Abstract
Despite high levels of MMR-II usage in the US, mumps outbreaks continue to occur. Evidence suggests that mumps vaccine-induced humoral immunity wanes over time. Relatively few studies have examined cell-mediated immunity or reported on sex-based differences. To better understand sex-based differences in the immune response to mumps vaccine, we measured neutralizing antibody titers and mumps-specific cytokine/chemokine responses in a cohort of 748 adolescents and young adults after two doses of MMR vaccine. We observed significantly higher neutralizing antibody titers in females than in males (120.8 IU/mL, 98.7IU/mL, p=0.038) but significantly higher secretion levels of MIP-1α, MIP-1β, TNFα, IL-6, IFNγ, and IL-1β in males compared to females. These data demonstrate that sex influences mumps-specific humoral and cell-mediated immune response outcomes, a phenomenon that should be considered during efforts to improve vaccines and prevent future outbreaks.
Keywords: Mumps, MMR Vaccine, Cytokine, Chemokine, Cellular Immunity, sex-based differences
Introduction
Humans serve as the only natural host for mumps virus. The current live attenuated mumps virus (MuV) vaccine in the US contains the Jeryl Lynn (JL) strain, and for more than 30 years, has been administered as a two-dose series in the U.S. as part of the trivalent measles-mumps-rubella (MMR) vaccine [1]. Although the introduction of vaccination against mumps substantially reduced disease incidence, recent outbreaks in vaccinated populations and subsequent study findings have demonstrated inadequate long-term mumps vaccine efficacy [1–5]. From 2015 to 2019, the CDC reported nearly 20,000 mumps cases throughout the United States [6]. Multiple studies report waning of MuV-specific antibody titers and a decline in seropositivity starting at 8 years to as late as 27 years after vaccination [3, 7–9]. The effect of waning immunity to mumps vaccine has been observed on college campuses, when 10–15 years have generally passed since students’ last immunization, and outbreaks have been observed [2, 3, 10, 11]. It is probable that disease prevalence has been underestimated, given that nearly one third of those infected remain asymptomatic [12][13].
Despite similar vaccination rates, a greater disease incidence has been reported in several studies in females compared to males [2, 14, 15]. Studies have demonstrated that female adolescents exhibit significantly higher mumps-specific IgG antibody titers than males [16–18]. Though mumps-specific sex-based differences in humoral immunity have been reported, little is known about the effect of sex on vaccine-induced cellular immunity. It has been suggested that cellular immunity may provide protection against mumps infection in individuals who experience exposure to natural infection but lack detectable mumps virus-specific antibodies [19]. It is known that mumps-specific T cell responses may be maintained longer than memory B cells and virus-specific long-lived plasma cells, which have been thought to confer protective immunity [1, 20, 21].
In this study, we sought to examine mumps-specific immune responses (i.e., neutralizing antibody and cytokine/chemokine responses) in healthy adolescents following two doses of MMR-II vaccine in order to understand inter-individual variation and sex-based differences in immune responses to mumps vaccination.
Methods
Study Design
As previously detailed [22–25], the 748-subject study cohort was formed from two independent, age-stratified cohorts from the Minnesota Independent School District 535 in Rochester, MN. Subjects were 11–19 years of age and had been vaccinated with two doses of MMR-II vaccine, with the second dose received 1–17 years prior to study participation. There were no known mumps outbreaks in the surrounding area during the lifetimes of participants prior to sample collection. All study participants provided written informed consent, and all study proceedings were endorsed by the Mayo Clinic Institutional Review Board. The methods described herein are similar or identical to those we have previously published for other mumps-specific studies [9, 26, 27].
PBMC isolation and storage
Blood collection and biospecimen processing and storage methods are identical to those reported in previous studies [28, 29, 30]. Briefly, collection of whole blood from subjects was performed using BD Vacutainer® CPT™ tubes containing sodium citrate. Peripheral blood mononuclear cells (PBMCs) were isolated according to the manufacturer’s protocol (BD; Franklin Lakes, NJ) and established laboratory SOPs. Purified PBMCs were re-suspended at 1×10^7 cells/ml in freezing media (GIBCO RPMI with L-glutamine [Invitrogen; Carlsbad, CA], 20% heat inactivated FCS [HyClone; Logan, UT [, 10% DMSO [Protide Pharmaceuticals Inc.; Lake Zurich], IL]) and stored in liquid nitrogen. Cells were thawed and cultured for the detection of mumps-specific cytokines as previously described [31, 32].
Mumps-specific neutralizing antibody assay
Neutralizing antibodies to mumps virus (JL strain) were quantified by a mumps-specific plaque-reduction neutralization assay established and performed by the Center for Biologics Evaluation and Research, U.S. Food and Drug Administration (FDA), as previously described [33]. Briefly, heat-inactivated sera were diluted with minimal essential media (MEM) 2-fold from 1:2 to 1:2,048 in 96-well microtiter plates. A standard control reference serum was also diluted to be used as a positive control. Equal volumes of media containing 100 plaque forming units (PFU) of MuV-JL were added to all wells. Virus was also added to medium-only wells (containing no serum), which served as a virus-only control. Plates were incubated for 1hr at 37°C/5%CO2. The inoculum was transferred in duplicate to 24-well plates with Vero cell monolayers. Following 1.5hr incubation at 37°C/5% CO2, the inoculum was aspirated and plates were supplemented with MEM containing 5% FBS, antibiotics and 2% carboxymethlycellulose. Following 5 days of incubation (37°C/5% CO2), Vero cell monolayers were stained with neutral red dye and incubated for additional 12–24hr. After the monolayers were fixed with formaldehyde and allowed to air dry, plaques were counted. The Karber method was used, and neutralizing antibody titers were determined as the highest serum dilution at which the number of plaques were diminished by 50% when compared to the virus-only control [33, 34]. According to the control reference mumps immune serum, the coefficient of variation for this assay was 7.95% [33].
