Summary
Transforming growth factor-β (TGF-β) is a potent effector in the liver, which is involved in a plethora of processes initiated upon liver injury. TGF-β affects parenchymal, non-parenchymal, and inflammatory cells in a highly context-dependent manner. Its bioavailability is critical for a fast response to various insults. In the liver – and probably in other organs – this is made possible by the deposition of a large portion of TGF-β in the extracellular matrix as an inactivated precursor form termed latent TGF-β (L-TGF-β). Several matrisomal proteins participate in matrix deposition, latent complex stabilisation, and activation of L-TGF-β. Extracellular matrix protein 1 (ECM1) was recently identified as a critical factor in maintaining the latency of deposited L-TGF-β in the healthy liver. Indeed, its depletion causes spontaneous TGF-β signalling activation with deleterious effects on liver architecture and function. This review article presents the current knowledge on intracellular L-TGF-β complex formation, secretion, matrix deposition, and activation and describes the proteins and processes involved. Further, we emphasise the therapeutic potential of toning down L-TGF-β activation in liver fibrosis and liver cancer.
Keywords: Latent TGF-β, ECM1, TGF-β activation, TGF-β signalling, Liver disease
Abbreviations: α-SMA, alpha-smooth muscle actin; BMDCs, bone marrow-derived dendritic cells; BMPs, bone morphogenetic proteins; cFn, cellular fibronectin; Co-Smad, co-mediator Smad; cRGD, cyclic arginine-glycine-aspartic acid peptide; ECM, extracellular matrix; ECM1, extracellular matrix protein 1; rECM1, recombinant ECM1 protein; HCC, hepatocellular carcinoma; HSCs, hepatic stellate cells; I-Smad, inhibitory Smad; LAP, latency-associated peptide; LLC, large latent complex; LTBP, latent TGF-β binding protein; L-TGF-β, latent transforming growth factor β; MMP, matrix metalloproteinase; PAI-1, plasminogen activator inhibitor-1; pFn, plasma fibronectin; RGD, arginine-glycine-aspartic acid; ROS, reactive oxygen species; R-Smad, receptor-regulated Smad; SLC, small latent complex; TGF-β, transforming growth factor β; TIMP-1, tissue inhibitor of metalloproteinase-1; TSP, thrombospondin
Key points.
-
•
Transforming growth factor-β (TGF-β) signalling from the cell surface to the nucleus is regulated at multiple levels, starting from ligand activation, receptor complex formation, endocytosis, and recycling, via downstream pathway branching and co-factor recruitment, to target gene promoter binding.
-
•
The pro-TGF-β dimer is cleaved into the TGF-β ligand and the latency-associated peptide (LAP) to form the small latent complex (SLC) via non-covalent interactions. The SLC is disulphide-bonded to the latent TGF-β binding protein (LTBP) to form the large latent complex (LLC). The LLC is secreted and stored as latent TGF-β (L-TGF-β) in the extracellular matrix via the LTBP.
-
•
L-TGF-β activation is mediated by several signals, including integrins, thrombospondin, proteases, as well as compounds and chemicals which act through proteolysis or induction of structural alterations, all resulting in dissociation of the LAP.
-
•
Extracellular matrix protein 1 (ECM1) protects L-TGF-β from integrin-mediated activation by competitively binding to the arginine-glycine-aspartic acid (RGD) motif present in most integrins.
-
•
ECM1 depletion in the liver leads to robust spontaneous L-TGF-β activation, the release of the active ligand, and massive R-Smads activation in target cells, especially hepatic stellate cells, leading to progressive fibrosis and rapid mortality, indicating ECM1 as a gatekeeper for homeostasis in a healthy liver.
TGF-β signalling pathway
Transforming growth factor β (TGF-β) was identified as an inducer of anchorage-independent growth1 and as the first member of the largest family of secreted morphogens in mammals. TGF-β family members are potent multifunctional cytokines that modulate many cellular processes, including cell differentiation and fate decisions of epithelial, mesenchymal, and inflammatory cell types; proliferation; extracellular matrix synthesis; production of inflammatory and fibrogenic cytokines/chemokines; tumorigenesis; and metastasis.2 The TGF-β family, comprising at least 33 different members, is divided into distinct classes, based on biological function: TGF-β ligands, activins, inhibins, bone morphogenetic proteins (BMPs), nodal, Müllerian-inhibiting substance,3,4 and growth differentiation factors (Fig. 1A). Perturbation of TGF-β family signalling may cause or facilitate various diseases, such as fibrosis, connective tissue, skeletal, cardiovascular, or autoimmune diseases, and finally, cancer.4 Upon activation, the secreted protein ligands exert their cellular function via transmembrane type I and type II serine/threonine kinase receptors5 and, canonically, intracellular Smad transcription factors.6
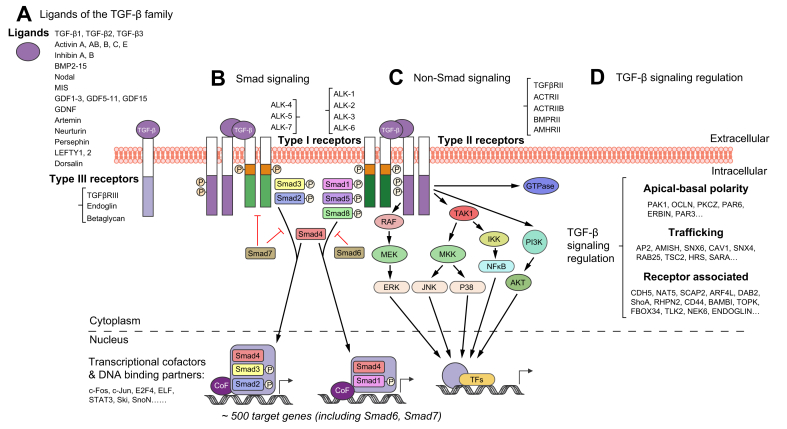
Fig. 1.
The TGF-β signalling pathway.
The scheme comprises different ligands and receptors of the TGF-β family, canonical Smad and non-canonical signalling pathways, and the network of regulatory interactions at multiple levels from the cell surface to the nucleus. (A) TGF-β family members, including TGF-β1, TGF-β2, TGF-β3, activins, inhibins, BMPs, nodal, MIS, and GDFs, initiate multiple cellular responses and cell fate decisions. (B) Secreted as protein ligands in latent and inactive forms, upon activation they may be presented by type III receptors (TGFβRIII or Endoglin) lacking kinase activity to the signalling receptor complex, consisting of heteromeric type II and type I receptors. Ligand binding to transmembrane type II receptors (e.g. TGFβRII, ACTRII, BMPRII) leads to autophosphorylation, recruitment and phosphorylation of type I receptor kinases (e.g. ALK-4, ALK-5, ALK-7 and ALK-1, ALK-2), thus forming a ligand-receptor complex to phosphorylate and activate the canonical Smad signalling pathway. Activated R-Smads (Smad2, Smad3 and Smad1, Smad5, Smad8, can be phosphorylated by ALK-4, ALK-5, ALK-7 and ALK-1, ALK-2, respectively) form hetero-oligomers with Co-Smads (Smad4), and the heteromeric complexes then translocate into the nucleus, where, in conjunction with variant co-factors and DNA-binding partners, such as c-Fos, c-Jun, ELF, or Ski, SnoN, and CEBP, they modulate chromatin decondensation and regulate target gene transcription. The I-Smads Smad6 and Smad7, which are direct transcriptional targets of Smad signalling, form crucial negative feedback loops to TGF-β signalling. (C) Non-Smad TGFβR signalling pathways are also frequently involved in regulating intracellular TGF-β signalling. TAK1 plays a central role, potentially coordinating the activation of MAPK signalling, including JNK, p38 MAPK, and NF-κB signalling. MEK-ERK, PI3K-AKT and some Rho family members like GTPases were also identified in the large TGF-β signalling crosstalk. (D) TGF-β signalling regulation occurs at multiple levels in extracellular and intracellular spaces. Thereby, the core components of the TGF-β-Smad signalling pathway are embedded within a huge network of hierarchical protein-protein interactions, leading to regulatory nodes at multiple levels. BMPs, bone morphogenetic proteins; Co-Smad, co-mediator Smad; I-Smad, inhibitory Smad; GDFs, growth differentiation factors; MIS, Müllerian-inhibiting substance; R-Smad, receptor-regulated Smad; TGF-β, transforming growth factor β.
TGF-β family cellular signal transduction is principally mediated by a linear pathway including ligand binding to the type II receptor, recruitment of the type I receptor kinase, activation of signalling mediators (Smads),7,8 translocation of Smad complexes into the nucleus, and regulation of target gene transcription.[9], [10], [11] Intracellular signal transduction by TGF-β family ligands is initiated by the binding and heteromeric formation of type II and type I receptors.5,12,13 In many cases, the ligand-receptor complex includes another less described type III receptor, endoglin or TβRIII, that displays no kinase activity but facilitates ligand binding to the TGF-βR type II.14 Receptor serine/threonine kinases currently comprise 12 members in the human genome – 7 type I and 5 type II receptors.15 TGF-β ligand binding to its receptors at the plasma membrane may elicit multiple cellular responses.5 Activated receptor complexes are usually internalised for signalling and subsequently undergo either proteasomal degradation or recycling to the cell surface.16,17 These processes have a significant impact on the signal duration and the cellular response. Mechanistically, ligand binding induces a conformational change in the type II receptor, leading to autophosphorylation, recruitment of the respective receptor I kinase, and phosphorylation of the latter on a characteristic SGSGSG sequence of its GS domain.5 This large active ligand-receptor complex initiates canonical signalling through phosphorylation of Smads.
Smad proteins consist of three classes with distinct functions, the receptor-regulated Smads (R-Smads), the co-mediator Smads (Co-Smads), and the inhibitory Smads (I-Smads). Ligand-activated type I kinase receptors phosphorylate R-Smads (Smad1, 2, 3, 5, and 8) at C-terminal serines. Phosphorylated R-Smads form hetero-oligomers with Co-Smads (Smad4).7 The activated heteromeric complexes then translocate into the nucleus, where they bind nuclear co-activators or co-repressors to direct chromatin decondensation and regulate target gene transcription in a context-dependent manner (Fig. 1B). Finally, the Smads may interact with a plethora of DNA-binding partners to initiate gene promoter activation or repression. Among the multiple potential target genes of Smad signalling are the I-Smads, Smad6 and Smad7.18,19 Both form negative feedback loops, inhibiting R-Smad activation and mediating proteasomal degradation of activated receptor complexes by recruiting the E3 ubiquitin ligases Smad ubiquitin regulatory factors 1/2 (Smurf 1/2) to the TGF-β-receptor complex.20 In addition, I-Smads interfere with R-Smad and Co-Smad interactions,21 thus preventing transcriptionally active complex formation.
Besides the canonical Smad pathway, TGF-β family ligands may also directly affect other major intracellular signalling mediators, including JNK, p38, ERK, PI3K-AKT, TAK1-IKK-NFκB, and Rho family GTPases22,23 (Fig. 1C). Activated by ligand-receptor complexes, non-Smad signalling transducers affect downstream cellular responses either by themselves or in crosstalk with the canonical Smad pathway.24 Non-Smad pathways significantly contribute to the complexity of TGF-β effects, providing multiple options for intracellular signal branching and mutual interactions at regulatory nodes, resulting in a strongly context-dependent cellular response22 (Fig. 1C-D).
Thus, although the TGF-β signalling pathway seems simple and linear, we know today that embedding the core components of the canonical TGF-β-Smad pathway within an extensive network of hierarchical protein-protein interactions leads to regulatory nodes at multiple levels that allow for substantial and highly context-dependent versatility.16,25 For example, at the receptor level, accessory receptors such as TGF-βRIII and endoglin facilitate ligand binding. Furthermore, TGF-β receptor trafficking has also emerged as an essential regulator of Smad signalling,26 indicating that the availability and activity of numerous components of the endosomal and trafficking system may have a critical impact. Finally, TGF-β signalling has cell type-specific outcomes. For example, TGF-β receptors modulate apical-basal polarity through Par6 and other components of the polarity complex in epithelial cell types, a feature that is disturbed during epithelial-to-mesenchymal transition (Fig. 1D). In fibroblasts, TGF-β drives cellular activation, in immune cells it is principally anti-inflammatory, whereas its effect on endothelial cells is pro-angiogenic.27
The present review will focus on the regulation of ligand bioavailability by intracellular and extracellular mechanisms at the TGF-β family protein level. We will describe the mechanisms and interactions that facilitate or interfere with the conversion of extracellular matrix (ECM)-deposited latent TGF-β (L-TGF-β) into an active signalling ligand (Table 1, Table 2).
