Summary
Background
Renal replacement therapy (RRT) is an effective rescue therapy for Type 1 cardiorenal syndrome (CRS). Previous studies have demonstrated that type 1 CRS patients with severe renal dysfunction were susceptible to sepsis, and that serum lactate has been correlated with the risk of mortality in patients with sepsis. However, the association between serum lactate level and the prognosis of type 1 CRS patients requiring RRT is unknown.
Methods
An inception cohort of 500 type 1 CRS patients who received RRT in a tertiary-care referral hospital in Taiwan from August 2011 to January 2018 were enrolled. The outcomes of interest were dialysis withdrawal and 90-day mortality. The results were further externally validated using sampling data of type 1 CRS patients requiring dialysis from multiple tertiary-care centers.
Findings
The 90-day mortality rate was 52.8% and the incidence rate of dialysis withdrawal was 34.8%. Lower pre-dialysis lactate was correlated with a higher rate of dialysis withdrawal and lower rate of mortality. Generalized additive model showed that 4.2 mmol/L was an adequate cut-off value of lactate to predict mortality. Taking mortality as a competing risk, Fine-Gray subdistribution hazard analysis further indicated that a low lactate level (≦ 4.2 mmol/L) was an independent predictor for the possibility of dialysis withdrawal, as also shown in external validation. The interaction of quick Sequential Organ Failure Assessment score and lactate was associated with dialysis dependence in a disease severity-dependent manner. Furthermore, the associations between hyperlactatemia and dialysis dependence were consistent in the patients with and without sepsis.
Interpretation
Serum lactate level is accurate and capable of forecasting the prognosis along with qSOFA severity for clinical decision-making for treating type 1 CRS patients. Further studies are needed to validate our results.
Funding
This study was supported by grants from Taiwan National Science Council [104–2314-B-002–125-MY3,106–2314-B-002–166-MY3,107–2314-B-002–026-MY3], National Taiwan University Hospital [106-FTN20,106-P02,UN106–014,106-S3582,107-S3809,107-T02,PC1246,VN109–09,109-S4634,UN109–041], Ministry of Science and Technology of the Republic of China [MOST106–2321-B-182–002,106-2314-B-182A-064,MOST107–2321-B-182–004,MOST107-2314-B-182A-138, MOST108–2321-B-182–003,MOST109–2321-B-182–001, MOST108-2314-B-182A-027], Chang Gung Memorial Hospital [CMRPG-2G0361,CMRPG-2H0161,CMRPG-2J0261, CMRPG-2K0091], and Ministry of Health and Welfare of the Republic of China [PMRPG-2L0011].
Keywords: Type 1 cardiorenal syndrome, renal replacement therapy, withdrawal, serum lactate, Prognosis
Research in context.
Evidence before this study
Limited studies provide objective parameters to evaluate the possibility of dialysis withdrawal or survival in type 1 cardiorenal syndrome (CRS) patients. Previous studies demonstrated that type 1 CRS patients with severe renal dysfunction were susceptible to sepsis, and that serum lactate has been correlated with the risk of mortality in patients with sepsis. However, the association between serum lactate level and the prognosis of type 1 CRS patients requiring renal replacement therapy (RRT) is unknown.
Added value of this study
We conducted a prospective study to explore the correlation of serum lactate level with the possibility of dialysis withdrawal in type 1 CRS patients who required RRT. The severity of illness was evaluated by quick Sequential Organ Failure Assessment (qSOFA) score. We found that the interaction between qSOFA score and lactate level was associated with dialysis dependence in a disease severity-dependent manner. Furthermore, the associations between hyperlactatemia and dialysis dependence were consistent in the patients with and without sepsis.
Implications of all the available evidence
The findings of the present study suggest that pre-dialysis serum lactate level could predict the possibility of withdrawal from acute dialysis in these patients. The serum lactate level is accurate and capable of forecasting the prognosis along with qSOFA severity for clinical decision-making for treating type 1 CRS patients.
Alt-text: Unlabelled box
Introduction
Type 1 cardiorenal syndrome (CRS) is characterized by a rapid worsening of cardiac function and marked systemic congestion that absolutely or relatively causes a secondary insult to kidney function.1, 2, 3 The appearance of CRS generally indicates a grave outcome, such as an increased risk of long-term mortality and end-stage renal disease.4 Emerging evidence has demonstrated a strong correlation between acute kidney injury (AKI) and systemic inflammation in type 1 CRS patients.5 In addition, even a moderate degree of kidney function impairment has been independently associated with an increased risk of mortality from any cause in patients with heart failure (HF).6 Treatment options for type 1 CRS are still limited, and renal replacement therapy (RRT) is regarded to be an effective rescue therapy when refractory HF symptoms persist.7 However, objective parameters to provide accurate information on disease severity and evaluate the possibility of dialysis withdrawal or survival in type 1 CRS patients are lacking.
Lactic acid is a biomarker of tissue hypoperfusion, and serum lactate has been demonstrated to be a good indicator of the severity of cardiac shock.8 Unlike other widely used markers in HF, lactate is unique because it reflects the dynamic energetic/metabolic status of these patients.9 Several investigations have indicated that increased serum lactate is a strong predictor of mortality in the setting of septic patients requiring RRT,10, 11, 12 however the relationship between serum lactate levels and the prognosis of type 1 CRS is still unknown. In critical care patients with considerable comorbidities, the competing risk of death is especially high and will confound the clinical analysis of dialysis withdrawal.
The aim of this study was to identify whether predialysis serum lactate can be potential prognostic to predict dialysis withdrawal, 90-day mortality and competing death in type 1 CRS patients who required dialysis for AKI.
Methods
Ethics statement and registration of the observational study
This study was conducted in full compliance with the ethical principles of the Declaration of Helsinki and was consistent with Good Clinical Practice guidelines and the applicable local regulatory requirements. The study was approved by the Institutional Review Board of National Taiwan University Hospital (201407076RINA). Patients who met the inclusion criteria were invited to participate in this study on their first day of admission to the intensive care unit (ICU). Trained physicians evaluated the patients’ mental status during the screening and proceeded to perform informed consent procedures. A written informed consent was obtained from all mentally competent subjects or next-of-kin of compromised ones prior to their participation.13, 14, 15, 16
Study design
We prospectively enrolled patients with type 1 CRS undergoing RRT for AKI in multiple intensive care units (ICUs) from the National Taiwan University Study Group on Acute Renal Failure.13,14,17, 18, 19 According to the consensus definition of the Acute Dialysis Quality Initiative (ADQI) group, type 1 CRS was defined as an abrupt worsening of cardiac function leading to acute impairment of kidney function.2 From August 2011 to January 2019, type 1 CRS patients who required RRT after ICU admission were prospectively enrolled. The exclusion criteria were: age 〈 18 years, previous nephrectomy, renal transplantation or prior RRT treatment, ICU or hospital length of stay of respectively < 2 days and 〉 180 days during the index hospitalization, and AKI caused by kidney surgically induced injury, vasculitis, obstruction, glomerulonephritis, interstitial nephritis, hemolytic uremic syndrome, or thrombotic thrombocytopenic purpura.
Patient enrollment and data collection
The cardiac reasons for ICU admission included: acute coronary syndrome, acute decompensated HF, or impending HF with the need for intensive monitoring. All data were collected prospectively, including data on the patients’ sex, age, comorbidities, the primary reason for ICU admission, underlying heart disease, disease severity and clinical parameters before dialysis.
Baseline serum creatinine (sCr) was defined as the nadir value obtained after the previous admission in those who had more than one admission within 1 year before the index admission, or the mean outpatient value over the 180 days before the index admission in those with no previous admission20,21 Sepsis and septic shock were defined by Sepsis-3.22 Drug related AKI was defined as an abrupt decrease in renal function after exposure to nephrotoxic agents.23 Pigment nephropathy was defined as an abrupt decrease in renal function due to rhabdomyolysis or hemolysis.24 Contrast nephropathy was defined as an absolute (≥ 0.5 mg/dl) or relative increase (≥ 25%) in serum creatinine value at 48–72 h after exposure to a contrast agent compared to baseline serum creatinine values, while alternative explanations for renal impairment have been excluded.25 The RRT modality in each patient was initially chosen by the attending physician and adapted according to hemodynamic evaluation and evolution by a critical care nephrologist.
