Abstract
The gap gene of Staphylococcus aureus, encoding glyceraldehyde-3-phosphate dehydrogenase, was used as a target to amplify a 933-bp DNA fragment by PCR with a pair of primers 26 and 25 nucleotides in length. PCR products, detected by agarose gel electrophoresis, were also amplified from 12 Staphylococcus spp. analyzed previously. Hybridization with an internal 279-bp DNA fragment probe was positive in all PCR-positive samples. No PCR products were amplified when other gram-positive and gram-negative bacterial genera were analyzed using the same pair of primers. AluI digestion of PCR-generated products gave 12 different restriction fragment length polymorphism (RFLP) patterns, one for each species analyzed. However, we could detect two intraspecies RFLP patterns in Staphylococcus epidermidis, Staphylococcus hominis, and Staphylococcus simulans which were different from the other species. An identical RFLP pattern was observed for 112 S. aureus isolates from humans, cows, and sheep. The sensitivity of the PCR assays was very high, with a detection limit for S. aureus cells of 20 CFU when cells were suspended in saline. PCR amplification of the gap gene has the potential for rapid identification of at least 12 species belonging to the genus Staphylococcus, as it is highly specific.
The staphylococci are the causative agents of many opportunistic human and animal infections and are considered among the most important pathogens isolated in the clinical microbiology laboratory (8, 14, 29). Staphylococcus aureus, which is frequently isolated from milk, is the leading cause of intramammary infections in cows, with major economic repercussions (1, 35). However, several coagulase-negative staphylococci are increasingly recognized as etiologic agents of infections in humans and animals (5, 13, 16, 28, 33). For this reason it is of crucial importance to isolate and identify the offending species in order to initiate appropriate antibiotic therapy. Several methods of identifying Staphylococcus spp. have been proposed, including those that detect traditional phenotypic properties, which are available in miniaturized form for automation and convenience (2, 10, 21, 34), and gas-liquid chromatography analysis of cellular fatty acids (31). However, many isolates are still poorly identified by these methods, and supplementary test are often required for a complete identification.
Molecular methods such as PCR-based DNA fingerprinting and hybridization have also been used successfully for staphylococcus identification at the species level (6, 9, 15, 20, 36). In general, rapid bacterial identification by either PCR or hybridization uses species-specific and ubiquitous DNA as a target (3, 4). However, the use of universal pathway genes and universal function genes, whose nucleotide sequences are fairly homologous in bacteria, is becoming more and more frequent as target DNAs for PCR amplification (9, 19, 26).
It has been described that S. aureus as well as a number of coagulase-negative staphylococci, including S. epidermidis, S. capitis, S. haemolyticus, and S. hominis, have a 42-kDa transferrin-binding protein (Tpn) in common located within the cell wall, which is a member of the newly emerging family of multifunctional cell wall-associated glycerahdehyde-3-phosphate dehydrogenases, which catalyze the conversion of glyceraldehyde-3-phosphate to 1,3-diphosphoglycerate and incorporate binding sites for both transferrin and the serine protease plasmin (22, 23, 24, 25). However, this protein is absent from the cell wall of S. saprophyticus and S. warneri, which are unable to bind human transferrin (24).