Table 1.
Compounds, nucleic acids and peptides described as extracellular modulators of TGF-β signalling activation (in the order of appearance).
| Molecules/compounds | Function | Ref. |
|---|---|---|
| MIR100HG | Long noncoding RNA, controls the magnitude of TGF-β signalling in carcinomas | 59 |
| GM6001 | Metalloprotease inhibitor, abrogates αvβ8-mediated TGF-β activation in airway fibroblasts and SW480 cells | 86,98 |
| c8 | Synthetic compound with inhibitory activity on αvβ1 integrin and downstream L-TGF-β activation in fibroblasts | 103 |
| CWHM12 | Small molecule inhibitor of αv integrins, potential anti-fibrotic effects | 105 |
| LSKL | Peptide that blocks TSP-1-mediated TGF-β signalling activation | 118 |
| KRFK | TSP-1-derived TGF-β1 activating peptide | 118 |
| NDMA | Organic chemical, induction of hepatic fibrosis in mouse model | 131 |
| LAP-DP | Valuable biomarkers for monitoring L-TGF-β activation and the clinical course of chronic liver diseases | 139 |
| cRGD | Synthetic peptide blocking the interaction of αv integrin and L-TGF-β | 182 |
LAP, latency-associated peptide; LAP-DP, LAP degradation product; L-TGF-β, latent TGF-β; NDMA, N-nitrosodimethylamine; TGF-β, transforming growth factor β; TSP-1, thrombospondin 1.
Table 2.
Physiological proteins able to modulate extracellular TGF-β signalling activation (in the order of appearance).
| Physiological proteins | Function | Ref. |
|---|---|---|
| Fibrillin-1 | ECM structural protein, facilitates the deposition of L-TGF-β | 63 |
| Fibronectin | ECM structural protein, facilitates the deposition of L-TGF-β | 73,74 |
| Decorin | Storage of TGF-β in the ECM, decreases TGF-β activity | 75,76 |
| rhDecorin | Inhibition of TGF-β activity in HSCs | 79 |
| αv Integrins | Cell adhesion molecules, activate L-TGF-β and downstream signalling | 85,88 |
| Thrombospondin | ECM protein, activates L-TGF-β | 114,116 |
| ADAMTS | Facilitates the activation of L-TGF-β in HSCs | 123 |
| ADAMTS16 | Activates L-TGF-β in cardiac fibroblasts | 124 |
| MMPs | Facilitate the activation of L-TGF-β | 126,127,131 |
| BMP1/tolloid MMPs | Regulate L-TGF-β activation via cleavage of LTBP | 134 |
| Kallikreins | Facilitate the activation of L-TGF-β | 136,137 |
| Prostate-specific antigen | Specifically activates L-TGF-β2 in prostate cells | 140 |
| Plasmin | Regulates the activation of L-TGF-β in co-cultures of pericytes and endothelial cells, in retinoid-treated endothelial cells, and on activated macrophages surfaces | 141,142,[145], [146], [147] |
| ECM1 | Inhibits αv integrin-mediated L-TGF-β activation | 179,183 |
| rECM1 | Inhibitory effect on L-TGF-β activation in BMDCs and HSCs | 179 |
ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; BMDCs, bone marrow-derived dendritic cells; BMP1, bone morphogenetic protein 1; ECM, extracellular matrix; ECM1, extracellular matrix protein 1; HSCs, hepatic stellate cells; L-TGF-β, latent TGF-β; MMPs, matrix metalloproteinases; rECM1, recombinant ECM1; rhDecorin, recombinant human Decorin; TGF-β, transforming growth factor β.
Intracellular formation and secretion of the latent TGF-β complex
TGF-β family mRNA is translated into pre-pro-TGF-β protein, which is transported into the endoplasmic reticulum (ER) after cleavage of the N-terminal signal peptide, where two pro-TGF-β polypeptide chains fold into a disulphide-bonded dimer.28 Upon trafficking into the trans-Golgi, furin or furin-like proteases remove the mature TGF-β family ligand by proteolytic cleavage from the pro-polypeptide, which, however, remains non-covalently associated[29], [30], [31], [32] (Fig. 2A). The dimeric complex consisting of the pro-peptides (latency-associated peptide [LAP]) and the TGF-β dimer is termed the small latent complex (SLC).30,33 The non-covalent association of the components in the SLC involves extensive but reversible structural changes in the LAP.34 Three cysteines in LAP are required for diverse functional interactions, Cys-223, Cys-225, and Cys-33. When transfected with a LAP plasmid encoding serine instead of cysteine at positions 223 and 225, cells express monomeric precursor proteins and release already activated TGF-β, suggesting that dimerisation of LAP is necessary for latency35 and that Cys-223 and -225 are required for interchain disulphide bond formation.
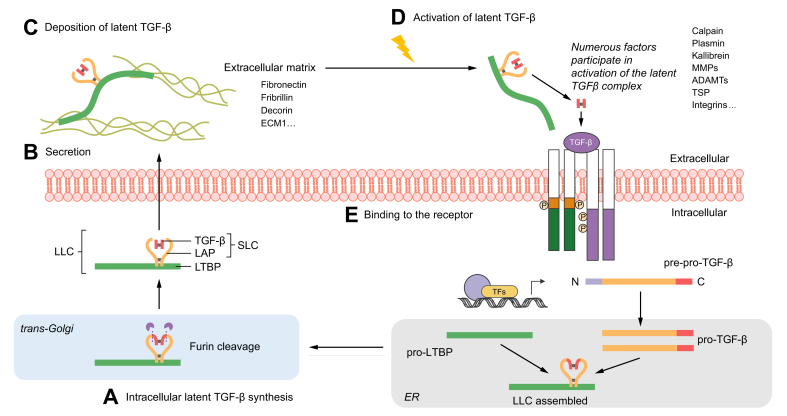
Fig. 2.
Graphical summary of TGF-β protein production, secretion, matrix deposition, and maturation.
(A) (starting at the bottom) After translation of pre-pro-TGF-β, the N-terminal signal peptide is cleaved to yield pro-TGF-β which translocates into the ER (lower right), where pro-TGF-β molecules dimerise and form disulphide bonds to LTBP, forming a ternary complex. The TGF-β dimer is cleaved from its pro-peptide in the trans-Golgi (lower left) but remains strongly associated with the LAP via non-covalent interactions. TGF-β and LAP form the SLC, which together with LTBP comprise the LLC. (B) Once secreted (middle left), the latent TGF-β is deposited into the extracellular matrix (C) (upper left), thanks to the high affinity of LTBP to various matrix components, such as fibronectin, fibrillin, and decorin. (D) (upper right) Numerous factors, like physiological proteins including proteases, thrombospondin and integrins, physical conditions and chemicals, including ROS and lactic acid, participate in activation of latent TGF-β, leading to the release of the mature TGF-β signalling ligand. The active ligand then binds to the cell surface receptors (E) (middle right) to regulate a wide variety of downstream signalling pathways and cellular responses.28 ER, endoplasmic reticulum; LAP, latency-associated peptide; LLC, large latent complex; LTBP, latent TGF-β binding protein; ROS, reactive oxygen species; SLC, small latent complex; TGF-β, transforming growth factor β.
The SLC in the ER is also covalently linked to the L-TGF-β binding protein (LTBP) via another pair of disulphide bonds, now between LTBP and LAP (Cys-33).[36], [37], [38] LTBPs are large multidomain glycoproteins that interact with fibrillin microfibrils in the ECM and build bridges with other matrix proteins, contributing to microfibril organisation and elastic fibre assembly.36,39 There are 4 LTBP isoforms (LTBP-1, -2, -3, and -4) described in the human genome, with various affinities for LAPs.40 LTBP-1 and LTBP-3 bind well to all three TGF-β isoforms; LTBP-4 binds only weakly to TGF-β1-LAP, whereas LTBP-2 does not bind at all. The tripartite complex of TGF-β, LAP, and LTBP is called large latent complex (LLC). LTBPs and LAP, thus play important roles in maintaining TGF-β latency.
LTBP isoforms are widely expressed in the normal and diseased human liver, as shown in liver specimens from patients with hepatitis B, hepatitis C, primary biliary cholangitis, and primary sclerosing cholangitis, and in cultured human liver myofibroblasts.41,42 Upregulated expression levels of LTBP proteins (e.g., LTBP-1) were described in cirrhosis and chronic hepatitis C.43,44 Splice variants of LTBPs have also been described. LTBP-1D is less sensitive to proteolytic degradation, thus protecting TGF-β from activation, and could be an important modulator of the biological activity of TGF-β in normal and diseased liver.42 Ltbp1 knockout (KO) mice exhibit reduced TGF-β activity and are less prone to hepatic fibrogenesis. Further, gene expression profiling of cultured hepatic stellate cells (HSCs) confirmed that Ltbp1 KO cells are less receptive to cellular activation and less prone to transdifferentiation into myofibroblasts, which indicates that LTBP-1 has essential functions in TGF-β-mediated HSC activation.45 In hepatocellular carcinoma (HCC), mRNA analysis and immunostaining showed that while LTBP-1 was upregulated, LTBP-1D was not detectable in the matrix.46 LTBP-1 in circulating plasma has thus been suggested as a novel biomarker for the early detection of HCC.43 Aryl hydrocarbon receptor (AhR) signalling negatively regulates LTBP-1 expression.47 Myofibroblasts from Ahr KO mice overexpress LTBP-1 and secrete 4-fold more active TGF-β than those from wild-type mice.48 mRNA and protein levels of LTBP-2 are significantly upregulated in HCC tissues compared to adjacent non-tumour tissue. Similarly, patients with HCC display higher serum LTBP-2 levels than controls, in line with the tumour’s malignant degree, progression, differentiation, size, and stage, and with hepatitis virus infection, suggesting that LTBP-2 has prognostic implications in patients with HCC.49,50 LTBP-4 exists as two N-terminally distinct isoforms, LTBP-4S (short) and LTBP-4L (long). The liver mainly expresses LTBP-4L. LTBP-4L is secreted as a complex with TGF-β1, whereas most LTBP-4S is free. Moreover, LTBP-4S is incorporated into the ECM, whereas LTBP-4L was not readily detectable in the ECM.51 LTBP-4 is present in muscle and was originally identified from a genome-wide search for genetic modifiers of muscular dystrophy in mice. Two different alleles were found: one, containing a 12 amino acid insertion in the hinge region, had an enhanced affinity for L-TGF-β and led to reduced muscle fibrosis. LTBP-4 protein lacking those 12 amino acids is more susceptible to proteolysis and L-TGF-β activation, further supporting the important role of the hinge region. Moreover, a monoclonal human anti-LTBP-4 antibody directed towards the hinge region bound LTBP-4, inhibited proteolytic cleavage, and was beneficial in a mouse model of muscular dystrophy. Combining treatment with anti-LTBP-4 plus prednisone, the standard of care treatment for Duchenne muscular dystrophy, further improved muscle function and protected against injury in mice.52
TGF-β and its isoforms are principally secreted from cells in the latent and inactive form, either as SLC or LLC (Fig. 2B). After the cleavage process in the trans-Golgi, the LLC is prepared for secretion. LTBPs serve as chaperones to enhance the folding and secretion of pro-TGF-β.53 While SLC secretion has sometimes been observed, it takes longer than for LLC due to the lack of LTBPs.53,54 Without LTBPs, most of the SLC stays in the Golgi apparatus in a latent and immature form.55 Tryptic digestion confirmed that without LTBPs, the precursor dimer contains improper disulphide bonding.53 Furthermore, transgenic mice with a LAP carrying a substitution of Cys-33, the cysteine required for the interaction with LTBP, exhibit an inflammatory and tumorigenic phenotype similar to Tgfb1 null mice.56 The secretion of LTBP-3 also depends on binding to TGF-β, whereas LTBP-1, LTBP-2, and LTBP-4 can be secreted without linkage to TGF-β.32,57 Secretion of L-TGF-β was also linked to autophagy since it was abrogated in fibroblasts and macrophages with depleted RHOA (autophagic component).58 Additionally, a long noncoding RNA, MIR100HG, induced by TGF-β, facilitates L-TGF-β1 secretion and controls the magnitude of TGF-β signalling, especially in carcinomas.59
Deposition of the latent TGF-β complex in the extracellular matrix
The secreted LLC is rapidly deposited in the ECM via the association of LTBPs with ECM proteins.60 Immunofluorescence studies revealed two ECM-binding regions of LTBP-1, one at the N-terminus and another at the C-terminus.61 In addition, interaction studies showed that LTBPs bind to fibrillin fibres, fibronectin, and decorin (Fig. 2C).
