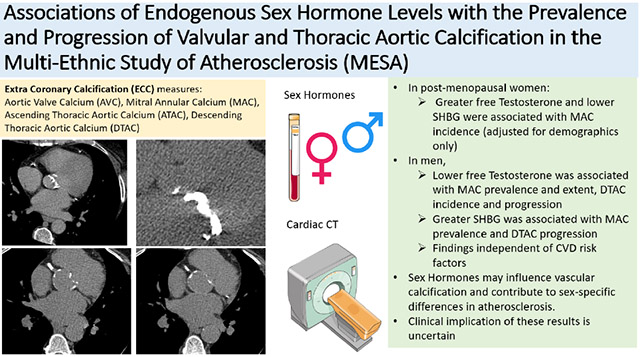
Abstract
Background and aims:
Sex hormones (SH) may contribute to sex differences in cardiovascular disease (CVD). High free testosterone (T) and low sex hormone binding globulin (SHBG) have been associated with progression of coronary artery calcification in women. We now examined the association of SH with extra-coronary calcification (ECC) prevalence and progression among MESA participants.
Methods:
We studied 2,737 postmenopausal women and 3,130 men free of clinical CVD with baseline SH levels. ECC measurements [ascending and descending thoracic aortic calcification (ATAC, DTAC), mitral annular calcification (MAC), aortic valve calcification (AVC)] were done by computed tomography at baseline and after 2.4±0.9 years. We used multivariable Poisson regression to evaluate associations with ECC prevalence and incidence (Agatston scores >0) and linear mixed effects models for ECC progression, per 1-SD increment in log(SH) in women and men separately.
Results:
The mean age was 65±9 and 62±10 years for women and men, respectively. In women, greater free T and lower SHBG were associated with MAC incidence in a demographic-adjusted model only. In men, lower free T was associated with MAC prevalence, DTAC incidence and progression, while greater SHBG was associated with MAC prevalence and DTAC progression after further adjusting for CVD risk factors.
Conclusions:
In this diverse cohort free of CVD, we found some associations of SH with ECC measures. In particular, free T was inversely associated with prevalent MAC and DTAC progression in men independent of CVD risk factors. SH may influence vascular calcification, but further work is needed to understand clinical implications of these findings.
Keywords: endogenous sex hormones, testosterone, sex hormone binding globulin, extra coronary calcification, mitral annular calcification, descending thoracic aortic calcification
Graphical Abstarct
1. Introduction
Despite advances in the prevention and management of cardiovascular diseases (CVD), CVD continues to be the leading cause of death in the United States (U.S.) and globally.1 Additionally, there are significant differences in the prevalence, risk factors and outcomes of CVD in men and women suggesting a role of sex hormones in disease mechanisms.2,3 Hence, the identification of mechanistic pathways that help in understanding and improved risk-stratification of individuals (both men and women) at risk for CVD are essential.
Markers of subclinical atherosclerosis, such as coronary artery calcium (CAC) and extra-coronary calcification (ECC), have been shown to be better predictors of incident clinical CVD events than traditional risk factors.4-13 While CAC and ECC both represent atherosclerosis, the location of calcification across the various vascular beds provides unique information about mortality risk.14,15 CAC is associated with ECC16,17 and shares many similar traditional CVD risk factors;18-20 however, risk factors for vascular calcification, including CAC and ECC, are not necessarily similar across the various vascular beds.21 ECC includes both the ascending and descending thoracic aortic calcification (ATAC, DTAC) along with left-sided valvular calcification (mitral annular calcification (MAC) and aortic valve calcification (AVC)). Earlier studies have shown that ECC and ECC progression are associated with increased risk of stroke, coronary heart disease (CHD), heart failure (HF), and all-cause mortality.6-13,22,23 Thus, the identification of ECC or its progression may represent an intermediate step in the pathogenesis leading to clinical CVD events.
Prior work from the Multi-Ethnic Study of Atherosclerosis (MESA) has found that post-menopausal women who have a more androgenic sex hormone profile of higher endogenous free testosterone (T) relative to estradiol (E2) levels had an increased risk for incident CVD events.24 Furthermore, post-menopausal women with higher free T and lower sex hormone binding globulin (SHBG) levels experienced greater progression of CAC over 10 years.25 However, there is limited evidence on the association of sex hormones with ECC prevalence and progression, specifically, requiring further study in this area. Understanding the association of sex hormones with each specific type of ECC may further our understanding of sex differences in CVD pathogenesis and improve sex-specific CVD risk stratification.
Therefore, our study was aimed to explore the association between endogenous sex hormones and ECC (ATAC, DTAC, MAC, AVC) prevalence and progression in MESA, stratified by sex. We hypothesized that unlike men, a higher androgenic profile [higher serum levels of free T and lower SHBG] in women will be associated with greater prevalence and extent of ECC at baseline as well as greater progression and incidence of ECC.
2. Patients and methods
2.1. Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a continuing prospective cohort study from six centers around the U.S. with the aim of exploring subclinical atherosclerosis in men and women aged 45-84 years without clinical CVD at baseline (2000-2002).26 Our study included men and post-menopausal women in MESA who had endogenous sex hormone levels measured at baseline and who had the outcome ECC (ATAC, DTAC, MAC and AVC) measured at baseline and at least one of the follow up MESA exams (Figure 1A (women), Figure 1B (men)). An established algorithm that has been reported earlier27 was used to determine menopausal status in women. Premenopausal women were excluded given their sex hormone profile was significantly different from post-menopausal women, in addition to their comparatively fewer numbers in MESA, limiting interpretation of results in this subgroup. Individuals were also excluded with missing data on the exposure (sex hormone), the outcome (ECC variables), or the covariates in our main model. Institutional Review Boards of all collaborating institutions and the MESA Coordinating Center approved the study, and voluntary participation with informed consent was ensured from each individual.
