Abstract
Objective:
Sequencing cell-free DNA now allows detection of large chromosomal abnormalities and dominant Mendelian disorders in the prenatal period. Improving upon these methods would allow newborn screening programs to begin with prenatal genetics, ultimately improving the management of rare genetic disorders.
Methods:
As a pilot study, we performed exome sequencing on the cell-free DNA from three mothers with singleton pregnancies to assess the viability of broad sequencing modalities in a noninvasive prenatal setting.
Results:
We found poor resolution of maternal and fetal genotypes due to both sampling and technical issues.
Conclusion:
We find broad sequencing modalities inefficient for noninvasive prenatal applications. Alternatively, we suggest a more targeted path forward for noninvasive prenatal genotyping.
1 |. INTRODUCTION
The beneficial health outcomes from newborn screening programs (NBS) are indisputable. We envision future NBS will begin with prenatal genetic testing to enable care in the immediate newborn period, and open up new possibilities for in utero and genetic therapies. During pregnancy, placental DNA (cell-free fetal DNA; cffDNA) is released into maternal circulation, enabling noninvasive interrogation of fetal genetics. cffDNA has well-established clinical utility in screening for common chromosomal abnormalities such as Down syndrome with high sensitivity and specificity.1 More recently, efforts have demonstrated sequencing-based testing for de novo pathogenic variants in a list of 30 genes associated with dominant Mendelian disorders2 and polymerase chain reaction (PCR)-based testing for a small number of recessive Mendelian disorders.3 Using relative haplotype dosage analysis (RHDO),4 multiple groups have successfully diagnosed single gene disorders5–7 including a new offering of noninvasive prenatal diagnosis for cystic fibrosis in the UK Public Health Service.8 RHDO typically relies on collecting parental, and ideally proband, genetic information to resolve parental haplotypes; Jang et al.7 demonstrated success in diagnosing Duchenne muscular dystrophy by estimating haplotypes solely from maternal long-read sequencing. Scotchman et al.9 provide a more detailed discussion about the development of noninvasive fetal genetic testing, and we refer interested readers to their work. To date, no one has reported reliable fetal genotyping purely from maternal cell-free DNA using a sequencing-based approach.
To begin NBS with prenatal genetic testing, we believe we first need a reliable noninvasive test only requiring a maternal sample. Others could reasonably argue the availability of carrier screening, and the immeasurably small risk of invasive testing,10 removes the need for noninvasive testing. Such an argument, however, dismisses (1) the ethical and practical issues surrounding the necessity of involving the biological father, (2) the fact that many genetic disorders arise due to de novo mutations, (3) the understandable fear and apprehension around invasive testing (especially for rare conditions), and (4) the cost and limited availability of invasive procedures. In addition, we believe the prenatal diagnosis community should focus work on sequencing-based (as opposed to PCR-based) approaches. Sequencing generalizes across disorders more easily than PCR techniques, allows multiplexing to a degree not feasible using PCR, and will only continue to decrease in cost.
In research settings, three groups have performed/attempted fetal genome sequencing from cell-free DNA.4,11,12 In a review of noninvasive fetal sequencing work, Snyder et al.13 illustrate the cost-infeasibility and suggest more targeted approaches such as exome sequencing (ES). As an exploratory exercise, we performed ES on cell-free DNA (cfES) from three pregnant women. All three women had singleton pregnancies with suspected genetic conditions identified by a genetic counselor. We hypothesized cfES would allow us to identify genetic etiologies.
2 |. METHODS
2.1 |. Participant selection
Genetic counselors identified pregnant women with suspected genetic disorders based either on family history or fetal sonographic findings. We enrolled three women, blinded to their family history and sonographic findings. All participants were consented and enrolled at UNC Hospitals by certified genetic counselors with approval from the UNC Institutional Review Board (IRB Number: 18-2618); we do not include any identifying information in this manuscript. Either due to family history of genetic condition or because of identified fetal abnormalities in the current pregnancy, the patients all pursued genetic workup through the genetic counselors separate from their participation in this study. After we completed our analyses, we obtained records of their genetic workup including diagnostic single gene sequencing of P3H1 (Case 2) and multiplex ligation-dependent probe amplification of ATP7A exons 1–23 (Case 1).