Indications for dialysis
Indications for initiating RRT included one or more of the following: (1) azotemia (i.e. blood urea nitrogen > 80 mg/dL and sCr > 2 mg/dL) with uremic symptoms (encephalopathy, pericarditis, or pleuritis); (2) oliguria (urine output < 400 mL/24 h) or anuria refractory to diuretics; (3) fluid overload refractory to diuretics with a central venous pressure > 12 mmHg or pulmonary edema with PaO2/FiO2 < 300 mmHg; (4) hyperkalemia (serum potassium level > 5.5 mmol/L) refractory to medical treatment; and (5) metabolic acidosis (pH < 7.2 in arterial blood).13,26,27
Outcome assessments
The primary clinical endpoints were 90-day mortality after hospital discharge and withdrawal from dialysis in the survivors. All patients were followed until death or for 90 days after discharge, whichever occurred first. Successful withdrawal from dialysis was defined as surviving without dialysis at the end of study. Dialysis dependence was defined not weaning from dialysis within 90 days.7 Patients who died within 14 days after being weaned from dialysis were classified into the mortality group rather than the dialysis withdrawal group, because biological dialysis withdrawal without survival is not patient-centered.28 Patients who received acute dialysis and planned to receive palliative care were excluded.
External validation
We used the database of the Nationwide Epidemiology and Prognosis of Dialysis-requiring Acute Kidney Injury (NEP-AKI-D) study for further external validation.29 The NEP-AKI-D study was designed to enroll critically ill adult patients with AKI-D receiving RRT in the ICUs of both tertiary medical centers and regional hospitals located in the four geographical regions (north, middle, south, and east) of Taiwan. The four months during which the participants were enrolled were October 2014, and January, April, and July 2015, reflecting the seasons in Taiwan. After the enrollment process, we followed the clinical courses and documented the outcomes of these enrolled participants until death or 90 days following hospital discharge. The study was approved by the National Research Program for Biopharmaceuticals (NRPB)-Institutional Review Board (IRB) (NRPB2014050014) and the IRBs of all participating hospitals.30
Statistical analysis
Continuous data were expressed as mean ± standard deviation. Categorical data were expressed as number (percentage), and the χ2 or Fisher's exact test was used for comparisons. All variables were tested for normal distribution using the Kolmogorov–Smirnov test. The Student's t-test was used to compare the means of continuous variables and normally distributed data; otherwise, the Mann–Whitney U test was used. The χ2 test was used to compare categorical data. A generalized additive model was plotted and adjusted for comorbidities, sex and age in individual patients.31,32 The model incorporated subject-specific random effects, expressed as the logarithm of the odd (logit), and the optimal cut-off value was defined as a log odds value of zero.33 The variables were assessed in multivariable analysis using a Fine-Gray subdistribution hazard model to estimate subdistribution hazard ratios (sHRs) for the possibility of dialysis withdrawal, taking mortality as a competing risk.34 The significance levels for entry (SLE) and stay (SLS) were conservatively set to 0.15. We computed E-values using the methodology proposed by VanderWeele and Ding.35 Specifically, the E-values quantify what the risk ratio would need to be for unmeasured confounders to explain away the observed associations of pre-dialytic qSOFA score and lactate for mortality in the present study. All statistical tests were two-tailed, and a p value of < 0.05 was considered to be statistically significant. All analyses were performed using SPSS software (version 20, IBM, Armonk, NY), R software (version 3.4.2, Free Software Foundation, Inc., Boston, MA), SAS software version 9.1.3 (SAS Institute Inc., Cary, NC), and MedCalc Statistical Software (version 15.11.3, MedCalc Software bvba, Oostende, Belgium; https://www.medcalc.org; 2015).
Role of the funding source
The funders had no role in the study design, data analysis, interpretation or decision to submit for publication. VC Wu had full access to the data and took the decision to submit for publication.
Results
Baseline characteristics of the study population
We enrolled 500 eligible patients in this study. The overall 90-day mortality rate was 52.8% (264/500). The patients were divided into three groups: mortality group (n = 264), dialysis dependence group (n = 62), and dialysis withdrawal group (n = 174), according to whether they died, were dependent on dialysis, or withdrew from dialysis within 90 days after the initiation of dialysis for AKI. The baseline demographic data and clinical characteristics are shown in Table 1. Overall, the mean age of the patients was 61.4 years; 360 patients were men (72.0%) and 140 were women (28.0%). Sepsis was the most common cause of CRS superimposed with dialysis requiring AKI (52.6%). The baseline SCr level of the dialysis withdrawal group was significantly lower than that of the dialysis dependence group (P < 0.001) but higher than that of the mortality group (P < 0.001).
Table 1.
Baseline Characteristics of enrollee.
| Total patient (n = 500) | Mortality (n = 264) | dialysis dependence (n = 62) | dialysis withdrawal (n = 174) | ANOVA | |
|---|---|---|---|---|---|
| Demographic factors | |||||
| Age, years | 61.4 ± 16.3 | 62.8 ± 16.2 | 63.4 ± 15.1 | 58.4 ± 16.4 | 0.011 |
| Male gender | 360 (72.0%) | 191 (72.4%) | 41 (66.1%) | 128 (73.6%) | 0.526 |
| Baseline SCr | 2.0 ± 1.7 | 1.8 ± 1.4 | 2.9 ± 2.3 | 1.9 ± 1.7 | < 0.001 |
| Smoking | 1 (20.8%) | 54 (20.5%) | 12 (19.4%) | 38 (21.8%) | 0.900 |
| Hypertension | 283 (56.6%) | 145 (54.9%) | 39 (62.9%) | 99 (56.9%) | 0.519 |
| Diabetes mellitus | 215 (43.0%) | 101 (38.3%) | 37 (59.7%) | 77 (44.3%) | 0.008 |
| Coronary artery disease | 196 (39.2%) | 102 (38.6%) | 28 (45.2%) | 66 (37.9%) | 0.584 |
| Congestive heart failure | 370 (74.0%) | 191 (72.4%) | 47 (75.8%) | 132 (75.9%) | 0.673 |
| NYHA FC 1 | 140 (28.0%) | 75 (28.4%) | 14 (22.6%) | 51 (29.3%) | 0.585 |
| NYHA FC 2 | 87 (17.4%) | 44 (16.7%) | 11 (17.7%) | 32 (18.4%) | 0.895 |
| NYHA FC 3 | 77 (15.4%) | 42 (15.9%) | 8 (12.9%) | 27 (15.5%) | 0.839 |
| NYHA FC 4 | 66 (13.2%) | 30 (11.4%) | 14 (22.6%) | 22 (12.7%) | 0.061 |
| Chalson score | 5.3 ± 2.5 | 4.5 ± 2.6 | 4.7 ± 2.3 | 4.0 ± 2.5 | 0.066 |
| History of PTCA | 137 (27.4%) | 70 (26.5%) | 21 (33.9%) | 46 (26.4%) | 0.475 |
| Single vessel | 13 (2.6%) | 7 (2.7%) | 1 (1.6%) | 5 (2.9%) | 1.000 |
| Two vessels | 29 (5.8%) | 10 (3.8%) | 6 (9.7%) | 13 (7.5%) | 0.103 |
| Three vessels | 95 (19.0%) | 53 (20.1%) | 14 (22.6%) | 28 (16.1%) | 0.434 |
| With stents | 22 (4.4%) | 10 (3.8) | 9 (14.5) | 13 (7.5) | 0.006 |
| History of CABG | 70 (14.0%) | 41 (15.5%) | 5 (8.1%) | 24 (13.8%) | 0.311 |
| Single vessel | 5 (1.0%) | 3 (1.1%) | 0 (0.0%) | 2 (1.2%) | 1.000 |
| Two vessels | 13 (2.6%) | 6 (2.3%) | 2 (3.2%) | 5 (2.9%) | 0.784 |
| Three vessels | 52 (10.4%) | 32 (12.1%) | 3 (4.8%) | 17 (9.8%) | 0.226 |
| ICU Admission SOFA | 10.5 ± 4.0 | 11.3 ± 4.0 | 9.4 ± 3.8 | 9.8 ± 3.9 | < 0.001 |