Figure 1:
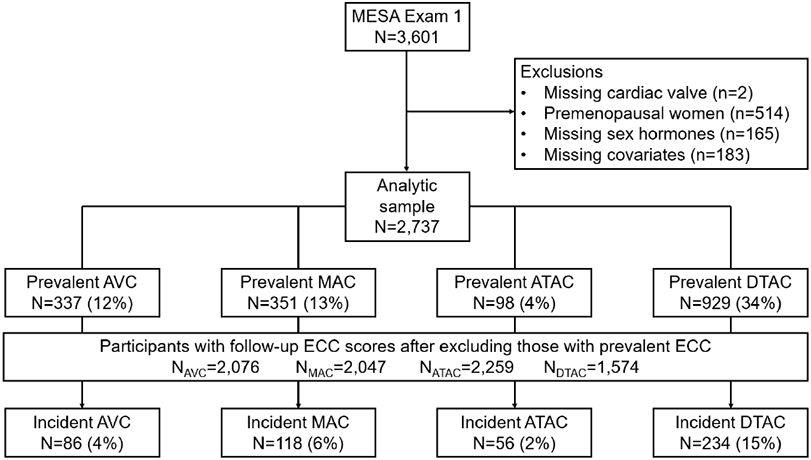
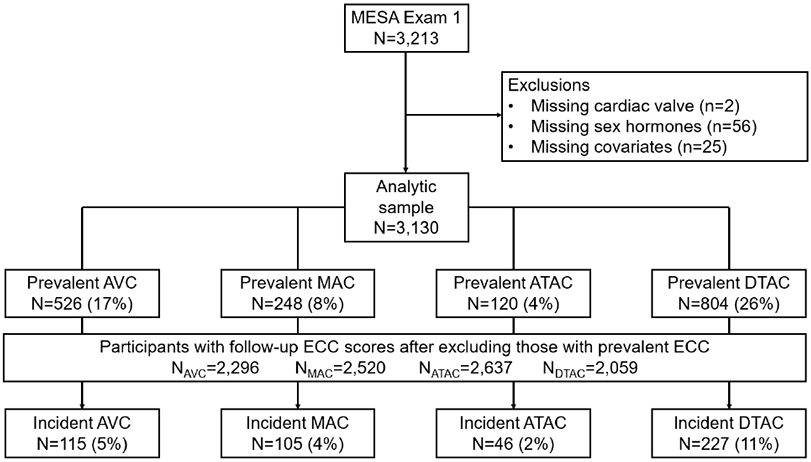
Flow diagram for participant inclusion/exclusion in study for women (A) and men (B)
2.2. Exposure assessment
The exposure variables included the endogenous sex hormone levels of total T, bioavailable T, free T, dehydroepiandrosterone (DHEA), estradiol (E2), and SHBG. These hormones were measured from fasting blood samples collected at the baseline exam and measurements were done at the Steroid Hormone Laboratory at the University of Massachusetts Medical Center (Worcester, MA). The assays and kits used to measure the hormone levels were an ultrasensitive radioimmunoassay kit for E2 (Diagnostic System Laboratories, Webster, TX), radioimmunoassay kits for total T and DHEA and a chemiluminescence enzyme immunometric assay using Immulite kits for SHBG (Diagnostic Products Corporation, Los Angeles, CA).25,27 The Sodergard equation28 was used to estimate free T from total T.
Given that our prior work in MESA has found that higher free T and low SHBG were the sex hormones most strongly associated with an adverse cardiovascular phenotype in women,25,27,29-31 we decided a priori to focus on those two hormones in our main analyses, to minimize number of statistical tests. However, in supplementary analyses, we also examined the association of the other hormones measured (Total T, E2, and DHEA) with ECC.
2.3. Covariates
Included covariates were the following demographic, behavioral and CVD risk factors measured at the baseline exam: age, sex, race/ethnicity, study site, education (<high school; high school or vocational school; college, graduate or professional school), body mass index (BMI) (as a continuous variable), smoking status (current, former or never), pack-years of smoking, physical activity level (MET hours/week of moderate or vigorous activity, continuous), systolic blood pressure (continuous), use of antihypertensive medications (yes, no), total cholesterol and HDL cholesterol (mg/dL, continuous), use of lipid lowering therapy (yes, no), diabetes (defined as fasting blood sugar ≥126 or non-fasting glucose ≥200 mg/dL or medication use), and estimated glomerular filtration rate (eGFR, continuous). In women, we additionally considered age at menopause and current use of hormone therapy. A standardized physical exam was performed to measure blood pressure, height and weight, and standardized laboratory measures were performed from serum after an overnight fast, as previously reported.26 A medication inventory approach was used to ascertain medication use, and demographic information, including education and smoking status, was obtained from questionnaire and interviews.
2.4. Outcome assessment
All MESA participants had an ECG-gated cardiac CT scan by either electron-beam CT [EBCT] or a four-slice multi-detector row helical CT [MDCT] at the baseline exam (2000-2002). Participants were then randomly assigned for a follow up CT scan at either Exam 2 (2002-2004) or Exam 3 (2004-2005).13,23 Scanning parameters were kept identical between the first three visits. Furthermore, prior work from MESA has shown good reproducibility of CAC and ECC measures across the CT scanners used in MESA.32,33 Although additional cardiac CTs were performed at later visits in MESA for CAC assessment, ECC results were not reported from those latter CTs. We included four ECC variables to study (ATAC, DTAC, MAC, AVC), ascertained from axial datasets of the cardiac CT scans. The Agatston scoring method was used for the quantification of ECC based on these CTs.34
MAC was measured at the mitral annulus; AVC was measured from the aortic valve to just before the aortic root. ATAC was measured from the aortic annulus to the lower edge of the pulmonary artery.35 DTAC was measured from the lower edge of pulmonary artery to the cardiac apex. The aortic arch was not seen on the scans. The primary outcomes analyzed were prevalence and severity of these 4 measures of ECC for cross-sectional analysis and incidence and progression of ECC for longitudinal analysis.
2.5. Statistical analysis
We stratified all analyses by sex, as the sex hormone distribution differs significantly between men and women and does not overlap. The baseline demographics and clinical characteristics of the study participants were determined. Continuous variables were expressed either as means (standard deviation (SD)) for normally distributed variables or median (interquartile range) for skewed variables while categorical variables were expressed as frequency (percentage). Sex hormones being positively skewed were log transformed and modeled as per one SD increment in sex hormone level per their sex distribution. ECC scores were also skewed and included many scores of 0. Thus, a ln(Agatston score+1) transformation of the outcome was used when using Agatston score as a continuous variable, as reported in previous works.25,36 Prevalent ECC was defined as baseline Agatston score >0 and incident ECC was determined when participants with baseline Agatston score of zero increased to a detectable score (>0) during follow up exam.