Fibrillin-1 is the main ECM structural protein in humans and comprises 43 calcium-binding epidermal growth factor-like domains, 7 TGF-β binding protein-like domains, and an integrin-binding region.62 The C-terminal domains of LTBP-1, LTBP-2, and LTBP-4 contain flexible linkers for binding to the N-terminus of fibrillin.63 LTBP-3 does not interact with fibrillin.63,64 In most cases, the assembly of the LLC with fibrillin fibres requires fibronectin, suggesting that a fibrillin/fibronectin network is responsible for the incorporation of LTBP/L-TGF-β into the ECM.65,66
Fibronectin (Fn) is an ECM glycoprotein that influences cell adhesion, migration, growth, and differentiation; it is particularly important in vertebrate development.[67], [68], [69] Fn is strongly expressed by multiple cell types and displays a profusion of interactions with collagens, integrins, fibrin, syndecans, and heparan sulphate proteoglycans, thus playing a crucial role in assembling complex fibrillar networks. Fn contains an arginine-glycine-aspartic acid (RGD) motif, the most common sequence required for cell attachment to the ECM,70 which supports its central position in fibrillogenesis through binding to αv-class integrins.69,71 Functional Fn is usually a dimer of 220 to 250 kDa subunits, covalently linked at their C-termini by a pair of disulphide bonds. Fn possesses two aggregated forms, the soluble plasma FN (pFn), and the insoluble cellular Fn (cFn). pFn is mainly synthesised in the liver by hepatocytes and circulates in the blood, while cFn is secreted by various cell types, including fibroblasts and HSCs.72 Upon synthesis and secretion, cFn joins the ECM. Colocalisation of Fn and LTBP-1 was documented by double immunofluorescence.73,74 Biochemical investigations indicate that Fn associates with a specific sequence of the LTBP-1 hinge domain, mediates localisation of the LLC into the ECM for storage, and is also required for subsequent activation by integrin αvβ6.
Decorin (DCN) is a small chondroitin-dermatan sulphate proteoglycan composed of a core protein with a single glycosaminoglycan chain near the N-terminus, 12 leucine-rich repeats, and N-glycosylation at three sites. The protein sequence comprises ten 24 amino acid repeats with a large proportion of leucine, also present in two other members of the DCN family, biglycan and fibromodulin. Decorin and biglycan do not bind L-TGF-β1, while all tested proteoglycans of the DCN family can bind the active forms of 3 TGF-β isoforms.75 Collagenase treatment induces the release of biologically active TGF-β1/2 from collagen-bound decorin, indicating that decorin participates in the storage of TGF-β in the ECM.76 Decorin decreases TGF-β activities in two ways, by sequestering the activated ligand into the ECM and by competitive inhibition of TGF-β binding to its receptors.75 In vivo, DCN plays a protective role in liver fibrogenesis insofar as its genetic ablation in mice leads to enhanced matrix deposition, impaired matrix degradation, and activation of HSCs. TGF-β1 exerts a more potent effect when DCN is absent.77 Blunting TGF-β1 in the Decorin KO setting is sufficient to reduce spontaneous collagen gel contraction activity, further confirming a regulatory interaction between decorin and TGF-β1.78 Recombinant human decorin (rhDecorin) treatment inhibits the proliferation of TGF-β1-stimulated LX-2 cells, as well as the expression of metalloproteinase-2 (MMP-2), tissue inhibitor of metalloproteinase-1 (TIMP-1), alpha-smooth muscle actin (α-SMA), collagen type III, and Smad2 phosphorylation.79 However, intrinsic expression of decorin along with TGF-β1 in liver tissue of 43 patients with chronic hepatitis and cirrhosis did not prevent liver fibrosis progression.80 Overall, decorin is a highly specific natural inhibitor of TGF-β.81
Activation of TGF-β signalling ligands from ECM storage
As mentioned, TGF-β is present in a latent complex in the ECM until a specific signal triggers its release.82 Several L-TGF-β regulators exist, including integrins, thrombospondin, proteases, and other compounds and chemicals which act via proteolysis or by altering the binding and dissociation of LAP (Fig. 2D-E).
Integrins are transmembrane cell adhesion molecules. Heterodimeric receptors, consisting of α and β subunits on the outside of the cell surface, they link the ECM with the intracellular actin cytoskeleton and regulate specific signal transduction cascades.83 Hence, they mediate communications between the ECM and cells, for instance, to trigger tissue fibrosis initiation, maintenance, and resolution. They possess the RGD tripeptide binding domain required for their interaction with L-TGF-β.[84], [85], [86], [87], [88], [89], [90] We currently know 24 integrin heterodimers. Eight of these, namely αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, α5β1, α8β1, and αIIbβ3, can bind the RGD motif. Mice deficient in integrin subunit genes Itgb3, Itgb5, or Itgb6, and selectively deficient in Itgb8 in haemopoietic cells are still sensitive to carbon tetrachloride (CCl4)-induced experimental liver fibrosis in mice, whereas mice with myofibroblasts deficient in the Itgav gene are protected.85 Exaggerated inflammatory diseases develop in the lungs and skin of mice with alterations in the integrin β6 subunit.91 Inhibition of αvβ6 and αvβ8 reproduces the developmental phenotype of mice deficient in TGF-β1 and TGF-β3,92 suggesting that these two integrins are required for most of these TGF-β isoforms’ functions during development. Although, in principle, all αv integrins have the potential to activate L-TGF-β, mice deficient in αvβ3 or αvβ5 do not phenocopy TGF-β1 deficiency. Both are weaker binding integrins but may activate TGF-β1 signalling to some extent in vitro.93,94
L-TGF-β activation by αvβ6 integrins requires LAP’s RGD motif and the integrin’s cytoplasmic sequences that anchor the actin cytoskeleton. Upon secretion, cell surface-bound integrin provides cell contractile forces to the L-TGF-β complex via binding to the LAP’s RGD motif. Since LTBP-1 – part of the complex – is attached to the stiff ECM, the binding of integrin creates counter traction deforming LAP, thereby facilitating the release of mature TGF-β.[95], [96], [97] This mechanism was experimentally confirmed by contracting myofibroblast cytoskeletons and integrins or anti-LAP antibody-coated ferromagnetic beads in a cell-free matrix.96 The activation of L-TGF-β by αvβ8 is different from αvβ6. αvβ8 activates L-TGF-β by presenting LAP complexes to cell surface matrix metalloproteinases (MMPs) for degradation, subsequently releasing TGF-β. αvβ8-mediated activation can be blocked by an MMP inhibitor (GM6001) and does not need integrin’s cytoplasmic domain, suggesting a traction-independent, probably protease-dependent mechanism involving the recruitment of MMPs to facilitate LAP cleavage.86,98 The physiological relevance of integrin-mediated L-TGF-β activation was confirmed in mice, where an RGE sequence was used to replace the RGD motif in TGF-β1-LAP, resulting in a phenotype similar to Tgfb1 KO mice with multiorgan inflammation and lack of epidermal Langerhans cells.92,99 The integrin-binding RGD sequence is thus essential for both αvβ6 and αvβ8 integrin-mediated L-TGF-β activation.
L-TGF-β activation by integrins is an important cell fate decision mechanism during development and in response to TGF-β-driven organ diseases, especially fibrosis,100 wherein depletion of integrins in mouse models attenuates disease severity in the liver, lungs, and kidneys.85,101 Integrin αvβ6 mRNA is increased in patients with fibrotic liver disease secondary to various aetiologies (primary biliary cholangitis, alcohol-induced liver disease, hepatitis B and C), and a correlation with fibrosis stage has been documented for hepatitis C.102 In contrast, no protective effect on liver fibrogenesis was found in the liver of αvβ6-deficient mice. c8, a synthetic compound with inhibitory activity on αvβ1 integrin, can interfere with the adhesion of TGF-β1 LAP to fibroblasts.103 Systemic delivery of c8 reduces CCl4-mediated liver fibrosis in mice.89 Current knowledge on the role of integrins in the induction of TGF-β activity suggests that RGD binding could be a promising target for antifibrotic therapies. CWHM12, a small molecule integrin antagonist that simultaneously and selectively targets several profibrogenic integrins, improved liver, lung, muscle, and pancreatic fibrosis in mouse injury models.104,105 Recently, hepatic steatosis, liver injury, inflammation, and fibrogenesis due to long-term high-fat diet feeding were decreased by CWHM12 treatment.106 Extensive in vitro assays using a two-cell integrin-dependent TGF-β reporter system could discriminate L-TGF-β activation mechanisms in TGF-β1, 2, and 3, suggesting that the latent forms of TGF-β2 and TGF-β3 can be activated by integrin-independent mechanisms, which is not the case for TGF-β1. The presence of integrin further increased TGF-β3-dependent reporter activity by another 51%, which was blunted by mutating the RGD motif. Since TGF-β2 lacks an RGD motif, its activation is probably completely independent of integrin. Further, the furin cleavage sites conserved in all three TGF-β isoforms are required for integrin-dependent as well as for integrin-independent L-TGF-β activation. Finally, it has been shown that LAP1 more efficiently inhibits receptor binding of all three TGF-β isoforms than LAP2 or LAP3, suggesting that the respective LAP domains are involved in the observed intrinsic activities of TGF-β2 and TGF-β3.107
Thrombospondin (TSP) is a secreted matrisomal protein deposited in the ECM or bound to cellular receptors found in connective tissues and platelets. During tissue injury and repair its expression is rapidly induced by growth factors, including TGF-β, leading to the conversion of L-TGF-β to its biologically active mature form through a non-proteolytic mechanism.108,109 This action, confirmed in different environments like in solution, at the cell surface, or in the extracellular milieu,110 is relevant to diverse health conditions, including wound healing, immune responses, renal fibrosis, diabetes, myocardial infarction, tumour progression, and experimental autoimmune uveoretinitis.111 TSPs are disulphide-linked homotrimers (subgroup A, comprising TSP-1 and TSP-2) or homopentamers (subgroup B, comprising TSP-3, TSP-4, TSP-5).112,113 The interaction between TSPs and TGF-β is specific to TSP subgroup A members.111 The WSHWSPW motif in the second thrombospondin type 1 repeat (TSR) of TSP-1 and TSP-2 can bind to VLAL motifs present in both TGF-β1 and the LAP,114,115 thus localising inactive TGF-β1 at specific sites within the ECM or in proximity to cell surfaces.111 Another interaction between a KRFK sequence of the second TSR and a conserved LSKL motif in the LAP can activate all three mammalian TGF-β isoforms by triggering their release from the SLC.114,116 TSP-2 cannot activate TGF-β due to the lack of a KRFK motif but can competitively antagonise TSP-1-mediated TGF-β activation.114,117
Colony formation assays showed that TSP is similarly efficient in activating the large (platelet-derived) and small forms of L-TGF-β. Gel permeation chromatography suggested a direct binding of TSP to the LAP. Furthermore, a polyclonal antibody specific for the N-terminal region of the LAP inhibits TSP-mediated activation of L-TGF-β.109 Young Tgfb1 KO and Tsp1 KO mice exhibit remarkably similar histological abnormalities in 9 organ systems, suggesting that TSP-1 is also largely responsible for TGF-β1 activation in vivo, in contrast to many other binding partners involved in storage and activation of L-TGF-β. Systemic treatment with a peptide (LSKL) blocking the activation of TGF-β1 by TSP-1 is sufficient to induce pathologies in the lungs and pancreas that are similar to those observed in Tgfb1 KO mice. In contrast, when using a TSP-1 peptide (KRFK) that activates TGF-β1, biologically active TGF-β can be detected in situ, and the abnormalities are reverted.118 In addition, the TSP-1 derived from antigen-presenting cells that activates L-TGF-β2 is essential for the adaptive regulatory immune response induced by TGF-β2-expressing antigen-presenting cells and subsequent peripheral immune tolerance in TGF-β2-expressing tissues.119
TSP-1 is expressed in quiescent and activated cultured HSCs, and to a minor extent in Kupffer cells and liver sinusoidal endothelial cells in normal livers, but not in hepatocytes. Platelet-derived growth factor BB, a profibrogenic mediator, and to a minor extent TNFα, induce TSP-1 expression in HSCs, thus enhancing TSP-1-dependent TGF-β/Smad signalling. In HSCs, an LSKL peptide, specifically blunting the interaction between TSP-1 and L-TGF-β or antibodies against TSP-1, similarly inhibits the activation of L-TGF-β and active TGF-β’s downstream effects.120 Liver TSP-1 levels were increased in experimental obese and insulin-resistant NAFLD/NASH mouse models and in obese patients with NASH. Tsp1 deletion in adipocytes does not protect mice from diet-induced NAFLD/NASH, whereas myeloid/macrophage-specific Tsp1 deletion protects mice against liver injury. Autocrine-acting macrophage-derived TSP-1 suppresses SMPDL3B expression, thereby increasing Toll-like receptor 4-mediated proinflammatory signalling and promoting NAFLD progression.121 Recently, BMP-1 was shown to be an activator of TSP-1 and an agonist of TGF-β signalling.122 Besides acting as a vital regulator of TGF-β activation, TSP also has TGF-β independent biological functions, e.g., on haemostasis, growth factor regulation, cell adhesion, migration, vascular barrier function, inflammation, inhibition of angiogenesis, and nitric oxide signalling through association with multiple integrins and other receptors.111
Several proteases belonging to different categories can activate L-TGF-β in vitro, including cysteine proteases like ADAMTS, and numerous serine proteases like MMPs, kallikreins, and plasmin. However, the relevance of these various proteases for L-TGF-β activation in vivo is unclear, as mice lacking individual proteases fail to show Tgfb KO phenotypes, perhaps due to functional redundancy.
