Abstract
Purpose
The prognosis of breast cancer (BC) patients who develop into brain metastases (BMs) is very poor. Thus, it is of great significance to explore the etiology of BMs in BC and identify the key genes involved in this process to improve the survival of BC patients with BMs.
Patients and methods
The gene expression data and the clinical information of BC patients were downloaded from TCGA and GEO database. Differentially expressed genes (DEGs) in TCGA-BRCA and GSE12276 were overlapped to find differentially expressed metastatic genes (DEMGs). The protein-protein interaction (PPI) network of DEMGs was constructed via STRING database. ClusterProfiler R package was applied to perform the gene ontology (GO) enrichment analysis of DEMGs. The univariate Cox regression analysis and the Kaplan-Meier (K-M) curves were plotted to screen DEMGs associated with the overall survival and the metastatic recurrence survival, which were identified as the key genes associated with the BMs in BC. The immune infiltration and the expressions of immune checkpoints for BC patients with brain relapses and BC patients with other relapses were analyzed respectively. The correlations among the expressions of key genes and the differently infiltrated immune cells or the differentially expressed immune checkpoints were calculated. The gene set enrichment analysis (GSEA) of each key gene was conducted to investigate the potential mechanisms of key genes involved in BC patients with BMs. Moreover, CTD database was used to predict the drug-gene interaction network of key genes.
Results
A total of 154 DEGs were identified in BC patients at M0 and M1 in TCGA database. A total of 667 DEGs were identified in BC patients with brain relapses and with other relapses. By overlapping these DEGs, 17 DEMGs were identified, which were enriched in the cell proliferation related biological processes and the immune related molecular functions. The univariate Cox regression analysis and the Kaplan-Meier curves revealed that CXCL9 and GPR171 were closely associated with the overall survival and the metastatic recurrence survival and were identified as key genes associated with BMs in BC. The analyses of immune infiltration and immune checkpoint expressions showed that there was a significant difference of the immune microenvironment between brain relapses and other relapses in BC. GSEA indicated that CXCL9 and GPR171 may regulate BMs in BC via the immune-related pathways.
Conclusion
Our study identified the key genes associated with BMs in BC patients and explore the underlying mechanisms involved in the etiology of BMs in BC. These findings may provide a promising approach for the treatments of BC patients with BMs.
Keywords: Breast cancer, Brain metastases, Tumor microenvironment, Bioinformatics
Abbreviations: BC, breast cancer; BMs, brain metastases; DEGs, differentially expressed genes; DEMGs, differentially expressed metastatic genes; PPI, protein-protein interaction; GO, gene ontology; GSEA, gene set enrichment analysis; BP, biological process; MF, molecular function; CC, cellular component
Highlights
-
•
CXCL9 and GPR171, as the key genes, were closely associated with the prognosis of brain metastases in breast cancer.
-
•
There was a significant difference of the immune microenvironment between brain and other metastases in breast cancer.
-
•
We revealed candidate drugs which associated with the key genes of breast cancer patients with brain metastases.
-
•
A series of bioinformatic analysis methods were used in this article.
1. Introduction
Brain metastases (BMs) are the most common intracranial tumor, with the number of cases 10 times than those of the primary brain tumor [1]. The common primary tumors associated with BMs include lung cancer, breast cancer (BC) and melanoma. With the improvements of the diagnosis and the treatments, patients suffered from cancers have longer survival period, which causes a higher morbidity of BMs in turn. BMs usually presents an extremely poor prognosis with the median survival of 1–4 months in spite of various treatment strategies [2,3].
According to the data from International Agency for Research on Cancer (IARC), BC is the most cancer in the world and the most frequent cancer diagnosed in women in worldwide. It is also considered to be the second frequent source of BMs. There is estimated to be 10–30% BC metastasis cases developing into BMs [4,5]. In the 12% of BC patients with distant metastases, the first site is brain tissue [6]. Previous studies suggested that the major risk factors relating BC to BMs are age, extracranial metastasis situation, number of BMs, and pathological type [7]. Due to the high heterogeneity of BC, the current clinical diagnoses and treatment therapies cannot detect or prevent BMs effectively [8]. It is necessary to find new genes or better molecular markers for the diagnoses and treatments of Breast Cancer Brain Metastasis(BCBMs).
Nowadays, the gene expression profiling analysis has promoted the clinical oncology research by investigating the tumor related genes, the molecular markers, the assessments of therapeutic effects, and the prognostic prediction [9,10]. Simultaneously, the multiple databases are used to verify different expression genes (DEGs), which contributes to the bioinformatics analysis. The interactions between the immune cells and the tumor cells also address our attentions, which shows a certain significance for the occurrence and the development of the malignant tumors. Previous studies showed that M2 alternative macrophages, which are significantly related to the tumor occurrences, can release many kinds of cytokines to induce tissue remodeling, angiogenesis, and the suppression of adaptive immunity [11]. Other researches also showed that the immune checkpoints, such as PD-1 and PD-L1, were differently expressed between the primary and the metastatic sites [12]. At the same time, these immune checkpoints were differently expressed between different metastatic lesions, which could affect the immune therapeutic effects. In this study, a series of bioinformatic analysis methods were used to screen out the different genes associated with BCBMs, providing the potential mRNA prognostic biomarkers, analyzing the correlation between the prognostic biomarkers and the immune cells, and contributing to the early diagnosis, the treatment and the prognosis of metastatic BC.
