Summary
Background
Little is known about the association between sarcopenia and cardiovascular disease (CVD) among middle-aged and older adults. Using the nationally representative data from the China Health and Retirement Longitudinal Study (CHARLS), we conducted cross-sectional and longitudinal analyses to investigate the association between sarcopenia status and CVD in middle-aged and older Chinese population.
Methods
The sample comprised 15,137 participants aged at least 45 years from the CHARLS 2015. Sarcopenia status was defined according to the Asian Working Group for Sarcopenia 2019 (AWGS 2019) criteria. CVD was defined as the presence of physician-diagnosed heart disease and/or stroke. A total of 11,863 participants without CVD were recruited from the CHARLS 2015 and were followed up in 2018. Cox proportional hazards regression models were conducted to examine the effect of sarcopenia on CVD.
Findings
The pre valence of CVD in total populations, no-sarcopenia, possible sarcopenia and sarcopenia individuals were 12.6% (1905/15,137), 10.0% (1026/10,280), 18.1% (668/3685), 18.0% (211/1172), respectively. Both possible sarcopenia [OR (95% CI): 1.29 (1.13–1.48)] and sarcopenia [1.72 (1.40–2.10)] were associated with CVD in total populations. During the 3.6 years of follow-up, 1,273 cases (10.7%) with incident CVD were identified. In the longitudinal analysis, individuals with the diagnosis of possible sarcopenia (HR:1.22, 95% CI: 1.05–1.43) and sarcopenia participants (HR:1.33, 95% CI: 1.04–1.71) were more likely to have new onset CVD than no-sarcopenia peers.
Interpretation
Both possible sarcopenia and sarcopenia, assessed using the AWGS 2019 criteria, were associated with higher CVD risk among middle-aged and older Chinese adults.
Funding
None.
Keywords: Sarcopenia, Possible sarcopenia, Cardiovascular diseases, Low muscle mass
Research in context.
Evidence before this study
We searched PubMed, Google Scholar, and China National Knowledge Infrastructure for studies published in English and Chinese from Jan 1, 1998, to May 1, 2021. We used the search terms “Sarcopenia”, and “cardiovascular disease (CVD)”, or “heart disease”, or “cardiac disease”, or “stroke”, and found that several recent studies have examined the cross-sectional relationship between sarcopenia and CVD, including coronary heart disease, atrial fibrillation, heart failure and stroke. However, there have been no large population-based studies exploring the longitudinal association between sarcopenia and CVD in middle-aged and older adults.
Added value of this study
We first found that both possible sarcopenia and sarcopenia, assessed using the Asian Working Group for Sarcopenia (AWGS) 2019 criteria, were associated with higher CVD risk among middle-aged and older Chinese adults. Our findings provided the new evidence supporting the longitudinal connection between sarcopenia and CVD, and suggested that preventing and/or improving both possible sarcopenia and sarcopenia may be beneficial for reducing CVD incidence and promoting healthy aging for middle-aged and older adults.
Implications of all the available evidence
This study contributes to extending our previous knowledge of the cardiometabolic importance of possible sarcopenia and sarcopenia. These findings may indicate that the assessment of both possible sarcopenia and sarcopenia in community-based health check‐ups and routine clinical practice might facilitate identification of those at greatest risk of incident CVD, who would benefit most from early intervention.
Alt-text: Unlabelled box
Introduction
Sarcopenia, a serious geriatric syndrome with loss of skeletal muscle mass, plus low muscle strength, and/or low physical performance, is a public health concern facing aging societies.1,2 Mounting evidence shows that sarcopenia is strongly associated with multiple adverse outcomes, such as falls, frailty, mortality and increasing healthcare utilization.1,3, 4, 5–6 Over the past decade, several consensus panels have created different algorithms to identify sarcopenia based on the combination of loss of muscle function and mass.7–8, 9 Subsequently, the research interest in sarcopenia has grown rapidly worldwide, Asia being no exception. Several studies have reported that the prevalence of sarcopenia among the elderly ranges from 6.8% to 25.7% in Asia.5,7,9, 10, 11–12 However, the fields of sarcopenia and routine clinical practice are still largely separated, and most clinicians remain unaware of the condition and the diagnostic methods needed to identify it.1,13
Advancing age is the strongest risk factor related to the development of cardiovascular disease (CVD), a major cause of mortality in older patients.14, 15–16 Globally, deaths from CVD increased by 14.5% between 2006 and 2016, though the age-standardized mortality rate from CVD decreased by 14.5% over this same time period. Ischaemic heart disease and stroke combined accounted for more than 85.1% of all CVD mortality in 2016.14 Recently, a growing body of research has examined the relationship between sarcopenia and CVD, such as coronary heart disease,17,18 atrial fibrillation,17 heart failure19,20 and stroke.21 One study22 utilized the representative Korean population data from the Korea National Health and Nutrition Examination Survey 2009, and reported that sarcopenia was associated with CVD independent of other well-documented risk factors among 1578 subjects aged 65 years and older. Fukuda et al23 found that sarcopenic obesity was significantly associated with incident CVD, including unstable angina, myocardial infarction and stroke, among 716 patients with type 2 diabetes. However, most studies were cross-sectional, with small sample sizes, or using different definitions of sarcopenia. Moreover, almost all studies have focused on the association between sarcopenia and various adverse cardiovascular events of patients with heart disease.18,24, 25–26 To date, there have been no large population-based studies exploring the longitudinal relationship between sarcopenia and CVD in middle-aged and older adults.
The definition and diagnosis of sarcopenia are still evolving as new findings challenge current understanding. In 2019, the Asian Working Group for Sarcopenia (AWGS) redefined the sarcopenia including possible sarcopenia, sarcopenia and severe sarcopenia.7 The AWGS 2019 retains the previous definition of sarcopenia in AWGS 2014,9 but revises the diagnostic algorithm and cutoff values for each component. The AWGS 2019 defines individuals with low muscle mass, low muscle strength, and low physical performance as having “severe sarcopenia”. To promote healthy aging, AWGS 2019 has defined a new entity of “possible sarcopenia” to facilitate timely lifestyle intervention in community health care and prevention settings, which will contribute to higher awareness of sarcopenia prevention and interventions in diverse health care settings.7 It was the first time to recommend the concept of possible sarcopenia: the presence of low muscle strength with or without reduced physical performance, as a basis on which to begin intervention in primary health care and preventive services. Hence, the results from previous studies using the former sarcopenia criteria may be no longer appropriate in Asia. To our knowledge, no studies have evaluated the relationship between possible sarcopenia and incident CVD. In addition, the impacts of muscle mass on CVD remains to be further investigated.