A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) may activate L-TGF-β leading to increased expression of TGF-β target genes in HSCs, which themselves produce more ADAMTS1 when activated. ADAMTS1 expression is also upregulated in mice with CCl4-induced liver fibrosis in parallel with type 1 collagen.123 Another protease of this family, ADAMTS16, may activate L-TGF-β in cardiac fibroblasts via its RRFR-motif.124
Matrix metalloproteinases (MMPs) are calcium-dependent zinc-containing endopeptidases.125 Despite documented activity on L-TGF-β, their actual physiological impact on TGFβ activation is not clear-cut. When presented to the tumour cell surfaces via CD44 during angiogenesis, MMP2 and MMP9 activate L-TGF-β.126 MMP3 from chondrocyte-derived vesicles can activate small L-TGF-β1,127 whereas MMP2, also present in the same vesicles, cannot. None of the many mice lacking these proteases clearly showed Tgfb KO phenotypes. MMP3 inactivation produces mice with no obvious abnormalities,128 and mice lacking MMP13 develop only minor skeletal defects.129 Mice lacking both MMP2 and MMP9 expression can survive,130 like mice lacking MMP9 and MMP13, although these mice are very small because of the defects in their growth plates.129 Levels of activated TGF-β and the fibrogenic markers connective tissue growth factor, α-SMA, type I collagen, and TIMP-1 are significantly decreased in Mmp13 KO mice compared to WT mice following N-nitrosodimethylamine treatment.131 Mice deficient in MMP14 display osteopenia, dwarfism, and fibrosis, but this is likely due to collagen turnover deficiencies rather than insufficient TGF-β activation.132 MMP2- and MMP14-deficient mice suffer from respiratory failure, abnormal vascular development, immature muscle fibres, and die soon after birth.133 However, it is difficult to match these phenotypes to defective TGF-β signalling. BMP1/tolloid MMPs also regulate L-TGF-β activation. Rather than degrading LAP directly, they promote the cleavage of LTBP-1 from the ECM, thereby freeing L-TGF-β bound to LTBP-1, which subsequently requires activation by other mediators, like MMPs. Bmp1 KO mice with additional depletion of the closely related tolloid1 (Tll1) protease exhibit excessive deposition of LTBP-1 and reduced positive staining of phospho-Smad2/3 in embryonic tissues, suggesting a defect in L-TGF-β activation.134 Mice deficient in both BMP1 and Tll1 expression die before birth.135 However, their impacts on various other matrix and signalling molecules, and their importance for L-TGF-β activation in vivo, warrant further investigations.
Kallikreins can activate L-TGF-β, for example, in the context of TGF-β-mediated immunosuppression136 and downstream of lipopolysaccharide during liver regeneration.137 TGF-β1 LAP can be cleaved by plasma kallikrein between Arg-58 and Leu-59. The LAP degradation products (LAP-DP) are increased in liver tissue of patients with hepatic fibrosis.138 Similarly, in patients with chronic liver diseases, Arg-58/LAP-DP were found around the sinusoids of fibrous regions (their levels were higher at fibrosis stage F1 than in more advanced stages). Similar observations in the plasma of patients suggest that plasma kallikrein-mediated TGF-β activation is an early step in liver fibrogenesis and that Arg-58 and Leu-59/LAP-DPs are valuable biomarkers for monitoring the clinical course of chronic liver diseases.139 The authors also observed significantly higher expression of tissue LAP-DP in liver samples of from patients with NAFLD compared to patients with HCV-induced liver fibrosis, indicating a potential role of aetiology. Prostate-specific antigen, another kallikrein family member, may activate L-TGF-β2, but not L-TGF-β1.140
Plasmin regulates TGF-β activation in co-cultures of pericytes and endothelial cells,141,142 an activation abrogated by antibodies against LTBP-1143 and inhibitors of the mannose-6-phosphate receptor.144 Similarly, plasmin activates L-TGF-β in cultures of retinoid-treated endothelial cells145 and on the surface of activated macrophages.146,147 The activation by macrophages depends on plasmin and L-TGF-β bound to the cell surface by TSP1 and CD36.148
Other binding proteins, including F-spondin,149 neuropilin,150,151 pregnancy-specific glycoprotein 1,152 tenascin-X,153 neuraminidase,154,155 and cadherin-11156 also, under certain conditions, have been identified as promoters of L-TGF-β activation, but more in-depth analyses are required. m-calpain‘s 80 kDa subunit, which requires chondroitinase ABC-sensitive proteoglycan for attachment to cell surfaces, may also activate L-TGF-β independently of plasmin.157
Activation by physical conditions and chemicals compounds
Exposure to specific physical or chemical conditions, such as detergents, chaotropic agents, ionising or UV radiation,158,159 reactive oxygen species (ROS),160 heat,161 physical shear stress,162 and extreme pH163 can activate L-TGF-β. For example, in cell culture and cell-free systems, ROS activate L-TGF-β efficiently.160 ROS also seem to regulate L-TGF-β activation caused by ionising160 and UV-B radiation.159 Low pH-mediated L-TGF-β activation may play a physiological role in some circumstances. For example, extracellular pH is reduced to 4.5 by osteoclasts during bone resorption, which leads to L-TGF-β activation.164 Lactic acid might promote TGF-β activity at specific concentrations and therefore has a physiological function in idiopathic pulmonary fibrosis.165 Physical shear or stirring can facilitate the activation of L-TGF-β released by platelets, depending on LTBP binding to LAP.162 Although there is convincing evidence that many physicochemical factors play essential roles in TGF-β activation, the detailed biological mechanisms are still unclear.
ECM1 – a new player in latent TGF-β activation
Extracellular matrix protein 1 (ECM1) is an 85-kDa glycoprotein166 widely distributed in human tissues. It is present within the epidermis and the dermis,167 where it maintains skin integrity and homeostasis.168 ECM1 dysfunction may result in two skin diseases,169 lipoid proteinosis (a rare autosomal recessive genodermatosis) and lichen sclerosus (a common acquired inflammatory skin disorder). Both have common clinicopathological features,169 including skin hyperkeratosis, a hyaline appearance of the papillary dermis, and disruption of basement membranes. In addition, ECM1 can also regulate endochondral bone formation170 or enhance endothelial cell proliferation.171 Most ECM1 functions are mediated via interaction with various extracellular and structural proteins, such as perlecan,172 fibulin-1C/D,173 and MMPs (e.g., MMP9).174 Specifically, ECM1 interacts via its C-terminal domain with the epidermal growth factor-like modules flanking the LG2 subdomain of perlecan’s domain V.172 Disruption of this association seems to be responsible for some pathological features observed in lipoid proteinosis and lichen sclerosus.172,175 ECM1’s second tandem repeat domain interacts with the main interactive domain of fibulin-1D and the C-terminal module of fibulin-1C.173 Fibulin-1D binding to ECM1 has been suggested to reduce endothelial cell proliferation and angiogenesis, thus reducing tumour transformation and invasion.176,177 ECM1’s C-terminal tandem repeat 2 interacts with MMP9, thereby inhibiting its proteolytic activity in vitro.174 In addition, ECM1 also interacts with αv integrin, one of the aforementioned physiological regulators of L-TGF-β activation.178 ECM1 inhibits differentiation of IL-17-producing T helper (Th17) cells and the development of experimental autoimmune encephalomyelitis through interference with αv integrin-mediated L-TGF-β activation,179 suggesting clinical importance in multiple sclerosis. Furthermore, ECM1 levels are increased in most malignant epithelial tumours180 and in tumours with metastases,177 indicating ECM1 involvement during cancer development.
Most evidence of crosstalk between ECM1 and TGF-β came from immunology. In order to elucidate the communication between ECM1 and L-TGF-β, Su et al. tested Tgfb mRNA expression in bone marrow-derived dendritic cells (BMDCs) grown in the presence of recombinant ECM1 protein (rECM1).179 Tgfb mRNA expression did not significantly change in BMDCs in the presence or absence of rECM1. However, when BMDCs in culture were treated with rECM1 protein and L-TGF-β, the level of active TGF-β in the culture supernatant was significantly reduced, as measured with a reporter cell line (HEK293T-cells stably transfected with a plasminogen activator inhibitor-1 (PAI1) promoter luciferase plasmid181). For the first time, these results indicated that ECM1 has an inhibitory effect on L-TGF-β activation. Th17 cell differentiation was induced during the co-culture of dendritic cells and CD4+ T cells in the presence of IL-6, IL-23, IL-1β, and one of IgG, ECM1, or cyclic arginine-glycine-aspartic acid peptide (cRGD). TGF-β or L-TGF-β were then added to the co-culture system. The Th17 cell proportion – determined by intracellular cytokine staining for IL-17 and FACS analyses – was significantly reduced by adding rECM1 to the culture system in the presence of L-TGF-β but did not change in the presence of active TGF-β. rECM1 has a similar inhibitory effect to cRGD, which inhibits L-TGF-β activation by blocking the interaction of αv integrin and L-TGF-β.182 Levels of active TGF-β in the supernatant decreased in the presence of rECM1, suggesting that ECM1 can suppress TGF-β maturation and Th17 cell differentiation as effectively as cRGD. Co-immunoprecipitation experiments identified a strong interaction between ECM1 and αv integrin, the latter being expressed on the surface of dendritic cells. When αv integrin was silenced in BMDCs, ECM1 could not inhibit Th17 differentiation in the co-culture system. Furthermore, in protein-protein interaction competition assays, ECM1 competed with the RGD peptide for αv integrin binding. ECM1 was thus identified as an inhibitor of L-TGF-β activation via binding to αv integrin, affecting TGF-β-driven Th17 cell differentiation.
We recently showed for the first time that ECM1 expression is a critical gatekeeper in the healthy liver, contributing to normal architecture and physiological homeostasis of cell-cell communication.183 Hepatocyte-specific Ecm1 KO leads to spontaneous and severe liver fibrosis. Via interaction with the αv integrin, ECM1 is critical for maintaining ECM-deposited TGF-β in its latent form (Fig. 3). Indeed, Ecm1 KO mice develop severe liver damage and fibrosis and die at between 8 and 12 weeks of age. Real time-PCR results showed no significant differences in Tgfb mRNA levels between Ecm1 KO and WT mice. However, in Ecm1 KO mice, TGF-β signalling activation was dramatically induced, as evidenced by strongly upregulated levels of Smad3 phosphorylation. Immunoprecipitation and immunofluorescent staining confirmed that – like in inflammatory cells – ECM1, αv integrin, and L-TGF-β interact and co-localise in the hepatic sinusoids. We tested TGF-β activation in vitro in the different liver cell types isolated from Ecm1 WT and KO mice. Co-culturing them with NIH3T3-TGF-β signalling reporter cells stably transfected with the PAI1-promoter-based luciferase plasmid, in the presence or absence of rECM1 or CWHM12 – an αv integrin inhibitor – showed that TGF-β is expressed and secreted primarily from HSCs and Kupffer cells, and that the amount of active TGF-β is much higher in cells isolated from Ecm1 KO mice. As with CWHM12 treatment, the addition of rECM1 significantly reduced active TGF-β levels. These data suggest that ECM1 also affects L-TGF-β in the liver, which translates into HSC activation and progressive fibrosis. Significantly, injecting Ecm1 KO mice with a vector expressing a soluble TGF-β type II receptor led to a decrease in collagen deposition, ACTA2-positive HSCs, and hydroxyproline levels and to a more or less normal liver architecture, rescuing the liver damage phenotype. Taken together, hepatic ECM1 depletion leads to spontaneous L-TGF-β activation and release of the active ligand, resulting in strongly enhanced TGF-β signalling, especially in HSCs, and is responsible for the onset of hepatic fibrosis in mice.
