Abstract
Background
Polyphenylene carboxymethylene (PPCM) sodium salt is a promising multipurpose technology for prevention of both sexually transmitted infections (STIs) and pregnancy. In preclinical studies, PPCM has demonstrated significant (1) antimicrobial activity against several important viral and bacterial pathogens and (2) contraceptive activity associated with premature acrosome loss.
Objective
To further evaluate a vaginal antimicrobial compound as a contraceptive agent in preclinical studies utilizing a repurposed hyaluronan binding assay (HBA).
Materials and methods
Semen samples containing either neat semen or washed spermatozoa were treated with increasing concentrations of PPCM or calcium ionophore A23187 (positive control). Sperm inactivation was measured by two methods: (1) double acrosome staining (AS), and (2) a hyaluronan binding assay (HBA®). Percentage of inactivated sperm was compared between untreated control sperm and those treated with PPCM or A23187.
Results
PPCM had a significant (p < 0.05) and dose‐dependent effect on sperm inactivation in both assays, with HBA detecting a higher proportion of inactivated sperm than AS. PPCM did not affect sperm motility and exhibited equivalent responses in the neat and washed samples.
Discussion
Both HBA and AS confirmed that spermatozoa were rapidly inactivated at PPCM concentrations likely present in the vagina under actual use conditions and in a time‐frame comparable to in vivo migration of spermatozoa out of seminal plasma into cervical mucus.
Conclusion
PPCM vaginal gel may provide contraceptive protection as well as help with STI prevention. HBA may be a sensitive and much needed biomarker for sperm activity in future contraceptive development.
Keywords: acrosome reaction, human spermatozoa, hyaluronan binding assay, polyphenylene carboxymethylene, vaginal contraceptive
1. INTRODUCTION
New non‐prescription, non‐hormonal vaginal methods of birth control may help overcome the disadvantages of barrier methods, hormonal treatments, and existing suboptimal vaginal products. While condoms provide both pregnancy and sexually transmitted infection (STI) protection, both male and female condoms require the cooperation of both partners, and many couples consider them difficult and unpleasant to use. 1 , 2 , 3 , 4 Hormonal and long‐acting reversible contraceptive (LARC) methods are highly effective, but do not protect against sexually transmitted infections (STIs). For many women, they are not a realistic option because they require medical services, are not easily accessible, and can be costly. 5 , 6 , 7 , 8 Furthermore, these systemically absorbed hormones can cause uncomfortable and potentially life‐threatening side effects. 9 Consequently, many women distrust hormone‐based methods while others do not want continuous protection. 10 , 11 Men may also prefer a non‐hormonal approach, one that is readily reversible and affords protection against STIs. 12 Current non‐hormonal vaginal products offer a reversible alternative, but most of them contain the spermicide nonoxynol‐9 (N‐9), which is cytotoxic to vaginal tissues and can cause significant tissue damage, even increasing risk of STIs, including HIV. 13 , 14 , 15 , 16 , 17 It is clear that safer contraceptive choices are needed, 11 , 18 , 19 including vaginal contraceptives that are not systemically absorbed, available over‐the‐counter (OTC), and can be used on an as‐needed basis. 20
Novel vaginal contraceptive products face a difficult development path. Vaginal products must be active in a complex environment. Coagulation, pH, semen proteins, and other factors in post‐coital genital fluids control the ability of spermatozoa to capacitate, move up the reproductive tract, and ultimately fertilize the egg. 21
In addition, the spermicidal activity of a contraceptive candidate is difficult to measure preclinically; it can be assessed by standard semen analysis in simulated genital secretions, but none of the measured parameters is a direct measure of the ability of spermatozoa to fertilize an ovum. Post‐coital cervical mucus testing (PCT; Sims–Huhner test) in women who are not at risk of pregnancy can also be used to evaluate contraceptive efficacy. This is a more direct way to identify motile sperm which have bypassed the vaginal contraceptive and reached the cervical os, indicating potential contraceptive failure. PCT has been used in the development of several vaginal contraceptives and may have some value for predicting clinical success. 22 However, in practical terms, it is difficult to use this procedure in a clinical trial and is typically limited to a small sample size, must be conducted immediately after coitus, and only during midcycle when fertile cervical mucus is present. Preclinical testing may also include contraceptive efficacy in animal models, primarily in the rabbit. However, animal models differ significantly from humans in basic reproductive physiology and vaginal environment and have limited ability to predict real‐world effectiveness in humans. 23 , 24 , 25
Human clinical trials in women at risk of pregnancy are difficult to conduct; women who participate are being asked to risk getting pregnant. Pregnancy is not only a clinical trial “failure,” but it may have life‐long consequences for the woman, her partner, and her family. In addition, non‐hormonal vaginal contraceptives are typically coitally dependent, and conducting a trial requiring correct use of a vaginal contraceptive method with each and every coital episode is difficult for both participants and investigators. Such trials can have poor compliance, difficult data collection and analysis, and significant underestimation of true efficacy. 26 , 27 , 28 , 29 Unfortunately, these challenges are often cited as evidence that any coitally dependent method should not be offered as a first‐line contraceptive option in the real world. 30 While it is likely that a vaginal method will always have a lower efficacy than LARCs even if used consistently and correctly, a higher risk of pregnancy may be more acceptable to many women than the problems associated with LARC use. 30 , 31
Because of these difficulties, vaginal contraceptive development has often leapfrogged from simple in vitro testing and efficacy in an animal model directly to human clinical efficacy trials. This is true for multiple N‐9 based products which were originally “grandfathered in” for rapid regulatory approval. More recent vaginal contraceptive development requires a stricter regulatory path to establish safety and efficacy, 32 but may still depend on PCT to guide clinical development. 22 There are no reliable preclinical data informing critical parameters such as optimal dose, onset, or duration of action in the vagina. Thus, any tool that measures sperm viability which can be used during both preclinical and clinical development of contraceptives may improve product design. Such a biomarker could be used in early clinical testing in women not at risk of pregnancy to optimize dosing and efficacy of a vaginal formulation. This would facilitate the design of subsequent “proof‐of‐concept” clinical testing where women are at risk of pregnancy and improve the likelihood of clinical success.
