Abstract
Background:
Postoperative delirium and postoperative cognitive dysfunction are the most common complications for older surgical patients. General anesthesia may contribute to the development of these conditions but there are little data on the association of age with cognitive recovery from anesthesia in the absence of surgery or underlying medical condition.
Methods:
We performed a single-center cohort study of healthy adult volunteers aged 40–80 years old (N = 71, mean age 58.5 years, 44% women) with no underlying cognitive dysfunction. Volunteers underwent cognitive testing before and at multiple time points after 2 hours of general anesthesia consisting of propofol induction and sevoflurane maintenance, akin to a general anesthetic for a surgical procedure although no procedure was performed. The primary outcome was time to recovery to cognitive baseline on the Postoperative Quality of Recovery Scale (PQRS) within 30 days of anesthesia. Secondary cognitive outcomes were time to recovery on in-depth neuropsychological batteries including the National Institutes of Health Toolbox and well-validated paper-and-pencil tests. The primary hypothesis is that time to recovery of cognitive function after general anesthesia increases across age decades from 40 to 80 years old. We examined this with discrete-time logit regression (for the primary outcome) and linear mixed models for interactions of age decade with time post-anesthesia (for secondary outcomes).
Results:
There was no association between age group and recovery to baseline on the PQRS; 36/69 (52%) recovered within 60 minutes post-anesthesia and 63/69 (91%) by day 1. Hazard ratios [95% confidence interval]) for each decade compared to 40–49 year olds were: 50–59 years, 1.41 [0.50, 4.03]; 60–69 years, 1.03 [0.35, 3.00]; 70–80 years, 0.69 [0.25, 1.88]. There were no significant differences between older decades relative to the 40–49 year reference decade in recovery to baseline on secondary cognitive measures.
Conclusions:
Recovery of cognitive function to baseline was rapid and did not differ between age decades of participants, although number in each decade were small. These results suggest that anesthesia alone may not be associated with cognitive recovery in healthy adults of any age decade.
Introduction
Postoperative neurocognitive disorders are the most common complications after surgery for older adults1. The two major types are postoperative delirium (POD), seen in 10–60%, and postoperative cognitive dysfunction (POCD), experienced by 15–30%2–6. The former is an acute attentional deficit and the latter is a decline in cognitive ability relative to pre-surgery levels. The etiology for both is unknown. Earlier literature postulated that anesthetic agents might be neurotoxic including the acceleration of the biochemistry of Alzheimer’s disease by common anesthetic agents7. However, postoperative neurocognitive disorders are not related to type of anesthetic drug or even the use of general versus regional anesthesia8,9 suggesting that some aspect of surgery or illness, rather than general anesthesia, is the critical risk factor. However, it is difficult to identify the specific role of anesthesia in postoperative neurocognitive disorders because previous studies have not separated the need for surgery and the surgical procedure, which may be risk factors in their own right, from the anesthetic agent.
Information regarding cognitive recovery is important for a population of patients at risk for postoperative cognitive alteration who are frequently sent home on the day of surgery with instructions for self-care. The controversy over delayed recovery of cognitive function after anesthesia may impact the willingness of patients to undertake surgery, preventing them from accessing appropriate life-enhancing therapies. From a research and intervention perspective, distinguishing the effects of anesthesia from those of surgery can provide a basis for interpreting studies of postoperative cognition in surgical populations. However, there is limited information regarding cognition after anesthesia alone.
To address the specific role of anesthesia in the absence of surgery on cognition in older adults, who have been identified at higher risk of postoperative neurocognitive disorders, we assessed cognitive recovery after general anesthesia in healthy adult volunteers by decade from 40–80 years old10. The primary hypothesis is that the time to recovery increases across age decades from 40 to 80 years old, based on evidence that postoperative neurocognitive disorders are related to surgical stress and not anesthesia. Specifically we examined whether there was an association between age group and time to recovery after adjusting for available confounding variables (gender, race, education). We conducted this study in healthy, non-surgical participants with normal cognition at baseline to focus on changes after anesthesia without surgery and without other confounds of frailty or preexisting cognitive impairment.
Methods
This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (New York, NY, USA; IRB@mssm.edu, 212–824-8200) and registered at ClinicalTrials.gov (NCT02275026, date of registration October 27, 2014, principal investigator first Jeffrey Silverstein MD, succeeded by Joshua Mincer, MD, PhD) prior to beginning participant recruitment. Full details of the study protocol are published including the statistical analysis plan10. Participants were recruited between February 2015 and April 2019 through local contacts and IRB-approved advertisements in local media and online. Potential participants were pre-screened by telephone by both research staff and a study anesthesiologist. Informed written consent was obtained by participants at the first in-person visit. Specific inclusion criteria were adults aged 40–80, American Society of Anesthesiologists (ASA) Physical Status 1 (no medical comorbidities) or 2 (one or more medical comorbidities which do not impact the patient’s function), and no underlying cognitive dysfunction as determined from baseline cognitive testing before general anesthesia. Exclusion criteria included contraindication to magnetic resonance imaging (MRI) scanning (implanted metal, presence of tattoos, claustrophobia), current smoking, use of illicit drugs, excessive use of alcohol, or other diseases that could affect response to anesthesia or alter brain physiology10. Participants were excluded after recruitment and consent if the scan prior to anesthesia revealed any of the following: cerebral microvascular disease, any mass, evidence of old infarct (even without clinical signs), atrophy and/or ventriculomegaly greater than expected for age in the neuroradiologist’s judgment. Age-appropriate changes, such as mild cortical atrophy, were not grounds for exclusion. Participants were also excluded if baseline neuropsychological testing suggested poor or abnormal baseline cognitive function in the judgment of the study neuropsychologist (M. Sewell).
Participants underwent a battery of cognitive tests prior to and at specific time points (detailed below) after exposure to 2 hours of general anesthesia. This duration of anesthesia was selected as it represents the duration of a wide range of surgical procedures. The primary outcome was recovery to baseline on the Postoperative Quality of Recovery Scale (PQRS) cognitive subtest, used for rapid assessment of short-term recovery11. The PQRS cognitive subtest consists of 5 items: name, place, and date of birth; forward digit span testing; backward digit span testing; recalling a list of words; and generating words beginning with a specific letter). This test is scored as a binary “recovered / not recovered” outcome based on whether post-anesthesia scores return to the pre-anesthesia level, within a certain tolerance range12. This test performs equivalently in psychometric terms when given in person or over the phone. Secondary outcomes were measures from in-depth neuropsychological testing that covered the domains of executive function and attention, episodic memory, language, processing speed, and working memory. Instruments for this testing were the National Institutes of Health (NIH) Toolbox Cognitive Battery13,14 and “paper-and-pencil” neuropsychological tests from the Alzheimer’s Disease Research Center Uniform Dataset Battery: Trail Making Test (parts A and B), Logical Memory (immediate and delayed recall), and Category Fluency15 as well as the California Verbal Learning Test (CVLT). For secondary measures, we used raw test scores on paper-pencil tests, and fully-adjusted T-scores (mean 50, standard deviation 10, adjusted for gender, age, level of education, and race/ethnicity) computed by the test software for each NIH Toolbox Cognitive Battery test and summed to create a composite score. We also analyzed the 7 subtests of the NIH Toolbox Cognitive Battery (dimensional change card sort, flanker inhibitory control and attention, picture sequence memory, list sorting working memory, oral reading recognition, picture vocabulary, and pattern comparison processing speed). Details of specific timing of test administration follow.
