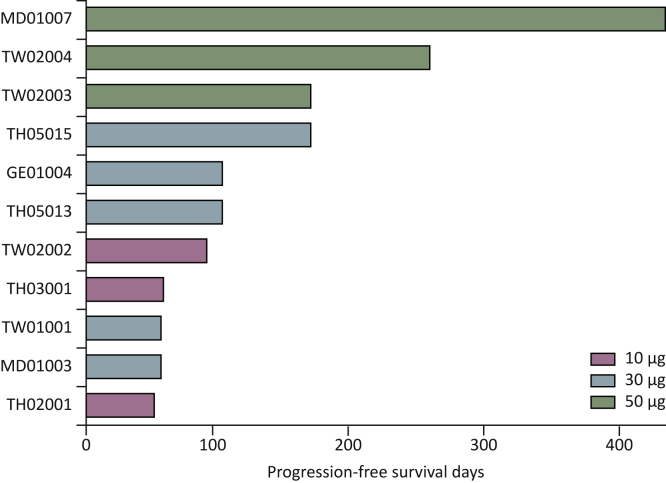Abstract
Immunotherapy has been a milestone in combatting cancer, by complementing or even replacing classic treatments like surgery, chemotherapy, radiation, and anti-hormonal therapy. In 15%-30% of breast cancers, overexpression of the human epidermal growth factor receptor 2 (Her-2/neu) is associated with more aggressive tumor development. Passive immunization/immunotherapy with the recombinantly produced Her-2/neu-targeting monoclonal antibodies (mAbs) pertuzumab and trastuzumab has been shown to effectively treat breast cancer and lead to a significantly better prognosis. However, allergic and hypersensitivity reactions, cardiotoxicity, development of resistance, lack of immunological memory which results in continuous application over a long period, and cost-intensiveness are among the drawbacks associated with this treatment. Furthermore, intrinsic or acquired resistance is associated with the application of therapeutic mAbs, leading to the disease recurrence. Conversely, these drawbacks could be potentially overcome by vaccination, i.e. an active immunization/immunotherapy approach by activating the patient’s own immune system to target cancer, along with inducing immunological memory. This review aims to summarize the main approaches investigated and undertaken for the production of Her-2/neu vaccine candidates, with the main focus on peptide-based vaccines and their evaluation in clinical settings.
Key words: cancer, Her-2/neu, vaccination, T-cell peptide vaccine, B-cell peptide vaccine/mimotopes
Highlights
-
•
Her-2/neu is overexpressed in 10%-30% of breast and gastric cancer patients and this correlates with poor clinical outcomes.
-
•
Passive application of trastuzumab and pertuzumab has outstandingly improved the Her-2/neu-related clinical outcomes.
-
•
Treatment with mAbs is associated with frequent administration, cost-intensiveness, and resistance.
-
•
Vaccination against Her-2/neu with e.g. mimotope- or peptide-based vaccines can alternatively overcome the mAbs’ drawbacks.
-
•
Such alternatives may pave the way to therapeutics which could be used as monotherapy or in combination therapies with mAbs.
Introduction
One of the leading causes of death among women is breast cancer, including the molecular sub-type with overexpressed Her-2/neu (ErbB2). Her-2/neu, a 185-kDa transmembrane protein, is a family member of the human epidermal growth factor receptors (EGFR), also including Her-1 (EGFR, ErbB1), Her-3 (ErbB3), and Her-4 (ErbB4).1 The amplification of Her-2/neu was first observed in 1985 where the human mammary carcinoma cells MAC117 were shown to have 5- to 10-fold overexpression of the receptor.2 The role of the Her-2/neu proto-oncogene in the pathogenesis and progression of human breast cancer was shown in 1987.3 Her-2/neu overexpression has been observed in 15%-30% of breast cancer patients4, 5, 6 resulting in a 100- to 200-fold increased concentration of the Her-2/neu protein in tumor versus normal tissue.7 Furthermore, the overexpression of the receptor correlates with poor clinical outcomes resulting in an aggressive tumor phenotype and reduced survival.8, 9, 10, 11 Therefore, due to the association of Her-2/neu overexpression with enhanced tumor progression, as well as weaker response to traditional chemotherapy, and consequently poor prognosis, the receptor has been an attractive target as a tumor-associated antigen (TAA) for cancer therapy.12, 13, 14, 15 Anticancer immunotherapies fall into the two categories of ‘passive’ or ‘active’ immunotherapy16; the emergence of both has been facilitated by the discovery of TAAs. By possessing an intrinsic antineoplastic activity, the application of therapeutic, tumor-targeting monoclonal antibodies (mAbs) is generally considered the passive form of immunotherapy.17,18 Conversely, anticancer vaccines under the category of active immunotherapy induce the engagement of the host immune system and the formation of immunological memory.19,20
Biological background
Immune dysregulation and dysfunction result in cancer progression and therefore the aim of cancer immunotherapy is to restore a specific anti-tumor immune response. In systems biology, the Her-2/neu pathway, or Her network, has been described as a complex and robust biological network.21,22 Unlike the other members, no ligand has been identified for Her-2/neu, i.e. an orphan receptor. The ligand binding to the extracellular domain (ECD) of Her-1, Her-3, and Her-4 results in the formation of catalytically active homo- and heterodimers. Her-2/neu, which is in an open position and naturally ready for dimerization, is a preferred partner for dimerization.23 Activation of the Her network as a result of the dimerization leads to auto-phosphorylation in C-terminal tyrosines of the receptors’ intracellular domain (ICD) and activation and recruitment of cytoplasmic signal transducers, such as Ras-Raf-MEK-MAPKs, phosphatidylinositol-3 kinase (PI3K)-Akt-ribosomal S6 kinase, and the signal transducers and activators of transcription.23 These consequently regulate cellular processes such as proliferation, differentiation, motility, adhesion, protection from apoptosis, and transformation.23,24 Over the past decades, due to the importance of the ECD and ICD of Her-2/neu, there has been remarkable progress in the regimens developed for the treatment of Her-2/neu-positive breast cancer.25, 26, 27