2. Material and methods
2.1. Data source
The gene expression profiles of 907 M0 and 22 M1 for patients with BC were downloaded from TCGA database. The gene expression data and the clinical information were downloaded from GSE12276, which comprised 16 BC patients with BMs and 188 BC patients with other metastases. The clinical information obtained from TCGA and GSE12276 were provided in Table S1.
2.2. Identification and functional analysis of DEMGs in BC
DEGs between M0 and M1 in TCGA were identified by limma R package to acquire the metastasis-related genes. Meanwhile, DEGs between BMs and other metastases in GSE12276 were also identified by limma R package to obtain the candidate important genes in BMs. The screening criteria of DEGs were |fold change| > 1.5 and P-value < 0.05. To get more robust metastasis-related genes involved in BMs, DEGs obtained from TCGA and GSE12276 were overlapped and defined as differentially expressed metastatic genes (DEMGs). The GO enrichment analysis of DEMGs, including biological process (BP), molecular function (MF) and cellular component (CC), were all performed using clusterProfiler R package. The protein-protein interaction (PPI) network of DEMGs was constructed via STRING database.
2.3. The overall survival and the metastatic recurrence survival analyses of DEMGs
DEMGs were first input to the univariate Cox regression analysis to screen out the DEMGs significantly associated with the survival of BC patients (p-value <0.05). Next, the patients were divided into the low- and high-expression groups based on the median expression of each DEMGs selected by the univariate Cox regression analysis. The Kaplan-Meier (K-M) curves were used to analyze the overall survival of patients in each group. The DEMGs with the significant survival difference between the low- and high-expression group were further selected for the following analysis. The bc-GenExMiner v4.6 online tool was applied in the metastatic recurrence survival analysis [13]. The parameters were as follows: baseline like (PAM50) and/or triple-negative BC (IHC) prognostic analysis; DNA microarray samples (n = 11,359); metastatic recurrence; segmentation criteria of median. The DEGs significantly associated with the overall survival and the metastatic recurrence survival was identified as the key DEMGs.
2.4. The evaluation of the immune microenvironment in BC patients with BMs and other metastases
To evaluate the relationship between the key DEMGs and the immune microenvironment, we analyzed and compared the immune infiltration between M0 and M1 patients and between patients with BMs and other metastases by CIBERSORT, respectively. Then we took the intersection of the differentially infiltrated immune cells for the downstream analysis. The infiltration of immune cells were evaluated by CIBERSORT, including naive B cells, memory B cells, plasma cells, CD8 T cells, naive CD4 T cells, resting memory CD4 T cells, activated memory CD4 T cells, follicular helper T cells, regulatory T cells, gamma delta T cells, resting NK cells, activated NK cells, monocytes, M0 macrophages, M1 macrophages, M2 macrophages, resting dendritic cells, activated dendritic cells, resting mast cells, activated mast cells, eosinophils and neutrophils. The immune checkpoints used in the current study were IDO1, PD-L1, PD-L2, TIM-3, TIGIT, PD-1, LAG3, CTLA4 and VTCN1. The Pearson's correlations were calculated among the expressions of the key DEMGs and the differentially infiltrated immune cells or the differentially expressed immune checkpoints.
2.5. GSEA
The patients were divided into the low- and the high-expression groups based on the median expression of each key DEMGs. The KEGG gene set was selected as the reference gene set and downloaded from MSigDB database.
2.6. Construction of gene-drug interaction network
The key DEMGs were searched in CTD database. The chemotherapeutic drugs that might elevate or decrease the expressions of DEMGs were selected with the interaction count higher than three. The gene-drug interaction networks were then visualized using Cytoscape software. The structures of selected drugs were searched in the DrugBank database.
2.7. Statistical analysis
All data was analyzed by R (version 4.0.0). Wilcoxon test was used to compare the data between two groups. Significant difference was considered as p-value < 0.05 unless specified.