Testing protocol and anesthesia exposure.
Within a week before anesthesia, participants underwent baseline cognitive testing (PQRS, NIH Toolbox Cognitive Battery, paper-and-pencil neuropsychological tests), and preanesthesia evaluation by an anesthesiologist. On the day of anesthesia, they first underwent a series of MRI scans while awake, including an MRI anatomical pre-anesthesia scan that was reviewed by a neuroradiologist for evidence of intracranial pathology as well as task-based and resting-state fMRI scans.
After the completion of pre-anesthesia scanning, a 22 gauge intravenous (IV) line was placed. Following application of standard ASA monitors and preoxygenation, anesthesia was induced in the MRI suite with intravenous propofol at a weight and age adjusted dose, after which a laryngeal mask airway (LMA) was placed. Anesthesia was maintained with inhaled sevoflurane at an age-adjusted depth of 1 minimum alveolar concentration (MAC). A Bispectral Index (BIS) level of 40–60 was obtained after LMA placement to aid in assessment of anesthetic depth while the participant was positioned and secured for MRI scanning, during which time inhaled sevoflurane equilibrated. After equilibration, the participant was returned to the MRI bore. Multiple MRI scan sequences were carried out over a ~2-hour period while general anesthesia was maintained. Ventilation was maintained to achieve a target end tidal CO2 of 30–35 mm Hg. Mean arterial blood pressure was maintained within 20% of baseline with bolus administration with ephedrine (5 mg IV or 25 mg IM) or phenylephrine (100 μg IV) as needed. No narcotics, benzodiazepines, or muscle relaxants were administered, so that any differences between age decades in recovery of cognitive function could be attributed to the general anesthetic drugs specifically. Ondansetron (4 mg, IV) was given prior to emergence for antiemetic prophylaxis. The LMA was removed at the end of the scan protocol in the MRI suite when the participant awakened.
Once the participant emerged (generally within 15 minutes), PQRS was performed. The participant was then returned to the MRI bore for scan acquisition, and approximately 1 hour after emergence from anesthesia PQRS was repeated. Participants were brought to the postanesthesia care recovery unit where they were monitored until discharge. Each participant performed follow-up cognitive testing and MRI scanning at 1 day and 7 days later, as well as additional in-person cognitive testing at 30 days, including the PQRS and all secondary cognitive measures. Additionally, the PQRS was administered via telephone at 3 days after anesthesia. Thus PQRS data were available at 6 time points after anesthesia (15 minutes, 60 minutes, 1 day, 3 days, 7 days, 30 days) for assessment of recovery to baseline within 30 days of anesthesia exposure, and secondary measures (NIH Toolbox Cognitive Battery and paper-and-pencil tests) were given at 1 day, 7 days, and 30 days after anesthesia, in addition to the baseline testing. Although the focus of the study was on cognitive recovery over the 30 days post anesthesia, the PQRS was administered at 6 and 12 months post anesthesia as well. This manuscript reports the full analyses of the cognitive primary and secondary endpoints for this study. Reports of neuroimaging findings will be published separately. We have already reported a pattern of anticorrelated resting state functional MRI activity in the early post-anesthesia recovery period that did not vary by age decade16.
Statistical Analysis.
Descriptive statistics by age decade were calculated. All analyses were adjusted for covariates: gender (male/female), level of education (less than 16 years / 16 years or more) and race (white / non-white). We treated age decade as a categorical variable (40–49 years referent decade, 50–59 years, 60–69 years, 70–80 years) for cognitive outcomes to allow for possible nonlinear (quadratic, cubic) associations with age decade. This decision was taken prior to data collection, although our power analysis conducted prior to beginning the study used a straightforward linear effect (see “Sample size calculation”). Discrete-time logit regression, adjusted for covariates, was used to test whether each age decade differed in the time to recovery relative to the youngest age decade (40 to 49) on the primary outcome (PQRS cognitive scale). Discrete-time logit regression can be applied when time is measured at a discrete (not continuous) time scale; thus, it accommodates multiple persons having the same apparent time the event occurs10,17,18. All participants with baseline PQRS data were included. For analyses of the time course of the secondary cognitive outcome measures, we used linear mixed models (LMMs) with a spatial power covariance structure of repeated observations within participant, and person-specific random intercepts. These models assume that the data are missing at random; thus, participants with missing observations contribute to the model estimation. LMMs for secondary outcomes included the same covariates, baseline and an interaction term for age decade by days post-anesthesia. This interaction term allowed for comparison to the referent decade at each post-anesthesia observation. Least squares mean score differences of each age decade to the referent decade were estimated for each day post-anesthesia. This allows for interpretation on the scale of each outcome. As a sensitivity analysis, we estimated discrete-time logit regression models, as for the primary outcome of the PQRS cognitive scale, on whether participants returned to their baseline score on each secondary measure. By treating age as categories based on decades, we were able to have non-linear effects without having to add quadratic or cubic terms to the statistical models. Analyses were performed in SAS® 9.4 (SAS Institute, Cary NC) with two-sided tests.
Sample size calculation.
Sample and power for primary and secondary outcomes were calculated with PASS1219. Returning to baseline PQRS over 30 days required 72 participants to detect a hazard ratio of 1.03 per year for age (β=0.033), assuming a standard deviation for age of 11.2 years, 80% power, a Type I error of 0.05, and adjusting for other characteristics expected to have a generalized R2 of 0.2. This can be interpreted as approximately 3% slower recovery per year relative to 40 year-old participants. For secondary outcomes, although there were fewer time points, the effect-size calculation is similar. We powered this study based on 7 secondary cognitive outcome measures (6 paper-and-pencil tests and the NIH Cognitive Battery composite score). Assuming all 72 participants would return to baseline over 30 days, a Bonferroni adjusted Type 1 error rate of 0.05/7=0.0071, and other covariates having a generalized R2 of 0.2 with age, a hazard ratio of 1.05 (β=0.05) could be detected with 80% power and a familywise Type 1 error of 0.05 (after Bonferroni adjustment 0.0071 for each outcome). Pilot data used for study planning had 3 of 4 participants return to baseline at 15 minutes after anesthesia and all 4 by 7 days. Based on these calculations, planned enrollment (based on completion of the anesthesia session) was 19 participants per age decade (40–49 years, 50–59 years, 60–69 years, 70–80 years), as 18 per age decade achieved adequate power and an additional participant per age decade was included in the event of a dropout.