2.2 |. ES and analysis
We collected cell-free DNA from maternal plasma, prepared sequencing libraries for the Illumina platform, and performed exome capture using the IDT xGen Exome Research Panel v1.0 (Cases 1 and 2) or Agilent SureSelect Human All Exon v7 (Case 3). Because existing germline variant calling pipelines would not account for the combination of read fractions from maternal and fetal DNA, we processed the data using a novel analytic pipeline developed in Snakemake14 using Anaconda environments for reproducibility (provided in supplemental materials). Briefly, sequencing reads were aligned to hg38 (excluding alternate contigs) using BWA-MEM,15 then base quality scores were recalibrated using GATK4.16–18 We only retained nonduplicate, properly paired reads with unambiguous mapping and mapping quality more than 30 for each read. We called variants using bcftools,19 requiring basepair quality scores more than 20. We suggest the review by Seaby et al.20 for more information on the specifics of collecting and processing ES data for clinical use. Analyses were restricted to the regions overlapping between the IDT and Agilent capture platforms. For cell-free analyses, we required five alternate allele-supporting read-pairs, and at least 80 total read-pairs. Using the identified single-nucleotide variants, we applied a novel empirical Bayesian procedure to estimate the fetal fraction (FF; the proportion of placental/fetal to maternal sequencing reads). We then estimated maternal and fetal genotypes using a maximal likelihood model incorporating the FF estimate and observed proportion of minor allele (alternate) reads (PMAR). The full details of the algorithm are provided in supplemental materials, including an R21 package reproducing our results for complete transparency: https://github.com/daynefiler/filer2020B.
2.3 |. Data availability
The data that support the findings of this study are available on request from the corresponding author. The raw sequencing data are not publicly available due to privacy or ethical restrictions. Allele depths, with the alleles masked and genomic location rounded to 10 kb are available in the self-contained R package reproducing the analysis herein (https://github.com/daynefiler/filer2020B).
3 |. RESULTS
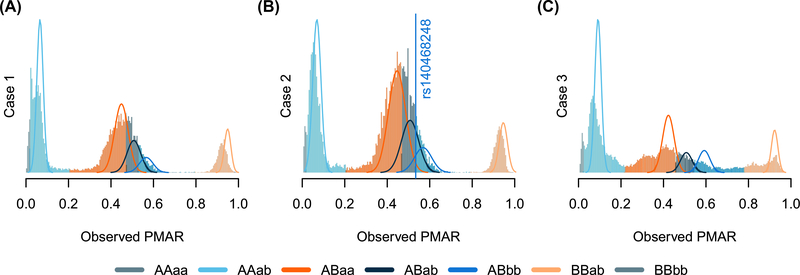
Using the final set of filtered reads, we analyzed single nucleotide loci with more than ×80 coverage and at least five reads supporting the alternate allele. At each analyzed site, we alternate allele sequencing depth and total sequencing depth to estimate the fetal fraction and maternal–fetal genotypes using our novel algorithm (Figure 1). Table 1 lists the known genetic diagnoses for the three cases presented. Genetic counselors recruited the three participants; investigators and cfES analysis was blinded to the eventual genetic diagnoses. In Cases 1 and 2, specific gene sequencing based on family history and sonographic findings, respectively, provided genetic diagnoses. To date, Case 3 does not have a genetic diagnosis. We learned the mother in Case 1 carries a deletion of exon 1 in the gene most-often responsible for Menke’s syndrome (ATP7A). Neither exome capture platform targets ATP7A exon 1; therefore, cfES could not have identified the diagnosis for Case 1 with the platform used. In Case 2, we identified the causal variant using cfES. In this case, we correctly genotyped the fetus, but lacked the power to make the genotyping call with any level of confidence acceptable for clinical use (Figure 1B, note the widely overlapping distributions at the causal variant). We did not identify any known pathogenic variants in the sequencing of Case 3, and despite performing genome sequencing on the newborn, we still do not have a genetic diagnosis for the family.
FIGURE 1.
Distribution of observed proportion of minor allele reads (PMAR) values for the three cases across the possible maternal-fetal genotype pairs. Uppercase letters give the estimated maternal genotype, lowercase letters give the estimated fetal genotype; “A/a” indicates the reference allele, “B/b” indicates the alternate allele. Solid lines show the normal approximation for the theoretical distribution of binomial probabilities, given the frequency of the estimated genotypes. The vertical line in (B) shows the observed PMAR for the known pathogenic variant, rs140468248
TABLE 1.
