Highlights
-
•
Leptomeningeal enhancement (LME) is a nonspecific imaging feature.
-
•
LME can be present in neoplastic, infectious, and primary neuroinflammatory diseases.
-
•
The presence of LME is associated with worse outcome measures in multiple sclerosis.
-
•
We recommend the ascertainment of LME in clinical practice.
-
•
Neuroinflammatory animal models can be used to investigate the pathophysiology of LME.
Keywords: Multiple sclerosis, Leptomeningeal enhancement, Leptomeningeal inflammation, Magnetic resonance imaging, Systematic review, Meta-analysis
Glossary: CIS, clinically isolated syndrome; CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; EDSS, Expanded Disability Status Scale; FLAIR, fluid-attenuated inversion recovery; Gd, gadolinium; LME, Leptomeningeal enhancement; MRI, magnetic resonance imaging; MS, multiple sclerosis (RR, relapsing-remitting); NMOSD, neuromyelitis optica spectrum disorder
Abstract
Background
The lack of systematic evidence on leptomeningeal enhancement (LME) on MRI in neurological diseases, including multiple sclerosis (MS), hampers its interpretation in clinical routine and research settings.
Purpose
To perform a systematic review and meta-analysis of MRI LME in MS and other neurological diseases.
Materials and Methods
In a comprehensive literature search in Medline, Scopus, and Embase, out of 2292 publications, 459 records assessing LME in neurological diseases were eligible for qualitative synthesis. Of these, 135 were included in a random-effects model meta-analysis with subgroup analyses for MS.
Results
Of eligible publications, 161 investigated LME in neoplastic neurological (n = 2392), 91 in neuroinfectious (n = 1890), and 75 in primary neuroinflammatory diseases (n = 4038). The LME-proportions for these disease classes were 0.47 [95%-CI: 0.37–0.57], 0.59 [95%-CI: 0.47–0.69], and 0.26 [95%-CI: 0.20–0.35], respectively. In a subgroup analysis comprising 1605 MS cases, LME proportion was 0.30 [95%-CI 0.21–0.42] with lower proportions in relapsing-remitting (0.19 [95%-CI 0.13–0.27]) compared to progressive MS (0.39 [95%-CI 0.30–0.49], p = 0.002) and higher proportions in studies imaging at 7 T (0.79 [95%-CI 0.64–0.89]) compared to lower field strengths (0.21 [95%-CI 0.15–0.29], p < 0.001). LME in MS was associated with longer disease duration (mean difference 2.2 years [95%-CI 0.2–4.2], p = 0.03), higher Expanded Disability Status Scale (mean difference 0.6 points [95%-CI 0.2–1.0], p = 0.006), higher T1 (mean difference 1.6 ml [95%-CI 0.1–3.0], p = 0.04) and T2 lesion load (mean difference 5.9 ml [95%-CI 3.2–8.6], p < 0.001), and lower cortical volume (mean difference −21.3 ml [95%-CI −34.7–-7.9], p = 0.002).
Conclusions
Our study provides high-grade evidence for the substantial presence of LME in MS and a comprehensive panel of other neurological diseases. Our data could facilitate differential diagnosis of LME in clinical settings. Additionally, our meta-analysis corroborates that LME is associated with key clinical and imaging features of MS.
PROSPERO No: CRD42021235026.
1. Introduction
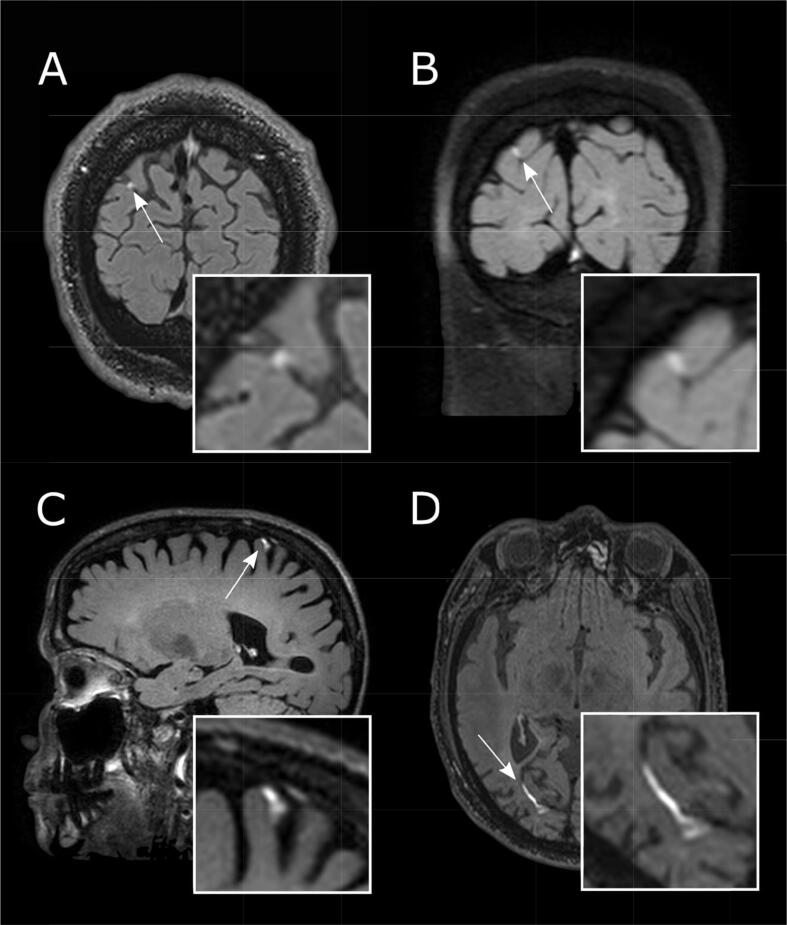
Abnormal meningeal contrast enhancement may take two distinct forms: pachymeningeal enhancement, referring to dural-arachnoidal enhancement, which follows the contour of the inner table of the skull and includes intradural veins and sinuses; and leptomeningeal enhancement (LME), which follows the pia-arachnoid abutting the cortical surface and extending into the sulci. LME is often caused by neoplastic or infectious processes. However, LME is also gaining attention as a putative imaging biomarker of meningeal inflammation in neuroinflammatory diseases, including MS and neurosarcoidosis (Fig. 1) (Zurawski et al., 2017).
Fig. 1.
Leptomeningeal enhancement (LME) across the spectrum of neurological diseases. Leptomeningeal enhancement (LME, white arrows) can be detected using post-gadolinium fluid-attenuated inversion recovery (FLAIR) T2-weighted magnetic resonance imaging and can be present in viral diseases such as HIV (A, at 3 T), in primary neuroinflammatory diseases such as Susac syndrome (B, at 3 T) and multiple sclerosis (C, at 7 T), and in aseptic meningitis (natalizumab-induced) (D, at 3 T).
Because LME can be present in a wide variety of neurological diseases (Absinta et al., 2017), differential diagnostic considerations are paramount for proper patient workup. However, there is a lack of systematic evidence on LME proportions in MS and other neurological diseases. Furthermore, a wide variety of methodological approaches to imaging LME has been published (Singh et al., 2000, Zivadinov et al., 2018), impeding the implementation of an appropriate imaging protocol for sensitive LME detection. With respect to MS, several studies have presented conflicting findings regarding the association of LME with clinical and imaging parameters (Absinta and Ontaneda, 2020), such that high-level evidence would benefit clinicians and researchers.
Based on these shortcomings, we set out to systematically summarize the available evidence on LME in neurological diseases with a focus on MS. This study had the following goals: (1) synthesize data on LME proportions in neurological diseases, including potentially distinct LME features such as phenotype or temporal evolution; (2) qualitatively and quantitatively summarize the potential association of LME with clinical and imaging features in MS; (3) propose an appropriate imaging protocol to detect LME in clinical and research practice; (4) summarize available data on pathological correlates of LME in neuroinflammation; (5) summarize available evidence on LME in animal models of neuroinflammation.
2. Materials and methods
We registered the study protocol in the International prospective register of systematic reviews (PROSPERO, CRD42021235026, https://www.crd.york.ac.uk/PROSPERO/) and used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Guidelines for reporting (Moher et al., 2015).
2.1. Search strategy
We searched for original studies published in full up to February 2, 2021, in PubMed, Scopus, and Ovid EMBASE. See Table S1 for the search string in each of these databases.
2.2. Inclusion and exclusion criteria
We included publications on human or animal data that reported on any outcome related to leptomeningeal inflammation on magnetic resonance imaging (MRI) in any neurological diseases. Case reports were also included in the systematic review. Exclusion criteria: conference abstracts, non-English articles, and publications that reiterated previously reported quantitative data. Reviews were excluded but retained as potential sources of additional records.
2.3. Study selection and data extraction
Titles and abstracts of studies were screened for their relevance in the web-based application Rayyan by two reviewers (CT and BVI), followed by full-text screening (Ouzzani et al., 2016). Subsequently, the following data were extracted: title, authors, publication year, study design, neurological disease, and number of subjects per group. For studies with ≥ 10 subjects, MRI sequences/field strength, LME location (spinal cord, convexities, basal, cerebellar, brainstem), main study findings (in narrative manner), pattern of LME (for example, nodular or linear), temporal dynamics of LME, and proportions of LME in experimental and control groups were also extracted. For missing data on MRI sequences to detect LME, corresponding authors of respective publications were contacted. In total, 41 e-mails were sent out, and the response rate was 27% (11 response e-mails).
2.4. Quality assessment
The quality of each study with ≥ 10 included subjects was assessed against predefined criteria by two reviewers (CT and BVI) using the Newcastle-Ottawa scale for evaluating risk of bias in nonrandomized studies (Wells et al., 2015). Discrepancies were resolved by discussion.
2.5. Data synthesis and analysis
Only diseases/disease classes with ≥ 2 publications describing ≥ 10 adult subjects each were included in the meta-analysis, and only summary-level data were used. As primary outcome, log-transformed proportions of LME were used. A random-effects model was fitted to the data. The amount of heterogeneity (τ2), was estimated using the DerSimonian-Laird estimator (DerSimonian and Laird, 1986). In addition, the Q-test for heterogeneity (Cochran, 1954) and the I2 statistic (Higgins and Thompson, 2002) are reported.
For subgroup analyses of clinical and imaging outcomes in MS, the analysis was carried out using the log-transformed proportions or mean difference as the outcome measure. Subgroup analyses were computed for MS to assess the proportion of LME in clinical MS phenotypes when ≥ 3 studies were available. Subgroup analyses in MS for clinical and imaging outcomes were computed when ≥ 3 studies reported at least mean, variance, and n for LME + and LME- groups on respective outcomes.
A two-tailed P value < 0.05 was considered statistically significant.
2.6. Publication bias
The rank correlation test and the regression test, using the standard error of the observed outcomes as predictor, were used to check for funnel plot asymmetry. The analysis was carried out using R (version 3.6.1) with the meta and metafor packages (version 2.4.0) (Viechtbauer, 2010).