| ICU Admission qSOFA | 1.3 ± 0.8 | 1.5 ± 0.8 | 1.1 ± 0.8 | 1.2 ± 0.8 | < 0.001 |
| CRS superimposed with dialysis requiring AKI | |||||
| Cardiogenic shock | 170 (34.0%) | 87 (33.0%) | 24 (38.1%) | 59 (33.9%) | 0.690 |
| Sepsis | 263 (52.6%) | 154 (58.3%) | 24 (38.1%) | 85 (48.9%) | 0.010 |
| Septic shock | 145 (29.0%) | 86 (32.5%) | 11 (17.74%) | 48 (27.6%) | 0.060 |
| Drug-related AKI | 184 (36.8%) | 106 (40.2%) | 24 (38.1%) | 54 (31.2%) | 0.162 |
| Pigment nephropathy | 34 (6.8%) | 18 (6.8%) | 2 (3.2%) | 14 (8.1%) | 0.414 |
| Contrast nephropathy | 68 (13.6%) | 29 (11.0%) | 9 (14.3%) | 30 (17.3%) | 0.163 |
| Clinical parameters before dialysis | |||||
| SBP, mmHg | 108.0 ± 26.8 | 102.1 ± 24.6 | 120.0 ± 30.3 | 112.5 ± 26.6 | < 0.001 |
| Daily UO (log), ml | 2.2 ± 0.9 | 2.0 ± 1.0 | 2.3 ± 0.9 | 2.4 ± 0.8 | < 0.001 |
| GCS | 9.6 ± 4.5 | 8.2 ± 4.5 | 11.6 ± 3.6 | 11.0 ± 4.3 | < 0.001 |
| Hgb, g/dL | 10.7 ± 2.3 | 10.7 ± 2.3 | 9.6 ± 1.8 | 11.0 ± 2.3 | < 0.001 |
| Na, mEq/L | 139.3 ± 7.7 | 139.4 ± 8.0 | 137.3 ± 6.2 | 139.8 ± 7.7 | 0.089 |
| K, mEq/L | 4.4 ± 0.9 | 4.3 ± 0.9 | 4.3 ± 0.6 | 4.4 ± 0.9 | 0.337 |
| Lactate, mmol/L | 5.7 ± 4.5 | 6.9 ± 4.8 | 3.7 ± 2.8 | 4.7 ± 3.9 | < 0.001 |
| SOFA | 12.1 ± 3.8 | 13.3 ± 3.5 | 10.0 ± 3.6 | 11.1 ± 3.6 | < 0.001 |
| qSOFA | 2.0 ± 0.6 | 2.1 ± 0.6 | 1.9 ± 0.7 | 1.8 ± 0.7 | < 0.001 |
| Clinical parameters at the end of the study | |||||
| SBP, mmHg | 92.0 ± 36.8 | 64.9 ± 25.9 | 125.2 ± 21.4 | 121.5 ± 18.6 | < 0.001 |
| Daily UO (log), ml | 2.4 ± 0.8 | 1.8 ± 0.7 | 2.5 ± 0.8 | 3.0 ± 0.3 | < 0.001 |
| GCS | 8.7 ± 5.7 | 3.6 ± 1.9 | 14.0 ± 2.4 | 14.6 ± 1.5 | < 0.001 |
| Hgb, g/dL | 9.8 ± 1.7 | 9.7 ± 1.7 | 9.3 ± 1.2 | 10.1 ± 1.7 | < 0.001 |
| Na, mEq/L | 137.3 ± 6.8 | 137.9 ± 8.2 | 135.1 ± 4.1 | 137.0 ± 4.5 | 0.009 |
| K, mEq/L | 4.3 ± 0.8 | 4.4 ± 0.9 | 4.1 ± 0.6 | 4.2 ± 0.6 | 0.003 |
| Lactate, mmol/L | 7.7 ± 6.9 | 8.2 ± 6.9 | 1.0 ± 0.0 | 1.4 ± 1.0 | < 0.001 |
| SOFA | 16.5 ± 5.3 | 17.9 ± 3.6 | 8.8 ± 2.4 | 5.0 ± 3.4 | < 0.001 |
Abbreviations: AKI, acute kidney injury; CABG, coronary artery bypass graft; FC, functional class; GCS, Glasgow coma scale; Hgb, hemoglobin; ICU, intensive care unit; MI, myocardial infarction; NYHA, New York Heart Association; PTCA, percutaneous transluminal coronary angioplasty; qSOFA, quick Sequential Organ Failure Assessment; SBP, systolic blood pressure; SCr, serum creatinine; SOFA, Sequential Organ Failure Assessment; TPN, total parental nutrition; UO, urine output.
Comparing the three groups, the mortality group had significantly higher Sequential Organ Failure Assessment (SOFA) (mortality group vs. dialysis dependence group: P < 0.001; mortality group vs. dialysis withdrawal group: P = 0.001) and quick Sequential Organ Failure Assessment (qSOFA) scores (mortality group vs. dialysis dependence group: P < 0.001; mortality group vs. dialysis withdrawal group: P = 0.006) on ICU admission than the other two groups; and the dialysis dependence group had higher age (mortality group vs. dialysis dependence group: P = 0.005; dialysis dependence group vs. dialysis withdrawal group: P = 0.034), and frequency of diabetes (mortality group vs. dialysis dependence group: P = 0.002; dialysis dependence group vs. dialysis withdrawal group: P = 0.037) than the other two groups. The dialysis withdrawal group was significantly associated with a higher probability of receiving total parenteral nutrition than the other two groups (mortality group vs. dialysis withdrawal group: P = 0.003; dialysis dependence group vs. dialysis withdrawal group: P = 0.005). The prevalence rates of hypertension, coronary artery disease, smoking, dementia, rheumatologic disease, hemiplegia, peptic ulcer disease, and malignancy, and the severity of congestive HF, involved number of vessels treated by percutaneous transluminal coronary angioplasty (PTCA) and coronary artery bypass graft and the urgency of receiving surgery were similar among the three groups (P > 0.05).
In this study, oliguria (66.6%) was the leading reason for receiving RRT. Before dialysis, the mortality group had significantly lower systolic blood pressure, daily urine output (UO) and Glasgow coma scale, but higher lactate levels, SOFA and qSOFA scores than the other two groups. In addition, the dialysis dependence group had significantly lower hgb levels than the other two groups (mortality group vs. dialysis dependence group: P = 0.002; dialysis dependence group vs. dialysis withdrawal group: P < 0.001). Comparisons of the indications for dialysis revealed that the mortality group had a significantly higher rate of receiving RRT due to metabolic acidosis than the other two groups (mortality group vs. dialysis dependence group: P = 0.037; mortality group vs. dialysis withdrawal group: P = 0.003). Comparisons of baseline characteristics and clinical parameters before dialysis across the outcome groups were expressed as correlation scatterplots with matrices and Seaborn violin plots (Figure S1–2).
Pre-dialysis lactate levels predicted dialysis withdrawal in the type 1 CRS patients receiving dialysis
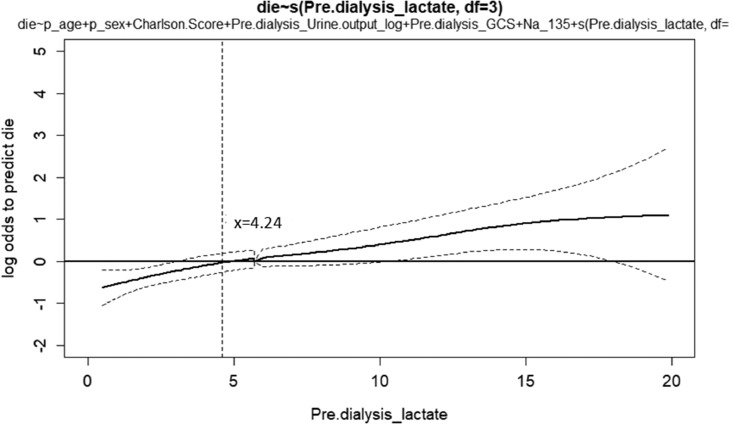
We used a nonlinear generalized additive model to identify adequate cut-off values of continuous parameters to predict mortality (Figure 1). All of the relevant covariates, including baseline characteristics, comorbidities, clinical parameters before dialysis such as qSOFA score, indications for dialysis, plasma lactate levels and other laboratory data listed in Table 1 were the selected variables. In our cohort, we found that lower pre-dialysis lactate (cut-off value: 4.2 mmol/L) was associated with a lower possibility of mortality. We further divided the patients according to their pre-dialysis lactate level (Table 2). There were no significant differences in age, sex, baseline characteristics and comorbidities between the high (pre-dialysis lactate > 4.2 mmol/L) and low lactate (pre-dialysis lactate ≦ 4.2 mmol/L) groups (P > 0.05). The 90-day mortality rate in the high lactate group was significantly higher than that in the low lactate group (P < 0.001). The prevalence rates of septic shock (P < 0.001) and SOFA score (P = 0.004) on ICU admission in the high lactate group were significantly higher than those in the low lactate group. Before dialysis, the high lactate group had significantly higher hgb (P < 0.001) and SOFA score (P < 0.001) but lower systolic blood pressure (P < 0.001), daily UO (P = 0.001) and Glasgow coma scale (P < 0.001) than the low lactate group.
Figure 1.
Generalized additive model plot for the probability of 90-day mortality against serum lactate levels at the initiation of dialysis. The generalized additive model plot was incorporated with subject-specific random effects expressed as the logarithm of the odds (logit). The probability of outcome events was constructed with lactate levels averaging zero over the range of the data, i.e. lactate = 4.24 ng/mL.
Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; qSOFA, quick Sequential Organ Failure Assessment.
¶Pre-dialysis lactate level ≦ 4.2 mmol/L was defined as low lactate.
Pre-dialysis lactate level > 4.2 mmol/L was defined as high lactate.