For now, the contribution of individual sarcopenia status to incident CVD is still unknown. In this study, using the nationally representative data from the China Health and Retirement Longitudinal Study (CHARLS), we conducted cross-sectional and longitudinal analyses to investigate the relation between sarcopenia status and CVD in middle-aged and older Chinese population.
Methods
Study population
The CHARLS, established in 2011, is an ongoing nationally representative longitudinal survey in China. In short, CHARLS collects high-quality data through one-to-one interviews with a structured questionnaire, from a nationally representative sample of Chinese population aged at least 45 years older, selected using multistage stratified probability-proportionate-to-size sampling. All participants underwent an assessment using a standardized questionnaire to collect data on sociodemographic and lifestyle factors and health-related information. In CHARLS 2011, a total of 17,708 participants in 10,257 households were recruited from 150 counties or districts and 450 villages within 28 provinces in China. All participants were followed up every 2 years after the baseline survey. The data included individual weighting variables to ensure that the survey sample was nationally representative. Detailed information about the study design of the CHARLS have been previously reported.27
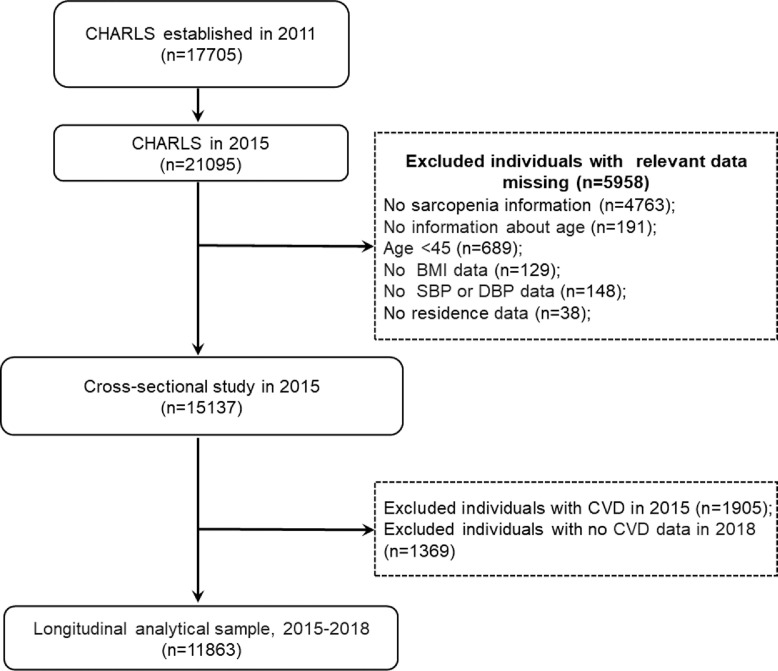
In the present study, we retrospectively analyzed data from the CHARLS 2015 and 2018. The inclusion criteria for the present study were: 1) individuals aged at least 45 years old in CHARLS 2015; 2) and having data regarding sarcopenia status. Exclusion criteria were: 1) missing data of sarcopenia status in CHARLS 2015; 2) no information about age; 3) persons aged less than 45 years old; 4) lack of residence, body mass index, and blood pressure data. This study was divided into two sections: (1) In the cross-sectional analysis, we used data from the large cohort that was followed up in CHARLS 2015. A total of 21,095 participants were interviewed in CHARLS 2015, 5958 individuals were excluded because of missing data on sarcopenia (n = 4763), no information about age (n = 191), aged less than 45 years (n = 689), lack of body mass index and blood pressure data (n = 277), and lack of residence data (n = 38), leaving 15,137 participants for cross-sectional analysis. (2) In the longitudinal analysis, we further excluded 1905 subjects with CVD in CHARLS 2015, and 1369 participants without CVD data in CHARLS 2018. Our final analytic sample included 11,863 persons, who had no CVD in CHARLS 2015 and were fully followed up in 2018. The detailed selection process is shown in Figure 1.
Figure 1.
Flow diagram for participants included in the study.
Abbreviation: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CVD, cardiovascular disease.
All participants provided informed consent, and the protocol was approved by the Ethical Review Committee of Peking University (approval number: IRB00001052–11,015). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.28
Assessment of sarcopenia status
Sarcopenia status was assessed according to the AWGS 2019 algorithm, which is consisted of three components: muscle strength, appendicular skeletal muscle mass (ASM), and physical performance.7
Sarcopenia is diagnosed when low muscle mass plus low muscle strength or low physical performance are detected. Handgrip strength (kg) was measured in the dominant hand and non-dominant hand, with the participant squeezing a YuejianTM WL-1000 dynamometer (Nantong Yuejian Physical Measure-ment Instrument Co., Ltd., Nantong, China) as hard as possible.27 The cut-off points for low grip strength for men and women were <28 and <18 kg, respectively. The ASM was estimated by a validated anthropometric equation in Chinese residents.29,30 Several studies have shown that the agreement of the ASM equation model and dual X-ray absorptiometry (DXA) was strong.29,30 The cut-off for defining low muscle mass was based on the sex-specific lowest 20% of the height-adjusted muscle mass (ASM/Ht2) among the study population.10,30,31 In our study, body weight and height were measured using a stadiometer and a digital floor scale to the nearest 0.1 cm and 0.1 kg, respectively; the body weight and height were measured in kilograms, and centimetres, respectively.10,30 Finally, the ASM/Ht2 values of <5.63 kg/m2 in women and <7.05 kg/m2 in men were considered as low muscle mass. In terms of physical performance, the gait speed and the chair stand test were performed using the method described by Wu et al.10 Further details about the definitions for sarcopenia components in the CHARLS have been described previously.10
In our study population, only 319 (2.1%) participants had severe sarcopenia. Therefore, we merged subjects with severe sarcopenia into the sarcopenia group and divided all participants into three groups: no-sarcopenia (n = 10,280), possible sarcopenia (n = 3685), and sarcopenia (n = 1172).
