Abstract
Background
COVID-19 pandemic spread around the world like an infectious disease that presents waved effects on patients. Some patients needed ICU and respiratory support. Some patients only had flu-like symptoms. Cytokine storm and elevated ROS were serious problems for treatment. Apoptotic genes and CYP Family are part of these mechanisms.
Aim
In this study, our aim was to examine the gene expression CYP2E1 and Caspase-3 in patients with COVID-19 infection.
Method
60 COVID-19(+) patients (ICU and non-ICU patients) and 30 healthy volunteers were enrolled to study. To measure the level of gene expression qPCR was used. The 2-ΔΔCt method was utilized to analyze gene expression.
Results
The expression of CYP2E1 and Caspase-3 genes showed a significant discrepancy between patients and healthy individuals. Caspase-3 expression increased (p=0,0041) but CYP2E1 expression decreased (p=0,0214) in COVID-19 patients compared to healthy individuals. Both levels of gene expression were lower in patients with affected lungs than patients with unaffected lungs (p<0,05). Laboratory findings including d-Dimer, LDH, platelet count, lymphocyte count were related to both gene expressions (p<0,05). We found no correlation between CYP2E1 and Caspase-3 expressions.
Conclusion
The expression of Caspase-3 demonstrated apoptotic situations of patients but was not related to the CYP2E1 expression level. CYP2E1 gene expression is an important actor to metabolize endogens and xenobiotics however, COVID-19 patients demonstrated decreased CYP2E1 expression. CYP2E1 and Caspase-3 gene expression levels may be used as a diagnostic tool for COVID-19 patients.
Keywords: CCYP2E1, COVID-19, Caspase-3, SARS-CoV-2
1. Introduction
Coronavirus disease-2019 (COVID-19) started in Wuhan, China expanded to world and effect millions (Lai et al., 2020). COVID-19 infection has been a critical disease that causes patients' death (Azkur et al., 2020) by declared and undeclared many reasons. Although hospitalized rate can change according to published studies, however clinic severity may change accompanied by age and the presence of comorbid disease (Richardson et al., 2020). In a recent study among hospitalized patients 14,2% needed to the intensive care unit (ICU), and 12,2% were provided by invasive mechanical ventilation (Richardson et al., 2020). Some patients need to intensive care unit (ICU) and some of them die, some exhibit just flu-like symptoms and quick recovery without any medicine and, some did not feel anything about infection when had COVID-19 virus (Becker, 2020).
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leads to releasing cytokines that cause cytokine storm, organ injury, and multiple organ failure (Jose and Manuel, 2020). This cytokine storm induced acute respiratory distress syndrome (ARDS) (Tisoncik et al., 2012). Cytokine storm may be a result of cell death in multiple organs. Apoptosis is one of the cell death processes shown in multiple diseases (Elmore, 2007).
Oxidative stress seen in the COVID-19 patients increases the production of reactive oxygen species (ROS) (Laforge et al., 2020). A high level of ROS production may results in opening mitochondrial membrane pores and activating the apoptosis process (Niizuma et al., 2009). Caspase-3 tends to lead to an apoptotic process. Caspase-3 is activated by the initiator caspase and active Caspase-3 catalyzes many cellular proteins which functions at DNA fragmentation, nuclear collapse, chromatin condensation (Elmore, 2007).
Cytochrome P450 (CYP) superfamily including heme-containing monooxygenase enzymes involved in oxidative transformation of drugs (Guengerich, 2008). These enzymes can be found mainly in the liver microsomes and extrahepatic tissues (Ding and Kaminsky, 2003). CYP2E1 metabolizes small organic molecules, endogenous compounds, clinically used drugs, toxic chemicals and environmental contaminants (Abdelmegeed et al., 2017; Novak and Woodcroft, 2000). Changes in gene expression levels of CYP2E1 in different diseases (Helmig, 2010) and its relation with cytokine activities have been shown (Cederbaum et al., 2012). Furthermore treatment of COVID-19 infection results in CYP downregulation (Fakhouri et al., 2020).
CYP2E1 and Caspase-3 are crucial proteins that lead to increase ROS (Hodges et al., 2007; Laforge et al., 2020; Niizuma et al., 2009; Schattenberg et al., 2004). CYP2E1 metabolizes clinically used drugs (Abdelmegeed et al., 2017) and enhanced CYP2E1 may cause increasing ROS production that leads to activate apoptotic genes (Hodges et al., 2007; Laforge et al., 2020; Schattenberg et al., 2004). So, we believed that CYP2E1 expression level may be a prognostic or diagnostic parameter to COVID-19 infection. Besides, changes in CYP2E1 expression may be related to Caspase-3 regulation through alteration of ROS level in COVID-19 patients. In this study we aimed to investigate Caspase-3 and CYP2E1 expression and to evaluate relation between gene expression and demographic-clinical data in patients with COVID-19 infection.