Trastuzumab, the first anti-Her-2/neu humanized mAb developed in 1990, interferes with Her-2/neu signaling via several mechanisms, including inhibition of ligand-independent dimerization, receptor internalization and/or degradation, inhibition of the PI3K–AKT signaling pathway, and antibody-dependent cellular cytotoxicity (ADCC).28,29 The addition of trastuzumab to chemotherapy was initially found to improve overall survival (OS) in women with Her-2/neu-positive metastatic breast cancer,30 and 1 year of treatment with trastuzumab after adjuvant chemotherapy was shown to significantly improve disease-free survival (DFS) among women with Her-2/neu-positive breast cancer.31 Therefore, trastuzumab has become a standard-of-care treatment of both metastatic and early-stage Her-2/neu-positive breast cancers for more than a decade. Pertuzumab, the second anti-Her-2/neu humanized mAb, was approved by the Food and Drug Administration in 2012 for the treatment of Her-2/neu-positive metastatic breast cancer.32 Pertuzumab, which has a different binding site than trastuzumab, binds to the dimerization domain 2 of Her-2/neu resulting in the inhibition of ligand-induced Her-2/neu heterodimerization.32,33 The combination of trastuzumab and pertuzumab has been shown to synergistically inhibit tumor growth in both in vitro and in vivo preclinical models.34,35 In the phase III CLEOPATRA trial, the combination of pertuzumab plus trastuzumab and docetaxel, compared with placebo plus trastuzumab and docetaxel, prolonged median OS by 15.7 months without any increase in the risk of cardiac toxicity.36 The improvement in clinical outcomes with the addition of pertuzumab to trastuzumab is seen as a result of its complementary mechanism of action to trastuzumab, resulting in maximal blockade of the Her-2/neu oncogenic pathway.32
Despite the tremendous success with its anti-tumor effect, immunotherapy by mAbs requires continuous application over a long period, and the half-life of the mAbs limits the duration of therapy, resulting in only temporary disease control, particularly once the tumor has metastasized.37, 38, 39, 40, 41, 42, 43, 44 Furthermore, the development of resistance to treatments with mAbs,45 immune-related adverse events, and hypersensitivity,46,47 which may be as a result of the mAbs administered doses to ensure their immediate bioavailability and potency48,49 and cardiotoxicity,50 are among the drawbacks of treatments with mAbs. Unlike mAbs or chemotherapy, TAA-based vaccines which induce the immune system to produce patients’ own antibodies toward the antigen may prevent the development or reduce the intensity of hypersensitivity reactions, can induce prolonged activation of the immune system, immunological memory, and potentially result in more efficient cancer control. Further advantages of vaccines include their less-frequent and easier administration and their relatively safer nature than chemotherapy.51
Anti-HER-2/NEU vaccines under development
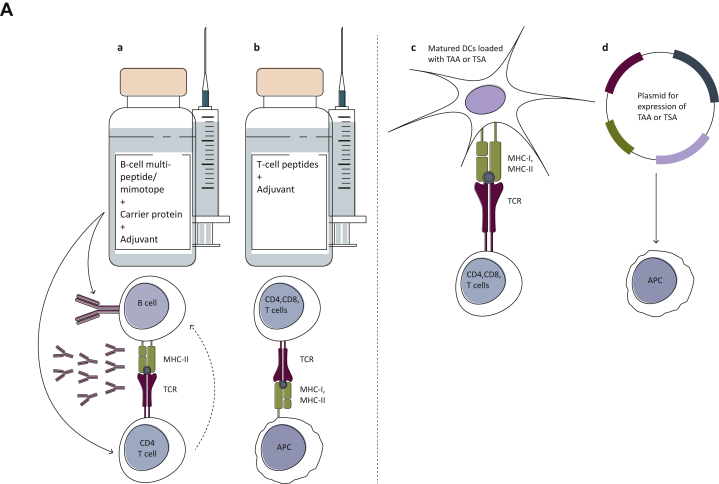
Different approaches have been undertaken to construct anti-Her-2/neu vaccines. As depicted in Figure 1A, these approaches include, B- or T-cell peptide-based vaccines, matured dendritic cells (DCs) loaded with TAA or tumor-specific antigens (TSA), which, as specialized antigen-presenting cells, possess a key role in the initiation and regulation of innate and adaptive immune responses by sensitizing antigen-specific CD4- and CD8-positive T cells,52, 53, 54 or DNA vaccination using plasmids delivering genes encoding the TAA or TSA to harness the immune system and elicit or augment the adaptive immune response against the respective tumor.55, 56, 57 This review focuses on peptide-based vaccine candidates targeting Her-2/neu (Figure 1A; Table 1).
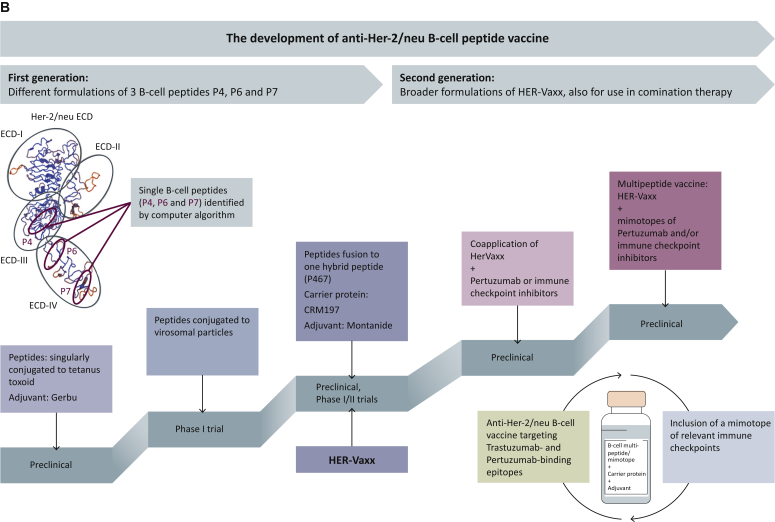
Figure 1.
(A) A diagram depicting different types of cancer vaccines against Her-2/neu. B-cell peptides representing the binding epitope of anti-Her-2/neu therapeutic monoclonal antibodies (mAbs), directly activate B cells for the production of Abs, corresponding to the therapeutic mAbs. The presence of T-cell epitopes in the carrier protein results in stimulation of T cells, which in turn further activates B cells for the production of antibodies. T-cell-based (b) and dendritic-cell (DC)-based (c) vaccines bind to either CD4+ or CD8+ T cells and result in their stimulation. DNA-based vaccines are also aimed to stimulate the T cells after being processed by APC (d). (B) A diagram depicting the development of the two generations of our Her-2/neu vaccine with the different examined formulations, and their evaluation in preclinical and clinical studies.68,70,72,73,76,77 The extracellular domain (ECD) of Her-2/neu was initially subjected to computer-aided algorithms and resulted in the identification of one B-cell peptide (P4) on the ECD-III and two additional B-cell peptides (P6 and P7) on the ECD-IV. These single peptides were the basis for the different indicated formulations of our Her-2/neu vaccine’s first generation, leading to the vaccine HER-Vaxx. The second generation of our vaccine candidate comprises the HER-Vaxx vaccine, formulated for co-application with pertuzumab and/or immune checkpoint inhibitors, or in combination with the mimotopes (B-cell epitopes) of pertuzumab and/or relevant immune checkpoint inhibitors.
APC, antigen-presenting cells; MHC-I/II, major histocompatibility complex I/II; TAA, tumor-associated antigen; TCR, T-cell receptor; TSA, tumor-specific antigen.
Table 1.
Developed or under development anti-Her-2/neu vaccines
| Platform | Vaccine |
Reference | |
|---|---|---|---|
| Peptide | Characteristics | ||
| T-cell peptide vaccines | E75 | CD8, MHC class I Extracellular domain of Her-2/neu (amino acids 369-377) |
59 |
| GP2 | CD8, MHC class I (HLA-A2- and A3-restricted) Transmembrane domain of Her-2/neu (amino acids 654-662) |
62 | |
| AE37 | CD4, MHC class II Intracellular domain of Her-2/neu (amino acids 776-790) |
63 | |
| B-cell peptide vaccines | HER-Vaxx | Three fused single peptides from the extracellular domain of Her-2/neu: PESFDGDPASNTAPLQPRVLQGLPREYVNARHSLPYMPIWKFPDEEGAC |
68,73 |
| JTMP | A 42-mer peptide as pertuzumab’s binding epitope on Her-2/neu (amino acids 260-301) | Reported here | |
| B-Vaxx | Trastuzumab-binding epitope (amino acids 597-626) and pertuzumab-binding epitope (amino acids 266-296) | 82,83 | |
| Liposome-based vaccine with B-cell peptides | Liposome-based vaccine comprising spatially separated B-cell epitope of the Her-2/neu targeted by pertuzumab, and ovalbumin peptide OVA | 85 | |
MHC, major histocompatibility complex.
