Supplemental Figure 7). The potential use of NbZnFP1 to confer Xoo resistance was assessed by transforming rice with NbZnFP1 under the control of its native promoter; however, we were unable to generate stable transgenic plantlets. This may have been caused by lethal effects of NbZnFP1 when expressed from its endogenous promoter or by other factors outside the scope of the present study.
C2H2-type ZnFPs are transcription factors that contain one or more potential DNA-binding regions comprising 25–30 amino acids (C-X2–4-C-X3-P-X5-L-X2-H-X3-H) (Han et al., 2020). In addition to roles in transcriptional activation, some ZnFPs also possess an EAR motif that can function as a transcriptional repressor (Han et al., 2020). In this study, the NbZnFP1 deduced protein sequence revealed a single C2H2 domain and an EAR motif at the C terminus. The subcellular localization experiments showed that both NbZnFP1 and AvrXa10 localized to the nucleus (Supplemental Figure 6C), suggesting that NbZnFP1 transcriptionally activates an unknown gene, exhibiting cross talk with other defense-related genes (Supplemental Figure 2). This possibility is worth investigating in the future.
C2H2 ZnFPs have been previously characterized in rice and function in development (Liu et al., 2018; Zhuang et al., 2020) and tolerance to abiotic stress, including drought, salt, and acidic soils (Iuchi et al., 2008; Xu et al., 2008; Sun et al., 2010). A growing number of studies have documented the contribution of C2H2 ZnFPs to both abiotic and biotic stress tolerance (Kim et al., 2004; Shi et al., 2014; Yu et al., 2016; Lawrence and Novak, 2018; Yin et al., 2020; Zhao et al., 2020). Furthermore, the potential use of C2H2 ZnFPs in enhancing pathogen resistance in transgenic plants has been successfully demonstrated using the ZFPs CaPIF1 and CaZFP, which were originally identified in pepper (Kim et al., 2004; Oh et al., 2005; Shi et al., 2014).
Certain R genes, like Xa23, Xa27, and Xa10, originated from O. rufipogon and Oryza minuta, which are wild species of domesticated rice (Gu et al., 2004; Wang et al., 2015; Zhang et al., 2001). Recently, a novel locus was identified in the wild species Oryza latifolia that conferred race-specific resistance to Xoo PXO339 (Angeles-Shim et al., 2020). A relevant example is the use of Rxo1 from the nonhost maize as a source of resistance to Xoc in rice, which indicates that NHR can be used in different crop species (Zhao et al., 2005). It is possible that TALE proteins may be occasionally trapped in nonhost plants, as in this study, in which AvrXa10 was trapped by the ZnFP gene in Nb (Figures 1 and 4) and tomato (Figure 7) to promote HR induction. Transient expression of NbZnFP1 and its homologs in rice protoplasts caused cell death (Figure 6C and Supplemental Figure 7C), suggesting that these ZnFP genes could potentially be used in rice resistance against Xoo/Xoc infection. The transfer of AvrXa10 into Xag strain ATCC43911 caused HR in Nb (Figure 5), which suggests that the promoter region of ZnFP1 could be engineered to confer a broad-spectrum resistance by inserting a major TALE EBE and could be used in a strategy that includes other R genes against Xoo (Zeng et al., 2015). Thus, it remains possible that NbZnFP1 could be used in a rice resistance breeding program if the barriers to stable transformation are overcome.
Methods
Bacterial strains, plasmids, plant materials, and DNA manipulation
The bacterial strains and plasmids used in this study are listed in Supplemental Table 2. Escherichia coli strains were grown in Luria-Bertani medium at 37°C (Sambrook and Russell, 2001), and Agrobacterium was grown in Luria-Bertani containing rifampicin at 28°C. When needed, antibiotics were added at the following final concentrations: ampicillin, 100 μg ml−1; rifampicin, 75 μg ml−1; and kanamycin, 25 μg ml−1. Protocols for DNA manipulation and plasmid construction are provided in Method S1.
Nb plants were cultivated in a growth chamber at 25°C with a 16 h light/8 h dark photoperiod. Four- to eight-week-old Nb plants were used for all experiments. Rice cv. Nipponbare (Oryza sativa subsp. japonica) was grown at 28°C in a greenhouse located at Shanghai Jiao Tong University with a 12 h light/dark photoperiod.