2. Material and methods
2.1. Patients and study design
This study was approved by the Ethics Committee of the Ataturk University Clinical Researches of Ethical Committee (NO;4) and carried out with the permission of the Ministry of Health .60 patients diagnosed with COVID-19(+) including 30 ICU patients and 30 non-ICU patients in Erzurum Regional Education and Research Hospital and 30 COVID-19 (-) healthy volunteers were enrolled. All volunteers had PCR tests to COVID-19. All patients and volunteers and/or their legally authorized representative educated about the study and gave written informed consent. Demographic data, clinical information and laboratory findings including White Blood Cell (WBC), neutrophil (Neu), lymphocyte(LYM), platelet(PLT), hemoglobin, hematocrit (HCT), albumin, alanine aminotransferase(ALT), aspartate aminotransferase (AST), bilirubin(total), lactate dehydrogenase(LDH), procalcitonin, C-reactive protein (CRP), D-dimer, creatinine, fibrinogen, blood urea nitrogen (BUN) of volunteers were collected from hospital database.
2.2. RNA isolation, cDNA synthesis, qPCR
Peripheral blood samples in ethylenediaminetetraacetic acid disodium salt (EDTA) tubes were collected from all patients and healthy volunteers. The samples were stored at +4°C for a short time. RNA isolation was performed by EcoPURE Total RNA Kit (Turkey) from 100μl peripheral blood samples. Total RNA was measured by an Epoch Spectrophotometer System and Take3 Plate (BioTek, USA). iScript cDNA Synthesis Kit (BioRad, USA) was used to cDNA synthesis. Gene expression experiments were carried out by Bio-Rad CFX-96 real time polymerase chain reactions (RT-PCR=qPCR) device. qPCR reactions were realized via SSoAdvanced Universal SYBR Green Supermix (BioRad, USA). β-Actin was utilized as an internal control gene. All reactions were triplicated. Gene expression analysis was evaluated by 2-ΔΔCt method (Livak and Schmittgen, 2001).
2.3. Statistically analysis
All experimental results were assessed with GraphPad Prism (version 5; San Diego, CA). To evaluate data distribution Kolmogorov-Smirnov was used. Two group analysis was realized by two-tailed student t-test, for normally distributed data, or Mann Whitney U, for not normally distrusted data. Categorical variables were evaluated by the χ2 test or Fisher's exact test. p value <0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical data of patients
60 COVID-19(+) patients and 30 healthy volunteers have enrolled the study. 30 patients were from the ICU and 13% of them died. Non-ICU patients had just flu-like symptoms but were not hospitalized. Laboratory findings were obtained from hospital database and summarized in Table 1 .
Table 1.
Laboratory findings of COVID-19 patients.
| Parameters (normal range) | Non-ICU patients (n (%)) | ICU patients (n (%)) | p-value | |
|---|---|---|---|---|
| White blood cell count (×109/L; 4,49-12,68) | ||||
| Low | 0 | 1 (4) | *0,0247 | |
| Normal | 21 (100) | 19 (76) | Ref | |
| High | 0 | 5 (20) | ||
| Neutrophils (×109/L; 2,04–7,54) | ||||
| Low | 0 | 0 | 0,0839 | |
| Normal | 19 (90) | 17 (68) | Ref | |
| High | 2 (10) | 8 (32) | ||
| Lymphocytes (×109/L; 1,21–3,77) | ||||
| Low | 1 (5) | 14 (56) | ***0,0003 | |
| Normal | 20 (95) | 11 (44) | Ref | |
| High | 0 | 0 | ||
| Platelets (×109/L; 152-383) | ||||
| Low | 1 (5) | 8 (32) | *0,0132 | |
| Normal | 20 (95) | 16 (64) | Ref | |
| High | 0 | 1 (4) | ||
| Hemoglobin (g/dL; 12,2-15,9) | ||||
| Low | 3 (14) | 8 (32) | Ref | 0,1878 |
| Normal | 12 (57) | 14 (56) | ||
| High | 6 (29) | 3 (12) | ||
| Hematocrit (%; 36,4-47,2) | ||||
| Low | 2 (10) | 6 (6) | 0,2981 | |