3. Results
3.1. Eligible publications
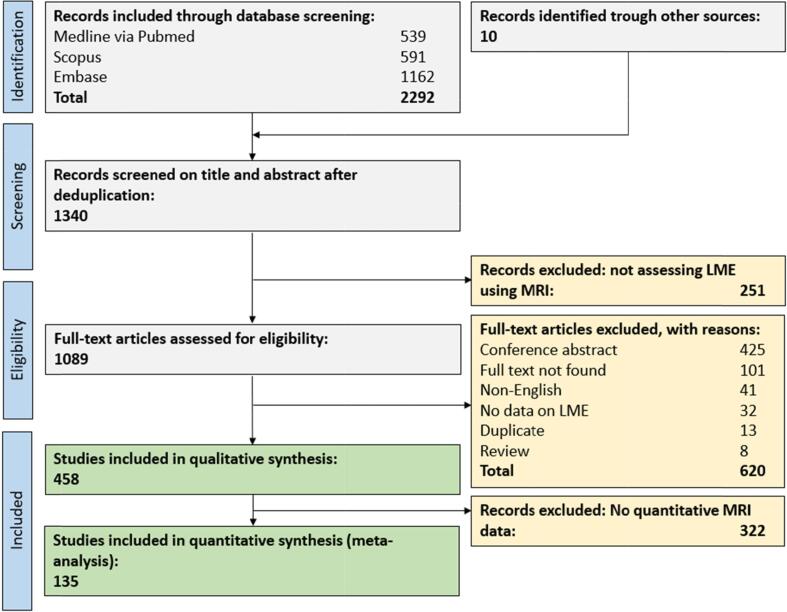
In total, 2292 original publications were retrieved from our comprehensive database search and an additional 10 publications from reference lists of reviews on related topics. After abstract and title screening, 1089 studies were eligible for full-text search. After screening the full text of these studies, 458 articles (35% of deduplicated references) were included for qualitative synthesis and 135 articles (10%) for quantitative synthesis (Figure S1).
3.2. General study characteristics
3.2.1. Included publications
Of the eligible publications, 144 investigated LME in neoplastic neurological diseases (2392 subjects including 183 children), 91 in infectious neurological diseases (1890 subjects including 48 children), and 76 in primary neuroinflammatory diseases (4038 subjects including 11 children). Additionally, 147 publications assessed LME in neurological diseases that did not belong to aforementioned categories (1961 subjects including 762 children). We also included 5 publications in animal models of neurological diseases (experimental autoimmune encephalomyelitis [EAE] in mice, bacterial meningitis in rats, bacterial CNS infection in a dog, subarachnoid diverticulum in a cat).
3.2.2. Risk of bias assessment
Most studies showed a low risk of bias for the selection domain (that is, whether patients and controls were defined according to acknowledged diagnostic criteria); see Table S2 and S3. Many studies did not report on adjusting their statistical analyses for subject age, sex, or other potential confounders (comparability domain), thus potentially inducing biases.
3.3. Leptomeningeal enhancement in neuroinflammatory diseases including multiple sclerosis
3.3.1. Primary neuroinflammatory diseases overall
3.3.1.1. Diseases
Studies reporting on LME in neuroinflammatory diseases were in neurosarcoidosis (20 publications), MS (17 publications), MOG-antibody diseases/encephalitis (8 publications), neuromyelitis optica spectrum disorder (NMOSD) (8 publications), primary angiitis of the CNS (6 publications), Susac syndrome (5 publications), anti-NMDA-receptor encephalitis (4 publications), Behçet syndrome (1 publication), and GFAP astrocytopathy (1 publication).
3.3.1.2. LME pattern
21 studies did not report on the LME pattern, whereas the remaining studies reported on different LME patterns. The pattern of LME has most extensively been described in MS in 12 publications, mostly as either nodular and/or laminar/spread-and-fill.(Harrison et al., 2017, Hildesheim et al., 2020, Ighani et al., 2020, Makshakov et al., 2017, Titelbaum et al., 2020, Zivadinov et al., 2017, Zurawski et al., 2020) Similar LME patterns have been described in Susac syndrome (Coulette et al., 2019) and neurosarcoidosis.(Junger et al., 1993, O'Connell et al., 2017) A spreading/laminar phenotype has been described in NMOSD,(Asgari et al., 2017, Fan et al., 2016, Long et al., 2014) anti-NMDA-receptor encephalitis,(Neo et al., 2020) and GFAP astrocytopathy.(Dubey et al., 2018)
3.3.1.3. MRSI acquisition of LME
Most studies employed a postcontrast T2w-FLAIR sequence to visualize LME (19 publications) followed by a postcontrast T1w sequence (7 publications). 10 studies did not report the sequences used to detect LME.
3.3.1.4. Meta-analysis
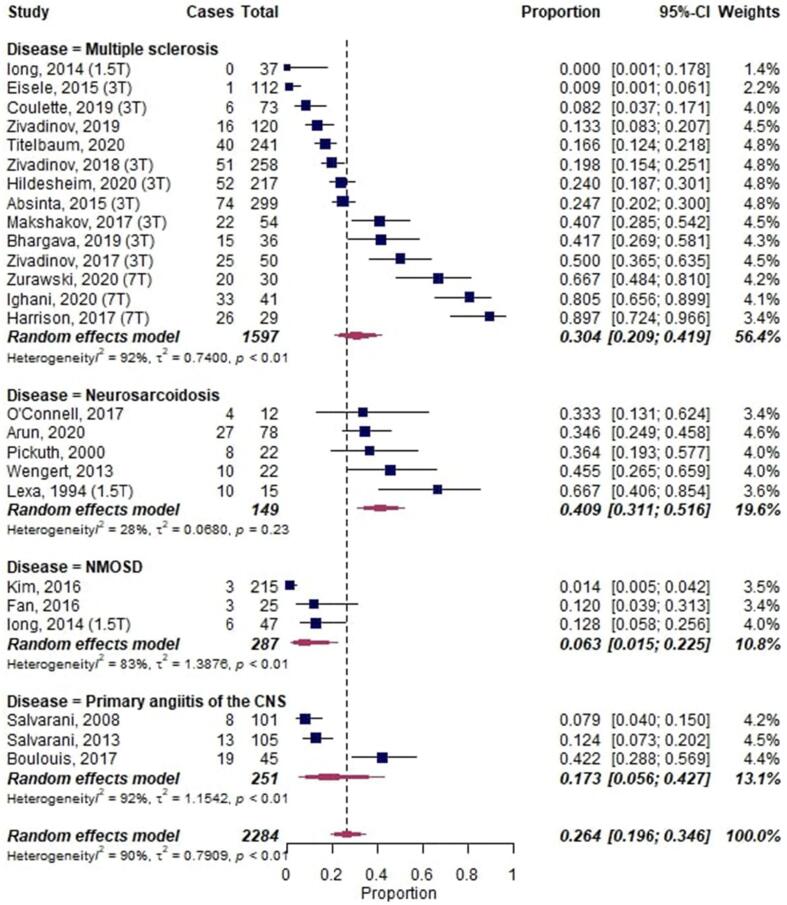
A meta-analysis of LME in primary neuroinflammatory diseases, including a total of 2284 patients, showed an overall proportion of 0.26 [95%-CI: 0.20–0.35] with substantial heterogeneity across studies (I2 = 90%, p < 0.001) (Fig. 2). NMOSD had the lowest proportion of LME with 0.06 [95%-CI 0.02–0.23], and neurosarcoidosis had the highest LME proportion with 0.41 [95%-CI 0.13–0.52].
Fig. 2.
Forest plot of leptomeningeal enhancement (LME) proportions in primary neuroinflammatory diseases. Pooled analyses of studies comparing the proportion of LME on MRI in neuroinflammatory diseases, stratified by diseases. Static magnetic field strength for MRI acquisition for respective studies are listed in brackets if reported. Proportions for LME were extracted and pooled using the random effects DerSimonian-Laird method. Abbreviations: CNS, central nervous system; CI, confidence interval; NMOSD, neuromyelitis optica spectrum disorder.
3.3.2. Multiple sclerosis
3.3.2.1. LME proportion in MS and subgroups
Two studies from 2015 first described LME in MS (Absinta et al., 2017, Eisele et al., 2015). These and subsequent studies comprised 1605 MS patients, 303 non-MS controls, and 126 healthy controls (Table 1).
Table 1.
Synopsis of studies assessing leptomeningeal enhancement (LME) in multiple sclerosis (MS).
| Study | n | Controls | Location | MRI field | MRI sequence | Pattern | Main findings |
|---|---|---|---|---|---|---|---|
| Eisele, 2015 | 112 | 5 stroke | Convexities | 3T | Pc. 2D T2w-FLAIR (at least 10 min delay), pc. 2D T1w | Nodular | Only 1/112 patient (<1%) showed evidence of LME in the right temporal lobe. This patient also showed 6 parenchymal contrast-enhancing lesions.Association of LME with imaging/clinical parameters:N/ATemporal dynamics of LME:N/A |
| Absinta, 2015 | 171 RRMS, 74 PPMS, 44 SPMS, 10 CIS | 37 HC | N/A | 3T in vivo, 7T postmortem | Pc. 3D T2w FLAIR (at least 10 min delay), pc. 3D T1w | Laminar, nodular | Focal LME was detected in the leptomeningeal compartment in 74 of 299 MS cases (25%) and in only 1 of 37 neurologically healthy controls (2.7%). Progressive MS showed around twice as much enhancement (39/118 cases, 33%) compared to relapsing-remitting MS (35/181, 19%). Association of LME with imaging/clinical parameters:Median age, disease duration, and EDSS were higher in LME+ MS patients compared to LME- patients. Relapsing or progressive phenotype were not associated with presence of LME. Whole brain and cortical volumes were lower in LME+ MS patients, but no difference for white matter lesions or white matter lesion volume. No association of LME with oligoclonal bands.Correlative histopathology showed perivascular lymphocytic and mononuclear infiltration in the enhancing areas in association with adjacent subpial cortical demyelination.Temporal dynamics of LME:Most LME foci (53/62, 85%) remained stable in shape and size throughout the evaluation period (up to 5.5 years). 1 LME focus disappeared; 6 new foci were detected in 4 patients. |
| Harrison, 2017 | 21 RRMS,4 SPMS,4 PPMS | 3 HC | Convexities | 7T | Pc. 3D T2w-MPFLAIR (20 min delay), pc. 3D T1w MP2RAGE (3 min delay) | Spread/fill (76% of subjects) and nodular (15% of subjects). Both types occurred simultaneously in 38% of patients. | LME on postcontrast 7T MPFLAIR is more prevalent than prior reports at 3T. T1w MP2RAGE images were consulted to exclude leptomeningeal vessels.Of note, no instances of spread/fill foci were seen in healthy controls. However, 2/3 healthy controls had nodular LME (normal variant?).Association of LME with imaging/clinical parameters:Spread/fill foci were associated with reduced cortical gray matter volumes. There were no differences in WM lesion, cerebral WM volume, thalamus, caudate, or putamen volume in those with and without spread/fill LME foci.Temporal dynamics of LME:N/A |
| Absinta, 2017 | 299 MS patients (same as in Absinta, 2015), | 189 non-MS, 66 HC | Convexities, infratentorial | 3T | Pc. 3D T2w FLAIR (at least 10 min delay), pc. 3D T1w | Nodular or linear | LME was observed in 56/254 non-MS patients (22%) compared to 74/299 (25%) of MS patients. LME was around 4-fold more common in non-MS inflammatory neurologic diseases (18/51 cases, 35%) than in noninflammatory neurologic diseases (3/38, 8%) and healthy volunteers (5/66, 8%). The highest prevalence of LME was detected in HTLV infections (17/38 cases, 45%), particularly in the setting of HTLV-associated myelopathy (14/25 cases, 56%). LME was also frequently detected in HIV infection (13/61 cases, 21%).Association of LME with imaging/clinical parameters:Unlike in MS, LME was not associated with lower brain and cortical volumes in non-MS inflammatory neurologic conditions, including HTLV and HIV infection.Temporal dynamics of LME:N/A |
| Makshakov, 2017 | 54 | 0 | Convexities (Mainly sulcal (65%) and less brain surface (35%) | 3T | Pc. 3D T2w-FLAIR (after T1w), pc. 3D T1w (lower sens for LME detection than T2w-FLAIR) | Linear (13%), plate-like (31%), nodular (54%) | LME was detected in 41% of MS patients.Association of LME with imaging/clinical parameters:LME+ patients had longer disease duration and higher EDSS score, but an equal relapse rate. No association of LME with higher frequency of contrast-enhancing lesions. LME+ patients had lower cortical volume, the total grey matter volume as well as total ventricular volume. No difference in oligoclonal bands or kappa-FLC.Temporal dynamics of LME:N/A |
| Xia, 2017 | 100 first-degree relatives of MS | 0 | N/A | 3T | Pc. 3D T2w FLAIR (at least 10 min delay), pc. 3D T1w | N/A | Higher-risk asymptomatic family members of patients with MS are more likely to have early subclinical manifestations of MS and deserve further monitoring. A subset of participants harboured LME, consistent with the hypothesis that these subjects ar at higher risk for developing MS.Association of LME with imaging/clinical parameters:N/ATemporal dynamics of LME:N/A |