For cross-sectional analysis at the baseline exam, multivariable-adjusted regression models were used to estimate the adjusted prevalence ratios of ECC (ATAC, DTAC, MAC, AVC), among men and post-menopausal women per 1 SD increment in sex hormone levels. We used linear regression to examine the association of endogenous sex hormone levels with extent or severity of ECC [ln(Agatston score+1)].
For longitudinal analysis, a multivariable Poisson model was used to estimate the relative risk of incident ECC (ATAC, DTAC, MAC, AVC) for those participants who have ECC=0 at baseline per 1SD increment in sex hormones among men and postmenopausal women, separately. Linear mixed effects models with random slopes and intercepts analysis determined the longitudinal associations of sex hormones with log (ECC+1) extent and progression. The time variable was scaled to derive the 2-year change. Percent change in ECC was derived from the linear mixed effect models and calculated by ([Exp (β) −1]*100).
In sex-stratified analyses, we applied progressively adjusted models for both cross-sectional and longitudinal data analysis as follows: Model 1 (demographics and field center) included age, race/ethnicity, MESA study site, Model 2 (+social and lifestyle factors) included Model 1 plus education, health insurance, physical activity, smoking status, pack-years of smoking, and BMI; Model 3 (+CVD risk factors) included Model 2 plus systolic blood pressure, use of anti-hypertensive medication, total cholesterol, HDL-cholesterol, use of lipid lowering medications, diabetes mellitus and eGFR. Relative risk ratios were additionally adjusted for time between scans in model 1. In women, Model 2 was also adjusted for current use of hormone therapy and age at menopause.
We further analyzed the prevalence and incidence ratios of ECC in a supplemental model 4 with sex hormones adjusted for each other including total T, E2, and SHBG in the same model. (Since bio T and free T are calculated from total T and SHBG, they were not included in the same model). In sensitivity analysis we explored further analysis excluding women taking hormone therapy at baseline for comparison of the different associations.
Note that we did perform some diagnostic checking on the models we performed. For the Poisson models, we checked for overdispersion by comparing the mean and variance of the outcome variances. Mean and variance were similar which means overdispersion was not present in the data. We used the post estimation test “estat gof” in STATA 15.0 to measure the goodness of fit of the model which showed the Poisson model was appropriate for the data as p >0.05. For the Linear mixed effects regression model, we checked for linearity by plotting a scatter plot of the dependent variables against independent variables. Normality of residuals was checked by plotting a kernel density estimate of the residuals. We checked for homoscedasticity of the residuals by plotting RVF plots. Multicollinearity was assessed by the variance inflation factor which was less than 3.0 for all the variables included in the model.
All analyses were performed using the STATA 15.0 version (StataCorp LP, College Station, TX). p values were two-sided, and significance level was set at 0.05 for primary results. Given that these results were meant to be exploratory and based on a prior hypothesis, we used a traditional p-value threshold <0.05 to be considered statistically significant. However, to address concerns regarding multiple testing, we also additionally noted results in our Tables that were considered statistically significant using a Bonferroni adjusted two-sided p <0.002.
3. Results
3.1. Baseline characteristics
The baseline characteristics are summarized in Table 1. There was a total of 5,867 participants in our study population including 2,737 post-menopausal women and 3,130 men. The mean (SD) age was 65 (9) years in women and 62 (10) in men, with 39% White, ~27% Black, ~23% Hispanic and 12% Chinese participants in both groups.
Table 1.
Baseline characteristics of study participants, MESA 2000-2002
| Women | Men | |
|---|---|---|
| N | 2,737 | 3,130 |
| Age, years | 65 (9) | 62 (10) |
| Race/ethnicity | ||
| White | 1,048 (38%) | 1,228 (39%) |
| Chinese American | 342 (13%) | 387 (12%) |
| Black | 737 (27%) | 801 (26%) |
| Hispanic | 610 (22%) | 714 (23%) |
| Education | ||
| <Bachelor’s degree | 1,966 (72%) | 1,841 (59%) |
| ≥Bachelor’s degree | 771 (28%) | 1,289 (41%) |
| Physical activity, MET-min/wk | 3,510 (1,710-6,435) | 4,470 (2,175-8,490) |
| Smoking status | ||
| Current smoker | 287 (10%) | 449 (14%) |
| Former smoker | 824 (30%) | 1,406 (45%) |
| Never smoker | 1,626 (59%) | 1,275 (41%) |
| Pack-years of smoking if >0 | 15 (6-31) | 18 (7-35) |
| BMI, kg/m2 | 29 (6) | 28 (4) |
| Health insurance | ||
| Yes | 2,507 (92%) | 2,851 (91%) |
| No | 230 (8%) | 279 (9%) |
| Systolic blood pressure, mmHg | 129 (24) | 126 (19) |
| Total cholesterol, mg/dL | 202 (36) | 188 (35) |
| HDL-cholesterol, mg/dL | 57 (15) | 45 (12) |
| Diabetes mellitus | 338 (12%) | 429 (14%) |
| eGFR, ml/min per 1.73m2 | 75 (16) | 78 (16) |
| Use of antihypertensive medication | 1,147 (42%) | 1,114 (36%) |
| Use of lipid-lowering medication | 522 (19%) | 511 (16%) |
| Total T (nmol/L) | 0.9 (0.6-1.3) | 14.2 (11.3-17.8) |
| Free T (%) | 1.3 (0.9-1.7) | 2.0 (1.7-2.3) |
| Estradiol (nmol/L) | 0.07 (0.05-0.15) | 0.11 (0.09-0.14) |
| DHEA (nmol/L) | 10.2 (6.9-14.5) | 12.5 (9.1-17.1) |
| SHBG (nmol/L) | 59.7 (40.6-93.9) | 40.8 (31.4-52.6) |
BMI, Body mass index; CT, Computed tomography; eGFR, Estimated glomerular filtration rate; DHEA, Dehydroepiandrosterone; HDL; High density lipoprotein; MESA, Multi-Ethnic Study of Atherosclerosis; MET-min/wk, Metabolic equivalent of task-minutes/week; SHBG, Sex hormone binding globulin; T, Testosterone.