| Normal | 12 (57) | 15 (60) | ||
| High | 7 (33) | 4 (16) | Ref | |
| Albumin (g/L; 32-48) | ||||
| Low | 0 | 4 (16) | **0,0022 | |
| Normal | 14 (67) | 21 (84) | ||
| High | 7 (33) | 0 | Ref | |
| Alanine aminotransferase (U/L; 7–40) | ||||
| Low | 0 | 0 | 0,7624 | |
| Normal | 14 (67) | 15 (60) | Ref | |
| High | 7 (33) | 10 (40) | ||
| Aspartate aminotransferase (U/L; 13–40) | ||||
| Low | 1 (5) | 0 | 0,0852 | |
| Normal | 13 (62) | 10 (40) | ||
| High | 7 (33) | 15 (60) | Ref | |
| Total bilirubin (mg/dL; 0,2–1,1) | ||||
| Low | 0 | 0 | *0,0274 | |
| Normal | 20 (95) | 17 (68) | Ref | |
| High | 1 (5) | 8 (32) | ||
| Lactate dehydrogenase (U/L; 120–246) | ||||
| Low | 1 (5) | 0 | 0,4390 | |
| Normal | 16 (76) | 22 (88) | Ref | |
| High | 4 (19) | 3 (12) | ||
| Procalcitonin (ng/mL; 0–0.5) | ||||
| Low | 0 | 0 | **0,0037 | |
| Normal | 8 (38) | 4 (16) | ||
| High | 5 (24) | 17 (68) | Ref | |
| C reactive protein (mg/L; 0–0,5) | ||||
| Low | 0 | 0 | *0,0365 | |
| Normal | 6 (29) | 1 (4) | ||
| High | 15 (71) | 24 (96) | Ref | |
| D-dimer (μg/ml; 0–500) | ||||
| Low | 0 | 0 | **0,0024 | |
| Normal | 14 (67) | 5 (20) | ||
| High | 7 (33) | 20 (80) | Ref | |
| Creatinine (mg/dL; 0,55-1,02) | ||||
| Low | 4 (19) | 1 (4) | ***0,0009 | |
| Normal | 16 (76) | 11 (44) | ||
| High | 1 (5) | 13 (52) | Ref | |
| Fibrinogen (mg/dL; 200-400) | ||||
| Low | 0 | 1 (4) | 0,1317 | |
| Normal | 10 (48) | 18 (72) | Ref | |
| High | 11 (52) | 6 (24) | ||
| BUN (mg/dL; 9-23) | ||||
| Low | 1 (5) | 0 | **0,008 | |
| Normal | 19 (90) | 13 (52) | Ref | |
| High | 1 (5) | 12 (48) | ||
p values comparing ICU care and non-ICU care are from χ2, Fisher's exact test. Laboratory values were not available for all patients. BUN; blood urea nitrogen; *p < 0,05 was considered statically significant. Bolded values are statically significant.
The mean age was 60±2,98 (mean±std) for patients (non-ICU 46±19,7 and ICU 72±11) and 48±3,45 (mean±std) for healthy individuals. Number of male and female individuals were equal. The most of infected patients had comorbidities (∼74%) including diabetes, hypertension, asthma, cardiovascular disease. A few healthy volunteers had disease (%18) like hypertension, chronic obstructive pulmonary disease and hepatitis C. Lung of the 65%of patients were affected by COVID-19 infection.
3.2. Comparison of Laboratory Findings Between non-ICU and ICU Patients
Laboratory findings of patients were summarized in Table 1 and data of fourteen patients were not accessible. Depend on patients' status as ICU and non-ICU, WBC cell count showed significant difference between cohorts. Number of patients with high WBC cell count was higher in ICU patients than non-ICU patients (p=0,0247). Lymphocytopenia and thrombocytopenia were more common in ICU patients than non-ICU patients (p=0,0003, p=0,0132, respectively). Patients with low levels of albumin was seen in just ICU patients (p = 0,0022). The number of patients with high level of bilirubin (total), procalcitonin, CRP, D-dimer, creatinine and BUN were more common in ICU patients than non-ICU patients (p=0,0274; p=0,0037; p=0,0365; p=0,0024; p=0.0009; p=0,008, respectively). The frequency of patients with high ALT, AST levels and high Neu cell count were higher in ICU patients than non-ICU patients (p=0,7624; p=0,0852; p=0,0839, respectively) The number of patients with low hemoglobin and hematocrit levels is higher in ICU patients than non-ICU patients (p=0,1878; p=0,2981, respectively). Higher frequency of patients with high fibrinogen and LDH levels were observed in non-ICU patients than ICU-patients (p=0,1317; p=0,4390, respectively).