| Zivadinov, 2017 | 27 RRMS, 23 SPMS | 0 | Convexities (mainly surface (79%) and less sulcal (21%) | 3T | Pc. 3D T2w-FLAIR (10 min delay), pc. 2D FLAIR (after Gd-injection), pc. 2D T1w | Most of the LME foci were nodular (49, 80%), linear, plate-like | In total, 25/50 MS patients (50%) showed LME at the 5-year follow-up. Of note, No LME foci were detected on 2D T2w-FLAIR or 2D T1w sequences.Association of LME with imaging/clinical parameters:SPMS presented with significantly more LME foci (12, 85.7%) compared to RRMS (2, 18.2%). LME+ MS patients had greater percentage decrease in total GM (−3.6% vs −2%) and cortical (−3.4% vs −1.8%) volumes and greater percentage increase in ventricular cerebrospinal fluid volume (22.8% vs 9.9%) over the follow-up compared to LME- MS patients. SPMS patients with LME showed higher T1 lesion volume increase compared to patients without LME. No difference in annual relapse rate or DMT.Temporal dynamics of LME:N/A |
| Jonas, 2018 | 21 RRMS, 7 SPMS, 3 PPMS | 0 | Convexities, mostly frontoparietal (75%) | 7T | Pc. 3D MP2RAGE (3 min delay, pc. MPFLAIR (20 min delay) | Subarachnoid spread/fill or nodular, vessel wall, dural (also including non-LME enhancement patterns) | 2-year follow up study. At baseline, 284 LME foci among 31 MS patients are reported., 25/50 MS patients (50%) showed LME at the 5-year follow-up. Of note, No LME foci were detected on 2D T2w-FLAIR or 2D T1w sequences.Association of LME with imaging/clinical parameters:No difference in the total number/proportion of longitudinallypersistent LME foci between those on or off treatment or between progressive versus relapsing MS. More persistent LME foci were present in EDSS progressors compared to non-EDSS progressors (median, 12; range 1–15 versus median, 7.5; range 1–24).Temporal dynamics of LME:In total, 15 additional LME foci developed within the follow-up period. LME patterns were: 6 subarachnoid spread/fill, 4 subarachnoid nodular, 2 vesselwall, and 3 dural foci. |
| Zivadinov, 2018 | 212 RRMS, 32 SPMS, 14 CIS | 0 | Convexities | 3T | Pc. 3D T2w-FLAIR (10 min delay; native, subtracted, or co-registered) | N/A | Study compared LME detection on 3D T2w-FLAIR postcontrast images in native space (method 1), on pre- and postcontrast 3D T2w-FLAIR images in native space (method 2), and on pre-/postcontrast 3D T2w-FLAIR co-registered and subtracted images (method 3).In total, 51 (20%) patients with MS showed LME using method 1; 39 (15%) using method 2; and 39 (15%), The mean time to analyze the 3D T2w-FLAIR images was lower with method 2 compared to the other 2 methods.Association of LME with imaging/clinical parameters:Higher T1 and T2 lesion volume in LME+ MS patients. Similar volume of contrast-enhancing lesions between LME+ and LME- patients. LME+ patients had higher EDSS and age but similar MS disease duration.Temporal dynamics of LME:N/A |
| Bergsland, 2019 | 43 RRMS, 15 SPMS | 0 | N/A | 3T | Pc. 3D T2w-FLAIR (10 min delay), Pc. 3D T1w | N/A | Focal LME is associated with reduced thickness of the adjacent cortex in patients with RRMS, but not in those with secondary-progressive MS. Association of LME with imaging/clinical parameters:See above.Temporal dynamics of LME:N/A |
| Bhargava, 2019 | 36 progressive MS | 0 | N/A | 3T | Pc. 3D T2w-FLAIR (10 min delay), Pc. 3D T1w | N/A | Study assessed safety of intrathecal rituximab on progressive MS and its potential effect on LME. Out of 36 screened patients, 15 had LME (42%). LME frequency did not change following intrathecal rituximab treatment.Association of LME with imaging/clinical parameters:N/ATemporal dynamics of LME:There was no change in the number or shape of LME during the 24-week follow-up period. There was no appearance of new LME over the course of the study. |
| Coulette, 2019 | 73 MS | 9 Susac | Convexities (MS), Cerebellar (Susac) | 3T | Pc. 3D T2w-FLAIR (3 min delay), Pc. 3D T1w | 2 nodular, 4 linear | Susac syndrome patients were more likely to present with LME: 5/9 (56%) versus 6/73 (8%) in the MS group.Association of LME with imaging/clinical parameters:N/ATemporal dynamics of LME:No evolution data on MS. |
| Zivadinov, 2019 | 120 RRMS | 0 | N/A | 3T | Pc. 3D T2w-FLAIR (10 min delay), pc. 3D T1w | N/A | No significant difference in LME between teriflunomide or dimethylfumarate treated patients. 12 out of 120 patients with LME (10%).Association of LME with imaging/clinical parameters:N/ATemporal dynamics of LME:Out of 8 dimethylfumarate-treated patients who presented with LME at baseline, 6 continued to show the same LME foci at 12 and 24 months, one other patient under dimethylfumarate developed a new LME focus over the follow-up. Of 4 teriflunomide-treated patients who presented with LME foci at baseline, 2 patients continued to present the same LME foci at follow-up, whereas one patient treated with teriflunomide developed a new LME focus over the follow-up |
| Bonnan, 2020 | 1 prog MS | 0 | Convexities | 3T | Pc. 3D T2w-FLAIR | laminar | Spontaneously remitting LME focus in 1 SPMS patient.Association of LME with imaging/clinical parameters:LME focus was associated with adjacent cortical thinning.Temporal dynamics of LME:Disappearance of focus at 3 and 9 months follow-up. |
| Hildesheim, 2020 | 193 RRMS, 24 progressive MS | 0 | Convexities | 3T | Pc. 3D T2w-FLAIR (10 min delay, subtraction),Pc. 3D T1w | Nodular (89%), filling-like (11%) | Fifty-three out of 217 MS patients (24%) had at least one LME focus. Association of LME with imaging/clinical parameters:No difference in LME between relapsing and progressive MS (23% vs. 33%). No difference in EDSS between LME+ and LME- MS patients when adjusted for age. LME+ MS patients showed higher CSF volume and more contrast-enhancing lesions compared to LME- patients at baseline. LME frequency was associated with higher age. LME was not associated with clinical or imaging markers of MS severity (T1 and T2 lesion volume).Temporal dynamics of LME:analyzed for persistence over 18 months follow-up. Of the 76 LME foci at baseline, 68 foci (90%) remained stable in shape and size. 8 LME foci resolved during the follow-up interval, a total of 14 LME foci were newly detected. |
| Ighani, 2020 | 31 RRMS, 5 SPMS, 5 PPMS | 5 HC | N/A | 7T | Pc. 3D T2w-MPFLAIR (unclear delay), pc. 3D MP2-RAGE T1w | Nodular, spread/fill-sulcal (59%), spread/fill-gyral (61%), spread/fill-infratentorial | 33/41 MS patients had LME (81%) and 27 had > 1 LME focus. One LME focus was found in 3/5 healthy controls (60%), one subject with a nodular LME and two cases with one spread/fill-sulcal LME each. None of the control subjects had >1 LME focus.Association of LME with imaging/clinical parameters:There was an association between spread/fill-sulcal LME and hippocampal lesion count in RRMS. Participants with RRMS had no correlation with cortical lesions, but significant correlations were detected between LME and hippocampal lesion count, normalized cortical gray matter volume, and mean cortical thickness. WM lesion volume was greater in patients with >1 focus of spread/fill-sulcal LME compared to those with ⩽1 focus.Temporal dynamics of LME:N/A |
| Titelbaum, 2020 | 241 | 100 non-MS | Convexities, rarely cerebellar | 1.5T (most patients), 3T | Pc. 3D T2w-FLAIR (delay 7 – 54 min) | Nodular, curvilinear | A total of 16.6% (40/241) of MS patients had LME compared to 8% (8/100) in non‐MS patients. There was no association with MS subtype, therapy, or disease activity. General Electric's version of 3D T2w-FLAIR (29%) was greater than with Siemen's 3D T2w-FLAIR (12%) at 1.5T. LME kinetics were heterogeneous, even within patients, without uniform optimal time for acquisition.Of note, Imaging pitfalls fell into three categories: contrast leakage of uncertain medical significance; enhancements related to cortical veins/anatomic structures; and imaging artifactsAssociation of LME with imaging/clinical parameters:N/ATemporal dynamics of LME:LME foci were overall persistent over the observation period (0.5 – 6 months) but resolved in 2 patients following high‐dose steroids. |
| Zurawski, 2020 | 30 RRMS | 15 HC | Convexities | 7T | Pc. 3D T2w-FLAIR (10 min delay), pc. 3D MP2RAGE T1w | nodular only n=12 (60%), any spread n=8 (40%) | Two thirds of MS patients had LME. Patients had a mean of 2.7 (± 1.5) LME foci.Association of LME with imaging/clinical parameters:LME+ patients had longer disease duration, a sixfold higher cortical lesion volume, and a higher T1/T2 lesion volume compared to LME- patients. The number of LME foci correlated with cortical and thalamic lesions.Patients with spread LME significantly higher cortical lesion volumes.Temporal dynamics of LME:N/A |
Studies are in chronological and alphabetical order. Abbreviations: FLAIR, fluid-attenuated inversion recovery (MP, magnetization-prepared); HC, healthy control; LME, leptomeningeal enhancement; MS, multiple sclerosis (RR relapsing-remitting, SP, secondary progressive; PP, primary progressive); pc, post-contrast.
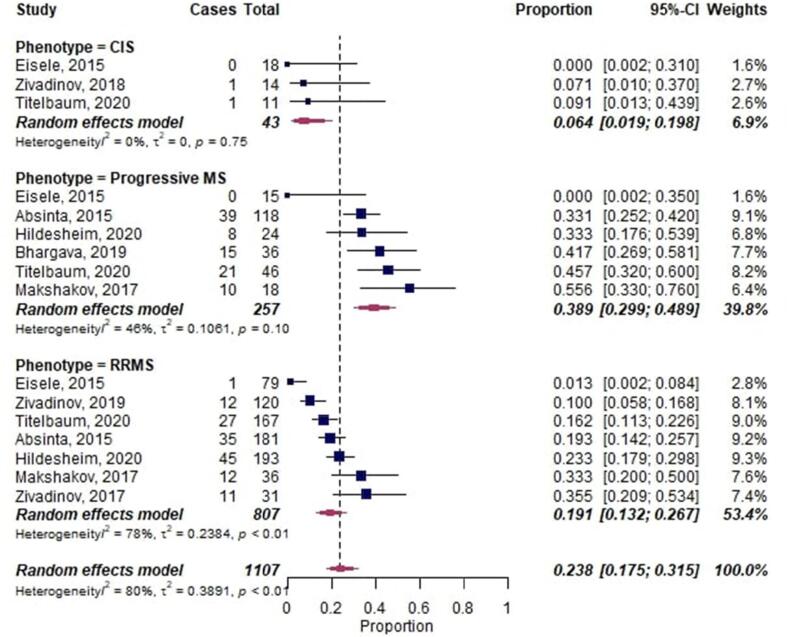
The overall proportion of LME in MS was 0.30 [95%-CI 0.21–0.42] (Fig. 3). However, LME proportions in MS patients with a relapsing-remitting clinical phenotype (0.19 [95%-CI 0.13–0.27]; 7 publications) and CIS patients (0.06 [95%-CI 0.02–0.20]; 3 publications) were significantly lower compared to progressive MS patients (0.39 [95%-CI 0.30–0.49], p = 0.002 and p = 0.003, respectively; 6 publications).