The hyaluronan binding assay (HBA) offers such a biomarker. The assay depends on the ability of intact sperm to bind to hyaluronan, thus immobilizing the cells on the assay slide. This mimics the binding of mature, capacitated, and acrosome‐intact sperm to the hyaluronan produced by cumulus cells in the zona pellucida, the matrix surrounding the oocyte plasma membrane. 33 , 34 Binding to hyaluronan causes the spermatozoa to acrosome react, releasing acrosomal enzymes which effect zona penetration and oocyte fertilization. 35 , 36 Only fully mature sperm have acrosomal receptors for hyaluronan 34 and bind tightly to the zona to initiate the acrosome reaction. 37 The HBA assesses this essential property of mature functional sperm. Importantly, spermatozoa that have undergone a premature acrosome reaction will not bind to solid‐state hyaluronan 33 on the HBA slide and are rendered infertile. 38 Hyaluronan‐binding sperm selection used during in vitro fertilization may permit selection of higher quality spermatozoa, leading to better rates of fertilization, embryo development, pregnancy, and reducing miscarriage risk. 39 , 40 , 41
We have repurposed a commercially available HBA to evaluate contraceptive activity of the candidate contraceptive microbicide, polyphenylene carboxymethylene (PPCM). PPCM is a unique polyanion under development as a multipurpose prevention technology providing both STI and contraceptive activity. It is structurally and functionally distinct from other polyanion candidate products and has important safety and effectiveness advantages. 42 The safety of PPCM has been shown in multiple in vitro and toxicology studies utilizing several cell lines, tissue explants, and animal models. 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52
As a contraceptive, PPCM has demonstrated significant contraceptive activity by causing premature acrosome loss (PAL), inactivating acrosomal enzymes (hyaluronidase and acrosin), and preventing fertilization in a rabbit model. 48 PPCM appears to block the binding of sperm‐specific adhesins to hyaluronan by inducing PAL. However, the specific interactions of PPCM with the sperm plasma membrane remain to be studied. Both zona‐pellucida‐induced acrosome loss and PPCM‐induced PAL are mediated through sperm‐specific Ca2+‐ selective ion channels. PPCM acts by increasing Ca2+ entry, effecting the acrosome reaction. 53 , 54 Since PPCM induces acrosomal loss in spermatozoa that are not near the oocyte (i.e., in the vaginal compartment), it may be expected to act in a contraceptive manner.
As a microbicide, PPCM may block similar interactions between the glycosaminoglycans of host cell surfaces and viral and bacterial adhesins, thus preventing infection. 55 , 56 , 57 , 58 Preclinical studies have demonstrated that PPCM prevents infection from several important STIs including human immunodeficiency virus (HIV), herpes simplex virus (HSV), Neisseria gonorrhoeae (Ng), and Chlamydia trachomatis. 44 , 45 , 48 , 49 , 50 , 51 , 52 Interestingly, PPCM also prevents infection by pathogenic strains of Ebola virus and SARS‐CoV‐2. 46 , 47
Using the HBA, we provide further evidence that PPCM disrupts sperm functions essential for in vivo fertilization. Other candidate contraceptives whose mechanism of action is disruption of sperm function, specifically sperm fertilizing ability, may be effectively validated using a biomarker such as the HBA, before advancing to clinical trials.
2. MATERIALS AND METHODS
2.1. Semen evaluation and sample preparation
Semen samples were collected by masturbation following 2‐5 days of abstinence from healthy patients visiting the University Andrology Laboratory at the University of Illinois at Chicago with Institutional Review Board approval. Samples were incubated at 37°C for 30 min to allow liquefaction and were then transferred to a conical tube for volume measurement. After thorough mixing, 5 µl of liquefied semen was loaded into a counting chamber (CASA chamber, depth 20 µm, CellVision Technologies, The Netherlands). Sperm concentration and motility were determined using a computer‐assisted semen analysis system (SCA Analyzer, MicroOptics, Barcelona, Spain). Sperm morphology was determined using CELL‐VU pre‐stained morphology slides (Millennium Sciences, Inc., Waltham, MA). Acceptable semen samples for the studies herein met the following criteria: sperm concentration > 20 × 106/ml; total count > 20 × 106; total motile sperm > 20 × 106; total motility > 60%; normal morphology > 30% normal forms by the WHO 3rd edition criteria, and absence of white blood cells. 59
Liquefied semen samples were individually tested as neat or washed specimens for all the studies presented. For washed spermatozoa, semen samples were diluted (1:1 v/v) with Biggers, Whitten, and Whittingham medium with no added protein, pH 7.4 (BWW; 14), and centrifuged at 675 g for 15 min. Post‐wash sperm samples were standardized to a final concentration of 20 × 106/ml using BWW and divided into smaller aliquots for testing. Aliquots of neat or washed spermatozoa were exposed to increasing concentrations of PPCM (Wilmington PharmaTech, Delaware), 1 nM calcium ionophore (A23187; Sigma‐Aldrich, St Louis, MO) as a positive control, or remained untreated as a negative control. All samples were incubated at 37°C until analyzed at the indicated times.
2.2. Premature acrosome loss assay
Within the context of this study, PAL refers to the disruption and loss of the human spermatozoa acrosome in response to treatment of the sperm suspension in BWW to calcium ionophore A23187 or PPCM. 52 , 60 Washed spermatozoa from the same individual specimens used for HBA studies (see below) were resuspended in protein‐free BWW to a concentration of 5 × 106 cells/ml and incubated at 37°C for 15 min in the presence of 1 nM A23187 or increasing concentrations of PPCM. After incubation, spermatozoa in BWW were fixed with an equal volume of 0.1 M cacodylate‐buffered 3% glutaraldehyde (pH 7.4), and fixed spermatozoa were stained with Bismarck Brown (0.8%) and Rose Bengal (0.8%) as previously described. 61 Fixing and staining reagents were purchased from Millipore‐Sigma (St. Louis, MO). Stained spermatozoa were examined for the presence/absence of acrosomes using bright‐field light microscopy at 1000× total magnification under oil immersion. Spermatozoa stained red/pink at the anterior portion of the sperm head and brown posterior to the equatorial segment were considered acrosome intact. Spermatozoa with a clear area over the anterior portion of the sperm head and brown posterior staining were considered to have lost their acrosomes. The percentage of spermatozoa without acrosomes at the indicated timepoints was calculated from 300‐400 spermatozoa examined per slide.
2.3. Hyaluronan binding assay (HBA®)
Commercially available HBA® kits (Sperm‐hyaluronan binding assay; Cooper Surgical, Trumbull, CT) were used according to the manufacturer's instructions to evaluate the ability of spermatozoa to bind hyaluronic acid, a process that requires an intact acrosome. In brief, a 7‐µl drop of neat semen or washed spermatozoa previously incubated as controls or exposed to A23187 or PPCM at varying doses and for specified periods of time was placed on the HBA® slide and a Cell‐Vu® gridded cover slip was immediately applied. The slides were incubated at room temperature for 10 min per manufacturer's instructions to allow sperm binding. Unbound and bound motile sperm were counted using a phase‐contrast microscope at 200x total magnification in the same number of grid squares. Between 100 and 200 spermatozoa were counted for each assessment, yielding a coefficient of variation of approximately 5%. The percentage of hyaluronan‐bound sperm was calculated at each time point.