3. Results
3.1. Identification and the functional enrichment analysis of 17 DEMGs in BC
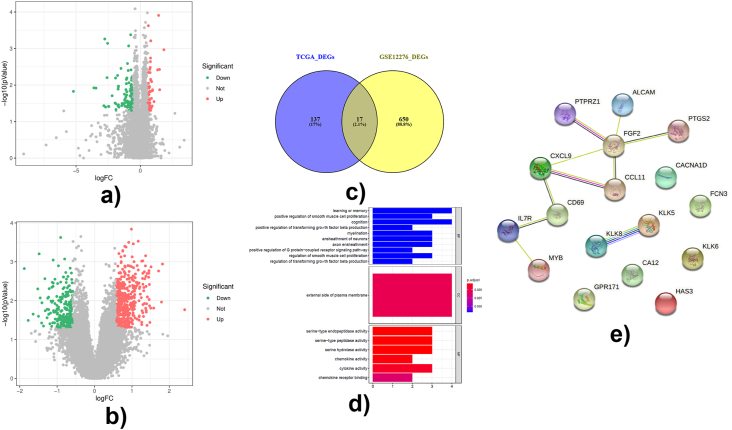
A total of 154 DEGs were identified between M0 and M1 BC patients in TCGA database (Fig. 1A). Meanwhile, a total of 667 DEGs were identified between BMs and other metastases in BC patients from GSE12276 (Fig. 1B). Then, the overlapped 17 DEGs in TCGA and GSE12276 were selected as DEMGs (Fig. 1C) in BC patients. The GO enrichment analysis of 17 DEMGs showed that they were involved in the BPs associated with the cell proliferation and the brain functions (e.g. learning and memory), the positive regulation of the smooth muscle cell proliferation, the ensheathment of neurons, the cognition and the positive regulation of G protein-coupled receptor signaling pathway, the CCs of external side of plasma membrane, and the MFs of chemokine receptor binding and the activities of serine-type endopeptidase, serine-type peptidase, serine hydrolase, chemokine and cytokine (Fig. 1D). Additionally, the molecular functions of 17 DEMGs were mainly associated with the immune activities, such as chemokine activity, cytokine activity and chemokine receptor binding (Fig. 1D). The PPI interaction network revealed that there were interactions among MYB, IL7R, CD69, CXCL9, CCL11, FGF2, PTGS2, ALCAM and PTPRZ1 (Fig. 1E).
Fig. 1.
Identification and functional analysis of differentially expressed metastatic genes. Differentially expressed genes were identified by limma R package using |fold change| > 1.5 and P-value < 0.05 as criteria. Functional analysis was performed by clusterProfiler R package.(A) Volcano plot of differentially expressed genes between 907 M0 and 22 M1 breast cancer samples. The red dots were upregulated DEGs, the green dots were downregulated DEGs, and the grey dots were genes without significant difference. (B)Volcano plot of differentially expressed genes between 16 breast cancer patients with brain metastases and 188 breast cancer patients with other metastases. The red dots were upregulated DEGs, the green dots were downregulated DEGs, and the grey dots were genes without significant difference. (C) Venn plot showed the intersection of 17 differentially expressed metastatic genes in breast cancer. (D) Top 10 GO terms of differentially expressed metastatic genes, including biological process (BP), cellular component (CC) and molecular function (MF) were identified by clusterProfiler R package and were shown in the bar chart. (E) The protein-protein interaction network of DEMGS analyzed by STRING database. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. The identification of CXCL9 and GPR171 as key DEMGs
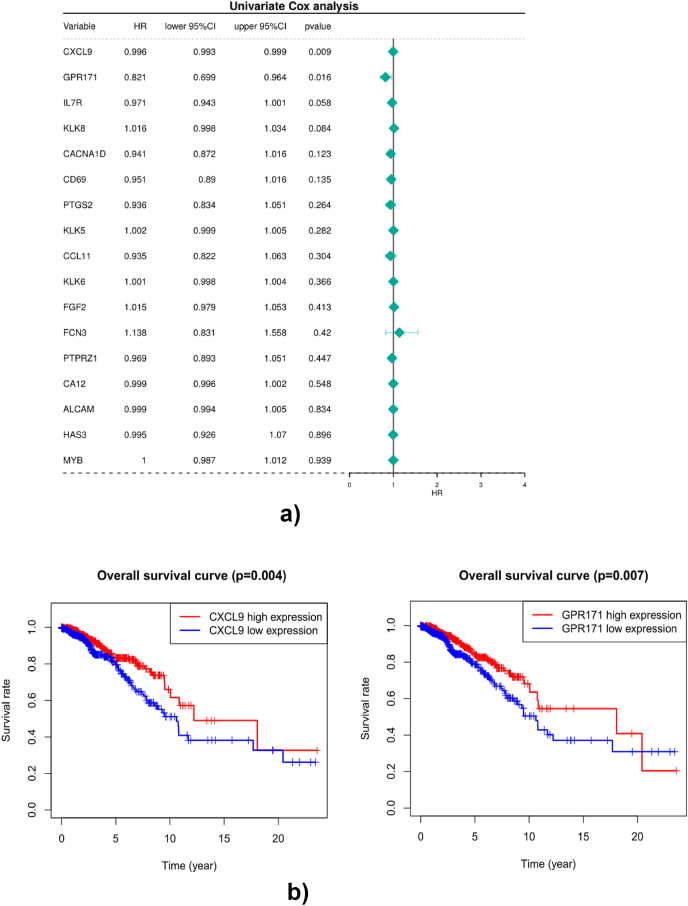
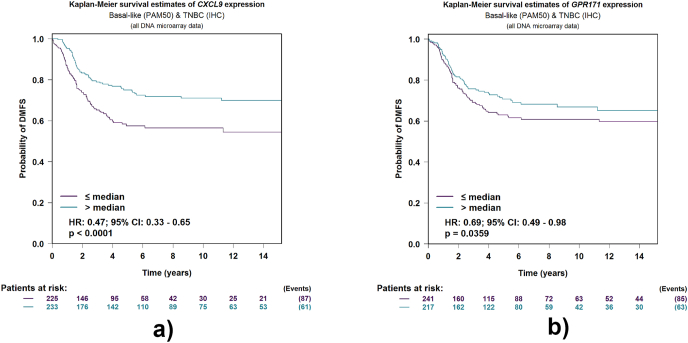
To investigate the prognostic role of 17 DEMGs in BC patients, we first performed the univariate Cox regression analysis and found that CXCL9 and GPR171 were significantly associated with the survival of BC patients (Fig. 2A). Then we performed the K-M analyses and found that patients in the high-expression groups of CXCL9 and GPR171 had better overall survival than those in the low-expression groups (Fig. 2B), indicating that CXCL9 and GPR171 were key DEMGs associated with the prognosis of BC patients. Thereafter, we further explored the role of CXCL9 and GPR171 in metastases. We found that the high-expressions of CXCL9 and GPR171 were more likely to have better distant metastasis-free survival (DMFS) (Fig. 3). Thus, CXCL9 and GPR171 were identified as key DEMGs in BCBMs.