Detection of cell death and ROS accumulation
Cell death was detected in Nb by Evans blue staining using established protocols (Wright et al., 2000). After excess dye was removed, Nb leaves were destained with 95% ethanol in a boiling water bath, stored in 65% ethanol, and photographed. Cell death in leaves was also estimated by measuring ion leakage as described previously (Wright et al., 2000), with minor modifications. Two days after agroinfiltration, five leaf discs (1 cm diameter) were immersed in deionized distilled water (5 ml) and incubated at 25°C with gentle agitation for 4 h. Ion leakage was measured with a DDS-12DW conductivity meter (Bante Instruments). All experiments were repeated three times.
ROS were detected in Nb leaves with 3,3′-diaminobenzidine at 32 and 48 hpi as described previously (Bindschedler et al., 2006). The experiment was repeated three times with three independent plants.
RNA sequencing
RNA sequencing was provided by Shanghai Ouyi Biomedical Technology Co. (Shanghai, China). Total RNA was extracted from Nb leaves inoculated with AvrXa10 or AvrXa10ΔCRR at 32 and 48 hpi using the mirVana miRNA Isolation Kit (Ambion) as recommended by the manufacturer. RNA integrity was evaluated using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), and samples with RNA integrity numbers ≥7 were used for library construction. RNA libraries were constructed using the TruSeq Stranded mRNA Sample Prep Kit (Illumina, San Diego, CA, USA) and sequenced using the Illumina platform (HiSeq 2500 or Illumina HiSeq X Ten); 125 bp/150 bp paired-end reads were generated. The Nb reference genome used for alignment was downloaded from ftp://ftp.solgenomics.net/genomes/Nicotiana_benthamiana.
Prediction of the EBE recognized by AvrXa10
The EBE recognized by AvrXa10 was predicted in a subset of AvrXa10-upregulated genes using the TALgetter tool (Grau et al., 2013). The Nb promoterome, beginning 2 kb upstream of the translation start site, was extracted from genome v.0.4.4 using the script described in Supplemental Dataset 1.
GUS reporter assays
The GUS reporter system was used to assess the transcriptional activation of selected target genes by AvrXa10. Promoter sequences located ∼1 kb upstream of the translational start site in 2913g, 36259g, 20731g, 44252g, 45656g, and 3692g were cloned into the binary GUS reporter construct pCAMBIA1381 using primers listed in Supplemental Table 3. The effector and reporter constructs were then co-expressed (OD600 0.8 for each strain) in 4- to 7-week-old Nb leaves via Agrobacterium-mediated transformation. In qualitative GUS assays, leaf discs (1 cm diameter) were sampled at 2 dpi, immersed in GUS staining solution for 6–8 h (100 mM phosphate buffer with 10 mM EDTA, 0.5% Triton X-100, 0.5 mM K3[Fe(CN)6], 0.5 mM K4[Fe(CN)6], and 0.5 mM X-gluc), and washed in 70% ethanol. In quantitative assays, three leaf discs (1 cm) were collected at 2 dpi, and GUS activity was measured using 4-methylumbelliferyl-β-glucuronide. Proteins were quantified using the Bradford method (Antony et al., 2010).
A novel in vivo reporter system was also utilized in which the gusA CDS was replaced with the CDS of target genes and fused with the Os8N3 promoter. The susceptibility gene Os8N3 is targeted by the effector PthXo1 in rice (Yang et al., 2006), and Os8N3 and PthXo1 were shown to interact when delivered in trans and co-expressed in Nb (Cai et al., 2017). This approach enabled us to test whether selected target genes triggered an HR in Nb. The reporter constructs were co-expressed with PthXo1 in Nb, and phenotypes were observed at 7 dpi. The experiment was repeated three times with five independent plants for each replicate.
Electrophoretic mobility-shift assays
Selected proteins were purified and used in EMSAs. AvrXa10 with a C-terminal 6×His-tag in the pET30a-AvrXa10 construct (Supplemental Table 2) was expressed in E. coli and purified as a histidine fusion protein (His-AvrXa10). Complementary pairs of EBE fragments (∼25–30 bp) were annealed and labeled with the Biotin 3′ End DNA Labeling Kit (Thermo Scientific, USA). EMSA was performed as described previously (Cai et al., 2017), and photos were taken with the ChemiScope 6000 series imaging system (Clinx Science Instruments Co., Ltd).
