Abstract
Progressive pancytopenia is a common feature observed in DNA crosslink repair deficiency disorder, Fanconi anemia (FA). However, this phenotype has not been recapitulated in single FA gene knockout animal models. In this study, we analyzed hematological characteristics in zebrafish null mutants for two FA genes, fanca and fanco. In adult mutants, we demonstrate age-associated reduction in blood cell counts for all lineages, resembling progressive pancytopenia in FA patients. In larval mutants, we demonstrate vascular injury-induced thrombosis defects, particularly upon treatment with crosslinking agent diepoxybutane (DEB), indicating DNA damage induced inefficiency of thrombocytes.
Keywords: Fanconi anemia, Zebrafish knockouts, Pancytopenia, Thrombocytopenia
1. Introduction
Progressive bone marrow failure resulting in depletion of all blood cell lineages leading to pancytopenia is the most common consequence of Fanconi anemia (FA). In addition, FA patients display congenital abnormalities and predisposition to develop hematological neoplasms and solid tumors [1, 2]. FA is a rare, primarily recessive disorder caused by mutations in any of the known 22 genes (FANCA-FANCW), wherein the encoding proteins orchestrate repair of DNA damage caused by interstrand crosslinks. In fact, sensitivity to DNA crosslinking agents such as diepoxybutane (DEB), and the ensuing increased chromosomal breakage, is a hallmark of FA cells and serves as a reliable diagnostic test for FA.
Single FA gene knockouts in mouse models have not recapitulated the human phenotype well, particularly hematological presentations of pancytopenia [3–5], except that Fancp (Slx4) null mice demonstrated a decrease in the number of WBC and platelets [6]. Zebrafish is being considered as a model organism for investigations on inherited bone marrow failure syndromes, which includes FA [7]. Zebrafish mutants of FA genes fancl [8], brca2/fancd1 [9–11] and rad5/fancr [12] have been characterized; however, pancytopenia has not been reported in any of these mutants. Recently, we employed CRISPR/Cas methodologies and generated null mutants in zebrafish for 17 FA genes and found that they survive to adulthood and the embryos are sensitive to DEB, consistent with inactivation of the FA repair pathway [13]. We chose zebrafish mutants for two FA genes, fanca−/− (hg41) and fanco−/− (hg65), which carry a 2 bp deletion and 13 bp insertion, respectively, to study hematological manifestations by the methods developed in our laboratory [14–17]. FANCA and FANCO mutations are responsible for approximately 65% and 0.2% of FA patients, respectively, and thus represent the two extremes of FA incidence [1, 18]. We found that the mutant fish, as they age, displayed a decrease in blood cell counts for all lineages. We also found vascular injury-induced thrombosis defects in larvae, particularly upon treatment with DEB.
2. Materials and methods
2.1. Zebrafish husbandry, ethics statement and generation of knockouts
All zebrafish experiments were performed in compliance with the University of North Texas-Institutional Animal Care and Use Committee (IACUC) approved protocols and the National Institutes of Health (NIH) guidelines for animal handling and research under NHGRI Animal Care and Use Committee (ACUC) approved protocol G-17–3 assigned to SCC. Zebrafish husbandry was performed as described previously [19]. Wildtype zebrafish strain TAB5 was used for generation of mutants. Knockout mutants of fanca and fanco were generated using CRISPR/Cas9 method as described [13].
2.2. Blood collection
Zebrafish were placed on a clean paper towel on the lateral side. The zebrafish head was covered with a wet Kimwipe, and the skin surface was gently wiped using the Kimwipe. Using 12 cm Noyes Scissors (World Precision Instruments, Sarasota, FL) with 15 mm blades, a lateral incision was made between the second and fourth black stripes located in between the ventral and dorsal fins [14]. The above procedure was approved by the Institutional Animal Care and Use Committee of the University of North Texas, and animal experiments were performed in compliance with the institutional guidelines.