Mumps-specific cytokine/chemokine secretion
The following cytokines (i.e., IL-2, IL-6, IL-10, IFNα2a, IFNγ, IL-1β, TNFα) and chemokines (i.e., IP-10, MCP-1, MIP-1α, MIP-1β) were measured using the Meso Scale Discovery’s electrochemiluminescence-based ELISA in cell culture supernatants, as previously described [35]. Briefly, PBMC cultures were stimulated with mumps virus (MuV) antigen (Enders strain, Bio-Rad/Abd Serotec cat. #PIP014) at 1:20 dilution, and PBMCs were incubated for 48 hours at 37°C/5% CO2. Cell culture supernatants were tested in duplicate in 96-well round bottom plates. Cell cultures for each subject included unstimulated wells (negative control) and phytohemagglutinin (PHA, 200ug/mL)-stimulated wells (positive control). Following incubation, supernatants were harvested and stored at −80°C until the electrochemiluminescence-based ELISA assays were performed. The coefficient of variation ranged from 10% - 31% depending on the analyte.
Statistical Methods and Analysis
Subjects were included for analysis depending on biospecimen availability and consent for use. Additionally, samples that failed quality control were excluded from data analysis. Self-reported ethnicity was used. Demographic data were expressed descriptively using the means and ranges for continuous variables (Table 1). The influence of demographic characteristics on mumps-specific immune response outcomes were assessed for significance using the Wilcoxon non-parametric test.
Table 1.
Demographic and clinical variables of the study population
| Variable | Overall (n=748) | Female (n=327) | Male (n=421) | Sex p-value* |
|---|---|---|---|---|
| Mean years (range) | 14.9 (11.0–19.0) | 14.9 (11.0–19.0) | 14.9 (11.0–19.0) | 0.97 |
| Mean months, (range) | 16.6 (11.0–185.0) | 17.0 (11.0–132.0) | 16.4 (11.0–185.0) | 0.550 |
| Mean years (range) | 8.3 (1.0–17.0) | 8.4 (1.0–17.0) | 8.3 (1.0–15.0) | 0.995 |
| Mean years, (range) | 6.5 (0.4–15.5) | 6.4 (0.6–15.4) | 6.6 (0.4–15.5) | 0.288 |
| Male N (%) | 421 (56.3%) |
The Wilcoxon rank-sum test was used to generate p-values measuring statistical difference between female and male groups.
Neutralizing antibody titer values were multiplied by each plate’s adjusting factor, resulting in the final adjusted outcome. Neutralizing antibody values were expressed using medians and the first and third interquartile range (Table 2).
Table 2.
MuV-specific humoral and cell-mediated immune outcomes overall and by sex
| Neutralizing Antibody/Cytokine/Chemokine | Overall Response (n = 748) (stim-unstim, pg/ml) Median (25%, 75% IQR) | Sex Female (n = 327) Median (25%, 75% IQR) | Sex Male (n = 421) Median (25%, 75% IQR) | Sex p-value |
|---|---|---|---|---|
| Neutralizing Antibody | 109.8 (43.9, 250.7) | 120.8 (49.7, 297.6) | 98.7 (40.6, 238.0) | 0.038 |
| MIP-1α | 780.6 (267.5, 1917.3) | 561.3 (201.3, 1566.9) | 922.4 (319.2, 2147.9) | 0.000044 |
| TNFα | 53.1 (27.2, 96.7) | 45.2 (25.0, 80.4) | 58.5 (30.0, 107.0) | 0.0003 |
| MIP-1β | 565.8 (263.2, 1097.8) | 494.7 (228.7, 958.9) | 618.1 (304.0, 1199.1) | 0.001 |
| IL-6 | 572.8 (320.6, 1011.1) | 519.0 (306.5, 874.1) | 603.6 (333.1, 1088.3) | 0.028 |
| IFNγ | 109.0 (42.4, 246.1) | 96.7 (37.8, 217.2) | 119.1 (47.3, 262.4) | 0.031 |
| IL-1β | 15.6 (6.3, 38.2) | 13.6 (5.6, 34.8) | 17.4 (7.2, 42.4) | 0.032 |
| IFNα2a | 71.2 (27.7, 145.4) | 71.4 (29.1, 146.8) | 70.7 (26.7, 139.5) | 0.28 |
| IL-10 | 4.9 (2.41, 9.62) | 4.8 (2.1, 9.4) | 5.2 (2.7, 9.8) | 0.29 |
| MCP-1 | 8848.5 (5114.2, 13533.7) | 8030.3 (4777.1, 12979.8) | 9292 (5191.9, 13632.2) | 0.31 |
| IL-2 | 13.6 (6.7, 25.6) | 13.2 (6.2, 25.7) | 13.9 (7.5, 25.3) | 0.35 |
| IP-10 | 902.2 (292.8, 2229.6) | 907.6 (264.9, 2252.5) | 887.8 (323.4, 2205.8) | 0.41 |
Outcomes and p-values significant for sex are indicated in bold. The p-value for neutralizing antibody was calculated using the Wilcoxon non-parametric test; cytokine/chemokine p-values are from coefficients of univariate regression.
Cytokine/chemokine readings below or above detection range were set to the minimum or maximum of the range, respectively. The average cytokine/chemokine value for each set of duplicates was then calculated, followed by background subtraction of subjects’ unstimulated wells. These values (stim – unstim) were expressed by the medians and first and third interquartile ranges (Table 2).
Rank-based inverse normal transformation of cytokine/chemokine outcomes were used in univariate regression analyses. For each outcome, the stim – unstim readings were ranked and then divided by the total number of readings. These values were then converted to standard normal z-scores by inverse normal transformation, which were treated as the final transformed value.