Table 2.
Basic characteristics of enrollee divided by pre-dialysis lactate level.
| Predictors | Pre-dialysis lactate > 4.2 mmol/L# (n = 288) | Pre-dialysis_ lactate ≦ 4.2 mmol/L# (n = 212) | P-value |
|---|---|---|---|
| Demographic factors | |||
| Age, years | 60.8 ± 16.9 | 62.0 ± 15.4 | 0.418 |
| Male gender | 205 (71.2%) | 155 (73.1%) | 0.634 |
| Baseline SCr, (mg/dL) | 1.9 ± 1.7 | 2.0 ± 1.6 | 0.468 |
| Smoking | 57 (19.8%) | 47 (22.2%) | 0.517 |
| Diabetes mellitus | 115 (39.9%) | 100 (47.2%) | 0.106 |
| Hypertension | 155 (53.8%) | 128 (60.4%) | 0.144 |
| Coronary artery disease | 105 (36.5%) | 91 (42.9%) | 0.143 |
| Congestive heart failure | 214 (74.3%) | 156 (73.6%) | 0.856 |
| NYHA FC 1 | 85 (29.5%) | 55 (25.9%) | 0.380 |
| NYHA FC 2 | 50 (17.4%) | 37 (17.5%) | 0.979 |
| NYHA FC 3 | 43 (14.9%) | 34 (16.0%) | 0.735 |
| NYHA FC 4 | 36 (12.5%) | 30 (14.2%) | 0.590 |
| Chalson score | 4.0 ± 2.5 | 4.5 ± 2.6 | 0.668 |
| History of PTCA | 74 (25.7%) | 63 (29.7%) | 0.319 |
| Single vessel | 5 (1.7%) | 8 (3.8%) | 0.157 |
| Two vessels | 14 (4.9%) | 15 (7.1%) | 0.295 |
| Three vessels | 55 (19.1%) | 40 (18.9)% | 0.949 |
| With stents | 15 (5.2%) | 17 (8.0%) | 0.205 |
| History of CABG | 40 (13.9%) | 30 (14.2%) | 0.934 |
| Single vessel | 1 (0.4%) | 4 (1.9%) | 0.168 |
| Two vessels | 7 (2.4%) | 6 (2.8%) | 0.781 |
| Three vessels | 32 (11.1%) | 20 (9.4%) | 0.544 |
| ICU Admission SOFA | 10.94±4.20 | 9.91±3.69 | 0.004 |
| ICU Admission qSOFA | 1.3 ± 0.9 | 1.3 ± 0.8 | 0.770 |
| CRS superimposed with dialysis requiring AKI | |||
| Cardiogenic shock | 88 (30.6%) | 82 (38.7%) | 0.058 |
| Sepsis | 157 (54.5%) | 106 (50.0%) | 0.318 |
| Septic shock | 111 (38.5%) | 34 (16.0%) | < 0.001 |
| Drug-related AKI | 76 (26.4%) | 79 (37.3%) | 0.359 |
| Pigment nephropathy | 18 (6.3%) | 10 (4.7%) | 0.051 |
| Contrast nephropathy | 21 (7.3%) | 33 (15.6%) | 0.305 |
| Clinical parameters before dialysis | |||
| SBP, mmHg | 103.5 ± 26.4 | 114.0 ± 26.2 | < 0.001 |
| Daily UO (log), ml | 2.1 ± 1.0 | 2.4 ± 0.9 | 0.001 |
| GCS | 8.8 ± 4.8 | 10.8 ± 4.0 | < 0.001 |
| Hgb, g/dL | 11.3 ± 2.6 | 10.4 ± 2.1 | < 0.001 |
| Na, mEq/L | 139.6 ± 7.0 | 138.9 ± 8.6 | 0.349 |
| K, mEq/L | 4.3 ± 0.9 | 4.4 ± 0.8 | 0.052 |
| SOFA | 12.8 ± 3.8 | 11.2 ± 3.5 | < 0.001 |
| qSOFA | 2.0 ± 0.6 | 2.0 ± 0.6 | 0.932 |
| Clinical parameters at the end of the study | |||
| SBP, mmHg | 115.4 ± 17.8 | 125.5 ± 17.8 | < 0.001 |
| Daily UO (log), ml | 3.1 ± 0.3 | 2.9 ± 0.5 | 0.064 |
| GCS | 14.7 ± 1.1 | 14.5 ± 1.7 | 0.347 |
| Hgb, g/dL | 10.3 ± 1.8 | 9.9 ± 1.5 | 0.100 |
| Na, mEq/L | 136.9 ± 4.4 | 136.6 ± 4.6 | 0.612 |
| K, mEq/L | 4.3 ± 0.6 | 4.2 ± 0.7 | 0.237 |
| SOFA | 5.7 ± 1.7 | 5.2 ± 4.1 | 0.630 |
| 90-day outcome | |||
| Mortality | 179 (62.2%) | 85 (40.1%) | < 0.001 |
| RRT dependence | 23 (8.0%) | 30 (14.2%) | 0.063 |
Abbreviations: AKI, acute kidney injury; CABG, coronary artery bypass graft; CRS, cardiorenal syndrome; FC, functional class; GCS, Glasgow coma scale; Hgb, hemoglobin; ICU, intensive care unit; MI, myocardial infarction; NYHA, New York Heart Association; PTCA, percutaneous transluminal coronary angioplasty; qSOFA, quick Sequential Organ Failure Assessment; SBP, systolic blood pressure; SCr, serum creatinine; SOFA, Sequential Organ Failure Assessment; TPN, total parental nutrition; UO, urine output.
Analysis of factors associated with the possibility of dialysis withdrawal
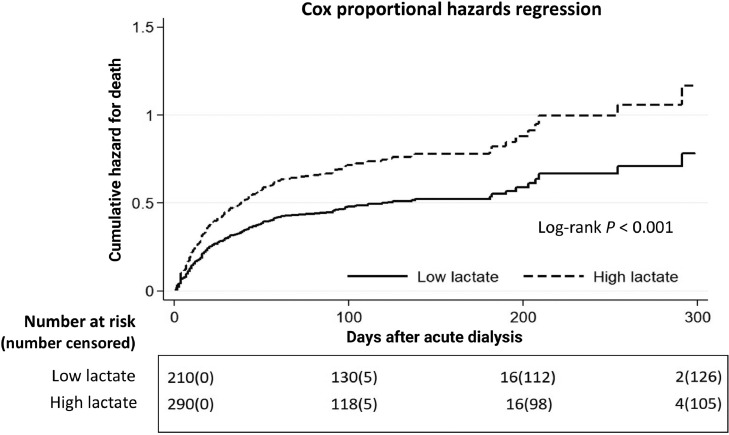
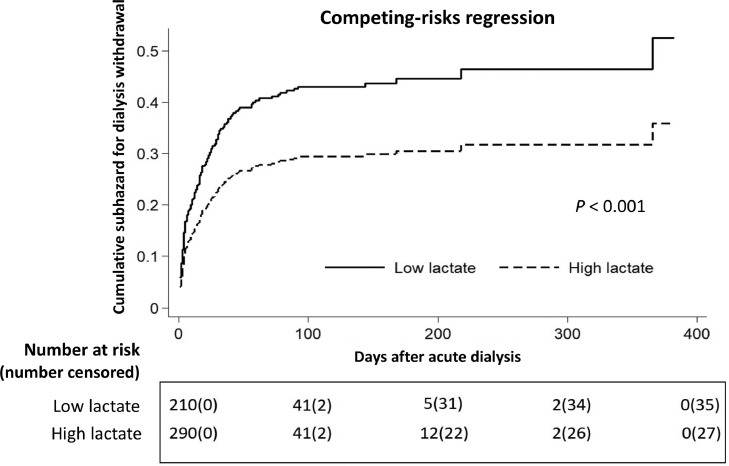
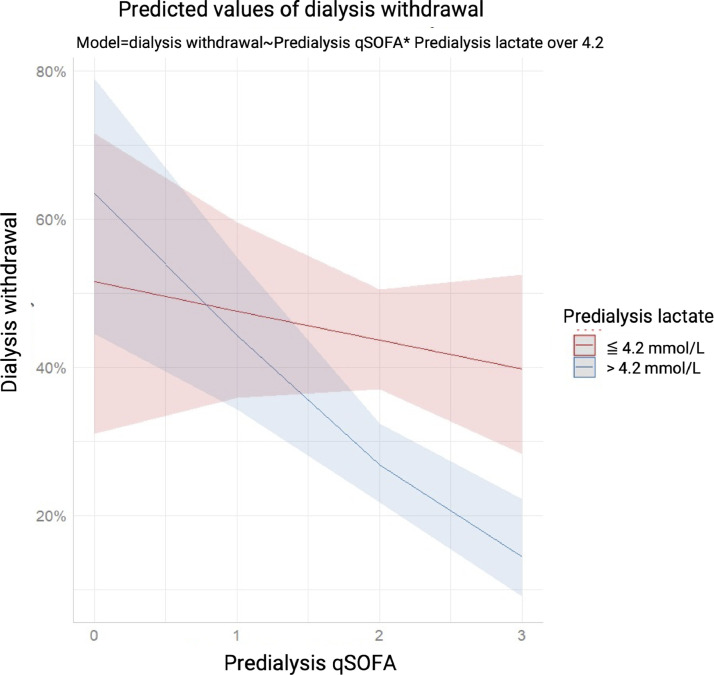
We also found that 12.4% of all of the patients and 26.3% of the survivors were dialysis dependent at the end of the study. Figure 2 illustrates stratified cumulative mortality rates according to pre-dialysis serum lactate level, and demonstrates that the low lactate group had a significantly lower cumulative mortality rate than the high lactate group (P < 0.001). In Fine-Gray subdistribution hazard multivariable analysis taking mortality as a competing risk, pre-dialysis daily UO (subdistribution hazard ratio [sHR] 1.28, P = 0.040), hgb (sHR 1.09, P = 0.043), Na (sHR 1.02, P = 0.045), lactate > 4.2 mmol/L (sHR 0.07, P = 0.009) and qSOFA score had independent prognostic significance for assessing the possibility of dialysis withdrawal. The sHRs for dialysis withdrawal calculated were severity dependent as per the stratification data of the pre-dialysis qSOFA scores and 0.14 (P = 0.001), 0.15 (P = 0.001), and 0.19 (P = 0.005) for qSOFA scores of 1, 2, and 3, respectively. We further examined the interaction between lactate > 4.2 and qSOFA score with regards to dialysis withdrawal. An attenuated trend of sHR was correlated with increasing qSOFA score in the patients with a serum lactate level > 4.2 mmol/L (Table 3). Figure 3 demonstrates the lower probability of dialysis withdrawal in the high lactate group compared to the low lactate group, taking mortality as a competing risk factor (P < 0.001). Figure 4 shows plots of marginal effects for the probability of dialysis withdrawal (y axis) against predialysis qSOFA score (x axis) according to a serum lactate level of > 4.2 mmol/L or ≦ 4.2 mmol/L. The interaction effects of lactate levels ≦ 4.2 mmol/L and > 4.2 mmol/L with regards to the possibility of dialysis withdrawal and the severity of pre-dialysis qSOFA level are shown in Figure 4. The crossed lines on the graph support that there was an interaction effect, for which the significant interactions between dialysis withdrawal and pre-dialysis qSOFA score could be specified. The graph shows that the dialysis withdrawal rate was 44.8% higher in the low lactate group compared to the high lactate group when the pre-dialysis qSOFA score was > 1.