Results
We met enrollment targets, based on completion of the anesthesia session, in all decades except the 60–69 year old participants. Recruitment lagged in this age decade because of difficulties in identifying prospective participants who met our inclusion criteria and were able to commit the time required to complete the study. Following consultation with the study Data and Safety Monitoring Board and the study biostatistician (H.A.) in April 2019, we closed enrollment for the study in May 2019 with 13 out of 19 planned 60–69 year old participants based on the fact that enrollment for the other three decades was already complete and recruitment of the remaining planned participants in the 60–69 year old decade would have been highly unlikely to have changed the primary outcome. A CONSORT diagram for the entire study is presented in Figure 1, and participant demographics and baseline characteristics in Table 1.
Figure 1.

CONSORT Diagram
Table 1.
Demographics of study participants
| Characteristics | Age Decade (years) Number of Participants (%) | P-value | ||||
|---|---|---|---|---|---|---|
| 40–49 | 50–59 | 60–69 | 70–80 | |||
| Total Enrolled: | 20 | 19 | 13 | 19 | ||
| Gender | Male | 9 (45.0) | 14 (73.7) | 7 (53.8) | 10 (52.6) | .32 |
| Female | 11 (55.0) | 5 (26.3) | 6 (46.2) | 9 (47.4) | ||
| Ethnicity | Hispanic or Latino | 5 (25.0) | 3 (15.8) | 1 (7.7) | 1(5.3) | .30 |
| Not Hispanic or Latino | 15 (75.0) | 16 (84.2) | 12 (92.3) | 18 (94.7) | ||
| Race | Black or African American | 12 (60.0) | 11 (57.9) | 3 (23.1) | 5 (26.3) | .014* |
| White | 6 (30.0) | 7 (36.8) | 9 (69.2) | 14 (73.7) | ||
| Other | 2 (10.0) | 1 (5.3) | 1 (7.7) | 0 | ||
| Education | High school (12 years) | 3 (15.0) | 3 (15.8) | 0 | 2 (10.5) | .007** |
| Some college (13–15 years) | 4 (20.0) | 11 (57.9) | 2 (15.4) | 5 (26.3) | ||
| College (16 years) | 8 (40.0) | 2 (10.5) | 8 (61.5) | 6 (31.6) | ||
| More than college (16+ years) | 5 (25.0) | 3 (15.8) | 3 (23.1) | 6 (31.6) | ||
| Age (years) | Mean | 45.5 | 54.9 | 64.9 | 73.4 | < .005 |
| Median | 45.57 | 54.95 | 64.1 | 72.6 | ||
| Standard Deviation | 2.7 | 2.9 | 3.3 | 3.4 | ||
| Minimum | 40.1 | 50.8 | 60.0 | 70.0 | ||
| Maximum | 49.9 | 59.8 | 69.5 | 80.7 | ||
| American Society of Anesthesiologists Status | 1 | 16 (80.0) | 17 (89.5) | 12 (92.3) | 12 (63.2) | .13 |
| 2 | 4 (20.0) | 2 (10.5) | 1 (7.7) | 7 (36.8) | ||
| Premorbid Medical Conditions | 0 | 16 (80.0) | 18 (94.7) | 9 (69.2) | 13 (68.4) | .18*** |
| 1 | 3 (15.0) | 1 (5.3) | 3 (23.1) | 4 (21.1) | ||
| 2 | 0 | 0 | 1 (7.7) | 2 (10.5) | ||
| 3+ | 1 (5.0) | 0 | 0 | 0 | ||
| Number of Medications on Initial Assessment | 0 | 18 (90.0) | 18 (94.7) | 9 (69.2) | 15 (79.9) | .19**** |
| 1 | 1 (5.0) | 0 | 2 (15.4) | 1 (5.2) | ||
| 2 | 1 (5.0) | 1 (5.3) | 1 (7.7) | 3 (15.8) | ||
| 3+ | 0 | 0 | 1 (7.7) | 0 | ||
white vs non-white
< 16 years vs >= 16 years
0 vs < 0
0 vs > 0
The paper-and-pencil and NIH Toolbox cognitive test composite scores showed similar preanesthesia baseline performance across age decades, confirming that even our oldest participants were cognitively healthy prior to anesthesia. The only test in which older participants performed significantly worse compared to 40–49 year old participants at baseline was Trails B. On average the oldest decade took longer to complete this test, although not greater than national norms for their age.
Primary outcome.
Two participants had missing or incomplete baseline PQRS data: one (40–49 year old decade) did not receive the PQRS at baseline because of a research coordinator error, and one (70–80 year old decade) refused one of the cognitive questions during the baseline and Day 1 tests, so time to recover on PQRS could not be determined for these participants. There was no overall association of age decade with time to recovery to baseline on the PQRS cognitive scale (N = 69 cases with complete data): Wald χ2(3 df) = 1.83, p = 0.609. None of the age decades significantly differed relative to the 40–49 year old participants; parameter estimates (hazard ratios) for each of the other decades compared to 40–49 year olds were: 50–59 years, (hazard ratio 1.41; 95% confidence interval [CI], 0.50 to 4.03; P = 0.517), 60–69 years (hazard ratio 1.03; 95% CI, 0.35 to 3.00; P = 0.963), or 70–80 years (hazard ratio 0.69; 95% CI; 0.25 to 1.88; P = 0.470). The vast majority (91%) of participants returned to baseline cognitive performance within 1 day of anesthesia (Table 2, Figure 2). The rapid rate of recovery to baseline accounts for the wide confidence intervals on these measures; a design with more measurement points within the first day would improve resolution, but such differences are unlikely to be clinically meaningful. Two participants did not recover to baseline by day 30. One of these (60–69 year old decade) returned within the tolerance limit of baseline performance for each of the 5 PQRS cognitive test items at least once during post-anesthesia testing, but never all 5 during the same test administration, the criterion for recovery to baseline. The other (40–49 year old decade) scored high on one cognitive test item (word generation) during the baseline test and did not return to this level in post-anesthesia testing. Performance on all the other neuropsychological tests for these two participants was equal to or better than age norms and within a standard deviation of their baseline performance, so there was no evidence of postoperative cognitive dysfunction in these two participants. Both of these participants had returned to baseline on the PQRS at the 6-month PQRS follow-up test.
Table 2.
Cumulative number and proportion of participants recovered on Postoperative Quality of Recovery Cognitive Scale at each study timepoint by age decade. Postoperative Quality of Recovery Cognitive Scale could not be determined for 2 participants (1 in the 40–49 year age decade, one in the 70–80 year age decade), bringing total sample to N = 69.
| Age decade | 15 min | 60 min | 1 day | 3 days | 7 days | 30 days |
|---|---|---|---|---|---|---|
| number / total number (%) | ||||||
| 40–49 | 3/19 (15.8) | 8/19 (42.1) | 17/19 (89.5) | 18/19 (94.7) | 18/19 (94.7) | 18/19 (94.7) |
| 50–59 | 5/19 (26.3) | 11/19 (57.9) | 18/19 (94.7) | 19/19 (100) | 19/19 (100) | 19/19 (100) |
| 60–69 | 1/13 (7.0) | 8/13 (61.5) | 12/13 (92.3) | 12/13 (92.3) | 12/13 (92.3) | 12/13 (92.3) |
| 70–80 | 3/18 (16.7) | 9/18 (50.0) | 16/18 (88.9) | 17/18 (94.4) | 17/18 (94.4) | 18/18 (100) |
Figure 2.