2.4. Sperm motility
For the analysis of PPCM effects on sperm motility, individual neat semen samples were incubated with A23187 or increasing doses of PPCM and aliquots removed at 20 min, 2, 6, and 18 h after exposure. Aliquots were placed in a Makler chamber and motility was assessed by the SCA sperm analysis system.
2.5. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). For assays performed in technical replicates, the average of the two values was used for an n of 1. Statistical analyses utilized ANOVA followed by Bonferroni post hoc tests using GraphPad Prism, version 7 software. A p‐value of < 0.05 or less was considered significant.
3. RESULTS
Four separate experiments were conducted to investigate PPCM activity on spermatozoa.
3.1. Comparison of the PAL assay and the HBA for evaluating the dose–response effects of PPCM on sperm acrosome status and binding activity
Washed spermatozoa from six individual semen samples were treated for 15 min with 1 nM A23187 Ca++ ionophore or 0.001 to 100 µg/ml PPCM, a concentration range bracketing the estimated IC50s (1‐10 µg/ml) found in prior preclinical studies on the effects of PPCM on spermatozoa and pathogenic microbes. 52 Sperm inactivation was measured both by PAL assay (acrosomal loss), and by HBA (hyaluronan binding) with technical replicates performed for each specimen and assay. PPCM exhibited a linear dose‐dependent relationship in both assays, with maximal loss of binding capacity at 100 µg/ml PPCM (Figure 1). The PAL assay directly quantitates acrosome loss, whereas the HBA quantitates the ability of spermatozoa to bind to hyaluronic acid (HA) and indicates that they are mature with unreacted, intact acrosomes. Loss of HA binding likely reflects loss of the acrosome following capacitation and acrosome reaction. The two assays showed a similar dose–response effect suggesting that they are measuring the same activity status of the spermatozoa. Of particular note, a larger percentage of inactivated sperm was detected by HBA than in the PAL assay for both PPCM and A23187 treatments. These data affirm those of Huszar, 34 that spermatozoa without acrosomes are unable to bind to solid‐state hyaluronan, and suggest that HBA is a more robust assay for detecting sperm inactivation than the PAL assay. Accordingly, the remainder of the PPCM tests were run using only the HBA.
FIGURE 1.

Comparison of sperm inactivation by PPCM measured by both the HBA (red bars) and the AS assay (black bars). Washed human spermatozoa (20 million/ml) were resuspended in BWW, divided into aliquots, and incubated with increasing concentrations of PPCM for 15 min. Control groups without PPCM were incubated in BWW (negative control) or with Ca++ ionophore A23187 (positive control). The “% Unbound to Hyaluronan” represents the fraction of spermatozoa inactivated by PPCM or A23187 and unable to bind to the hyaluronan‐coated slide. The “% Acrosome Reaction” represents the inactivated spermatozoa with premature acrosome loss caused by PPCM or A23187. Both assays demonstrated dose‐dependent sperm inactivation (contraceptive activity) after exposure to PPCM. The HBA exhibited a higher sensitivity for PPCM‐induced hyaluronan binding loss with a maximal effect at the 100 µg/ml dose resulting in 80% binding reduction, comparable to A23187. The AS assay at its peak showed maximal activity of only 27% acrosome‐reacted sperm, comparable to A23187 effects. Bars represent the mean ± SEM, n = 6/treatment group. #p < 0.01 PPCM at 100, 10, 1.0, and 0.1 versus no PPCM in the HBA assay. *p < 0.01 PPCM at 100, 10, 1.0, 0.1 µg/ml versus no PPCM in the AS assay. (Reprinted by permission of the J Biol Reprod, Oxford University Press. Weitzel, North, Waller, 2020, 103[2], 299‐309)
3.2. Comparison of the time course and strength of PPCM‐induced sperm inactivation in washed spermatozoa versus neat semen using the HBA
The results above documented the PPCM‐induced acrosomal disruption of washed spermatozoa. This PPCM activity was equal to the disruption caused by A2318, which acts as a positive control for the fraction of the sperm population able to capacitate, acrosome react, and ultimately fertilize the ovum. 62 However, testing in neat semen is also important to evaluate its efficacy as a vaginal contraceptive. Moreover, a time course of the response to PPCM is necessary to determine whether inactivation aligns with the time frame that spermatozoa reside in the vaginal environment. In this set of experiments, a new set of fresh semen samples (n = 3) was utilized. The samples were divided, with one half remaining neat and the other washed as described. Aliquots of neat semen and washed spermatozoa were treated with 10 µg/ml PPCM or 1 nM A23187 and sperm inactivation evaluated using the HBA at 1, 5, 10, and 15 min. The percentage of bound sperm detected by HBA for untreated washed spermatozoa and untreated semen control samples remained constant, indicating sperm viability was maintained throughout the time course in the control samples. In both neat and washed samples, inactivation of binding occurred rapidly with A23187‐induced acrosome reaction essentially complete by 10 min (Figure 2). Importantly, PPCM reduced hyaluronan binding in both washed spermatozoa and neat semen with equal potency and identical time courses. This contrasts with other polyanions which appear to lose effectiveness in coital secretions containing fresh ejaculate. 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 The rapid onset of PPCM activity, initiating by 1 min with maximal activity at 10 min, is critical for a vaginal contraceptive, given that upon ejaculation into the vagina, human spermatozoa may be able to migrate out of the seminal plasma and into cervical mucus in as little as 90 seconds. 71 , 72 , 73
FIGURE 2.