Fig. 2.
Overall survival analyses of DEMGs. (A) Univariate Cox regression analysis was performed to screen differentially expressed genes significantly associated with the survival of breast cancer patients (p-value<0.05). (B) Association between DEMGs and overall survival in patients with breast cancer. Survival analysis of CXCL9 and GPR171 genes. Red lines represent high expression and blue lines represent low expression of theDEMGs. CXCL9, C-X-C motif chemokine ligand 9; GPR171, G protein-coupled receptor 171. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Kaplan-Meier curves for distance metastases free survival (DMFS) according to different expression of DEMGs by using “TNBC (IHC) and/or Basal-like (PAM50) prognostic analysis” module. (A) K-M survival estimates of CXCL9 expression. (B) K-M survival estimates of GPR171 expression.
3.3. Different immune microenvironment between BMs and other metastases in BC patients
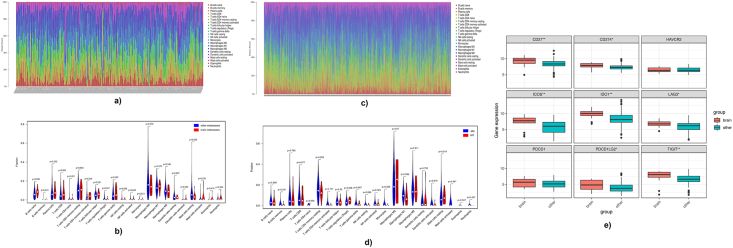
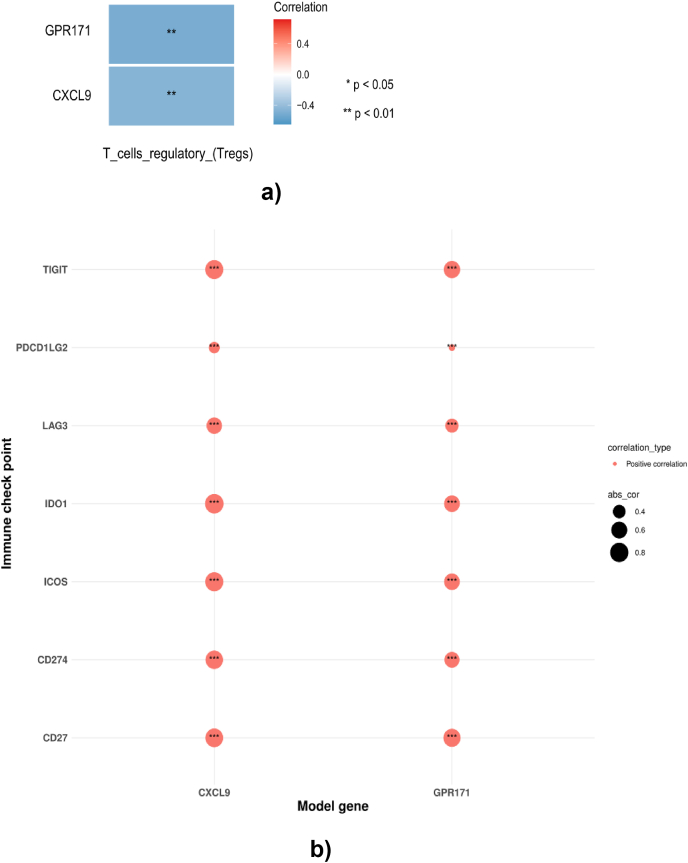
The increasing number of evidence show that the immune microenvironment has a close relationship with cancer metastases [14,15]. Thus, we investigated whether the metastasis altered the immune microenvironment and whether the immune microenvironment in BMs was different from other metastases in BC. Firstly, the immune infiltration of each sample from brain and other metastases was examined by CIBERSORT (Fig. 4A). Results showed that the abundance of activated memory CD4 T cells, regulatory T cells and resting mast cells were significantly different between brain and other metastases (Fig. 4B). Meanwhile, results also indicated that the infiltration of memory B cells and regulatory T cells were significantly different in M0 and M1 patients (Fig. 4C and D). Secondly, the expressions of immune checkpoints in brain and other metastases were checked and results showed that the expressions of CD27, CD274, ICOS, IDO1, LAG3, PDCD1LG2 and TIGIT (Fig. 4E) were significantly higher in BMs compared to those in other metastases. The above results demonstrated that the metastasis affected the immune microenvironment. The immune microenvironments between BMs and other metastases were also different, indicating the important roles of the differentially infiltrated immune cells and the expressed immune checkpoints in BCBMs. Thereafter, the relationship between key DEMGs and regulatory T cells was assessed, which were both differentially infiltrated between M0 and M1 patients and between patients with BMs and other metastases. Interestingly, the expressions of GPR171 and CXCL9 presented a moderate negative relationship with the regulatory T cells (p <0.01, Fig. 5A). In addition, it can be found that the expressions of CXCL9 and GPR171 were moderately or strongly positively associated with the differentially expressed immune checkpoints (Fig. 5B). These results suggest that the key DEMGs may interact with the immune microenvironment to affect the BMs in BC.