Assessment of CVD events
The study outcome was CVD events, including heart disease and stroke. Similar to previous studies,32,33 CVD events were assessed by the following questions: “Have you been told by a doctor that you have been diagnosed with a heart attack, angina, coronary heart disease, heart failure, or other heart problems?” or “Have you been told by a doctor that you have been diagnosed with a stroke?”. Participants who reported heart disease or stroke were defined as having CVD.32
Covariates
At baseline, trained interviewers collected information on socio-demographic status and health-related factors using a structured questionnaire. Sociodemographic variables included age, sex, education (elementary school and below, secondary school, and college and above), marital status (married and others), and residence (rural, urban). Health-related factors included body mass index (BMI), smoking and drinking status (yes or no), hypertension, diabetes, self-reported physician-diagnosed dyslipidemia and kidney disease, use of medications for hypertension, diabetes, and dyslipidemia. Subjects were diagnosed as hypertensive when the systolic blood pressure was ≥140 mmHg and the diastolic pressure was ≥90 mmHg or if antihypertensive medications were currently used. Diabetes was defined based on the use of insulin or oral hypoglycaemic agents, and plasma glucose ≥200 mg/dL. BMI was divided into three groups: underweight (BMI <18.5 kg/m2), normal weight (BMI =18.5 kg/m2 to 24 kg/m2), and overweight or obesity (BMI ≥24 kg/m2).10
In the cross-sectional analysis, a subgroup of 12,318 individuals underwent measurements of metabolic biomarkers, including total cholesterol, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and serum creatinine. The estimated glomerular filtration rate was calculated according to the Chronic Kidney Disease Epidemiology Collaboration's 2009 creatinine equation.34
Statistical analysis
Data are presented as means ± standard deviation (SD) or median and interquartile range for continuous variables and percentages for categorical variables. First, baseline characteristics in the cross-sectional and longitudinal analytical samples were summarized based on sarcopenia status and compared between participants using the chi-squared test, analysis of variance, Tukey's test or the Kruskal-Wallis test, as appropriate. Secondly, logistic regression analysis was used to estimate the associations between possible sarcopenia, sarcopenia, and CVD and its components in the cross-sectional analysis. Thirdly, in the longitudinal analysis, we calculated the incidence rates of CVD per 1000 person-years in CHARLS 2018. We also measured the follow-up time as the time elapsed from the date of the last interview to either the date of diagnosis of CVD or the date of the latest interview (March 2019) in which the individual participated. To estimate the relationship between baseline sarcopenia status and incident CVD, Cox proportional hazards models were used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs). Finally, considering the gender differences in cut-off points for low muscle mass, we also evaluated the association between low muscle mass alone and CVD stratified by sex.
In the cross-sectional and longitudinal analyses, three models were estimated: in model 1, age and sex were adjusted; in model 2, age, sex, marital status, residence, educational level, smoking, drinking and BMI were adjusted; model 3 was adjusted as model 2 with further adjustment for systolic blood pressure, diastolic blood pressure, hypertension, dyslipidemia, diabetes and chronic kidney disease, and use of hypertension medications, diabetes medications, and lipid-lowering therapy. In addition, we further adjusted for metabolic biomarkers in the subgroup of participants who underwent metabolic examinations (12,318 and 9753 participants in the cross-sectional and longitudinal analyses, respectively). All statistical analysis was performed retrospectively with SPSS 25.0 (SPSS, Inc., Chicago, IL), and R version 3.5.1 (R Foundation for Statistical Computing). In all cases, P < 0.05 was considered statistically significant.
Role of the funding sources
There was no funding for this study.
Results
Characteristics of participants in the cross‑sectional and longitudinal study
Table 1 shows the characteristics of the participants according to sarcopenia status. The mean (SD) age of the study population was 60.6 (9.9) years, and 7179 (47.4%) were females. Among these 15,137 middle-aged and older adults, the prevalence of possible sarcopenia and sarcopenia was 24.3% (3685/15,137) and 7.7% (1172/15,137), respectively. Compared with those with no-sarcopenia, persons with sarcopenia were more likely to be older (mean age, 68.5 vs 58.0 years) and not married (23.0% vs 8.8%), be male (56.4% vs 49.6%), live in rural setting (93.7% vs 86.8%), had higher prevalence of hypertension (34.5% vs 29.3%), kidney disease (7.9% vs 5.7%), and medications for hypertension (25.2% vs 20.3%) (all P < 0.01). Table S1 also presents the baseline characteristics of 11,863 participants without CVD (52.3% women; mean age 59.7 ± 9.6) in the longitudinal study stratified by sarcopenia status.
Table 1.
Baseline characteristics of all participants by sarcopenia status.