2.3. Mepacrine labelling and FACS analysis of blood cells
Two μL of blood welling from the wounded site was added to a tube containing 500 μL 1X PBS and 1 μL of 20 mM mepacrine. Labelled cells were subjected to flow cytometry using BD Accuri C6 Plus flow cytometer, and the blood cells with mepacrine fluorescence were separated. The graph was plotted with FITC fluorescence (FITC A) on X-axis and side scatter (SSC) on Y-axis. The dots representing different fluorescence intensities were gated appropriately [15]. The same gates were used for all experiments.
2.4. Hematocrit assay
The caudal artery of zebrafish was clipped as described above in 2.3. section. A microhematocrit capillary tube (Fisher Scientific, Hampton, NH) was placed on the blood welling from the blood vessel and allowed to flow into the tube by capillary action. The end of the capillary tube containing blood was then placed vertically on the Critoseal sealer (Oxford Labware, St Louis, MO) such that the blood faced it. The tube was then pressed gently such that sealing was complete. It was then centrifuged for 5 min in a microcentrifuge (MicroMB, International Medical Equipment, North Andover, MA) to separate the plasma from RBC. After centrifugation, the capillary tubes were photographed and loaded into a PowerPoint file. The pictures were magnified such that the width of each tube was identical. Straight lines were drawn to the boundaries of the plasma and the lengths of the RBC layer alone, and the plasma and RBC layers together were measured by calculating line size using the PowerPoint program. From these values, the RBC percentages were calculated [14].
2.5. Laser thrombosis assay
Five days post-fertilization (dpf) larvae in 500 μL aquaria water were anesthetized by adding 10 μL of 10 mM MS222. After larvae were anesthetized, 500 μL of 1.6% low melting agarose maintained at 35°C was added and the contents mixed by inverting gently 2–3 times. The agarose along with larvae were then poured into a rectangular chamber, made using a rectangular rubber gasket pressed on to a thin coat of petroleum jelly on the microscopic slide. The slide carrying larvae adjusted to lay on their lateral sides were focused under microscope using 20X objective. The nitrogen-pulsed laser routed through coumarin 440 dye with 445 nm (Micro Point Laser, Stanford Research Systems Inc., Sunnyvale, CA) set at 12 hits per cycle was delivered through the fluorescence port such that it hit either a dorsal aorta or caudal vein area around 5–6 somites away from the anal pore towards the tail end [16]. The laser injury-initiated vessel occlusion was measured by time to occlusion (TTO), which is the time taken to occlude the vessel from the time the laser hits the vessel until the vessel was completely occluded. If the vessels did not occlude after 120 sec, TTO was recorded as 120 sec. For DEB treatment experiments, the embryos were treated with 0.2 μg/mL DEB (Sigma-Aldrich, St. Louis, MO) in egg water with methylene blue at 1 dpf and incubated until 5 dpf.
2.6. Rescue experiment
Zebrafish embryo staging and microinjections were performed as described previously [19]. Primers with SP6 promoter and poly(A) tail overhangs (Table 1) were used to amplify the sense and anti-sense coding region of zebrafish fanco utilizing a wildtype fish cDNA template. mMESSAGE mMACHINE™ SP6 Transcription Kit (Thermo Fisher Scientific) was used for in vitro synthesis of capped RNA as per manufacturer’s instructions. Synthesized RNAs were purified by lithium chloride precipitation method and checked for stability and size on agarose gel prior to injection. A 200 pg dose of sense or anti-sense RNA was injected into one-cell stage embryos collected from facno null incrosses.
Table 1:
Primers used for amplification of sense and anti-sense strands of zebrafish fanco.