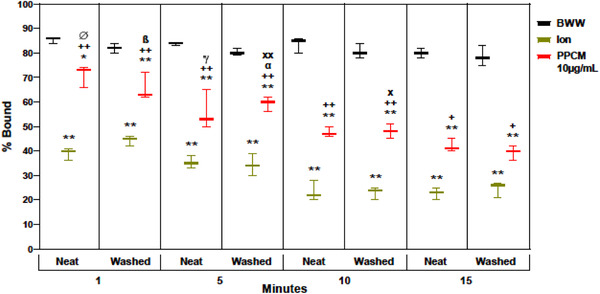
Time course analysis of 10 µg/ml PPCM inhibition of sperm binding in the HBA used neat semen and washed spermatozoa. The HBA activity of spermatozoa incubated for 1, 5, 10, and 15 min in BWW (negative control), the Ca++ ionophore (Ion) A23187 (positive control) and PPCM (10 µg/ml) was examined in split semen specimens (n = 5); one sperm portion remained in seminal plasma (neat) while the second portion was washed free of seminal plasma and resuspended in BWW. Bars represent the mean ± SEM
3.3. Assessment of PPCM activity at clinically relevant vaginal doses
The vaginal dose of PPCM is expected to be approximately 3‐5 ml of a bioadhesive gel formulation containing 4% w/v PPCM. 52 The vaginal concentration of PPCM is predicted to be ∼10‐20 mg/ml after vaginal application and dilution with genital secretions. This concentration is consistent with levels utilized in clinical studies of other vaginal polyanions and is expected to provide maximal effectiveness 74 , 75 , 76 , 77 , 78 while maintaining a high selectivity index for safety. 42 PPCM activity at these high (clinical) concentrations was assessed in a new set of individual neat semen samples (n = 3), using the HBA at 1, 5, 10, and 15 min. PPCM exposures caused significant and rapid sperm inactivation in neat semen at concentrations expected to be present in real world vaginal doses (Figure 3). Since this occurred in as early as 1 min of exposure to PPCM, spermatozoa may be inactivated in vivo prior to reaching the cervical os and entering the upper reproductive tract.
FIGURE 3.
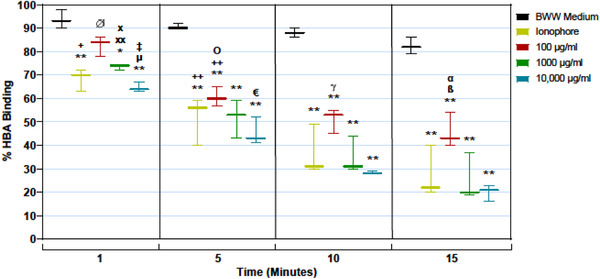
Analysis of high‐dose PPCM effects on sperm binding in the HBA over time. Spermatozoa in neat semen were exposed to BWW (negative control), the Ca++ ionophore (Ion) A23187 (positive control) and increasing doses of PPCM (100, 1000, and 10,000 µg/ml) and spermatozoa bound to hyaluronan‐coated slides were quantified at for 1, 5, 10, and 15 min. Bars represent the mean ± SEM, n = 3
3.4. Evaluation of PPCM effects on sperm motility
Sperm motility was evaluated after incubation of the unwashed spermatozoa with PPCM (from study 3) for up to 18 h. There was a general decline of motility in control samples over the 18 h time course, and treatment with PPCM did not significantly accelerate this trend (Figure 4). Spermatozoa exposed to PPCM in the vagina retain motility and may move into the upper reproductive tract, but are unlikely to bind to hyaluronan surrounding the ovum and fertilize the egg due to premature acrosome reaction. This is consistent with PPCM's presumptive mechanism of action of inhibiting normal acrosome function rather than a general cytotoxic effect. 43 , 52
FIGURE 4.
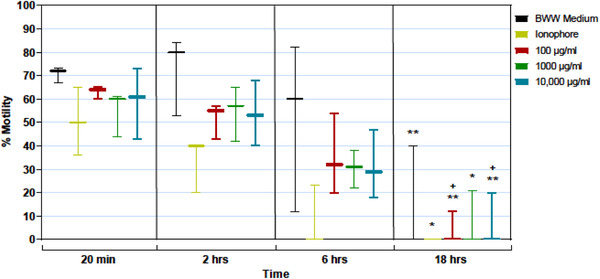
Effect of high‐dose PPCM exposure over time on sperm motility (%). Unwashed spermatozoa exposed to BWW (negative control), the Ca++ ionophore (Ion) A23187 (positive control) and increasing doses of PPCM (100, 1000, and 10,000 µg/ml) were assessed for % motility at 20 min, 2, 6, and 18 h after exposure. Sperm motility slowly declined in all exposed spermatozoa over time at the same rate, with no significant differences between treatment groups at each time point. Bars represent the mean ± SEM, n = 3 semen samples
4. DISCUSSION
Vaginal contraceptive development is challenging. The vagina is a unique environment; it may have an active inflammatory response to toxins or injury, and may secrete water‐ soluble natural defenses against pathogens—all in a complex interaction with a diverse microbiome. Seminal fluids from coitus add another level of complexity. 63 , 79 The effectiveness of any vaginal product can be affected by all these factors, and it is difficult to mimic these conditions in preclinical testing.
Additionally, clinical testing is challenging, since contraceptive failure is a major life event with significant consequences for the pregnant woman. This is particularly true with coitally dependent vaginal contraceptives that must be used regularly and correctly. However, these non‐systemic methods offer many advantages, including having fewer adverse side effects and lower cost than hormonal treatments, as well as over‐the‐counter accessibility and the potential for both contraceptive and STI protection. Biomarkers to assess the contraceptive activity of vaginal products that could streamline their development are lacking. 80
By contrast, due to the extensive efforts to develop a vaginally applied microbicide for HIV prevention, a comprehensive preclinical toolbox has been developed to assess these vaginal products, and the failure of early clinical trials to clearly demonstrate the effectiveness of these vaginal microbicides have prompted further refinement in preclinical testing. As a result, numerous biomarkers have been developed to assess the microbicidal activity of vaginal microbicides. 49 , 81 , 82 , 83 , 84 , 85 A similar situation is found in the assisted reproductive technology field, where biomarkers for predicting fertility are the subject of continuing research, with the goal of improving pregnancy outcomes. 86 , 87
Our preliminary studies support the use of the HBA as a surrogate biomarker for vaginal contraceptive activity of PPCM and other vaginal contraceptives acting by preventing sperm binding to the ovum. The HBA is selective of mature, viable spermatozoa capable of fertilization. 37 , 88 , 89 Since hyaluronan binding is necessary for fertilization, this assay may offer a more sensitive and convenient measure of vaginal contraceptive activity than other methods such as PCT.