Construction of designer TALEs
TAL Effector-Nucleotide Targeter 2.0 (TALE-NT 2.0) (Doyle et al., 2012) was used to construct dTALEs that targeted 18–19 bp promoter regions in the tobacco genes Nb20731g (dTAL-A), Nb44252g (dTAL-B), and Nb45656g (dTAL-C). A library of four basic repeats encoding RVDs NG, NI, HD, and NN, which correspond to target nucleotides T, A, C, and G, respectively, was used. The repeat regions of artificial dTALEs were assembled using the RVDs corresponding to the targeted nucleotides in the promoter regions of Nb20731g, Nb44252g, and Nb45656g. The CRRs of dTALEs were then synthesized by ViewSolid Biotechnology (Beijing, China) using the TALE construction kit (Catalog no. VK006-02) and then cloned into pUC57. Detailed information on the dTALEs, including RVDs and targeted EBE sequences, is provided in Supplemental Figure 5A. For transient expression in Nb, the dTALEs were replaced with AvrXa10 in pHB-AvrXa10 at the SphI site, giving rise to pHB-dTALE (Supplemental Figure 5B).
Agro-mediated VIGS of target gene Nb20731g
On-spot VIGS was used to downregulate the expression of the target gene Nb20731g (NbZnFP1) in Nb leaves using the tobacco rattle virus (TRV) bipartite system. A 285-bp fragment of NbZnFP1 was amplified from Nb cDNA using primers NbZnFP1(VIGS)-F/R (Supplemental Table 3); the PCR product was then cloned into the TRV RNA2-containing vector pYL156 as an XbaI/BamHI fragment, resulting in pYL156-NbZnFP1. For VIGS, Agrobacterium strains containing the helper vector pTRV-RNA1 and pYL156 (EV) or pYL156-NbZnFP1 were cultured overnight, collected, washed once with infiltration buffer (10 mM MgCl2, 0.2 mM acetosyringone, and 200 mM MES [pH 5.6]), and then resuspended in infiltration buffer to OD600 1.0. The pTRV-RNA1 culture suspension was mixed with suspensions containing pYL156 or pYL156-20731g at a 1:1 ratio and then combined with an Agrobacterium suspension containing pHB-AvrXa10. Cultures were mixed and used to infiltrate fully expanded leaves of 5-week-old Nb. The phenotypes were observed at 3 dpi and photographed, and one image was chosen from three independent replicates.
Cell death assays in rice protoplasts
The role of NbZnFP1 (Nb20731g) and its four rice orthologs in mediating cell death in rice was explored using a luciferase (LUC) reporter system. Protoplasts were isolated from 10-day-old seedlings of rice cv. Nipponbare as described previously (He et al., 2016). The pRTVcVC-LUC construct, containing LUC fused with the C terminus of mVenus (Xu et al., 2021), was used as a source of luciferase (Supplemental Table 2). NbZnFP1 (Nb20731g) and Xa10 were cloned into pRTVcHA (He et al., 2018) as described in Supplemental Methods 1. Two micrograms of pRTVcHA-NbZnFP1, pRTVcHA-Xa10, or pRTVcHA (EV) was co-transfected with pRTVcVC-LUC (2 μg) into rice protoplasts as described previously (He et al., 2016). After a 24-h incubation period, LUC activity and fluorescence were detected using a luminometer (Tecan M200), CCD imaging system (IVIS Spectrum, PerkinElmer, USA), and the Luciferase Assay System (Promega). Co-expression of pRTVcHA-Xa10 (Xa10) or pRTVcHA (EV) with the LUC construct served as positive and negative controls, respectively.
Funding
This work was supported by the National Natural Science Foundation of China (31830072), the National Key Research and Development Program of China (2016YFD0100601), and the National Transgenic Major Program (2016ZX08001-002).
Author contributions
F.H. and G.C. designed the experiments. F.H. performed the experiments. X.X. and W.M. provided technical assistance. S.M.A.S., L.L., B.Z., and L.Z. contributed materials. F.H. and G.C. wrote the manuscript. G.C. supervised the project.
Acknowledgments
We thank Professor Xin Zhong Cai for providing the VIGS vectors. No conflict of interest is declared.
Published: October 14, 2021
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information can be found online at Plant Communications Online.