3.3. Gene expression of CYP2E1 and Caspase3
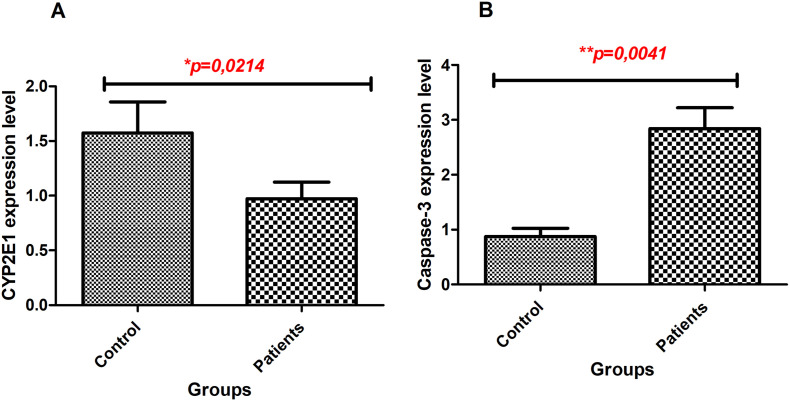
In COVID-19(+) patients (ICU and non-ICU) had lower gene expression of CYP2E1 than COVID-19(-) individuals (healthy volunteers) (Fig. 1A) (p=0,0214). Higher level of Caspase-3 expression was determined in COVID-19(+) patients than COVID-19 (-) individuals (Fig. 1B) (p=0,0041).
Fig. 1.
Alteration of mRNA expression levels between groups. A. CYP2E1 gene expression, B. Caspase-3 gene expression.
3.4. Correlation analysis of mRNA expression of CYP2E1 and Caspase-3
Our results showed that decreased CYP2E1 and increased Caspase-3 expression in COVID-19(+) patients. However, correlation analysis results demonstrated very weak correlation between CYP2E1 and Caspase-3 expression in COVID-19(+), not significant (p=0,570, r=0,09).
3.5. Relation of gene expression and clinical parameters
Relation between gene expressions and laboratory findings showed different results for each gene (Table 2 ). Increased CYP2E1 gene expression level have a relation in patients with younger than 50 years old (p=0,0012). Female patients had lower CYP2E1 level than male patients (p=0,4419). Patients with unaffected lung had higher CYP2E1 expression than patients with affected lungs (p=0,0001). Patients with low level of lymphocyte and platelet showed lower CYP2E1 expressions than patients with normal range of lymphocyte and platelet (p=0,0070; p=0,0111, respectively). Patients had high levels of AST, bilirubin (total), LDH, procalcitonin, CRP, creatinine, BUN were related with lower level of CYP2E1 expression (p=0,0293; p=0,03378; p=0,0172; p=0,0129; p=0,0218; p=0,0201; p=0,0052, respectively). We did not find significant CYP2E1 gene expression according to WBC, Neu, hemoglobin, HCT, albumin, ALT, D-dimer, fibrinogen (p=0,0682; p=0,1015; p=0,3572; p=0,9589; p=0,1127; p=0,2848; p=0,1632; p=0,5582).
Table 2.
Clinical parameters and gene expressions of covid-19 patients.
| Parameters (normal range) | CYP2E1 (2–∆∆Ct) (Mean) |
p | Caspase-3 (2–∆∆Ct) (Mean) |
p |
|---|---|---|---|---|
| Age (years) | ||||
| <50 | 2.03157 | **0.0012 | 3.09647 | **0.0095 |
| ≥50 | 0.628595 | 1.52784 | ||
| Gender | ||||
| Female | 0.982168 | 0.4419 | 2.03781 | 0.5235 |
| Male | 1.21901 | 1.97269 | ||
| Effected lung | 0.623731 | ***0.0001 | 1.42367 | **0.0030 |
| Unaffected lung | 1.86532 | 3.09569 | ||
| White blood cell count (x109/L;4.49–12.68) | ||||
| Normal | 1.18295 | 0.0682 | 2.16677 | 0.0678 |
| High | 0.241276 | 0.56765 | ||
| Neutrophils (×109/L; 2.04–7.54) | ||||
| Normal | 1.15792 | 0.1015 | 2.22466 | 0.1959 |
| High | 0.687199 | 1.21537 | ||
| Lymphocytes (×109/L; 1.21–3.77) | ||||
| Low | 0.585651 | **0.0070 | 0.859153 | ***0.0009 |
| Normal | 1.26119 | 2.50666 | ||
| Platelets (×109/L; 152–383) | ||||
| Low | 0.412991 | ⁎0.0111 | 0.897469 | ⁎0.0277 |
| Normal | 1.23811 | 2.3335 | ||
| Hemoglobin (g/dL; 12.2–15.9) | ||||
| Low | 0.596569 | 0.3572 | 1.69127 | 0.3899 |
| Normal | 1.19509 | 2.26348 | ||
| Hematocrit (%; 36.4–47.2) | ||||
| Normal | 1.05042 | 0.9589 | 2.13097 | 0.2064 |
| High | 1.07203 | 1.6052 | ||
| Albumin (g/L; normal range 32–48) | ||||
| Normal | 0.982731 | 0.1127 | 1.97088 | 0.2505 |
| High | 1.89832 | 2.97677 | ||
| Alanine aminotransferase (U/L; 7–40) | ||||
| Normal | 1.15722 | 0.2848 | 2.304 | 0.1428 |
| High | 0.882214 | 1.495 | ||
| Aspartate aminotransferase (U/L;13–40) | ||||