We have successfully applied the HBA to further evaluate the contraceptive activity of PPCM. The HBA demonstrated the ability of PPCM to inactivate up to 80% of the sperm population, which is consistent with spermatozoa in fresh semen inactivated by the ionophore A23187 and represents the fraction of mature spermatozoa capable of capacitation and fertilization. In contrast, the AS detected PPCM inactivation of only about 20%‐30% of the sperm population and may represent differences in method sensitivity. The AS requires more complex processing and may be unable to detect early steps in acrosome inactivation that prevent hyaluronan binding but are not yet reflected by changes in staining characteristics. An earlier study showed a similar discrepancy in the proportion of acrosome‐reacted sperm in which the fraction of acrosome‐reacted sperm assessed by hyaluronan bound to the zona pellucida was greater than sperm treatment with A23187 utilizing a staining technique. 90
The use of hyaluronan binding to assess PPCM activity is consistent with its mechanisms of action. As a contraceptive, PPCM activates Ca2+ channels resulting in PAL. This, in turn, prevents the hyaluronan binding needed for fertilization. As a microbicide, PPCM is classified as a fusion and entry inhibitor which prevents infection by several microbial pathogens via a similar mechanism by blocking their attachment to host cell receptor sites. Such receptor sites are composed of glycosaminoglycans similar to hyaluronan. 55 , 56 , 58 , 91
The actual contraceptive effectiveness of PPCM when applied precoitally may be limited by the complex physical and chemical environment of the human vagina. However, HBA‐measured contraceptive activity of PPCM is identical in washed spermatozoa or neat fresh semen. This is particularly important when evaluating vaginal products. Semen is known to interfere with microbicidal activity of numerous vaginal products and may be partly responsible for their poor efficacy in early HIV prevention clinical trials. 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 The potential inhibitory activity of seminal fluid, particularly the protein components, 70 should be evaluated early in development of any vaginal product used during coitus. Our results show that the sensitivity of the HBA was not adversely affected by the presence of proteins or any other component of neat semen, and the efficacy of PPCM was not reduced.
These data confirm the ability of PPCM to inhibit sperm function in a clinically significant manner. PPCM is effective at doses likely to be used in clinical settings (10 mg/ml) and its activity is observed at 1 min and is sustained for at least 30 min. Additionally, PPCM did not affect sperm motility at doses ranging from 0.01 ug/ml to clinically relevant concentrations (10 mg/ml), suggesting no overall cytotoxicity to spermatozoa cells. This is consistent with multiple in vitro studies confirming the safety of PPCM. 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52
Our studies also support the use of the HBA in vitro to evaluate contraceptive effectiveness under various conditions, as well as in early clinical testing to assess ex vivo contraceptive activity in genital fluids collected both pre‐ and post‐coitally. This could provide valuable pharmacokinetic information on contraceptive effectiveness in the post‐coital vaginal environment. Thus, dose‐ranging information, time of onset, time to maximum activity, duration of activity, and other parameters can be measured to optimize formulation design and optimize usage. This may encourage the development of new vaginal contraceptives by providing a much‐needed biomarker for contraception and by reducing the need for early‐phase clinical trials that put women at risk of pregnancy.
CONFLICT OF INTEREST
G. S. Prins, C. J. De Jonge, L. A. Birch, W. X. Birch, and K. A. Feathergill have no conflict of interest to declare. None of the authors have a financial interest with the vendor of the HBA®. The patent for PPCM is licensed to Yaso Therapeutics, Inc. B. B. North, M. B. Weitzel, and D. P. Waller are employees of and have equity interest in Yaso Therapeutics, Inc.
AUTHOR CONTRIBUTIONS
G. S. Prins supervised and performed the studies in her clinical laboratory and was a major contributor to all aspects of the work. B. B. North, M. B. Weitzel, C. J. De Jonge, and D. P. Waller assisted in study design and interpreted the results. W. X. Birch and K. A. Feathergill conducted the laboratory studies. L. A. Birch performed data analysis and prepared the figures. B. B. North, G. S. Prins, M. B. Weitzel, and C. J. De Jonge wrote the manuscript. B. B. North revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENT
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development R44 HD092206 “Preclinical Development of PPCM Vaginal Contraceptive to Submit IND.”
North BB, Weitzel MB, Waller DP, et al. Evaluation of the novel vaginal contraceptive agent PPCM in preclinical studies using sperm hyaluronan binding and acrosome status assays. Andrology. 2022;10:367–376. 10.1111/andr.13110
REFERENCES
- 1. Ezire O, Oluigbo O, Archibong V, Ifeanyi O, Anyanti J. Barriers to repeated use of female condom among women and men of reproductive age in Nigeria. J AIDS HIV Res. 2013;5(6):206‐213. [Google Scholar]
- 2. Guerra F, Simbayi L. Prevalence of knowledge and use of the female condom in South Africa. AIDS Behav. 2014;18:146‐158. [DOI] [PubMed] [Google Scholar]
- 3. Copen CE. Condom use during sexual intercourse among women and men aged 15‐44 in the United States: 2011‐2015 National Survey of Family Growth. Natl Health Stat Report. 2017;(105):1‐18. [PubMed] [Google Scholar]
- 4. Peters A, Jansen W, van Driel F. The female condom: the international denial of a strong potential. Reprod Health. 2010;18(35):119‐128. 10.1016/S0968-8080(10)35499-1 [DOI] [PubMed] [Google Scholar]
- 5. Castle S, Askew I. Contraception discontinuations: reasons, challenges, and solutions. Family Planning 2020, Population Council 2015: Report. http://www.familyplanning2020.org/microsite/contraceptive‐discontinuation
- 6.Power to Decide: Birth Control Access. Contraceptive Deserts. Secondary Power to Decide: Birth Control Access. Contraceptive Deserts. 2020. https://powertodecide.org/what-we-do/access/birth-control-access
- 7. Janiak ECJ, Bartz D, Langer A, Gottlieb B. Barriers and pathways to providing long‐acting reversible contraceptives in Massachusetts community health centers: a qualitative exploration. Perspect Sexual Reprod Health. 2018;50(3):111‐118. [DOI] [PubMed] [Google Scholar]
- 8. Moreau C, Cleland K, Trussell J. Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception. 2007;76(4):267‐272. https://pubmed.ncbi.nlm.nih.gov/17900435/ [DOI] [PubMed] [Google Scholar]
- 9.FDA. Drug Interactions with hormonal contraceptives: public health and drug development implications. Center for Drug Evaluation and Research: Public Meeting, November 9, 2015. http://www.fda.gov/downloads/Drugs/NewsEvents/UCM493767.pdf
- 10. Bradley SEK, Schwandt HM, Khan S. Levels, trends, and reasons for contraceptive discontinuation. DHS Analytical Studies No. 20. Calverton, Maryland, USA: ICF Macro; 2009.