Case summaries
| GA | Clinical findings | Genetic diagnosis | FF | Depth | % Dup | %Filt | |
|---|---|---|---|---|---|---|---|
| 1 | 32w2d | 5 prior pregnancies affected with X-linked recessive Menke’s syndrome | Menke’s syndrome; del. ATP7A exon 1 | 0.117 | 241 | 42.8 | 21.96 |
| 2 | 24w5d | Fetal sonogram at 21w5d showed femoral bowing with shortened length (<3% for GA) bilaterally | Osteogenesis imperfecta type VIII; P3H1 c.1120G > T (rs140468248) | 0.122 | 152 | 33.32 | 22.09 |
| 3 | 34w0d | Fetal sonogram at 19w0d showed bilateral club foot with bilateral upper limb arthrogryposis | None, to date, despite exome and genome sequencing of newborn | 0.169 | 330 | 53.67 | 32.65 |
Abbreviations: depth, median depth used to estimate genotypes (does not include duplicated/filtered reads); FF, estimated fetal fraction; GA, gestational age at the time of blood draw for cfES; %Dup, percentage of total mapped read pairs discarded as PCR and/or optical duplicates; %Filt, percentage of total mapped read pairs discarded for improper pairing and/or mapping quality.
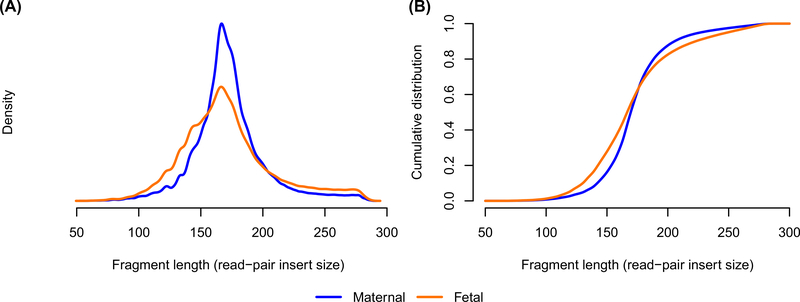
In Case 3, in addition to cfES, we performed ES on fetal, maternal, and paternal samples. Based on previous work demonstrating the differential length of maternal and fetal fragments,22–25 we interrogated the distribution of presumed maternal and fetal reads (Figure 2). We identified maternal and fetal reads by identifying sites with unique heterozygosity in the direct maternal and fetal ES results; at the informative sites, we extracted reads supporting the allele unique to the mother or fetus. In total, we identified 654,619 maternal reads and 279,508 fetal reads. We found, as others have, a higher proportion of fetal reads falling below 150 bp; however, we also note the increased observed dispersion of fetal read length led to a higher proportion of fetal reads longer than 200 bp (Figure 2). Limited to the single case, we cannot comment on the significance or generalizability of the increased dispersion.
FIGURE 2.
Distribution of maternal versus fetal fragment length in Case 3. (A) The density. (B) The emperic cumulative distribution. The horiztonal axis shows the fragment length (insert size taken from aligned read-pairs). Blue lines show maternal reads, orange lines show fetal reads. We only included cfES reads supporting alleles unique to the mother or fetus, as identified from the direct maternal and fetal ES
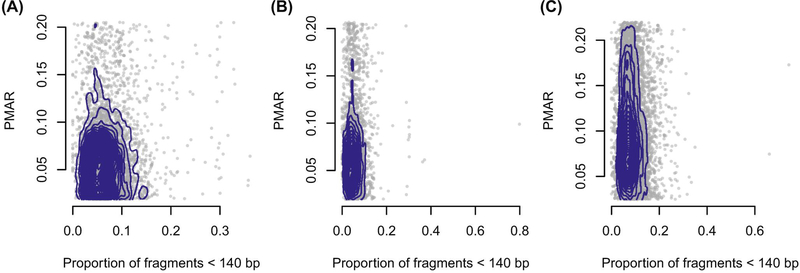
Rabinowitz et al.25 proposed the Hoobari method that incorporates fragment lengths into fetal genotype estimates, finding the difference in accuracy varied from −0.25% to 1.89% when using versus not using fragment length in their exome analyses. To explore the utility of correcting for fragment length in our analysis, we interrogated the PMAR as a function of the short read proportion (fraction of reads with insert sizes less than 140 bp; Figure 3). We selected 140 as the cut-off based on the Hoobari algorithm. Overall, we found no meaningful relationship between the short read proportion and the observed PMAR and chose not to incorporate fragment length into our genotype estimates.
FIGURE 3.