T-cell peptide vaccines
Therapeutic peptide-based vaccines can reduce tumor growth by generating tumor-specific CD8 cytotoxic T lymphocytes (CTLs) or CD4 helper T lymphocytes. Activation of T cells is achieved by both antigen-specific signaling, i.e. interaction between T-cell receptor and the antigen in the context of human leukocyte antigen (HLA) classes I and II of the antigen-presenting cells (APCs), to form a peptide–HLA complex, and CD28 co-stimulation, i.e. interaction between co-stimulatory receptors like CD28 with CD80 or CD86 on the APC.20,58 It was proposed that Her-2/neu may serve as a TAA since the processing of overexpressed Her-2/neu protein would theoretically result in an increased peptide supply which could occupy a significant number of major histocompatibilty complex (MHC) molecules in competition with other peptides.7
The 9-mer peptide E75, derived from the ECD of Her-2/neu (amino acids 369-377), is among the most studied peptide vaccine candidates against Her-2/neu.59 The peptide was identified as one of the common immunogenic epitopes of Her-2/neu that were recognized by CD3+ CD4− CD8+ ovarian-specific CTL lines, and is based on the anchor motif of the HLA-A2 allele7 as well as HLA-A3 allele.60
GP2, an MHC class I, nine–amino acid-long peptide (IISAVVGIL), is a fragment (amino acids 654-662) of the transmembrane domain of Her-2/neu which binds to the HLA-A2 molecule and activates CTLs.61,62
AE37, an MHC class II peptide, activates CD4 T helper cells and is a modified version of the naturally occurring AE36 wild-type peptide (Her-2/neu, amino acids 776-790) derived from the ICD of Her-2/neu.63 AE37 was generated by linking AE36 to Ii-Key (LRMK), a portion of the MHC class II-associated invariant chain (Ii protein), to enhance the peptide’s potency in activating CD4+ T cells. The Ii-Key hybrid AE37, generated by linking LRMK to the known Her-2/neu MHC class II epitope (amino acids 776-790), has been shown to generate robust, long-lasting Her-2/neu-specific immune responses both in patients with breast cancer and prostate cancer.64,65
B-cell peptide vaccines
The tremendous success of passive immunotherapy with trastuzumab has paved the way for more interest in the identification of B-cell peptides/binding epitope of mAbs for use as anti-Her-2/neu vaccine antigens.66 This was further reinforced by the proven efficacy of the dozens of additional approved therapeutic mAbs.67 Vaccines including B-cell peptides induce an immune response specific to the TAA or TSA and, unlike T-cell peptides, B-cell peptides are not restricted to a specific HLA, advantageously allowing a broader application across all HLA types.20 This becomes, in particular, important regarding the reduction of expression of MHC-I molecules as tumor evasion mechanisms.20
In line with this approach, our group has been working on developing B-cell peptide-based Her-2/neu vaccines, formulated differently with regard to their immunogenicity, adjuvanticity, and their evaluation in several preclinical and clinical studies, as depicted in Figure 1B and described below.
By the means of computer algorithms, as the first generation of our vaccine, we identified three single peptides representing B-cell epitopes located in the ECD-III (P4; amino acids 378-394) and ECD-IV (P6 and P7; amino acids 545-560 and 610-623, respectively).68 Immunization with the single peptides, conjugated to tetanus toxoid and mixed together with Gerbu, a veterinary adjuvant based on the immunomodulator glycopeptide from the cell wall of the bacterium Lactobacillus bulgaricus,69 led to induction of antibodies with the capacity in inhibiting tumor growth in vitro, as shown by proliferation assays and complement-dependent and antibody-dependent cell cytotoxicity assays.68 In a breast cancer mouse model with an activated c-neu oncogene, immunization with the aforementioned formulation was shown to delay tumor onset and reduce tumor growth progression. Moreover, co-application of the vaccine with IL-12 resulted in a Th1-polarized immune response with elevated Her-2/neu-specific IgG levels and increased in vitro production of interferon-γ by splenocytes.70 For clinical evaluation, the single peptides were conjugated to virosomes, possessing an intrinsic adjuvant activity,71 and examined in a phase I study for evaluating the safety and immunogenicity in breast cancer patients in the advanced stage of the disease.72 While the study showed good immunogenicity as well as an excellent safety profile,72 drawbacks with regard to solubility and limited stability after coupling the single peptides to virosomes were associated with the formulation. This consequently led to the development of an improved formulation,73 comprising the three single peptides fused into the hybrid peptide P467 (PESFDGDPASNTAPLQPRVLQGLPREYVNARHSLPYMPIWKFPDEEGAC), conjugated to the carrier protein CRM197 (CRM; cross-linking materials) and administered in conjunction with the Th1/Th2-driving adjuvant Montanide. As an enzymatically inactive and nontoxic (toxoid) form of diphtheria toxin,74 CRM197 rapidly activates CD4+ T cells with a heterogeneous Th1 and Th2 cytokine profile and consequently activates B cells and regulates the quantity of the induced antibodies,75 justifying its use with our vaccine. In preclinical evaluations, the hybrid peptide was shown to retain the B-cell epitopes of the single peptides with a strong capacity to induce long-lasting antibody responses. Additionally, the fusion of the single peptides had further generated a T-cell immunodominant epitope in the hybrid peptide, inducing cellular responses as shown by T-cell proliferation of splenocytes from mice.73 This formulated vaccine, i.e. HER-Vaxx (Figure 1B), was evaluated in vivo for its anti-tumor effect, showing that active immunization with the vaccine significantly inhibits tumor growth in our mouse syngeneic model with solid tumors following engraftment of BALB/c mammary carcinoma cells expressing human Her-2/neu (D2F2/E2).76 Applying the mouse model, we have also shown that the combination of HER-Vaxx with immune checkpoint (PD-1) blockade enhanced the vaccine’s anti-tumor effect.76 As described below, HER-Vaxx has been evaluated in phase Ib77 and phase II trials78 involving patients with Her-2/neu-overexpressing metastatic or advanced adenocarcinoma of the stomach or gastroesophageal junction.
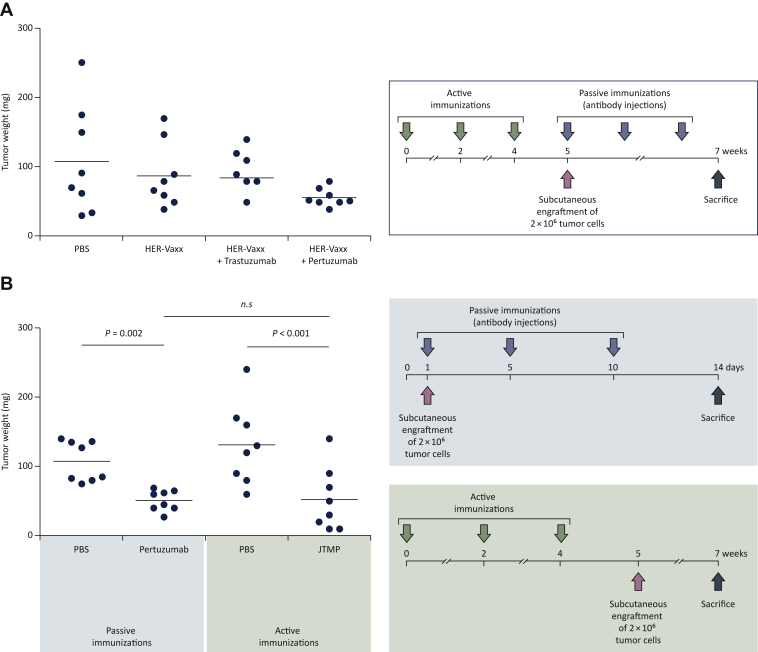
We have recently established a platform for the identification of mimotopes (B-cell peptides) of therapeutic mAbs.76 Toward the construction of a multi-peptide vaccine targeting the binding epitopes of trastuzumab and pertuzumab (Figure 1B), we aimed to identify the binding epitope of pertuzumab by the application of the mimotope platform. This was supported following in vivo evaluation of the anti-tumor effect of HER-Vaxx in combination with trastuzumab or pertuzumab in the aforementioned syngeneic solid tumor mouse model.76 As shown in Figure 2A, the combination of HER-Vaxx with trastuzumab did not enhance the vaccine’s anti-tumor effect, which is attributed to the fact that HER-Vaxx comprises the binding-site epitope of trastuzumab and therefore no synergistic effect was observed. However, in combination with pertuzumab, a significantly enhanced anti-tumor effect of HER-Vaxx, when compared with the vaccine alone, was shown (Figure 2A).
Figure 2.
Evaluation of anti-tumor capacity by active or passive immunization with HER-Vaxx, Trastuzumab, Pertuzumab, or the mimotope of Pertuzumab (JTMP) in a syngeneic tumor mouse model.