In this paper we describe a rapid, sensitive, and specific nucleic acid-based procedure allowing the identification of 12 Staphylococcus species analyzed previously when PCR and restriction fragment length polymorphism (RFLP), using AluI restriction endonuclease, were combined. We selected the gap gene as a target for PCR amplification. The gap gene is a part of the glycolytic operon in S. aureus, whose product, Tpn, is widely distributed and very well conserved in bacteria. We also show evidence for the presence of gap genetic information in S. saprophyticus and S. warneri in spite of their failure to bind human transferrin.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are detailed in Table 1. Forty-four S. aureus strains were isolated from milk from both cows and ewes (36) with acute clinical mastitis, all of which came from farms in the northwest of Spain. The 59 S. aureus human isolates were made available through F. Tenover (Centers for Disease Control and Prevention, Atlanta, Ga.) (30, 32). Clinical isolates from humans and animals, CI-1 to CI-6, were tested in our laboratory by standard procedures. Reference strains used in this study were selected from the Spanish Type Culture Collection (CECT; other reference is given when known): S. aureus CECT 86 (ATCC 12600), S. xylosus CECT 237, S. hominis CECT 234, S. saprophyticum CECT 235, S. epidermidis CECT 232, S. warneri CECT 236, S. capitis CECT 233, S. simulans CECT 4538, S. auricularis CECT 4052, and S. carnosus CECT 4491. Other reference strains were S. intermedius ATCC 49052, and S. haemolyticus. Strains were grown on Luria agar or Luria broth (LB) and mannitol salt agar. Other gram-positive and gram-negative bacteria, used as negative controls in PCR assays, were grown on LB and are listed in Table 1. Cells were incubated at 37°C except for Aeromonas hydrophila and Yersinia ruckeri, which were grown at 28°C. Human and animal staphylococcus isolates were obtained from clinical samples and identified using a commercial identification system (Api-Staph; Biomerieux).
TABLE 1.
Bacterial strains used in this study
| Strain | gap PCR amplification | Source or reference |
|---|---|---|
| Staphylococcus aureus | + | CECT 86 (ATCC 12600) |
| + | CECT 240 (ATCC 6538P) | |
| + | CECT 435 (ATCC 25923) | |
| + | CECT 469 (ATCC 29737) | |
| + | 59 human isolates (29, 30) | |
| + | 44 cow and sheep isolates (19) | |
| Staphylococcus epidermidis | + | CECT 232 (ATCC 14990) |
| + | CECT 231 (ATCC 12228) | |
| + | 6 human isolates (this study) | |
| + | 27 cow and sheep isolates (this study) | |
| Staphylococcus capitis | + | CECT 233 (ATCC 27840) |
| Staphylococcus hominis | + | CECT 234 (ATCC 27844) |
| + | CI-2 (human isolate, this study) | |
| Staphylococcus saprophyticus | + | CECT 235 (ATCC 15305) |
| Staphylococcus warneri | + | CECT 236 (ATCC 27836) |
| Staphylococcus sp. | + | CI-3 (human isolate, this study) |
| Staphylococcus xylosus | + | CECT 237 (ATCC 29971) |
| Staphylococcus sp. | + | CI-5 (sheep isolate, this study) |
| Staphylococcus auricularis | + | CECT 4052 (ATCC 33753) |
| Staphylococcus carnosus | + | CECT 4491 |
| Staphylococcus simulans | + | CECT 4538 (ATCC 27848) |
| + | CI-4 (sheep isolate, this study) | |
| Staphylococcus intermedius | + | ATCC 49052 |
| Staphylococcus haemolyticus | + | CI-6 (human isolate, this study) |
| Streptococcus sp. | − | Sheep isolate (this study) |
| Streptococcus agalactiae | − | CECT 183 (ATCC 13813) |
| Streptococcus bovis | − | CECT 213 (ATCC 33317) |
| Streptococcus dysagalactiae | − | CECT 758 (NCTC 4335) |
| Streptococcus suis | − | CECT 958 (ATCC 43765) |
| Bacillus cereus | − | CECT 193 (ATCC 11778) |
| Enterococcus faecalis | − | CECT 795 (ATCC 29212) |
| Micrococcus luteus | − | CECT 241 (ATCC 9341) |
| Aeromonas hydrophila | − | CECT 839 (ATCC 7966) |
| Escherichia coli | − | CECT 434 (ATCC 25922) |
| Salmonella cholerasuis | − | CECT 556 |
| Yersina ruckeri | − | CECT 4319 (ATCC 29473) |
Chromosomal DNA isolation and manipulation.
Chromosomal DNA from bacteria other than Staphylococcus spp. was obtained from overnight cultures grown in LB-agar. Samples to be analyzed (a colony) were suspended in 100 μl of 1 × PCR buffer (10 mM Tris-HCl [pH 8.3], 2 mM MgCl, 50 mM KCl) and incubated at 95°C for 15 min. A 1-μl amount of the samples was used for PCR analysis. Chromosomal DNA from Staphylococcus strains was extracted following the procedure detailed elsewhere (36), and 5 μl of each sample was used for PCR analysis.