Neutralizing antibody log2 transformed values were used to assess correlations with cytokine/chemokine stim – unstim raw values. Pearson correlation and Spearman p values were used. Univariate regression analysis was employed to complete covariate analysis. Log2 transformed neutralizing antibody and inverse normal transformed values for cytokines/chemokines were used. The effect of sex was further assessed using raw stim – unstim median values and ranges for both sexes (Table 2). Each covariate was examined, and the analysis took any effects into account.
Results
Study subjects
The demographics of our study cohort (n=748) are detailed in Table 1. The cohort had an average age of 14.9 years, and the majority of subjects were non-Hispanic/white (97.3%). Males were slightly over-represented, comprising 56% of our study cohort. Overall, the median age was 1.3 years of age for receipt of the first dose of MMR-II and 8.4 years of age for the second dose of MMR-II. Biospecimen collection was performed an average of 6.5 years post-second immunization. There were no significant demographic and vaccine history differences between male and female subjects (Table 1).
Neutralizing antibody responses
Neutralizing antibody titers were measured in all study participants (n= 748). The median neutralizing antibody titer was 109.8 IU/mL, with an interquartile range of 43.9 – 250.7IU/mL. The distribution of antibody titers (using log2 transformed values) is shown in Supplemental Figure 2C.
Mumps-specific cytokine/chemokine responses
Cytokine/chemokine responses were robust for MIP-1α, MIP-1β, IL-6, MCP-1, and IP-10. There was moderate secretion of TNFα, IFNγ, and IFNα2a in response to mumps virus stimulation. IL-1β, IL-10 and IL-2 exhibited minimal secretion. IL-13 was also included in the panel but was excluded from further analysis because the majority of the samples tested were below the lower limit of detection. These immune outcomes are summarized in Table 2.
Mumps-specific immune response outcome correlations
Correlations between individual cytokines and chemokines are illustrated in Supplemental Figure 1. There was at least a slight positive correlation between all cytokines and chemokines of interest except for IL-1β and IL-2, which demonstrated a significant but weak negative correlation of r= −0.034 (p=0.00008). Several cytokine and chemokine outcomes demonstrated highly significant (p<2e−16) positive correlations: MIP-1α with MIP-1β, IL-10, IL-1β, and TNFα; IL-1β with IL-10, TNFα, and IL-6; TNFα with IL-6.
Covariate influence of sex on immune outcomes
All immune response outcomes were evaluated for correlations with sex as a covariate. Neutralizing antibody titer was significantly associated with sex; higher median values were observed in females when compared to males (Table 2, Figure 1A). Correlations between sex and cytokine/chemokine outcomes were found to be significant for the following analytes: MIP-1α, TNFα, MIP-1β, IL-6, IFNγ, and IL-1β (Table 2). Immune outcomes (median values, IQR) were presented by sex and compared for statistically significant differences between sexes (Table 2). All cytokine/chemokine median responses (except IFNα2a and IP-10) were higher in males when compared to females (Table 2, Figure 1B).
Figure 1.
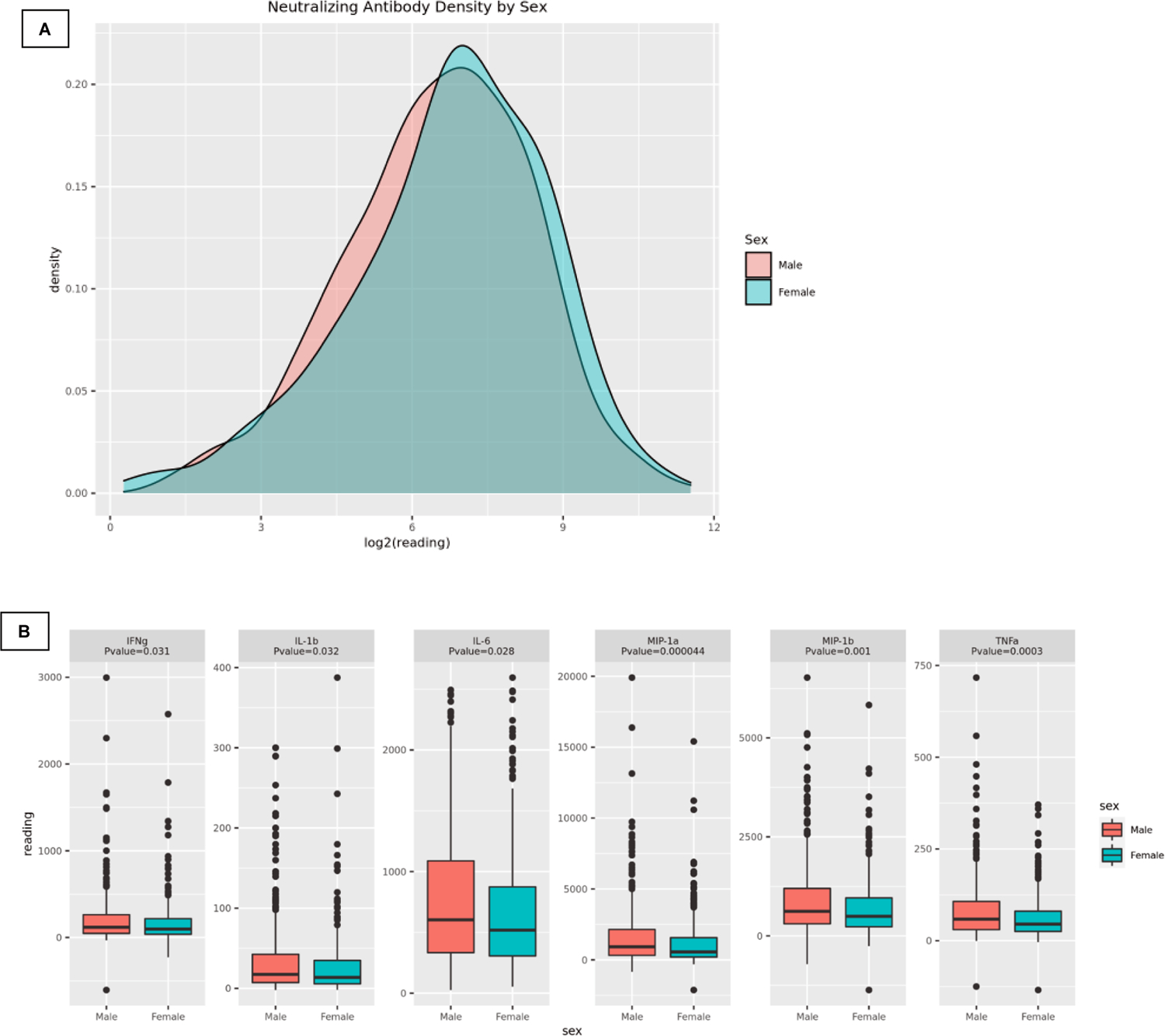
(A) MuV-specific neutralizing antibody density plot by sex. (B) MuV-specific cell-mediated immune outcome box plots (stim-unstim) by sex with associated p-values.