| Primer | Sequence (5’–> 3’) |
|---|---|
| fanco_sense_SP6 | ATTTAGGTGACACTATAGAAGAGGCCACCATGCACAGGACTGTACCGAG |
| fanco_sense_polyA | TTTTTTTTTTTTTTTTTTT TATAGACCCTCAAGACAGATC |
| fanco_anti-sense_SP6 | ATTTAGGTGACACTATAGAAGAG TATAGACCCTCAAGACAGATC |
| fanco_anti-sense_polyA | TTTTTTTTTTTTTTTTTTTGCCACCATGCACAGGACTGTACCGAG |
3. Results and discussion
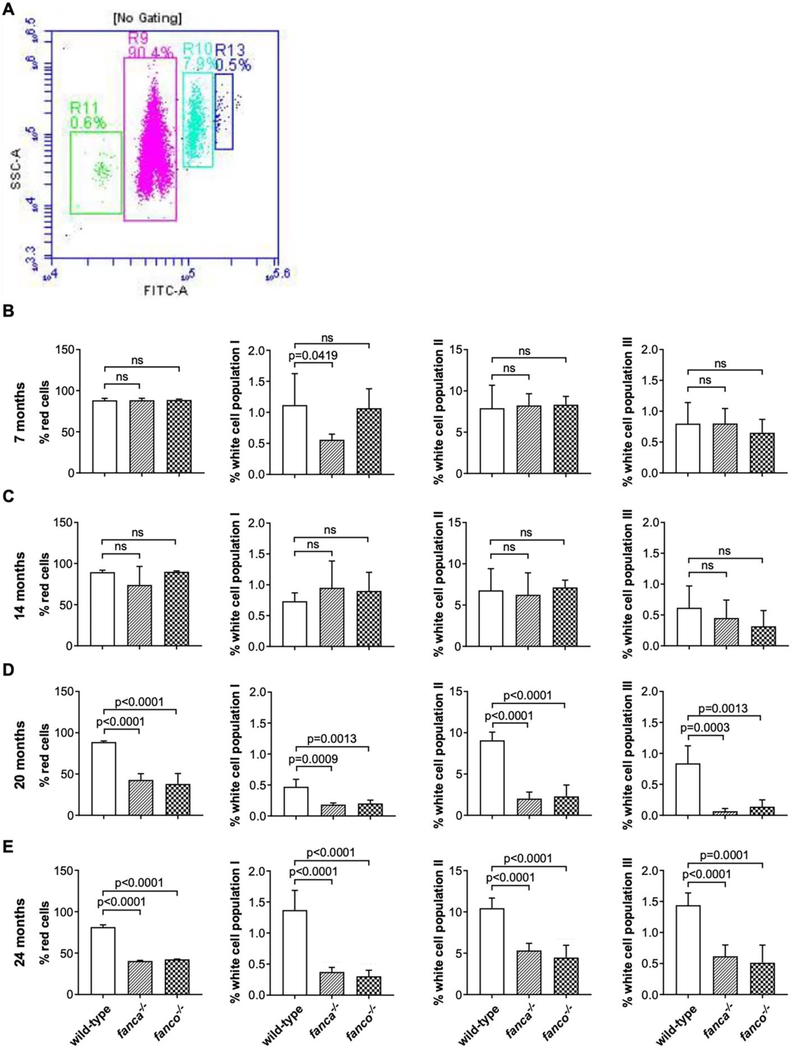
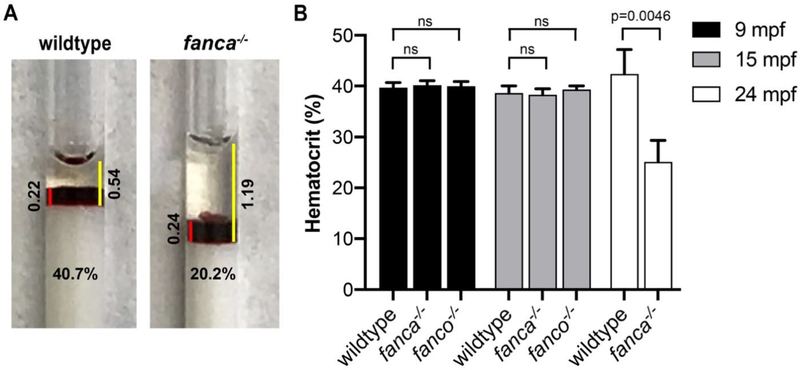
To test for pancytopenia, we analyzed mature blood from fanca−/− and fanco−/− null mutants at 7-months post-fertilization (mpf). Mepacrine labelled blood cells were subjected to flow cytometry to measure percentages of four different populations of cells corresponding to red cells, white cell population I, white cell population II and white cell population III as previously described [15, 17]. A representative image of the four gated population of cells is shown in Fig. 1A. We found no difference in the blood cell populations between mutants and wildtype controls (Fig. 1B). We then examined the mutants at 14, 20, and 24 mpf to check whether aging has an effect. While we again found no difference in blood cell populations between mutants and wildtype at 14 mpf (Fig. 1C), we found a greater than 50% reduction in all four cell populations in both 20 and 24 mpf mutants compared to wildtype controls (Fig. 1D–E). To corroborate the above findings, we measured hematocrits in 9, 15, and 24 mpf mutants to calculate RBC content using microhematocrit capillary tubes as previously described [14]. Representative images of the hematocrit for wildtype and fanca−/− mutant (24 mpf) are shown in Fig. 2A. The hematocrits did not show any difference between mutants and wildtype controls at 9 and 15 mpf; however, in 24 mpf mutants, the hematocrit values were significantly reduced (~50%) in fanca−/− mutants compared to wildtype controls (Fig. 2B). These results are consistent with flow cytometry observations.