Blotting and hybridization were performed by standard procedures, and DNA labeling was carried out by random priming with digoxigenin-dUTP. Hybrids were detected by enzyme immunoassay following the manufacturer's instructions (Boehringer GmbH, Mannheim, Germany). For digestion of PCR products, a 5-μl sample was used. Restriction endonucleases were purchased from Boehringer GmbH.
PCR-RFLP assays.
PCR amplification tests were performed using a pair of primers selected on the basis of the gap gene nucleotide sequence of S. aureus (933-bp long, from the GenBank database under accession number AJ133520). A 26-nucleotide forward primer, GF-1 (5′-ATGGTTTTGGTAGAATTGGTCGTTTA-3′), corresponding to positions 22 to 47 of the gap gene, and a 25-nucleotide reverse primer, GR-2 (5′-GACATTTCGTTATCATACCAAGCTG-3′), corresponding to positions 956 to 932 of the previously mentioned gene, were selected. For comparative studies, another pair of primers were used: G1 (5′-GAAGTCGTAACAAGG-3′) and L1 (5′-CAAGGCATCCACCGT-3′), which were selected as described (20) so as to analyze the staphylococcal 16S-23S ribosomal DNA (rDNA) intergenic spacer region. Primers were synthesized by British Bio-Technological Products (Avingdon, England). PCR amplification was carried out with a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.) by using a PCR kit (Boehringer GmbH) and following the instructions of the manufacturer with some modifications. Briefly, the reaction mixture consisted of 5 μl of DNA-containing sample, 1.25 U of Taq DNA polymerase, 5 μl of 10× PCR amplification buffer, 0.8 μM each primer, 0.4 mM deoxynucleoside triphosphate, and double-distilled water to a final volume of 50 μl. In order to prevent evaporation, 50 μl of mineral oil was added to the mixture. DNA was denatured at 94°C for 2 min. A total of 40 PCR cycles were run under the following conditions: DNA denaturation at 94°C for 20 s, primer annealing at 55°C for 30 s, and DNA extension at 72°C for 40 s. After the final cycle, reactions were terminated by an extra run at 72°C for 5 min. PCR products were analyzed by agarose gel electrophoresis (3% agarose gels were prepared with MetaPhor agarose for fine separation and resolution of small nucleic acids; FMC Bioproducts, Rockland, Maine) in Tris-borate-EDTA (TBE) buffer.
The RFLP procedure was carried out by digesting PCR-amplified products with AluI endonuclease and analyzing them by agarose gel electrophoresis as previously mentioned. The procedure mentioned above (DNA extraction, PCR amplification, and RFLP) was done in triplicate, obtaining identical results. To determine the sensitivity of the PCR assays for staphylococci, 10-fold serial dilutions (from 106 to 0 bacteria) in saline were tested. One hundred microliters of each dilution was processed as previously mentioned, and 5 μl of each sample was used for PCR amplification. Viable cells were counted as CFU by triplicate plating of samples on Luria agar, and colony counts after incubation at 37°C for 24 h were determined. When nucleic acids were used, the sensitivity of the PCR was determined by amplifying 5 μl of 10-fold serial dilutions (1 ng to 0.1 pg).
RESULTS
PCR-RFLP analysis.