| Characteristics | No sarcopenia (n = 10,280) | Possible sarcopenia (n = 3685) | Sarcopenia (n = 1172) | P |
|---|---|---|---|---|
| Age, y | 58.0 ± 8.5 | 65.2 ± 10.1 ⁎⁎⁎ | 68.5 ± 10.4 ⁎⁎⁎ | <0.001 |
| Male, n (%) | 5104 (49.6) | 1414 (38.4) ⁎⁎⁎ | 661 (56.4) ⁎⁎⁎ | <0.001 |
| Married (vs others) | 9376 (91.2) | 2872 (77.9) ⁎⁎⁎ | 902 (77.0) ⁎⁎⁎ | <0.001 |
| Rural (vs urban) | 8922 (86.8) | 3271 (88.8) ⁎⁎ | 1098 (93.7) ⁎⁎⁎ | <0.001 |
| Smokinga | 2957 (33.7) | 890 (26.5) ⁎⁎⁎ | 445 (40.6) ⁎⁎⁎ | <0.001 |
| Drinkinga | 2659 (36.3) | 765 (25.9) ⁎⁎⁎ | 312 (32.2) ⁎⁎ | <0.001 |
| Educational levela | <0.001 | |||
| Elementary school or below | 5463 (66.3) | 2616 (81.8) ⁎⁎⁎ | 906 (86.6) ⁎⁎⁎ | |
| Secondary school | 2637 (32.0) | 568 (17.8) ⁎⁎⁎ | 132 (12.6) ⁎⁎⁎ | |
| College and above | 138 (1.7) | 16 (0.5) ⁎⁎⁎ | 8 (0.8) * | |
| Height, cm | 159.3 ± 8.3 | 155.2 ± 9.1 ⁎⁎⁎ | 157.3 ± 7.9 ⁎⁎⁎ | <0.001 |
| Weight, kg | 61.3 ± 11.4 | 58.8 ± 12.8 ⁎⁎⁎ | 51.8 ± 9.5 ⁎⁎⁎ | <0.001 |
| BMI, kg/m2 | 24.1 ± 3.6 | 24.2 ± 3.9 | 20.9 ± 3.7 ⁎⁎⁎ | <0.001 |
| BMI category, n (%) | <0.001 | |||
| Underweight | 427 (4.2) | 187 (5.1) * | 283 (24.1) ⁎⁎⁎ | |
| Normal weight | 4901 (47.7) | 1727 (46.9) | 675 (57.6) ⁎⁎⁎ | |
| Overweight or obesity | 4952 (48.2) | 1771 (48.1) | 214 (18.3) ⁎⁎⁎ | |
| Blood pressure, mm Hg | ||||
| Systolic | 127 ± 19 | 132 ± 22 ⁎⁎⁎ | 129 ± 22 ⁎⁎ | <0.001 |
| Diastolic | 76 ± 12 | 75 ± 12 | 73 ± 12 ⁎⁎⁎ | <0.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 3015 (29.3) | 1576 (42.8) ⁎⁎⁎ | 404 (34.5) ⁎⁎⁎ | <0.001 |
| Dyslipidemia | 1244 (12.1) | 570 (15.5) ⁎⁎⁎ | 113 (9.6) * | <0.001 |
| Diabetes | 788 (7.7) | 422 (11.5) ⁎⁎⁎ | 109 (9.3) | <0.001 |
| Kidney disease | 588 (5.7) | 284 (7.7) ⁎⁎⁎ | 93 (7.9) ⁎⁎ | <0.001 |
| History of medication use, n (%) | ||||
| Diabetes medicationsa | 531 (5.2) | 308 (8.5) ⁎⁎⁎ | 68 (5.9) | <0.001 |
| Hypertension medicationsa | 2049 (20.3) | 1257 (34.9) ⁎⁎⁎ | 287 (25.2) ⁎⁎⁎ | <0.001 |
| Lipid-lowering therapya | 739 (7.3) | 365 (10.2) ⁎⁎⁎ | 78 (6.8) | <0.001 |
| Metabolic biomarkersb | ||||
| Total cholesterol, mg/dL | 184.6 ± 35.9 | 184.6 ± 36.9 | 177.0 ± 34.9 ⁎⁎⁎ | <0.001 |
| Triglycerides, mg/dL | 115.0 (83.2, 172.6) | 122.1 (87.6, 176.1) | 92.0 (70.8, 135.4) ⁎⁎⁎ | <0.001 |
| LDL cholesterol, mg/dL | 102.5 ± 28.7 | 103 ± 29.1 | 97.4 ± 27.3 ⁎⁎⁎ | <0.001 |
| HDL cholesterol, mg/dL | 51.3 ± 11.2 | 50.3 ± 11.6 ⁎⁎⁎ | 53.3 ± 13.3 ⁎⁎⁎ | <0.001 |
| eGFR, mL/min/1.73 m2 | 96.4 ± 21.5 | 93.1 ± 24.0 ⁎⁎⁎ | 93.4 ± 25.0 ⁎⁎⁎ | <0.001 |
| Handgrip strength, kg | 32.9 ± 9.2 | 21.5 ± 9.8 ⁎⁎⁎ | 21.7 ± 7.7 ⁎⁎⁎ | <0.001 |
| ASM/Ht,2 kg/m2 | 7.2 ± 1.5 | 7.5 ± 1.0 ⁎⁎⁎ | 5.6 ± 2.2 ⁎⁎⁎ | <0.001 |
| Gait speed, m/s | 0.86 ± 0.19 | 0.66 ± 0.23 ⁎⁎⁎ | 0.67 ± 0.21 ⁎⁎⁎ | <0.001 |
| 5-time chair stand test, s | 8.1 ± 1.9 | 12.7 ± 4.7 ⁎⁎⁎ | 12.6 ± 5.1 ⁎⁎⁎ | <0.001 |
Data are shown as means ± standard deviation, median (interquartile range), or numbers (percentages).
Missing data: 1896 for smoking, 3880 for drinking, 2653 for educational level, 170 for diabetes medications,293 for hypertension medications and 322 for lipid-lowering therapy.
Measured in subpopulation of 12,318 participants.
Abbreviation: ASM, appendicular skeletal muscle; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate.
Significant at *P < 0.05, **P < 0.01 and ⁎⁎⁎P < 0.001 compared to no sarcopenia group.
Cross-sectional associations of possible sarcopenia, sarcopenia with cardiovascular disease
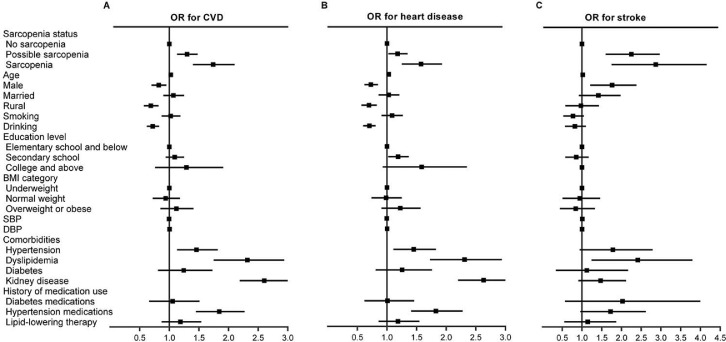
In the cross‑sectional study, the prevalence of CVD in total populations, no-sarcopenia, possible sarcopenia and sarcopenia individuals were 12.6% (1905/15,137), 10.0% (1026/10,280), 18.1% (668/3685), 18.0% (211/1172), respectively (P for trend < 0.001, Table S2). After adjustment for socio-demographic characteristics and health-related factors, both possible sarcopenia [OR (95% CI): 1.29 (1.13–1.48)] and sarcopenia [1.72 (1.40–2.10)] were significantly associated with CVD (both P < 0.001, Figure 2). The cross‑sectional relationship between sarcopenia status and CVD was not significantly changed after further adjusting for metabolic biomarkers in the subpopulations of 12,318 participants (Table S3).
Figure 2.