Accession numbers
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental information
References
- Angeles-Shim R.B., Shim J., Vinarao R.B., Lapis R.S., Singleton J.J. A novel locus from the wild allotetraploid rice species Oryza latifolia Desv. confers bacterial blight (Xanthomonas oryzae pv. oryzae) resistance in rice (O. sativa) PLoS One. 2020;15:e0229155. doi: 10.1371/journal.pone.0229155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony G., Zhou J., Huang S., Li T., Liu B., White F., Yang B. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. The Plant Cell. 2010;22:3864–3876. doi: 10.1105/tpc.110.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai S., Yoshioka H. Nitric oxide as a partner of reactive oxygen species participates in disease resistance to necrotrophic pathogen Botrytis cinerea in Nicotiana benthamiana. Mol. Plant-Microbe Interact. 2009;22:619–629. doi: 10.1094/MPMI-22-6-0619. [DOI] [PubMed] [Google Scholar]
- Bindschedler L.V., Dewdney J., Blee K.A., Stone J.M., Asai T., Plotnikov J., Denoux C., Hayes T., Gerrish C., Davies D.R., et al. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006;47:851–863. doi: 10.1111/j.1365-313X.2006.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch J., Bonas U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu. Rev. Phytopathol. 2010;48:419–436. doi: 10.1146/annurev-phyto-080508-081936. [DOI] [PubMed] [Google Scholar]
- Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., Lahaye T., Nickstadt A., Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- Bogdanove A.J., Schornack S., Lahaye T. TAL effectors: finding plant genes for disease and defense. Curr. Opin. Plant Biol. 2010;13:394–401. doi: 10.1016/j.pbi.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Cai L., Cao Y., Xu Z., Ma W., Zakria M., Zou L., Cheng Z., Chen G. A transcription activator-like effector Tal7 of Xanthomonas oryzae pv. oryzicola activates rice gene Os09g29100 to suppress rice immunity. Sci. Rep. 2017;7:5089. doi: 10.1038/s41598-017-04800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernadas R.A., Doyle E.L., Niño-Liu D.O., Wilkins K.E., Bancroft T., Wang L., Schmidt C.L., Caldo R., Yang B., White F.F., et al. Code-assisted discovery of TAL effector targets in bacterial leaf streak of rice reveals contrast with bacterial blight and a novel susceptibility gene. PLoS Path. 2014;10:e1003972. doi: 10.1371/journal.ppat.1003972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Liu P., Mei L., He X., Chen L., Liu H., Shen S., Ji Z., Zheng X., Zhang Y. Xa7, a new executor R gene that confers durable and broad-spectrum resistance to bacteria-blight disease in rice. Plant Commun. 2021:100143. doi: 10.1016/j.xplc.2021.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M., Bart R.S., Shybut M., Dahlbeck D., Gomez M., Morbitzer R., Hou B.-H., Frommer W.B., Lahaye T., Staskawicz B.J. Xanthomonas axonopodis virulence is promoted by a transcription activator-like effector–mediated induction of a SWEET sugar transporter in cassava. Mol. Plant-microbe Interact. 2014;27:1186–1198. doi: 10.1094/MPMI-06-14-0161-R. [DOI] [PubMed] [Google Scholar]
- Cox K.L., Meng F., Wilkins K.E., Li F., Wang P., Booher N.J., Carpenter S.C., Chen L.-Q., Zheng H., Gao X. TAL effector driven induction of a SWEET gene confers susceptibility to bacterial blight of cotton. Nat. Commun. 2017;8:1–14. doi: 10.1038/ncomms15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb D., Anderson R.G., How-Yew-Kin T., Tyler B.M., McDowell J.M. Conserved RxLR effectors from oomycetes Hyaloperonospora arabidopsidis and Phytophthora sojae suppress PAMP-and effector-triggered immunity in diverse plants. Mol. Plant-microbe Interact. 2018;31:374–385. doi: 10.1094/MPMI-07-17-0169-FI. [DOI] [PubMed] [Google Scholar]