Covariate influence of ethnicity, age, and vaccination schedule on immune outcomes
All immune response outcomes were evaluated for correlations with other covariates; significant associations were noted between age at enrollment and the following mumps-specific immune outcomes: IL-2, IFNγ, IP-10, and MCP-1. Age at second immunization and the time interval between the last immunization and sample collection were both significantly associated with two mumps-specific immune outcomes: MIP-1α and MIP-1β (Supplemental Table 1). The influence of ethnicity on mumps-specific immune outcomes could not be assessed due to limited ethnic diversity in the study cohort.
Our study cohort involves school-age children and therefore puberty may be a factor in the observed immune responses. In order to assess this, we split the cohort by age in years and sex. We noted that immune outcomes displayed some variation by year (See Supplemental Figure 4). As we did not obtain any information on puberty onset in our study cohort, we could not classify subjects as pre- or post-pubescent, therefore we used age as an approximate indicator. We added 2 years to the average age of puberty in boys and girls in order to more fully capture hormonal maturation. For girls, we compared those 13 and younger (n=109) with those 14 and older (n=218). For boys, we compared those 14 and younger (n=186) with those 15 and older (n=235). Using these cut-offs we compared mumps-specific immune outcomes between older and younger participants and found several statistically significant differences for boys and for girls (Supplemental Table 2), suggesting that puberty may affect immune responses to mumps. Given these differences, we next compared mumps-specific immune outcomes between the younger boys and younger girls and performed a similar comparison between older participants. The results (reported in Table 3) demonstrate that sex-based differences are present in the older participants but not the younger participants, indicating that puberty and hormonal changes are a potential cause of the observed differences in immune outcomes.
Table 3.
Sex Differences in Mumps Immune Outcomes by Age
| Younger Participants (Females <14 and Males <15) | |||||
|---|---|---|---|---|---|
| Females (pg/ml IQR) | Males (pg/ml IQR) | p-value | |||
| Neutralizing antibody | 52.9–302.0 | 35.6–266.3 | 0.22 | ||
| MIP-1α | 295–1, 942 | 284.9–1, 902.8 | 0.64 | ||
| MIP-1β | 253.8–1, 042.0 | 263.1–1, 039.9 | 0.92 | ||
| IFNγ | 29.4–195.5 | 41.3–221.5 | 0.68 | ||
| IL-1β | 6.2–36.0 | 5.9–39.1 | 0.87 | ||
| IL-6 | 331.1–941.6 | 307.0–999.9 | 0.86 | ||
| TNF α | 27.3–84.5 | 26.2–90.9 | 0.68 | ||
| Older Participants (Females ≥14 and Males ≥15) | |||||
| Females (pg/ml IQR) | Males (pg/ml IQR) | p-value | |||
| Neutralizing antibody | 46.7–287.6 | 43.1–207.0 | 0.08 | ||
| MIP-1α | 179.7–1, 452.5 | 362.2–2, 313.5 | <0.001 | ||
| MIP-1β | 226.5–912.9 | 338.3–1, 252.3 | <0.001 | ||
| IFNγ | 43.1–223.8 | 53.6–293.8 | 0.003 | ||
| IL-1β | 5.4–33.5 | 7.9–44.3 | 0.003 | ||
| IL-6 | 297.4–856.0 | 397.9–1, 193.5 | <0.001 | ||
| TNF α | 24.7–78.9 | 34.4–125.3 | <0.001 | ||
Discussion
In this study we investigated mumps-specific humoral and markers of cellular immune response after two doses of MMR-II vaccine. We identified correlations between mumps-specific immune response outcomes and covariates (e.g., sex) potentially influencing individual immune responses in a study cohort of 748 healthy individuals. Though race has been found to impact Th1 responses to measles, another paramyxovirus, racial differences could not be assessed in this study due to the limited racial diversity of the cohort [36].
Overall cellular immune response outcomes were pro-inflammatory in nature, as characterized by increased secretion of: TNFα, IL-1β, IL-6, and IFNγ (Table 2). Secretion of IL-10 was minimal and IL-13 was below the limit of detection, suggesting that our cohort did not have Th2-biased responses. We also noted considerable inter-individual variation in the cytokine and chemokine response to in vitro mumps virus stimulation (Supplemental Figure 2. A, B). The finding that females have higher IgG neutralizing antibody titers compared to males (p=0.038) is significant and is supported by other studies which have found higher mumps-specific total IgG responses in females [16, 37]. Mumps-specific cytokines and chemokines significantly associated with sex included the following: MIP-1α, TNFα, MIP-1β, IL-6, IFNγ, and IL-1β, suggesting the potential impact of sex on mumps vaccine-induced cellular immunity and inflammatory response. These cytokine/chemokine median secretion values were higher in males compared to females and were also highly correlated with each other (Supplemental Figure 1). A prior study did not find significant associations between measures of cellular and humoral immunity [38] while other studies have identified associations between specific T cell subsets and antibody responses to measles [39, 40]. Those data and the results reported here suggest that cellular and humoral immunity are regulated by overlapping but not identical processes. Further investigation into the linkages between coordination between these arms of the immune system after measles vaccination is warranted.