Fig. 4.
Evaluation of immune microenvironment in breast cancer with metastases. (A–B) Immune microenvironment analysis between brain metastases and other metastases in breast cancer patients. (A) The immune infiltration in each samples. (B) Violin diagram of the proportion of 22 types of immune cells showed the difference in infiltration between brain metastases and other metastases in breast caner patients. (C–D) Immune microenvironment analysis between M0 and M1 in breast cancer patients. (C) The immune infiltration in each samples. (D) Violin diagram of the proportion of 22 types of immune cells showed the difference in infiltration between M0 and M1 in breast caner patients. (E) Different gene expressions of immune checkpoints between brain metastases and other metastases. *p-value <0.05 **p-value<0.01.
Fig. 5.
Associations between key DEMGs and immune cells and checkpoints. (A) The expressions of key DEMGs have significant negative relationship with regulatory T cells. (B) The expressions of key DEMGs have moderate or strong positive correlation with differentially expressed immune checkpoints.
3.4. The key DEMGs involved in BC via the immune-related pathways
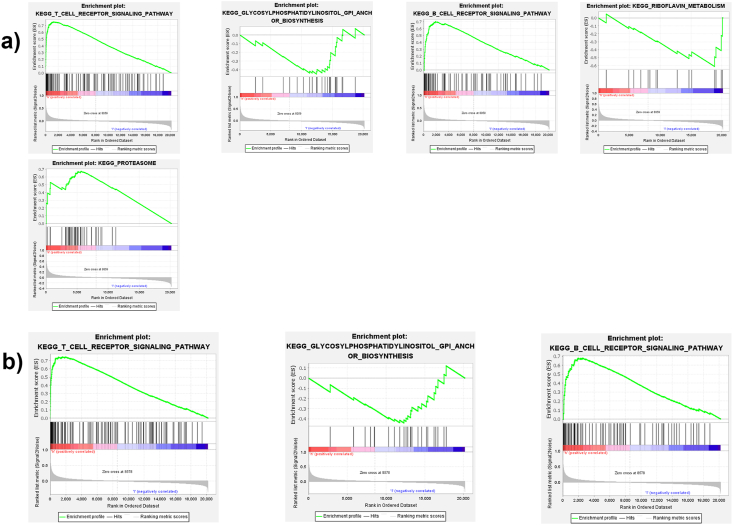
To explore the pathways associated with the key DEMGs, GSEA of each key DEMG was performed. Notably, results showed that there were some common immune related pathways enriched in the high-expression groups of the key DEMGs, such as the B cell receptor signaling pathway, the glycosylphosphatidylinositol anchor biosynthesis and the T cell receptor signaling pathway (Fig. 6A and B). In addition, the distinct KEGG pathways were also enriched into the high-CXCL9 expression group, such as the proteasome and the riboflavin metabolism (Fig. 6A)
Fig. 6.
Signaling pathways associated with key DEMGs predicted by GSEA. (A) GSEA-based KEGG-enrichment plots of CXCL9: T cell receptor signaling pathway, B cell receptor signaling pathway, GPI anchor biosynthesis, proteasome and riboflavin metabolism; (B) GSEA-based KEGG-enrichment plots of GPR171: T cell receptor signaling pathway, B cell receptor signaling pathway, GPI anchor biosynthesis. GSEA-gene set enrichment analysis. GPI-glycosylphosphatidylinositol.