- Dou D., Zhou J.-M. Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host & Microbe. 2012;12:484–495. doi: 10.1016/j.chom.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Doyle E.L., Booher N.J., Standage D.S., Voytas D.F., Brendel V.P., VanDyk J.K., Bogdanove A.J. TAL effector-nucleotide targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 2012;40:W117–W122. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau J., Wolf A., Reschke M., Bonas U., Posch S., Boch J. Computational predictions provide insights into the biology of TAL effector target sites. Plos Comp. Biol. 2013;9:e1002962. doi: 10.1371/journal.pcbi.1002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu K., Tian D., Yang F., Wu L., Sreekala C., Wang D., Wang G.-L., Yin Z. High-resolution genetic mapping of Xa27 (t), a new bacterial blight resistance gene in rice, Oryza sativa L. Theor. Appl. Genet. 2004;108:800–807. doi: 10.1007/s00122-003-1491-x. [DOI] [PubMed] [Google Scholar]
- Gupta S.K., Rai A.K., Kanwar S.S., Sharma T.R. Comparative analysis of zinc finger proteins involved in plant disease resistance. PLoS One. 2012;7:e42578. doi: 10.1371/journal.pone.0042578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G., Lu C., Guo J., Qiao Z., Sui N., Qiu N., Wang B. C2H2 zinc finger proteins: master regulators of abiotic stress responses in plants. Front. Plant Sci. 2020;11:115. doi: 10.3389/fpls.2020.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq F., Xie S., Huang K., Shah S.M.A., Ma W., Cai L., Xu X., Xu Z., Wang S., Zou L. Identification of a virulence tal gene in the cotton pathogen, Xanthomonas citri pv. malvacearum strain Xss-V2–18. BMC Microbiol. 2020;20:1–13. doi: 10.1186/s12866-020-01783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Chen S., Ning Y., Wang G.L. Rice (Oryza sativa) protoplast isolation and its application for transient expression analysis. Curr. Protoc. Plant Biol. 2016;1:373–383. doi: 10.1002/cppb.20026. [DOI] [PubMed] [Google Scholar]
- Hu Y., Zhang J., Jia H., Sosso D., Li T., Frommer W.B., Yang B., White F.F., Wang N., Jones J.B. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA. 2014;111:E521–E529. doi: 10.1073/pnas.1313271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Kobayashi Y., Koyama H., Kobayashi M. STOP1, a Cys2/His2 type zinc-finger protein, plays critical role in acid soil tolerance in Arabidopsis. Plant Signal. Behav. 2008;3:128–130. doi: 10.4161/psb.3.2.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen L.K., Carrington J.C. Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol. 2001;126:930–938. doi: 10.1104/pp.126.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Jones J.D., Vance R.E., Dangl J.L. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354:aaf6395. doi: 10.1126/science.aaf6395. [DOI] [PubMed] [Google Scholar]
- Kay S., Hahn S., Marois E., Hause G., Bonas U. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science. 2007;318:648–651. doi: 10.1126/science.1144956. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Hong J.K., Lee S.C., Sohn K.H., Jung H.W., Hwang B.K. CAZFP1, Cys2/His2-type zinc-finger transcription factor gene functions as a pathogen-induced early-defense gene in Capsicum annuum. Plant Mol. Biol. 2004;55:883–904. doi: 10.1007/s11103-004-2151-5. [DOI] [PubMed] [Google Scholar]
- Koguchi M., Yamasaki K., Hirano T., Sato M.H. Vascular plant one-zinc-finger protein 2 is localized both to the nucleus and stress granules under heat stress in Arabidopsis. Plant Signal. Behav. 2017;12:e1295907. doi: 10.1080/15592324.2017.1295907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbach C., Schultheiss H., Rosendahl M., Tresch N., Conrath U., Goellner K. Interspecies gene transfer provides soybean resistance to a fungal pathogen. Plant Biotechnol. J. 2016;14:699–708. doi: 10.1111/pbi.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence S.D., Novak N.G. The remarkable plethora of infestation-responsive Q-type C2H2 transcription factors in potato. BMC Res. Notes. 2018;11:1–7. doi: 10.1186/s13104-018-3503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Xu Y.-P., Zhang Z.-X., Cao W.-Y., Li F., Zhou X., Chen G.-Y., Cai X.