| Normal | 1.21686 | ⁎0.0293 | 2.08155 | 0.9547 |
| High | 0.824631 | 1.85276 | ||
| Total bilirubin (mg/dL; 0.2–1.1) | ||||
| Normal | 1.23365 | ⁎0.0378 | 2.33736 | ⁎0.0278 |
| High | 0.414558 | 0.809643 | ||
| Lactate dehydrogenase (U/L; 120–246) | ||||
| Normal | 1.25268 | ⁎0.0172 | 2.51968 | ⁎0.0550 |
| High | 0.903975 | 1.60952 | ||
| Procalcitonin (ng/mL; 0–0.5) | ||||
| Normal | 1.31972 | ⁎0.0129 | 2.43965 | 0.4595 |
| High | 0.814779 | 1.96406 | ||
| C reactive protein (mg/L; 0–0.5) | ||||
| Normal | 1.88604 | ⁎0.0218 | 2.84864 | 0.3122 |
| High | 0.906532 | 1.85387 | ||
| D-dimer(μg/ml; 0–500) | ||||
| Normal | 1.41717 | 0.1632 | 2.72276 | ⁎0.0149 |
| High | 0.801143 | 1.50033 | ||
| Creatinine (mg/dL; 0.55–1.02 | ||||
| Normal | 1.21048 | ⁎0.0201 | 1.95996 | 0.2869 |
| High | 0.424441 | 1.41275 | ||
| Fibrinogen (mg/dL; 200–400) | ||||
| Normal | 1.04387 | 0.5582 | 1.7354 | 0.3842 |
| High | 0.11917 | 2.023916 | ||
| BUN (mg/dL; 9–23) | ||||
| Normal | 1.34861 | **0.0052 | 2.4447 | 0.1763 |
| High | 0.397366 | 1.30929 |
BUN; blood urea nitrogen; Mann Whitney U test was used to all parameters.
p < 0.05 was considered statically significant.
Caspase-3 gene expression was higher in patients with younger than 50 years old and unaffected lungs (p = 0,0095; p = 0,003, respectively). Patients with low level of lymphocyte and platelet showed decreased level of Caspase-3 expression (p = 0,0009; p = 0,0277). Patients with high level of bilirubin (total), LDH, D-dimer showed decreased Caspase-3 expression (p = 0,0278; p = 0,0550; p = 0,0149, respectively). Patients with high levels of WBC, Neutrophil, HCT, ALT, AST, procalcitonin, CRP, creatinine, BUN had lower level of Caspase-3 expression but statically not significant (p > 0,05). Patients with high level of albumin and fibrinogen showed high level of Caspase-3 expression (p > 0,05). Patients who had low hemoglobin level demonstrated expression of low Caspase-3 level (p > 0,05).
We have banded together the laboratory findings that were significantly associated with both gene levels in Table 3 . Patients who is younger than 50 years old and patients had unaffected lungs showed increased both gene expressions. Patients with low lymphocytes and platelet counts and patients with high level of bilirubin and LDH showed low level of gene expressions to CYP2E1 and Caspase-3.
Table 3.
Significant relation between clinical parameters and gene expressions of CYP2E1 and Caspase-3.
| Parameters (normal range) | CYP2E1 (2–∆∆Ct) (Mean) |
p | Caspase-3 (2–∆∆Ct) (Mean) |
p |
|---|---|---|---|---|
| Age (years) | ||||
| <50 | 2.03157 | **0.0012 | 3.09647 | **0.0095 |
| ≥50 | 0.628595 | 1.52784 | ||
| Effected lung | 0.623731 | ***0.0001 | 1.42367 | **0.0030 |
| Unaffected lung | 1.86532 | 3.09569 | ||
| Lymphocytes (×109/L; 1.21–3.77) | ||||
| Low | 0.585651 | **0.0070 | 0.859153 | ***0.0009 |
| Normal | 1.26119 | 2.50666 | ||
| Platelets (×109/L; 152–383) | ||||
| Low | 0.412991 | ⁎0.0111 | 0.897469 | ⁎0.0277 |
| Normal | 1.23811 | 2.3335 | ||
| High | 0.824631 | 1.85276 | ||
| Total bilirubin (mg/dL; 0.2–1.1) | ||||
| Normal | 1.23365 | ⁎0.0378 | 2.33736 | ⁎0.0278 |
| High | 0.414558 | 0.809643 | ||
| Lactate dehydrogenase (U/L; 120–246) | ||||
| Normal | 1.25268 | ⁎0.0172 | 2.51968 | ⁎0.0550 |
| High | 0.903975 | 1.60952 |
BUN; blood urea nitrogen; Mann Whitney U test was used to all parameters.