Proportion of minor allele reads (PMAR) as a function of the short read proportion for genotypes estimated as “AAab.” Short reads defined as fragments less than 140 basepairs. (A–C) Cases 1–3. Gray points show the individual sites; blue contour lines show the two-dimensional distribution of values
Returning to Case 3, we interrogated the fetal genotyping accuracy at all sites with cell-free genotype estimates and reliable calls from the direct fetal sample. Overall, we found a 50.91% accuracy (Table 2). We provide the complete set of calls within the R package, and a summary within the supplemental document.
TABLE 2.
Case 3 fetal versus cell‐free calls
| Cell-free |
||||
|---|---|---|---|---|
| aa | ab | bb | ||
| Fetal | aa | 1063 | 1857 | 9 |
| ab | 3598 | 7079 | 1454 | |
| bb | 76 | 2197 | 1391 | |
Note: “a” represents the major allele; “b” represents the minor allele. Sites with cell-free estimates and reliable direct fetal calls included (reliable defied as passing all quality checks and having a total sequencing depth greater than 30).
4 |. DISCUSSION
To move NBS into the fetal period we must develop noninvasive methodologies agnostic to paternity. Having prenatal genetic diagnoses will allow for optimized care in the immediate newborn period and the further development of in utero therapies. Invasive testing confers very minimal risk when performed by properly trained specialists,10 but procedure costs and limited availability prohibit universal access for patients. We must also recognize the ethical issues with requiring the biological father in neonatal genetic screening programs; many family structures do not include the biological father and requiring both parents to miss work for multiple appointments may exclude low-income parents. From our own clinical experience, we find women hesitant to undergo invasive testing even in the presence of severe fetal findings.
Without the ability to reliably exclude maternal DNA fragments, noninvasive sequencing-based methods to genotype the fetus either require additional sequencing of parental samples or distinguishing genotypes by the PMAR. Here, we make no attempt to utilize parental genetic information and demonstrate the difficulty of inferring the genotypes directly from the PMAR. We model the PMAR as a binomial proportion; given the fetal fraction, one can prove the true PMAR defines the maternal and fetal genotypes (Supporting Information S1).
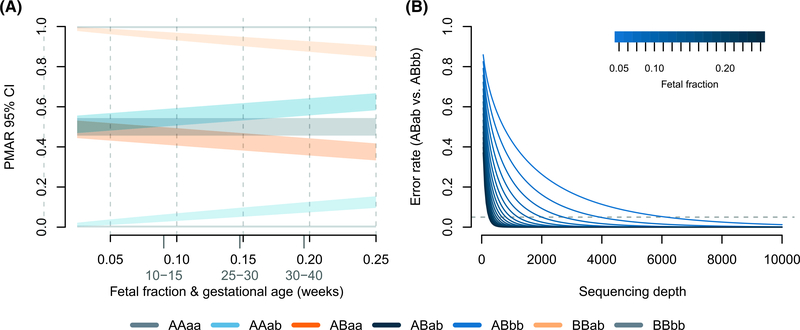
The theoretical bounds of the binomial distribution, therefore, confine our ability to discriminate maternal-fetal genotypes. Using the normal approximation for the binomial variance (valid when the number of observations, i.e., sequencing depth, times the binomial proportion, i.e., PMAR, is >10), we can clearly explain the poor results we observed (Figure 4). At sequencing depths up to ×500, the 95% confidence intervals on PMAR distributions still overlap for fetal fractions up to roughly 0.17 (Figure 4A). When we calculate the degree of distribution overlap (a proxy for classification error rate), we see required sequencing depths in excess of ×8000 for low fetal fraction samples (Figure 4B). We, therefore, cannot expect cell-free sequencing to reliably differentiate genotypes without substantially higher depth or additional genetic information. No amount of cleverness in the analysis can overcome the fundamental variance bounds when estimating binomial proportions.
FIGURE 4.
Binomial distribution limitations. (A) 95% Confidence intervals on the binomial proportions for possible maternal-fetal genotype pairs across increasing fetal fractions; represents a sequencing depth of ×500. Average fetal fractions by gestational age (in weeks) given in light gray.26 (B) Expected misclassification rate (Weitzman overlapping coefficient; i.e., the area of overlapping distributions in A) considering ABab versus ABbb as a function of sequencing depth and fetal fraction. The dashed horizontal line shows 5% error. The theoretical error rates for ABab versus ABaa are symmetric and equal; however, the frequency of errors will depend on the population frequency of the reference versus alternate allele
We acknowledge the three cases included do not provide information about diagnostic yield and only partially address feasibility. Genetic counselors referred the cases to our study prior to knowing the genetic diagnosis. Of the three cases, only Case 2 presented the opportunity to identify the genetic diagnosis using cfES. One case had an exon deletion not covered by the exome panel used, and one case still does not have a genetic diagnosis. Even had the exome panel covered the exon deletion in Case 1, detecting copy number variants falls outside the scope of allele ratio analysis. The cases presented do highlight key issues with cfES.