The pair of primers used in this study, GF-1 and GR-2, selected from the S. aureus gap gene sequence successfully primed the synthesis of an expected 933-bp fragment, which represents most of the gap gene sequence (Fig. 1A), when DNAs from 12 species of Staphylococcus tested in this study were used as a target, although a limited number of strains from each species were tested. However, no PCR amplification products were obtained when other genera were used as sources of high-molecular-weight target DNA (Fig. 1B). A single 933-bp hybridization band was also obtained when PCR products hybridized with the AluI 279-bp internal fragment of the gap gene, which was cloned from the S. aureus CECT 86 PCR-amplified product and digested with AluI endonuclease (data not shown). These results demonstrate that PCR amplification of the gap gene could be a useful tool for the rapid identification of staphylococci not only from bacterial cells but also from biological materials, such as milk. The sensitivity of our PCR assay was 25 viable cells or 40 pg of extracted DNA when S. aureus was used as the source of DNA and serial dilutions were conducted in saline. However, when S. aureus was serially diluted in sterilized whole milk, the lower detection limit was about 500 CFU. Similar results were obtained with the other 11 Staphylococcus species.
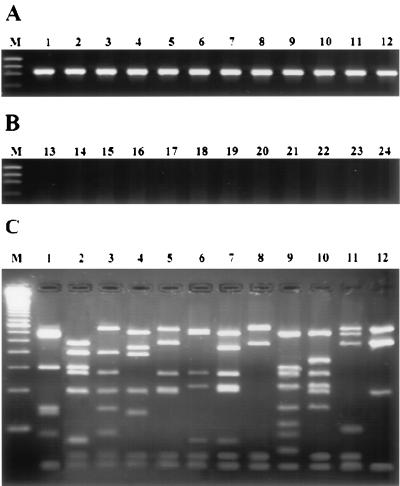
FIG. 1.
Agarose gel electrophoresis of the 933-bp PCR amplification products from chromosomal DNA from staphylococcal species reference strains using primers GF-1 and GR-2 (A), from other genera used as negative control (B), and fragments produced by AluI endonuclease digestion of PCR amplification products (C). Lanes: 1, S. aureus CECT 86; 2, S. epidermidis CECT 232; 3, S. capitis CECT 233; 4, S. hominis CECT 234; 5, S. saprophyticus CECT 235; 6, S. warneri CECT 236; 7, S. xylosus CECT 237; 8, S. auricularis CECT 4052; 9, S. carnosus CECT 4491; 10, S. simulans CECT 4538; 11, S. intermedius ATCC 49052; 12, S. haemolyticus human isolate; 13, Streptococcus sp. mastitis isolate; 14, S. agalactiae CECT 183; 15, S. dysagalactiae CECT 758; 16, S. suis CECT 958; 17, B. cereus CECT 193; 18, E. faecalis CECT 795; 19, M. luteus CECT 241; 20, A. hydrophila CECT 839; 21, E. coli CECT 434; 22, S. cholerasuis CECT 556; 23, Y. ruckeri CECT 4319; 24, reaction mixture with no DNA. Lanes M, standard DNA size markers (φX174 digested with HaeIII), (A and B, from top to bottom: 1,353, 1,078, 872, and 603 bp) and 50-bp ladder (C); bottom band, 50 bp.
The 933-bp PCR products of the 12 Staphylococcus spp. used in this study were AluI digested, and the resulting fragments were separated by MetaPhor agarose gel electrophoresis. A distinctive RFLP pattern was obtained for every species analyzed (Fig. 1C). A total of 103 S. aureus strains (59 human isolates and 44 animal isolates) were analyzed in this study, all of them showing an identical RFLP pattern (Fig. 1C, lane 1), indicating intraspecific uniformity. All clinical strains of S. epidermidis (6 strains isolated from humans and 27 strains isolated from animals) presented the same RFLP pattern as the reference strain CECT 232 (Fig. 1C, lane 2) except for two human isolates, termed CI-1, which presented a very similar RFLP pattern (Fig. 2A, lane 2), also different from the other staphylococcus species RFLP patterns. S. epidermidis phenotypic identification by the API ID32 Staph system was made with reasonable accuracy. These two strains were confirmed as S. epidermidis by 16S-23S intergenic spacer PCR (ITS-PCR) identification following the method described elsewhere (20).