p < 0.05 was considered statically significant
4. Discussion
COVID-19 infection may destroy not only respiratory tract but also the liver and gastrointestinal tract and other organs and thus it may be considered a systemic and inflammatory disease (H. Li et al., 2020; Wiersinga et al., 2020; Zhang et al., 2020). Blood tests are nonspecific to COVID-19 infection but they show patients' prognosis. Like hematological and biochemical findings many molecular changes were seen in COVID-19 infection. CYP enzyme levels (Fakhouri et al., 2020) and expression of Caspase genes (Ren et al., 2020) may be a part of these changes. Viral infection, increased cytokine levels decreased CYP enzymes(Kim et al., 2012). Caspase-3 is an apoptotic gene and may give information about organ injury. Treatment of COVID-19 is needed for multiple drug usage. Besides the disease, medical treatment affects patients sophisticatedly. For this reason, we measured the CYP2E1 and Caspase-3 mRNA expression and investigated their relation with laboratory findings in patients with COVID-19 infection.
Coagulation abnormalities and elevated levels of D-dimer result in high mortality among COVID-19 patients (Tang et al., 2020). The study by Javanian et al. showed non-survivor patients have a higher WBC count than survivor patients. They related lymphopenia and increased WBC count with a high level of CRP and increased mortality (Javanian et al., 2020). Increasing D-dimer level lead to excessive thrombin generation and fibrinolysis were shown in several COVID-19 studies (Q. Li et al., 2020). Li et al. showed some factors including CRP, LDH, platelet, fibrinogen and old age linked with mortality of COVID-19 infection (Q. Li et al., 2020). Blood lymphocyte amount was linked to COVID-19 severity. Pormohammad et al. analyzed laboratory results of COVID-19 patients and demonstrated some rates are that 61% to thrombocytosis, 57.5% to lymphopenia and 79% to elevated CRP. Besides that case fatality rate was 39,5% to patients older than 50 (Pormohammad et al., 2020). Ten et al. showed that the percentage of blood lymphocytes (LYM%) is the signature of disease prognosis (Tan et al., 2020). Huang et al. indicated that comorbidities, lymphopenia, and hypoalbuminemia are determinants of COVID-19 mortality (Huang et al., 2020). In harmony with the recent studies we revealed that higher WBC cell count, bilirubin(total), procalcitonin, CRP, D-dimer, creatinine, and BUN levels in ICU patients than non-ICU patients. However, ICU patients had lower levels of lymphocyte, platelets, albumin than non-ICU patients.
Cytokine storm is a major problem for the treatment of COVID-19 infection. Cytokine including interleukin (IL)-6 leads to downregulation of cytochrome p450 enzymes during viral infection (Kim et al., 2012). COVID-19 virus binds to ACE-2 receptors and enters cells and diminishes the amount and function of hemoprotein including hemoglobin, myoglobin, CYP enzymes (Fakhouri et al., 2020). Deficiency of hemoproteins like CYP is related to tissue inflammation, organ damage, and prothrombotic state (Fakhouri et al., 2020). Proteins including heme present a substrate to SARS-CoV-2 viruses to enter the cell (Wenzhong and Hualan, 2020). Drugs used to treat COVID-19 infections downregulate CYP and so heme production (Fakhouri et al., 2020). CYP2E1 is expressed primarily in liver and other mammalian tissues (Norris et al., 2010; Novak and Woodcroft, 2000). CYP2E1 is involved in the biotransformation of many compounds like small organic molecules, endogenous compounds, clinically used drugs, toxic chemicals and environmental contaminants (Abdelmegeed et al., 2017; Novak and Woodcroft, 2000). Accelerated CYP2E1 activation may lead to ROS generation through NADPH oxidase activity of CYP2E1 and thus to macromolecular damage and pathologic conditions (Lu and Cederbaum, 2008). Kim et al. demonstrated decreased level of CYP2E1 protein and mRNA level in the cecal ligation and puncture model of rats (Kim et al., 2011). Many reasons may cause CYP downregulation, which one primer curator is unknown. However, this study showed that CYP2E1 mRNA expression level was lower in COVID-19(+) patients than healthy individuals. Drugs, oxidative stress, inflammation, or renal impairment are factors that may cause to downregulation of CYP2E1 expression.