The sequencing herein suffers from three problems: (1) inadequate sequencing depth; (2) biased PMAR values from the removal of duplicate reads; (3) errors in sequencing and/or PCR. We have already illustrated the inadequate depth, but emphasize that the theoretical results we present speak to the final depths (not the raw sequencing depth). In our three cases, we excluded over half the reads taken off the sequencer due to sequencing quality thresholds (Table 1). We observe the evidence of problems (2) and (3) by observing the high proportion of both duplicate reads and PMAR values outside the theoretic distributions. In addition, we observed very poor accuracy in the Case 3 genotype estimates.
Typical sequencing workflows start with randomly fragmenting DNA molecules to build sequencing libraries. Standard bioinformatic practices suggest we remove read-pairs with identical endpoints, because the duplicate read-pairs more likely represent PCR amplification of a single molecule than two molecules with the same fragmentation. Cell-free DNA molecules are shorter than nuclear DNA, not requiring manual fragmentation, and have a non-random distribution of endpoints.23 Therefore, compared to standard sequencing libraries, the likelihood of observing true duplicates in cell-free libraries increases and we cannot necessarily assume duplicates represent PCR amplification. However, for this work we have no way of differentiating reads representing true duplicate molecules versus PCR duplicates and thus excluded duplicate reads from our analysis.
Assuming adequate depth and appropriate handling of duplicate reads and sequencing errors, incorporating the fragment length into the statistical model may prove more beneficial. The high variability of the binomial distribution for small n obfuscates any meaningful relationship between fragment length and PMAR in our data. We reiterate, however, incorporating fragment length may give better estimates of the binomial proportion but cannot decrease variance beyond the distribution bounds.
To solve the above issues, we advocate a more targeted approach with much greater sequencing depth and unique molecular identifiers. Unique molecular identifiers allow identification of sequencing errors and differentiate true versus artifactual duplicate reads. Given the depth requirements for estimating fetal genotypes by the PMAR, and the challenge of variants of uncertain clinical significance, we advocate against broad sequencing modalities on noninvasive samples. Recognizing that all capture methods introduce bias in the relative sequencing efficiency of different targeted regions,20 the sequencing depths needed for noninvasive fetal genotyping necessitate a targeted approach. Despite the challenges raised by this work, we believe assessing hundreds to thousands of basepairs, rather than the tens of millions targeted in ES, will prove economical and clinically reliable. Doing so, we hope, will foster population-level screening for Mendelian disorders during the prenatal period and, ultimately, unlock new avenues in the treatment of these disorders.
Supplementary Material
Key points.
What’s already known about this topic?
Sequencing-based noninvasive testing detects large copy number abnormalities and some single-gene disorders.
Fetal exome sequencing (ES) provides 10%–20% diagnostic yield for structural abnormalities after normal karyotype and microarray.
What does this study add?
Cell-free ES in three gravid patients with suspected genetic disease in the fetus.
Discussion of probability theory underlying noninvasive fetal genotyping.
We demonstrate broad sequencing approaches are limited by sampling and technical difficulties, concluding broad sequencing is currently inappropriate for noninvasive testing.
ACKNOWLEDGMENTS
The authors thank Dr. James Evans for providing review and feedback of this manuscript. Neeta Vora and this work was supported by NICHD (K23HD088742). Dayne Filer was supported by NICHD (F30HD101228) and by NIGMS (5T32GM067553).