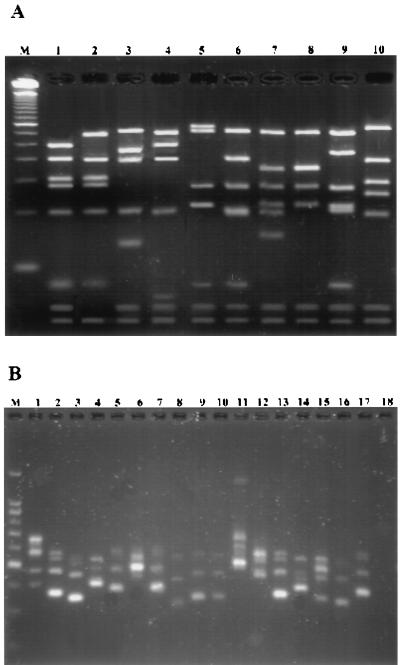
FIG. 2.
Agarose gel electrophoresis of fragments produced by AluI digestion of the 933-bp PCR amplification products (A) and PCR-amplified 16S-23S rDNA spacer regions (B) from Staphylococcus spp. (A) Lanes: 1, S. epidermidis CECT 232; 2, S. epidermidis CI-1; 3, S. hominis CECT 234; 4, S. hominis CI-2; 5, S. warneri CECT 236; 6, Staphylococcus sp. CI-3; 7, S. simulans CECT 4538; 8, S. simulans CI-4; 9, S. xylosus CECT 237; 10, Staphylococcus sp. CI-5. (B) Lanes: 1, S. aureus CECT 86; 2, S. epidermidis CECT 232; 3, S. capitis CECT 233; 4, S. hominis CECT 234; 5, S. saprophyticus CECT 235; 6, S. warneri CECT 236; 7, S. xylosus CECT 237; 8, S. auricularis CECT 4052; 9, S. carnosus CECT 4491; 10, S. simulans CECT 4538; 11, S. intermedius ATCC 49052; 12, S. haemolyticus human isolate; 13 to 17, CI-1 to CI-5, respectively. Lanes M, DNA molecular mass markers, 50-bp ladder (A); bottom band, 50 bp; and 100-bp ladder (B); bottom band, 200 bp.
The CI-2 isolate showed an RFLP pattern very similar to that of S. hominis CECT 234 (Fig. 2A, lanes 3 and 4), and both strains presented identical ITS-PCR patterns when compared. However, phenotypic methods did not allow a correct CI-2 identification. It is known that S. hominis, which is one of the most common species after S. epidermidis and S. haemolyticus, is usually identified with a low accuracy by the API ID32 system (10, 11, 27).
Isolate CI-3 was phenotypically identified as S. warneri with a low accuracy. However, the RFLP pattern was quite different from that of the reference strain S. warneri CECT 236 (Fig. 2A, lanes 5 and 6). CI-3 also presented a different ITS-PCR pattern when compared with the reference strain. Probably, this isolate could be considered a Staphylococcus spp. which is highly related to S. capitis CECT 233 by both RFLP-PCR and ITS-PCR (Fig. 1C and 2A). Similar results were obtained by others (18).
CI-4 isolate was identified as S. simulans with a low accuracy by phenotypic identification. The RFLP-PCR pattern was very similar to that of the reference strain S. simulans CECT 4538 (Fig. 2A, lanes 7 and 8). CA-4 had an ITS-PCR pattern identical to that of the reference strain mentioned (Fig. 2B, lanes 10 and 17).
Isolate CI-5 was identified as S. xylosus by biochemical properties with high accuracy. However, biochemical identification did not agree with our RFLP-PCR assay. Very different RFLP patterns were obtained when CA-5 and the reference strain S. xylosus CECT 237 were compared (Fig. 2A, lanes 9 and 10), although identical ITS-PCR patterns were obtained when these two strains were compared (Fig. 2B, lanes 7 and 17). Our results would agree with what is easily observed, such as growth and pigment production. Reference strains grew faster than isolate CA-5 and presented slightly yellowish colonies while isolate CA-5 presented almost white colonies when both strains were grown on LB agar medium.