(A) Female BALB/c mice (Charles River, Germany, 6-8 weeks of age at the time of delivery) were used in immunization experiments involving a syngeneic solid tumor mouse model (Tobias et al.76). The experiment, representative of at least two experiments, consisted of four groups of mice (n = 8) subcutaneously injected with phosphate-buffered saline (PBS) (control mice), with HER-Vaxx alone or followed by intraperitoneal injection (100 μg/dose) with either trastuzumab or pertuzumab, as depicted in the immunization schedule. Two weeks after the tumor cell grafting, mice were sacrificed, the tumors were excised, and their weight was measured. (B) Female BALB/c mice (Charles River, Germany, 6-8 weeks of age at the time of delivery) were used in immunization experiments involving a syngeneic solid tumor mouse model (Tobias et al.76). The experiment, representative of at least two experiments, consisted of two settings. In the passive immunization setting, mice (n = 8) were either intraperitoneally injected with PBS or with pertuzumab (100 μg/dose), as depicted in the immunization schedule. In the active immunization setting, mice (n = 8) were either subcutaneously injected with PBS or with JTMP, the mimotope of pertuzumab (50 μg/dose), as depicted in the immunization schedule. Two weeks after the tumor cell grafting, mice were sacrificed, the tumors were excised, and their weight was measured. Tumor mass data were analyzed by a General Linear Model with a log link. Residuals were tested for normality by Kolmogorov–Smirnov tests and homogeneity of variances by the Brown–Forsythe test. Comparisons to controls and between active and passive immunization were done by linear contrasts. Significant differences are indicated by the respective P values. n.s., not significant.
By applying our established platform for identification of mimotopes of therapeutic mAbs,76 a 42-mer peptide (HSGICELHCPALVTYNTDTFESMPNPEGRYTFGASCVTACPY), located at the positions 260-301 on Her-2/neu, was designed. Based on solid phase-based inhibition ELISA assay, the mimotope was shown to significantly and dose-dependently inhibit the mAb’s binding to recombinant Her-2/neu (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100361). Furthermore, using the breast cancer cell line SKBR-3, endogenously expressing high levels of Her-2/neu as well as Her-3,79,80 mimotope-specific mouse IgG antibodies were shown to modulate the phosphorylation of intracellular Her-2/neu as well the phosphorylation of the protein kinase Akt, at the positions 308 and 473, in a similar manner as pertuzumab (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100361); the heterodimerization of Her-2/neu and Her-3 is considered to be the realm of oncologic signaling through the potent activation of the Akt signaling pathway.81
By employing the syngeneic solid tumor mouse model, we have shown that active immunization with the mimotope induces an anti-tumor effect, reflected by a significant tumor growth reduction compared with the corresponding control mice. Additionally, the level of the anti-tumor effect mediated by the mimotope-induced Abs was shown to be comparable with that following passive immunization of mice with pertuzumab (Figure 2B).
In an established mouse model for Her-2/neu-expressing lung metastases, reflecting the metastatic stage of the disease and the adjuvant setting in the clinic, active immunization with the mimotope has shown a significant capacity in preventing lung metastases (unpublished data). Furthermore, the potential enhancement of our multi-peptide vaccine’s anti-tumor effect when combined with relevant immune checkpoint inhibitors or their respective mimotopes is currently being investigated in this model.
By the means of X-ray structures of the Her-2/neu–trastuzumab and Her-2/neu–pertuzumab complexes, another research group identified the rationally designed B-cell peptides as pertuzumab’s binding epitope (amino acids 266-296, overlapping with the sequence of our pertuzumab’s mimotope JTMP), and trastuzumab’s binding epitope (amino acids 597-626).82,83 The peptides are individually fused at their N-terminus, by a linker consisting of GPSL, to a measles virus fusion protein (MVF), a carrier protein representing a ‘promiscuous’ T helper cell epitope. This allows MCH class II-mediated activation of multiple T helper cells, leading to increased production of also tumor peptide-specific Ab production by B cells.84 A vaccine, B-Vaxx, consisting of a mixture of the two chimeric constructs in combination with a potent adjuvant nor-muramyl dipeptide (n-MDP) and emulsified in Montanide ISA 720, was formulated.84
In a recent study, the use of a liposomal system for the delivery of B-cell peptide of Her-2/neu was also investigated.85 The liposomal system was composed of a spatially separated B-cell epitope of the Her-2/neu ECD targeted by pertuzumab, and ovalbumin peptide OVA (amino acids 323-339), to provide non-cognate T-cell support.85 Immunization of mice with the liposome-based vaccine was shown to induce significant humoral responses, specific for the Her-2/neu peptide, in vivo. The generated antibodies were shown to induce cell death in vitro using the mouse mammary carcinoma cell line TUBO overexpressing rat Her-2.85
Completed or current ongoing clinical trials—T-cell peptide vaccines
E75, GP2, AE37, multi-peptide
The T-cell peptide-based vaccine E75, otherwise known as nelipepimut-S or NeuVAX (Galena Biopharma/SELLAS Life Sciences Group, Inc., New York, NY), has so far been the most extensively studied vaccine in several clinical trials (Table 2). The earliest phase I pilot study involved patients with breast or ovarian cancer that expressed HLA-A2 and Her-2/neu, or epithelial mucin MUC1, which is aberrantly overexpressed in various cancers in human epithelia.86 Autologous DCs were pulsed with E75 or with MUC1-derived peptides and used for the vaccination which was well tolerated with no side effects.87 The vaccination was also shown to induce the production of peptide-specific CTLs which lasted for >6 months.87
Table 2.
Completed or current ongoing clinical trials with T- and B-cell peptide vaccines
| T-cell peptide vaccines | |||||||
|---|---|---|---|---|---|---|---|
| Examined vaccine | Phase | Adjuvant | Systemic intervention | Patients | Setting | NCT/trial’s identifier | Reference |
| E75 | I | GM-CSF | None | Breast cancer | Experimental (vaccine) versus no intervention (control) | NCT00841399 | 88 |
| I | GM-CSF | None | Breast cancer | Experimental (vaccine) versus no intervention (control) | NCT00854789 | 88 | |
| II | GM-CSF | Trastuzumab | Breast cancer | Vaccine + trastuzumab versus trastuzumab + GM-CSF | NCT01570036 | 89 | |
| III | GM-CSF | None | Breast cancer (low to intermediate Her-2/neu expression) | Vaccine versus GM-CSF only | NCT01479244 | 90 | |
| II | GM-CSF | None | Breast cancer | Vaccine versus GM-CSF only | NCT02636582 | 88 | |
| II | GM-CSF | Trastuzumab | Breast cancer | Vaccine + trastuzumab versus trastuzumab + GM.-CSF | NCT02297698 | 88 | |
| GP2 | I | GM-CSF | None | Breast cancer | Dose escalation | #11 730a | 92 |
| II | GM-CSF | None | Breast cancer | Vaccine versus GM-CSF only | NCT00524277 | 93 | |
| Ib | GM-CSF | Trastuzumab | Breast cancer | Vaccine + trastuzumab versus trastuzumab | NCT03014076 | 94 | |
| AE37 | I | GM-CSF | None | Breast cancer | Dose escalation | #12 229a | 95 |
| II | GM-CSF | None | Breast cancer | Vaccine versus GM-CSF only | NCT00524277 | 96 | |
| HER2/neu peptide | I/II | Breast and ovarian cancers | Trastuzumab | Breast or ovarian cancer | Active, not recruiting | NCT00194714 | 98 |
| B-cell peptide vaccines | |||||||
|---|---|---|---|---|---|---|---|
| Examined vaccine | Phase | Adjuvant | Systemic intervention | Patients | Setting | NCT/trial’s identifier | Reference |
| HER-Vaxx (IMU-131) | Ib | Montanide | Chemotherapy | Her-2 + gastric cancer | Dose escalation | 77 | |
| II | Montanide | Chemotherapy | Her-2 + gastric cancer | Vaccine + chemotherapy versus chemotherapy alone | Ongoing study | ||
| B-vaxx | I | None | Metastatic solid tumors | Dose escalation | 102 | ||
| II | None | Metastatic solid tumors (breast, ovarian and gastrointestinal cancers) | Extension trial | Ongoing study | |||
GM-CSF, granulocyte–macrophage colony-stimulating factor.
Walter Reed Army Medical Center.