- 11. Sedgh G, Ashford L, Hussain R. Unmet need for contraception in developing countries: examining women's reasons for not using a method. Guttmacher Institute, New York 2016: Report. https://www.guttmacher.org/sites/default/files/report_pdf/unmet‐need‐for‐contraception‐in‐developing‐countries‐report.pdf
- 12. Friedman M, Nickels L, Sokal D, et al. Interest among U.S. men for new male contraceptive options consumer research study. Male Contraceptive Initiative. February 2019. http://www.malecontraceptive.org/uploads/1/3/1/9/131958006/mci_consumerresearchstudy.pdf
- 13. Fichorova R, Tucker L, Anderson D. The molecular basis of nonoxynol‐9‐induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184(4):418‐428. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11471099 [DOI] [PubMed] [Google Scholar]
- 14. Hillier S, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of Nonoxynol 9. J Acquir Immune Defic Syndr. 2005;39(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 15. Hoffman I, Taha T, Padian N, et al. Nonoxynol‐9 100 mg gel: multi‐site safety study from sub‐Saharan Africa. AIDS. 2004;18(16):2191‐2195. http://www.ncbi.nlm.nih.gov/pubmed/15577653?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DiscoveryPanel.Pubmed_Discovery_RA&linkpos=4&log$=relatedarticles&logdbfrom=pubmed [DOI] [PubMed] [Google Scholar]
- 16. Stafford M, Ward H, Flanagan A, et al. Safety study of nonoxynol‐9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(4):327‐331. http://www.ncbi.nlm.nih.gov/pubmed/9525433?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum [DOI] [PubMed] [Google Scholar]
- 17.USFDA. FDA mandates new warning for nonoxynol 9 OTC contraceptive products. Label must warn consumers products do not protect against STDs and HIV/AIDS. FDA News 2007. http://www.fda.gov/bbs/topics/NEWS/2007/NEW01758.html
- 18. Brady M, Tolley E. Aligning product development and user perspectives: social‐behavioural dimensions of multipurpose prevention technologies. BJOG. 2014;121(5):70‐78. [DOI] [PubMed] [Google Scholar]
- 19. Ross JS. Use of modern contraception increases when more methods become available: analysis of evidence from 1982‐2009. Glob Health Sci Pract. 2013;1(2):203‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hemmerling A, Christopher E, Holt B. Towards a roadmap to advance non‐hormonal contraceptive multipurpose prevention technologies: strategic insights from key stakeholders. Biol Reprod. 2020;103(2):289‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Jonge C. Biological basis for human capacitation‐revisited. Hum Reprod Update. 2017;23(3):289‐299. [DOI] [PubMed] [Google Scholar]
- 22. Mauck C, Vincent K. The postcoital test in the development of new vaginal contraceptives. Biol Reprod. 2020;103(2):437‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castle P, Hoen T, Whaley K, Cone R. Contraceptive testing of vaginal agents in rabbits. Contraception. 1998;58:51‐60. [DOI] [PubMed] [Google Scholar]
- 24. Hartman C. Unsuitability of the rabbit for in vivo testing of contraceptive jellies and creams. Fert Steril. 1961;12:170‐171. [DOI] [PubMed] [Google Scholar]
- 25. Zeitlin L, Hoen T, Achilles S, et al. Tests of BufferGel for contraception and prevention of sexually transmitted diseases in animal models. Sex Trans Dis. 2001;28(7):417‐423. [DOI] [PubMed] [Google Scholar]
- 26. Masse B, Boily M, Dimitrov D, Desai K. Efficacy dilution in randomized placebo‐controlled vaginal microbicide trials. Emerg Themes Epidemiol. 2009;6:5. 10.1186/1742-7622-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Notario‐Pérez F, Caro R, Veiga‐Ochoa M. Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: from past failures to future hopes. Drug Des Devel Ther. 2017;11:1167‐17787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trussell J. Understanding contraceptive failure. Best Pract Res Clin Obstet Gynaecol. 2009;23:199‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turner A, De Kock A, Meehan‐Ritter A, et al. Many vaginal microbicide trial participants acknowledged they had misreported sensitive sexual behavior in face‐to‐face interviews. J Clin Epidemiol. 2009;62:759‐765. [DOI] [PubMed] [Google Scholar]
- 30. Raymond E, Trussell J, Weaver M, Reeves M. Estimating contraceptive efficacy: the case of spermicides. Contraception. 2013;87:134‐137. [DOI] [PubMed] [Google Scholar]
- 31. Wolgemuth T, Judge‐Golden C, Callegari L, Zhao X, Mor M, Borrero S. Associations between pregnancy intention, attitudes, and contraceptive use among women veterans in the ECUUN Study. Women's Health Issues. 2018;28‐6:480‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FDA. Rulemaking history for OTC vaginal contraceptive drug products. 2017; Pending Final Monograph (21 CFR part 351): Vaginal Contraceptive Drug Products for Over‐the‐Counter Human Use. https://www.fda.gov/drugs/status‐otc‐rulemakings/rulemaking‐history‐otc‐vaginal‐contraceptive‐drug‐products
- 33. Liu D, Garrett C, Baker H. Acrosome‐reacted human sperm in insemination medium do not bind to the zona pellucida of human oocytes. Int J Androl. 2006;29(4):475‐481. [DOI] [PubMed] [Google Scholar]
- 34. Huszar G, Ozenci C, Cayli S, Zavaczki Z, Hansch E, Vigue L. Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and normal chromatin structure. Fertil Steril. 2003;79:1616‐1624. [DOI] [PubMed] [Google Scholar]
- 35. Erberelli RSR, Pereira D, Wolff P. Hyaluronan‐binding system for sperm selection enhances pregnancy rates in ICSI cycles associated with male factor infertility. JBRA Assist Reprod. 2017;21(1):02‐06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim E, Yamashita M, Kimura M, Honda A, Kashiwabara S, Baba T. Sperm penetration through cumulus mass and zona pellucida. Int J Dev Biol. 2008;52:677‐682. [DOI] [PubMed] [Google Scholar]
- 37. Huszar G. Objective Biomarkers of Sperm Development and Fertility: Assessment of Sperm‐Zona Pellucida Binding Ability and Hyaluronic Acid‐Mediated Selection of Sperm for ICSI Fertilization. Springer Science+Business Media; 2013. [Google Scholar]