We and others have previously reported sex-based differences in humoral immune responses to mumps vaccine [8, 17, 41]; however, no studies have assessed MuV-specific neutralizing antibody in a cohort of this size. Though a significant sex-based difference was observed, it is unclear whether a difference in titer of ~20 IU/mL (Table 2) is clinically meaningful in terms of vaccine effectiveness or durability of immunity, nor do our results link these differences in antibody titer to differential susceptibility to infection between males and females. It is possible that such a difference does reflect and is of importance for maintaining longer-term immunity for subjects at the higher end of the antibody-response spectrum. Interpreting the neutralizing antibody results is further complicated because an antibody correlate of protection has yet to be established for mumps, though neutralizing antibody is generally considered to be required for protection [42, 43]. Several studies have indicated that females experience mumps infection more often than males, yet some have reported the opposite trend [2, 14, 17, 44]. Though commonly employed, neutralizing antibody measurements only partially represent the full range of possible immunologic-effector mechanisms that antibodies are capable of in vivo [42]. Additional studies that more comprehensively examine the humoral immune response to mumps may be needed to determine if sex differences in mumps vaccine-induced neutralizing antibody titers have a clinical impact on protection from disease. Our cohort was also predominantly Caucasian and was drawn from a community with high vaccine coverage and no endemic disease, thus eliminating/reducing contamination of our results due to wild virus boosting. The results may be quite different in areas of the world with varying immunization policies and/or disease prevalence.
Our study is the first to demonstrate a sex-specific difference in innate, inflammatory, and T cell cytokine/chemokine responses to mumps vaccine. Sex-associated cytokine/chemokine outcomes (MIP-1α, MIP-1β, IFNγ, IL-1β, IL-6, and TNFα) all demonstrated significantly (p<0.05) greater median values for males compared to females (Table 2, Figure 1B). To our knowledge, no studies have explored sex-based differences in markers of mumps-specific cell-mediated immunity with such a comprehensive panel of nearly a dozen cytokines/chemokines. Jokinen et al. studied a small cohort (n=50) of Finnish participants 21 years after the first of two MMR-II immunizations and found that mumps-specific IFNγ production was significantly greater in females than males [45]. The results from this study are different from our findings. An explanation as to why this discrepancy exists could be due to sample size (50 subjects in Jokinen et al.’s study vs 748 subjects in our study), age at first and second immunizations (First dose: 1.5 or 6 years in Jokinen et al. vs 1.5 years in our study; Second dose: 6 or 11–13 years in Jokinen et al. vs 8 years in our study), time since vaccination (21 years in Jokinen et al. vs 1–17 years in our study), disease prevalence during the lifetimes of the study cohorts (and possibilities of subclinical infection and immune boosting), differences in IFNγ cytokine assay and measurements, or even biases in subject-recruitment (Jokinen et al. sought seronegative or low antibody titer subjects, while our study did not screen subjects). Others have used IFNγ measurements as a marker of mumps-specific cell-mediated immunity but do not assess sex-specific differences [46–48]. Despite clear evidence that sex affects the immune response to both vaccination and infection, very few studies actually report results by sex [49, 50]. Because of this, the National Institutes of Health has emphasized the study of sex as an important biological variable. We have identified a trend toward higher vaccine-induced neutralizing antibody titers in women and significant differences in markers of cellular immunity. Follow-up studies are necessary to confirm these findings and to more accurately measure the effect of sex on mumps vaccine-induced immunity.
The results from this study specifically demonstrated that the immune response to mumps in our cohort had a distinct Th1 bias and that multiple cytokines were secreted at higher levels in males compared to females. Our data also indicate that puberty may play a role in these sex-based differences, as the immune responses in younger males were not significantly different from that of younger females. While Villacres et. al. noted higher baseline IFNg production in females, Girón-González et. al. reported an increased IFNg:IL-4 ratio in mitogen-stimulated PBMCs[51, 52]. This result is in-line with our results demonstrating a more robust Th1 response males following antigenic-stimulation of PBMCs with MuV [52]. Challenges exist in characterizing Th1/Th2 sex-specific responses, especially when considering distinct cell subsets, pathogen/stimulant exposure, timing of infection, and/or model differences (i.e., human, rodent, in vitro cell studies) [50, 53]. The clinical relevance of our findings remains to be determined; however the findings are interesting given the propensity for more serious illness in males – perhaps reflective of a stronger inflammatory response at sites of infection. A potential follow-up to this study would be to compare the relative number of mumps virus-specific activated IFNγ+, CD69+, CD25+ effector T cells and IFNγ-central memory T cells in males compared to females [54, 55]. This would allow for a better understanding of the magnitude (number of MuV-specific T cells) and durability (number of central memory cells compared to effector cells) of MuV-specific Th1 response in males versus females. Another area for follow-up is a more careful examination of the role of puberty in controlling the immune response to mumps vaccination and/or infection.
While mumps vaccine-specific studies examining the explicit roles of each of the cytokines/chemokines assessed remain to be completed, general in-vitro data suggests possible functions of MIP-1α and MIP-1β at the secretion levels we have observed [56]. MIP-1β has been shown to attract CD4+ T cells in-vitro, while the in-vitro chemoattractant effect of MIP-1α activity has chemo-attractive effects on B cells and CD8+ T cells at ~100pg/mL and CD4+ T cells at concentrations >10,000pg/mL [56]. The median MIP-1α and MIP-1β concentrations for male subjects in our study (Table 2) would suggest that CD4+ T cells (due to MIP-1β), CD8+ T cells, and B cells (both due to MIP-1 α) could be more abundantly recruited to the vaccination site in males. It is possible that the chemoattractant effects of MIP-1α and MIP-1β may help establish stronger mumps-specific T cell memory in men, potentiating a longer durability of mumps vaccine-induced immunity in males.