Figure 2.
Cox proportional hazard plots stratified by pre-dialysis serum lactate level for assessing the probability of mortality. All relevant covariates, including characteristics, comorbidities and laboratory data at ICU admission, etiology of AKI, indication for dialysis, dialysis modality, qSOFA score, and plasma lactate level at dialysis, and some of their interactions including those listed in Table 1 were put on a selected variable list to predict the outcome of interest. Cumulative hazard for mortality rates differed significantly for patients with a high lactate level (lactate level > 4.2 mmol/L) and those with a low lactate level (lactate level ≦ 4.2 mmol/L) before dialysis (Log-rank P < 0.001).
Table 3.
Fine-Gray subdistribution hazard model depicting the possibility of dialysis withdrawal, taking mortality as a competing risk.
| Predictors | sHR with 95%CI | P-value |
|---|---|---|
| Demographic factors | ||
| Age | 0.99 (0.98–1.00) | 0.084 |
| Male gender | 1.03 (0.70–1.52) | 0.885 |
| Baseline SCr | 0.95 (0.87–1.05) | 0.351 |
| Smoking | 0.97 (0.62–1.51) | 0.889 |
| Hypertension | 0.99 (0.69–1.42) | 0.961 |
| Diabetes mellitus | 1.31 (0.90–1.91) | 0.157 |
| Coronary artery disease | 0.89 (0.62–1.28) | 0.531 |
| Charlson score | 0.95 (0.88–1.03) | 0.194 |
| History of PTCA with stents | 1.24 (0.54–2.83) | 0.607 |
| History of CABG | 1.11 (0.88–1.40) | 0.364 |
| ICU admission SOFA | 1.01 (0.95–1.08) | 0.765 |
| ICU admission qSOFA | 0.89 (0.68–1.17) | 0.409 |
| CRS superimposed with dialysis requiring AKI | ||
| Cardiogenic shock | 0.88 (0.52–1.47) | 0.617 |
| Sepsis | 1.00 (0.81–1.24) | 0.983 |
| Clinical parameters before dialysis | ||
| SBP, mmHg | 1.01 (1.00–1.01) | 0.141 |
| Daily UO (log), ml | 1.28 (1.01–1.61) | 0.040 |
| GCS | 1.05 (0.98–1.11) | 0.145 |
| Hgb, g/dL | 1.09 (1.00–1.18) | 0.043 |
| Na, mEq/L | 1.02 (1.00–1.04) | 0.045 |
| K, mEq/L | 1.20 (0.97–1.47) | 0.088 |
| Lactate > 4.2 mmol/L | 0.07 (0.01–0.50) | 0.009 |
| SOFA | 0.97 (0.88–1.07) | 0.528 |
| qSOFA | ||
| 1 | 0.14 (0.04–0.44) | 0.001 |
| 2 | 0.15 (0.05–0.45) | 0.001 |
| 3 | 0.19 (0.06–0.61) | 0.005 |
| qSOFA x high Lactate¶ | ||
| qSOFA = 1, high v.s. low Lacate | 17.36 (2.13–141.25) | 0.008 |
| qSOFA = 2, high v.s. low Lacate | 9.35 (1.13–77.63) | 0.038 |
| qSOFA = 3, high v.s. low Lacate | 6.17 (0.66–57.37) | 0.110 |
Abbreviations: AKI, acute kidney injury; CABG, coronary artery bypass graft; CRS, cardiorenal syndrome; FC, functional class; GCS, Glasgow coma scale; Hgb, hemoglobin; ICU, intensive care unit; MI, myocardial infarction; NYHA, New York Heart Association; PTCA, percutaneous transluminal coronary angioplasty; qSOFA, quick Sequential Organ Failure Assessment; SBP, systolic blood pressure; SCr, serum creatinine; SOFA, Sequential Organ Failure Assessment; TPN, total parental nutrition; UO, urine output.
Pre-dialysis lactate ≦ 4.2 mmol/L was defined as low lactate
Pre-dialysis lactate > 4.2 mmol/L was defined as high lactate.
Figure 3.
Cox proportional hazard plots stratified by pre-dialysis serum lactate level for assessing probability of dialysis withdrawal, taking mortality as a competing risk¶. All relevant covariates, including characteristics, comorbidities and laboratory data at ICU admission, etiology of AKI, indication for dialysis, dialysis modality, qSOFA score, and plasma lactate level at dialysis, and some of their interactions including those listed in Table 1 were put on a selected variable list to predict the outcome of interest. Cumulative subhazard for dialysis withdrawal differed significantly for patients with a high lactate level (lactate level > 4.2 mmol/L) and those with a low lactate level (lactate level ≦ 4.2 mmol/L) before dialysis (P < 0.001).
¶Pre-dialysis lactate level ≦ 4.2 mmol/L was defined as low lactate
Pre-dialysis lactate level > 4.2 mmol/L was defined as high lactate.
Figure 4.
Marginal effects of the interaction between probability of dialysis withdrawal and predialysis qSOFA score according to a high (> 4.2 mmol/L) or low (≦ 4.2 mmol/L) predialysis serum lactate level. The crossed blue and red lines supported that there was an interaction effect, in which an impressive interaction between the probability of dialysis withdrawal and predialysis qSOFA score was confirmed under the influence of a high and low lactate level. The graph showed that the probability of dialysis withdrawal was significantly higher for the acute dialysis patients with a pre-dialysis serum lactate level ≦ 4.2 mmol/L (P < 0.001).
Sensitivity analyses
Table 4 shows the E-values for the point estimate and lower limit of the CI for pre-dialytic qSOFA score and lactate for mortality, separately. This analysis indicated no substantial unmeasured confounding (E-values for the point estimates [lower limits of the CI] were 2.82 [1.00], 2.66 [1.00], 3.17 [1.00], and 2.32 [1.56] for qSOFA scores of 1, 2, 3, and lactate, respectively.
Table 4.