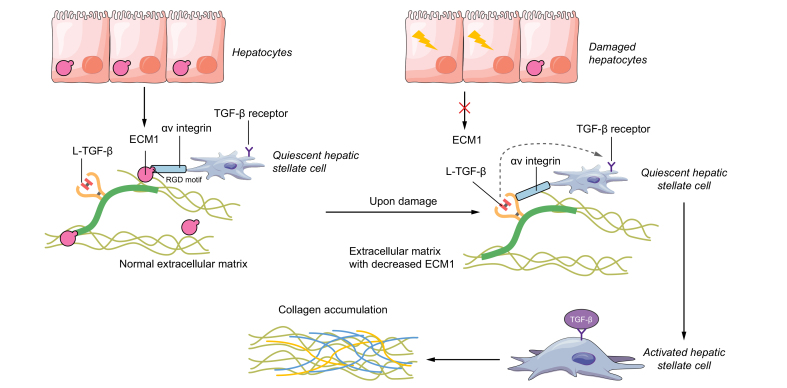
Fig. 3.
Scheme showing how ECM1 sequesters the latent TGF-β from integrin activation.
We recently found that ECM1, a glycoprotein in the extracellular matrix of the liver, is critical in maintaining normal architecture and physiological homeostasis. Under normal conditions, ECM1 binds to the RGD motif, which commonly exists in the LAP and most integrins, thus inhibiting the activation between integrins and latent TGF-β. The presence of ECM1 is critical to keep extracellular matrix-deposited TGF-β in a latent and quiescent form, preventing HSC activation and liver fibrosis. Conversely, depleting the liver of ECM1 results in spontaneous release of active TGF-β ligand from the latent complex, leading to strongly enhanced TGF-β signalling, HSC activation, collagen accumulation, ultimately promoting spontaneous architectural disturbances and severe liver fibrosis. ECM1, extracellular matrix protein 1; HSC, hepatic stellate cell; LAP, latency-associated peptide; L-TGF-β, latent TGF-β; RGD, arginine-glycine-aspartic acid; TGF-β, transforming growth factor β.
Therapeutic strategies targeting TGF-β signalling
The profibrogenic, anti-inflammatory, and pro-oncogenic impact of the TGF-β signalling pathway has encouraged the development of targeted therapeutic approaches for fibrosis and cancer. Tools have been generated to interfere with various levels of the TGF-β signalling cascade, including antisense oligonucleotides,184,185 TGF-β ligand-targeting antibodies (e.g. fresolimumab186 and 1D11187), ligand traps such as soluble TGF-β receptor II or AVID200,188 as well as small molecule inhibitors directed to the internal part of the signalling receptors27 or targeting Smad3 (SIS3).189,190
Results from preclinical studies targeting TGF-β in fibrosis or cancer have been very promising.191,192 However, translating these approaches for clinical use has been and remains a challenge. Indeed, TGF-β signalling is critical for maintaining tissue homeostasis, and TGF-β displays pleiotropic effects. In addition to the “diseased” target cells, healthy tissues can be compromised by systemic or organ-wide delivery of TGF-β signalling-directed drugs, which can and frequently do lead to unwanted side effects and safety concerns.
To overcome these drawbacks and tap the full potential of TGF-β as a therapeutic target while increasing safety and efficacy, we need i) a better understanding of the molecular mechanisms by which TGF-β signalling controls homeostatic and disease processes, ii) predictive biomarkers from tissue and blood signatures to stratify patients that may benefit from TGF-β targeting,193,194 iii) careful dosing or pulsatile treatment windows, and iv) a selection of potential targets from specific branches of TGF-β signalling pathways as is nicely summarised in the review article of ten Dijke and colleagues.27 Targeting the mechanisms underpinning L-TGF-β activation is one such approach for a more fine-tuned interference with TGF-β. For example, EMD527040, a selective antibody against αvβ6 integrin, blocks the activation of L-TGF-β and inhibits fibrogenesis in bile duct ligated rats and Mdr2 KO mice.195 Similarly, antibodies against αvβ6 or αvβ8 integrin also display anti-tumour activity in preclinical mouse models through suppression of TGF-β signalling,196,197 and clinical translation for patients with cancer is currently envisaged. Small molecules to target L-TGF-β integrin interactions are also available. For example, the cyclic RGD pentapeptide cilengitide (EMD121974) was efficiently used in several preclinical models of solid tumours198 and is now being tested in clinical studies in patients with cancer, as summarised in ten Dijke’s review.27 Furthermore, the small molecule CWHM12 pharmacologically blocks αv-containing integrins, thus attenuating fibrosis in the liver and lung.85 We hypothesise that delivery of ECM1 protein to the chronically damaged liver may be another promising approach that can delay the process of disease progression without major side effects.
Outlook
The bioavailability of TGF-β and TGF-β family members is just one of the multiple levels of regulation to fine-tune initiation, strength, duration, branching, crosstalk, and target gene regulation of TGF-β family signalling. Multiple major components can modulate TGF-β signalling in complex and diverse ways, as indicated in the Snapshot on TGF-β signalling regulation by Wrana and Taylor.25 This complexity is the primary reason for the highly context-dependent impacts of activated TGF-β signalling on cell types and the difficulties of developing specific therapeutics that do not induce unmanageable side effects. Our current knowledge on TGF-β bioavailability shows that, at least in the liver, a significant amount of L-TGF-β is deposited in the ECM and can be activated immediately without the need for de novo synthesis. We can assume that this will also be true for other tissues, although that must be proven. Thus, mRNA measurements might, in many cases, reflect a second wave of pathophysiological responses, e.g., to replenish the emptied ECM deposits. It also seems that such an available reservoir of L-TGF-β is required for a spatially restricted response, e.g., at the place of injury, where immediate action of the cytokine might be necessary. In such cases, local activation is controlled by changes in the cell-cell and cell-ECM communication that lead to direct interactions between the L-TGF-β, structural proteins of the ECM, and components located at the cell surface of neighbouring cells. Proteolytic activity and mechanical forces will lead to L-TGF-β activation and cell signalling initiation. Importantly, local interference with L-TGF-β activation might be a safer therapeutic strategy than targeting the activated ligand, since it may tone down instead of totally blunting the effects of TGF-β.
Financial support
This work was supported by the Federal Ministry of Education and Research (BMBF) Programs [Liver Systems Medicine (LiSyM), grant number PTJ-031L0043 and LiSyM-HCC, grant number PTJ-031L0257A; to SD], Deutsche Forschungsgemeinschaft (DFG) [grant number DO 373/20-1 SD; to SW], [grant number DO 373/19-1 SD; to YL].
Authors’ contributions
YL, FL, SW, and SD wrote the original draft. YL, WF, SW, and SD reviewed and edited the manuscript. SW and SD share the co-corresponding authorship.
Conflict of interest
The authors declare that they have no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
We thank Nicolas Gambardella for support in editing the manuscript.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100397.
Contributor Information
Sai Wang, Email: sai.wang@medma.uni-heidelberg.de.
Steven Dooley, Email: steven.dooley@medma.uni-heidelberg.de.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Roberts A.B., Anzano M.A., Lamb L.C., Smith J.M., Sporn M.B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981;78:5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taipale J., Saharinen J., Keski-Oja J. Extracellular matrix-associated transforming growth factor-beta: role in cancer cell growth and invasion. Adv Cancer Res. 1998;75:87–134. doi: 10.1016/s0065-230x(08)60740-x. [DOI] [PubMed] [Google Scholar]
- 3.Laiho M., Keski-Oja J. Transforming growth factors-beta as regulators of cellular growth and phenotype. Crit Rev Oncog. 1992;3:1–26. [PubMed] [Google Scholar]
- 4.Morikawa M., Derynck R., Miyazono K. TGF-beta and the TGF-beta family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 6.Nickel J., Ten Dijke P., Mueller T.D. TGF-beta family co-receptor function and signaling. Acta Biochim Biophys Sin (Shanghai) 2018;50:12–36. doi: 10.1093/abbs/gmx126. [DOI] [PubMed] [Google Scholar]
- 7.Heldin C.H., Miyazono K., ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 8.Huse M., Muir T.W., Xu L., Chen Y.G., Kuriyan J., Massague J. The TGF beta receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell. 2001;8:671–682. doi: 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 9.Itoh S., Itoh F., Goumans M.J., Ten Dijke P. Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- 10.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 11.Moustakas A., Souchelnytskyi S., Heldin C.H. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 12.Franzen P., ten Dijke P., Ichijo H., Yamashita H., Schulz P., Heldin C.H., et al. Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. Cell. 1993;75:681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- 13.ten Dijke P., Franzen P., Yamashita H., Ichijo H., Heldin C.H., Miyazono K. Serine/threonine kinase receptors. Prog Growth Factor Res. 1994;5:55–72. doi: 10.1016/0955-2235(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 14.Vander Ark A., Cao J., Li X. TGF-beta receptors: in and beyond TGF-beta signaling. Cell Signal. 2018;52:112–120. doi: 10.1016/j.cellsig.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 16.Derynck R., Budi E.H. Specificity, versatility, and control of TGF-beta family signaling. Sci Signal. 2019;12 doi: 10.1126/scisignal.aav5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y.G. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi H., Abdollah S., Qiu Y., Cai J., Xu Y.Y., Grinnell B.W., et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 19.Nakao A., Afrakhte M., Moren A., Nakayama T., Christian J.L., Heuchel R., et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 20.Izzi L., Attisano L. Regulation of the TGFbeta signalling pathway by ubiquitin-mediated degradation. Oncogene. 2004;23:2071–2078. doi: 10.1038/sj.onc.1207412. [DOI] [PubMed] [Google Scholar]
- 21.Miyazawa K., Miyazono K. Regulation of TGF-beta family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol. 2017;9:a022095. doi: 10.1101/cshperspect.a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moustakas A., Heldin C.H. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y.E. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y.E. Non-Smad signaling pathways of the TGF-beta family. Cold Spring Harb Perspect Biol. 2017;9:a022129. doi: 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor I.W., Wrana J.L. SnapShot: the TGFbeta pathway interactome. Cell. 2008;133:378 e371. doi: 10.1016/j.cell.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Le Roy C., Wrana J.L. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 27.Liu S., Ren J., Ten Dijke P. Targeting TGFbeta signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6:8. doi: 10.1038/s41392-020-00436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson I.B., Rifkin D.B. Regulation of the bioavailability of TGF-beta and TGF-beta-related proteins. Cold Spring Harb Perspect Biol. 2016;8:a021907. doi: 10.1101/cshperspect.a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubois C.M., Laprise M.H., Blanchette F., Gentry L.E., Leduc R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem. 1995;270:10618–10624. doi: 10.1074/jbc.270.18.10618. [DOI] [PubMed] [Google Scholar]
- 30.Gentry L.E., Lioubin M.N., Purchio A.F., Marquardt H. Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor beta to the mature polypeptide. Mol Cell Biol. 1988;8:4162–4168. doi: 10.1128/mcb.8.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray A.M., Mason A.J. Requirement for activin A and transforming growth factor--beta 1 pro-regions in homodimer assembly. Science. 1990;247:1328–1330. doi: 10.1126/science.2315700. [DOI] [PubMed] [Google Scholar]
- 32.Saharinen J., Hyytiainen M., Taipale J., Keski-Oja J. Latent transforming growth factor-beta binding proteins (LTBPs)--structural extracellular matrix proteins for targeting TGF-beta action. Cytokine Growth Factor Rev. 1999;10:99–117. doi: 10.1016/s1359-6101(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence D.A., Pircher R., Kryceve-Martinerie C., Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984;121:184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- 34.McMahon G.A., Dignam J.D., Gentry L.E. Structural characterization of the latent complex between transforming growth factor beta 1 and beta 1-latency-associated peptide. Biochem J. 1996;313(Pt 1):343–351. doi: 10.1042/bj3130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunner A.M., Marquardt H., Malacko A.R., Lioubin M.N., Purchio A.F. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor beta 1 precursor. Expression and characterization of mutant proteins. J Biol Chem. 1989;264:13660–13664. [PubMed] [Google Scholar]