Of note, all cytokines and chemokines significantly associated with sex were also highly correlated with each other (Supplemental Figure 1). It is clear that these six signaling molecules, (i.e., MIP-1α, MIP-1β, IFNγ, IL-1β, IL-6, and TNFα) are involved in one or several signaling pathways in the male response to mumps vaccine. Signaling pathways involving these cytokines/chemokines could be partially regulated by prominent sex-specific differences like sex chromosomes and hormones [16, 50, 53]. However, it is certain that upstream immune pathways and other transcriptional regulators are also influencing the observed immune response outcomes. Among the many functions of transcription factor NF-κB in viral immune responses is the role of inducing expression of HLA I and HLA II genes as well as genes encoding IFNγ, IL-1β, IL-6, and TNFα [57]. NF-κB is likely integral in the cellular immune response to mumps vaccine. The transcription factor T-bet, a known regulator of Th1 cell differentiation or the transducer STAT-1, an activator of the Type II Interferon response and subsequent cytokine production, are also likely involved [58–60]. It is probable that these transcriptional regulators and their pathways, in addition to those not described, contribute to the production of differing responses in men compared to women. Functional studies examining these molecular mechanisms remain to be performed in the context of mumps vaccine-induced immunity.
Limitations of our study include study cohort recruitment from one area of the US and the lack of diversity in racial and ethnic groups. Furthermore, the immunological challenge employed whole inactivated mumps virus in order to focus on the immune response to the viral antigens without the confounding effect of viral infection. These conditions may not produce the same results as cell stimulation with live mumps virus. The large size of our cohort, in addition to the inclusive profile of numerous cytokines and chemokines, which to our knowledge have not been this comprehensively reported, are major strengths of this study. Furthermore, study participants were in the age range within which the majority of recent mumps outbreaks are occurring [45, 61].
Conclusion
In conclusion, our study demonstrated a pronounced impact of sex on markers of cellular and humoral immunity to mumps vaccine. It is imperative that immunological variations induced by basic attributes, like sex, be thoroughly assessed when studying infectious diseases and vaccine-induced immunity as these variations could reveal specific mechanisms that underly the differences observed. Sex-based differences to mumps vaccine could be better understood through (1) functional studies evaluating the mumps-specific activity of transcription factors and signaling pathways, (2) assessing differences in the mumps-specific activity of these transcription factors and pathways between the sexes, (3) investigating variations in mumps-specific cell subsets between sexes. These studies could inform the development of future vaccine candidates that better target upstream effector mechanisms and induce greater neutralizing antibody and memory T cell production in males and females. These improvements could help make mumps vaccines more effective at preventing infection/outbreaks and could help combat waning immunity in both sexes by inducing more durable mumps vaccine-induced immune responses.
Supplementary Material
Highlights.
Even with high vaccine coverage, mumps outbreaks still occur in the U.S.
MuV-specific neutralizing antibody titers significantly higher in females (p=0.038)
Significantly enhanced secretion of MuV-specific MIP-1α, MIP-1β, TNFα, IL-6, and IL-1β in males
MuV-specific cellular immunity should be considered when developing more effective mumps vaccines
Acknowledgements
We thank the children and adolescents who served as subjects in this study and their families. We are grateful to the Mayo Clinic Immunochemical core lab as well as Dr. Steven A. Rubin and the Center for Biologics Evaluation and Research at the U.S. Food and Drug Administration for their assistance in laboratory assays and analysis. Within the Mayo Clinic Vaccine Research Group, Ms. Caroline L. Vitse provided excellent editorial work. We thank Mrs. Diane E. Grill and Mr. Nathaniel D. Warner for their skilled statistical contributions. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI-127365. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
Dr. Poland offers consultative advice to Johnson & Johnson/Janssen Global Services LLC, and is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland also offers consultative advice on vaccine development to Merck & Co., Medicago, GlaxoSmithKline, Sanofi Pasteur, Emergent Biosolutions, Dynavax, Genentech, Eli Lilly and Company, Kentucky Bioprocessing, Bavarian Nordic, AstraZeneca, Exelixis, Regeneron, Janssen, Vyriad, Moderna, and Genevant Sciences, Inc. Drs. Poland and Ovsyannikova hold patents related to vaccinia and measles peptide vaccines. Drs. Kennedy, Poland, and Ovsyannikova hold a patent related to vaccinia peptide vaccines. Drs. Poland, Kennedy, and Ovsyannikova have received grant funding from ICW Ventures for preclinical studies on a peptide-based COVID-19 vaccine. Dr. Kennedy has received funding from Merck Research Laboratories to study waning immunity to mumps vaccine. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubin S, Kennedy R, and Poland G, Emerging Mumps Infection. The Pediatric infectious disease journal, 2016. 35(7): p. 799–801. [DOI] [PubMed] [Google Scholar]
- 2.Dayan GH, et al. , Recent resurgence of mumps in the United States. N.Engl.J Med, 2008. 358(15): p. 1580–1589. [DOI] [PubMed] [Google Scholar]
- 3.Lewnard JA and Grad YH, Vaccine waning and mumps re-emergence in the United States. Sci Transl Med, 2018. 10(433). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barskey AE, et al. , Mumps outbreak in Orthodox Jewish communities in the United States. The New England Journal of Medicine, 2012. 367(18): p. 1704–13. [DOI] [PubMed] [Google Scholar]
- 5.Fields VS, et al. , Mumps in a highly vaccinated Marshallese community in Arkansas, USA: an outbreak report. Lancet Infect Dis, 2019. 19(2): p. 185–192. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease, C. and Prevention. Mumps. https://www.cdc.gov/mumps/outbreaks.html. 2020. February 11, 2020.