Data were presented as mean (SD) for normally distributed variables, median (IQR) for skewed variables and count (percentage) for categorical variables.
3.2. Cross-sectional analysis
The prevalence of ECC for ATAC, DTAC, MAC and AVC among female participants at baseline was 4%, 34%, 13% and 12% (Figure 1A) and for male participants was 4%, 26%, 8% and 17%, respectively (Figure 1B). The prevalence ratios (95% CI) of the ECC per 1 SD increment of sex hormones (free T and SHBG) in men and women are shown in Table 2 and the percent difference in extent of ECC by sex hormone levels are shown in Table 3.
Table 2.
Association between sex hormones and prevalent extra-coronary calcification at MESA exam 1 (2000-2002).
| Women | Men | |||
|---|---|---|---|---|
| Free Testosterone |
SHBG | Free Testosterone |
SHBG | |
| Prevalence ratio (95% CI) | ||||
| Aortic valve | ||||
| Model 1 | 1.09 (0.98, 1.21) | 0.91 (0.82, 1.01) | 1.05 (0.97, 1.15) | 0.95 (0.87, 1.03) |
| Model 2 | 1.05 (0.94, 1.18) | 0.94 (0.84, 1.05) | 1.04 (0.95, 1.14) | 0.97 (0.88, 1.06) |
| Model 3 | 0.96 (0.85, 1.08) | 1.03 (0.91, 1.17) | 0.99 (0.91, 1.09) | 1.02 (0.93, 1.11) |
| Mitral valve | ||||
| Model 1 | 1.03 (0.94, 1.14) | 0.96 (0.87, 1.06) | 0.89 (0.79, 1.01) | 1.13 (0.99, 1.30) |
| Model 2 | 0.93 (0.83, 1.05) | 1.06 (0.95, 1.19) | 0.86 (0.75, 0.97) | 1.20 (1.04, 1.38) |
| Model 3 | 0.89 (0.78, 1.01) | 1.11 (0.98, 1.27) | 0.83 (0.73, 0.95) | 1.24 (1.08, 1.43) |
| Ascending aorta | ||||
| Model 1 | 1.17 (0.95, 1.43) | 0.87 (0.72, 1.06) | 0.91 (0.75, 1.10) | 1.08 (0.87, 1.33) |
| Model 2 | 1.09 (0.84, 1.41) | 0.94 (0.74, 1.21) | 0.94 (0.78, 1.15) | 1.04 (0.83, 1.29) |
| Model 3 | 0.98 (0.74, 1.29) | 1.05 (0.81, 1.37) | 0.91 (0.74, 1.11) | 1.08 (0.87, 1.35) |
| Descending aorta | ||||
| Model 1 | 1.03 (0.98, 1.08) | 0.98 (0.92, 1.02) | 1.01 (0.95, 1.07) | 0.98 (0.92, 1.05) |
| Model 2 | 1.02 (0.97, 1.08) | 0.98 (0.92, 1.03) | 1.01 (0.95, 1.08) | 0.98 (0.92, 1.05) |
| Model 3 | 0.97 (0.91, 1.03) | 1.04 (0.97, 1.10) | 0.98 (0.93, 1.04) | 1.02 (0.95, 1.08) |
CI, Confidence intervals; MESA, Multi-Ethnic Study of Atherosclerosis; SHBG, Sex hormone binding globulin.
Prevalence ratio was derived from Poisson regression with robust variance estimation for extra-coronary calcification greater than zero at baseline.
The independent variables (sex hormones) were natural log-transformed and modeled per one standard deviation.
Results in bold font indicate statistical significance at p <0.05.
Results in bold font with asterisk indicate Bonferroni corrected statistical significance at p <0.002.
Model 1: adjusted for age, race/ethnicity and MESA study site.
Model 2: adjusted for model 1 covariates plus education, physical activity, smoking, pack-years of smoking, BMI and health insurance.
Model 3: adjusted for model 2 covariates plus systolic blood pressure, anti-hypertensive medication, total cholesterol, HDL-C, lipid-lowering medication, diabetes mellitus, and eGFR.
In women, model 2 was additionally adjusted for age at menopause and current use of hormone therapy.
Table 3.
Cross sectional association between sex hormones and extra-coronary calcification at MESA exam 1 (2000-2002)
| Women | Men | |||
|---|---|---|---|---|
| Free testosterone | SHBG | Free testosterone | SHBG | |
| Percent difference (95% CI) | ||||
| Aortic valve | ||||
| Model 1 | 6 (1, 11) | −6 (−11, −1) | 0 (−6, 7) | −1 (−7, 5) |
| Model 2 | 4 (−2, 10) | −4 (−10, 1) | −1 (−8, 5) | 1 (−5, 8) |
| Model 3 | −1 (−7, 5) | 0 (−6, 6) | −5 (−11, 2) | 4 (−2, 11) |
| Mitral valve | ||||
| Model 1 | 7 (0, 13) | −6 (−12, 0) | −3 (−8, 2) | 3 (−2, 7) |
| Model 2 | −2 (−9, 5) | 2 (−5, 10) | −5 (−10, 0) | 5 (0, 11) |
| Model 3 | −7 (−14, 0) | 7 (−1, 16) | −5 (−10, 0) | 5 (0, 11) |
| Ascending aorta | ||||
| Model 1 | 3 (0, 5) | −2 (−5, 0) | −2 (−5, 1) | 2 (−1,5) |
| Model 2 | 1 (−2, 5) | −1 (−4, 3) | −1 (−4, 2) | 1 (−2, 4) |
| Model 3 | −1 (−4, 3) | 1 (−2, 5) | −2 (−5, 1) | 2 (−1,5) |
| Descending aorta | ||||
| Model 1 | 7 (−2, 17) | −6 (−14, 2) | 1 (−7, 11) | −3 (−10, 6) |
| Model 2 | 4 (−6, 16) | −4 (−14, 7) | 3 (−5, 13) | −5 (−12, 4) |
| Model 3 | −8 (−18, 3) | 9 (−2, 22) | −1 (−9, 8) | −1 (−9, 7) |
CI, Confidence intervals; MESA, Multi-Ethnic Study of Atherosclerosis SHBG, Sex hormone binding globulin.