In two follow-up paired sets of early phase I clinical trials (NCT00841399 and NCT00854789; Table 2), Her-2/neu-expressing breast cancer patients were vaccinated with E75 and the vaccine adjuvant granulocyte–macrophage colony-stimulating factor (GM-CSF). The vaccine was shown to be safe, and an 89.7% DFS in the vaccinated group versus 80.2% in the control group were observed, indicating the vaccine’s clinical efficacy.88 In a phase IIb combination trial (NCT01570036), the two arms of trastuzumab plus nelipepimut-S or trastuzumab plus GM-CSF were evaluated.89 The primary outcome in this study was a 24-month DFS, and a 36-month DFS, safety, and immunologic responses were the study’s secondary outcomes. Based on the results, no clinicopathologic differences between groups were observed and the concurrent treatment with trastuzumab and the vaccine was shown to be safe compared to the control group. No significant difference in the DFS at a median follow-up of 25.7 months was found between the vaccinated and the control groups. The results of this study indicated the safety of both nelipepimut-S and GM-CSF in combination with trastuzumab, findings that warranted further investigation in a phase III randomized trial.
The phase III trial (NCT01479244; Table 2), with nelipepimut-S as a single agent, involved 758 patients with early-stage, node-positive breast cancer with low to intermediate Her-2/neu expression. Patients received nelipepimut-S plus GM-CSF or GM-CSF alone, over the course of six intradermal injections followed by boosting every 6 months for 3 years. The interim analysis of the results demonstrated no overall difference in DFS between arms at 16 months’ follow-up.90 Based on the results from this phase III trial, combination studies were designed.91
In an ongoing phase II trial, patients with ductal carcinoma in situ of the breast (NCT02636582; Table 2) were treated either with nelipepimut-S or with sargramostim (the recombinant GM-CSF). The aim of this study, with pending results, is to evaluate the level of circulating immune response at 6 months after vaccination, as well as toxicity and safety.88 In an additional ongoing randomized phase II trial (NCT02297698; Table 2),88 patients with non-metastatic Her-2/neu overexpression (immunohistochemistry 3+) were vaccinated with nelipepimut-S + GM-CSF after completion of infusion with trastuzumab, or vaccinated only with GM-CSF and simultaneously treated with trastuzumab. Based on interim results, nelipepimut-S was shown to be well tolerated and no significant differences in side-effect profile or cardiac ejection fractions were observed between the two arms of the study.88
In a phase I dose-escalation trial (Walter Reed Army Medical Center, Washington DC; drug application BB-IND #11,730) involving disease-free, lymph node-negative, HLA-A2-positive breast cancer patients, the peptide GP2 in combination with GM-CSF was found to be safe and well tolerated with minimal local/systemic toxicity (Table 2). GP2 elicited Her-2/neu-specific immune responses, including epitope spreading in high-risk, lymph node-negative breast cancer patients.92 However, in a follow-up phase II trial (NCT00524277) involving clinically disease-free HLA-A2-positive patients with Her-2/neu-expressing tumors, the vaccinated group did not show a statistically significant difference in the rate of recurrence compared to the control group. The trial confirmed that the GP2 vaccine is safe, suggesting that the vaccination may have clinical activity, particularly in patients with Her-2/neu overexpression who received the full vaccine series.93 In an additional phase Ib trial (NCT03014076) involving Her-2/neu-overexpressing breast cancer patients, the combination of GP2 with trastuzumab was evaluated showing that the vaccine is safe and stimulates an immunologic response when given concurrently with trastuzumab.94
In a phase I clinical trial (Walter Reed Army Medical Center, Washington, DC; drug application #12 229), the peptide AE37 in conjunction with GM-CSF was shown to be safe and well tolerated with minimal toxicity in breast cancer patients and induced Her-2/neu-specific immune response without the use of an immunoadjuvant95 (Table 2). In a follow-up phase II adjuvant trial (NCT00524277) involving clinically disease-free node-positive and high-risk node-negative breast cancer patients with tumors showing any degree of Her-2/neu expression (immunohistochemistry 1+ to 3+), the efficacy of AE37 in conjunction with GM-CSF was evaluated.96 Although no benefit of vaccination was observed, the study confirmed the safety of the vaccine and its clinical benefit in patients with low Her-2/neu-expressing tumors.96
A vaccine consisting of putative Her-2/neu helper T-cell epitopes (369-384, 688-703, and 971-984) as well as HLA-A2–binding motifs (369-377, 689-697, and 971-979)97 was recently examined in phase I and II clinical trials (NCT00194714) involving patients with stage IV Her-2/neu-positive breast cancer (Table 2). The safety and immunogenicity of combination therapy consisting of concurrent vaccination and treatment with trastuzumab were the primary objectives of the trials, and the therapy was found to be associated with minimal toxicity and resulted in prolonged and robust antigen-specific immune responses in treated patients.98
Completed or current ongoing clinical trials—B-cell peptide vaccines
HER-Vaxx
The vaccine was recently evaluated in a multicenter phase Ib clinical trial (NCT02795988) involving 14 patients with advanced and metastatic Her-2/neu-overexpressing gastroesophageal adenocarcinomas in three dose-escalated cohorts of 10, 30, and 50 μg, who were also treated with chemotherapy77 (Table 2). Considering the immunosuppressive effect of cytotoxic chemotherapies on lymphocytes,99 particularly B cells,100 the first vaccination was initiated before the onset of chemotherapy.77 This scheduling was shown to effectively prime the immune response and patients develop good immune responses and thus the vaccine is immunogenic enough for concomitant treatments with chemotherapy.77 The results also indicated that all the patients who had received the highest dose of the vaccine mounted substantial Her-2/neu-specific IgG. In addition to the observed tolerability and safety of the vaccine at the highest dose, the progression-free survival (PFS) in this cohort was prolonged to 450 days (Figure 3) and was shown to be correlated with vaccine-specific humoral and cellular responses.77 Based on clinical laboratory findings along with vital signs, electrocardiograms, and physical examinations no dose-limiting toxicities were observed, indicating the vaccine’s safety and good tolerability.77 This is in particular a positive important observation, as the association of treatments with trastuzumab and cardiotoxicity poses challenges for both clinicians and patients.101 Based on the success of this evaluation, the highest dose (50 μg) was chosen for further evaluation of the vaccine in an ongoing phase II clinical trial.
Figure 3.
Progression-free survival (in days, swimmer plot per treatment group).
Progression-free survival in days is shown for each patient enrolled in the trial (NCT02795988), following the administration of HER-Vaxx at the indicated doses. With permission from the publishing journal Clinical Cancer Research.77
This phase II trial has included patients with Her-2/neu-overexpressing metastatic or advanced adenocarcinoma of the stomach or gastroesophageal junction, randomized to those receiving HER-Vaxx plus standard chemotherapy (arm 1) or the standard chemotherapy alone (arm 2).78 The interim analysis results have shown that the OS and the PFS rates are higher in patients who received HER-Vaxx plus chemotherapy, with hazard ratios of 0.418 (OS) and 0.532 (PFS), corresponding also with the overall response rates in patients who received the vaccine. Furthermore, patients who received the vaccine plus chemotherapy had more reduction in tumor size compared to the patients who were treated only with chemotherapy, correlating with the observed OS results.78
Overall, the results of these trials have shown that HER-Vaxx is safe, as reflected by intensive preclinical toxicity studies in different species which showed no signs of toxicity and inflammation. Moreover, the vaccine is immunogenic, clinically efficacious, and leads to increased OS and PFS rates among vaccinated patients.