DISCUSSION
The use of nucleic acid amplification by PCR has applications in many fields, especially for the rapid identification of bacteria. In this study we were able to differentiate among 12 reference species of Staphylococcus as well as to discriminate between strains belonging to the same species by a combination of gap gene PCR amplification and RFLP with AluI. The pair of primers used in this study did not recognize the other bacterial genera tested. The sensitivity of PCR analysis accords with that described for other bacteria, that is, between 1 and 20 CFU, or between 1 and 100 pg of DNA extracted from Staphylococcus spp. (3, 4, 17, 36).
The gap gene, encoding glyceraldehyde-3-phosphate dehydrogenase, has proved to be a very well conserved gene that may be a useful tool in an RFLP-PCR assay for differentiating staphylococcal species. Genetic uniformity was found in S. aureus strains analyzed by our procedure, although they could be grouped by RFLP-PCR amplification of the aroA gene (36) and by using other protocols (7, 12, 15). RFLP-PCR of the gap gene allowed detection of intraspecies polymorphism among S. epidermidis, S. hominis, and S. simulans strains. However, two isolates, CI-3 and CI-5, presumably identified as S. warnieri and S. xylosus, respectively, presented RFLP-PCR patterns quite different from those of their reference strains. Although CI-5 could be assigned to S. xylosus by ITS-PCR, colony morphology and pigments are very different from those of the reference strain S. xylosus CECT 237.
The gap gene product, glyceraldehyde-3-phosphate dehydrogenase, has been discovered to be located within the cell wall of S. aureus and other coagulase-negative staphylococcal species (24). Although this enzyme was absent from the S. warneri and S. saprophyticus cell wall (24), genetic information coding for it does exist, since a PCR product with high nucleotide sequence homology (data not shown) was amplified from these two species. These results suggest that glyceraldehyde-3-phosphate dehydrogenase may be located in the cytoplasm of these two species.
In conclusion, our results indicate that the RFLP-PCR protocol used in this study is relatively accurate in the identification of at least 12 species of Staphylococcus and may be useful for differentiating clinical isolates of staphylococci, especially for those which often do not allow correct phenotypic identification.
ACKNOWLEDGMENTS
This work was supported by a grant from the Spanish Ministerio de Educación y Cultura (DGICYT AGF98-0187). J.Y. is a fellowship holder of the University of León.
REFERENCES
- 1.Bartlett P C, Miller G Y, Lancet S E, Heider L E. Clinical mastitis and intramammary infections on Ohio dairy farms. Prev Vet Med. 1992;12:59–71. [Google Scholar]
- 2.Bes M, Brun Y, Gayral J P, Fleurette J, Laban P. Improvement of the API Staph gallery and identification of new species of staphylococci. Zentralbl Bakteriol. 1985;14:169–171. [Google Scholar]
- 3.Cascón A, Anguita J, Hernanz C, Sánchez M, Fernández M, Naharro G. Identification of Aeromonas hydrophila hybridization group 1 by PCR assays. Appl Environ Microbiol. 1996;62:1167–1170. doi: 10.1128/aem.62.4.1167-1170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cascón A, Anguita J, Hernanz C, Sánchez M, Yugueros J, Naharro G. RFLP-PCR analysis of the aroA gene as a taxonomic tool for the genus Aeromonas. FEMS Microbiol Lett. 1997;156:199–204. doi: 10.1111/j.1574-6968.1997.tb12727.x. [DOI] [PubMed] [Google Scholar]
- 5.Costa E O, Benites N R, Guerra J L, Melville P A. Antimicrobial susceptibility of Staphylococcus spp. isolated from mammary parenchymas of slaughtered dairy cows. Zentralbl Veterinarmed B. 2000;47:99–103. doi: 10.1046/j.1439-0450.2000.00319.x. [DOI] [PubMed] [Google Scholar]