-Z. Identification of genes required for nonhost resistance to Xanthomonas oryzae pv. oryzae reveals novel signaling components. PLoS One. 2012;7:e42796. doi: 10.1371/journal.pone.0042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-R., Ma W.-X., Che Y.-Z., Zou L.-F., Zakria M., Zou H.-S., Chen G.-Y. A highly-conserved single-stranded DNA-binding protein in Xanthomonas functions as a harpin-like protein to trigger plant immunity. PLoS One. 2013;8:e56240. doi: 10.1371/journal.pone.0056240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zou L., Ye G., Xiong L., Ji Z., Zakria M., Hong N., Wang G., Chen G. A potential disease susceptibility gene CsLOB of citrus is targeted by a major virulence effector PthA of Xanthomonas citri subsp. Citri. Mol. Plant. 2014;7:912–915. doi: 10.1093/mp/sst176. [DOI] [PubMed] [Google Scholar]
- Liu L., Ma W.-X., Zou L.-F., Chen X.-B., Chen G.-Y. Comparative analysis of type-III secretion system and T3SS-secreted effectors among seven quarantine Xanthomonas pathovars. Acta Phytopathol. Sin. 2016;46:37–46. [Google Scholar]
- Liu X., Li D., Zhang D., Yin D., Zhao Y., Ji C., Zhao X., Li X., He Q., Chen R. A novel antisense long noncoding RNA, TWISTED LEAF, maintains leaf blade flattening by regulating its associated sense R2R3-MYB gene in rice. New Phytol. 2018;218:774–788. doi: 10.1111/nph.15023. [DOI] [PubMed] [Google Scholar]
- Luo D., Huguet-Tapia J.C., Raborn R.T., White F.F., Brendel V.P., Yang B. The Xa7 resistance gene guards the susceptibility gene SWEET14 of rice against exploitation by bacterial blight pathogen. Plant Commun. 2021:100164. doi: 10.1016/j.xplc.2021.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Xu X., Cai L., Cao Y., Haq F., Alfano J.R., Zhu B., Zou L., Chen G. A Xanthomonas oryzae type III effector XopL causes cell death through mediating ferredoxin degradation in Nicotiana benthamiana. Phytopathology Res. 2020;2:1–12. [Google Scholar]
- Moscou M.J., Bogdanove A.J. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- Ogata T., Kida Y., Arai T., Kishi Y., Manago Y., Murai M., Matsushita Y. Overexpression of tobacco ethylene response factor NtERF3 gene and its homologues from tobacco and rice induces hypersensitive response-like cell death in tobacco. J. Gen. Plant Pathol. 2012;78:8–17. [Google Scholar]
- Oh S.K., Park J.M., Joung Y.H., Lee S., Chung E., KIM S.Y., Yu S.H., Choi D. A plant EPF-type zinc-finger protein, CaPIF1, involved in defence against pathogens. Mol. Plant Pathol. 2005;6:269–285. doi: 10.1111/j.1364-3703.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- Peng Z., Hu Y., Zhang J., Huguet-Tapia J.C., Block A.K., Park S., Sapkota S., Liu Z., Liu S., White F.F. Xanthomonas translucens commandeers the host rate-limiting step in ABA biosynthesis for disease susceptibility. Proc. Natl. Acad. Sci. USA. 2019;116:20938–20946. doi: 10.1073/pnas.1911660116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer P., Hahn S., Jordan T., Strauß T., Bonas U., Lahaye T. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science. 2007;318:645–648. doi: 10.1126/science.1144958. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Russell D.W. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. Molecular Cloning - A Laboratory Manual. [Google Scholar]
- Schultink A., Qi T., Lee A., Steinbrenner A.D., Staskawicz B. Roq1 mediates recognition of the Xanthomonas and Pseudomonas effector proteins XopQ and HopQ1. Plant J. 2017;92:787–795. doi: 10.1111/tpj.13715. [DOI] [PubMed] [Google Scholar]
- Schwartz A.R., Potnis N., Timilsina S., Wilson M., Patané J., Martins J., Jr., Minsavage G.V., Dahlbeck D., Akhunova A., Almeida N. Phylogenomics of Xanthomonas field strains infecting pepper and tomato reveals diversity in effector repertoires and identifies determinants of host specificity. Front. Microbiol. 2015;6:535. doi: 10.3389/fmicb.2015.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B., Ronald P.C. Plant innate immunity: perception of conserved microbial signatures. Annu. Rev. Plant Biol. 2012;63:451–482. doi: 10.1146/annurev-arplant-042811-105518. [DOI] [PubMed] [Google Scholar]