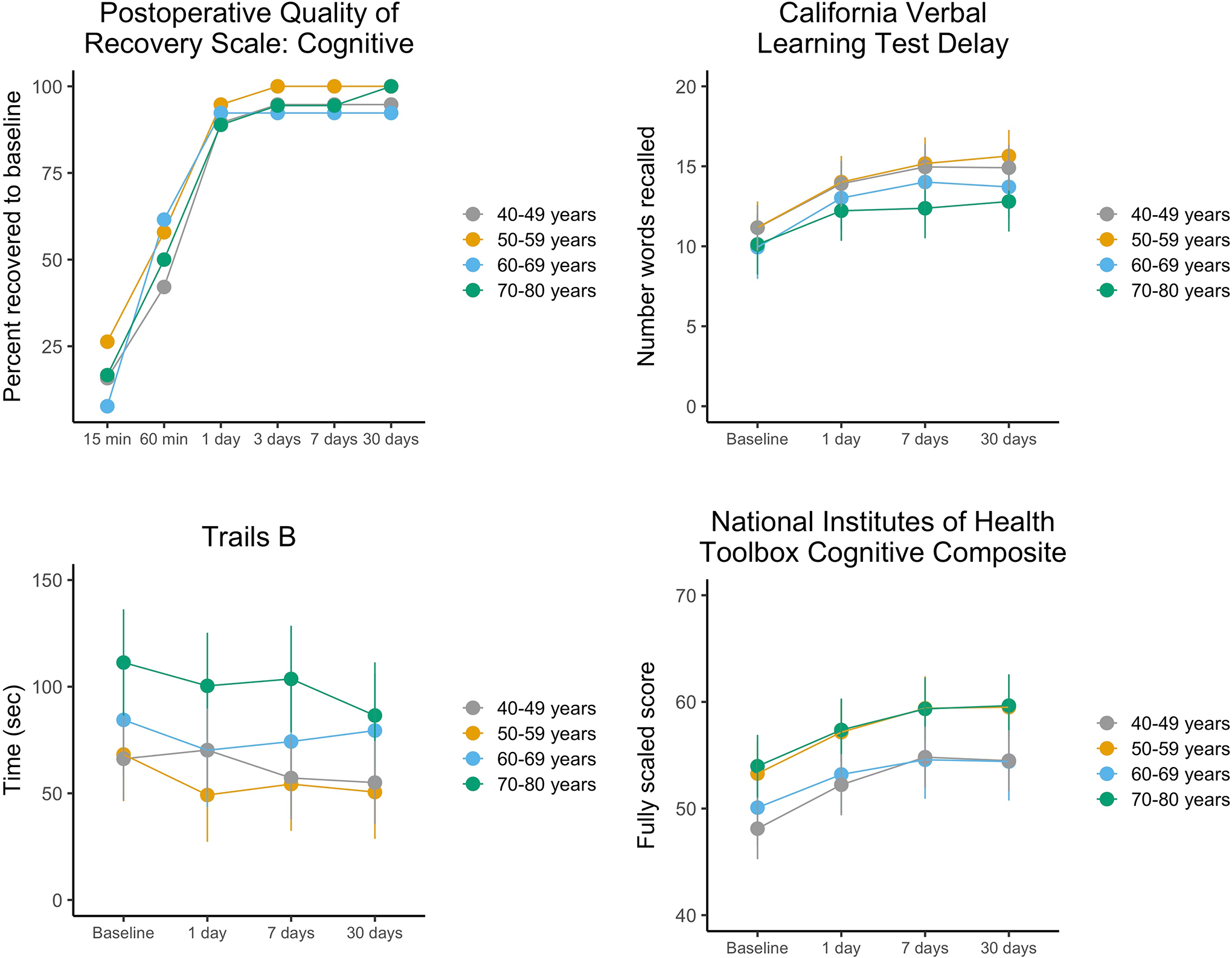
Performance on the primary outcome (time to recover to baseline on the PQRS) and selected secondary cognitive measures adjusted for gender, education and race and within-participant correlations over time. Adjustment for multiple comparisons were made with the Bonferroni procedure to preserve an overall two-sided Type 1 error rate at 0.05. The least squares means and error bars indicating the associated 95% confidence intervals (1.96 times the standard error of the least squares means) are shown. Higher scores reflect better performance on CVLT and NIH Toolbox, and lower (faster) scores reflect better performance on Trails B. All age decade by time interactions were non-significant.
Secondary outcomes.
For the NIH toolbox composite, there was no evidence that age decade was associated with post-anesthesia test performance as a function of time, as would be the case if older participants tended to decline (or improve more slowly) in post-anesthesia testing as compared to 40–49 year old participants. The interactions between age decade and time point on the NIH Toolbox Cognitive composite score (mean across all 7 tests) and the paper-and-pencil tests were not significant (Ps > 0.16; Table 3). There were also no significant associations of age decade with the proportion of patients returning to their baseline score on any of these measures (Ps > 0.6, Table 4). Time to recovery on the PQRS (the primary outcome) and covariate-adjusted least-squares means for three selected cognitive measures (CVLT A delayed recall, Trail Making Test B time to complete, NIH Toolbox Cognitive Battery composite score) representing different cognitive domains and test modalities are illustrated in Figure 2, showing similar improvement in performance across time on all three secondary measures for all the age decades. We also analyzed each of the 7 subtests of the NIH Toolbox Cognitive Battery, which showed no associations of age decade with time to return to performance equal to or greater than their baseline score on any of the subtest measures. For scores on 6 of the 7 subtests, interactions between age decade and time were non-significant. For the Picture Sequence Memory Test, the interaction was significant (P = 0.0017). This test requires participants to place a sequence of images in order based on a verbal narrative. It includes 3 forms (different narratives and pictures), but our participants saw the same pictures on each of the 4 test occasions. As such, the age by time interaction on this test may reflect chance, a real residual effect of anesthesia, or age differences in strategy on this specific test. Detailed statistical results for analyses of primary and secondary outcomes are presented in Table 3 (LMMs for secondary outcomes) and Table 4 (time to recovery analyses for primary and secondary outcomes).
Table 3.