ORs and 95% CIs of CVD and its components by sarcopenia status in the cross-sectional analysis.
Forest plots show odds ratios (ORs) and 95%CIs for A) CVD, B) heart disease, and C) stroke adjusted for age, sex, residence, marital status, educational level, smoking status, drinking status, BMI, SBP, DBP; history of hypertension, dyslipidemia, diabetes, chronic kidney disease; and use hypertension medications, diabetes medications, and lipid-lowering therapy.
ORs, odds ratios; CVD, cardiovascular disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
The associations between sarcopenia status and different CVD components are shown in Table S2 and Figure 2. After adjusting for covariates, both possible sarcopenia and sarcopenia were found to be positively associated with the CVD components, including heart disease [1.17 (1.01–1.35) in possible sarcopenia persons and 1.55 (1.25–1.93) in sarcopenia participants] and stroke [2.18 (1.60–2.97) in possible sarcopenia persons and 2.70 (1.75–4.16) in sarcopenia participants] (all P < 0.05, Table S2). Among the subpopulations of 12,318 participants with completed metabolic biomarkers measurements, similar results were observed in sarcopenia participants after further adjusting for metabolic biomarkers. However, we found that participants with possible sarcopenia were significantly associated with stroke [2.31 (1.65–3.24)], but not heart disease [1.14 (0.97–1.33)] (Table S3).
Longitudinal association between baseline sarcopenia status and incident cardiovascular disease at follow-up, 2015–2018
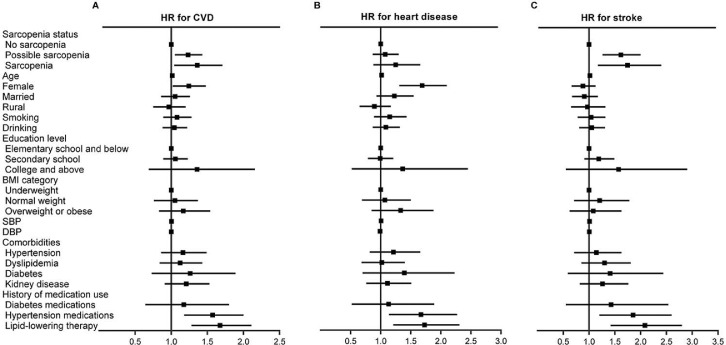
During the 3.6 years of follow-up, 1273 cases (10.7%) with incident CVD events were identified. In the longitudinal analysis, the incidence rate of CVD was 25.74 per 1000 person-years among persons with no-sarcopenia, 39.67 per 1000 person-years among possible sarcopenia group and 36.99 per 1000 person-years among participants with sarcopenia. Table 2 shows the relationship between baseline sarcopenia status and incident CVD. After adjusting for covariates in Models 1–3, individuals with the diagnosed possible sarcopenia [HR (95% CI): 1.22 (1.05–1.43)] and sarcopenia participants [1.33 (1.04–1.71)] were more likely to have new onset CVD than those with no-sarcopenia (both P < 0.05, Figure 3). The longitudinal associations between possible sarcopenia, sarcopenia, and CVD were not significantly changed after further adjusting for metabolic biomarkers among the subpopulations of 9753 subjects with metabolic biomarkers measurements (Table S4). The presence of possible sarcopenia was associated with a 21.0% increased risk of CVD incidence [1.21 (1.03–1.43), P < 0.05]. Individuals with sarcopenia had a 39.0% increased risk of incident CVD compared to those with no-sarcopenia [1.39 (1.06–1.81), P < 0.05].
Table 2.
Incidence of CVD according to baseline sarcopenia status, 2015–2018.
| Outcome | Cases, No. | Incidence Rate, per 1000 Person-Years | HR (95% CI) |
||
|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||
| CVD | |||||
| No sarcopenia | 789 | 25.74 | Reference | Reference | Reference |
| Possible sarcopenia | 376 | 39.67 | 1.31 (1.15, 1.50) ⁎⁎⁎ | 1.28 (1.10, 1.48) ⁎⁎ | 1.22 (1.05, 1.43) * |
| Sarcopenia | 108 | 36.99 | 1.18 (0.96, 1.46) + | 1.29 (1.02, 1.65) * | 1.33 (1.04, 1.71) * |
| Heart disease | |||||
| No sarcopenia | 550 | 17.83 | Reference | Reference | Reference |
| Possible sarcopenia | 217 | 22.67 | 1.14 (0.96, 1.34) | 1.15 (0.94, 1.39) | 1.06 (0.87, 1.30) |
| Sarcopenia | 66 | 22.35 | 1.12 (0.86, 1.47) | 1.26 (0.93, 1.71) | 1.21 (0.88, 1.66) |
| Stroke | |||||
| No sarcopenia | 273 | 8.77 | Reference | Reference | Reference |
| Possible sarcopenia | 184 | 19.18 | 1.77 (1.45, 2.17) ⁎⁎⁎ | 1.61 (1.29, 2.02) ⁎⁎⁎ | 1.59 (1.26, 2.00) ⁎⁎⁎ |
| Sarcopenia | 51 | 17.17 | 1.37 (1.01, 1.89) * | 1.48 (1.03, 2.11) * | 1.67 (1.17, 2.40) ⁎⁎ |
Abbreviation: HR, hazard ratio; CVD, cardiovascular disease.
Model 1 was adjusted for age, sex.
Model 2 was adjusted for age, sex, residence, marital status, educational level, smoking status, drinking status and body mass index.
Model 3 was adjusted as model 2 with further adjustment for systolic blood pressure, diastolic blood pressure; history of hypertension, dyslipidemia, diabetes and chronic kidney disease; and use hypertension medications, diabetes medications, and lipid-lowering therapy.
P < 0.1.
P < 0.05.
P < 0.01.
P < 0.001.
Figure 3.
Longitudinal association of baseline sarcopenia status with incident CVD, 2015–2018.
Graph shows hazard ratios (HRs) and 95%CIs for A) CVD, B) heart disease, and C) stroke adjusted for age, sex, residence, marital status, educational level, smoking status, drinking status, BMI, SBP, DBP; history of hypertension, dyslipidemia, diabetes, chronic kidney disease; and use hypertension medications, diabetes medications, and lipid-lowering therapy.