- 7.Davidkin I, Valle M, and Julkunen I, Persistence of anti-mumps virus antibodies after a two-dose MMR vaccination. A nine-year follow-up. Vaccine, 1995. 13(16): p. 1617–1622. [DOI] [PubMed] [Google Scholar]
- 8.Davidkin I, et al. , Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis, 2008. 197(7): p. 950–956. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy RB, et al. , Differential durability of immune responses to measles and mumps following MMR vaccination. Vaccine, 2019. 37(13): p. 1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mumps outbreak on a university campus--California, 2011. MMWR Morb Mortal Wkly Rep, 2012. 61(48): p. 986–9. [PubMed] [Google Scholar]
- 11.Albertson JP, et al. , Mumps Outbreak at a University and Recommendation for a Third Dose of Measles-Mumps-Rubella Vaccine - Illinois, 2015–2016. MMWR. Morbidity and mortality weekly report, 2016. 65(29): p. 731–4. [DOI] [PubMed] [Google Scholar]
- 12.Meissner HC What you need to know about mumps. https://www.aappublications.org/news/2016/10/03/IDSnapshot100316. 2016.
- 13.Henle G, Henle W, and et al. , Isolation of mumps virus from human beings with induced apparent or inapparent infections. J Exp Med, 1948. 88(2): p. 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wharton M, et al. , A large outbreak of mumps in the postvaccine era. Journal of Infectious Diseases, 1988. 158: p. 1253–1260. [DOI] [PubMed] [Google Scholar]
- 15.Cheek JE, et al. , Mumps outbreak in a highly vaccinated school population. Evidence for large-scale vaccination failure. Archives of Pediatric Adolescent Medicine, 1995. 149: p. 774–778. [DOI] [PubMed] [Google Scholar]
- 16.Klein SL, Marriott I, and Fish EN, Sex-based differences in immune function and responses to vaccination. Transactions of the Royal Society of Tropical Medicine and Hygiene, 2015. 109(1): p. 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ovsyannikova IG, et al. , Human leukocyte antigen and cytokine receptor gene polymorphisms associated with heterogeneous immune responses to mumps viral vaccine. Pediatrics, 2008. 121(5): p. e1091–e1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez A, et al. , Seroprevalence of measles, rubella, and mumps antibodies in Catalonia, Spain: results of a cross-sectional study. Eur.J Clin.Microbiol.Infect Dis, 2006. 25(5): p. 310–317. [DOI] [PubMed] [Google Scholar]
- 19.Weibel RE, et al. , Live, attenuated mumps-virus vaccine. 3. Clinical and serologic aspects in a field evaluation. New England Journal of Medicine, 1967. 276: p. 245–251. [DOI] [PubMed] [Google Scholar]
- 20.Vandermeulen C, Leroux-Roels G, and Hoppenbrouwers K, Mumps outbreaks in highly vaccinated populations: What makes good even better? Human Vaccines, 2009. 5(7): p. 494–6. [DOI] [PubMed] [Google Scholar]
- 21.Gourley TS, et al. , Generation and maintenance of immunological memory. Semin Immunol, 2004. 16(5): p. 323–33. [DOI] [PubMed] [Google Scholar]
- 22.Ovsyannikova IG, et al. , Influence of host genetic variation on rubella-specific T cell cytokine responses following rubella vaccination. Vaccine, 2009. 27: p. 3359–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhiman N, et al. , SNP/haplotype associations in cytokine and cytokine receptor genes and immunity to rubella vaccine. Immunogenetics, 2010. 62(4): p. 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy RB, et al. , Genetic polymorphisms associated with rubella virus-specific cellular immunity following MMR vaccination. Human Genetics, 2014. 133(11): p. 1407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haralambieva IH, et al. , Characterization of rubella-specific humoral immunity following two doses of MMR vaccine using proteome microarray technology. PLos ONE, 2017. 12(11): p. e0188149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crooke SN, et al. , Durability of humoral immune responses to rubella following MMR vaccination. Vaccine, 2020. 38(51): p. 8185–8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crooke SN, et al. , Associations between markers of cellular and humoral immunity to rubella virus following a third dose of measles-mumps-rubella vaccine. Vaccine, 2020. 38(50): p. 7897–7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haralambieva IH, Kennedy RB, Simon WL, Goergen KM, Grill DE, Ovsyannikova IG, Poland GA. Differential miRNA expression in B cells is associated with inter-individual differences in humoral immune response to measles vaccination. PLoS One. 2018. Jan 30;13(1):e0191812. doi: 10.1371/journal.pone.0191812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voigt EA, Haralambieva IH, Larrabee BL, Kennedy RB, Ovsyannikova IG, Schaid DJ, Poland GA. Polymorphisms in the Wilms Tumor Gene Are Associated With Interindividual Variations in Rubella Virus-Specific Cellular Immunity After MeaslesMumps-Rubella II Vaccination. J Infect Dis. 2018. Jan 30;217(4):560–566. doi: 10.1093/infdis/jix538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haralambieva IH, Ovsyannikova IG, Kennedy RB, Larrabee BR, Zimmermann MT, Grill DE, Schaid DJ, Poland GA. Genome-wide associations of CD46 and IFI44L genetic variants with neutralizing antibody response to measles vaccine. Hum Genet. 2017. Apr;136(4):421–435. doi: 10.1007/s00439-017-1768-9. Epub 2017 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ovsyannikova IG, et al. , HLA class II alleles and measles virus-specific cytokine immune response following two doses of measles vaccine. Immunogenetics, 2005. 56(11): p. 798–807. [DOI] [PubMed] [Google Scholar]
- 32.Ovsyannikova IG, et al. , Immunologic significance of HLA class I genes in measles virus-specific IFN-gamma and IL-4 cytokine immune responses. Immunogenetics, 2005. 57(11): p. 828–836. [DOI] [PubMed] [Google Scholar]