Incidence rate ratios and E-values of pre-dialytic qSOFA score and lactate for mortality in type 1 CRS patients.
| Parameter | Total | Event | Mean follow up days (SD) | Incidence rate/100,000 PY | Incidence rate ratio (95% CI) | E-value (E-value for lower limit of CI) |
|---|---|---|---|---|---|---|
| qSOFA = 1 | 103 | 30 | 107.13 (64.74) | 271.89 | 0.58 (0.18–2.99) | 2.82 (1.00) |
| qSOFA = 2 | 291 | 168 | 81.66 (74.17) | 706.98 | 1.52 (0.510–7.43) | 2.66 (1.00) |
| qSOFA = 3 | 100 | 63 | 77.61 (82.33) | 811.75 | 1.74 (0.570–8.68) | 3.17 (1.00) |
| Lactate | 288 | 180 | 74.56 (74.90) | 838.30 | 1.38 (1.147–1.65) | 2.32 (1.56) |
Abbreviations: qSOFA, quick Sequential Organ Failure Assessment.
Subgroup analysis
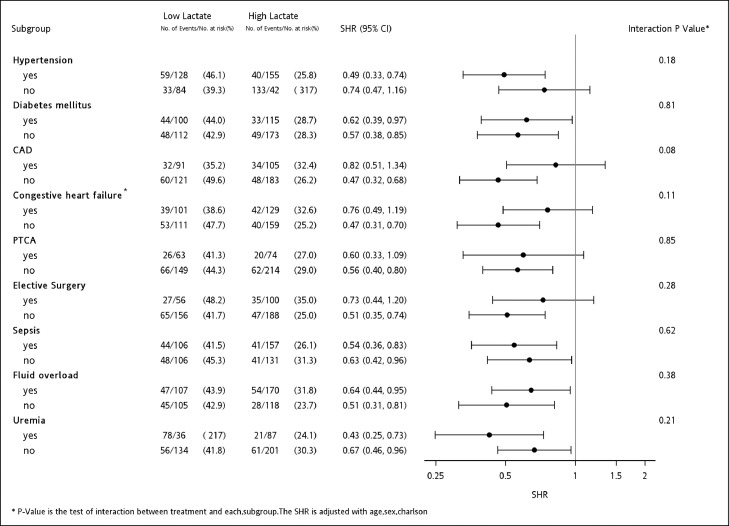
In subgroups analysis, the association between low lactate level and a lower risk of dialysis dependence remained consistent across diabetes, sepsis, fluid overload and uremia subgroups as the indication for initiating dialysis (P > 0.05), while this association was more significant in the patients with hypertension (P = 0.001) and without coronary artery disease (P < 0.001), severe congestive HF (NYHA functional class 2–4) (P < 0.001), history of PTCA (P = 0.001) or elective surgery (P < 0.001) (Figure 5).
Figure 5.
Forest plot depicting subgroup analysis of dialysis withdrawal compared with high and low pre-dialysis serum levels of lactate, taking mortality as a competing risk¶. The association between low lactate level and a lower risk of dialysis dependence remained consistent across diabetes, sepsis, fluid overload and uremia subgroups as the indication for initiating dialysis (P > 0.05), while this association was more significant in the patients with hypertension (P = 0.001) and without coronary artery disease (P < 0.001), severe congestive HF (NYHA functional class 2–4) (P < 0.001), history of PTCA (P = 0.001) or elective surgery (P < 0.001).
Abbreviations: CAD, coronary artery disease; PTCA, percutaneous transluminal coronary angioplasty;
¶ Pre-dialysis lactate level ≦ 4.2 mmol/L was defined as low lactate
Pre-dialysis lactate level > 4.2 mmol/L was defined as high lactate
*Congestive heart failure NYHA functional class ≥ 2.
External validation
The results were then validated using the NEP-AKI-D database. A total of 240 type 1 CRS patients requiring dialysis were enrolled. In this multi-center database, the incidence rates of mortality, dialysis dependence and dialysis withdrawal at 90 days were 60.4% (145/240), 11.3% (27/240), and 28.3% (68/240), respectively. Multivarible analysis indicated that pre-dialysis serum lactate level was an independent predictor of 90-day mortality (OR 2.276, P = 0.027) and dialysis withdrawal (sHR 0.490, P = 0.047) (Table S1–4).
Discussion
In this study, the 90-day mortality rate was 52.8% and the incidence rate of RRT dependence was 12.4% among type 1 CRS patents requiring dialysis for AKI. A lower pre-dialysis serum lactate level was associated with both a higher rate of survival and also a higher rate of dialysis withdrawal. Our results showed the considerable potential of serum lactate as a predictive biomarker for the probability of being successfully weaned from dialysis for AKI in type 1 CRS patents after considering death as a competing risk. We also showed that the interaction between qSOFA score and lactate level was associated with dialysis dependence in a disease severity-dependent manner. The results were consistent and further validated using multi-center registration data (Table S1–4). qSOFA score is a useful clinical tool for identifying infected patients likely to have life-threatening organ dysfunction. The third international consensus definitions for sepsis proposed that both qSOFA score and serum lactate levels are crucial clinical variables that can reflect the severity of acute illness in critically ill sepsis patients.22 Recently, Wagner et al. reported a correlation between qSOFA score and the risk of worse outcomes in critically ill patients with acute decompensated HF.36 We found that in addition to septic disease, the interaction between qSOFA and lactate level could predict the outcomes in type 1 CRS patients. In our model, less unmeasured confounding is needed to explain away the observed association (Table 4).
To estimate the occurrence of dialysis dependence, we used a competing risk model, which could more accurately estimate the risk of dialysis dependence while considering that a high percentage of patients may die before the end of the 90-day study follow-up period.37 The early recognition of patients at risk of RRT dependence and mortality may allow for timely and targeted interventions for these patients. Our results identified that daily UO, Hgb, Na, lactate > 4.2 mmol/L and qSOFA score determined before dialysis were independent predictors for the possibility of dialysis withdrawal (Table 3).
Lactic acid is produced in hypoxic conditions and high glycolytic activity38,39 Multiple inflammatory mechanisms are involved in the accumulation of lactate acid, including direct cellular injury, inflammation-induced injury, microcirculatory dysfunction, arterial hypotension and reduced oxygen extraction from surrounding tissues11,40 In type 1 CRS, the principal causes of kidney abnormalities are low cardiac output, decreased renal blood flow and secondary tubular hypoxic injury.41 The level of serum lactate may reflect both the severity of disease and also the degree of anaerobic metabolism, further leading to different renal outcomes in this clinical setting (Figure 4).40
Emerging evidences have indicated that AKI impacts the cytokine regulation and cellular immune system.42 Previous study had demonstrated that type 1 CRS with severe AKI were susceptible to infection43,44 In the current study, the prevalence of sepsis was 52.6%, which was consistent with that of the external validated database of type 1 CRS from the NEP-AKI-D study, that is a large number of representative AKI patients throughout Taiwan, including regional and medical centers. Our data in line with the evidence by Thakar et al. and Pistolesi et al. that the prevalence of sepsis was 58.5% and 52.5% in the setting of type 1 CRS with severe AKI requiring RRT43,45 The Third International Consensus Definitions for Sepsis and Septic Shock had proposed that elevated serum lactate levels represent an important marker of “cryptic shock” in the absence of hypotension, and the clinical diagnosis of septic shock should be based on the combination of hyperlactatemia with fluid-resistant hypotension.22 Recently, the association between the elevation of serum lactate levels and ventricular dysfunction in septic patients was reported.46 Nevertheless, to the best of our knowledge, there is still lacking of information about the prognostic value for hyperlactatemia in type 1 CRS patient. Our study demonstrated the patients with a high and low pre-dialysis serum lactate level had significantly different mortality rates and cumulative dialysis withdrawal rates (Table 2 and Figure 3). Moreover, we found that the associations between hyperlactatemia and dialysis dependence were consistent in the patients with and without sepsis (Figure 5), which signifies the generalizability of our findings.
In the literature, there is currently no general consensus on the correlation between exact lactate levels and poor prognosis.47 An increase in serum lactate > 2 mmol/L had been reported to play a key role in the diagnosis of cardiogenic shock and septic shock.47 We further found that a serum lactate level > 4.2 mmol/L could reflect the dynamic aspects of disease severity and provide superior information on the prognosis of our patients with type 1 CRS (Figure 1). The cut-off serum lactate value in our study is similar to the component of IABP-SHOCK II risk score of 5 mmol/L for severe cardiogenic shock.48 Cardiac dysfunction results in systemic congestion, which in turn causes absolute or relative arterial hypovolemia and upregulates the sympathetic system and the release of arginine vasopressin, thereby exacerbating positive fluid balance and further impairing cardiac function because of a progressive increase in cardiac filling pressure. Type 1 CRS patients are susceptible to a vicious circle of reduced cardiac performance and fluid overload.41,49 Lactate is a unique HF marker because it reflects the dynamic metabolic status of the patient rather than the static degree of myocardial damage or dysfunction (such as NT-proBNP or troponin I).50 Interestingly, the subgroup analysis in our study showed that the association between low lactate level and a lower risk of dialysis dependence was more prominent in the patients without coronary artery disease, severe congestive HF (NYHA functional class 2–4), history of PTCA or elective surgery. This finding suggests that serum lactate level may be more predictive in patients without a history of prior cardiovascular diseases (Figure 5).