Percent difference was derived from linear mixed effect models and calculated by ([Exp (β) −1]*100).
Extra-coronary calcification was analyzed as ln(ECC+1).
The independent variables (sex hormones) were natural log-transformed and modeled per one standard deviation.
Results in bold font indicate statistical significance at p <0.05.
Results in bold font with asterisk indicate Bonferroni corrected statistical significance at p <0.002.
Model 1: adjusted for age, race/ethnicity and MESA study site.
Model 2: adjusted for model 1 covariates plus education, physical activity, smoking, pack-years of smoking, BMI and health insurance.
Model 3: adjusted for model 2 covariates plus systolic blood pressure, use of anti-hypertensive medication, total cholesterol, HDL-C, use of lipid-lowering medication, diabetes mellitus and eGFR.
In women, model 2 was additionally adjusted for age at menopause and current use of hormone therapy.
3.2.1. ATAC:
There was no statistically significant association found between endogenous sex hormones and prevalence or extent of ATAC at baseline among MESA participants (Tables 2 and 3).
3.2.2. DTAC:
There was no statistically significant association found between endogenous sex hormones and prevalence or extent of DTAC at baseline among MESA participants (Tables 2 and 3).
3.2.3. AVC:
There was no statistically significant association found between endogenous sex hormones and prevalence of AVC at baseline among MESA participants (Table 2). Among women, SHBG was inversely associated with AVC extent only in the demographic model 1 (Table 3), which was no longer statistically significant in further adjusted models.
3.2.4. MAC:
In men, after adjustment for demographic and lifestyle factors (model 2), greater SHBG and lower free T were associated with prevalent MAC (>0) that remained statistically significant in model 3, which was further adjusted for intermediary CVD risk factors (SHBG: prevalence ratio 1.24 (95% CI 1.08, 1.43); Free T: 0.83 (0.73, 0.95)) (Table 2). Additionally in men, higher free T was associated with less MAC extent [−5% (−10, 0)] while higher SBHG was associated with greater MAC extent [5% (0, 11)] (Table 3). On the contrary, there was no association of sex hormones with prevalent MAC in women. However, greater free T and lower SHBG were associated with MAC extent in demographic-adjusted model in women, but this association was not significant in additionally adjusted models (Table 3).
3.3. Longitudinal analysis
The mean follow-up time between CT scans was 2.4 (0.9) years. After excluding participants with ECC score >0 at baseline, the percentage of study participants that developed incident ECC >0 for ATAC, DTAC, AVC and MAC was 2%, 15%, 4% and 6% in women (Figure 1A) and 2%, 11%, 5% and 4% in men, respectively (Figure 1B).
3.3.1. ATAC:
For both men and women, we observed no association between sex hormones and incident ATAC, as well as 2-year change in ATAC in all our models (Tables 4 and 5).
Table 4.
Association between sex hormones and incident extra-coronary calcification at exam 2/3 (2002–2005)
| Women | Men | |||
|---|---|---|---|---|
| Free Testosterone |
SHBG | Free Testosterone | SHBG | |
| Incidence rate ratios (95% CI) | ||||
| Aortic valve | ||||
| Model 1 | 0.96 (0.76, 1.21) | 1.03 (0.81, 1.29) | 1.13 (0.91, 1.39) | 0.87 (0.70, 1.08) |
| Model 2 | 0.96 (0.73, 1.26) | 1.02 (0.78, 1.34) | 1.07 (0.86, 1.33) | 0.92 (0.73, 1.15) |
| Model 3 | 0.86 (0.64, 1.15) | 1.14 (0.85, 1.53) | 1.04 (0.84, 1.28) | 0.95 (0.76, 1.20) |
| Mitral valve | ||||
| Model 1 | 1.27 (1.05, 1.53) | 0.79 (0.66, 0.95) | 1.16 (0.92, 1.47) | 0.81 (0.64, 1.03) |
| Model 2 | 1.21 (0.97, 1.52) | 0.82 (0.66, 1.03) | 1.13 (0.90, 1.42) | 0.85 (0.68, 1.07) |
| Model 3 | 1.09 (0.86, 1.37) | 0.92 (0.73, 1.16) | 1.12 (0.89, 1.41) | 0.86 (0.68, 1.09) |
| Ascending aorta | ||||
| Model 1 | 0.94 (0.72, 1.25) | 1.07 (0.82, 1.40) | 0.87 (0.59, 1.29) | 1.11 (0.74, 1.67) |
| Model 2 | 0.84 (0.60, 1.16) | 1.21 (0.88, 1.67) | 0.85 (0.56, 1.27) | 1.14 (0.75, 1.75) |
| Model 3 | 0.73 (0.52, 1.04) | 1.40 (0.99, 1.98) | 0.77 (0.50, 1.18) | 1.28 (0.81, 2.01) |
| Descending aorta | ||||
| Model 1 | 1.09 (0.97, 1.24) | 0.91 (0.80, 1.03) | 0.95 (0.83, 1.08) | 1.02 (0.89, 1.17) |
| Model 2 | 1.13 (0.98, 1.30) | 0.88 (0.77, 1.01) | 0.94 (0.82, 1.07) | 1.04 (0.91, 1.20) |
| Model 3 | 1.06 (0.91, 1.24) | 0.94 (0.81, 1.09) | 0.86 (0.75, 0.99) | 1.13 (0.97, 1.32) |
CI, Confidence intervals; MESA, Multi-Ethnic Study of Atherosclerosis; SHBG, Sex hormone binding globulin.
Incidence rate ratio was derived from Poisson regression with robust variance estimation for extra-coronary calcification greater than zero at exam 2/3 among participants with zero extra-coronary calcification at baseline.
The independent variables (sex hormones) were natural log-transformed and modeled per one standard deviation.
Results in bold font indicate statistical significance at p <0.05.
Results in bold font with asterisk indicate Bonferroni corrected statistical significance at p <0.002.