- 38. Liu D, Garrett C, Baker H. Acrosome‐reacted human sperm in insemination medium do not bind to the zona pellucida of human oocytes. Inter J Androl. 2006;29:475‐481. [DOI] [PubMed] [Google Scholar]
- 39. Huszar G, Ozkavukcu S, Jakab A, Celik‐Ozenci C, Sati G, Cayli S. Hyaluronic acid‐binding ability of human sperm reflects cellular maturity and fertilizing potential: selection of sperm for intracytoplasmic sperm injection. Curr Opin Obstet Gynecol. 2006;18(3):260‐267. [DOI] [PubMed] [Google Scholar]
- 40. Kirkman‐Brown J, Pavitt S, Khalaf Y, et al. Sperm selection for assisted reproduction by prior hyaluronan binding: the HABSelect RCT. Southampton (UK): NIHR Journals Library; 2019 Feb. (Efficacy and Mechanism Evaluation, No. 6.1.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK537400/ doi: 10.3310/eme06010 [PubMed]
- 41. Oehninger S, Franken R, Ombelet W. Sperm functional tests. Fert Ster. 2014;102(6):1528‐1533. [DOI] [PubMed] [Google Scholar]
- 42. Weitzel M, North B, Waller D. Development of multipurpose technologies products for pregnancy and STI prevention: update on polyphenylene carboxymethylene MPT gel development. Biol Reprod. 2020;103(2):299‐309. 10.1093/biolre/ioaa087 https://academic.oup.com/biolreprod/article/103/2/299/5848264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson R, Brown D, Jackson E, et al. Feasibility of repurposing the polyanionic microbicide, PPCM, for prophylaxis against HIV transmission during ART. ISRN Obstet Gynecol. 2011;2011:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang T, Teleshova N, Rapista A, et al. SAMMA, a mandelic acid condensation polymer, inhibits dendritic cell‐mediated HIV transmission. FEBS Lett. 2007;581:4596‐4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheshenko N, Keller M, MasCasullo V, et al. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell‐to‐cell spread. Antimicrob Agent Chemother. 2004;48:2025‐2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Escaffre O, Freiberg AN. Polyphenylene carboxymethylene (PPCM) microbicide repurposed as antiviral against SARS‐CoV‐2. Proof of concept in primary human undifferentiated epithelial cells. Antiviral Res. 2021;194:105162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Escaffre O, Juelich T, Freiberg A. Polyphenylene carboxymethylene (PPCM) in vitro antiviral efficacy against Ebola virus in the context of a sexually transmitted infection. Antiviral Res. 2019;170:104567. 10.1016/j.antiviral.2019.104567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Herold B, Scordi‐Bello I, Cheshenko N, et al. Mandelic acid condensation polymer: novel candidate microbicide for prevention of human immunodeficiency virus and herpes simplex virus entry. J Virol. 2002;76:11236‐11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keller M, Klotman M, Herold B. Rigorous pre‐clinical evaluation of topical microbicides to prevent transmission of human immunodeficiency virus. J Antimicrob Chemother. 2003;51:1099‐1102. [DOI] [PubMed] [Google Scholar]
- 50. Mesquita P, Wilson S, Manlow P, et al. Candidate microbicide PPCM blocks human immunodeficiency virus type 1 infection in cell and tissue cultures and prevents genital herpes in a murine model. J Virol. 2008;82(13):6576‐6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pilligua‐Lucas M, Waller D, North B, Weitzel M, Jerse A. Polyphenylene carboxymethylene (PPCM) is a highly effective topical microbicide against Neisseria gonorrhoeae. CDC STD Prevention Conference. Virtual, 2020. https://s6.goeshow.com/ncsd/prevention/2020/profile.cfm?profile_name=session&master_key=A70ED4CE‐BF7F‐1B46‐8974‐E3ED4AE4DB7B&page_key=D379DFE4‐E7D5‐41D9‐8DC1‐8E1CEF5E729A&xtemplate&userLGNKEY=0&hide_role=Co‐Author
- 52. Zaneveld L, Anderson R, Diao X‐H, et al. Use of mandelic acid condensation polymer (SAMMA), a new antimicrobial contraceptive agent, for vaginal prophylaxis. Fertil Steril. 2002;78:1107‐1115 [DOI] [PubMed] [Google Scholar]
- 53. Anderson R, Feathergill K, Chany C II, Jain S, Krunic A. Nitric oxide‐dependent human acrosomal loss induced by PPCM (SAMMA) and by nitric oxide donors occurs by independent pathways: basis for synthesis of an improved contraceptive microbicide. J Androl. 2009;30(2):168‐182. [DOI] [PubMed] [Google Scholar]
- 54. Anderson RA, Feathergill KA, Waller DP, Zaneveld LJD. SAMMA induces premature human acrosomal loss by Ca2+ signaling dysregulation. J Androl. 2006;27:568‐577. [DOI] [PubMed] [Google Scholar]
- 55. Chen Y, Götte M, Liu J, Par P. Microbial subversion of heparan sulfate proteoglycans. Mol Cells. 2008;26:415‐426. [PubMed] [Google Scholar]
- 56. Doncel G. Exploiting common targets in human fertilization and HIV infection: development of novel contraceptive microbicides. Hum Reprod Update. 2006;12(2):103‐117. [DOI] [PubMed] [Google Scholar]
- 57. García B, Fernández‐Vega I, García‐Suárez O, Castañón S, Quirós L. The role of heparan sulfate proteoglycans in bacterial infections. J Med Microb Diagn. 2014;3(4):7. [Google Scholar]
- 58. Park P, Reizes O, Bernfield M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand‐receptor encounters. J Biol Chem. 2000;275(39):29923‐29926. [DOI] [PubMed] [Google Scholar]
- 59. WHO . WHO Laboratory Manual for the Examination of Human Semenand Sperm–Cervical Mucus Interaction. 3rd ed. Cambridge University Press; 1992.. [Google Scholar]
- 60. Anderson R, Feathergill K, de Jonge C, Mack R, Zaneveld L. Facilitative effect of pulsed addition of dibutyryl cAMP on the acrosome reaction of noncapacitated human spermatozoa. J Androl. 1992;13:398‐408. [PubMed] [Google Scholar]
- 61. De Jonge C, Mack S, Zaneveld L. Synchronous assay for human sperm capacitation and the acrosome reaction. J Androl. 1989;10(3):232‐239. [DOI] [PubMed] [Google Scholar]
- 62. Liu D, Baker H. Inducing the human acrosome reaction with a calcium ionophore A23187 decreases sperm‐zona pellucida binding with oocytes that failed to fertilize in vitro. J Reprod Fertil. 1990;89(1):127‐134. [DOI] [PubMed] [Google Scholar]