In spite of the encouraging results observed in this study, the serum lactate level should only be used as a prognostic marker. The decision process for starting or avoiding RRT in AKI patient should be individualized. Furthermore, several potential limitations should also be recognized. First, the fact that our study involved patients of the same ethnicity limits the generalizability of the findings to other hospitals with different patient populations. Second, the predictive value of lactate clearance in the risk of mortality has been well documented in many clinical scenarios.12,39 However, serum lactate level was not measured sequentially in this study, and the role of sequential measurements of serum lactate with the highest and lowest record and lactate clearance may be more useful predictive markers than initial lactate alone. While the highest or lowest serum lactate levels did not have a specific time point of appearance, so it is difficult to apply clinically. Therefore, we recorded the serum lactate level at the time point before initializing of dialysis because that is the most critical time point of the patients. Thirdly, we also acknowledge that the observational nature of the study without a pre-specified protocol for the intervention cannot conclude causal relationships. Clinical decisions regarding when to wean from dialysis were not based on standardized criteria; rather, decisions were made by attending physicians according to their clinical judgment. However, this study was conducted by the team that have historically cooperated for over 10 years and collaborated on many research studies.13, 14, 15,51 This long-term collaboration may have partially reduced the heterogeneity in clinical practice styles with regards to ceasing and re-initiating dialysis. Finally, medication could be an essential causal factor for the prognosis of the patients. However, we did not record the information about the drugs in this study. Therefore, we can only speculate that serum lactate level may be a prognostic variable, and further studies are needed to validate our results. We recorded the worst SOFA score within 24 h before the initiation of dialysis. This approach is consistent with clinical practice, the daily SOFA or qSOFA score may not reflect the value immediately before the initiation of RRT.
More than half of the type 1 CRS patients in this study died and 34.8% were successfully weaned from dialysis for AKI. Our results showed that a serum lactate level > 4.2 mmol/L before dialysis could be considered as an independent risk factor for 90-day mortality and dialysis withdrawal, regardless of the presence or absence of sepsis. We suggested that serum lactate level is accurate and capable of forecasting the prognosis along with qSOFA severity for clinical decision-making for treating type 1 CRS patients.
Contributors
VCW contributed to the conception and study design. TMH, NKC, CHT, and TSL provided patient information and participated in the design. HCP, CYS, and FYY contributed to collecting data, data analysis and statistical analysis. CYS, YMC, and VCW provided supervision or mentorship. HCP, TMH, CYS, NKC, CHT, FYY, TSL, YMC, and VCW contributed to provide intellectual content of the work and were involved in editing and revising the manuscript. All authors discussed, contributed to, and approved the final manuscript version.
Declaration of interests
HCP, TMH, CYS, NKC, CHT, FYY, TSL, YMC, and VCW reported grants from Taiwan National Science Council, National Health Research Institutes, National Taiwan University Hospital, and Ministry of Science and Technology (MOST) of the Republic of China (Taiwan). No other disclosures were reported.
Acknowledgments
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The authors also thank all participants of NSARF and CAKs as well as the staff of the National Health Research Institute and Harvard Statistics. (The details of the members of CAKs can be downloaded here: http://links.lww.com/MD/B298).
Funding
This study was supported by grants from Taiwan National Science Council [104–2314-B-002–125-MY3,106–2314-B-002–166-MY3,107–2314-B-002–026-MY3], National Taiwan University Hospital [106-FTN20,106-P02, UN106–014, 106-S3582, 107-S3809, 107-T02, PC1246, VN109–09, 109-S4634, UN109–041], Ministry of Science and Technology of the Republic of China [MOST106–2321-B-182–002, 106-2314-B-182A-064, MOST107–2321-B-182–004, MOST 107-2314-B-182A-138, MOST108–2321-B-182–003,MOST109–2321-B-182–001, MOST 108-2314-B-182A-027] , Chang Gung Memorial Hospital [CMRPG-2G0361, CMRPG-2H0161, CMRPG-2J0261, CMRPG-2K0091], and Ministry of Health and Welfare of the Republic of China [PMRPG-2L0011].
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101232.
Appendix. Supplementary materials
References
- 1.Initiative ADQ. consensus group Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Europ Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronco C., Cicoira M., McCullough P.A. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60(12):1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 3.Li J., Sheng X., Cheng D., Wang F., Jian G., Li Y., et al. Is the mean platelet volume a predictive marker of a high in-hospital mortality of acute cardiorenal syndrome patients receiving continuous renal replacement therapy? Medicine (Baltimore) 2018;97(25) doi: 10.1097/MD.0000000000011180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shirakabe A., Hata N., Kobayashi N., Shinada T., Tomita K., Tsurumi M., et al. Long-term prognostic impact after acute kidney injury in patients with acute heart failure. Int Heart J. 2012;53(5):313–319. doi: 10.1536/ihj.53.313. [DOI] [PubMed] [Google Scholar]
- 5.Wellen K.E., Hotamisligil G.S. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dries D.L., Exner D.V., Domanski M.J., Greenberg B., Stevenson L.W. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35(3):681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 7.Wu B., Yan W., Li X., Kong X., Yu X., Zhu Y., et al. Initiation and cessation timing of renal replacement therapy in patients with type 1 cardiorenal syndrome: an observational study. Cardiorenal Med. 2017;7(2):118–127. doi: 10.1159/000454932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitaka C., Masuda T., Kido K., Uchida T., Abe S., Miyasho T., et al. Polymyxin B hemoperfusion prevents acute kidney injury in sepsis model. J Surg Res. 2016;201(1):59–68. doi: 10.1016/j.jss.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Metra M., Nodari S., Parrinello G., Specchia C., Brentana L., Rocca P., et al. The role of plasma biomarkers in acute heart failure. Serial changes and independent prognostic value of NT-proBNP and cardiac troponin-T. Eur J Heart Fail. 2007;9(8):776–786. doi: 10.1016/j.ejheart.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Wittayachamnankul B., Chentanakij B., Sruamsiri K., Chattipakorn N. The role of central venous oxygen saturation, blood lactate, and central venous-to-arterial carbon dioxide partial pressure difference as a goal and prognosis of sepsis treatment. J Crit Care. 2016;36:223–229. doi: 10.1016/j.jcrc.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Albright C.M., Ali T.N., Lopes V., Rouse D.J., Anderson B.L. Lactic acid measurement to identify risk of morbidity from sepsis in pregnancy. Am J Perinatol. 2015;32(05):481–486. doi: 10.1055/s-0034-1395477. [DOI] [PubMed] [Google Scholar]
- 12.da Hora Passos R., Ramos J.G.R., Gobatto A., Mendonça E.J.B., Miranda E.A., Dutra F.R.D., et al. Lactate clearance is associated with mortality in septic patients with acute kidney injury requiring continuous renal replacement therapy: a cohort study. Medicine (Baltimore) 2016;95(40) doi: 10.1097/MD.0000000000005112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu V.C., Ko W.J., Chang H.W., Chen Y.S., Chen Y.W., Chen Y.M., et al. Early renal replacement therapy in patients with postoperative acute liver failure associated with acute renal failure: effect on postoperative outcomes. J Am Coll Surg. 2007;205(2):266–276. doi: 10.1016/j.jamcollsurg.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Wu V.C., Ko W.J., Chang H.W., Chen Y.W., Lin Y.F., Shiao C.C., et al. Risk factors of early redialysis after weaning from postoperative acute renal replacement therapy. Intensive Care Med. 2008;34(1):101–108. doi: 10.1007/s00134-007-0813-x. [DOI] [PubMed] [Google Scholar]
- 15.Wu V.C., Young G.H., Huang P.H., Lo S.C., Wang K.C., Sun C.Y., et al. In acute kidney injury, indoxyl sulfate impairs human endothelial progenitor cells: modulation by statin. Angiogenesis. 2013;16(3):609–624. doi: 10.1007/s10456-013-9339-8. [DOI] [PubMed] [Google Scholar]