Fig. 3.
Forest plot of leptomeningeal enhancement (LME) proportions in multiple sclerosis (MS). Pooled analyses of studies comparing the proportion of LME on MRI in MS, stratified by clinical phenotype. Proportions for LME were extracted and pooled using the random effects DerSimonian-Laird method. Abbreviations: CI confidence interval; CIS, clinically isolated syndrome; RRMS, relapsing-remitting multiple sclerosis.
3.3.2.2. MRI acquisition of LME
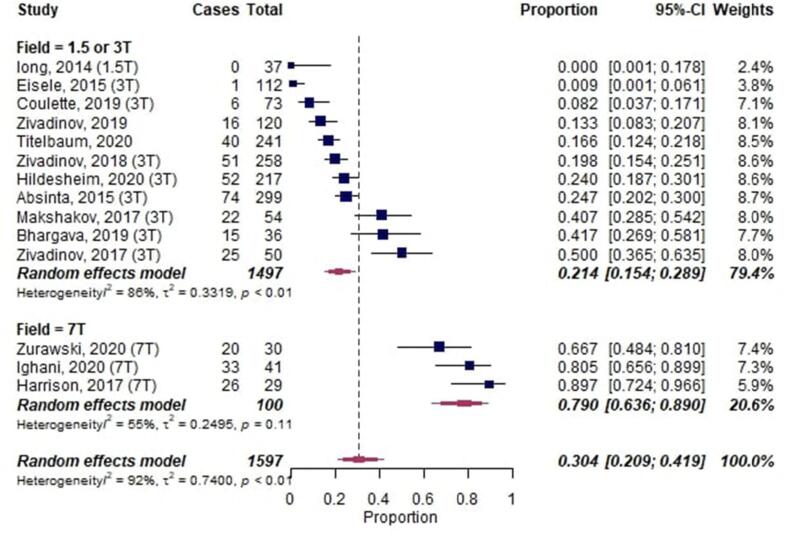
14 studies acquired MRI at 3 T (or complementing 1.5 T) and 3 studies at 7 T (Harrison et al., 2017, Ighani et al., 2020, Zurawski et al., 2020). In a meta-subgroup-analysis with the B0 magnetic field strength as moderator, LME proportions were higher in studies imaging at 7 T (0.79 [95%-CI 0.64–0.89]; 3 publications) compared to studies imaging at 1.5/3T (0.21 [95%-CI 0.15–0.29], p < 0.001; 11 publications) (Fig. 4).
Fig. 4.
Forest plot of leptomeningeal enhancement (LME) proportions in multiple sclerosis (MS) with B0 magnetic field strength as moderator. Pooled analyses of studies comparing the proportion of LME on MRI in MS, stratified by magnetic field strength. Proportions for LME were extracted and pooled using the random effects DerSimonian-Laird method. Abbreviations: CI confidence interval.
All 17 studies investigating LME in MS employed both a postcontrast 3D T2w-FLAIR and a postcontrast 3D T1w sequence to detect LME. Of note, 1 study acquired 2D scans instead of 3D, showing the lowest LME proportion among all MS studies (<0.01, 1/112 cases) (Eisele et al., 2015). The acquisition of the 3D T2w-FLAIR sequence was mostly 10 min after Gd-injection, with two studies reporting either earlier (3 min) (Coulette et al., 2019) or later acquisition (up to 54 min) (Titelbaum et al., 2020). It is noteworthy that latter study suggested that individual LME foci might have different enhancement kinetics and thus different peak enhancement time points, and that differences in LME detection between scanner types could be due to differences in 3D T2w-FLAIR sequences.
In a non-MS cohort, it has been shown that 3D T2w-FLAIR can have higher sensitivity to detect gadolinium enhancement compared to T1w imaging, particularly for superficial enhancement (Mathews et al., 1999). Along these lines, one MS study found lower sensitivity for LME detection using T1w sequences compared to T2w-FLAIR (Makshakov et al., 2017). Another study compared LME detection on 3D T2w-FLAIR postcontrast images in native space (method 1), on pre- and postcontrast 3D T2w-FLAIR images in native space (method 2), and on pre-/postcontrast 3D T2w-FLAIR co-registered and subtracted images (method 3) (Zivadinov et al., 2018). In total, 51 (20%) MS cases showed LME using method 1; 39 (15%) using method 2; and 39 (15%) using method 3. The mean time to analyze the 3D T2w-FLAIR images was lower with method 2 compared to the other 2 methods.
3.3.2.3. LME phenotypes
Overall, two configurations of LME have been described, albeit with inhomogeneous nomenclature: nodular and “spread-fill” (subsuming linear, laminar, and plate-like). Most studies described these two phenotypes (12/17 publications) (Absinta et al., 2017, Absinta et al., 2015, Coulette et al., 2019, Harrison et al., 2017, Hildesheim et al., 2020, Ighani et al., 2020, Makshakov et al., 2017, Titelbaum et al., 2020, Zivadinov et al., 2017, Zurawski et al., 2020). 1 early publication with a very low LME proportion of < 0.01 exclusively described a nodular LME phenotype (Eisele et al., 2015). 5 publications did not report on the LME pattern.
The prevalence of these patterns varied considerably between studies: 4 publications observed higher frequencies of nodular LME: 60% (vs. spread 40%) (Zurawski et al., 2020), 54% (vs. 13% linear and 31% plate-like) (Makshakov et al., 2017), 80% (vs. linear and plate-like) (Zivadinov et al., 2017), and 89% (vs. 11% filling-like) (Hildesheim et al., 2020). Two publications described higher frequencies of linear/spread-fill LME: 59–61% for spread-fill sulcal or gyral (vs. nodular) (Ighani et al., 2020) and 76% spread-fill (vs. 15% nodular) (Harrison et al., 2017b). The latter study observed simultaneous presence of both LME phenotypes in 38% of MS cases.
3.3.2.4. Temporal evolution of LME
Eleven studies did not include longitudinal MRI data and did thus not assess temporal evolution of LME. The remaining 6 studies consistently reported mostly stable LME foci over several years. One study with a follow-up period of up to 5.5 years reported that 85% of LME foci remained stable and that only 6 new LME foci were detected over this observation period (in 4 of 299 MS patients) (Absinta et al., 2015). Similar high percentages of stable LME foci have been observed in other studies: 75% stable over 24 months (and 2 new LME foci in 2/120 patients) (Zivadinov et al., 2019), 90% stable over 18 months (and 14 new LME foci) (Hildesheim et al., 2020), 100% stable over 24 weeks (Bhargava et al., 2019), and 73–100% stable over 24 months (Jonas et al., 2018). The latter study also included non-LME enhancement patterns and found that subarachnoid nodular and spread/fill LME patterns persisted less often than dural or vessel wall foci, as well as that MS patients with EDSS progression showed more persistent LME foci.
3.3.2.5. Association of LME with clinical and imaging parameters
Three studies found an association between LME and age and/or disease duration (Absinta et al., 2015, Hildesheim et al., 2020, Makshakov et al., 2017), which was not confirmed for disease duration in 1 study (Zivadinov et al., 2018). An association between LME and Expanded Disability Status Scale (EDSS) was described in 3 studies (Absinta et al., 2015, Makshakov et al., 2017, Zivadinov et al., 2018) but was not confirmed in 1 study after adjusting for age (Hildesheim et al., 2020). MS relapse rate was not associated with LME in 2 studies (Makshakov et al., 2017, Zivadinov et al., 2017).
Five studies assessed the association between LME and T1 or T2 lesion volume. Three studies found such an association (Zivadinov et al., 2018, Zivadinov et al., 2017, Zurawski et al., 2020), whereas 2 did not (Harrison et al., 2017, Hildesheim et al., 2020). Six studies consistently reported lower cortical gray matter volume and/or thickness in MS cases with LME (Bergsland et al., 2019, Harrison et al., 2017, Ighani et al., 2020, Makshakov et al., 2017, Zivadinov et al., 2017, Zurawski et al., 2020). Two studies assessed the association of LME with cortical MS lesions at 7 T; one of these studies found no such association (Ighani et al., 2020) while the other did (Zurawski et al., 2020). Both studies found an association of LME with subcortical gray matter MS lesions (thalamic and hippocampal, respectively).
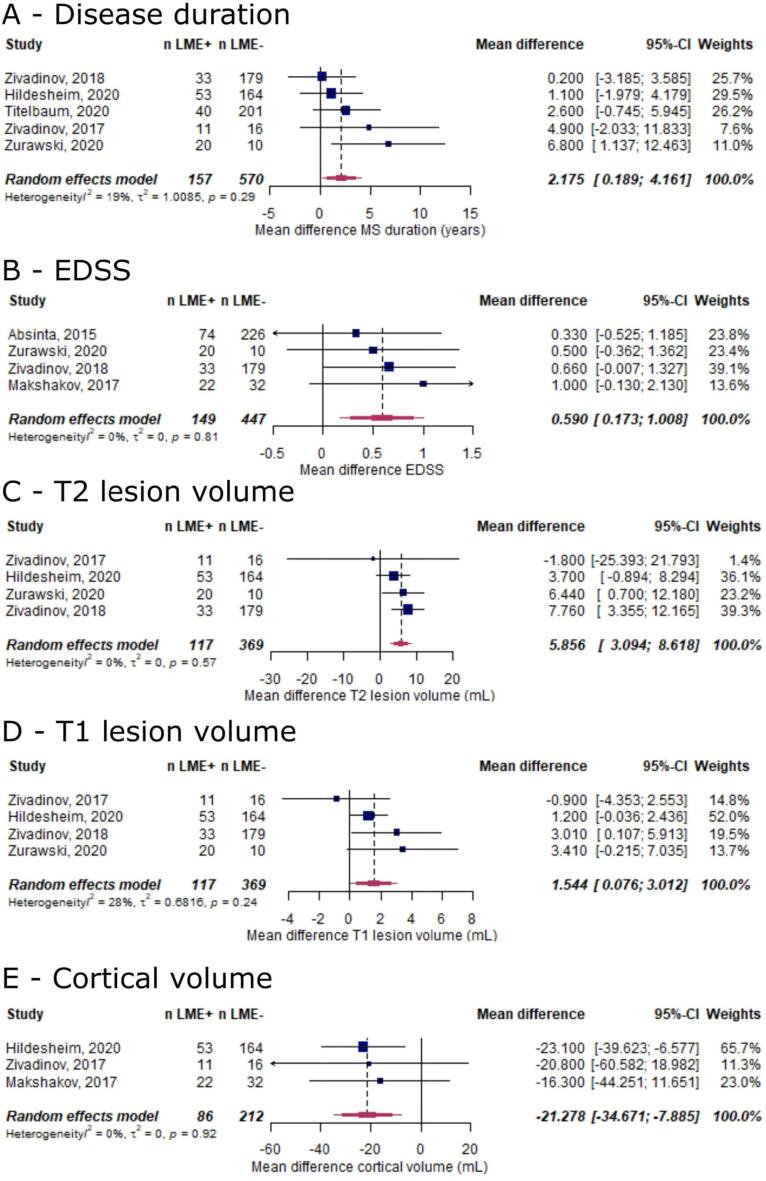
In view of the inconsistency regarding the association of LME with clinical and imaging parameters in MS, we assessed these effects in a meta-analysis (for all outcomes reported in ≥ 3 publications). In this analysis, the presence of LME was associated with longer disease duration (mean difference 2.2 years [95%-CI 0.2–4.2], p = 0.03, Fig. 5A) and higher EDSS (mean difference 0.6 EDSS points [95%-CI 0.2–1.0], p = 0.006, Fig. 5B). Additionally, MS cases with LME showed higher T1 lesion volume (mean difference 1.5 ml [95%-CI 0.1–3.0], p = 0.04, Fig. 5C), higher T2 lesion volume (mean difference 5.9 ml [95%-CI 3.2–8.6], p < 0.001, Fig. 5D), and lower cortical volume (mean difference −21.3 ml [95%-CI −34.7–-7.89], p = 0.002, Fig. 5E).