- 36.Gleizes P.E., Beavis R.C., Mazzieri R., Shen B., Rifkin D.B. Identification and characterization of an eight-cysteine repeat of the latent transforming growth factor-beta binding protein-1 that mediates bonding to the latent transforming growth factor-beta1. J Biol Chem. 1996;271:29891–29896. doi: 10.1074/jbc.271.47.29891. [DOI] [PubMed] [Google Scholar]
- 37.Miyazono K., Hellman U., Wernstedt C., Heldin C.H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988;263:6407–6415. [PubMed] [Google Scholar]
- 38.Saharinen J., Taipale J., Keski-Oja J. Association of the small latent transforming growth factor-beta with an eight cysteine repeat of its binding protein LTBP-1. EMBO J. 1996;15:245–253. [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson I.B., Horiguchi M., Zilberberg L., Dabovic B., Hadjiolova K., Rifkin D.B. Latent TGF-beta-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saharinen J., Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell. 2000;11:2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangasser-Stephan K., Gartung C., Lahme B., Gressner A.M. Expression of isoforms and splice variants of the latent transforming growth factor beta binding protein (LTBP) in cultured human liver myofibroblasts. Liver. 2001;21:105–113. doi: 10.1034/j.1600-0676.2001.021002105.x. [DOI] [PubMed] [Google Scholar]
- 42.Michel K., Roth S., Trautwein C., Gong W., Flemming P., Gressner A.M. Analysis of the expression pattern of the latent transforming growth factor beta binding protein isoforms in normal and diseased human liver reveals a new splice variant missing the proteinase-sensitive hinge region. Hepatology. 1998;27:1592–1599. doi: 10.1002/hep.510270619. [DOI] [PubMed] [Google Scholar]
- 43.Cao B., Yang L., Rong W., Feng L., Han N., Zhang K., et al. Latent transforming growth factor-beta binding protein-1 in circulating plasma as a novel biomarker for early detection of hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:16046–16054. [PMC free article] [PubMed] [Google Scholar]
- 44.Kinnman N., Andersson U., Hultcrantz R. In situ expression of transforming growth factor-beta1-3, latent transforming growth factor-beta binding protein and tumor necrosis factor-alpha in liver tissue from patients with chronic hepatitis C. Scand J Gastroenterol. 2000;35:1294–1300. doi: 10.1080/003655200453656. [DOI] [PubMed] [Google Scholar]
- 45.Drews F., Knobel S., Moser M., Muhlack K.G., Mohren S., Stoll C., et al. Disruption of the latent transforming growth factor-beta binding protein-1 gene causes alteration in facial structure and influences TGF-beta bioavailability. Biochim Biophys Acta. 2008;1783:34–48. doi: 10.1016/j.bbamcr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Roth-Eichhorn S., Heitmann B., Flemming P., Kubicka S., Trautwein C. Evidence for the decreased expression of the latent TGF-beta binding protein and its splice form in human liver tumours. Scand J Gastroenterol. 2001;36:1204–1210. doi: 10.1080/00365520152584851. [DOI] [PubMed] [Google Scholar]
- 47.Santiago-Josefat B., Mulero-Navarro S., Dallas S.L., Fernandez-Salguero P.M. Overexpression of latent transforming growth factor-beta binding protein 1 (LTBP-1) in dioxin receptor-null mouse embryo fibroblasts. J Cell Sci. 2004;117:849–859. doi: 10.1242/jcs.00932. [DOI] [PubMed] [Google Scholar]
- 48.Corchero J., Martin-Partido G., Dallas S.L., Fernandez-Salguero P.M. Liver portal fibrosis in dioxin receptor-null mice that overexpress the latent transforming growth factor-beta-binding protein-1. Int J Exp Pathol. 2004;85:295–302. doi: 10.1111/j.0959-9673.2004.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J., Gao G., Wang H., Ye X., Zhou J., Lin J. Expression and clinical significance of latent-transforming growth factor beta-binding protein 2 in primary hepatocellular carcinoma. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000017216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.da Costa A.N., Plymoth A., Santos-Silva D., Ortiz-Cuaran S., Camey S., Guilloreau P., et al. Osteopontin and latent-TGF beta binding-protein 2 as potential diagnostic markers for HBV-related hepatocellular carcinoma. Int J Cancer. 2015;136:172–181. doi: 10.1002/ijc.28953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kantola A.K., Ryynanen M.J., Lhota F., Keski-Oja J., Koli K. Independent regulation of short and long forms of latent TGF-beta binding protein (LTBP)-4 in cultured fibroblasts and human tissues. J Cell Physiol. 2010;223:727–736. doi: 10.1002/jcp.22082. [DOI] [PubMed] [Google Scholar]
- 52.Demonbreun A., Fallon K.S., Oosterbaan C.C., Vaught L.A., Reiser N.L., Bogdanovic E., et al. Anti-latent TGF beta binding protein 4 antibody improves muscle function and reduces muscle fibrosis in muscular dystrophy. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abf0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyazono K., Olofsson A., Colosetti P., Heldin C.H. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taipale J., Miyazono K., Heldin C.H., Keski-Oja J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyazono K., Thyberg J., Heldin C.H. Retention of the transforming growth factor-beta 1 precursor in the Golgi complex in a latent endoglycosidase H-sensitive form. J Biol Chem. 1992;267:5668–5675. [PubMed] [Google Scholar]
- 56.Yoshinaga K., Obata H., Jurukovski V., Mazzieri R., Chen Y., Zilberberg L., et al. Perturbation of transforming growth factor (TGF)-beta1 association with latent TGF-beta binding protein yields inflammation and tumors. Proc Natl Acad Sci U S A. 2008;105:18758–18763. doi: 10.1073/pnas.0805411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Penttinen C., Saharinen J., Weikkolainen K., Hyytiainen M., Keski-Oja J. Secretion of human latent TGF-beta-binding protein-3 (LTBP-3) is dependent on co-expression of TGF-beta. J Cell Sci. 2002;115:3457–3468. doi: 10.1242/jcs.115.17.3457. [DOI] [PubMed] [Google Scholar]
- 58.Nuchel J., Ghatak S., Zuk A.V., Illerhaus A., Morgelin M., Schonborn K., et al. TGFB1 is secreted through an unconventional pathway dependent on the autophagic machinery and cytoskeletal regulators. Autophagy. 2018;14:465–486. doi: 10.1080/15548627.2017.1422850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papoutsoglou P., Rodrigues-Junior D.M., Moren A., Bergman A., Ponten F., Coulouarn C., et al. The noncoding MIR100HG RNA enhances the autocrine function of transforming growth factor beta signaling. Oncogene. 2021;40:3748–3765. doi: 10.1038/s41388-021-01803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koli K., Hyytiainen M., Ryynanen M.J., Keski-Oja J. Sequential deposition of latent TGF-beta binding proteins (LTBPs) during formation of the extracellular matrix in human lung fibroblasts. Exp Cell Res. 2005;310:370–382. doi: 10.1016/j.yexcr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 61.Unsold C., Hyytiainen M., Bruckner-Tuderman L., Keski-Oja J. Latent TGF-beta binding protein LTBP-1 contains three potential extracellular matrix interacting domains. J Cell Sci. 2001;114:187–197. doi: 10.1242/jcs.114.1.187. [DOI] [PubMed] [Google Scholar]
- 62.Lee S.S., Knott V., Jovanovic J., Harlos K., Grimes J.M., Choulier L., et al. Structure of the integrin binding fragment from fibrillin-1 gives new insights into microfibril organization. Structure. 2004;12:717–729. doi: 10.1016/j.str.2004.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robertson I.B., Handford P.A., Redfield C. NMR spectroscopic and bioinformatic analyses of the LTBP1 C-terminus reveal a highly dynamic domain organisation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robertson I., Jensen S., Handford P. TB domain proteins: evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem J. 2011;433:263–276. doi: 10.1042/BJ20101320. [DOI] [PubMed] [Google Scholar]
- 65.Kinsey R., Williamson M.R., Chaudhry S., Mellody K.T., McGovern A., Takahashi S., et al. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- 66.Sabatier L., Chen D., Fagotto-Kaufmann C., Hubmacher D., McKee M.D., Annis D.S., et al. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.George E.L., Georges-Labouesse E.N., Patel-King R.S., Rayburn H., Hynes R.O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 68.Hynes R.O., Yamada K.M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982;95:369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pankov R., Yamada K.M. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 70.Plow E.F., Haas T.A., Zhang L., Loftus J., Smith J.W. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 71.Benito-Jardon M., Strohmeyer N., Ortega-Sanchis S., Bharadwaj M., Moser M., Muller D.J., et al. alphav-Class integrin binding to fibronectin is solely mediated by RGD and unaffected by an RGE mutation. J Cell Biol. 2020;219 doi: 10.1083/jcb.202004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White E.S., Baralle F.E., Muro A.F. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dallas S.L., Sivakumar P., Jones C.J., Chen Q., Peters D.M., Mosher D.F., et al. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- 74.Taipale J., Saharinen J., Hedman K., Keski-Oja J. Latent transforming growth factor-beta 1 and its binding protein are components of extracellular matrix microfibrils. J Histochem Cytochem. 1996;44:875–889. doi: 10.1177/44.8.8756760. [DOI] [PubMed] [Google Scholar]
- 75.Hildebrand A., Romaris M., Rasmussen L.M., Heinegard D., Twardzik D.R., Border W.A., et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schonherr E., Broszat M., Brandan E., Bruckner P., Kresse H. Decorin core protein fragment Leu155-Val260 interacts with TGF-beta but does not compete for decorin binding to type I collagen. Arch Biochem Biophys. 1998;355:241–248. doi: 10.1006/abbi.1998.0720. [DOI] [PubMed] [Google Scholar]
- 77.Baghy K., Iozzo R.V., Kovalszky I. Decorin-TGFbeta axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem. 2012;60:262–268. doi: 10.1369/0022155412438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferdous Z., Wei V.M., Iozzo R., Hook M., Grande-Allen K.J. Decorin-transforming growth factor- interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 2007;282:35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- 79.Shi Y.F., Zhang Q., Cheung P.Y., Shi L., Fong C.C., Zhang Y., et al. Effects of rhDecorin on TGF-beta1 induced human hepatic stellate cells LX-2 activation. Biochim Biophys Acta. 2006;1760:1587–1595. doi: 10.1016/j.bbagen.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 80.Dudas J., Kovalszky I., Gallai M., Nagy J.O., Schaff Z., Knittel T., et al. Expression of decorin, transforming growth factor-beta 1, tissue inhibitor metalloproteinase 1 and 2, and type IV collagenases in chronic hepatitis. Am J Clin Pathol. 2001;115:725–735. doi: 10.1309/J8CD-E9C8-X4NG-GTVG. [DOI] [PubMed] [Google Scholar]
- 81.Noble N.A., Harper J.R., Border W.A. In vivo interactions of TGF-beta and extracellular matrix. Prog Growth Factor Res. 1992;4:369–382. doi: 10.1016/0955-2235(92)90017-c. [DOI] [PubMed] [Google Scholar]