Figure 1: Age-dependent pancytopenia observed in fanca and fanco null fish.
Mapacrine labelled zebrafish blood (2 μL) from caudal artery of fanca−/− and fanco−/− mutants along with age matched wildtype controls were subjected to flow cytometry using BD Accuri C6 Plus Flow Cytometer. (A) Representative dot plot shows percentages of blood cells that are gated and shown in pink (R9), green (R11), sky blue (R10) and navy blue (R13) representing red cells, white cell population I, white cell population II, and white cell population III, respectively. The fluorescence is given on x-axis as FITC-A and the side scatter is shown on y-axis as SSC-A. (B-E) Bar graphs showing the percentage of blood cells for all four populations in 7, 14, 20, and 24 mpf fish. The panels from left to right show the percentage of red cells, white cell population I, white cell population II, and white cell population III, respectively. Statistical significance was assessed by one-way ANOVA followed by Dunnett’s multiple comparison test. n=6, p-value <0.05 was considered significant; ns=not significant.
Figure 2: Reduced hematocrit levels observed in older fanca null fish.
Microhematocrit capillary tube with zebrafish blood from caudal artery of wildtype control, fanca−/− and fanco−/− mutants were sealed and centrifuged to separate the plasma from RBC. (A) Representative photographs of microcapillary tubes showing hematocrit for wildtype and fanca−/− (24 mpf) mutant. Red line indicates the length of RBC layer and yellow line indicates the length of RBC plus plasma layer. The length of the lines was used to calculate hematocrit levels. (B) Bar graphs showing the hematocrit levels in 9, 15 and 24 mpf fish. Hematocrit levels for fanco−/− fish at 24 mpf could not be collected due to unavailability of fish. Statistical significance was assessed by one-way ANOVA followed by Dunnett’s multiple comparison test. p-value <0.05 was considered significant, n=4.
These findings in zebrafish FA gene mutants clearly demonstrate reduction in various blood cell lineages, which is consistent with pancytopenia observed in FA patients. Reduction in thrombocytes precursor cells was observed in rad51/fancr mutant larvae [12]. However, in contrast to the larval studies, our results demonstrate pancytopenia in adult mutants by measuring the mature blood cells in circulation. Our results also show that pancytopenia in FA gene mutants is an age-dependent phenotype, consistent with progressive bone marrow failure observed in FA patients. It is likely that in aged zebrafish, the accumulation of other mutations may exacerbate the effects of FA gene mutations. Interestingly, since the phenotype recapitulated in mutants of two independent FA genes, the aging has a similar effect in the absence of efficient FA pathway in both these mutant lines.