Model 1: adjusted for age, race/ethnicity, MESA study site and time between scans.
Model 2: adjusted for model 1 covariates plus education, physical activity, smoking, pack-years of smoking, BMI and health insurance.
Model 3: adjusted for model 2 covariates plus systolic blood pressure, use of anti-hypertensive medication, total cholesterol, HDL-C, use of lipid-lowering medication, diabetes mellitus and eGFR.
In women, model 2 was additionally adjusted for age at menopause and current use of hormone therapy.
Table 5.
Association between sex hormones and 2-year change in extra-coronary calcification from MESA exam 1 (2000–2002) to exam 2/3 (2002–2005)
| Women | Men | |||
|---|---|---|---|---|
| Free testosterone | SHBG | Free testosterone |
SHBG | |
| Percent change (95% CI) | ||||
| Aortic valve | ||||
| Model 1 | 1 (−2, 4) | −1 (−4, 2) | 0 (−3, 3) | 0 (−3, 3) |
| Model 2 | 1 (−2, 4) | −1 (−4, 2) | 0 (−3, 3) | 0 (−3, 3) |
| Model 3 | 1 (−2, 4) | −1 (−4, 2) | 0 (−3, 3) | 0 (−3, 3) |
| Mitral valve | ||||
| Model 1 | 1 (−2, 4) | −1 (−4, 2) | −2 (−4, 1) | 1 (−2, 3) |
| Model 2 | 1 (−2, 4) | −1 (−4, 2) | −1 (−4, 2) | 0 (−2, 3) |
| Model 3 | 1 (−2, 4) | −1 (−4, 2) | −1 (−4, 2) | 0 (−2, 3) |
| Ascending aorta | ||||
| Model 1 | −1 (−3, 1) | 1 (−1, 3) | 0 (−2, 2) | 0 (−2, 2) |
| Model 2 | −1 (−3, 1) | 1 (−1, 3) | 0 (−2, 2) | 0 (−2, 2) |
| Model 3 | −1 (−3, 1) | 1 (−1, 3) | 0 (−2, 2) | 0 (−2, 2) |
| Descending aorta | ||||
| Model 1 | 3 (−2, 8) | −3 (−7, 1) | −8 (−11, −4)* | 6 (2, 11) |
| Model 2 | 3 (−2, 8) | −3 (−7, 1) | −7 (−11, −3)* | 6 (2, 10) |
| Model 3 | 3 (−2, 7) | −3 (−7, 1) | −7 (−11, −3)* | 6 (2, 10) |
CI, Confidence intervals; MESA, Multi-Ethnic Study of Atherosclerosis; SHBG, Sex hormone binding globulin.
Percent change was derived from linear mixed effect models and calculated by ([Exp (β) −1]*100).
Extra-coronary calcification was analyzed as ln(ECC+1).
The independent variables (sex hormones) were natural log-transformed and modeled per one standard deviation.
Results in bold font indicate statistical significance at p <0.05.
Results in bold font with asterisk indicate Bonferroni corrected statistical significance at p <0.002.
Model 1: adjusted for age, race/ethnicity and MESA study site.
Model 2: adjusted for model 1 covariates plus education, physical activity, smoking, pack-years of smoking, BMI and health insurance.
Model 3: adjusted for model 2 covariates plus systolic blood pressure, use of anti-hypertensive medication, total cholesterol, HDL-C, use of lipid-lowering medication, diabetes mellitus and eGFR.
In women, model 2 was additionally adjusted for age at menopause and current use of hormone therapy.
3.3.2. DTAC:
Though the association between sex hormones and incidence and progression of DTAC was not significant in women, the strongest relationship of sex hormones among all ECC markers was observed with DTAC in men. Among men with no DTAC (score=0) at baseline, higher free T (per 1-SD) was significantly associated with a lower risk of incident DTAC in model 3 adjusted for lifestyle and CVD risk factors with an incident ratio of 0.86 (0.75, 0.99) (Table 4). Furthermore, low free T and higher SHBG was associated with 2-year progression of DTAC, a finding that remained unchanged and significant in further adjusted models (Table 5). Per 1-SD increment in free T, there was 7% less DTAC progression [−7% (−11, −3)] and per 1-SD increment in SHBG, there was 6% greater DTAC progression [6% (2, 10)].
3.3.3. AVC:
Our analysis found no association between sex hormones and incident AVC or 2-year change in AVC in any model for both women and men (Table 4 and 5).
3.3.4. MAC:
In women, higher free T and lower SBHG were positively associated with incident MAC in model 1, but these associations were attenuated in subsequent models adjusted for lifestyle factors, CVD risk factors and medications (Table 4). Furthermore, no association was observed between sex hormones and 2-year change in MAC score in women. For men, there was no association of MAC incidence or progression with free T or SHBG.
3.4. Other comparisons and sensitivity analysis
We conducted a sensitivity analysis for the association of sex hormones with incident ECC and 2-year progression after excluding women who were currently on hormone therapy. This showed similar results for free T and SHBG for DTAC, AVC, and MAC, except for the incidence ratios for SHBG for ATAC which is now statistically significant in model 3 (Supplemental Table 1).
We also examined the associations of the other sex hormones of total T, E2, and DHEA with ECC and did find some associations in the most-adjusted models (Supplemental Tables 2-5). Notably, lower DHEA was associated with MAC prevalence in women. Higher E2 was associated with decreased prevalent DTAC, decreased incident MAC, and increased incident ATAC in women. Total T was associated with decreased incident DTAC. In men, higher DHEA was associated with less AVC extent in men, and higher total T being associated with less DTAC extent. In men, higher total T and DHEA were both associated with decreased progression of MAC and DTAC, and higher E2 associated with decreased progression of DTAC. Again, we consider these exploratory analyses since our a priori hypotheses focused on free T and SHBG, presented in main results.
We also conducted a supplemental model analysis with the sex hormones (Total T, E2 and SHBG) adjusted for each other and all included in the same model (Supplemental Tables 6 and 7). There were some statistically significant associations noted; although no consistent pattern of sex hormones across the various ECC measures.