3.5. The establishment of the key DEMG-drug interaction network for BC patients
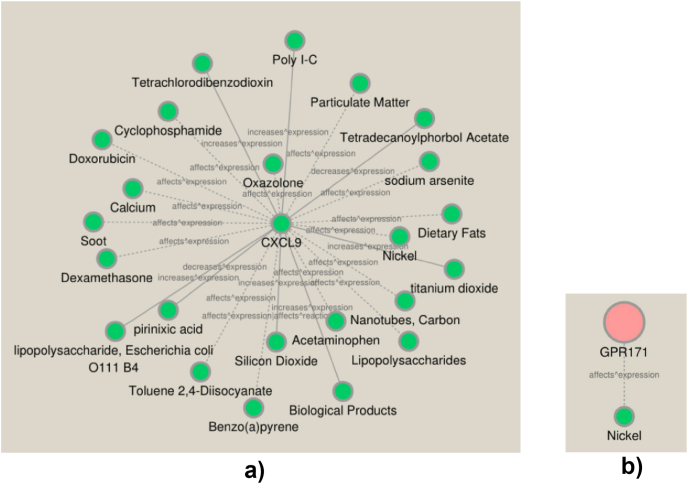
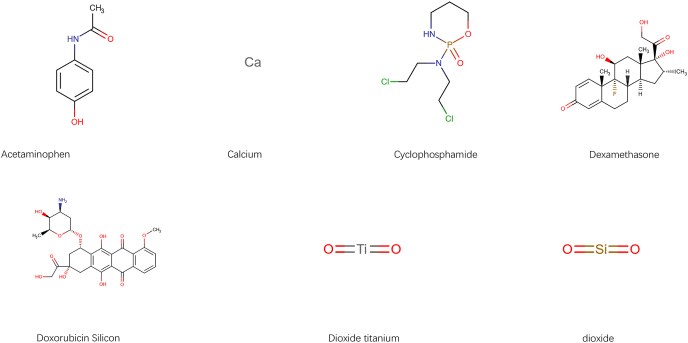
Eventually, to deliver the useful information on the treatments of BCBMs patients, the CTD database was used to search the compounds that may elevate or reduce the expression of key DEMGs. There were 23 compounds interacted with CXCL9 and one compound interacted with GPR171 respectively and the corresponding gene-drug interaction networks were also established (Fig. 7A and B). Then we displayed the structures of 7 drugs in the network via DrugBank database (Fig. 8).
Fig. 7.
Compounds interaction with key DEMGs respectively.(A) Interactions of compounds with CXCL9. (B) Interaction of compound with GPR171. The full line represents increase/decrease expression of gene affected by compounds. The dotted line represents that compounds affect expression of genes but without specific direction.
Fig. 8.
Chemical structure of isolated and identified compounds which affects the expression of key genes.
4. Discussion
There are approximately 20% patients with cancers eventually diagnosed BMs [16]. Patients with BMs commonly present the serious neurological dysfunction and the poor prognosis in spite of the minor lesions and the short median survival. The three most common sources of BMs are from lung (20–56%), breast (5–20%) and melanoma (7–16%) [7]. The risk factors of developing into BMs include age, geographic location, tumor source and molecular subtype. The median time from the initially diagnosis of primary tumor to the development of BMs is varied from 11 to 34 months [17,18]. These differences might be attributed to the cancer subtype or the stage of primary tumor at the diagnosis. Moreover, the molecular subtypes across the primary tumor and the metastasis lesion may also contribute to it.
BC is the most common cancer in worldwide according to IARC 2020. Most BC metastases spread in same organs while BMs usually occur at the advanced stage [19]. Therefore, the earlier prevention and diagnosis are significant for BMs from BC. According to the previous studies, the risk factors for BMs include age, pathological types, stage of primary tumor, and driver mutations of genes [7,20]. However, the results are inconsistence. The gene expression analyses have identified four intrinsic BC subtypes: luminal A, luminal B, HER2 positive, and triple negative (TNBC). HER2+ and TNBC are most likely to metastasize to brain and TNBC demonstrates a high risk of death after BMs [21]. Some studies indicate that HER2 positive tumors present a well treatment response to trastuzumab and thus inhibit the extracranial metastases while the risk of developing into BMs increases due to the prolonged survival [22]. In recent years, the gene profiling analysis provides a basis for the precision medicine. The high-throughput data provided by next-generation sequencing (NGS) can help identify the potential biomarkers, guide the new drug development, and establish the prediction models. Pangeni et al. investigated that the methylations of three genes, GALNT9, CCDC8 and BNC1, are related to the BMs process of BC [23]. This study showed that the methylation of CCDC8 occurs at the early stage of the metastatic evolution while the methylations of other two genes occur at the later stage. Gao et al. established a clinical prediction model for BCBMs, suggesting that two genes, DLG3 and GFI1, are strongly associated with BMs [24]. The immune response in the cell proliferation PI3K–AKT pathway was also shown to be significantly associated with the overall survival outcomes in patients with BCBMs [25]. Further investigations of the immune microenvironment and molecular differences facilitate the understanding of the mechanism for the pathogenesis and the development of BM and it is also of great significance for the development of the novel targeted therapies.