- 82.Annes J.P., Munger J.S., Rifkin D.B. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 83.Takada Y., Ye X., Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Annes J.P., Rifkin D.B., Munger J.S. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 2002;511:65–68. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- 85.Henderson N.C., Arnold T.D., Katamura Y., Giacomini M.M., Rodriguez J.D., McCarty J.H., et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mu D., Cambier S., Fjellbirkeland L., Baron J.L., Munger J.S., Kawakatsu H., et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munger J.S., Huang X., Kawakatsu H., Griffiths M.J., Dalton S.L., Wu J., et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 88.Nishimura S.L. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol. 2009;175:1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reed N.I., Jo H., Chen C., Tsujino K., Arnold T.D., DeGrado W.F., et al. The alphavbeta1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Worthington J.J., Klementowicz J.E., Travis M.A. TGFbeta: a sleeping giant awoken by integrins. Trends Biochem Sci. 2011;36:47–54. doi: 10.1016/j.tibs.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 91.Huang X.Z., Wu J.F., Cass D., Erle D.J., Corry D., Young S.G., et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aluwihare P., Mu Z., Zhao Z., Yu D., Weinreb P.H., Horan G.S., et al. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci. 2009;122:227–232. doi: 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Asano Y., Ihn H., Yamane K., Kubo M., Tamaki K. Increased expression levels of integrin alphavbeta5 on scleroderma fibroblasts. Am J Pathol. 2004;164:1275–1292. doi: 10.1016/s0002-9440(10)63215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sarrazy V., Koehler A., Chow M.L., Zimina E., Li C.X., Kato H., et al. Integrins alphavbeta5 and alphavbeta3 promote latent TGF-beta1 activation by human cardiac fibroblast contraction. Cardiovasc Res. 2014;102:407–417. doi: 10.1093/cvr/cvu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Annes J.P., Chen Y., Munger J.S., Rifkin D.B. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buscemi L., Ramonet D., Klingberg F., Formey A., Smith-Clerc J., Meister J.J., et al. The single-molecule mechanics of the latent TGF-beta1 complex. Curr Biol. 2011;21:2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 97.Wipff P.J., Rifkin D.B., Meister J.J., Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Araya J., Cambier S., Markovics J.A., Wolters P., Jablons D., Hill A., et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Z., Mu Z., Dabovic B., Jurukovski V., Yu D., Sung J., et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leask A., Abraham D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 101.Puthawala K., Hadjiangelis N., Jacoby S.C., Bayongan E., Zhao Z., Yang Z., et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Popov Y., Patsenker E., Stickel F., Zaks J., Bhaskar K.R., Niedobitek G., et al. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol. 2008;48:453–464. doi: 10.1016/j.jhep.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 103.Conroy K.P., Kitto L.J., Henderson N.C. alphav integrins: key regulators of tissue fibrosis. Cell Tissue Res. 2016;365:511–519. doi: 10.1007/s00441-016-2407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murray I.R., Gonzalez Z.N., Baily J., Dobie R., Wallace R.J., Mackinnon A.C., et al. alphav integrins on mesenchymal cells regulate skeletal and cardiac muscle fibrosis. Nat Commun. 2017;8:1118. doi: 10.1038/s41467-017-01097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ulmasov B., Neuschwander-Tetri B.A., Lai J., Monastyrskiy V., Bhat T., Yates M.P., et al. Inhibitors of Arg-Gly-Asp-binding integrins reduce development of pancreatic fibrosis in mice. Cell Mol Gastroenterol Hepatol. 2016;2:499–518. doi: 10.1016/j.jcmgh.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ulmasov B., Noritake H., Carmichael P., Oshima K., Griggs D.W., Neuschwander-Tetri B.A. An inhibitor of arginine-glycine-aspartate-binding integrins reverses fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatol Commun. 2019;3:246–261. doi: 10.1002/hep4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun T., Huang Z., Liang W.C., Yin J., Lin W.Y., Wu J., et al. TGFbeta2 and TGFbeta3 isoforms drive fibrotic disease pathogenesis. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abe0407. [DOI] [PubMed] [Google Scholar]
- 108.Schultz-Cherry S., Murphy-Ullrich J.E. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schultz-Cherry S., Ribeiro S., Gentry L., Murphy-Ullrich J.E. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-beta in a chemically defined system. J Biol Chem. 1994;269:26775–26782. [PubMed] [Google Scholar]
- 110.Sweetwyne M.T., Murphy-Ullrich J.E. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Adams J.C., Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lawler J., McHenry K., Duquette M., Derick L. Characterization of human thrombospondin-4. J Biol Chem. 1995;270:2809–2814. doi: 10.1074/jbc.270.6.2809. [DOI] [PubMed] [Google Scholar]
- 113.Qabar A., Derick L., Lawler J., Dixit V. Thrombospondin 3 is a pentameric molecule held together by interchain disulfide linkage involving two cysteine residues. J Biol Chem. 1995;270:12725–12729. doi: 10.1074/jbc.270.21.12725. [DOI] [PubMed] [Google Scholar]
- 114.Schultz-Cherry S., Chen H., Mosher D.F., Misenheimer T.M., Krutzsch H.C., Roberts D.D., et al. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- 115.Young G.D., Murphy-Ullrich J.E. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-beta complex. J Biol Chem. 2004;279:47633–47642. doi: 10.1074/jbc.M404918200. [DOI] [PubMed] [Google Scholar]
- 116.Ribeiro S.M., Poczatek M., Schultz-Cherry S., Villain M., Murphy-Ullrich J.E. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 117.Daniel C., Wagner A., Hohenstein B., Hugo C. Thrombospondin-2 therapy ameliorates experimental glomerulonephritis via inhibition of cell proliferation, inflammation, and TGF-beta activation. Am J Physiol Ren Physiol. 2009;297:F1299–F1309. doi: 10.1152/ajprenal.00254.2009. [DOI] [PubMed] [Google Scholar]
- 118.Crawford S.E., Stellmach V., Murphy-Ullrich J.E., Ribeiro S.M., Lawler J., Hynes R.O., et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 119.Mir F.A., Contreras-Ruiz L., Masli S. Thrombospondin-1-dependent immune regulation by transforming growth factor-beta2-exposed antigen-presenting cells. Immunology. 2015;146:547–556. doi: 10.1111/imm.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Breitkopf K., Sawitza I., Westhoff J.H., Wickert L., Dooley S., Gressner A.M. Thrombospondin 1 acts as a strong promoter of transforming growth factor beta effects via two distinct mechanisms in hepatic stellate cells. Gut. 2005;54:673–681. doi: 10.1136/gut.2004.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gwag T., Reddy Mooli R.G., Li D., Lee S., Lee E.Y., Wang S. Macrophage-derived thrombospondin 1 promotes obesity-associated non-alcoholic fatty liver disease. JHEP Rep. 2021;3:100193. doi: 10.1016/j.jhepr.2020.100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anastasi C., Rousselle P., Talantikite M., Tessier A., Cluzel C., Bachmann A., et al. BMP-1 disrupts cell adhesion and enhances TGF-beta activation through cleavage of the matricellular protein thrombospondin-1. Sci Signal. 2020;13 doi: 10.1126/scisignal.aba3880. [DOI] [PubMed] [Google Scholar]
- 123.Bourd-Boittin K., Bonnier D., Leyme A., Mari B., Tuffery P., Samson M., et al. Protease profiling of liver fibrosis reveals the ADAM metallopeptidase with thrombospondin type 1 motif, 1 as a central activator of transforming growth factor beta. Hepatology. 2011;54:2173–2184. doi: 10.1002/hep.24598. [DOI] [PubMed] [Google Scholar]
- 124.Yao Y., Hu C., Song Q., Li Y., Da X., Yu Y., et al. ADAMTS16 activates latent TGF-beta, accentuating fibrosis and dysfunction of the pressure-overloaded heart. Cardiovasc Res. 2020;116:956–969. doi: 10.1093/cvr/cvz187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Verma R.P., Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15:2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 126.Yu Q., Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 127.Maeda S., Dean D.D., Gay I., Schwartz Z., Boyan B.D. Activation of latent transforming growth factor beta1 by stromelysin 1 in extracts of growth plate chondrocyte-derived matrix vesicles. J Bone Miner Res. 2001;16:1281–1290. doi: 10.1359/jbmr.2001.16.7.1281. [DOI] [PubMed] [Google Scholar]
- 128.Johnson J.L., Dwivedi A., Somerville M., George S.J., Newby A.C. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler Thromb Vasc Biol. 2011;31:e35–e44. doi: 10.1161/ATVBAHA.111.225623. [DOI] [PubMed] [Google Scholar]
- 129.Stickens D., Behonick D.J., Ortega N., Heyer B., Hartenstein B., Yu Y., et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garg P., Vijay-Kumar M., Wang L., Gewirtz A.T., Merlin D., Sitaraman S.V. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G175–G184. doi: 10.1152/ajpgi.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.George J., Tsutsumi M., Tsuchishima M. MMP-13 deletion decreases profibrogenic molecules and attenuates N-nitrosodimethylamine-induced liver injury and fibrosis in mice. J Cell Mol Med. 2017;21:3821–3835. doi: 10.1111/jcmm.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S.A., et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 133.Oh J., Takahashi R., Adachi E., Kondo S., Kuratomi S., Noma A., et al. Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene. 2004;23:5041–5048. doi: 10.1038/sj.onc.1207688. [DOI] [PubMed] [Google Scholar]
- 134.Ge G., Greenspan D.S. BMP1 controls TGFbeta1 activation via cleavage of latent TGFbeta-binding protein. J Cell Biol. 2006;175:111–120. doi: 10.1083/jcb.200606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pappano W.N., Steiglitz B.M., Scott I.C., Keene D.R., Greenspan D.S. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol Cell Biol. 2003;23:4428–4438. doi: 10.1128/MCB.23.13.4428-4438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Emami N., Diamandis E.P. Potential role of multiple members of the kallikrein-related peptidase family of serine proteases in activating latent TGF beta 1 in semen. Biol Chem. 2010;391:85–95. doi: 10.1515/BC.2010.007. [DOI] [PubMed] [Google Scholar]
- 137.Akita K., Okuno M., Enya M., Imai S., Moriwaki H., Kawada N., et al. Impaired liver regeneration in mice by lipopolysaccharide via TNF-alpha/kallikrein-mediated activation of latent TGF-beta. Gastroenterology. 2002;123:352–364. doi: 10.1053/gast.2002.34234. [DOI] [PubMed] [Google Scholar]
- 138.Hara M., Kirita A., Kondo W., Matsuura T., Nagatsuma K., Dohmae N., et al. LAP degradation product reflects plasma kallikrein-dependent TGF-beta activation in patients with hepatic fibrosis. Springerplus. 2014;3:221. doi: 10.1186/2193-1801-3-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yokoyama H., Masaki T., Inoue I., Nakamura M., Mezaki Y., Saeki C., et al. Histological and biochemical evaluation of transforming growth factor-beta activation and its clinical significance in patients with chronic liver disease. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dallas S.L., Zhao S., Cramer S.D., Chen Z., Peehl D.M., Bonewald L.F. Preferential production of latent transforming growth factor beta-2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. J Cell Physiol. 2005;202:361–370. doi: 10.1002/jcp.20147. [DOI] [PubMed] [Google Scholar]
- 141.Antonelli-Orlidge A., Saunders K.B., Smith S.R., D'Amore P.A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989;86:4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sato Y., Rifkin D.B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Flaumenhaft R., Abe M., Sato Y., Miyazono K., Harpel J., Heldin C.H., et al. Role of the latent TGF-beta binding protein in the activation of latent TGF-beta by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993;120:995–1002. doi: 10.1083/jcb.120.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dennis P.A., Rifkin D.B. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci U S A. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kojima S., Rifkin D.B. Mechanism of retinoid-induced activation of latent transforming growth factor-beta in bovine endothelial cells. J Cell Physiol. 1993;155:323–332. doi: 10.1002/jcp.1041550213. [DOI] [PubMed] [Google Scholar]
- 146.Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol. 2008;40:1068–1078. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 147.Nunes I., Shapiro R.L., Rifkin D.B. Characterization of latent TGF-beta activation by murine peritoneal macrophages. J Immunol. 1995;155:1450–1459. [PubMed] [Google Scholar]