Fig. 5.
Forest plot for subgroup analysis for the association between LME and clinical and imaging outcomes in MS. Pooled analyses of studies comparing the mean differences of clinical and imaging outcomes between LME-positive and LME-negative MS cases (A, disease duration [years]; B, EDSS [in EDSS points]; C, T1 lesion volume [in mL]; D, T2 lesion volume [in mL]; E, cortical gray matter volume [in mL]). Mean differences were extracted and pooled using the random effects DerSimonian-Laird method. Abbreviations: CI confidence interval; EDSS, Expanded Disability Status Scale; LME, leptomeningeal enhancement.
3.3.2.6. Histopathological validation of LME
One study performed histopathological validation of three LME foci in two progressive MS cases (Absinta et al., 2015). The gyri adjacent to the LME foci were affected by confluent cortical demyelination and/or subpial cortical demyelination. Leptomeningeal perivascular inflammation, including T cells, B cells, and macrophages, was detected in these areas.
3.3.2.7. LME in MS animal models
We included two studies that assessed LME in murine EAE using postcontrast T2w-FLAIR. The first study employing 9.4 T MRI found that all 13 inoculated mice showed LME foci, compared to none of the control mice (Pol et al., 2019). Peak LME intensity was at 10 days post induction and correlated with weight loss and clinical symptoms. In a histopathological analysis, LME foci were associated with high densitiy of Iba-1 positive microglia cells as well as T and B cells, which were absent in control mice. The second study at 11.7 T showed that mice treated with a Bruton tyrosine kinase-inhibitor (evobrutinib) had a reduced number of LME foci, while anti-CD20 therapy had no effect on LME (Bhargava et al., 2021). The pathological tissue substrate showed that this corresponded to a reduction in B cells within regions of meningeal inflammation as well as reduced astrocytosis in the adjacent cortex. Interestingly, myeloid cell infiltrates seemed to persist despite B cell depletion.
3.3.2.8. Therapeutic impact on LME
Four studies assessed the impact of drug treatment on LME resolution. One study found similar LME persistence rates in MS patients with/without disease-modifying therapy (DMT) (Jonas et al., 2018). Another study assessing the efficacy of dimethyl fumarate or teriflunomide on LME reported no differences in LME resolution between treatment groups (8 of 12 patients showed stable LME, and 2 patients developed new LME) (Zivadinov et al., 2019). One study assessing intrathecal rituximab treatment in 15 progressive MS patients observed stable LME foci in all patients over the 24-week follow-up period (Bhargava et al., 2019). In contrast to these studies with relatively small sample size and consequently lower statistical power, one study including 241 MS patients observed resolution of LME in 2 patients after high-dose steroid treatment within 6 months follow-up (Titelbaum et al., 2020).
3.4. Leptomeningeal enhancement in other neurological diseases
3.4.1. Infectious CNS diseases
3.4.1.1. Diseases
Studies reported on LME in infectious (encephalo-)meningitis caused by various pathogens: bacterial (36 publications: tuberculosis, Bacillus anthracis/Anthrax, Borrelia, Clostridium, E. coli, group B streptococcus, Listeria), parasitic (20 publications, among them Angiostrongylus cantonensis, amoeba, Cryptococcus, Toxocariasis, and Toxoplasmosis), viral (18 publications: HIV, HTLV, SARS-CoV-2, Epstein-Barr virus, Murray Valley encephalitis, tick-borne encephalitis virus, Nipah virus, respiratory viruses [various strains], West Nile virus, and Enterovirus), and fungal (14 publications: Candida, Coccidioides, Blastomyces, and Histoplasma).
3.4.1.2. LME pattern
32 studies did not report on the LME pattern, while 4 studies did (2 spread, 2 nodular). 33 studies did not report on the LME evolution over follow-up, while 3 studies reported LME increase with clinical worsening, and 1 study in cryptococcal meningitis reported LME resolution with clinical improvement (Sarkis et al., 2015).
3.4.1.3. Imaging
Most studies employed a postcontrast T1w sequence to visualize LME (21 publications), followed by T2w-FLAIR (7 publications). 6 studies did not report which sequences were used for LME detection.
3.4.1.4. Meta-analysis
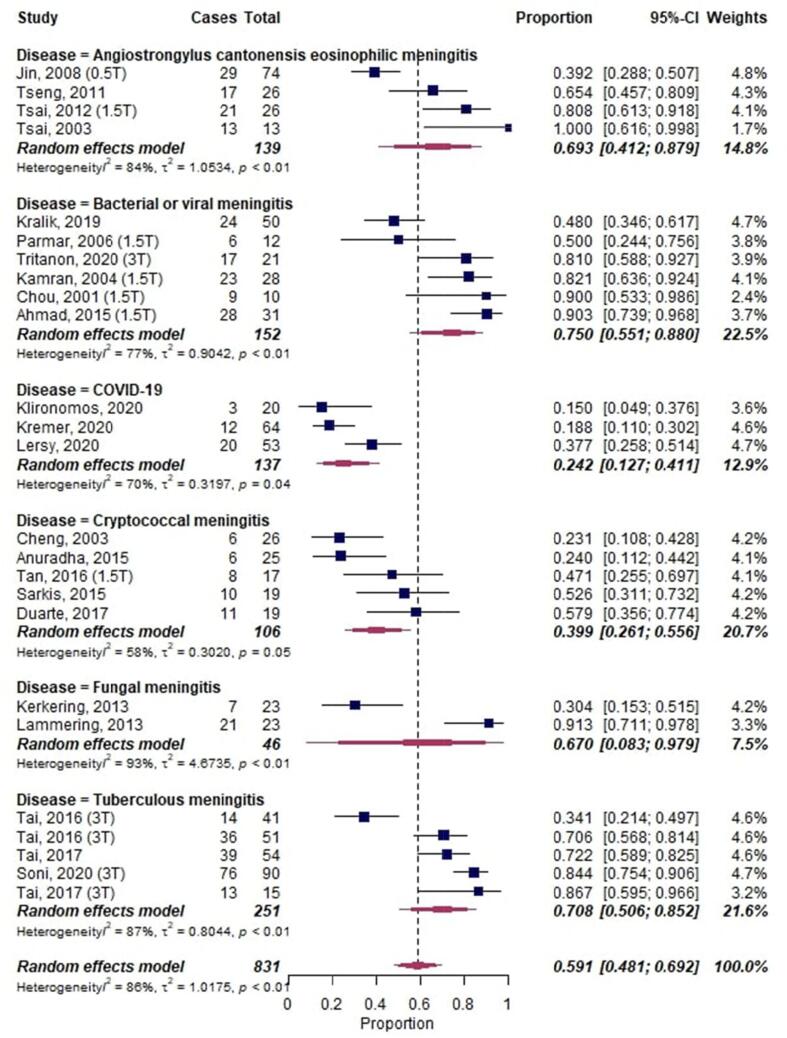
A meta-analysis on LME in infectious diseases, including a total of 831 cases, showed an overall proportion of 0.59 [95%-CI: 0.47–0.69] with substantial heterogeneity across studies (I2 = 86%, p < 0.01) (Fig. 6). COVID-19 had the lowest proportion of LME with 0.24 [95%-CI 0.13–0.41].
Fig. 6.
Forest plot of leptomeningeal enhancement (LME) proportions in infectious neurological diseases. Pooled analyses of studies comparing the proportion of LME on MRI in infectious neurological diseases. Static magnetic field strength for MRI acquisition for respective studies are listed in brackets if reported. Proportions for LME were extracted and pooled using the random effects DerSimonian-Laird method. Abbreviations: CI confidence interval.
3.5. Neoplastic CNS diseases
3.5.1. Diseases
Studies reporting on LME in neoplastic CNS diseases were associated with primary CNS tumors (92 publications: CNS lymphoma, choroid plexus papilloma, meningioma, germinoma, lipoma, primitive neuroectodermal tumors (PNET), diffuse leptomeningeal glioneuronal tumor, midline glioma, hemangioblastoma, glioblastoma/high-grade astrocytoma, Hodgkin lymphoma, xanthogranuloma, medulloblastoma, melanoma, oligodendroglioma, pilocytic astrocytoma, anaplastic astrocytoma, and giant cell astrocytoma), leptomeningeal metastases (47 publications: metastases from breast cancer, acute myeloid leukemia, rhabdomyosarcoma, gastric cancer, lung adenocarcinoma, small-cell lung cancer, pancreatic cancer, melanoma, Waldenstrom macroglobulinemia [Bing-Neel syndrome], and multiple myeloma), and hereditary tumor syndromes (5 publications: von Hippel-Lindau syndrome, Klippel-Trenaunay-Weber syndrome, and tuberous sclerosis).
3.5.2. LME pattern
28 studies did not report on the LME pattern, while 8 studies did (3 spread, 3 nodular, 2 laminar/linear). 33 studies did not report on LME evolution over follow-up, while 1 study in glioblastoma reported persistent LME at follow-up MRI (up to two years later).(Kim et al., 2018)
3.5.3. Imaging
Most studies employed a postcontrast T1w sequence to visualize LME (23 publications) followed by a postcontrast T2w-FLAIR (6 publications). 9 studies did not report which sequences were used for LME detection.