COVID-19 infection affects multiple systems including cell death programs of patients (Elmore, 2007). Maleki et al. showed that seminal plasma of COVID-19 patients showed that higher Caspase-3 expression than healthy control (Hajizadeh Maleki and Tartibian, 2021). According to a recent study, plasma-derived extracellular vesicles of severe COVID-19 patients increased Caspase-3 activity and decreased survival in human pulmonary microvascular endothelial cells (Krishnamachary et al., 2020). Increased Caspase-3 expression in lungs of COVID-19 patients was shown before (Magro et al., 2021). ORF3a expressing HEK293T cells showed that SARS-CoV-2 ORF3a leads to induce apoptosis via extrinsic pathway and cleavage/activate Caspase-9 and elevate Caspase-3 expression (Ren et al., 2020). In vitro study demonstrated that SARS-CoV-2-infected lung epithelial cells presence of a cleaved form of Caspase-3 (S. Li et al., 2020). In harmony with the studies, our patients showed higher Caspase-3 expression than the healthy volunteers. Apoptosis rate depends on viral replication (S. Li et al., 2020) so collecting the blood of patients presented different apoptotic ranges which is depends on at advancing period of the disease.
This study had some limitations. We could find limited COVID-19 patients and healthy individuals to this study. Other CYP members and apoptotic genes could not be studied. Some patients' clinical and demographic information was not accessible.
This is the first study that investigates CYP2E1 and Caspase-3 expressions, together in COVID-19 patients. COVID-19 patients had decreased CYP2E1 and increased Caspase-3 gene expression levels. Besides, gene expressions and some laboratory findings were related. Coagulation irregularity, organ damages, and increased inflammation seen in COVID-19 infection (Jose and Manuel, 2020) may effect CYP2E1 and Caspase-3 levels. Downregulation of CYP2E1, considering its role on metabolism, may result in dysregulation of many biological pathways which results in the accumulation of toxic molecules and decreasing biosynthesis of endogen molecules (Abdelmegeed et al., 2017; Novak and Woodcroft, 2000) that may affect patients' survival. Laboratory findings related with COVID-19 severity may change Caspase-3 gene expression. Drugs that cause CYPs downregulation (Fakhouri et al., 2020) should be given more attention when using to treat COVID-19 patients. Besides, CYP2E1 gene expression levels may be used to estimate patient prognosis and manage the treatment.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Research involving human participants and/or animals
Research involves only human participants.
CRediT authorship contribution statement
S. Karabulut Uzuncakmak: Conceptualization, Methodology, Investigation. E. Dirican: Investigation, Visualization. M.E. Naldan: Investigation. F. Kesmez Can: Investigation. Z. Halici: Writing – review & editing.
Declaration of competing interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abdelmegeed M.A., Ha S.-K., Choi Y., Akbar M., Song B.-J. Role of CYP2E1 in mitochondrial dysfunction and hepatic injury by alcohol and non-alcoholic substances. Curr. Mol. Pharmacol. 2017;10:207–225. doi: 10.2174/1874467208666150817111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azkur A.K., Akdis M., Azkur D., Sokolowska M., Veen W., Brüggen M., O’Mahony L., Gao Y., Nadeau K., Akdis C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R.C. COVID-19 update: Covid-19-associated coagulopathy. J. Thromb. Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum A.I., Yang L., Wang X., Wu D. CYP2E1 sensitizes the liver to LPS- and TNF α -induced toxicity via elevated oxidative and nitrosative stress and activation of ASK-1 and JNK mitogen-activated kinases. Int. J. Hepatol. 2012;2012:1–19. doi: 10.1155/2012/582790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Kaminsky L.S. H UMAN E XTRAHEPATIC C YTOCHROMES P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu. Rev. Pharmacol. Toxicol. 2003;43:149–173. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri E.W., Peterson S.J., Kothari J., Alex R., Shapiro J.I., Abraham N.G. Genetic polymorphisms complicate COVID-19 therapy: pivotal role of HO-1 in cytokine storm. Antioxidants. 2020;9:636. doi: 10.3390/antiox9070636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F.P. Cytochrome P450 and chemical toxicology. Chem. Res. Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- Hajizadeh Maleki B., Tartibian B. COVID-19 and male reproductive function: a prospective, longitudinal cohort study. Reproduction. 2021;161:319–331. doi: 10.1530/REP-20-0382. [DOI] [PubMed] [Google Scholar]