- 63. Herold B, Mesquita P, Madan R, Keller M. Female genital tract secretions and semen impact the development of microbicides for the prevention of HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65(3):325‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johnson J, Flores M, Rosa J, et al. The high content of fructose in human semen competitively inhibits broad and potent antivirals that target high‐mannose glycans. J Virol. 2020;94(9):e01749‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Keller M, Mesquita P, Torres N, et al. Postcoital bioavailability and antiviral activity of 0.5% PRO 2000 gel: implications for future microbicide clinical trials. PLOS ONE. 2010;5(1):e8781. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0008781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Neurath R, Strick N, Li Y. Role of seminal plasma in the anti‐HIV‐1 activity of candidate microbicides. BMC Infect Dis. 2006;6:150. http://www.biomedcentral.com/content/pdf/1471‐2334‐6‐150.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Patel S, Hazrati E, Cheshenko N, et al. Seminal plasma reduces the effectiveness of topical polyanionic microbicdes. J Infect Dis. 2007;196:1394‐1402. [DOI] [PubMed] [Google Scholar]
- 68. Tan S, Lu L, Li L, et al. Polyanionic candidate microbicides accelerate the formation of semen‐derived amyloid fibrils to enhance HIV‐1 infection. PLOS One. 2013;8(3):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zirafi O, Kim K, Roan N, et al. Semen enhances HIV infectivity and impairs the antiviral efficacy of microbicides. Sci Transl Med. 2014;6(262):262ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Röcker A, Roan N, Yadav J, Fändrich M, Münch J. Structure, function, and antagonism of semen amyloids. Chem Commun (Camb). 2018;54(55):7557‐7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Suarez S, Wolfner M. Seminal plasma plays important roles in fertility. In: Yanagimachi R, De Jonge C,Barratt C, eds. The Sperm Cell: Production, Maturation, Fertilization, Regeneration. Cambridge University Press; 2017:88‐108. [Google Scholar]
- 72. Sobrero A, Macleod J. The immediate postcoital test. Fertil Steril. 1962;13:184‐189. [DOI] [PubMed] [Google Scholar]
- 73. Tredway D. The postcoital test. Global Library of Women's Medicine: International Federation of Gynecology and Obstetrics; 2020. https://www.glowm.com/section_view/heading/The%20Postcoital%20Test/item/321
- 74. Karim SSA, Richardson BA, Ramjee G, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25(7):957‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Barnhart KT, Rosenberg MJ, MacKay HT, et al. Contraceptive efficacy of a novel spermicidal microbicide used with a diaphragm: a randomized controlled trial. Obstet Gynecol. 2007;110(3):577‐586. [DOI] [PubMed] [Google Scholar]
- 76. Lacey CJ, Wright A, Weber JN, Profy AT. Direct measurement of in‐vivo vaginal microbicide levels of PRO 2000 achieved in a human safety study. AIDS. 2006;20(7):1027‐1030. [DOI] [PubMed] [Google Scholar]
- 77. McCormack S, Ramjee G, Kamali A, et al. PRO2000 vaginal gel for prevention of HIV‐1 infection (Microbicides Development Programme 301): a phase 3, randomised, double‐blind, parallel‐group trial. Lancet. 2010;376(9749):1329‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Williams DL, Newman DR, Ballagh SA, et al. Phase I safety trial of two vaginal microbicide gels (Acidform or BufferGel) used with a diaphragm compared to KY jelly used with a diaphragm. Sex Transm Dis. 2007;34(12):977‐984. [DOI] [PubMed] [Google Scholar]
- 79. Gupta S, Kakkar V, Bhushan I. Crosstalk between vaginal microbiome and female health: a review. Microb Pathog. 2019;136:103696. [DOI] [PubMed] [Google Scholar]
- 80. Mauck C. Biomarkers for evaluating vaginal microbicides and contraceptives: discovery and early validation. Sex Trans Dis. 2009;36(3):S73‐S75. [DOI] [PubMed] [Google Scholar]
- 81. Cummins J, Guarner J, Flowers L, et al. Preclinical testing of candidate topical microbicides for anti‐human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51(5):1770‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Doncel G, Chandra N, Fichorova R. Preclinical assessment of the proinflammatory potential of microbicide candidates. J Acquir Immune Defic Syndr. 2004;37:s174‐s180. [PubMed] [Google Scholar]
- 83. Keller M, Herold B. Understanding basic mechanisms and optimizing assays to evaluate the efficacy of vaginal microbicides. Sex Tran Dis. 2009;36(3):S92‐S95. [DOI] [PubMed] [Google Scholar]
- 84. Keller MJ, Herold BC. Impact of microbicides and sexually transmitted infections on mucosal immunity in the female genital tract. Am J Reprod Immunol. 2006;56(5‐6):356‐363. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17076680 [DOI] [PubMed] [Google Scholar]
- 85. Herrera C. The pre‐clinical toolbox of pharmacokinetics and pharmacodynamics: in vitro and ex vivo models. Front Pharmacol. 2019;10:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pathak U, Gabrielsen J, Lipshultz L. Cutting‐edge evaluation of male infertility. Urol Clin North Am. 2020;47(2):129‐138. [DOI] [PubMed] [Google Scholar]
- 87. Zarinara A, Zeraati H, Kamali K, Mohammad K, Shahnazari P, Akhondi M. Models predicting success of infertility treatment: a systematic review. J Reprod Infertil. 2016;17(2):68‐81. [PMC free article] [PubMed] [Google Scholar]
- 88. Fen C, Lee S, Lim M, Yu S. Relationship between sperm hyaluronan‐binding assay (HBA) scores on embryo development, fertilisation, and pregnancy rate in patients undergoing intra‐cytoplasmic sperm injection (ICSI). Proc Singapore Healthcare. 2013;22(2):120‐124. [Google Scholar]
- 89. Vandevoort C, Cherr G, Overstreet J. Hyaluronic acid enhances the zona pellucida‐induced acrosome reaction of macaque sperm. J Androl. 1997;18:1‐5. [PubMed] [Google Scholar]
- 90. Liu D, Baker H. Relationship between the zona pellucida (ZP) and ionophore A23187‐induced acrosome reaction and the ability of sperm to penetrate the ZP in men with normal sperm‐ZP binding. Fert Ster. 1996;66(2):312‐315. [DOI] [PubMed] [Google Scholar]
- 91. García B, Merayo‐Lloves J, Martin C, Alcalde I, Quirós L, Vazquez F. Surface proteoglycans as mediators in bacterial pathogens infections. Front Microbiol. 2016;7(220):11. [DOI] [PMC free article] [PubMed] [Google Scholar]