3.5.4. Meta-analysis
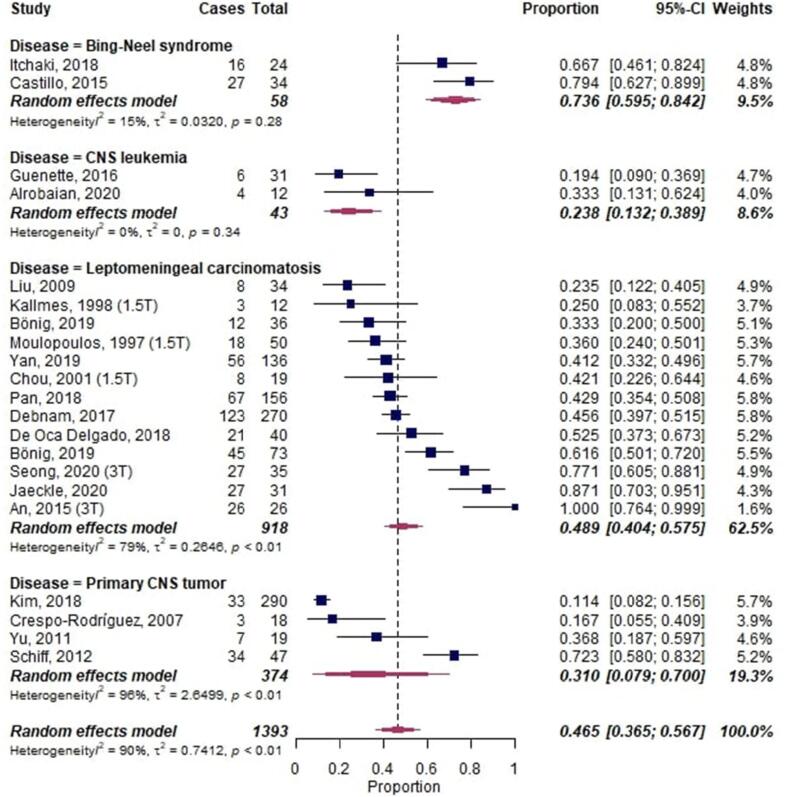
A meta-analysis on LME in neoplastic diseases, including a total of 1393 cases, showed an overall proportion of 0.47 [95%-CI: 0.37–0.57] with substantial heterogeneity across studies (I2 = 90%, p < 0.01) (Fig. 7). CNS leukemia had the lowest proportion of LME with 0.24 [95%-CI 0.13–0.39]. Bing-Neel syndrome (Waldenstrom macroglobulinemia) showed the highest proportion of LME with 0.74 [95%-CI 0.60–0.84].
Fig. 7.
Forest plot of leptomeningeal enhancement (LME) proportions in neoplastic neurological diseases. Pooled analyses of studies comparing the proportion of LME on MRI in infectious neurological diseases. Static magnetic field strength for MRI acquisition for respective studies are listed in brackets if reported. Proportions for LME were extracted and pooled using the random effects DerSimonian-Laird method. Abbreviations: CI confidence interval; CNS, central nervous system.
3.6. Other neurological diseases including vascular diseases
3.6.1. Diseases
Of 147 publications, the most notable neurological diseases not belonging to the classes above were: rheumatoid arthritis with meningitis (16 publications), Sturge-Weber syndrome (16 publications), familial leptomeningeal amyloidosis/polyneuropathy (11 publications), ischemic (reversible cerebral vasoconstriction syndrome, stroke, post interventional revascularization, severe carotid stenosis, 11 publications), cerebral amyloid angiopathy (7 publications), epileptic seizures (6 publications), Moyamoya disease (6 publications), intoxications/drug-induced LME (abrin, ibuprofen, ipilimumab, propofol [in children], tacrolimus; 5 publications), hemophagocytic lymphohistiocytosis (4 publications), posterior reversible encephalopathy syndrome (PRES) (4 publications), Rosai-Dorfman disease (3 publications), hepatic encephalopathy (3 publications), Sjogren syndrome (2 publications), and traumatic brain injury (2 publications).
3.6.2. Meta-analysis
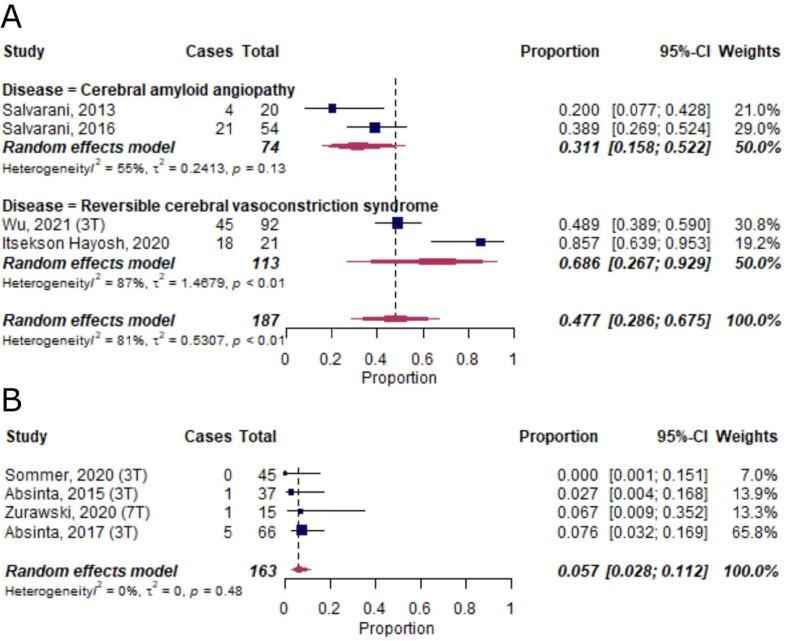
A meta-analysis on diseases with ≥ 2 publications and ≥ 10 subjects per study, including a total of 187 cases, showed an LME proportion of 0.31 [95%-CI 0.16–0.52] in cerebral amyloid angiopathy and 0.69 [95%-CI 0.27–0.93] in reversible cerebral vasoconstriction syndrome (Fig. 8A).(Itsekson Hayosh et al., 2020, Wu et al., 2021)
Fig. 8.
Forest plot of leptomeningeal enhancement (LME) proportions in neurological diseases not classified into the other groups and in healthy controls. Pooled analyses of studies comparing the proportion of LME on MRI in other neurological diseases (A) and in healthy control subjects (B). Static magnetic field strength for MRI acquisition for respective studies are listed in brackets if reported. Proportions for LME were extracted and pooled using the random effects DerSimonian-Laird method. Abbreviations: CI confidence interval.
3.7. Leptomeningeal enhancement in healthy controls
LME has also been reported in healthy subjects (6 publications). A meta-analysis of 4 publications, including a total of 163 individuals, corroborated the presence of LME in this group, albeit at a low overall proportion of around 0.06 [95%-CI 0.03–0.11] (Fig. 8B). In addition to the low proportions of LME, it has been shown in 2 publications that none of the LME-positive control subjects had more than 1 LME focus.(Ighani et al., 2020, Zurawski et al., 2020) In another publication, the 2 LME-positive controls exclusively presented with more than one nodular LME foci.(Harrison et al., 2017a)
4. Discussion
4.1. Main findings
This study aims to provide systematic evidence on LME proportion in neurological diseases, including MS, and to determine whether the role of LME as a prognostic biomarker for MS is substantiated in the literature. Overall, primary neuroinflammatory diseases showed lower LME proportion (0.26 [95%-CI: 0.20–0.35]) compared to neoplastic (0.47 [95%-CI: 0.37–0.57]) or infectious neurological diseases (0.59 [95%-CI: 0.47–0.69]). Additionally, the presence of LME was associated with worse clinical and imaging parameters in MS, that is, on average MS patients with LME had 2 years longer disease duration (p = 0.03), higher EDSS by 0.7 points (p = 0.006), 21 ml less cortical volume (p = 0.002), 5.9 ml more T2 lesion volume (p < 0.001), and 1.6 ml more T1 lesion volume (p = 0.04) compared to MS patients without LME. Finally, based on a few histopathological validation studies in MS and neuroinflammatory animal models, LME corresponds to meningeal inflammatory infiltrates as well as microglial activation in the adjacent cortex. However, the evidence supporting the association of LME with cortical MS pathology remains conflicting.
4.2. Findings in the context of existing evidence
A wide variety of disorders may present with LME, including neoplastic and infectious neurological diseases, making LME a highly nonspecific imaging finding. However, proportions of LME have considerable ranges across different neurological diseases. High LME proportions (on the order of 0.75), have been observed in Bing-Neel syndrome (a rare complication of Waldenstrom's macroglobulinemia) (Castillo et al., 2015, Itchaki et al., 2018) and infectious meningitis (Ahmad et al., 2015, Kralik et al., 2019, Soni et al., 2020). Interestingly, a subset of smaller studies employing ultrahigh-field (7 T) static magnetic field strengths also found proportions of LME around 0.8 in MS (Harrison et al., 2017, Ighani et al., 2020, Zurawski et al., 2020), which may indicate a need for higher static magnetic field strengths to facilitate LME detection.
Other notable diseases with LME include ischemic neurological diseases, such as reversible cerebral vasoconstriction syndrome (proportion around 0.7) (Itsekson Hayosh et al., 2020, Wu et al., 2021) and stroke (not included in the meta-analysis) (Henning et al., 2008, Latour et al., 2004, Warach and Latour, 2004). The presence of LME in brain ischemia also suggests a relevant role of the leptomeningeal compartment in its pathogenesis. Here, LME on post-contrast T2w-FLAIR has been attributed to early blood–brain-barrier disruption (Latour et al., 2004), also being associated with hemorrhagic transformation and worse clinical outcomes (Latour et al., 2004). Furthermore, poor leptomeningeal collateral flow has been associated with worse clinical outcome in acute stroke (Menon et al., 2013). Finally, also COVID-19 has been reported to present with LME, albeit with low proportions around 0.25 (Klironomos et al., 2020, Kremer et al., 2020, Lersy et al., 2020).
Several primary neuroinflammatory diseases can also present with LME, among them neurosarcoidosis (proportion around 0.4), MS (0.3), primary angiitis of the CNS (0.2), NMOSD (0.06), and Susac’s syndrome (not included in the meta-analysis) (Susac et al., 2003). Of note, in MS, LME proportions seem to vary among clinical phenotypes, with relapsing-remitting having lower proportions than progressive MS (0.2 vs. 0.4). However, overall longer disease duration in progressive MS could be a confounding factor.
Our meta-analysis substantiates the role of LME as prognostic biomarker in MS. The presence of LME was associated with worse physical disability and higher lesion burden as well as lower cortical volumes — the latter being also associated with worse clinical MS outcomes (reviewed in (Calabrese et al., 2015)). With this, our study highlights the relevance of including LME in routine clinical imaging. Along these lines, ultrahigh-field imaging at 7 T might substantially improve the sensitivity to detect LME in MS in the clinical setting, as shown by our meta-analysis.
One major remaining question about LME is its underlying tissue signature. In neoplastic and infectious CNS diseases, LME likely corresponds to increased local blood supply and/or extravasation of gadolinium. However, in neuroinflammatory diseases, the pathological substrate of LME is much less clear. Based on limited EAE and MS histopathology data, LME corresponds to meningeal inflammatory infiltrates and/or tertiary lymphoid follicles (reviewed in (Zurawski et al., 2017)) (Absinta et al., 2015, Bhargava et al., 2021). Despite conflicting evidence as to whether LME is spatially associated with cortical pathology in MS (Absinta and Ontaneda, 2020), there has been a consistent association of LME with low cortical volumes across studies, also substantiated by our meta-analysis. This indicates that the pathology underlying L ME could exert a diffusely deleterious effect on cortical gray matter.
Different LME patterns have been described in MS (Harrison et al., 2017a) and, to a much lesser extent, in other neuroinflammatory diseases such as Susac syndrome (Coulette et al., 2019), neurosarcoidosis (Junger et al., 1993, O'Connell et al., 2017), and NMOSD (Asgari et al., 2017, Fan et al., 2016, Long et al., 2014). LME patterns were very rarely reported in non-inflammatory neurological diseases, mostly as diffuse LME in neoplastic neurological diseases (Schluterman et al., 2004). In MS, the prevalence of different LME patterns, their nomenclature as well as their association to clinical measures were highly inconsistent among studies. Nevertheless, different LME patterns could represent distinct pathophysiological features, also emphasized by the observation that healthy controls may present with nodular but not non-nodular LME (Harrison et al., 2017a). With this, more data and a more stringent nomenclature is needed to describe LME phenotypes and their potential association to clinical disability and disease phenotypes. We favor the nomenclature convention of nodular versus linear LME.