- Helmig Decreased Cyp2E1 mRNA expression in human leucocytes in patients with fibrotic and inflammatory lung diseases. Int. J. Mol. Med. 2010:26. doi: 10.3892/ijmm_00000446. [DOI] [PubMed] [Google Scholar]
- Hodges N.J., Green R.M., Chipman J.K., Graham M. Induction of DNA strand breaks and oxidative stress in HeLa cells by ethanol is dependent on CYP2E1 expression. Mutagenesis. 2007;22:189–194. doi: 10.1093/mutage/gem001. [DOI] [PubMed] [Google Scholar]
- Huang J., Cheng A., Kumar R., Fang Y., Chen G., Zhu Y., Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J. Med. Virol. 2020;92:2152–2158. doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanian M., Bayani M., Shokri M., Sadeghi-Haddad-Zavareh M., Babazadeh A., Yeganeh B., Mohseni S., Mehraeen R., Sepidarkish M., Bijani A., Rostami A., Shahbazi M., Tabari A.M., Shabani A., Masrour-Roudsari J., Hasanpour A.H., Gholinejad H.E., Ghorbani H., Ebrahimpour S. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol north of Iran: a retrospective cohort study. Rom. J. Intern. Med. 2020;58:161–167. doi: 10.2478/rjim-2020-0013. [DOI] [PubMed] [Google Scholar]
- Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.-H., Lee S.-H., Lee S.-M. Role of kupffer cells in pathogenesis of sepsis-induced drug metabolizing dysfunction. FEBS J. 2011;278:2307–2317. doi: 10.1111/j.1742-4658.2011.08148.x. [DOI] [PubMed] [Google Scholar]
- Kim S., Östör A.J.K., Nisar M.K. Interleukin-6 and cytochrome-P450, reason for concern? Rheumatol. Int. 2012;32:2601–2604. doi: 10.1007/s00296-012-2423-3. [DOI] [PubMed] [Google Scholar]
- Krishnamachary B., Cook C., Spikes L., Chalise P., Dhillon N.K. medRxiv Prepr. Serv. Heal. Sci; 2020. The Potential Role of Extracellular Vesicles in COVID-19 Associated Endothelial Injury and Pro-inflammation. [DOI] [Google Scholar]
- Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.-J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Cao Y., Chen Lei, Wu D., Yu J., Wang H., He W., Chen Li, Dong F., Chen Weiqun, Chen Wenlan, Li L., Ran Q., Liu Q., Ren W., Gao F., Chen Z., Gale R.P., Hu Y. Hematological features of persons with COVID-19. Leukemia. 2020;34:2163–2172. doi: 10.1038/s41375-020-0910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhang Y., Guan Z., Li H., Ye M., Chen X., Shen J., Zhou Y., Shi Z.-L., Zhou P., Peng K. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 2020;5:235. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu Y., Cederbaum A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C.M., Mulvey J., Kubiak J., Mikhail S., Suster D., Crowson A.N., Laurence J., Nuovo G. Severe COVID-19: a multifaceted viral vasculopathy syndrome. Ann. Diagn. Pathol. 2021;50 doi: 10.1016/j.anndiagpath.2020.151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizuma K., Endo H., Chan P.H. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurochem. 2009;109:133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S.M., Bombardier E., Smith I.C., Vigna C., Tupling A.R. ATP consumption by sarcoplasmic reticulum ca 2+ pumps accounts for 50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. Am. J. Physiol. Physiol. 2010;298:C521–C529. doi: 10.1152/ajpcell.00479.2009. [DOI] [PubMed] [Google Scholar]
- Novak R.F., Woodcroft K.J. The alcohol-inducible form of cytochrome P450 (CYP2E1): role in toxicology and regulation of expression. Arch. Pharm. Res. 2000;23:267–282. doi: 10.1007/BF02975435. [DOI] [PubMed] [Google Scholar]
- Pormohammad A., Ghorbani S., Baradaran B., Khatami A., Turner R.J., Mansournia M.A., Kyriacou D.N., Idrovo J.-P., Bahr N.C. Clinical characteristics, laboratory findings, radiographic signs and outcomes of 61,742 patients with confirmed COVID-19 infection: a systematic review and meta-analysis. Microb. Pathog. 2020;147 doi: 10.1016/j.micpath.2020.104390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Shu T., Wu D., Mu J., Wang C., Huang M., Han Y., Zhang X.-Y., Zhou W., Qiu Y., Zhou X. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell. Mol. Immunol. 2020;17:881–883. doi: 10.1038/s41423-020-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattenberg J.M., Wang Y., Rigoli R.M., Koop D.R., Czaja M.J. CYP2E1 overexpression alters hepatocyte death from menadione and fatty acids by activation of ERK1/2 signaling. Hepatology. 2004;39:444–455. doi: 10.1002/hep.20067. [DOI] [PubMed] [Google Scholar]
- Tan L., Wang Qi, Zhang D., Ding J., Huang Q., Tang Y.-Q., Wang Qiongshu, Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target. Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzhong Liu, Hualan L. ChemRxiv. Prepr; 2020. COVID-19:Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. [DOI] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19) JAMA. 2020;324:782. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]