- Senthil-Kumar M., Mysore K.S. Nonhost resistance against bacterial pathogens: retrospectives and prospects. Annu. Rev. Phytopathol. 2013;51:407–427. doi: 10.1146/annurev-phyto-082712-102319. [DOI] [PubMed] [Google Scholar]
- Shi H., Wang X., Ye T., Chen F., Deng J., Yang P., Zhang Y., Chan Z. The Cysteine2/Histidine2-Type transcription factor ZINC FINGER OF ARABIDOPSIS THALIANA6 modulates biotic and abiotic stress responses by activating salicylic acid-related genes and C-REPEAT-BINDING FACTOR genes in Arabidopsis. Plant Physiol. 2014;165:1367–1379. doi: 10.1104/pp.114.242404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauß T., van Poecke R.M., Strauß A., Römer P., Minsavage G.V., Singh S., Wolf C., Strauß A., Kim S., Lee H.-A. RNA-seq pinpoints a Xanthomonas TAL-effector activated resistance gene in a large-crop genome. Proc. Natl. Acad. Sci. USA. 2012;109:19480–19485. doi: 10.1073/pnas.1212415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.-J., Guo S.-Q., Yang X., Bao Y.-M., Tang H.-J., Sun H., Huang J., Zhang H.-S. Functional analysis of a novel Cys2/His2-type zinc finger protein involved in salt tolerance in rice. J. Exp. Bot. 2010;61:2807–2818. doi: 10.1093/jxb/erq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Wang G., Zhou J.-M. Receptor kinases in plant-pathogen interactions: more than pattern recognition. The Plant Cell. 2017;29:618–637. doi: 10.1105/tpc.16.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., Wang J., Zeng X., Gu K., Qiu C., Yang X., Zhou Z., Goh M., Luo Y., Murata-Hori M., et al. The rice TAL effector–dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. The Plant Cell. 2014;26:497–515. doi: 10.1105/tpc.113.119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uma B., Rani T.S., Podile A.R. Warriors at the gate that never sleep: non-host resistance in plants. J. Plant Physiol. 2011;168:2141–2152. doi: 10.1016/j.jplph.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhang X., Fan Y., Gao Y., Zhu Q., Zheng C., Qin T., Li Y., Che J., Zhang M., et al. XA23 is an executor R protein and confers broad-spectrum disease resistance in rice. Mol. Plant. 2015;8:290–302. doi: 10.1016/j.molp.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Wang J., Tian D., Gu K., Yang X., Wang L., Zeng X., Yin Z. Induction of Xa10-like genes in rice cultivar Nipponbare confers disease resistance to rice bacterial blight. Mol. Plant-microbe Interact. 2017;30:466–477. doi: 10.1094/MPMI-11-16-0229-R. [DOI] [PubMed] [Google Scholar]
- Wei C.-F., Kvitko B.H., Shimizu R., Crabill E., Alfano J.R., Lin N.-C., Martin G.B., Huang H.-C., Collmer A. A Pseudomonas syringae pv. tomato DC3000 mutant lacking the type III effector HopQ1-1 is able to cause disease in the model plant Nicotiana benthamiana. Plant J. 2007;51:32–46. doi: 10.1111/j.1365-313X.2007.03126.x. [DOI] [PubMed] [Google Scholar]
- Wright K.M., Duncan G.H., Pradel K.S., Carr F., Wood S., Oparka K.J., Santa Cruz S. Analysis of the N gene hypersensitive response induced by a fluorescently tagged tobacco mosaic virus. Plant Physiol. 2000;123:1375–1386. doi: 10.1104/pp.123.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.-Q., Huang J., Guo S.-Q., Yang X., Bao Y.-M., Tang H.-J., Zhang H.-S. Overexpression of a TFIIIA-type zinc finger protein gene ZFP252 enhances drought and salt tolerance in rice (Oryza sativa L.) FEBS Lett. 2008;582:1037–1043. doi: 10.1016/j.febslet.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Xu X., Xu Z., Ma W., Haq F., Li Y., Shah S.M.A., Zhu B., Zhu C., Zou L., Chen G. TALE-triggered and iTALE-suppressed Xa1 resistance to bacterial blight is independent of OsTFIIAγ1 or OsTFIIAγ5 in rice. J. Exp. Bot. 2021 doi: 10.1093/jxb/erab054. [DOI] [PubMed] [Google Scholar]
- Xu Z., Xu X., Gong Q., Li Z., Li Y., Wang S., Yang Y., Ma W., Liu L., Zhu B., et al. Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable TALE-binding elements of multiple susceptibility genes in rice. Mol. Plant. 2019;12:1434–1446. doi: 10.1016/j.molp.2019.08.006. [DOI] [PubMed] [Google Scholar]