It is interesting that LME has also been observed in healthy controls, albeit at low proportions (0.06) (Absinta et al., 2017, Absinta et al., 2015, Sommer et al., 2020, Zurawski et al., 2020). The etiology of LME foci in healthy subjects is still a matter of debate. However, one potential cause could be minor traumatic brain injuries. It should be emphasized that LME foci can be very subtle and can easily be misinterpreted on MRI. Hence, several imaging pitfalls for LME should be taken into account, among them: gadolinium leakage of indeterminate biological significance, enhancement related to slow blood flow of cortical veins, or anatomic structures as well as imaging artifacts (Titelbaum et al., 2020).
Finally, data from clinical studies do not suggest a therapeutic effect of DMTs on LME in MS. However, most of these studies have a small sample size and might thus be insufficiently powered to detect a potential therapeutic effect. In addition, newer DMTs such as Bruton tyrosine kinase inhibitors (Sellebjerg and Weber, 2021) have not been assessed in this regard, even though these drugs led to a resolution of LME in one rodent study employing a neuroinflammatory model (Bhargava et al., 2021). Hence, more data is needed on potential therapeutic impact of DMT on LME resolution.
5. Limitations
Our study has some limitations: First, a wide variety of imaging methods have been employed to detect LME, for which we only partially corrected our analysis (e.g., static magnetic field strengths). Notably, the use of either T1w or T2w-FLAIR postcontrast sequences was not considered, with the latter generally having higher sensitivity to detect LME (Makshakov et al., 2017, Singh et al., 2000). This could have led to an underestimation of LME proportions in studies acquiring T1w sequences. However, studies in neoplastic and infectious neurological diseases with mostly bulk LME mainly employed T1w sequences, potentially counterbalancing this effect. Along these lines, we also point out that a substantial number of studies did not report on which MRI sequences they employed (T1w versus T2w-FLAIR) and only scant additional data were obtainable by directly contacting study authors. Second, for assessing the prognostic value of LME, we pooled studies with various methodological backgrounds for summary estimates. Nonetheless, the outcome measures reported were surprisingly uniform, even allowing for the use of mean differences in our meta-analysis.
6. Conclusions
Our study provides systematic evidence for LME proportions in a comprehensive panel of neurological diseases, including MS. This high-level evidence also corroborates the prognostic value of LME in MS. With this, LME qualifies to be included as a standard imaging feature in clinical MS imaging in our opinion, not least to enhance the knowledge and experience of radiologists and referring neurologists on this matter (Okar and Reich, 2021). Furthermore, our systematic review identified several methodological factors which need to be considered when assessing LME, including technical parameters such as magnetic field strength, type of 3D T2w-FLAIR sequence, and scanner type but also timing of acquisition as well as exclusion of LME imaging mimicks (Titelbaum et al., 2020). We also identified knowledge gaps: First, more data is needed on the potential therapeutic impact on LME in MS. Second, more evidence on the pathological substrate of LME and on its potential association with cortical pathology in neuroinflammation is warranted to further improve our understanding for this MRI feature and to strengthen its role in the clinical and research settings.
7. Summary statement
Our systematic review and meta-analysis synthesize leptomeningeal enhancement proportions across a comprehensive panel of neurological diseases, including multiple sclerosis, and assesses its prognostic value in multiple sclerosis.
8. Summary data
•Leptomeningeal enhancement (LME) is a nonspecific imaging feature present across many neurological disorders, including neoplasm, infection, and primary neuroinflammation.
•The presence of LME is associated with worse clinical and imaging outcomes in multiple sclerosis, justifying its ascertainment in clinical practice.
•Neuroinflammatory animal models can be used to further investigate the pathophysiology of LME, including its pathological tissue signature and/or its association with cortical pathology.
Funding
BVI was supported by the Forschungskredit from the University of Zurich. CT was supported by the Swiss National Science Foundation (Grant number: 320030_156860), the Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung, as well as from the University of Basel (Grant numbers: 3MS1020 and 3MS1049). MA is supported by the Conrad N. Hilton Foundation (Marylin Hilton Bridging Award for Physician-Scientists, grant #17313), the Roche Foundation for Independent Research, the Cariplo Foundation (grant #1677) and the FRRB Early Career Award (grant #1750327). The study was partially supported by the Intramural Research Program of NINDS, NIH.
CRediT authorship contribution statement
Benjamin V. Ineichen: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization. Charidimos Tsagkas: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization. Martina Absinta: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization. Daniel S. Reich: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Irene Cortese, Govind Nair, Avindra Nath, and Bryan Smith for facilitating MRI acquisition. We also thank the Intramural Research Program of National Institute of Neurological Disorders and Stroke for financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.102939.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- Absinta M., Cortese I.C.M., Vuolo L., Nair G., de Alwis M.P., Ohayon J., Meani A., Martinelli V., Scotti R., Falini A., Smith B.R., Nath A., Jacobson S., Filippi M., Reich D.S. Leptomeningeal gadolinium enhancement across the spectrum of chronic neuroinflammatory diseases. Neurology. 2017;88(15):1439–1444. doi: 10.1212/WNL.0000000000003820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta, M., Ontaneda, D., 2020. Controversial association between leptomeningeal enhancement and demyelinated cortical lesions in multiple sclerosis. SAGE Publications Sage UK: London, England. [DOI] [PubMed]
- Absinta M., Vuolo L., Rao A., Nair G., Sati P., Cortese I.C.M., Ohayon J., Fenton K., Reyes-Mantilla M.I., Maric D., Calabresi P.A., Butman J.A., Pardo C.A., Reich D.S. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology. 2015;85(1):18–28. doi: 10.1212/WNL.0000000000001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Azad S., Azad R. Differentiation of leptomeningeal and vascular enhancement on post-contrast FLAIR MRI sequence: role in early detection of infectious meningitis. J. Clin. Diagn. Res. 2015;9:TC08-TC12. doi: 10.7860/JCDR/2015/11519.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari, N., Flanagan, E.P., Fujihara, K., Kim, H.J., Skejoe, H.P., Wuerfel, J., Kuroda, H., Kim, S.H., Maillart, E., Marignier, R., Pittock, S.J., Paul, F., Weinshenker, B.G., 2017. Disruption of the leptomeningeal blood barrier in neuromyelitis optica spectrum disorder. Neurol. Neuroimmunol. NeuroInflammation 4. [DOI] [PMC free article] [PubMed]
- Bergsland N., Ramasamy D., Tavazzi E., Hojnacki D., Weinstock-Guttman B., Zivadinov R. Leptomeningeal contrast enhancement is related to focal cortical thinning in relapsing-remitting multiple sclerosis: a cross-sectional MRI study. Am. J. Neuroradiol. 2019;40:620–625. doi: 10.3174/ajnr.A6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P., Kim S., Reyes A.A., Grenningloh R., Boschert U., Absinta M., Pardo C., Zijl P.V., Zhang J., Calabresi P.A. Imaging meningeal inflammation in CNS autoimmunity identifies a therapeutic role for BTK inhibition. Brain. 2021 doi: 10.1093/brain/awab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P., Wicken C., Smith M.D., Strowd R.E., Cortese I., Reich D.S., Calabresi P.A., Mowry E.M. Trial of intrathecal rituximab in progressive multiple sclerosis patients with evidence of leptomeningeal contrast enhancement. Multiple Sclerosis Relat. Disord. 2019;30:136–140. doi: 10.1016/j.msard.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese M., Magliozzi R., Ciccarelli O., Geurts J.J.G., Reynolds R., Martin R. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 2015;16(3):147–158. doi: 10.1038/nrn3900. [DOI] [PubMed] [Google Scholar]
- Castillo J.J., D'Sa S., Lunn M.P., Minnema M.C., Tedeschi A., Lansigan F., Palomba M.L., Varettoni M., Garcia-Sanz R., Nayak L., Treon S.P. Bing-neel syndrome: a multi-institutional retrospective study. Hematol. Oncol. 2015;33:168. doi: 10.1111/bjh.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10(1):101. doi: 10.2307/3001666. [DOI] [Google Scholar]
- Coulette S., Lecler A., Saragoussi E., Zuber K., Savatovsky J., Deschamps R., Gout O., Sabben C., Aboab J., Affortit A., Charbonneau F., Obadia M. Diagnosis and prediction of relapses in susac syndrome: a new use for MR postcontrast FLAIR leptomeningeal enhancement. Am. J. Neuroradiol. 2019;40(7):1184–1190. doi: 10.3174/ajnr.A6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dubey, D., Hinson, S., Zekeridou, A., Flanagan, E., Pittock, S., Basal, E., Drubach, D., Lachance, D., Lennon, V., McKeon, A., 2018. Autoimmune GFAP astrocytopathy: prospective evaluation of 90 patients in 1 year. Neurology 90. [DOI] [PubMed]
- Eisele P., Griebe M., Szabo K., Wolf M.E., Alonso A., Engelhardt B., Hennerici M.G., Gass A. Investigation of leptomeningeal enhancement in MS: a postcontrast FLAIR MRI study. Neurology. 2015;84(8):770–775. doi: 10.1212/WNL.0000000000001286. [DOI] [PubMed] [Google Scholar]
- Fan Y., Shan F., Lin S.-P., long Y., Liang B., Gao C., Gao Q. Dynamic change in magnetic resonance imaging of patients with neuromyelitis optica. Int. J. Neurosci. 2016;126(5):448–454. doi: 10.3109/00207454.2015.1055356. [DOI] [PubMed] [Google Scholar]
- Harrison D.M., Wang K.Y., Fiol J., Naunton K., Royal W., Hua J., Izbudak I. Leptomeningeal enhancement at 7T in multiple sclerosis: frequency, morphology, and relationship to cortical volume. J. Neuroimaging. 2017;27(5):461–468. doi: 10.1111/jon.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning E.C., Latour L.L., Warach S. Verification of enhancement of the CSF space, not parenchyma, in acute stroke patients with early blood-brain barrier disruption. J. Cereb. Blood Flow Metab. 2008;28(5):882–886. doi: 10.1038/sj.jcbfm.9600598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hildesheim F.E., Ramasamy D.P., Bergsland N., Jakimovski D., Dwyer M.G., Hojnacki D., Lizarraga A.A., Kolb C., Eckert S., Weinstock-Guttman B., Zivadinov R. Leptomeningeal, dura mater and meningeal vessel wall enhancements in multiple sclerosis. Mult. Scler. Relat. Disord. 2020;47:102653. doi: 10.1016/j.msard.2020.102653. [DOI] [PubMed] [Google Scholar]