In this study, we used TCGA and GEO to identify 17 genes as DEMs for BCBMs. Subsequently, based on the survival analysis, CXCL9 and GPR171 were found to be associated with BC patients’ survival. By analyzing bc-GenExMiner database, it can be also found that CXCL9 and GPR171 was associated with the metastasis and the recurrence of BC. CXC motif chemokine ligand 9 (CXCL9), a monokine induced by gamma-interferon (MIG), can be produced during the inflammation within the tumor microenvironment. Its expression is associated with many tumors, such as BC, nasopharyngeal carcinoma, prostate cancer, and ovarian cancer [[26], [27], [28]]. Razis et al. demonstrated that the high CXCL9 expression was a favorable prognosticator for both DFS and OS of triple negative BC [29]. Neo et al. found that the overexpression of CXCL9 could reduce the tumor progression and metastasis via the inhibition of angiogenesis [30]. Wu et al. found that the higher CXCL9 expression was a significant prognostic factor for the colorectal carcinoma patients with a higher overall survival rate [31]. However, in other studies, the overexpression of CXCL9 results in the Akt signaling pathway activation and the cytoskeleton rearrangement, which could promote the invasion and metastasis [32]. GPR171 is activated by BigLEN and the BigLEN-GPR171 system plays an important role in feeding and metabolism, which could be a potential target for anti-obesity [33]. In addition, obesity is the risk factor for many cancer types and associated with the poor outcomes. Therefore, GPR171 may regulate the development of cancer. Dho et al. discovered that the inhibition of GPR171 could synergistically enhance the tumoricidal activity of EGFR inhibitor in lung cancer, which could provide a promising anti-neoplastic strategy [34].
We also used the Cibersort algorithm to evaluate the composition of immune cells in each sample from GSE12276 data set and analyze the differences of the immune cells between BMs and other metastatic groups. We found that the activated memory CD4 T cells, regulatory T cells and resting mast cells present significant differences between BMs and others and then found that these DEMGs may regulate the occurrence and the development of BMs by interacting with the immune microenvironment. CTD contains many precise data describing the chemical gene/proteins interactions across species, the chemical-disease relationship and the gene-compounds. This data helps to understand the network of interactions between genes and proteins. We used CTD to search for compounds associated with CXCL9 and GPR171. The seven drug structures have been found in Drugbank Database and those compounds may serve as candidate drugs in the treatment of BC patients with BMs.
There are several limitations in our study. First, the above results were analyzed based on TCGA, GEO and other online databases and were not verified using the fresh specimens. Second, further experiments will be performed to identify the expression levels of these genes.
5. Conclusion
There are two biomarker genes explored by the systematic bioinformatics analyses based on the public databases, providing a theoretical support for the BCBMs studies. The results of this study provide the basis and the reference for the clinical diagnosis and therapy for BCBMs. Further experiments are required to clarify the roles of these biomarkers.
Disclosure
The author reports no conflicts of interest in this work.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2022.101203.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Davis F.G., Dolecek T.A., McCarthy B.J., Villano J.L. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012 Sep;14(9):1171–1177. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freilich R.J., Seidman A.D., DeAngelis L.M. Central nervous system progression of metastatic breast cancer in patients treated with paclitaxel. Cancer. 1995 Jul 15;76(2):232–236. doi: 10.1002/1097-0142(19950715)76:2<232::aid-cncr2820760212>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Mustillo A., Ayoub J.P., Charpentier D., et al. Prognosis in young women less than 40 years of age with brain metastasis from breast cancer. Curr. Oncol. 2020 Feb;27(1):39–45. doi: 10.3747/co.27.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos P.D., Zhang X.H., Nadal C., et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009 Jun 18;459(7249):1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin N.U., Bellon J.R., Winer E.P. CNS metastasis in breast cancer. J. Clin. Oncol. 2004 Sep 1;22(17):3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 6.Altundag K., Bondy M.L., Mirza N.Q., et al. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer. 2007 Dec 15;110(12):2640–2647. doi: 10.1002/cncr.23088. [DOI] [PubMed] [Google Scholar]
- 7.Achrol A.S., Rennert R.C., Anders C., et al. Brain metastasis. Nat. Rev. Dis. Prim. 2019 Jan 17;5(1):5. doi: 10.1038/s41572-018-0055-y. [DOI] [PubMed] [Google Scholar]
- 8.Zubair M., Wang S., Ali N. Advanced approaches to breast cancer classification and diagnosis. Front. Pharmacol. 2021 Feb 26;11:632079. doi: 10.3389/fphar.2020.632079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Escudero R., Paramio J.M. Gene expression profiling as a tool for basic analysis and clinical application of human cancer. Mol. Carcinog. 2008 Aug;47(8):573–579. doi: 10.1002/mc.20430. [DOI] [PubMed] [Google Scholar]