B-Vaxx
In a first-in-human phase I clinical trial (NCT01376505; Table 2) involving patients with metastatic solid tumors, the safety and optimal immunologic/biological dose of the vaccine were evaluated (Table 2). The results of the study indicated that the vaccine is well tolerated and able to generate a sustained anti-Her-2/neu immune response. Additionally, the patients’ antibodies, generated in response to the vaccine, were shown to possess anti-tumor effects and trigger defense mechanisms in vitro (i.e. induction of ADCC and apoptosis, inhibition of proliferation, and phosphorylation).102
Conclusions
In the past decades, the dramatically evolving concept of cancer immunotherapy has been a milestone in the treatment of several types of advanced cancers, including the application of mAbs targeting Her-2/neu-expressing tumors in various cancer entities. However, to overcome the disadvantages associated with such treatments, peptide-based vaccines are progressively becoming promising alternatives, considering the comprehensive involvement of the patient’s immune system and also the easier and cheaper means for their production. Supported by the successful approach of active immunization with HER-Vaxx, a new formulation of the vaccine, broadened to a B-cell-based multi-peptide vaccine including the mimotope of pertuzumab, is aimed for prevention of metastasis and tumor recurrence (i.e. adjuvant setting) (Manuscript in preparation). The breast tumor microenvironment (TME) has been shown to be an important and a long understudied barrier to the efficacy of therapeutic vaccines.103 Therefore, combination strategies are being evaluated to add additional strategies for modulating the TME and killing the tumor cells, for example, by combining a tumor-specific cancer vaccine with immune checkpoint inhibitors.76,104, 105, 106, 107 Our group has taken this concept of combination therapy one step forward and has recently identified the mimotope of the immune checkpoint PD-1 and has shown the enhanced anti-tumor effect of HER-Vaxx in combination with this mimotope76,107; along this line, the combination of a PD-1 peptide (PD1-Vaxx) and B-Vaxx is planned to be evaluated in a clinical trial.108 Preclinical studies by our group are also ongoing to evaluate the effect of vaccination in the prevention of metastasis development. Based on the results of the clinical trials reviewed here, new therapies against Her-2/neu-expressing cancers are on the horizon. Such immune system-based therapies may potentially pave the way for treatments overcoming the disadvantages of mAbs administration, which could be used as monotherapy, or in combination with the latter. Furthermore, these vaccination strategies will always be carried out in combination with other treatments such as chemotherapy or even with checkpoint inhibitors as described elsewhere,76,107 in regimens adapted to the type, stage, and progression phase of the tumor.
Acknowledgements
The authors cordially acknowledge Prof. C. Zielinski who initiated the idea which led to the construction of our Her-2/neu B-cell peptide vaccine (patent US20090269364A1). The authors also cordially thank Prof. Michael Kundi for carrying out the statistical analyses related to the original data presented here. The authors also thank Ms Katarina Ambroz for her valuable assistance in the development of the syngeneic solid tumor mouse model.
Funding
The preclinical studies presented here were supported by a research grant from Imugene Ltd (until 31 October 2020) to the Medical University of Vienna (no grant number), and the phase Ib and II trials were supported by Imugene Ltd (no grant number).
Disclosure
UW was CSO of Imugene until September 2018, and has received funding to the Institute from GSK, Pfizer, and Themis. All other authors have declared no conflicts of interest.
Contributor Information
J. Tobias, Email: joshua.tobias@meduniwien.ac.at.
U. Wiedermann, Email: ursula.wiedermann@meduniwien.ac.at.
Supplementary data
References
- 1.Roskoski R., Jr. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319:1–11. doi: 10.1016/j.bbrc.2004.04.150. [DOI] [PubMed] [Google Scholar]
- 2.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.King C.R., Kraus M.H., Aaronson S.A. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229:974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 4.Ross J.S., Slodkowska E.A., Symmans W.F., Pusztai L., Ravdin P.M., Hortobagyi G.N. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal N., Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. doi: 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh D.Y., Bang Y.J. HER2-targeted therapies – a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33–48. doi: 10.1038/s41571-019-0268-3. [DOI] [PubMed] [Google Scholar]
- 7.Fisk B., Blevins T.L., Wharton J.T., Ioannides C.G. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;181:2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arienti C., Pignatta S., Tesei A. Epidermal growth factor receptor family and its role in gastric cancer. Front Oncol. 2019;9:1308. doi: 10.3389/fonc.2019.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hynes N.E., Stern D.F. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 10.Slamon D.J., Godolphin W., Jones L.A., et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 11.Yu D., Hung M.C. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6115–6121. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 12.Cui N., Shi J., Yang C. HER2-based immunotherapy for breast cancer. Cancer Biother Radiopharm. 2018;33:169–175. doi: 10.1089/cbr.2017.2327. [DOI] [PubMed] [Google Scholar]
- 13.Tai W., Mahato R., Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146:264–275. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witsch E.J., Mahlknecht G., Wakim J., et al. Generation and characterization of peptide mimotopes specific for anti ErbB-2 monoclonal antibodies. Int Immunol. 2011;23:391–403. doi: 10.1093/intimm/dxr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneble E., Jinga D.C., Peoples G. Breast cancer immunotherapy. Maedica (Buchar) 2015;10:185–191. [PMC free article] [PubMed] [Google Scholar]
- 16.Lesterhuis W.J., Haanen J.B., Punt C.J. Cancer immunotherapy – revisited. Nat Rev Drug Discov. 2011;10:591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- 17.Costa R.L.B., Czerniecki B.J. Clinical development of immunotherapies for HER2(+) breast cancer: a review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer. 2020;6:10. doi: 10.1038/s41523-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strebhardt K., Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 19.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiedermann U., Davis A.B., Zielinski C.C. Vaccination for the prevention and treatment of breast cancer with special focus on Her-2/neu peptide vaccines. Breast Cancer Res Treat. 2013;138:1–12. doi: 10.1007/s10549-013-2410-8. [DOI] [PubMed] [Google Scholar]
- 21.Citri A., Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 22.Moasser M.M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarden Y., Sliwkowski M.X. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 24.Hsu J.L., Hung M.C. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35:575–588. doi: 10.1007/s10555-016-9649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Exman P., Tolaney S.M. HER2-positive metastatic breast cancer: a comprehensive review. Clin Adv Hematol Oncol. 2021;19:40–50. [PubMed] [Google Scholar]
- 26.Pallerla S., Abdul A., Comeau J., Jois S. Cancer vaccines, treatment of the future: with emphasis on HER2-positive breast cancer. Int J Mol Sci. 2021;22:779. doi: 10.3390/ijms22020779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct Target Ther. 2019;4:34. doi: 10.1038/s41392-019-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klapper L.N., Vaisman N., Hurwitz E., Pinkas-Kramarski R., Yarden Y., Sela M. A subclass of tumor-inhibitory monoclonal antibodies to ErbB-2/HER2 blocks crosstalk with growth factor receptors. Oncogene. 1997;14:2099–2109. doi: 10.1038/sj.onc.1201029. [DOI] [PubMed] [Google Scholar]
- 29.Klapper L.N., Waterman H., Sela M., Yarden Y. Tumor-inhibitory antibodies to HER-2/ErbB-2 may act by recruiting c-Cbl and enhancing ubiquitination of HER-2. Cancer Res. 2000;60:3384–3388. [PubMed] [Google Scholar]