Summary of results for time point by age decade analyses (linear mixed models) of scores on each secondary outcome as well as the subtests of the NIH Toolbox Cognitive Battery. (The primary outcome is not designed for evaluation of raw score). Least-squares means are covariate-adjusted (gender, race, education). P-values shown are not adjusted for multiple comparisons.
| Test Scores | Least-squares mean (standard error) | |||||
|---|---|---|---|---|---|---|
| Age decade × time interaction | 40–49 years | 50–59 years | 60–69 years | 70–80 years | ||
| Secondary outcomes | N = 20 | N = 19 | N = 13 | N = 19 | ||
| NIH Toolbox Composite1 | F(9, 197) = 0.53, p = 0.85 | Baseline | 48.1 (1.5) | 53.3 (1.5) | 50.1 (1.9) | 54 (1.5) |
| 1 day | 52.2 (1.5) | 57.2 (1.5) | 53.2 (1.9) | 57.4 (1.5) | ||
| 7 days | 54.8 (1.5) | 59.4 (1.5) | 54.6 (1.9) | 59.3 (1.5) | ||
| 30 days | 54.5 (1.5) | 59.5 (1.5) | 54.4 (1.9) | 59.6 (1.5) | ||
| Logical memory immediate | F(9, 201) = 0.81, p = 0.61 | Baseline | 14.6 (0.8) | 14.4 (0.8) | 12.9 (1) | 14.2 (0.8) |
| 1 day | 17.9 (0.8) | 17.4 (0.8) | 15.8 (1) | 17.4 (0.8) | ||
| 7 days | 19.8 (0.8) | 19.5 (0.8) | 18 (1) | 18.2 (0.8) | ||
| 30 days | 19.8 (0.8) | 19.8 (0.8) | 17.4 (1) | 19.4 (0.8) | ||
| Category fluency - animals | F(9, 201) = 0.53, p = 0.85 | Baseline | 23.1 (1.6) | 22.8 (1.6) | 19.2 (2) | 19.9 (1.6) |
| 1 day | 23.6 (1.6) | 24.2 (1.6) | 21 (2) | 21.4 (1.6) | ||
| 7 days | 24.5 (1.6) | 23.3 (1.6) | 20.7 (2) | 21 (1.6) | ||
| 30 days | 24 (1.6) | 24.9 (1.6) | 20.5 (2) | 21.9 (1.6) | ||
| Trails A time | F(9, 201) = 0.47, p = 0.90 | Baseline | 30.1 (2.5) | 28.6 (2.6) | 28 (3.2) | 37.9 (2.6) |
| 1 day | 25.1 (2.5) | 26.5 (2.6) | 25.9 (3.2) | 35.3 (2.6) | ||
| 7 days | 25.6 (2.5) | 25.6 (2.6) | 23.8 (3.2) | 33.2 (2.6) | ||
| 30 days | 22.5 (2.5) | 23.9 (2.6) | 24.8 (3.2) | 32.1 (2.6) | ||
| Trails B time | F(9, 201) = 1.11, p = 0.36 | Baseline | 65.7 (8.5) | 68.1 (8.8) | 86 (10.7) | 112.5 (8.7) |
| 1 day | 69.8 (8.5) | 49.1 (8.8) | 71.7 (10.7) | 101.6 (8.7) | ||
| 7 days | 56.7 (8.5) | 54.2 (8.8) | 75.8 (10.7) | 104.8 (8.7) | ||
| 30 days | 54.6 (8.5) | 50.5 (8.8) | 81 (10.7) | 87.7 (8.7) | ||
| Logical memory delayed | F(9, 201) = 1.31, p = 0.24 | Baseline | 14.3 (0.8) | 13.1 (0.9) | 12.1 (1) | 13.7 (0.8) |
| 1 day | 17.3 (0.8) | 17.2 (0.9) | 14.6 (1) | 17 (0.8) | ||
| 7 days | 19.2 (0.8) | 18.9 (0.9) | 16.9 (1) | 18.1 (0.8) | ||
| 30 days | 18.8 (0.8) | 19.3 (0.9) | 18.1 (1) | 19 (0.8) | ||
| CVLT delayed recall | F(9, 201) = 1.46, p = 0.16 | Baseline | 10.7 (0.6) | 10.7 (0.6) | 9.2 (0.8) | 9.4 (0.6) |
| 1 day | 13.4 (0.6) | 13.5 (0.6) | 12.2 (0.8) | 11.5 (0.6) | ||
| 7 days | 14.5 (0.6) | 14.7 (0.6) | 13.2 (0.8) | 11.7 (0.6) | ||
| 30 days | 14.4 (0.6) | 15.1 (0.6) | 12.9 (0.8) | 12.1 (0.6) | ||
| NIH Toolbox subtests | ||||||
| Dimensional change card sort1 | F(9, 197) = 0.62, p = 0.78 | Baseline | 40.9 (2.5) | 46.1 (2.6) | 54.1 (3.2) | 50.3 (2.6) |
| 1 day | 47.1 (2.5) | 47.8 (2.6) | 54.6 (3.2) | 53.1 (2.6) | ||
| 7 days | 48 (2.5) | 50.1 (2.7) | 57.2 (3.2) | 55 (2.6) | ||
| 30 days | 47.3 (2.5) | 52.1 (2.6) | 58.9 (3.2) | 54.3 (2.6) | ||
| Flanker inhibitory control and attention1 | F(9, 197) = 0.66, p = 0.74 | Baseline | 39.3 (2.7) | 43.2 (2.9) | 42 (3.5) | 55.6 (2.8) |
| 1 day | 39.7 (2.7) | 43.7 (2.9) | 41.7 (3.5) | 59.7 (2.8) | ||
| 7 days | 42.8 (2.7) | 46.3 (2.9) | 44.3 (3.5) | 59.3 (2.8) | ||
| 30 days | 42.7 (2.7) | 47.2 (2.9) | 45.3 (3.5) | 61.1 (2.8) | ||
| Picture sequence memory1 | F(9, 197) = 3.09, p = 0.0017 | Baseline | 50 (2.4) | 51.8 (2.5) | 45.3 (3) | 44.9 (2.4) |
| 1 day | 59.7 (2.4) | 63.5 (2.5) | 54.6 (3) | 46.7 (2.4) | ||
| 7 days | 66 (2.4) | 66.2 (2.5) | 55.1 (3.1) | 49.1 (2.4) | ||
| 30 days | 65.8 (2.4) | 63.4 (2.5) | 51.9 (3.1) | 52 (2.4) | ||
| List sorting working memory1 | F(9, 197) = 0.66, p = 0.75 | Baseline | 50.1 (2.2) | 54.3 (2.3) | 52.7 (2.8) | 50 (2.2) |
| 1 day | 52.6 (2.2) | 56.8 (2.3) | 54.7 (2.8) | 53.7 (2.2) | ||
| 7 days | 55.2 (2.2) | 58.8 (2.3) | 53.3 (2.8) | 54.9 (2.2) | ||
| 30 days | 54.4 (2.2) | 58.9 (2.3) | 54.9 (2.8) | 52.3 (2.2) | ||
| Oral reading recognition2 | F(9, 196) = 1.04, p = 0.41 | Baseline | 57.9 (2.4) | 62.7 (2.5) | 51.5 (3.1) | 58.9 (2.5) |
| 1 day | 58.4 (2.4) | 62.3 (2.5) | 52.5 (3.1) | 61 (2.5) | ||
| 7 days | 57.1 (2.4) | 63 (2.5) | 52.8 (3.1) | 61.2 (2.5) | ||
| 30 days | 58.4 (2.4) | 62.9 (2.5) | 51.6 (3.1) | 63.9 (2.5) | ||
| Picture vocabulary3 | F(9, 192) = 1.59, p = 0.12 | Baseline | 54.1 (3.3) | 61.5 (3.4) | 50.6 (4.2) | 64.9 (3.4) |
| 1 day | 53.8 (3.3) | 64 (3.5) | 52.9 (4.2) | 65.9 (3.4) | ||
| 7 days | 56.1 (3.3) | 63.6 (3.5) | 52.9 (4.2) | 66 (3.4) | ||
| 30 days | 54.2 (3.3) | 66.9 (3.4) | 51.3 (4.2) | 64.9 (3.4) | ||
| Pattern comparison processing speed4 | F(9, 196) = 0.54, p = 0.85 | Baseline | 44.4 (3.1) | 53.4 (3.2) | 54.2 (3.9) | 53.1 (3.1) |
| 1 day | 54.1 (3.1) | 62.3 (3.2) | 61.2 (3.9) | 62.8 (3.1) | ||
| 7 days | 58.5 (3.1) | 67.9 (3.2) | 66 (3.9) | 70.2 (3.1) | ||
| 30 days | 58.5 (3.1) | 64.9 (3.2) | 66.7 (3.9) | 70 (3.1) | ||
Data were missing for all NIH Toolbox tests for 2 participants at 1 occasion, and for 1 participants at 2 occasions. N was 18 for 50–59 years at 1 day and 7 days, and 12 for 60–69 years at 7 days and 30 days.