- 6.Cuny C, Witte W. Typing of Staphylococcus aureus by PCR for DNA sequences flanked by Tn916 target region and ribosomal binding site. J Clin Microbiol. 1996;34:1502–1505. doi: 10.1128/jcm.34.6.1502-1505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolzani L, Tonin E, Lagatolla C, Minti-Bragadin C. Typing of Staphylococcus aureus by amplification of the 16S–23S rRNA intergeneic spacer sequences. FEMS Microbiol Lett. 1994;119:167–174. doi: 10.1111/j.1574-6968.1994.tb06884.x. [DOI] [PubMed] [Google Scholar]
- 8.Fidalgo S, Vasques F, Mendoza M C, Pérez F, Méndez F J. Bacteremia due to Staphylococcus epidermidis: microbiological, epidemiologic, clinical, and prognostic features. Rev Infect Dis. 1990;12:520–528. doi: 10.1093/clinids/12.3.520. [DOI] [PubMed] [Google Scholar]
- 9.Goh S H, Santucci Z, Kloos W E, Faltyn M, George C G, Driedger D, Hemmingsen S M. Identification of Staphylococcus species and subspecies by the chaperonin 60 gene identification method and reverse checkerboard hybridization. J Clin Microbiol. 1997;35:3116–3121. doi: 10.1128/jcm.35.12.3116-3121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iven M, Verhoeven J, Pattyn S R, Goossens H. Rapid and economical method for species identification of clinically significant coagulase-negative staphylococci. J Clin Microbiol. 1995;33:1060–1063. doi: 10.1128/jcm.33.5.1060-1063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janda W M, Ristow K, Novak D. Evaluation of RapidDEC Staph for identification of Staphylococcus aureus, Staphylococcus epidermidis, and Staphylococcus saprophyticus. J Clin Microbiol. 1994;32:2056–2059. doi: 10.1128/jcm.32.9.2056-2059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen M A, Webster J A, Straus N. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol. 1993;59:945–952. doi: 10.1128/aem.59.4.945-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloos W, Bannerman T L. Update on clinical significance of coagulase-negative staphylococci. Clin Microbiol Rev. 1994;7:117–140. doi: 10.1128/cmr.7.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloos W E, Bannerman T L. Staphylococcus and Micrococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 282–298. [Google Scholar]
- 15.Kumari D N P, Keer V, Hawkey P M, Parnell P, Joseph N, Richardson J F, Cookson B. Comparison and application of ribosome spacer DNA amplicon polymorphisms and pulsed-field gel electrophoresis for differentiation of methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 1997;35:881–885. doi: 10.1128/jcm.35.4.881-885.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lattore M, Rojo P M, Unrago M J, Cisterna R. Staphylococcus schleiferi: a new opportunistic pathogen. Clin Infect Dis. 1993;16:589–590. doi: 10.1093/clind/16.4.589. [DOI] [PubMed] [Google Scholar]
- 17.Lebech A M, Hindersson P, Vuust J, Hansen K. Comparison of in vitro culture and polymerase chain reaction for detection of Borrelia burgdorferi in tissue from experimentally infected animals. J Clin Microbiol. 1991;29:731–737. doi: 10.1128/jcm.29.4.731-737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes N, Gheldre Y, Ryck R, Vaneechoutte M, Meugnier H, Etienne J, Struelens M. Rapid identification of Staphylococcus species by tRNA intergenic spacer length polymorphism analysis. J Clin Microbiol. 1997;35:2477–2481. doi: 10.1128/jcm.35.10.2477-2481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martineau F, Picard F J, Roy P H, Ouellette M, Bergeron M G. Species-specific and ubiquitous DNA-based assays for rapid identification of Staphylococcus aureus. J Clin Microbiol. 1998;36:618–623. doi: 10.1128/jcm.36.3.618-623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendoza M, Meugnier H, Bes M, Etienne J, Freney J. Identification of Staphylococcus species by 16S–23S rDNA intergenic spacer PCR analysis. Int J Syst Bacteriol. 1998;48:1049–1055. doi: 10.1099/00207713-48-3-1049. [DOI] [PubMed] [Google Scholar]