- Ighani M., Jonas S., Izbudak I., Choi S., Lema-Dopico A., Hua J., O’Connor E.E., Harrison D.M. No association between cortical lesions and leptomeningeal enhancement on 7-Tesla MRI in multiple sclerosis. Mult. Scler. J. 2020;26(2):165–176. doi: 10.1177/1352458519876037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itchaki, G., Paludo, J., Palomba, M.L., Varettoni, M., Talaulikar, D., Chavez, J.C., Buske, C., Tedeschi, A., Simpson, D., Tam, C.S., Issa, S., Ansell, S.M., Treon, S.P., Castillo, J.J., 2018. Ibrutinib for the treatment of bing-neel syndrome. Blood 132. [DOI] [PubMed]
- Itsekson Hayosh Z., Tsarfati G., Greenberg G., Sharon M., Bakon M., Wohl A., Chapman J., Orion D. Early FLAIR enhancement in reversible cerebral vasoconstriction syndrome. Eur. J. Neurol. 2020;27:126. doi: 10.5853/jos.2020.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S.N., Izbudak I., Frazier A.A., Harrison D.M. Longitudinal persistence of meningeal enhancement on postcontrast 7T 3D-FLAIR MRI in multiple sclerosis. Am. J. Neuroradiol. 2018;39(10):1799–1805. doi: 10.3174/ajnr.A5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger S.S., Stern B.J., Levine S.R., Sipos E., Marti-Masso J.F. Intramedullary spinal sarcoidosis: clinical and magnetic resonance imaging characteristics. Neurology. 1993;43(2):333. doi: 10.1212/wnl.43.2.333. [DOI] [PubMed] [Google Scholar]
- Kim H., Lim D.H., Kim T.G., Lee J.-I., Nam D.-H., Seol H.J., Kong D.-S., Choi J.W., Suh Y.-L., Kim S.T. Leptomeningeal enhancement on preoperative brain MRI in patients with glioblastoma and its clinical impact. Asia-Pacific J. Clin. Oncol. 2018;14(5):e366–e373. doi: 10.1111/ajco.12861. [DOI] [PubMed] [Google Scholar]
- Klironomos S., Tzortzakakis A., Kits A., Öhberg C., Kollia E., Ahoromazdae A., Almqvist H., Aspelin Å., Martin H., Ouellette R., Al-Saadi J., Hasselberg M., Haghgou M., Pedersen M., Petersson S., Finnsson J., Lundberg J., Falk Delgado A., Granberg T. Nervous system involvement in coronavirus disease 2019: results from a retrospective consecutive neuroimaging cohort. Radiology. 2020;297(3):E324–E334. doi: 10.1148/radiol.2020202791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralik S.F., Kukreja M.K., Paldino M.J., Desai N.K., Vallejo J.G. Comparison of CSF and MRI findings among neonates and infants with E coli or Group B Streptococcal Meningitis. Am. J. Neuroradiol. 2019;40(8):1413–1417. doi: 10.3174/ajnr.A6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer S., Lersy F., Anheim M., Merdji H., Schenck M., Oesterlé H., Bolognini F., Messie J., Khalil A., Gaudemer A., Carré S., Alleg M., Lecocq C., Schmitt E., Anxionnat R., Zhu F., Jager L., Nesser P., Mba Y.T., Hmeydia G., Benzakoun J., Oppenheim C., Ferré J.-C., Maamar A., Carsin-Nicol B., Comby P.-O., Ricolfi F., Thouant P., Boutet C., Fabre X., Forestier G., de Beaurepaire I., Bornet G., Desal H., Boulouis G., Berge J., Kazémi A., Pyatigorskaya N., Lecler A., Saleme S., Edjlali-Goujon M., Kerleroux B., Constans J.-M., Zorn P.-E., Mathieu M., Baloglu S., Ardellier F.-D., Willaume T., Brisset J.-C., Caillard S., Collange O., Mertes P.M., Schneider F., Fafi-Kremer S., Ohana M., Meziani F., Meyer N., Helms J., Cotton F. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology. 2020;95(13):e1868–e1882. doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- Latour L.L., Kang D.-W., Ezzeddine M.A., Chalela J.A., Warach S. Early blood–brain barrier disruption in human focal brain ischemia. Ann. Neurol. 2004;56(4):468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Lersy F., Benotmane I., Helms J., Collange O., Schenck M., Brisset J.C., Chammas A., Willaume T., Lefebvre N., Solis M., Hansmann Y., Fabacher T., Caillard S., Mertes P.M., Pottecher J., Schneider F., Meziani F., Fafi-Kremer S., Kremer S. Cerebrospinal fluid features in COVID-19 patients with neurologic manifestations: correlation with brain MRI findings in 58 patients. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y., Chen M., Zhang B., Gao C., Zheng Y., Xie L., Gao Q., Yin J. Brain gadolinium enhancement along the ventricular and leptomeningeal regions in patients with aquaporin-4 antibodies in cerebral spinal fluid. J. Neuroimmunol. 2014;269(1-2):62–67. doi: 10.1016/j.jneuroim.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Makshakov, G., Magonov, E., Totolyan, N., Nazarov, V., Lapin, S., Mazing, A., Verbitskaya, E., Trofimova, T., Krasnov, V., Shumilina, M., Skoromets, A., Evdoshenko, E., 2017. Leptomeningeal contrast enhancement is associated with disability progression and grey matter atrophy in multiple sclerosis. Neurol. Res. Int. 2017. [DOI] [PMC free article] [PubMed]
- Mathews V.P., Caldemeyer K.S., Lowe M.J., Greenspan S.L., Weber D.M., Ulmer J.L. Brain: gadolinium-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology. 1999;211(1):257–263. doi: 10.1148/radiology.211.1.r99mr25257. [DOI] [PubMed] [Google Scholar]
- Menon B.K., O'Brien B., Bivard A., Spratt N.J., Demchuk A.M., Miteff F., Lu X., Levi C., Parsons M.W. Assessment of leptomeningeal collaterals using dynamic CT angiography in patients with acute ischemic stroke. J. Cereb. Blood Flow Metab. 2013;33(3):365–371. doi: 10.1038/jcbfm.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(1) doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neo S., Yeo T., Chen Z., Ngiam N.H.W., Lim E.-X., Tan K., Lim T.C.C. Acute radiological features facilitate diagnosis and prognosis of anti-N-methyl-d-aspartate receptor (NMDAR) and anti-voltage-gated potassium channel (VGKC) encephalitis in adults. J. Neurol. Sci. 2020;419:117216. doi: 10.1016/j.jns.2020.117216. [DOI] [PubMed] [Google Scholar]
- O’Connell K., Williams L., Jones J., McCabe D.J.H., Murphy D., Killeen R., Tubridy N., O’Riordan S., McGuigan C. Neurosarcoidosis: clinical presentations and changing treatment patterns in an Irish Caucasian population. Ir. J. Med. Sci. 2017;186(3):759–766. doi: 10.1007/s11845-016-1539-y. [DOI] [PubMed] [Google Scholar]
- Okar S.V., Reich D.S. Routine gadolinium use for MRI follow-up of multiple sclerosis: Point—The role of leptomeningeal enhancement. Am. J. Roentgenol. 2021 doi: 10.2214/AJR.21.26999. [DOI] [PubMed] [Google Scholar]
- Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol S., Schweser F., Bertolino N., Preda M., Sveinsson M., Sudyn M., Babek N., Zivadinov R. Characterization of leptomeningeal inflammation in rodent experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis. Exp. Neurol. 2019;314:82–90. doi: 10.1016/j.expneurol.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Sarkis R.A., Mays M., Isada C., Ahmed M. MRI findings in cryptococcal meningitis of the non-HIV population. Neurologist. 2015;19:40–45. doi: 10.1097/NRL.0000000000000000. [DOI] [PubMed] [Google Scholar]
- Schluterman K.O., Fassas A.-T., Van Hemert R.L., Harik S.I. Multiple myeloma invasion of the central nervous system. Arch. Neurol. 2004;61(9):1423. doi: 10.1001/archneur.61.9.1423. [DOI] [PubMed] [Google Scholar]
- Sellebjerg F., Weber M.S. Targeting B cells in multiple sclerosis. Curr. Opin. Neurol. 2021;34:295–302. doi: 10.1097/WCO.0000000000000938. [DOI] [PubMed] [Google Scholar]
- Singh S.K., Agris J.M., Leeds N.E., Ginsberg L.E. Intracranial leptomeningeal metastases: comparison of depiction at FLAIR and contrast-enhanced MR imaging. Radiology. 2000;217(1):50–53. doi: 10.1148/radiology.217.1.r00oc3550. [DOI] [PubMed] [Google Scholar]
- Sommer N.N., Pons Lucas R., Coppenrath E., Kooijman H., Galiè F., Hesse N., Sommer W.H., Treitl K.M., Saam T., Froelich M.F. Contrast-enhanced modified 3D T1-weighted TSE black-blood imaging can improve detection of infectious and neoplastic meningitis. Eur. Radiol. 2020;30(2):866–876. doi: 10.1007/s00330-019-06475-3. [DOI] [PubMed] [Google Scholar]
- Soni N., Kumar S., Shimle A., Ora M., Bathla G., Mishra P. Cerebrovascular complications in tuberculous meningitis—A magnetic resonance imaging study in 90 patients from a tertiary care hospital. Neuroradiol. J. 2020;33(1):3–16. doi: 10.1177/1971400919881188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susac J.O., Murtagh F.R., Egan R.A., Berger J.R., Bakshi R., Lincoff N., Gean A.D., Galetta S.L., Fox R.J., Costello F.E., Lee A.G., Clark J., Layzer R.B., Daroff R.B. MRI findings in Susac's syndrome. Neurology. 2003;61(12):1783–1787. doi: 10.1212/01.wnl.0000103880.29693.48. [DOI] [PubMed] [Google Scholar]
- Titelbaum D.S., Engisch R., Schwartz E.D., Napoli S.Q., Sloane J.A., Samaan S., Katz J.D., Lathi E.S. Leptomeningeal enhancement on 3D-FLAIR MRI in multiple sclerosis: systematic observations in clinical practice. J. Neuroimaging. 2020;30(6):917–929. doi: 10.1111/jon.12774. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48. [Google Scholar]
- Warach S., Latour L.L. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood–brain barrier disruption. Stroke. 2004;35(11_suppl_1):2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- Wells, G.A., Tugwell, P., O’Connell, D., Welch, V., Peterson, J., Shea, B., Losos, M., 2015. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses.
- Wu C.-H., Lirng J.-F., Ling Y.-H., Wang Y.-F., Wu H.-M., Fuh J.-L., Lin P.-C., Wang S.-J., Chen S.-P. Noninvasive characterization of human glymphatics and meningeal lymphatics in an in vivo model of blood-brain barrier leakage. Ann. Neurol. 2021;89(1):111–124. doi: 10.1002/ana.25928. [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Bergsland N., Carl E., Ramasamy D., Hagemeier J., Dwyer M., Lizarraga A., Kolb C., Hojnacki D., Weinstock-Guttman B. Effect of teriflunomide and dimethyl fumarate on cortical atrophy and leptomeningeal inflammation in multiple sclerosis: a retrospective, observational, case-control pilot study. J. Clin. Med. 2019;8(3):344. doi: 10.3390/jcm8030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R., Ramasamy D.P., Hagemeier J., Kolb C., Bergsland N., Schweser F., Dwyer M.G., Weinstock-Guttman B., Hojnacki D. Evaluation of leptomeningeal contrast enhancement using pre-and postcontrast subtraction 3D-FLAIR imaging in multiple sclerosis. Am. J. Neuroradiol. 2018;39(4):642–647. doi: 10.3174/ajnr.A5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R., Ramasamy D.P., Vaneckova M., Gandhi S., Chandra A., Hagemeier J., Bergsland N., Polak P., Benedict R.HB., Hojnacki D., Weinstock-Guttman B. Leptomeningeal contrast enhancement is associated with progression of cortical atrophy in MS: a retrospective, pilot, observational longitudinal study. Mult. Scler. 2017;23(10):1336–1345. doi: 10.1177/1352458516678083. [DOI] [PubMed] [Google Scholar]
- Zurawski J., Lassmann H., Bakshi R. Use of magnetic resonance imaging to visualize leptomeningeal inflammation in patients with multiple sclerosis: a review. JAMA Neurol. 2017;74(1):100. doi: 10.1001/jamaneurol.2016.4237. [DOI] [PubMed] [Google Scholar]
- Zurawski J., Tauhid S., Chu R., Khalid F., Healy B.C., Weiner H.L., Bakshi R. 7T MRI cerebral leptomeningeal enhancement is common in relapsing-remitting multiple sclerosis and is associated with cortical and thalamic lesions. Mult. Scler. J. 2020;26(2):177–187. doi: 10.1177/1352458519885106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.