Data were missing for an additional participant in the 70–80 years group on the 30 day assessment. N was 18 for 50–59 years at 1 day and 7 days, 12 for 60–69 years at 7 days and 30 days, and 18 for 70–80 years at 30 days.
Data were missing for 5 additional participants at one time point for each participant. N was 18 for 50–59 years at 1 day, N was 17 for 50–59 years at 7 days, N was 12 for 60–69 years at 7 days and 30 days, N was 17 for 70–80 years at 1 day, N was 18 for 70–80 years at 7 days, and N was 18 for 70–80 years at 30 days.
Data were missing for an additional participant in the 50–59 years group on the 30 day assessment. N was 18 for 50–59 years at 1 day, 7 days, and 30 days, and 12 for 60–69 years at 7 days and 30 days.
Table 4.
Summary of results for time-to-recovery analyses (discrete-time logit regression) for the primary outcome, secondary outcomes, and subtests of the NIH Toolbox Cognitive Battery. P-values shown are not adjusted for multiple comparisons.
| Time to Recovery | HR vs 40–49 year old (N = 20) reference decade (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Overall effect of age decade | 50–59 years | p | 60–69 years | p | 70–80 years | p | |
| N = 19 | N = 13 | N = 19 | |||||
| Primary outcome | |||||||
| PQRS1 | χ2(3) = 1.83, p = .61 | 1.41 (0.50,4.03) | 0.52 | 1.03 (0.35, 3.00) | 0.96 | 0.69 (0.25, 1.88) | 0.69 |
| Secondary outcomes | |||||||
| NIH Toolbox Composite2 | χ2(3) = 0.92, p = .82 | 0.40 (0.05, 3.03) | 0.37 | 0.47 (0.06, 4.07) | 0.50 | 0.44 (0.06, 3.37) | 0.43 |
| Logical memory immediate | χ2(3) = 0, p = 1.00 | 1.00 (0.51, 1.95) | 1.00 | 1.00 (0.49, 2.08) | 1.00 | 1.00 (0.51, 1.95) | 1.00 |
| Category fluency - animals | χ2(3) = 0.57, p = 0.90 | 1.10 (0.55, 2.20) | 0.78 | 1.29 (0.61, 2.74) | 0.50 | 1.25 (0.63, 2.49) | 0.53 |
| Trails A time | χ2(3) = 0.05, p = 0.997 | 1.01 (0.52, 1.98) | 0.97 | 1.07 (0.51, 2.23) | 0.86 | 1.06 (0.53, 2.11) | 0.87 |
| Trails B time | χ2(3) = 1.85, p = 0.60 | 1.59 (0.79, 3.20) | 0.20 | 1.37 (0.64, 2.95) | 0.42 | 1.18 (0.60, 2.33) | 0.64 |
| Logical memory delayed | χ2(3) = 0, p = 1.00 | 1.00 (0.51, 1.95) | 1.00 | 1.00 (0.48, 2.08) | 1.00 | 1.00 (0.51, 1.95) | 1.00 |
| CVLT delayed recall | χ2(3) = 0, p = 1.00 | 1.00 (0.51, 1.95) | 1.00 | 1.00 (0.48, 2.08) | 1.00 | 1.00 (0.51, 1.95) | 1.00 |
| NIH Toolbox subtests 2 | |||||||
| Dimensional change card sort | χ2(3) = 2.17, p = .54 | 0.67 (0.20, 2.20) | 0.50 | 1.02 (0.26, 3.99) | 0.98 | 1.89 (0.49, 7.32) | 0.35 |
| Flanker inhibitory control and attention | χ2(3) = 0.54, p = .91 | 1.23 (0.38, 4.02) | 0.73 | 0.98 (0.23, 4.19) | 0.97 | 1.55 (0.43, 5.53) | 0.50 |
| Picture sequence memory | χ2(3) = 6.62, p = .085 | 0.27 (0.02, 3.03) | 0.29 | 0.27 (0.02, 3.55) | 0.32 | 0.08 (0.01, 0.76) | 0.03 |
| List sorting working memory | χ2(3) = 0.33, p = .95 | 1.08 (0.28, 4.20) | 0.91 | 0.78 (0.19, 3.19) | 0.73 | 1.23 (0.30, 5.12) | 0.78 |
| Oral reading recognition | χ2(3) = 3.33, p = .34 | 1.51 (0.53, 4.28) | 0.44 | 3.44 (0.89, 13.29) | 0.07 | 1.70 (0.57, 5.04) | 0.34 |
| Picture vocabulary | χ2(3) = 1.27, p = .74 | 1.63 (0.46, 5.79) | 0.45 | 0.83 (0.22, 3.16) | 0.78 | 1.57 (0.43, 5.80) | 0.49 |
| Pattern comparison processing speed | χ2(3) = 1.05, p = .79 | 2.51 (0.33, 18.93) | 0.37 | 1.00 (0.12, 7.99) | 0.998 | 1.87 (0.25 14.16) | 0.55 |
N = 19 for 40–49 years and N = 18 for 70–80 years (due to missing data at baseline assessment).