4. Discussion
Our study demonstrated certain associations of sex hormones with measures of ECC in this community-based cohort of individuals free of baseline CVD. In women, greater free T and low SHBG were associated with incident MAC in a demographic-adjusted model but were no longer statistically significant after adjustment for CVD risk factors. In men, greater SHBG and low free T were associated with prevalent MAC, low free T with incident DTAC, and greater SHBG and low free T with DTAC progression in the most adjusted models. No association was found between sex hormones and AVC or ATAC in either men or women.
Prior studies have implicated sex hormones to be related to CVD risk. Among post-menopausal women, a more androgenic profile was found to be associated with increased concentric left ventricular remodeling over 10 years,29 greater prevalence of endothelial dysfunction,30 increased aortic stiffness,31 and higher NT-proBNP levels.27 Moreover, among postmenopausal women in MESA, the more androgenic profile of higher T/E2 ratio was associated with an increased risk for incident total CVD, CHD, and HF events, while higher estradiol was associated with a decreased risk for CHD and HF with reduced ejection fraction.24 These same associations were not found or were attenuated in men.29,30 Thus, sex differences in endogenous sex hormone profiles may explain the observed differences in cardiovascular outcomes among women and men.37
Regarding the relationship of sex hormones and subclinical atherosclerosis, specifically, prior work in MESA has also examined the cross-sectional relation between sex hormones and markers of atherosclerosis and found higher T and lower SHBG to be associated with carotid artery intimal-medial thickness and CAC in post-menopausal women.38 While in that same cross-sectional study, higher SHBG and lower T were associated with greater baseline CAC score among women with CAC >0.38 Another study evaluated the cross-sectional association between sex hormones and abdominal aortic calcification (AAC) and suggested an inverse association of SHBG with the presence and extent of AAC in women.39 Those studies were limited by cross-sectional design. In a prospective follow-up in MESA, we previously found that higher free T and lower SHBG were associated with CAC progression over 10 years among postmenopausal women.25 Thus, given the close association of CAC with ECC, we focused primarily on the association of these two hormones (free T and SHBG) with ECC progression as well. Although some significant associations were found between sex hormones and ECC presence and/or progression that were independent of CVD risk factors, the relationships were not consistent between cross-sectional and longitudinal analyses, and also there were inconsistencies in the associations depending on how we modeled sex hormones in our sensitivity analyses. These findings suggest the role of sex hormones and atherosclerosis is complex, and ECC progression could not easily be attributed to one hormone pattern vs another. Also, ECC, particularly valvular calcification, is not as closely tied to atherosclerosis as CAC, and this may be another reason we did not find the same associations of free T and SHBG with ECC progression that we previously found with CAC.25
Even if sex hormone levels were causally linked to atherosclerosis progression, it is unclear what the best intervention is to modify sex hormone levels for risk reduction. Contrary to observational studies, randomized clinical trials failed to show a beneficial effect of hormone therapy on CVD risk in generally older post-menopausal women.40-42 In contrast, among younger post-menopausal women age 50-59 years who were closer to the menopausal transition, estrogen therapy during the Women’s Health Initiative trial was associated with lower burden of CAC at trial completion.43 However, given overall unfavorable results in these trials, currently hormone therapy is not recommended for the sole purpose of CVD prevention. While a sex hormone profile might be a useful marker in identifying those at risk for atherosclerosis progression, our findings are not robust enough to recommend sex hormone screening in clinical practice for the purposes of CVD risk stratification. Nevertheless, we undertook this study to try to better understand sex-differences in atherosclerosis progression and CVD risk from a mechanistic standpoint as the relationship of sex hormones with atherosclerosis progression across vascular beds had not previously been well established.
4.1. Limitations and strengths
Our findings should be considered with several limitations. Sex hormones were measured only once at baseline, so we could not examine for change in sex hormone levels. There were very few pre-menopausal women in MESA, whom we excluded from our analyses, so we could not examine the relationship of sex hormones with ECC in the pre-menopausal period. The selection bias and temporal bias are limitations for observational analysis. There may be survival bias in this prospective cohort, although we examined ECC change over a relatively short period of 2.4 years. We performed multiple statistical tests of several sex hormones with multiple measures of ECC, so findings may be due to chance. However, we tried to focus on two sex hormones in particular based on strength of evidence from prior work. Furthermore, we considered all associations with cautious interpretation as exploratory, and we hope the patterns we found can be confirmed in further research.
Despite these limitations, our study had many strengths. To our knowledge, this is the first study that comprehensively examined sex hormone levels with longitudinal changes with 4 distinct ECC measures (AVC, MAC, DTAC, and ATAC), which each have different prognostic value for CVD events. We examined these relationships among both men and women in an ethnically diverse cohort of individuals who had comprehensive assessment of risk factors for covariate adjustment.
4.2. Conclusion
In sum, our study helped further characterize sex differences in CVD risk by assessing the association between sex hormones level and ECC prevalence and progression in men and women. Although the clinical implication of these results is uncertain, these findings add to our existing knowledge on sex-specific CVD risk that may shape future work in this area.
Supplementary Material
Highlights.
Sex hormones (SH) may explain sex differences in cardiovascular disease (CVD)
Extra coronary calcium (ECC) and its progression are markers of subclinical atherosclerosis and prognostic of CVD risk.
In a multi-ethnic cohort free of baseline CVD, we examined the association of SH with ECC measured by cardiac CT.
We found some associations of SH with ECC measures which differed by sex and differed across vascular beds, although the clinical significance is uncertain.
Our findings suggest SH may influence vascular calcification.