- 30.Slamon D.J., Leyland-Jones B., Shak S., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 31.Piccart-Gebhart M.J., Procter M., Leyland-Jones B., et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 32.Smith M.B., Reardon J., Olson E.M. Pertuzumab for the treatment of patients with previously untreated HER2-positive metastatic breast cancer. Drugs Today (Barc) 2012;48:713–722. doi: 10.1358/dot.2012.48.11.1885879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai K., Yokote H., Murakami-Murofushi K., Tamura T., Saijo N., Nishio K. Pertuzumab, a novel HER dimerization inhibitor, inhibits the growth of human lung cancer cells mediated by the HER3 signaling pathway. Cancer Sci. 2007;98:1498–1503. doi: 10.1111/j.1349-7006.2007.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahta R., Hung M.C., Esteva F.J. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 35.Scheuer W., Friess T., Burtscher H., Bossenmaier B., Endl J., Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 36.Baselga J., Cortes J., Kim S.B., et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scolnik P.A. mAbs: a business perspective. MAbs. 2009;1:179–184. doi: 10.4161/mabs.1.2.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chames P., Van Regenmortel M., Weiss E., Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins F., Sofiya L., Sykiotis G.P., et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 40.Fares C.M., Van Allen E.M., Drake C.G., Allison J.P., Hu-Lieskovan S. Mechanisms of Resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book. 2019;39:147–164. doi: 10.1200/EDBK_240837. [DOI] [PubMed] [Google Scholar]
- 41.Verma V., Sprave T., Haque W., et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer. 2018;6:128. doi: 10.1186/s40425-018-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Holstein Y., Kapiteijn E., Bastiaannet E., van den Bos F., Portielje J., de Glas N.A. Efficacy and adverse events of immunotherapy with checkpoint inhibitors in older patients with cancer. Drugs Aging. 2019;36:927–938. doi: 10.1007/s40266-019-00697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishii K., Morii N., Yamashiro H. Pertuzumab in the treatment of HER2-positive breast cancer: an evidence-based review of its safety, efficacy, and place in therapy. Core Evid. 2019;14:51–70. doi: 10.2147/CE.S217848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bettaieb A., Paul C., Plenchette S., Shan J., Chouchane L., Ghiringhelli F. Precision medicine in breast cancer: reality or utopia? J Transl Med. 2017;15:139. doi: 10.1186/s12967-017-1239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torka P., Barth M., Ferdman R., Hernandez-Ilizaliturri F.J. Mechanisms of resistance to monoclonal antibodies (mAbs) in lymphoid malignancies. Curr Hematol Malig Rep. 2019;14:426–438. doi: 10.1007/s11899-019-00542-8. [DOI] [PubMed] [Google Scholar]
- 46.Isabwe G.A.C., Garcia Neuer M., de Las Vecillas Sanchez L., Lynch D.M., Marquis K., Castells M. Hypersensitivity reactions to therapeutic monoclonal antibodies: phenotypes and endotypes. J Allergy Clin Immunol. 2018;142:159–170.e2. doi: 10.1016/j.jaci.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Pintea I., Petricau C., Dumitrascu D., et al. Hypersensitivity reactions to monoclonal antibodies: classification and treatment approach (Review) Exp Ther Med. 2021;22:949. doi: 10.3892/etm.2021.10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendrikx J., Haanen J., Voest E.E., Schellens J.H.M., Huitema A.D.R., Beijnen J.H. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22:1212–1221. doi: 10.1634/theoncologist.2017-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price L., Brunt A.M. Trastuzumab infusion reactions in breast cancer. Should we routinely observe after the first dose? Eur J Hosp Pharm. 2018;25:331–333. doi: 10.1136/ejhpharm-2016-001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z.I., Ai D.I. Cardiotoxicity associated with targeted cancer therapies. Mol Clin Oncol. 2016;4:675–681. doi: 10.3892/mco.2016.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lollini P.L., Cavallo F., Nanni P., Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6:204–216. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- 52.Datta J., Terhune J.H., Lowenfeld L., et al. Optimizing dendritic cell-based approaches for cancer immunotherapy. Yale J Biol Med. 2014;87:491–518. [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta-Damani A., Markowicz S., Engleman E.G. Generation of antigen-specific CD8+ CTLs from naive precursors. J Immunol. 1994;153:996–1003. [PubMed] [Google Scholar]
- 54.Mehta-Damani A., Markowicz S., Engleman E.G. Generation of antigen-specific CD4+ T cell lines from naive precursors. Eur J Immunol. 1995;25:1206–1211. doi: 10.1002/eji.1830250511. [DOI] [PubMed] [Google Scholar]
- 55.Norell H., Poschke I., Charo J., et al. Vaccination with a plasmid DNA encoding HER-2/neu together with low doses of GM-CSF and IL-2 in patients with metastatic breast carcinoma: a pilot clinical trial. J Transl Med. 2010;8:53. doi: 10.1186/1479-5876-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quaglino E., Riccardo F., Macagno M., et al. Chimeric DNA vaccines against ErbB2+ carcinomas: from mice to humans. Cancers (Basel) 2011;3:3225–3241. doi: 10.3390/cancers3033225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riccardo F., Bolli E., Macagno M., Arigoni M., Cavallo F., Quaglino E. Chimeric DNA vaccines: an effective way to overcome immune tolerance. Curr Top Microbiol Immunol. 2017;405:99–122. doi: 10.1007/82_2014_426. [DOI] [PubMed] [Google Scholar]
- 58.Sharpe A.H. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mittendorf E.A., Holmes J.P., Ponniah S., Peoples G.E. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother. 2008;57:1511–1521. doi: 10.1007/s00262-008-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patil R., Clifton G.T., Holmes J.P., et al. Clinical and immunologic responses of HLA-A3+ breast cancer patients vaccinated with the HER2/neu-derived peptide vaccine, E75, in a phase I/II clinical trial. J Am Coll Surg. 2010;210:140–147. doi: 10.1016/j.jamcollsurg.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Ayoub N.M., Al-Shami K.M., Yaghan R.J. Immunotherapy for HER2-positive breast cancer: recent advances and combination therapeutic approaches. Breast Cancer (Dove Med Press) 2019;11:53–69. doi: 10.2147/BCTT.S175360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clive K.S., Tyler J.A., Clifton G.T., et al. The GP2 peptide: a HER2/neu-based breast cancer vaccine. J Surg Oncol. 2012;105:452–458. doi: 10.1002/jso.21723. [DOI] [PubMed] [Google Scholar]
- 63.Sears A.K., Perez S.A., Clifton G.T., et al. AE37: a novel T-cell-eliciting vaccine for breast cancer. Expert Opin Biol Ther. 2011;11:1543–1550. doi: 10.1517/14712598.2011.616889. [DOI] [PubMed] [Google Scholar]
- 64.Gillogly M.E., Kallinteris N.L., Xu M., Gulfo J.V., Humphreys R.E., Murray J.L. Ii-Key/HER-2/neu MHC class-II antigenic epitope vaccine peptide for breast cancer. Cancer Immunol Immunother. 2004;53:490–496. doi: 10.1007/s00262-003-0463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu M., Kallinteris N.L., von Hofe E. CD4+ T-cell activation for immunotherapy of malignancies using Ii-Key/MHC class II epitope hybrid vaccines. Vaccine. 2012;30:2805–2810. doi: 10.1016/j.vaccine.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 66.Ladjemi M.Z., Jacot W., Chardes T., Pelegrin A., Navarro-Teulon I. Anti-HER2 vaccines: new prospects for breast cancer therapy. Cancer Immunol Immunother. 2010;59:1295–1312. doi: 10.1007/s00262-010-0869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu R.M., Hwang Y.C., Liu I.J., et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27:1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jasinska J., Wagner S., Radauer C., et al. Inhibition of tumor cell growth by antibodies induced after vaccination with peptides derived from the extracellular domain of Her-2/neu. Int J Cancer. 2003;107:976–983. doi: 10.1002/ijc.11485. [DOI] [PubMed] [Google Scholar]
- 69.Schwarzkopf C., Thiele B. Effectivity of alternative adjuvants in comparison to Freund's complete adjuvant. ALTEX. 1996;13:22–25. [PubMed] [Google Scholar]
- 70.Wagner S., Jasinska J., Breiteneder H., et al. Delayed tumor onset and reduced tumor growth progression after immunization with a Her-2/neu multi-peptide vaccine and IL-12 in c-neu transgenic mice. Breast Cancer Res Treat. 2007;106:29–38. doi: 10.1007/s10549-006-9469-4. [DOI] [PubMed] [Google Scholar]
- 71.Moser C., Muller M., Kaeser M.D., Weydemann U., Amacker M. Influenza virosomes as vaccine adjuvant and carrier system. Expert Rev Vaccines. 2013;12:779–791. doi: 10.1586/14760584.2013.811195. [DOI] [PubMed] [Google Scholar]
- 72.Wiedermann U., Wiltschke C., Jasinska J., et al. A virosomal formulated Her-2/neu multi-peptide vaccine induces Her-2/neu-specific immune responses in patients with metastatic breast cancer: a phase I study. Breast Cancer Res Treat. 2010;119:673–683. doi: 10.1007/s10549-009-0666-9. [DOI] [PubMed] [Google Scholar]