As noted in Table 3, some participants had missing data for individual NIH Toolbox assessments at some time points. Times to recovery for these participants were determined based on available data.
Discussion
In this study we found no association between age decade and time to cognitive recovery within 30 days from anesthesia in healthy volunteers without dementia. In general recovery was rapid, with 91% of participants recovered to baseline on the primary endpoint measure within 1 day of anesthesia and 97% recovered on this measure within 30 days. There were no differences between older participants (50–59, 60–69, or 70–80 years old) relative to 40–49 year old participants. There was no indication of differences among age decades on post-anesthesia cognitive function on any of the computerized or paper-and-pencil cognitive tests that were our secondary measures, with participants maintaining or improving their performance on these tests post-anesthesia with no differences in post-anesthesia cognitive performance as a function of age (with the exception of one of 7 subtests of the NIH Toolbox Cognitive Battery). Strengths of this study include our ability to model changes in recovery and cognitive performance after anesthesia without the bias of comorbid condition or of surgical intervention, both of which are likely to play a role in cognitive recovery. An additional strength is the use of comprehensive pre- and post-anesthesia cognitive testing, including a brief assessment used in the peri-anesthesia setting; well-validated paper-and-pencil neuropsychological tests, and a computerized test battery. Moreover, the participants in our study included two categories of individuals in the age range most vulnerable to POD and POCD2,20. We also were able in a secondary study from this sample to determine that general anesthesia did not affect plasma biomarkers of neural injury or Alzheimer’s disease21, providing further evidence that advanced age per se is not associated with neurobiological impairment after general anesthesia.
There are also important limitations of our study. The use of healthy, non-surgical participants with normal cognition at baseline may limit the applicability to clinical populations, who may have significant comorbidities and/or poor preoperative cognition which are risk factors for postoperative neurocognitive disorders22. This also limited our ability to include ASA status and number of medications as covariates in analyses because of small N. The anesthetic administered was dose-adjusted for age, and depth of anesthesia was monitored and limited to 2 hours. Many elderly patients are overdosed in general practice, not monitored for anesthetic depth, and surgeries can last for many hours. Thus the effect of overdose of anesthetics in elderly patients is unclear and it is unlikely for ethical reasons that such a study will be conducted. We only examined sevoflurane general anesthesia, so our results may not generalize to other anesthetic agents. However, available evidence suggests that volatile versus intravenous anesthesia either does not affect the incidence of postoperative neurocognitive disorders or that risk is increased after volatile anesthesia23–26. Our design employed repeated testing such that practice effects may have obscured subtle differences. Indeed, participants generally improved on tests with time, suggestive of practice effects. We lacked a comparison group that did not receive anesthesia, however all participants underwent testing on the same schedule, which would have allowed us to separate interactions of age decade with anesthesia from practice effects on the neuropsychological tests. Although this is an important consideration, clear age-related increases in incidence of POD and POCD2,20 make age differences by decade in time to recovery the primary question of interest, which we were able to investigate without a non-anesthesia comparison group. Finally, the interpretation of a lack of difference between age decades can be challenging. The need to recruit healthy participants and expose them to two hours of general anesthesia for research purposes was balanced against the sample size required for statistical power to detect a reasonable association of age with rate of cognitive recovery after anesthesia. Although the PQRS cognitive scale was very sensitive to the time post-anesthesia in all age decades, recovery did not vary across the age decades. We cannot rule out a more subtle effect of age that we were not adequately powered to detect with our sample size, which included a total of 71 participants of whom 32 were age 60 or over. However, after day 1 there were only 2 persons 40–49, 1 person 50–59, 1 60–69 and 2 70–80 years old yet to recover. Thus, rather than more persons enrolled, we would need a design with more measures within the first day to refine the estimate of time to recovery, but such small intervals may not reflect clinically important differences.
We found no association between age category and time to cognitive recovery after general anesthesia, although the strength of this inference is limited by our sample size. Given that surgery under general anesthesia elevates biomarkers of neural injury27, surgical stress and inflammation may be the primary culprit, although it is a logical possibility that anesthesia might exacerbate these responses even if it does not cause them on its own21 or that anesthetic depth during surgery may moderate neurophysiology in meaningful ways that affect postoperative cognition28–30. Finally, we cannot exclude that there are vulnerable subgroups, based on preoperative comorbidity or the presence of geriatric syndromes such as frailty or cognitive impairment. Future studies may seek to focus on these patients to determine whether there is an association of anesthetic technique with cognitive recovery in patients with preexisting geriatric syndromes.
Key points:
Question: Is time to recovery of baseline cognitive function after exposure to general anesthesia, without surgery, associated with age group?
Findings: Recovery of cognitive function to baseline was rapid and did not differ by age decade.
Meaning: General anesthesia alone is not associated with time to cognitive recovery in healthy adults of any age decade.
Acknowledgments
Dr. Jeffrey Silverstein MD (Icahn School of Medicine at Mount Sinai, original primary investigator, deceased), many thanks to Rachelle Jacoby, Kirklyn Escondo, Jong Kim, Matthew Hartnett, Carolyn Fan, and Jim Leader (all of the Department of Anesthesiology, Perioperative and Pain Medicine, Icahn School of Medicine at Mount Sinai) for their assistance with this study.
Funding statement:
Grant support from this study was from National Institute on Aging R01 AG046634. PJM and JSM acknowledge additional support from National Cancer Institute Cancer Center Support Grant P30 CA008748.
Glossary:
- ASA
American Society of Anesthesiologists
- BIS
bispectral index
- CVLT
California Verbal Learning Test
- IV
intravenous
- LMA
laryngeal mask airway
- LMM
linear mixed model
- MAC
minimum alveolar concentration
- MRI
magnetic resonance imaging
- NIH
National Institutes of Health
- POCD
postoperative cognitive dysfunction
- POD
postoperative delirium
- PQRS
Postoperative Quality of Recovery Scale
Footnotes
Clinical trial registration: NCT02275026, ClinicalTrials.gov, http://clinicaltrials.gov
Conflicts of interest: The authors disclose the following relationships, none related to the work presented in this paper: JSM, JWB, AS, HA, TN, MI, M. Sewell, HGA, CMR nothing to disclose, MGB has been a consultant for Unity Biotechnology; PJM’s spouse holds equity in Johnson & Johnson; M. Sano has been a consultant for VTV Therapeutics, Hoffman-LaRoche, Biogen, CogRx, Bracket, Eisai, Eli Lilly and Company, member of the DSMB for AZTherapies and for NIA ”ASPREE”, adjudicator for Trial Endpoint for Takeda Pharmaceutical; SGD has served as a consultant for Merck, Covidien, received product support from Covidien and CASMED (processed electroencephalogram and oximetry monitors, sensors) and as an expert witness for legal affairs.