Funding information
National Institute of General Medical Sciences, Grant/Award Number: 5T32GM067553; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: F30HD101228, K23HD088742
Footnotes
CONFLICT OF INTEREST
The authors declare that there is are conflict of interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Mackie FL, Hemming K, Allen S, Morris R, Kilby M. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124(1):32–46. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Li J, Saucier JB, et al. Non-invasive prenatal sequencing for multiple Mendelian monogenic disorders using circulating cell-free fetal DNA. Nat Med. 2019;25(3):439–447. [DOI] [PubMed] [Google Scholar]
- 3.Tsao DS, Silas S, Landry BP, et al. A novel high-throughput molecular counting method with single base-pair resolution enables accurate single-gene NIPT. Sci Rep. 2019;9(1):14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis Lo YM, Allen Chan KC, Sun H, et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med. 2010;2(61):61ra91. [DOI] [PubMed] [Google Scholar]
- 5.Hui WWI, Jiang P, Tong YK, et al. Universal haplotype-based noninvasive prenatal testing for single gene diseases. Clin Chem. 2017;63(2):513–524. [DOI] [PubMed] [Google Scholar]
- 6.Vermeulen C, Geeven G, de Wit E, et al. Sensitive monogenic noninvasive prenatal diagnosis by targeted haplotyping. Am J Hum Genet. 2017;101(3):326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang SS, Lim BC, Yoo S-K, et al. Targeted linked-read sequencing for direct haplotype phasing of maternal DMD alleles: a practical and reliable method for noninvasive prenatal diagnosis. Sci Rep. 2018; 8(1):8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandler NJ, Ahlfors H, Drury S, et al. Noninvasive prenatal diagnosis for cystic fibrosis: implementation, uptake, outcome, and implications. Clin Chem. 2020;66(1):207–216. [DOI] [PubMed] [Google Scholar]
- 9.Scotchman E, Chandler NJ, Mellis R, Chitty LS. Noninvasive prenatal diagnosis of single-gene diseases: the next frontier. Clin Chem. 2020; 66(1):53–60. [DOI] [PubMed] [Google Scholar]
- 10.Salomon LJ, Sotiriadis A, Wulff CB, Odibo A, Akolekar R. Risk of miscarriage following amniocentesis or chorionic villus sampling: systematic review of literature and updated meta-analysis. Ultrasound Obstet Gynecol. 2019;54(4):442–451. [DOI] [PubMed] [Google Scholar]
- 11.Kitzman JO, Snyder MW, Ventura M, et al. Noninvasive whole-genome sequencing of a human fetus. Sci Transl Med. 2012;4(137): 137ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan HC, Gu W, Wang J, Blumenfeld YJ, El-Sayed YY, Quake SR. Noninvasive prenatal measurement of the fetal genome. Nature. 2012;487(7407):320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder MW, Simmons LVE, Kitzman JO, et al. Noninvasive fetal genome sequencing: a primer. Prenat Diagn. 2013;33(6): 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koster J, Rahmann S. Snakemake—a scalable bioinformatics workflow engine. Bioinformatics. 2012;28(19):2520–2522. [DOI] [PubMed] [Google Scholar]
- 15.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv:1303.3997. 2013. https://arxiv.org/abs/1303.3997 [Google Scholar]
- 16.McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Auwera GA, Carneiro MO, Hartl C, et al. From FastQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43(1110): 11.10.1–11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poplin R, Ruano-Rubio V, DePristo MA, et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv. 2018. 10.1101/201178 [DOI] [Google Scholar]
- 19.Li H A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27(21): 2987–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seaby EG, Pengelly RJ, Ennis S. Exome sequencing explained: a practical guide to its clinical application. Brief Funct Genomics. 2016; 15(5):374–384. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 22.Allen Chan KC, Zhang J, Hui ABY, et al. Size distributions of maternal and fetal DNA in maternal plasma. Clin Chem. 2004;50(1): 88–92. [DOI] [PubMed] [Google Scholar]
- 23.Allen Chan KC, Jiang P, Sun K, et al. Second generation noninvasive fetal genome analysis reveals de novo mutations, single-base parental inheritance, and preferred DNA ends. Proc Natl Acad Sci U S A. 2016;113(50):E8159–E8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang P, Dennis Lo YM. The long and short of circulating cell-free DNA and the ins and outs of molecular diagnostics. Trends Genet. 2016;32(6):360–371. [DOI] [PubMed] [Google Scholar]
- 25.Rabinowitz T, Polsky A, Golan D, et al. Bayesian-based noninvasive prenatal diagnosis of single-gene disorders. Genome Res. 2019;29(3): 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinnings SL, Geis JA, Almasri E, et al. Factors affecting levels of circulating cell-free fetal DNA in maternal plasma and their implications for noninvasive prenatal testing. Prenat Diagn. 2015;35(8):816–822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The raw sequencing data are not publicly available due to privacy or ethical restrictions. Allele depths, with the alleles masked and genomic location rounded to 10 kb are available in the self-contained R package reproducing the analysis herein (https://github.com/daynefiler/filer2020B).