- 73.Tobias J., Jasinska J., Baier K., et al. Enhanced and long term immunogenicity of a Her-2/neu multi-epitope vaccine conjugated to the carrier CRM197 in conjunction with the adjuvant Montanide. BMC Cancer. 2017;17:118. doi: 10.1186/s12885-017-3098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malito E., Bursulaya B., Chen C., et al. Structural basis for lack of toxicity of the diphtheria toxin mutant CRM197. Proc Natl Acad Sci U S A. 2012;109:5229–5234. doi: 10.1073/pnas.1201964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamboj K.K., King C.L., Greenspan N.S., Kirchner H.L., Schreiber J.R. Immunization with Haemophilus influenzae type b-CRM(197) conjugate vaccine elicits a mixed Th1 and Th2 CD(4+) T cell cytokine response that correlates with the isotype of antipolysaccharide antibody. J Infect Dis. 2001;184:931–935. doi: 10.1086/323342. [DOI] [PubMed] [Google Scholar]
- 76.Tobias J., Battin C., De Sousa Linhares A., et al. A New strategy toward B cell-based cancer vaccines by active immunization with mimotopes of immune checkpoint inhibitors. Front Immunol. 2020;11:895. doi: 10.3389/fimmu.2020.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiedermann U., Garner-Spitzer E., Chao Y., et al. Clinical and immunologic responses to a B-cell epitope vaccine in patients with HER2/neu-overexpressing advanced gastric cancer-results from phase Ib trial IMU.ACS.001. Clin Cancer Res. 2021;27:3649–3660. doi: 10.1158/1078-0432.CCR-20-3742. [DOI] [PubMed] [Google Scholar]
- 78.Maglakelidze M., Ryspayeva D., Bulat I., et al. A phase 1b/2 open-label study with randomization in phase 2 of Imu-131 Her2/Neu peptide vaccine plus standard of care chemotherapy in patients with Her2/Neu overexpressing metastatic or advanced adenocarcinoma of the stomach or gastroesophageal junction. Cancer Res. 2021;81 [Google Scholar]
- 79.Lewis G.D., Figari I., Fendly B., et al. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol Immunother. 1993;37:255–263. doi: 10.1007/BF01518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato Y., Yashiro M., Takakura N. Heregulin induces resistance to lapatinib-mediated growth inhibition of HER2-amplified cancer cells. Cancer Sci. 2013;104:1618–1625. doi: 10.1111/cas.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Majumder A., Sandhu M., Banerji D., Steri V., Olshen A., Moasser M.M. The role of HER2 and HER3 in HER2-amplified cancers beyond breast cancers. Sci Rep. 2021;11:9091. doi: 10.1038/s41598-021-88683-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allen S.D., Garrett J.T., Rawale S.V., et al. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J Immunol. 2007;179:472–482. doi: 10.4049/jimmunol.179.1.472. [DOI] [PubMed] [Google Scholar]
- 83.Garrett J.T., Rawale S., Allen S.D., et al. Novel engineered trastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER-2/neu. J Immunol. 2007;178:7120–7131. doi: 10.4049/jimmunol.178.11.7120. [DOI] [PubMed] [Google Scholar]
- 84.Kaumaya P.T. B-cell epitope peptide cancer vaccines: a new paradigm for combination immunotherapies with novel checkpoint peptide vaccine. Future Oncol. 2020;16:1767–1791. doi: 10.2217/fon-2020-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wallis J., Katti P., Martin A.M., et al. A liposome-based cancer vaccine for a rapid and high-titre anti-ErbB-2 antibody response. Eur J Pharm Sci. 2020;152:105456. doi: 10.1016/j.ejps.2020.105456. [DOI] [PubMed] [Google Scholar]
- 86.Okarvi S.M., AlJammaz I. Development of the tumor-specific antigen-derived synthetic peptides as potential candidates for targeting breast and other possible human carcinomas. Molecules. 2019;24:3142. doi: 10.3390/molecules24173142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brossart P., Wirths S., Stuhler G., Reichardt V.L., Kanz L., Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 88.Mittendorf E.A., Clifton G.T., Holmes J.P., et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014;25:1735–1742. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clifton G.T., Hale D., Vreeland T.J., et al. Results of a randomized phase IIb trial of nelipepimut-S + trastuzumab versus trastuzumab to prevent recurrences in patients with high-risk HER2 low-expressing breast cancer. Clin Cancer Res. 2020;26:2515–2523. doi: 10.1158/1078-0432.CCR-19-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mittendorf E.A., Lu B., Melisko M., et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin Cancer Res. 2019;25:4248–4254. doi: 10.1158/1078-0432.CCR-18-2867. [DOI] [PubMed] [Google Scholar]
- 91.Dillon P.M., Brenin C.M., Slingluff C.L., Jr. Evaluating nelipepimut-S in the treatment of breast cancer: a short report on the emerging data. Breast Cancer (Dove Med Press) 2020;12:69–75. doi: 10.2147/BCTT.S224758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carmichael M.G., Benavides L.C., Holmes J.P., et al. Results of the first phase 1 clinical trial of the HER-2/neu peptide (GP2) vaccine in disease-free breast cancer patients: United States Military Cancer Institute Clinical Trials Group Study I-04. Cancer. 2010;116:292–301. doi: 10.1002/cncr.24756. [DOI] [PubMed] [Google Scholar]
- 93.Mittendorf E.A., Ardavanis A., Litton J.K., et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget. 2016;7:66192–66201. doi: 10.18632/oncotarget.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clifton G.T., Litton J.K., Arrington K., et al. Results of a phase Ib trial of combination immunotherapy with a CD8+ T cell eliciting vaccine and trastuzumab in breast cancer patients. Ann Surg Oncol. 2017;24:2161–2167. doi: 10.1245/s10434-017-5844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holmes J.P., Benavides L.C., Gates J.D., et al. Results of the first phase I clinical trial of the novel II-key hybrid preventive HER-2/neu peptide (AE37) vaccine. J Clin Oncol. 2008;26:3426–3433. doi: 10.1200/JCO.2007.15.7842. [DOI] [PubMed] [Google Scholar]
- 96.Mittendorf E.A., Ardavanis A., Symanowski J., et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol. 2016;27:1241–1248. doi: 10.1093/annonc/mdw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knutson K.L., Schiffman K., Disis M.L. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Disis M.L., Wallace D.R., Gooley T.A., et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park Y.H., Lal S., Lee J.E., et al. Chemotherapy induces dynamic immune responses in breast cancers that impact treatment outcome. Nat Commun. 2020;11:6175. doi: 10.1038/s41467-020-19933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waidhauser J., Schuh A., Trepel M., Schmalter A.K., Rank A. Chemotherapy markedly reduces B cells but not T cells and NK cells in patients with cancer. Cancer Immunol Immunother. 2020;69:147–157. doi: 10.1007/s00262-019-02449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohan N., Jiang J., Dokmanovic M., Wu W.J. Trastuzumab-mediated cardiotoxicity: current understanding, challenges, and frontiers. Antib Ther. 2018;1:13–17. doi: 10.1093/abt/tby003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bekaii-Saab T., Wesolowski R., Ahn D.H., et al. Phase I immunotherapy trial with two chimeric HER-2 B-cell peptide vaccines emulsified in montanide ISA 720VG and nor-MDP adjuvant in patients with advanced solid tumors. Clin Cancer Res. 2019;25:3495–3507. doi: 10.1158/1078-0432.CCR-18-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gordon B., Gadi V.K. The role of the tumor microenvironment in developing successful therapeutic and secondary prophylactic breast cancer vaccines. Vaccines (Basel) 2020;8:529. doi: 10.3390/vaccines8030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y., Miao W., He D., et al. Recent progress on immunotherapy for breast cancer: tumor microenvironment, nanotechnology and more. Front Bioeng Biotechnol. 2021;9:680315. doi: 10.3389/fbioe.2021.680315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crosby E.J., Lyerly H.K., Hartman Z.C. Cancer vaccines: the importance of targeting oncogenic drivers and the utility of combinations with immune checkpoint inhibitors. Oncotarget. 2021;12:1–3. doi: 10.18632/oncotarget.27861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mougel A., Terme M., Tanchot C. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front Immunol. 2019;10:467. doi: 10.3389/fimmu.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tobias J., Steinberger P., Drinic M., Wiedermann U. Emerging targets for anti-cancer vaccination: PD-1. ESMO Open. 2021;6:100278. doi: 10.1016/j.esmoop.2021.100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo L., Kaumaya P.T.P. First prototype checkpoint inhibitor B-cell epitope vaccine (PD1-Vaxx) en route to human Phase 1 clinical trial in Australia and USA: exploiting future novel synergistic vaccine combinations. Br J Cancer. 2021;6:100278. doi: 10.1038/s41416-021-01342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.