- Xue J., Lu Z., Liu W., Wang S., Lu D., Wang X., He X. The genetic arms race between plant and Xanthomonas: lessons learned from TALE biology. Sci. China Life Sci. 2020;64:51–65. doi: 10.1007/s11427-020-1699-4. [DOI] [PubMed] [Google Scholar]
- Yang B., White F.F. Diverse members of the AvrBs3/PthA family of type III effectors are major virulence determinants in bacterial blight disease of rice. Mol. Plant-microbe Interact. 2004;17:1192–1200. doi: 10.1094/MPMI.2004.17.11.1192. [DOI] [PubMed] [Google Scholar]
- Yang B., Sugio A., White F.F. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA. 2006;103:10503–10508. doi: 10.1073/pnas.0604088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Wang L., Zhao J., Li Y., Huang R., Jiang X., Zhou X., Zhu X., He Y., He Y. Genome-wide characterization of the C2H2 zinc-finger genes in Cucumis sativus and functional analyses of four CsZFPs in response to stresses. BMC Plant Biol. 2020;20:1–22. doi: 10.1186/s12870-020-02575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.-H., Li X.-Z., Wu Z.-J., Chen D.-X., Li G.-R., Li X.-Q., Zhang G.-H. VvZFP11, a Cys2His2-type zinc finger transcription factor, is involved in defense responses in Vitis vinifera. Biol. Plant. 2016;60:292–298. [Google Scholar]
- Zeng X., Tian D., Gu K., Zhou Z., Yang X., Luo Y., White F.F., Yin Z. Genetic engineering of the Xa10 promoter for broad-spectrum and durable resistance to Xanthomonas oryzae pv. Oryzae. Plant Biotechnol. J. 2015;13:993–1001. doi: 10.1111/pbi.12342. [DOI] [PubMed] [Google Scholar]
- Zhang H., Gao X., Zhi Y., Li X., Zhang Q., Niu J., Wang J., Zhai H., Zhao N., Li J. A non-tandem CCCH-type zinc-finger protein, IbC3H18, functions as a nuclear transcriptional activator and enhances abiotic stress tolerance in sweet potato. New Phytol. 2019;223:1918–1936. doi: 10.1111/nph.15925. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yin Z., White F. TAL effectors and the executor R genes. Front. Plant Sci. 2015;6:641. doi: 10.3389/fpls.2015.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wang C., Zhao K., Zhao Y., Caslana V., Zhu X., Li D., Jiang Q. The effectiveness of advanced rice lines with new resistance gene Xa23 to rice bacterial blight. Rice Genet. Newsl. 2001;18:71–72. [Google Scholar]
- Zhao B., Lin X., Poland J., Trick H., Leach J., Hulbert S. A maize resistance gene functions against bacterial streak disease in rice. Proc. Natl. Acad. Sci. USA. 2005;102:15383–15388. doi: 10.1073/pnas.0503023102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Ardales E.Y., Raymundo A., Bai J., Trick H.N., Leach J.E., Hulbert S.H. The avrRxo1 gene from the rice pathogen Xanthomonas oryzae pv. oryzicola confers a nonhost defense reaction on maize with resistance gene Rxo1. Mol. Plant-microbe Interact. 2004;17:771–779. doi: 10.1094/MPMI.2004.17.7.771. [DOI] [PubMed] [Google Scholar]
- Zhao T., Wu T., Zhang J., Wang Z., Pei T., Yang H., Li J., Xu X. Genome-wide analyses of the genetic screening of C2H2-type zinc finger transcription factors and abiotic and biotic stress responses in tomato (Solanum lycopersicum) based on RNA-seq data. Front. Genet. 2020;11:540. doi: 10.3389/fgene.2020.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H., Wang H.-L., Zhang T., Zeng X.-Q., Chen H., Wang Z.-W., Zhang J., Zheng H., Tang J., Ling Y.-H. NONSTOP GLUMES1 encodes a C2H2 zinc finger protein that regulates spikelet development in rice. The Plant Cell. 2020;32:392–413. doi: 10.1105/tpc.19.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Wang X., Xiang Y., Zhang B., Li Y.-R., Xiao Y., Wang J., Walmsley A.R., Chen G. Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl. Environ. Microbiol. 2006;72:6212–6224. doi: 10.1128/AEM.00511-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Zhang F., Sun W., Ning Y., Wang G. A versatile vector toolkit for functional analysis of rice genes. Rice. 2018;11:1–10. doi: 10.1186/s12284-018-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.