References
- 1.Evered LA, Silbert BS. Postoperative Cognitive Dysfunction and Noncardiac Surgery. Anesthesia & Analgesia 2018;127:496–505. [DOI] [PubMed] [Google Scholar]
- 2.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. The Lancet 2014;383:911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in Elderly Patients and the Risk of Postdischarge Mortality, Institutionalization, and Dementia: A Meta-analysis. JAMA 2010;304:443–51. [DOI] [PubMed] [Google Scholar]
- 4.Daiello LA, Racine AM, Gou RY, Marcantonio ER, Xie Z, Kunze LJ, Vlassakov KV, Inouye SK, Jones RN. Postoperative Delirium and Postoperative Cognitive Dysfunction: Overlap and Divergence. Anesthesiology 2019;131:477–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudolph JL, Marcantonio ER, Culley DJ, Silverstein JH, Rasmussen LS, Crosby GJ, Inouye SK. Delirium is associated with early postoperative cognitive dysfunction. Anaesthesia 2008;63:941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudolph James L, Jones Richard N., Levkoff Sue E., Rockett Christopher, Inouye Sharon K., Sellke Frank W., Khuri Shukri F., Lipsitz Lewis A., Ramlawi Basel, Levitsky Sidney, Marcantonio Edward R. Derivation and Validation of a Preoperative Prediction Rule for Delirium After Cardiac Surgery. Circulation 2009;119:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang JX, Eckenhoff MF. Anesthetic effects in Alzheimer transgenic mouse models. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2013;47:167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiologica Scandinavica 2003;47:260–6. [DOI] [PubMed] [Google Scholar]
- 9.Evered L, Scott DA, Silbert B, Maruff P. Postoperative Cognitive Dysfunction Is Independent of Type of Surgery and Anesthetic. Anesthesia & Analgesia 2011;112:1179–85. [DOI] [PubMed] [Google Scholar]
- 10.Mincer JS, Baxter MG, McCormick PJ, Sano M, Schwartz AE, Brallier JW, Allore HG, Delman BN, Sewell MC, Kundu P, Tang CY, Sanchez A, Deiner SG. Delineating the Trajectory of Cognitive Recovery From General Anesthesia in Older Adults: Design and Rationale of the TORIE (Trajectory of Recovery in the Elderly) Project. Anesthesia & Analgesia 2018;126:1675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royse CF, Newman S, Chung F, Stygall J, McKay RE, Boldt J, Servin FS, Hurtado I, Hannallah R, Yu B, Wilkinson DJ. Development and Feasibility of a Scale to Assess Postoperative RecoveryThe Post-operative Quality Recovery Scale. Anesthesiology 2010;113:892–905. [DOI] [PubMed] [Google Scholar]
- 12.Royse CF, Newman S, Williams Z, Wilkinson DJ. A Human Volunteer Study to Identify Variability in Performance in the Cognitive Domain of the Postoperative Quality of Recovery Scale. Anesthesiology 2013;119:576–81. [DOI] [PubMed] [Google Scholar]
- 13.Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, Beaumont J, Casaletto KB, Conway K, Slotkin J, Gershon R. Reliability and Validity of Composite Scores from the NIH Toolbox Cognition Battery in Adults. Journal of the International Neuropsychological Society 2014;20:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, Havlik R, Conway K, Edwards E, Freund L, King JW, Moy C, Witt E, Gershon RC. Cognition assessment using the NIH Toolbox. Neurology 2013;80:S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The Neuropsychologic Test Battery. Alzheimer Disease & Associated Disorders 2009;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nir T, Jacob Y, Huang K-H, Schwartz AE, Brallier JW, Ahn H, Kundu P, Tang CY, Delman BN, McCormick PJ, Sano M, Deiner S, Baxter MG, Mincer JS. Resting-state functional connectivity in early postanaesthesia recovery is characterised by globally reduced anticorrelations. British Journal of Anaesthesia 2020;0. Available at: https://bjanaesthesia.org/article/S0007-0912(20)30552-3/abstract. Accessed September 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison PD. Discrete-Time Methods for the Analysis of Event Histories. Sociological Methodology 1982;13:61. [Google Scholar]
- 18.Singer JD, Willett JB. It’s about Time: Using Discrete-Time Survival Analysis to Study Duration and the Timing of Events. Journal of Educational Statistics 1993;18:155–95. [Google Scholar]
- 19.PASS 12 Power Analysis and Sample Size Software. Kaysville, Utah: NCSS, LLC, 2012. [Google Scholar]
- 20.Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT. The assessment of postoperative cognitive function. Acta Anaesthesiologica Scandinavica 2001;45:275–89. [DOI] [PubMed] [Google Scholar]
- 21.Deiner S, Baxter MG, Mincer JS, Sano M, Hall J, Mohammed I, O’Bryant S, Zetterberg H, Blennow K, Eckenhoff R. Human plasma biomarker responses to inhalational general anaesthesia without surgery. British Journal of Anaesthesia 2020. Available at: http://www.sciencedirect.com/science/article/pii/S0007091220303391. Accessed July 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devore EE, Fong TG, Marcantonio ER, Schmitt EM, Travison TG, Jones RN, Inouye SK. Prediction of Long-term Cognitive Decline Following Postoperative Delirium in Older Adults. J Gerontol A Biol Sci Med Sci 2017;72:1697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Shan G-J, Zhang Y-X, Cao S-J, Zhu S-N, Li H-J, Ma D, Wang D-X. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. British Journal of Anaesthesia 2018;121:595–604. [DOI] [PubMed] [Google Scholar]
- 24.Miller D, Lewis SR, Pritchard MW, Schofield-Robinson OJ, Shelton CL, Alderson P, Smith AF. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database of Systematic Reviews 2018. Available at: https://www-cochranelibrary-com.eresources.mssm.edu/cdsr/doi/10.1002/14651858.CD012317.pub2/full. Accessed July 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao Y, Feng H, Zhao T, Yan H, Zhang H, Zhao X. Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol 2015;15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain M, Berger M, Eckenhoff RG, Seitz DP. General anesthetic and the risk of dementia in elderly patients: current insights. Clinical Interventions in Aging 2014;9:1619–28. Available at: https://www.dovepress.com/general-anesthetic-and-the-risk-of-dementia-in-elderly-patients-curren-peer-reviewed-article-CIA. Accessed July 15, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evered L, Silbert B, Scott DA, Zetterberg H, Blennow K. Association of Changes in Plasma Neurofilament Light and Tau Levels With Anesthesia and Surgery: Results From the CAPACITY and ARCADIAN Studies. JAMA Neurol 2018;75:542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deiner S, Luo X, Silverstein JH, Sano M. Can Intraoperative Processed EEG Predict Postoperative Cognitive Dysfunction in the Elderly? Clinical Therapeutics 2015;37:2700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hesse S, Kreuzer M, Hight D, Gaskell A, Devari P, Singh D, Taylor NB, Whalin MK, Lee S, Sleigh JW, García PS. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: an early sign of postoperative complications. British Journal of Anaesthesia 2019;122:622–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soehle M, Dittmann A, Ellerkmann RK, Baumgarten G, Putensen C, Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol 2015;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