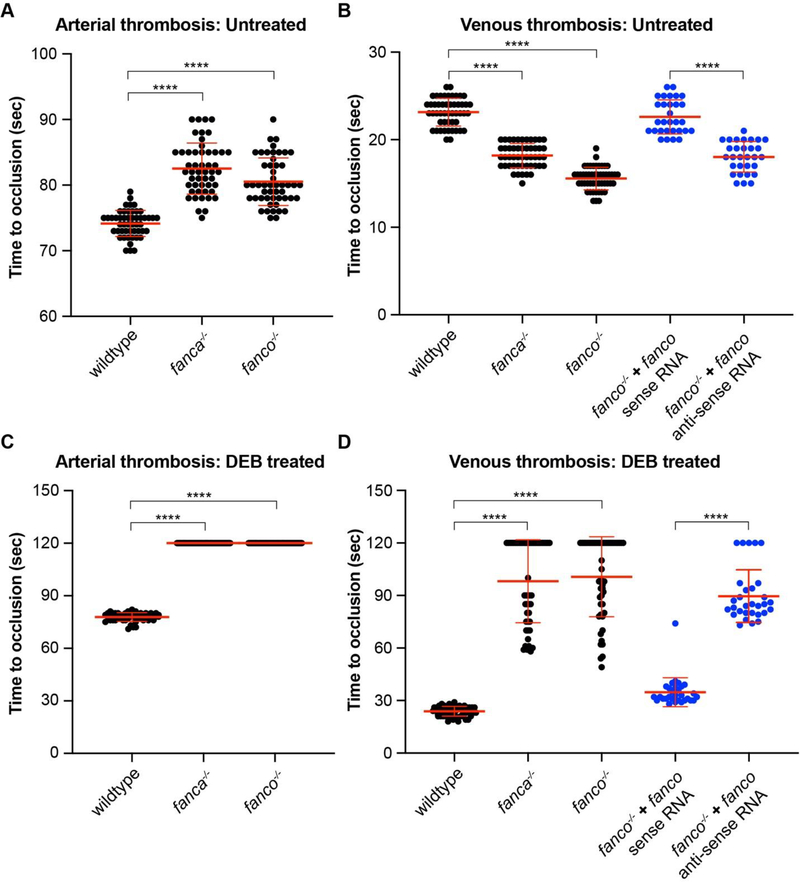
Since pancytopenia, including thrombocytopenia, was observed in older fanca and fanco mutant fish, we hypothesized that young larvae might not be thrombocytopenic. We tested this using arterial and venous thrombosis assay in 5-day post-fertilization larvae by measuring time to occlusion (TTO) of blood upon laser injury as previously described [16]. Surprisingly, we found a modest prolongation of arterial thrombosis in both fanca and fanco mutant larvae, suggesting marginal thrombocytopenia (Fig. 3A). In contrast, the venous thrombosis in mutant larvae was found to have modestly shortened TTO (Fig. 3B). The shortening of venous TTO in untreated mutant larvae cannot be explained by reduction in number of thrombocytes and this could be due to red cell lysis in these mutant larvae probably due to complement-sensitive red cell lysis as observed in aplastic anemia [20].
Figure 3: Laser-induced thrombosis assay reveals defective clotting in fanca and fanco null larvae.
Laser thrombosis assay measuring time to occlusion (TTO) upon laser injury of caudal vein or artery was employed to collect data from 5-day post-fertilization larvae of wildtype controls, fanca−/− and fanco−/− mutants. TTO longer than 120 seconds was taken as 120 seconds for plotting. (A-D) Scatter dot plots showing TTOs measured from arterial and venous thrombosis assay. (A) Arterial thrombosis measured as TTO are plotted for untreated larvae, n=50. (B) Venous thrombosis measured as TTO are plotted for untreated larvae (black), n=50. (C) Arterial thrombosis measured as TTO are plotted for DEB treated larvae, n=50. (D) Venous thrombosis measured as TTO are plotted for DEB treated larvae (black), n=50. (B and D) Rescue experiment for venous thrombosis defect in fanco−/− mutants by injecting 200 pg of either sense or anti-sense fanco transcript into one-cell stage embryos (blue), n=30. Statistical significance was assessed by one-way ANOVA followed by Dunnett’s multiple comparison test. p-value <0.05 was considered significant. **** represents p ≤ 0.0001.
We also measured arterial and venous thrombosis upon laser injury in DEB treated larvae to check its effect on thrombosis. DEB treatment was administered between day 1 and 5 at 0.2 μg/mL concentrations that do not cause any visible abnormalities in embryo/larvae [13]. Strikingly, DEB treatment resulted in exacerbated prolongation of both arterial (Fig. 3C) and venous thrombosis (Fig. 3D). These results are consistent with severe thrombocytopenia phenotype, although DEB could cause other changes in the genome that could result in defective thrombocyte aggregation. Since DEB is well-known to cause DNA damage by crosslinking, it is expected that it will enhance the effect of DNA repair defects in these mutants. This probably resulted in thrombocytopenia mimicking the phenotype of older FA zebrafish adults.