CVD, cardiovascular disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
For CVD components, individuals with both possible sarcopenia [1.59 (1.26–2.00)] and sarcopenia [1.67 (1.17–2.40)] had higher risks of stroke, but not heart disease (Table 2 and Figure 3). After further adjusting for metabolic biomarkers, individuals with the diagnosed possible sarcopenia [1.60 (1.25–2.05)] and sarcopenia participants [1.77 (1.21–2.59)] were more likely to have incident stroke than those with no-sarcopenia (both P < 0.01). Subjects with possible sarcopenia or sarcopenia were also not significantly associated with an increased risk of heart disease compared with those with no-sarcopenia (Table S4). In addition, we also assessed the risk of newly onset CVD between the possible sarcopenia and sarcopenia subgroups. As shown in Table S5, we found that compared with individuals with possible sarcopenia, those having sarcopenia were not significantly associated with increased risk of incident CVD (P>0.05).
Cross-sectional and longitudinal associations of low muscle mass alone with cardiovascular disease
In the cross‑sectional analysis, we further excluded 3130 subjects with low physical performance, and 1727 participants with low grip strength in CHARLS 2015. Our final samples included 10,280 subjects, among which 2101 (20.4%) had low muscle mass alone with neither low handgrip strength nor low physical performance. The prevalence of CVD among individuals without any sarcopenia components and individuals with low muscle mass alone were 9.2% and 7.6% in men, and 10.9% and 11.7% in women, respectively (Table S6). Individuals with low muscle mass alone were not significantly associated with CVD and its components (all P>0.05). In the longitudinal analysis, those with low muscle mass alone, but without low grip strength and low physical performance, were also not significantly associated with an increased risk of incident CVD and its components in both male and female participants (Table S7, all P>0.05).
Discussion
In the present study, we found that both possible sarcopenia and sarcopenia, assessed using the AWGS 2019 algorithm, were independently and positively associated with CVD in the cross-sectional analysis. Importantly, individuals with diagnosed possible sarcopenia or sarcopenia were at higher risk of incident CVD among middle-aged and older adults. There was no significant increased risk of CVD for participants with low muscle mass alone in the absence of low grip strength and low physical performance.
Several cross-sectional studies suggest that the presence of sarcopenia was correlated with higher odds of heart disease and stroke.17,21,22 Xia et al.17 demonstrated that sarcopenia was positively associated with increased risk of carotid atherosclerosis, myocardial infarction and atrial fibrillation among 2432 middle-aged and elderly adults. Park et al.21 reported that CVD prevalence was positively associated with sarcopenia, and men aged ≥50 years with sarcopenia showed elevated prevalence of CVDs, especially stroke. These cross-sectional findings are largely consistent with our results, although the criteria for diagnosis of sarcopenia differ markedly. In the cross‑sectional study, we found that sarcopenia was significantly associated with CVD, including heart disease and stroke. However, there is a paucity of research on the longitudinal association between sarcopenia and CVD event among middle-aged and older general population. To our knowledge, this is the first large, population-based longitudinal study that has found sarcopenia, assessed using the AWGS 2019 criteria, is positively associated with an increased incidence of CVD in Asia. We found that sarcopenic individuals had a 33% higher risk of new onset CVD compared with the no-sarcopenia group. Our findings may indicate that the assessment of sarcopenia in community-based health check‐ups and routine clinical practice might facilitate identification of those at greatest risk of incident CVD, who would benefit most from early intervention. The underlying mechanisms of the relation between sarcopenia and CVD are multifactorial, involving several pathophysiological changes in sarcopenic patients,1,2,17,35 including muscle mitochondria dysfunction, oxidative stress, hyper-inflammation status, microvascular endothelial dysfunction and multiple metabolic disorders (insulin resistance and non-alcoholic fatty liver disease), and similar lifestyle factors,1,2,36 such as malnutrition and insufficient physical activity.
No studies have assessed the association between possible sarcopenia, assessed by the AWGS 2019 algorithm, and CVD in Asia. In addition to sarcopenia, the current study also demonstrated interesting findings regarding the effect of possible sarcopenia on CVD. Compared with no sarcopenic participants, the prevalence of CVD and its components were significantly increased in persons with possible sarcopenia. More importantly, we first found that individuals with the diagnosed possible sarcopenia were associated with significantly increased risk of incident CVD, especially stroke. A few previous studies have separately reported the associations between grip strength, walking pace and the occurrence of CVD among the general population.37–39 A recent meta-analysis study comprising of 42 observational studies conducted by Wu et al.37 showed that lower handgrip strength, one component of possible sarcopenia, was an independent predictor of CVD and stroke in community-dwelling populations. A Mendelian randomization study also reported that increased handgrip strength was causally related to lower risk of incident CVD in UK individuals.38 Besides, Gill et al.39 have proven that walking speed, another component of possible sarcopenia, was associated with the incidence of self-reported CVD, and those who reported a slow walking pace were at higher risk of CVD and mortality compared to those reporting brisk walking speed. Our findings supported the validity of current major criteria of possible sarcopenia by the AWGS 2019, and suggested that maintaining enough muscle strength and/or physical performance could be beneficial for the prevention of CVD for middle-age and older adults. Meanwhile, early diagnosis, lifestyle interventions, and treatment of possible sarcopenia in routine clinical practice should be taken as a factor in fighting against CVD and promoting healthy aging.
To the best of our knowledge, several previous studies have evaluated the association between muscle mass and incident CVD; however, no consistent conclusions have been reached.40–44 Knowles et al.40 reported that the ASM was positively associated with CVD incidence in men and there was a curvilinear association in women. Tyrovolas et al.41 found that baseline skeletal muscle mass, using ASM standardized by BMI, showed a negative association with the 10-years CVD incidence among adults 45+ years old. However, Lee demonstrated that higher estimated 10-years CVD risk was associated with combined phenotypes of low muscle mass and high fat in men but not in women.44 In addition, several studies have illustrated that muscle mass was not significantly correlated with increased risk of CVD.42,43 As part of the results, we also found that participants with low muscle mass alone were not significantly associated CVD in both men and women. The possible causes underlying these conflicting results might be confounded by body fat mass, differences in muscle mass measurement methods, and subtypes of CVD (stroke, heart failure, coronary artery disease and arrhythmia).43 Therefore, the relationship of muscle mass with CVD risk and its potential mechanisms are still needed to be further investigated. In addition, the longitudinal associations between possible sarcopenia, sarcopenia, and heart disease may be undetected because of the short study period.