- 16.Cheng C.-.L., Lee C.-.H., Chen P.-.S., Li Y.-.H., Lin S.-.J., Yang Y.-.H.K. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol. 2014;24(6):500–507. doi: 10.2188/jea.JE20140076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiao C.C., Wu V.C., Li W.Y., Lin Y.F., Hu F.C., Young G.H., et al. Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care. 2009;13(5):R171. doi: 10.1186/cc8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu V.C., Wang C.H., Wang W.J., Lin Y.F., Hu F.C., Chen Y.W., et al. Sustained low-efficiency dialysis versus continuous veno-venous hemofiltration for postsurgical acute renal failure. Am J Surg. 2010;199(4):466–476. doi: 10.1016/j.amjsurg.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Huang T.M., Wu V.C., Young G.H., Lin Y.F., Shiao C.C., Wu P.C., et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol. 2011;22(1):156–163. doi: 10.1681/ASN.2010050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu V.C., Huang T.M., Lai C.F., Shiao C.C., Lin Y.F., Chu T.S., et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int. 2011;80(11):1222–1230. doi: 10.1038/ki.2011.259. [DOI] [PubMed] [Google Scholar]
- 21.Shu K.H., Wang C.H., Wu C.H., Huang T.M., Wu P.C., Lai C.H., et al. Urinary pi-glutathione S-transferase predicts advanced acute kidney injury following cardiovascular surgery. Sci Rep. 2016;6:26335. doi: 10.1038/srep26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pannu N., Nadim M.K. An overview of drug-induced acute kidney injury. Crit Care Med. 2008;36(4):S216–S223. doi: 10.1097/CCM.0b013e318168e375. [DOI] [PubMed] [Google Scholar]
- 24.Sakthirajan R., Dhanapriya J., Varghese A., Saravanakumar K., Dineshkumar T., Balasubramaniyan T., et al. Clinical profile and outcome of pigment-induced nephropathy. Clin Kidney J. 2018;11(3):348–352. doi: 10.1093/ckj/sfx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golshahi J., Nasri H., Gharipour M. Contrast-induced nephropathy: a literature review. J Nephropathol. 2014;3(2):51. doi: 10.12860/jnp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y.F., Ko W.J., Wu V.C., Chen Y.S., Chen Y.M., Hu F.C., et al. A modified sequential organ failure assessment score to predict hospital mortality of postoperative acute renal failure patients requiring renal replacement therapy. Blood Purif. 2008;26(6):547–554. doi: 10.1159/000178771. [DOI] [PubMed] [Google Scholar]
- 27.Shiao C.C., Ko W.J., Wu V.C., Huang T.M., Lai C.F., Lin Y.F., et al. U-curve association between timing of renal replacement therapy initiation and in-hospital mortality in postoperative acute kidney injury. PLoS ONE. 2012;7(8):e42952. doi: 10.1371/journal.pone.0042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kellum J.A. How can we define recovery after acute kidney injury? Considerations from epidemiology and clinical trial design. Nephron Clin Pract. 2014;127(1–4):81–88. doi: 10.1159/000363681. [DOI] [PubMed] [Google Scholar]
- 29.Shiao C.C., Wu P.C., Wu V.C., Lin J.H., Pan H.C., Yang Y.F., et al. Nationwide epidemiology and prognosis of dialysis-requiring acute kidney injury (NEP-AKI-D) study: design and methods. Nephrology. 2016;21(9):758–764. doi: 10.1111/nep.12670. [DOI] [PubMed] [Google Scholar]
- 30.Shiao C.-.C., Chang Y.-.H., Yang Y.-.F., Lin E.-.T., Pan H.-.C., Chang C.-.H., et al. Association between regional economic status and renal recovery of dialysis-requiring acute kidney injury among critically ill patients. Sci Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-71540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu V.C., Lo S.C., Chen Y.L., Huang P.H., Tsai C.T., Liang C.J., et al. Endothelial progenitor cells in primary aldosteronism: a biomarker of severity for aldosterone vasculopathy and prognosis. J Clin Endocrinol Metab. 2011;96(10):3175–3183. doi: 10.1210/jc.2011-1135. [DOI] [PubMed] [Google Scholar]
- 32.Wu V.C., Lai C.F., Shiao C.C., Lin Y.F., Wu P.C., Chao C.T., et al. Effect of diuretic use on 30-day postdialysis mortality in critically ill patients receiving acute dialysis. PLoS ONE. 2012;7(3):e30836. doi: 10.1371/journal.pone.0030836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hin L.Y., Lau T.K., Rogers M.S., Chang A.M. Dichotomization of continuous measurements using generalized additive modelling–application in predicting intrapartum caesarean delivery. Stat Med. 1999;18(9):1101–1110. doi: 10.1002/(sici)1097-0258(19990515)18:9<1101::aid-sim99>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Austin P.C., Latouche A., Fine J.P. A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat Med. 2020;39(2):103–113. doi: 10.1002/sim.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 36.Wagner T., Sinning C., Haumann J., Magnussen C., Blankenberg S., Reichenspurner H., et al. qSOFA score is useful to assess disease severity in patients with heart failure in the setting of a heart failure unit (HFU) Front Cardiovas Med. 2020;7 doi: 10.3389/fcvm.2020.574768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noordzij M., Leffondré K., van Stralen K.J., Zoccali C., Dekker F.W., Jager K.J. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dialysis Transplant. 2013;28(11):2670–2677. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 38.Pucino V., Cucchi D., Mauro C. Lactate transporters as therapeutic targets in cancer and inflammatory diseases. Expert Opin Ther Targets. 2018;22(9):735–743. doi: 10.1080/14728222.2018.1511706. [DOI] [PubMed] [Google Scholar]
- 39.Ryoo S.M., Lee J., Lee Y.-.S., Lee J.H., Lim K.S., Huh J.W., et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit Care Med. 2018;46(6):e489–e495. doi: 10.1097/CCM.0000000000003030. [DOI] [PubMed] [Google Scholar]
- 40.Prowle J.R., Kirwan C.J., Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10(1):37. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- 41.Valika A.A., Costanzo M.R. The acute cardiorenal syndrome type I: considerations on physiology, epidemiology, and therapy. Curr Heart Fail Rep. 2014;11(4):382–392. doi: 10.1007/s11897-014-0224-6. [DOI] [PubMed] [Google Scholar]
- 42.Bonavia A., Singbartl K. A review of the role of immune cells in acute kidney injury. Pediatr Nephrol. 2018;33(10):1629–1639. doi: 10.1007/s00467-017-3774-5. [DOI] [PubMed] [Google Scholar]
- 43.Thakar C.V., Yared J.-.P., Worley S., Cotman K., Paganini E.P. Renal dysfunction and serious infections after open-heart surgery. Kidney Int. 2003;64(1):239–246. doi: 10.1046/j.1523-1755.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosner M.H., Okusa M.D. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 45.Pistolesi V., Di Napoli A., Fiaccadori E., Zeppilli L., Polistena F., Sacco M.I., et al. Severe acute kidney injury following cardiac surgery: short-term outcomes in patients undergoing continuous renal replacement therapy (CRRT) J Nephrol. 2016;29(2):229–239. doi: 10.1007/s40620-015-0213-1. [DOI] [PubMed] [Google Scholar]
- 46.Innocenti F., Palmieri V., Stefanone V.T., D'Argenzio F., Cigana M., Montuori M., et al. Prognostic stratification in septic patients with overt and cryptic shock by speckle tracking echocardiography. Intern Emerg Med. 2020:1–8. doi: 10.1007/s11739-020-02545-3. [DOI] [PubMed] [Google Scholar]
- 47.Van Diepen S., Katz J.N., Albert N.M., Henry T.D., Jacobs A.K., Kapur N.K., et al. Contemporary management of cardiogenic shock: a scientific statement from the American heart association. Circulation. 2017;136(16):e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 48.Thiele H., Zeymer U., Thelemann N., Neumann F.-.J., Hausleiter J., Abdel-Wahab M., et al. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation. 2019;139(3):395–403. doi: 10.1161/CIRCULATIONAHA.118.038201. [DOI] [PubMed] [Google Scholar]
- 49.McCullough P.A., Kellum J.A., Haase M., Mueller C., Damman K., Murray P.T., et al. ADQI consensus on AKI biomarkers and cardiorenal syndromes. Karger Publishers; 2013. Pathophysiology of the cardiorenal syndromes: executive summary from the eleventh consensus conference of the acute dialysis quality initiative (ADQI) pp. 82–98. 182. [Google Scholar]
- 50.Biegus J., Zymliński R., Gajewski P., Sokolski M., Siwołowski P., Sokolska J., et al. Persistent hyperlactataemia is related to high rates of in-hospital adverse events and poor outcome in acute heart failure. Kardiol Pol. 2019;77(3):355–362. doi: 10.5603/KP.a2019.0030. [DOI] [PubMed] [Google Scholar]
- 51.Wu V.-.C., Wu C.-.H., Huang T.-.M., Wang C.-.Y., Lai C.-.F., Shiao C.-.C., et al. Long-term risk of coronary events after AKI. J American Soc Nephrol. 2014;25(3):595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.