We then investigated whether lack of Fanco has resulted in altered thrombosis in fanco−/− larvae by measuring venous thrombosis in both untreated and DEB treated larvae after complementation with either sense or anti-sense transcripts of zebrafish fanco. Primers with SP6 promoter and poly(A) tail overhangs (Table 1) were used to amplify the sense and anti-sense coding region of fanco utilizing a wildtype zebrafish cDNA template. mMESSAGE mMACHINE™ SP6 Transcription Kit (Thermo Fisher Scientific) was used for in vitro synthesis of capped RNA as per manufacturer’s instructions and purified by lithium chloride precipitation method. A 200 pg dose of sense or anti-sense RNA was injected into one-cell stage embryos collected from facno null incross. Sense RNA injection resulted in near normal venous TTO in both untreated (Fig. 3B) and DEB treated (Fig. 3D) larvae, suggesting that the altered thrombosis is due to loss of Fanco protein function. As expected, anti-sense RNA injection did not rescue abnormal venous thrombosis.
4. Conclusions
The results described here established that FA gene mutations cause pancytopenia in adult zebrafish. This is the first demonstration of pancytopenia among zebrafish FA gene mutants. In larvae, these mutations cause modest increase in arterial TTO that is exacerbated by DEB treatment. Thus, taken together, our results support that zebrafish could be used to model human hematological manifestations in FA. This model would be useful in the identification of genetic suppressors by ENU mutagenesis or by genome-wide piggyback knockdowns [21]. Additionally, it should be possible to study molecular consequences caused by DEB treatment in FA gene mutant embryo/larvae.
Funding
The work was supported by National Institutes of Health (grant number DK117384 to P.J.) and by the intramural research program of the National Human Genome Research Institute, National Institutes of Health (R.R-B., M.R., B.C., and S.C.C.).
Footnotes
Declaration of competing interest
The authors have no competing interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Niraj J, Farkkila A, D’Andrea AD, The Fanconi Anemia Pathway in Cancer, Annu Rev Cancer Biol 3 (2019) 457–478, 10.1146/annurev-cancerbio-030617-050422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kottemann MC, Smogorzewska A, Fanconi anaemia and the repair of Watson and Crick DNA crosslinks, Nature 493 (7432) (2013) 356–63, 10.1038/nature11863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guitton-Sert L, Gao Y, Masson JY, Animal models of Fanconi anemia: A developmental and therapeutic perspective on a multifaceted disease, Semin Cell Dev Biol 113 (2021) 113–131, 10.1016/j.semcdb.2020.11.010 [DOI] [PubMed] [Google Scholar]
- [4].Bakker ST, de Winter JP, te Riele H, Learning from a paradox: recent insights into Fanconi anaemia through studying mouse models, Dis Model Mech 6 (1) (2013) 40–7, 10.1242/dmm.009795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Parmar K, D’Andrea A, Niedernhofer LJ, Mouse models of Fanconi anemia, Mutat Res 668 (1–2) (2009) 133–40, 10.1016/j.mrfmmm.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Crossan GP, van der Weyden L, Rosado IV, Langevin F, Gaillard PHL, McIntyre RE, Sanger Mouse Genetics P, Gallagher F, Kettunen MI, Lewis DY, Brindle K, Arends MJ, Adams DJ, Patel KJ, Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia, Nat Genet 43 (2) (2011) 147–52, 10.1038/ng.752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oyarbide U, Topczewski J, Corey SJ, Peering through zebrafish to understand inherited bone marrow failure syndromes, Haematologica 104 (1) (2019) 13–24, 10.3324/haematol.2018.196105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rodriguez-Mari