- 21.Miller J M, Biddle J W, Quenzer V K, Mclaughlin J C. Evaluation of Biolog for identification of members of the family Micrococcaceae. J Clin Microbiol. 1993;31:3170–3173. doi: 10.1128/jcm.31.12.3170-3173.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modun B, Cockayne A, Finch R G, Williams P. The Staphylococcus aureus and Staphylococcus epidermidis transferrin-binding proteins are expressed in vivo during infection. Microbiology. 1998;144:1005–1012. doi: 10.1099/00221287-144-4-1005. [DOI] [PubMed] [Google Scholar]
- 23.Modun B, Evans R W, Joannou J I, Williams P. Receptor-mediated recognition and uptake of iron from human transferrin by Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun. 1998;66:3591–3596. doi: 10.1128/iai.66.8.3591-3596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modun B, Kendall D, Williams P. Staphylococci express a receptor for human transferrin: identification of a 42-kilodalton cell wall transferrin-binding protein. Infect Immun. 1994;62:3850–3858. doi: 10.1128/iai.62.9.3850-3858.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modun B, Williams P. The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect Immun. 1999;67:1086–1092. doi: 10.1128/iai.67.3.1086-1092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mollet C, Drancourt M, Raoult D. rpoB sequence analysis as a novel basis for bacterial identification. Mol Microbiol. 1997;26:1005–1011. doi: 10.1046/j.1365-2958.1997.6382009.x. [DOI] [PubMed] [Google Scholar]
- 27.Piccolomini R, Catamo G, Picciani C, D'Antonio D. Evaluation of Staf-System 18-R for identification of staphylococcal clinical isolates to the species level. J Clin Microbiol. 1994;32:649–653. doi: 10.1128/jcm.32.3.649-653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Refsahl K, Andersen B M. Clinical significance of coagulase-negative staphylococci: identification and resistance patterns. J Hosp Infect. 1992;22:19–31. doi: 10.1016/0195-6701(92)90127-8. [DOI] [PubMed] [Google Scholar]
- 29.Roberts F J, Gere I W, Coldman A. A three-year study of positive blood cultures, with emphasis on prognosis. Rev Infect Dis. 1991;13:34–46. doi: 10.1093/clinids/13.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Smeltzer M S, Gillaspy A, Pratt F L, Thames M D. Comparative evaluation of use of cna, fnbA, fnbB, and hlb for genomic fingerprinting in the epidemiological typing of Staphylococcus aureus. J Clin Microbiol. 1997;35:2444–2449. doi: 10.1128/jcm.35.10.2444-2449.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoakes L, John M A, Lannigan R, Schieven B C, Ramos M, Harley D, Hussain Z. Gas-liquid chromatography of cellular fatty acids for identification of staphylococci. J Clin Microbiol. 1994;32:1908–1910. doi: 10.1128/jcm.32.8.1908-1910.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenover F C, Arbeit R, Archer G, Biddle J, Byrne S, Goering R, Hancock G, Hébert G A, Hill B, Hollis R, Jarvis W R, Kreiswirth B, Eisner W, Maslow J, McDougal L K, Michael J, Mulligan M, Pfaller M A. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J Clin Microbiol. 1994;32:407–415. doi: 10.1128/jcm.32.2.407-415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandenesch F, Eykyn S J, Etienne J. Infections caused by newly-described species of coagulase-negative staphylococci. Rev Med Microbiol. 1995;6:94–100. [Google Scholar]
- 34.Watts J L, Washburn P J. Evaluation of the Staph-Zym system with staphylococci isolated from bovine intramammary infection. J Clin Microbiol. 1991;29:59–61. doi: 10.1128/jcm.29.1.59-61.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witold A F, Davis W C, Hamilton M J, Park Y H, Deobald C F, Fox L, Bohach G. Activation of bovine lymphocyte subpopulations by staphylococcal enterotoxin C. Infect Immun. 1998;66:573–580. doi: 10.1128/iai.66.2.573-580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yugueros J, Cascón A, Sánchez M, Hernanz C, Suárez S, Smeltzer M S, Naharro G. Rapid identification and typing of Staphylococcus aureus by PCR-restriction fragment length polymorphism analysis of the aroA gene. J Clin Microbiol. 1999;37:570–574. doi: 10.1128/jcm.37.3.570-574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]