- 10.Weeraratna A.T. Discovering causes and cures for cancer from gene expression analysis. Ageing Res. Rev. 2005 Nov;4(4):548–563. doi: 10.1016/j.arr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Gocheva V., Wang H.W., Gadea B.B., et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010 Feb 1;24(3):241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X., Yin X., Zhang H., et al. Differential expressions of PD-1, PD-L1 and PD-L2 between primary and metastatic sites in renal cell carcinoma. BMC Cancer. 2019 Apr 16;19(1):360. doi: 10.1186/s12885-019-5578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jézéquel P., Frénel J.S., Campion L., et al. bc-GenExMiner 3.0: new mining module computes breast cancer gene expression correlation analyses. Database. 2013 Jan 15:bas060. doi: 10.1093/database/bas060. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altorki N.K., Markowitz G.J., Gao D., et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer. 2019 Jan;19(1):9–31. doi: 10.1038/s41568-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Kenawi A., Hänggi K., Ruffell B. The immune microenvironment and cancer metastasis. Cold Spring Harb Perspect Med. 2020 Apr 1;10(4):a037424. doi: 10.1101/cshperspect.a037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nayak L., Lee E.Q., Wen P.Y. Epidemiology of brain metastasis. Curr. Oncol. Rep. 2012 Feb;14(1):48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 17.Rostami R., Mittal S., Rostami P., et al. Brain metastasis in breast cancer: a comprehensive literature review. J. Neuro Oncol. 2016 May;127(3):407–414. doi: 10.1007/s11060-016-2075-3. [DOI] [PubMed] [Google Scholar]
- 18.Chang E.L., Lo S. Diagnosis and management of central nervous system metastasis from breast cancer. Oncol. 2003;8(5):398–410. doi: 10.1634/theoncologist.8-5-398. [DOI] [PubMed] [Google Scholar]
- 19.Weil R.J., Palmieri D.C., Bronder J.L., et al. Breast cancer metastasis to the central nervous system. Am. J. Pathol. 2005 Oct;167(4):913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mustafa D.A.M., Pedrosa R.M.S.M., Smid M., et al. T lymphocytes facilitate brain metastasis of breast cancer by inducing Guanylate-Binding Protein 1 expression. Acta Neuropathol. 2018 Apr;135(4):581–599. doi: 10.1007/s00401-018-1806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anders C.K., Deal A.M., Miller C.R., et al. The prognostic contribution of clinical breast cancer subtype, age, and race among patients with breast cancer brain metastasis. Cancer. 2011 Apr 15;117(8):1602–1611. doi: 10.1002/cncr.25746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson E.M., Abdel-Rasoul M., Maly J., et al. Incidence and risk of central nervous system metastasis as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann. Oncol. 2013 Jun;24(6):1526–1533. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pangeni R.P., Channathodiyil P., Huen D.S., et al. The GALNT9, BNC1 and CCDC8 genes are frequently epigenetically dysregulated in breast tumours that metastasise to the brain. Clin. Epigenet. 2015 May 27;7(1):57. doi: 10.1186/s13148-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y., Liu J., Qian X., He X. Identification of markers associated with brain metastasis from breast cancer through bioinformatics analysis and verification in clinical samples. Gland Surg. 2021 Mar;10(3):924–942. doi: 10.21037/gs-20-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamo B., Deal A.M., Burrows E., et al. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastasis. Breast Cancer Res. 2011;13(6):R125. doi: 10.1186/bcr3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei H., Liu D., Sun J., et al. Circular RNA circ_0008450 upregulates CXCL9 expression by targeting miR-577 to regulate cell proliferation and invasion in nasopharyngeal carcinoma. Exp. Mol. Pathol. 2019 Oct;110:104288. doi: 10.1016/j.yexmp.2019.104288. [DOI] [PubMed] [Google Scholar]
- 27.Tan S., Wang K., Sun F., Li Y., Gao Y. CXCL9 promotes prostate cancer progression through inhibition of cytokines from T cells. Mol. Med. Rep. 2018 Aug;18(2):1305–1310. doi: 10.3892/mmr.2018.9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bronger H., Singer J., Windmüller C., et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br. J. Cancer. 2016 Aug 23;115(5):553–563. doi: 10.1038/bjc.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razis E., Kalogeras K.T., Kotsantis I., et al. The role of CXCL13 and CXCL9 in early breast cancer. Clin. Breast Cancer. 2020 Feb;20(1):e36–e53. doi: 10.1016/j.clbc.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Neo S.Y., Lundqvist A. The multifaceted roles of CXCL9 within the tumor microenvironment. Adv. Exp. Med. Biol. 2020;1231:45–51. doi: 10.1007/978-3-030-36667-4_5. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z., Huang X., Han X., et al. The chemokine CXCL9 expression is associated with better prognosis for colorectal carcinoma patients. Biomed. Pharmacother. 2016 Mar;78:8–13. doi: 10.1016/j.biopha.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Li Z., Liu J., Li L., et al. Epithelial mesenchymal transition induced by the CXCL9/CXCR3 axis through AKT activation promotes invasion and metastasis in tongue squamous cell carcinoma. Oncol. Rep. 2018 Mar;39(3):1356–1368. doi: 10.3892/or.2017.6169. [DOI] [PubMed] [Google Scholar]
- 33.Gomes I., et al. GPR171 is a hypothalamic G protein-coupled receptor for BigLEN, a neuropeptide involved in feeding. Proc. Natl. Acad. Sci. - PNAS. 2013;110(40):16211–16216. doi: 10.1073/pnas.1312938110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dho S.H., Lee K.P., Jeong D., et al. GPR171 expression enhances proliferation and metastasis of lung cancer cells. Oncotarget. 2016 Feb 16;7(7):7856–7865. doi: 10.18632/oncotarget.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.