A, Canestro C, Bremiller RA, Nguyen-Johnson A, Asakawa K, Kawakami K, Postlethwait JH, Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis, PLoS Genet 6 (7) (2010) e1001034, 10.1371/journal.pgen.1001034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rodriguez-Mari A, Wilson C, Titus TA, Canestro C, BreMiller RA, Yan YL, Nanda I, Johnston A, Kanki JP, Gray EM, He X, Spitsbergen J, Schindler D, Postlethwait JH, Roles of brca2 (fancd1) in oocyte nuclear architecture, gametogenesis, gonad tumors, and genome stability in zebrafish, PLoS Genet 7 (3) (2011) e1001357, 10.1371/journal.pgen.1001357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shive HR, West RR, Embree LJ, Azuma M, Sood R, Liu P, Hickstein DD, brca2 in zebrafish ovarian development, spermatogenesis, and tumorigenesis, Proc Natl Acad Sci U S A 107 (45) (2010) 19350–5, 10.1073/pnas.1011630107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kroeger PT Jr., Drummond BE, Miceli R, McKernan M, Gerlach GF, Marra AN, Fox A, McCampbell KK, Leshchiner I, Rodriguez-Mari A, BreMiller R, Thummel R, Davidson AJ, Postlethwait J, Goessling W, Wingert RA, The zebrafish kidney mutant zeppelin reveals that brca2/fancd1 is essential for pronephros development, Dev Biol 428 (1) (2017) 148–163, 10.1016/j.ydbio.2017.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Botthof JG, Bielczyk-Maczynska E, Ferreira L, Cvejic A, Loss of the homologous recombination gene rad51 leads to Fanconi anemia-like symptoms in zebrafish, Proc Natl Acad Sci U S A 114 (22) (2017) E4452–E4461, 10.1073/pnas.1620631114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ramanagoudr-Bhojappa R, Carrington B, Ramaswami M, Bishop K, Robbins GM, Jones M, Harper U, Frederickson SC, Kimble DC, Sood R, Chandrasekharappa SC, Multiplexed CRISPR/Cas9-mediated knockout of 19 Fanconi anemia pathway genes in zebrafish revealed their roles in growth, sexual development and fertility, PLoS Genet 14 (12) (2018) e1007821, 10.1371/journal.pgen.1007821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Deebani A, Iyer N, Raman R, Jagadeeswaran P, Effect of MS222 on Hemostasis in Zebrafish, J Am Assoc Lab Anim Sci 58 (3) (2019) 390–396, 10.30802/AALAS-JAALAS-18-000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sundaramoorthi H, Panapakam R, Jagadeeswaran P, Zebrafish thrombocyte aggregation by whole blood aggregometry and flow cytometry, Platelets 26 (7) (2015) 613–9, 10.3109/09537104.2015.1018879 [DOI] [PubMed] [Google Scholar]
- [16].Jagadeeswaran P, Carrillo M, Radhakrishnan UP, Rajpurohit SK, Kim S, Laser-induced thrombosis in zebrafish, Methods Cell Biol 101 (2011) 197–203, 10.1016/B978-0-12-387036-0.00009-8 [DOI] [PubMed] [Google Scholar]
- [17].Thattaliyath B, Cykowski M, Jagadeeswaran P, Young thrombocytes initiate the formation of arterial thrombi in zebrafish, Blood 106 (1) (2005) 118–24, 10.1182/blood-2004-10-4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang AT, Smogorzewska A, SnapShot: Fanconi anemia and associated proteins, Cell 160 (1–2) (2015) 354–354 e1, 10.1016/j.cell.2014.12.031 [DOI] [PubMed] [Google Scholar]
- [19].Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, Stages of embryonic development of the zebrafish, Dev Dyn 203 (3) (1995) 253–310, 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- [20].Ben-Bassat I, Brok-Simoni F, Ramot B, Complement-sensitive red cells in aplastic anemia, Blood 46 (3) (1975) 357–61, [PubMed] [Google Scholar]
- [21].Raman R, Ryon M, Jagadeeswaran P, RNaseH-mediated simultaneous piggyback knockdown of multiple genes in adult zebrafish, Sci Rep 10 (1) (2020) 20187, 10.1038/s41598-020-76655-5 [DOI] [PMC free article] [PubMed] [Google Scholar]