The present study has several strengths. First, this study includes a large and nationally representative sample size, thereby allowing for broad generalizability of our findings to middle-aged and older Chinese general population. Second, unlike most previous cross-sectional studies, this is the first study to explore the longitudinal relationships between possible sarcopenia, sarcopenia and CVD in Asia. More importantly, our findings supported the validity of current major algorithm of possible sarcopenia by the AWGS 2019, and suggested that preventing and/or improving both possible sarcopenia and sarcopenia may reduce the risk of CVD.
However, there are several limitations in the current study. Firstly, in our study, information about the Short Physical Performance Battery score were completely missing, so the estimates may be biased. Secondly, this study used observational data, which may have biased the observed relations by introducing confounding factors. To reduce such bias, we considered as many related factors as possible in the analysis. However, other potential confounding factors, such as body fat mass and physical inactivity, cannot be ruled out. Thirdly, consistent with other previous studies,32,45,46 the diagnosis of CVD was self-reported physician-diagnosed. Medical records were not available in the CHARLS, thus the use of self-reported measures of chronic diseases including CVD may also have some degree of bias. However, Xie et al.33 reported that 77.5% of self-reported incident coronary heart disease were confirmed by the English Longitudinal Study of Ageing researchers according to medical records. Fourth, due to the lack of detailed classification of CVD, we have not been able to analyze the association between sarcopenia and specific heart disease in depth. Fifth, due to the lack of late-onset sarcopenia data in the structured questionnaire of CHARLS 2018, we were unable to estimate the concomitant effect of late-onset sarcopenia on CVD and the role of CVD in the new onset of sarcopenia. Future studies are needed to pinpoint these important questions. In addition, some types of selection bias, such as potential volunteer bias and non-response bias, should also be taken into account when interpreting and extrapolating our results. Despite these limitations, this study contributes to extending our previous knowledge of the cardiometabolic importance of possible sarcopenia and sarcopenia. Our findings indicate that the assessment of both sarcopenia and possible sarcopenia should be introduced to community-based health check‐ups and routine clinical practice in order to reduce the incidence of CVD.
In conclusion, both possible sarcopenia and sarcopenia, assessed using the AWGS 2019 criteria, were associated with higher CVD risk among middle-aged and older Chinese adults. Our findings provided the new evidence supporting the longitudinal connection between sarcopenia and CVD, and suggested that preventing and/or improving both possible sarcopenia and sarcopenia may be beneficial for reducing CVD incidence and promoting healthy aging for middle-aged and older adults.
Declaration of interests
The authors declare no competing interests.
Acknowledgments
Acknowledgments
This study is based on the baseline of the China Health and Retirement Longitudinal Study (CHARLS). We would like to thank the CHARLS research team, the field team, and every respondent for their time and efforts that they have devoted to the CHARLS project.
Funding
None.
Contributors
D.Z., and K.G. conceived the protocol, K.G., L.-F.C., T.L., Y.-J.G., W.-Z.M., M.-S.L., and J.Z. contributed to analysis and interpretation of data. K.G. and L.-F.C. grafted the manuscript. D.Z., T.L. and W.-Z.M. critically revised the manuscript. All authors agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript. The corresponding author had full access to all data in the study and assumed final responsibility for the decision to submit the manuscript for publication.
Data sharing statement
The datasets generated for this study are available on request to the corresponding author.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101264.
Appendix. Supplementary materials
References
- 1.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;10191:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 2.Bauer J., Morley J.E., Schols A., et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle. 2019;5:956–961. doi: 10.1002/jcsm.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;1:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cawthon P.M., Lui L.Y., Taylor B.C., et al. Clinical definitions of sarcopenia and risk of hospitalization in community-dwelling older men: the osteoporotic fractures in men study. J Gerontol A Biol Sci Med Sci. 2017;10:1383–1389. doi: 10.1093/gerona/glw327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitamura A., Seino S., Abe T., et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. 2021;1:30–38. doi: 10.1002/jcsm.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X., Huang P., Dou Q., et al. Falls among older adults with sarcopenia dwelling in nursing home or community: a meta-analysis. Clin Nutr. 2020;1:33–39. doi: 10.1016/j.clnu.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Chen L.K., Woo J., Assantachai P., et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;3 doi: 10.1016/j.jamda.2019.12.012. 300-7.e2. [DOI] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., et al. Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing. 2010;4:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L.K., Liu L.K., Woo J., et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;2:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Wu X., Li X., Xu M., Zhang Z., He L., Li Y. Sarcopenia prevalence and associated factors among older Chinese population: findings from the China health and retirement longitudinal study. PLoS ONE. 2021;3 doi: 10.1371/journal.pone.0247617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanimoto Y., Watanabe M., Sun W., et al. Association between sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Arch Gerontol Geriatr. 2012;2:e9–13. doi: 10.1016/j.archger.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y.S., Lee Y., Chung Y.S., et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the fourth Korean national health and nutritional examination surveys. J Gerontol A Biol Sci Med Sci. 2012;10:1107–1113. doi: 10.1093/gerona/gls071. [DOI] [PubMed] [Google Scholar]
- 13.Sayer A.A. Sarcopenia. BMJ. 2010:c4097. doi: 10.1136/bmj.c4097. [DOI] [PubMed] [Google Scholar]