Acknowledgements
The authors thank the other investigators, the staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Financial support
This research was supported by R01 HL071739, R01 HL074406, and R01 HL074338. MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI), and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. Drs. Michos is additionally supported by the Amato Fund for Women’s Cardiovascular Health research at Johns Hopkins University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
Unrelated to this work, Dr. Michos reports Advisory Boards for Astra Zeneca, Amarin, Esperion, Bayer, Novartis, and Novo Nordisk. Dr. Budoff receives grant support from General Electric. The other authors have no disclosures to make.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241(1):211–218. [DOI] [PubMed] [Google Scholar]
- 3.McKibben RA, Al Rifai M, Mathews LM, Michos ED. Primary Prevention of Atherosclerotic Cardiovascular Disease in Women. Curr Cardiovasc Risk Rep. 2016;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michos ED, Blaha MJ, Blumenthal RS. Use of the Coronary Artery Calcium Score in Discussion of Initiation of Statin Therapy in Primary Prevention. Mayo Clin Proceed. 2017;92(12):1831–1841. [DOI] [PubMed] [Google Scholar]
- 5.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kizer JR, Wiebers DO, Whisnant JP, et al. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study. Stroke. 2005;36(12):2533–2537. [DOI] [PubMed] [Google Scholar]
- 7.Fox CS, Vasan RS, Parise H, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107(11):1492–1496. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Plehn JF, D'Agostino RB, et al. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327(6):374–379. [DOI] [PubMed] [Google Scholar]
- 9.Owens DS, Budoff MJ, Katz R, et al. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. 2012;5(6):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budoff MJ, Nasir K, Katz R, et al. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 2011;215(1):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann DM, Lehmann N, Gronewold J, et al. Thoracic aortic calcification is associated with incident stroke in the general population in addition to established risk factors. Eur Heart J Cardiovasc Imaging. 2015;16(6):684–690. [DOI] [PubMed] [Google Scholar]
- 12.Tison GH, Guo M, Blaha MJ, et al. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2015;9(5):406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fashanu OE, Bizanti A, Al-Abdouh A, et al. Progression of valvular calcification and risk of incident stroke: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2020;307:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison MA, Hsi S, Wassel CL, et al. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32(1):140–146. [DOI] [PubMed] [Google Scholar]
- 15.Bos D, Leening MJ, Kavousi M, et al. Comparison of Atherosclerotic Calcification in Major Vessel Beds on the Risk of All-Cause and Cause-Specific Mortality: The Rotterdam Study. Circ Cardiovasc Imaging. 2015;8(12). [DOI] [PubMed] [Google Scholar]
- 16.Jacobs PC, Prokop M, van der Graaf Y, et al. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high-risk population of heavy smokers. Atherosclerosis. 2010;209(2):455–462. [DOI] [PubMed] [Google Scholar]
- 17.Hamirani YS, Nasir K, Blumenthal RS, et al. Relation of mitral annular calcium and coronary calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2011;107(9):1291–1294. [DOI] [PubMed] [Google Scholar]
- 18.Elmariah S, Budoff MJ, Delaney JA, et al. Risk factors associated with the incidence and progression of mitral annulus calcification: the multi-ethnic study of atherosclerosis. Am Heart J. 2013;166(5):904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral Annulus Calcification. J Am Coll Cardiol. 2015;66(17):1934–1941. [DOI] [PubMed] [Google Scholar]
- 20.Kanjanauthai S, Nasir K, Katz R, et al. Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2010;213(2):558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dirrichs T, Penzkofer T, Reinartz SD, Kraus T, Mahnken AH, Kuhl CK. Extracoronary Thoracic and Coronary Artery Calcifications on Chest CT for Lung Cancer Screening: Association with Established Cardiovascular Risk Factors - The "CT-Risk" Trial. Acad Radiol. 2015;22(7):880–889. [DOI] [PubMed] [Google Scholar]
- 22.Kalsch H, Mahabadi AA, Moebus S, et al. Association of progressive thoracic aortic calcification with future cardiovascular events and all-cause mortality: ability to improve risk prediction? Results of the Heinz Nixdorf Recall (HNR) study. Eur Heart J Cardiovasc Imaging. 2019;20(6):709–717. [DOI] [PubMed] [Google Scholar]
- 23.Fashanu OE, Upadhrasta S, Zhao D, et al. Effect of Progression of Valvular Calcification on Left Ventricular Structure and Frequency of Incident Heart Failure (from the Multiethnic Study of Atherosclerosis). Am J Cardiol. 2020;134:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao D, Guallar E, Ouyang P, et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J Am Coll Cardiol. 2018;71(22):2555–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanya V, Zhao D, Ouyang P, et al. Association of endogenous sex hormone levels with coronary artery calcium progression among post-menopausal women in the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomogr. 2019;13(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 27.Ying W, Zhao D, Ouyang P, et al. Sex Hormones and Change in N-Terminal Pro-B-Type Natriuretic Peptide Levels: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2018;103(11):4304–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. [DOI] [PubMed] [Google Scholar]
- 29.Subramanya V, Zhao D, Ouyang P, et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas. 2018;108:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathews L, Subramanya V, Zhao D, et al. Endogenous Sex Hormones and Endothelial Function in Postmenopausal Women and Men: The Multi-Ethnic Study of Atherosclerosis. J Womens Health. 2019;28(7):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanya V, Ambale-Venkatesh B, Ohyama Y, et al. Relation of Sex Hormone Levels With Prevalent and 10-Year Change in Aortic Distensibility Assessed by MRI: The Multi-Ethnic Study of Atherosclerosis. Am J Hypertens. 2018;31(7):774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budoff MJ, McClelland RL, Chung H, et al. Reproducibility of coronary artery calcified plaque with cardiac 64-MDCT: the Multi-Ethnic Study of Atherosclerosis. AJR Am J Roentgenol. 2009;192(3):613–617. [DOI] [PubMed] [Google Scholar]
- 33.Budoff MJ, Takasu J, Katz R, et al. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13(2):166–172. [DOI] [PubMed] [Google Scholar]
- 34.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 35.Kianoush S, Al Rifai M, Cainzos-Achirica M, et al. Thoracic extra-coronary calcification for the prediction of stroke: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2017;267:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezeigwe A, Fashanu OE, Zhao D, et al. The novel inflammatory marker GlycA and the prevalence and progression of valvular and thoracic aortic calcification: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019;282:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6(8):532–542. [DOI] [PubMed] [Google Scholar]
- 38.Ouyang P, Vaidya D, Dobs A, et al. Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;204(1):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michos ED, Vaidya D, Gapstur SM, et al. Sex hormones, sex hormone binding globulin, and abdominal aortic calcification in women and men in the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 2008;200(2):432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. [DOI] [PubMed] [Google Scholar]
- 41.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 42.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–1477. [DOI] [PubMed] [Google Scholar]
- 43.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–2602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