- 148.Yehualaeshet T., O'Connor R., Green-Johnson J., Mai S., Silverstein R., Murphy-Ullrich J.E., et al. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol. 1999;155:841–851. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Attur M.G., Palmer G.D., Al-Mussawir H.E., Dave M., Teixeira C.C., Rifkin D.B., et al. F-spondin, a neuroregulatory protein, is up-regulated in osteoarthritis and regulates cartilage metabolism via TGF-beta activation. FASEB J. 2009;23:79–89. doi: 10.1096/fj.08-114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Glinka Y., Prud'homme G.J. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302–310. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Grandclement C., Pallandre J.R., Valmary Degano S., Viel E., Bouard A., Balland J., et al. Neuropilin-2 expression promotes TGF-beta1-mediated epithelial to mesenchymal transition in colorectal cancer cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blois S.M., Sulkowski G., Tirado-Gonzalez I., Warren J., Freitag N., Klapp B.F., et al. Pregnancy-specific glycoprotein 1 (PSG1) activates TGF-beta and prevents dextran sodium sulfate (DSS)-induced colitis in mice. Mucosal Immunol. 2014;7:348–358. doi: 10.1038/mi.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alcaraz L.B., Exposito J.Y., Chuvin N., Pommier R.M., Cluzel C., Martel S., et al. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-beta. J Cell Biol. 2014;205:409–428. doi: 10.1083/jcb.201308031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carlson C.M., Turpin E.A., Moser L.A., O'Brien K.B., Cline T.D., Jones J.C., et al. Transforming growth factor-beta: activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. Plos Pathog. 2010;6 doi: 10.1371/journal.ppat.1001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schultz-Cherry S., Hinshaw V.S. Influenza virus neuraminidase activates latent transforming growth factor beta. J Virol. 1996;70:8624–8629. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lodyga M., Cambridge E., Karvonen H.M., Pakshir P., Wu B., Boo S., et al. Cadherin-11-mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-beta. Sci Signal. 2019;12 doi: 10.1126/scisignal.aao3469. [DOI] [PubMed] [Google Scholar]
- 157.Abe M., Oda N., Sato Y. Cell-associated activation of latent transforming growth factor-beta by calpain. J Cell Physiol. 1998;174:186–193. doi: 10.1002/(SICI)1097-4652(199802)174:2<186::AID-JCP6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 158.Biswas S., Guix M., Rinehart C., Dugger T.C., Chytil A., Moses H.L., et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305–1313. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang H., Kochevar I.E. Involvement of UVB-induced reactive oxygen species in TGF-beta biosynthesis and activation in keratinocytes. Free Radic Biol Med. 2005;38:890–897. doi: 10.1016/j.freeradbiomed.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 160.Barcellos-Hoff M.H., Dix T.A. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 161.Brown P.D., Wakefield L.M., Levinson A.D., Sporn M.B. Physicochemical activation of recombinant latent transforming growth factor-beta's 1, 2, and 3. Growth Factors. 1990;3:35–43. doi: 10.3109/08977199009037500. [DOI] [PubMed] [Google Scholar]
- 162.Ahamed J., Burg N., Yoshinaga K., Janczak C.A., Rifkin D.B., Coller B.S. In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-beta1. Blood. 2008;112:3650–3660. doi: 10.1182/blood-2008-04-151753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lyons R.M., Keski-Oja J., Moses H.L. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oreffo R.O., Mundy G.R., Seyedin S.M., Bonewald L.F. Activation of the bone-derived latent TGF beta complex by isolated osteoclasts. Biochem Biophys Res Commun. 1989;158:817–823. doi: 10.1016/0006-291x(89)92795-2. [DOI] [PubMed] [Google Scholar]
- 165.Kottmann R.M., Kulkarni A.A., Smolnycki K.A., Lyda E., Dahanayake T., Salibi R., et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am J Respir Crit Care Med. 2012;186:740–751. doi: 10.1164/rccm.201201-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mathieu E., Meheus L., Raymackers J., Merregaert J. Characterization of the osteogenic stromal cell line MN7: identification of secreted MN7 proteins using two-dimensional polyacrylamide gel electrophoresis, western blotting, and microsequencing. J Bone Miner Res. 1994;9:903–913. doi: 10.1002/jbmr.5650090616. [DOI] [PubMed] [Google Scholar]
- 167.Smits P., Poumay Y., Karperien M., Tylzanowski P., Wauters J., Huylebroeck D., et al. Differentiation-dependent alternative splicing and expression of the extracellular matrix protein 1 gene in human keratinocytes. J Invest Dermatol. 2000;114:718–724. doi: 10.1046/j.1523-1747.2000.00916.x. [DOI] [PubMed] [Google Scholar]
- 168.Sercu S., Lambeir A.M., Steenackers E., El Ghalbzouri A., Geentjens K., Sasaki T., et al. ECM1 interacts with fibulin-3 and the beta 3 chain of laminin 332 through its serum albumin subdomain-like 2 domain. Matrix Biol. 2009;28:160–169. doi: 10.1016/j.matbio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 169.Chan I. The role of extracellular matrix protein 1 in human skin. Clin Exp Dermatol. 2004;29:52–56. doi: 10.1111/j.1365-2230.2004.01440.x. [DOI] [PubMed] [Google Scholar]
- 170.Deckers M.M., Smits P., Karperien M., Ni J., Tylzanowski P., Feng P., et al. Recombinant human extracellular matrix protein 1 inhibits alkaline phosphatase activity and mineralization of mouse embryonic metatarsals in vitro. Bone. 2001;28:14–20. doi: 10.1016/s8756-3282(00)00428-2. [DOI] [PubMed] [Google Scholar]
- 171.Han Z., Ni J., Smits P., Underhill C.B., Xie B., Chen Y., et al. Extracellular matrix protein 1 (ECM1) has angiogenic properties and is expressed by breast tumor cells. FASEB J. 2001;15:988–994. doi: 10.1096/fj.99-0934com. [DOI] [PubMed] [Google Scholar]
- 172.Mongiat M., Fu J., Oldershaw R., Greenhalgh R., Gown A.M., Iozzo R.V. Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis. J Biol Chem. 2003;278:17491–17499. doi: 10.1074/jbc.M210529200. [DOI] [PubMed] [Google Scholar]
- 173.Fujimoto N., Terlizzi J., Brittingham R., Fertala A., McGrath J.A., Uitto J. Extracellular matrix protein 1 interacts with the domain III of fibulin-1C and 1D variants through its central tandem repeat 2. Biochem Biophys Res Commun. 2005;333:1327–1333. doi: 10.1016/j.bbrc.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 174.Fujimoto N., Terlizzi J., Aho S., Brittingham R., Fertala A., Oyama N., et al. Extracellular matrix protein 1 inhibits the activity of matrix metalloproteinase 9 through high-affinity protein/protein interactions. Exp Dermatol. 2006;15:300–307. doi: 10.1111/j.0906-6705.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 175.Oyama N., Chan I., Neill S.M., Hamada T., South A.P., Wessagowit V., et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet. 2003;362:118–123. doi: 10.1016/S0140-6736(03)13863-9. [DOI] [PubMed] [Google Scholar]
- 176.Qing J., Maher V.M., Tran H., Argraves W.S., Dunstan R.W., McCormick J.J. Suppression of anchorage-independent growth and matrigel invasion and delayed tumor formation by elevated expression of fibulin-1D in human fibrosarcoma-derived cell lines. Oncogene. 1997;15:2159–2168. doi: 10.1038/sj.onc.1201385. [DOI] [PubMed] [Google Scholar]
- 177.Sercu S., Zhang L., Merregaert J. The extracellular matrix protein 1: its molecular interaction and implication in tumor progression. Cancer Invest. 2008;26:375–384. doi: 10.1080/07357900701788148. [DOI] [PubMed] [Google Scholar]
- 178.Wipff P.J., Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 179.Su P., Chen S., Zheng Y.H., Zhou H.Y., Yan C.H., Yu F., et al. Novel function of extracellular matrix protein 1 in suppressing Th17 cell development in experimental autoimmune encephalomyelitis. J Immunol. 2016;197:1054–1064. doi: 10.4049/jimmunol.1502457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang L., Yu J., Ni J., Xu X.M., Wang J., Ning H., et al. Extracellular matrix protein 1 (ECM1) is over-expressed in malignant epithelial tumors. Cancer Lett. 2003;200:57–67. doi: 10.1016/s0304-3835(03)00350-1. [DOI] [PubMed] [Google Scholar]
- 181.Abe M., Harpel J.G., Metz C.N., Nunes I., Loskutoff D.J., Rifkin D.B. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 182.Fondevila C., Shen X.D., Moore C., Busuttil R.W., Coito A.J. Cyclic RGD peptides with high affinity for alpha5beta1 integrin protect genetically fat Zucker rat livers from cold ischemia/reperfusion injury. Transpl Proc. 2005;37:1679–1681. doi: 10.1016/j.transproceed.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 183.Fan W., Liu T., Chen W., Hammad S., Longerich T., Hausser I., et al. ECM1 prevents activation of transforming growth factor beta, hepatic stellate cells, and fibrogenesis in mice. Gastroenterology. 2019;157:1352–1367 e1313. doi: 10.1053/j.gastro.2019.07.036. [DOI] [PubMed] [Google Scholar]
- 184.Schlingensiepen R., Goldbrunner M., Szyrach M.N., Stauder G., Jachimczak P., Bogdahn U., et al. Intracerebral and intrathecal infusion of the TGF-beta 2-specific antisense phosphorothioate oligonucleotide AP 12009 in rabbits and primates: toxicology and safety. Oligonucleotides. 2005;15:94–104. doi: 10.1089/oli.2005.15.94. [DOI] [PubMed] [Google Scholar]
- 185.Dropmann A., Dooley S., Dewidar B., Hammad S., Dediulia T., Werle J., et al. TGF-beta2 silencing to target biliary-derived liver diseases. Gut. 2020;69:1677–1690. doi: 10.1136/gutjnl-2019-319091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rice L.M., Padilla C.M., McLaughlin S.R., Mathes A., Ziemek J., Goummih S., et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125:2795–2807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dahly A.J., Hoagland K.M., Flasch A.K., Jha S., Ledbetter S.R., Roman R.J. Antihypertensive effects of chronic anti-TGF-beta antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R757–R767. doi: 10.1152/ajpregu.00098.2002. [DOI] [PubMed] [Google Scholar]
- 188.Varricchio L., Mascarenhas J., Migliaccio A.R., O’Connor-McCourt M., Tremblay G., Denis J.-F., et al. AVID200, a potent trap for TGF-β ligands inhibits TGF-β1 signaling in human myelofbrosis. Blood. 2018;132:1791. [Google Scholar]
- 189.Ji X., Wang H., Wu Z., Zhong X., Zhu M., Zhang Y., et al. Specific inhibitor of Smad3 (SIS3) attenuates fibrosis, apoptosis, and inflammation in unilateral ureteral obstruction kidneys by inhibition of transforming growth factor beta (TGF-beta)/Smad3 signaling. Med Sci Monit. 2018;24:1633–1641. doi: 10.12659/MSM.909236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shou J., Cao J., Zhang S., Sun R., Zhao M., Chen K., et al. SIS3, a specific inhibitor of smad3, attenuates bleomycin-induced pulmonary fibrosis in mice. Biochem Biophys Res Commun. 2018;503:757–762. doi: 10.1016/j.bbrc.2018.06.072. [DOI] [PubMed] [Google Scholar]
- 191.Colak S., Ten Dijke P. Targeting TGF-beta signaling in cancer. Trends Cancer. 2017;3:56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 192.Huynh L.K., Hipolito C.J., Ten Dijke P. A perspective on the development of TGF-beta inhibitors for cancer treatment. Biomolecules. 2019;9:743. doi: 10.3390/biom9110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cao Y., Agarwal R., Dituri F., Lupo L., Trerotoli P., Mancarella S., et al. NGS-based transcriptome profiling reveals biomarkers for companion diagnostics of the TGF-beta receptor blocker galunisertib in HCC. Cell Death Dis. 2017;8:e2634. doi: 10.1038/cddis.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Farrington D.L., Yingling J.M., Fill J.A., Yan L., Qian Y.W., Shou J., et al. Development and validation of a phosphorylated SMAD ex vivo stimulation assay. Biomarkers. 2007;12:313–330. doi: 10.1080/13547500601162441. [DOI] [PubMed] [Google Scholar]
- 195.Patsenker E., Popov Y., Stickel F., Jonczyk A., Goodman S.L., Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135:660–670. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Takasaka N., Seed R.I., Cormier A., Bondesson A.J., Lou J., Elattma A., et al. Integrin alphavbeta8-expressing tumor cells evade host immunity by regulating TGF-beta activation in immune cells. JCI Insight. 2018;3 doi: 10.1172/jci.insight.122591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Reader C.S., Vallath S., Steele C.W., Haider S., Brentnall A., Desai A., et al. The integrin alphavbeta6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J Pathol. 2019;249:332–342. doi: 10.1002/path.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Weller M., Silginer M., Goodman S.L., Hasenbach K., Thies S., Tabatabai P.S., et al. Effect of the integrin inhibitor cilengitide on TGF-β signaling. J Clin Oncol. 2012;30:2055. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.