- 14.Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;10100:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbett S., Courtiol A., Lummaa V., Moorad J., Stearns S. The transition to modernity and chronic disease: mismatch and natural selection. Nat Rev Genet. 2018;7:419–430. doi: 10.1038/s41576-018-0012-3. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar S.B., Khavjou O.A., Bakas T., et al. Projected costs of informal caregiving for cardiovascular disease: 2015 to 2035: a policy statement from the American heart association. CirculationCirculation. 2018;19:e558–ee77. doi: 10.1161/CIR.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 17.Xia M.F., Chen L.Y., Wu L., et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: a cross-sectional study. Clin Nutr. 2021;2:571–580. doi: 10.1016/j.clnu.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Santana N.M., Mendes R.M.L., Silva N.F.D., Pinho C.P.S. Sarcopenia and sarcopenic obesity as prognostic predictors in hospitalized elderly patients with acute myocardial infarction. Einstein. 2019;4:eAO4632. doi: 10.31744/einstein_journal/2019AO4632. (Sao Paulo) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curcio F., Testa G., Liguori I., et al. Sarcopenia and heart failure. Nutrients. 2020;1 doi: 10.3390/nu12010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki T., Palus S., Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018;6:1099–1107. doi: 10.1002/ehf2.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park S., Ham J.O., Lee B.K. A positive association between stroke risk and sarcopenia in men aged ≥ 50 years, but not women: results from the Korean national health and nutrition examination survey 2008-2010. J Nutr Health Aging. 2014;9:806–812. doi: 10.1007/s12603-014-0553-x. [DOI] [PubMed] [Google Scholar]
- 22.Chin S.O., Rhee S.Y., Chon S., et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea national health and nutrition examination survey (KNHANES) from 2009. PLoS ONE. 2013;3:e60119. doi: 10.1371/journal.pone.0060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda T., Bouchi R., Takeuchi T., et al. Sarcopenic obesity assessed using dual energy X-ray absorptiometry (DXA) can predict cardiovascular disease in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol. 2018;1:55. doi: 10.1186/s12933-018-0700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Requena Calleja M.A., Arenas Miquélez A., Díez-Manglano J., et al. Sarcopenia, frailty, cognitive impairment and mortality in elderly patients with non-valvular atrial fibrillation. Rev Clin Esp (Barc) 2019;8:424–432. doi: 10.1016/j.rce.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Zhang N., Zhu W.L., Liu X.H., et al. Prevalence and prognostic implications of sarcopenia in older patients with coronary heart disease. J Geriatr Cardiol. 2019;10:756–763. doi: 10.11909/j.issn.1671-5411.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han P., Chen X., Yu X., et al. The predictive value of sarcopenia and its individual criteria for cardiovascular and all-cause mortality in suburb-dwelling older Chinese. J Nutr Health Aging. 2020;7:765–771. doi: 10.1007/s12603-020-1390-8. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y., Hu Y., Smith J.P., Strauss J., Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS) Int J Epidemiol. 2014;1:61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;6:800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 29.Wen X., Wang M., Jiang C.M., Zhang Y.M. Anthropometric equation for estimation of appendicular skeletal muscle mass in Chinese adults. Asia Pac J Clin Nutr. 2011;4:551–556. [PubMed] [Google Scholar]
- 30.Yang M., Hu X., Wang H., Zhang L., Hao Q., Dong B. Sarcopenia predicts readmission and mortality in elderly patients in acute care wards: a prospective study. J Cachexia Sarcopenia Muscle. 2017;2:251–258. doi: 10.1002/jcsm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexandre Tda S., Duarte Y.A., Santos J.L., Wong R., Lebrão M.L. Sarcopenia according to the European working group on sarcopenia in older people (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J Nutr Health Aging. 2014;8:751–756. doi: 10.1007/s12603-014-0540-2. [DOI] [PubMed] [Google Scholar]
- 32.Li H., Zheng D., Li Z., et al. Association of depressive symptoms with incident cardiovascular diseases in middle-aged and older Chinese adults. JAMA Netw Open. 2019;12 doi: 10.1001/jamanetworkopen.2019.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie W., Zheng F., Yan L., Zhong B. Cognitive decline before and after incident coronary events. J Am Coll Cardiol. 2019;24:3041–3050. doi: 10.1016/j.jacc.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;9:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boengler K., Kosiol M., Mayr M., Schulz R., Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle. 2017;3:349–369. doi: 10.1002/jcsm.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen S., Nathan J.A., Goldberg A.L. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;1:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y., Wang W., Liu T., Zhang D. Association of grip strength with risk of all-cause mortality, cardiovascular diseases, and cancer in community-dwelling populations: a meta-analysis of prospective cohort studies. J Am Med Dir Assoc. 2017;6:551. doi: 10.1016/j.jamda.2017.03.011. e17-.e35. [DOI] [PubMed] [Google Scholar]
- 38.Tikkanen E., Gustafsson S., Ingelsson E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK biobank study. Circulation. 2018;24:2583–2591. doi: 10.1161/CIRCULATIONAHA.117.032432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celis-Morales C.A., Gray S., Petermann F., et al. Walking pace is associated with lower risk of all-cause and cause-specific mortality. Med Sci Sports Exerc. 2019;3:472–480. doi: 10.1249/MSS.0000000000001795. [DOI] [PubMed] [Google Scholar]
- 40.Knowles R., Carter J., Jebb S.A., Bennett D., Lewington S., Piernas C. Associations of skeletal muscle mass and fat mass with incident cardiovascular disease and all-cause mortality: a prospective cohort study of UK biobank participants. J Am Heart Assoc. 2021;9 doi: 10.1161/JAHA.120.019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyrovolas S., Panagiotakos D., Georgousopoulou E., et al. Skeletal muscle mass in relation to 10 year cardiovascular disease incidence among middle aged and older adults: the ATTICA study. J Epidemiol Commun Health. 2020;1:26–31. doi: 10.1136/jech-2019-212268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farmer R.E., Mathur R., Schmidt A.F., et al. Associations between measures of sarcopenic obesity and risk of cardiovascular disease and mortality: a cohort study and mendelian randomization analysis using the UK siobank. J Am Heart Assoc. 2019;13 doi: 10.1161/JAHA.118.011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H.M., Zhang Q., Shen W.D., et al. Sarcopenia-related traits and coronary artery disease: a bi-directional Mendelian randomization study. Aging. 2020;4:3340–3353. doi: 10.18632/aging.102815. (Albany NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee K. Muscle mass and body fat in relation to cardiovascular risk estimation and lipid-lowering eligibility. J Clin Densitom. 2017;2:247–255. doi: 10.1016/j.jocd.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Shi Z., Nicholls S.J., Taylor A.W., Magliano D.J., Appleton S., Zimmet P. Early life exposure to Chinese famine modifies the association between hypertension and cardiovascular disease. J Hypertens. 2018;1:54–60. doi: 10.1097/HJH.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y., Atun R., Oldenburg B., et al. Physical multimorbidity, health service use, and catastrophic health expenditure by socioeconomic groups in China: an analysis of population-based panel data. Lancet Glob Health. 2020;6 doi: 10.1016/S2214-109X(20)30127-3. e840-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.