- 33.Rubin SA, et al. , Antibody induced by immunization with the Jeryl Lynn mumps vaccine strain effectively neutralizes a heterologous wild-type mumps virus associated with a large outbreak. J Infect.Dis, 2008. 198(4): p. 508–515. [DOI] [PubMed] [Google Scholar]
- 34.Karber G, Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Arch.Exp.Pathol.Pharmakol, 1931. 162 p. 480–483. [Google Scholar]
- 35.Umlauf BJ, et al. , Associations between demographic variables and multiple measles-specific innate and cell-mediated immune responses after measles vaccination. Viral Immunology, 2012. 25(1): p. 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voigt EA, et al. , Genetically defined race, but not sex, is associated with higher humoral and cellular immune responses to measles vaccination. Vaccine, 2016. 34(41): p. 4913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dominguez A, et al. , Rubella elimination programme strengthened through measles elimination programme in Catalonia. Vaccine, 2006. 24(9): p. 1433–1437. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson RM, Ovsyannikova IG, Vierkant RA, Pankratz VS, Poland GA. Independence of measles-specific humoral and cellular immune responses to vaccination. Hum Immunol. 2012. May;73(5):474–9. doi: 10.1016/j.humimm.2012.02.016. Epub 2012 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen W, Ye H, Zhang X, Huo L, Shen J, Zhu L, Wang X, Cui D. Elevated expansion of follicular helper T cells in peripheral blood from children with acute measles infection. BMC Immunol. 2020. Sep 1;21(1):49. doi: 10.1186/s12865-020-00379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong EZ, Gan ES, de Alwis R, Wijaya L, Ong XM, Zhang M, Wong AW, Cheung YB, Zellweger RM, Ooi EE, Low JG. Genomic signature of early T-cell response is associated with lower antibody titer threshold for sterilizing immunity. Antiviral Res. 2019. Jun;166:35–41. doi: 10.1016/j.antiviral.2019.03.013. Epub 2019 Mar 30. [DOI] [PubMed] [Google Scholar]
- 41.Dhiman N, et al. , Associations between cytokine/cytokine receptor single nucleotide polymorphisms and humoral immunity to measles, mumps and rubella in a Somali population. Tissue Antigens, 2008. 72(3): p. 211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortese MM, et al. , Mumps antibody levels among students before a mumps outbreak: in search of a correlate of immunity. The Journal of Infectious Diseases, 2011. 204(9): p. 1413–22. [DOI] [PubMed] [Google Scholar]
- 43.Plotkin SA, Correlates of protection induced by vaccination. Clin.Vaccine Immunol, 2010. 17(7): p. 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, et al. , IL-6 and IFNgamma are elevated in severe mumps cases: a study of 960 mumps patients in China. Journal of infection in developing countries, 2014. 8(2): p. 208–14. [DOI] [PubMed] [Google Scholar]
- 45.Jokinen S, et al. , Cellular immunity to mumps virus in young adults 21 years after measles-mumps-rubella vaccination. J Infect Dis - Note not in file - see duplicate record 10801 for in file copy, 2007. 196(6): p. 861–867. [DOI] [PubMed] [Google Scholar]
- 46.Gans H, et al. , Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis, 2001. 184(7): p. 817–826. [DOI] [PubMed] [Google Scholar]
- 47.Nakayama T, et al. , Evaluation of live trivalent vaccine of measles AIK-C strain, mumps Hoshino strain and rubella Takahashi strain, by virus-specific interferon-gamma production and antibody response. Microbiology and Immunology, 1990. 34(6): p. 497–508. [DOI] [PubMed] [Google Scholar]
- 48.Hanna-Wakim R, et al. , Immune responses to mumps vaccine in adults who were vaccinated in childhood. J Infect Dis, 2008. 197(12): p. 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beery AK and Zucker I, Sex bias in neuroscience and biomedical research. Neuroscience and biobehavioral reviews, 2011. 35(3): p. 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein SL and Flanagan KL, Sex differences in immune responses. Nat Rev Immunol, 2016. 16(10): p. 626–38. [DOI] [PubMed] [Google Scholar]
- 51.Villacres MC, et al. , Predominant type 1 CMV-specific memory T-helper response in humans: evidence for gender differences in cytokine secretion. Hum Immunol, 2004. 65(5): p. 476–85. [DOI] [PubMed] [Google Scholar]
- 52.Girón-González JA, et al. , Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol, 2000. 143(1): p. 31–6. [DOI] [PubMed] [Google Scholar]
- 53.Klein SL, Jedlicka A, and Pekosz A, The Xs and Y of immune responses to viral vaccines. Lancet Infect.Dis, 2010. 10(5): p. 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dooms H and Abbas AK, Life and death in effector T cells. Nat Immunol, 2002. 3(9): p. 797–8. [DOI] [PubMed] [Google Scholar]
- 55.Wu C. y., et al. , Distinct lineages of TH1 cells have differential capacities for memory cell generation in vivo. Nature Immunology, 2002. 3(9): p. 852–858. [DOI] [PubMed] [Google Scholar]
- 56.Schall TJ, et al. , Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med, 1993. 177(6): p. 1821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siebenlist U, Franzoso G, and Brown K, Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol, 1994. 10: p. 405–55. [DOI] [PubMed] [Google Scholar]
- 58.Szabo SJ, et al. , A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell, 2000. 100(6): p. 655–69. [DOI] [PubMed] [Google Scholar]
- 59.Lazarevic V, Glimcher LH, and Lord GM, T-bet: a bridge between innate and adaptive immunity. Nature Reviews Immunology, 2013. 13(11): p. 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee AJ and Ashkar AA, The Dual Nature of Type I and Type II Interferons. Frontiers in Immunology, 2018. 9(2061). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubin S, Mumps Vaccines, in Plotkin’s VACCINES, Plotkin S, et al. , Editors. 2017, Elsevier: Philadelphia, PA. p. pp. 663–687. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


