Highlights
-
•
The preoperative application of denosumab can reduce tumor blood supply.
-
•
The decrease of blood supply was the most significant in the initial stage of treatment.
-
•
It is not recommended to apply denosumab long before surgical treatment.
Keywords: Giant cell tumor of bone, Denosumab, Preoperative treatment time, Tumor blood supply
Abstract
Background
The changes in the characteristics of the tumor blood supply of giant cell tumor of bone over time after treatment with denosumab remain unclear. The purpose of this study was to evaluate the change in the blood supply imaging characteristics of giant cell tumor of bone after preoperative denosumab treatment and to provide evidence for evaluating the reasonable time for preoperative treatment.
Methods
A total of 59 patients with giant cell tumor of bone who were treated in our hospital from 2014 to 2019 were enrolled in the study. All patients underwent enhanced CT examination of the tumor site before denosumab treatment and every month after treatment. The plain CT value and enhanced CT value of the tumor were measured, and the CT enhancement rate of the tumor was calculated. The change in the CT enhancement rate of the tumor over time after denosumab treatment was analyzed.
Results
The average tumor enhancement rates were 2.14 (1.22–4.05), 1.60 (1.12–2.53), 1.38 (1.02–2.24), and 1.25 (1–2.11) before denosumab treatment and one month, three months, and six months after treatment, respectively. After denosumab treatment, the average monthly CT enhancement rate decreased as follows: 0.54 (25.2%) in the first month, 0.11 (5.15%) in the second to third months, and 0.04 (1.87%) in the fourth to sixth months. The tumor enhancement rate was no longer significantly reduced three months post-treatment. There was a significant correlation between the reduction in the CT enhancement rate and the initial CT enhancement rate (P = 0.000).
Conclusion
The preoperative application of denosumab can reduce tumor blood supply. The decrease in the blood supply is the most significant in the initial stage of treatment. Following treatment, the decrease in the blood supply gradually reduces over time. Therefore, for the purpose of reducing intra-operative bleeding and facilitating surgery, application of denosumab treatment is not recommended more than three months before surgery.
1. Introduction
Although giant cell tumor of bone (GCTB) is histologically benign, it often presents with local invasion and has a high recurrence rate. A study of 349 cases of GCTB [1] reported a recurrence rate of 16% after curettage. In one study, the reported recurrence rate exceeded 50% after surgical treatment without auxiliary management [2]. A study of 621 cases of giant cell tumor of the extremities showed that the local recurrence rate could be reduced to 8.6% with the application of extended curettage [3]. However, for tumors with complex anatomical structures, such as tumors of the sacrum, pelvis and spine, the complication and recurrence rates of surgical treatment remain high [4], [5]. Moreover, due to the rich blood supply to the tumor, there can be significant blood loss during surgery on sacral or pelvic tumors, which can threaten the perioperative safety of patients [6]. Thus, effective reduction of intraoperative blood loss by reducing the tumor blood supply of GCTB before surgery is a critical problem that deserves further research attention.
In recent years, the application of denosumab for the treatment of GCTB has gained popularity and studies of the preoperative adjuvant treatment effect on surgical and oncological prognosis have been published [7], [8], [9]. A phase II clinical trial involving 281 patients showed that the pain symptoms of the majority of patients were significantly relieved within two months of treatment [7]. Another phase II clinical trial involving 222 cases showed that nearly 40% of the patients achieved surgical down-staging and a considerable number underwent successful joint-sparing curettage surgery [8]. Therefore, previous studies suggest that preoperative denosumab treatment can bring clinical benefits to many patients.
However, there remain several controversies regarding preoperative treatment. Some reports [9], [10], [11] suggest that preoperative application of denosumab can increase the difficulty of the surgery and the risk of recurrence. Excessive sclerosis of the lesion after denosumab treatment can also increase the difficulty of curettage and interfere with the judgment of the boundary between the tumor and normal tissue [12], [13]. Moreover, residual sclerotic lesion after surgery may become a risk for postoperative recurrence [14], [15]. In our previous studies, we obtained good clinical results, especially for sacral tumors. It is hoped that by reducing the blood supply to the tumor through preoperative medication, decreased intraoperative bleeding and reduced perioperative risk can be achieved. However, the changes in the characteristics of tumor blood supply over time are not clear. Therefore, we performed a clinical study to evaluate the characteristics of tumor blood supply and the changes over time after treatment. Moreover, the correlation between changes in the tumor blood supply and time after denosumab treatment was analyzed. The overarching goal of this study was to provide a quantitative analysis of image changes over time after preoperative application of denosumab in order to determine the appropriate surgery time.
2. Materials and methods
2.1. General study characteristics
This was a retrospective study of patients with GCTB treated with denosumab in our institution. The ethics committee of our institution reviewed and approved this study. All clinical data were collected from the musculoskeletal tumor database of our institution. The inclusion criteria were as follows: histologically confirmed GCTB (biopsy or postoperative pathology); Campanacci stage 3; adults or skeletally-mature adolescents (≥12 years of age); unresectable lesion/joint or important structure that cannot be retained. The exclusion criteria were as follows: malignant GCTB or malignant transformation of GCTB; GCTB combined with other bone tumors; arterial embolization, radiotherapy or other treatment that may affect tumor blood supply; history of osteonecrosis or osteomyelitis. After application of the above inclusion and exclusion criteria, 59 patients who were treated in our hospital from January 2014 to December 2019 were enrolled in this study (Table 1). The average age of the patients was 32.1 years (16–63 years), among which there were 26 males and 33 females. The lesion sites were as follows: sacrum or pelvis in 24 cases (sacrum in 20 cases, pelvis in 4 cases) and limb bone in 35 cases (tibia in 9 cases, femur in 5 cases, radius in 6 cases, fibula in 6 cases, humerus in 5 cases, ulna in 3 cases, metacarpal in 1 case).
Table 1.
General characteristics of patients.
| Variable | Patients | P value | |||
|---|---|---|---|---|---|
| n | % | ||||
| Gender | |||||
| Male | 26 | 44.1 | |||
| Female | 33 | 55.9 | |||
| Age (years) | |||||
| Mean | 32.1 | ||||
| Range | 16–63 | ||||
| Campanacci stage | |||||
| 1 | 0 | 0 | |||
| 2 | 0 | 0 | |||
| 3 | 59 | 100 | |||
| Tumor location | |||||
| Sacrum or Pelvis | 24 | 40.7 | |||
| Sacrum | 20 | 33.9 | |||
| Pelvis | 4 | 6.8 | |||
| Limb bone | 35 | 59.3 | |||
| Tibia | 9 | 15.3 | |||
| Femur | 5 | 8.5 | |||
| Radius | 6 | 10.2 | |||
| Fibula | 6 | 10.2 | |||
| Humerus | 5 | 8.5 | |||
| Ulna | 3 | 5.1 | |||
| Metacarpal | 1 | 1.7 | |||
| The VAS scores | 0.000 | ||||
| Pre-treatment | Mean | 4.0 | |||
| Range | 1–8 | ||||
| Post-treatment | Mean | 0.4 | |||
| Range | 0–2 | ||||
| The MSTS scores | 0.000 | ||||
| Pre-treatment | Mean | 23.8 | |||
| Range | 17–27 | ||||
| Post-treatment | Mean | 26.4 | |||
| Range | 22–28 | ||||
| CT enhancement rate of main vessels | 0.623 | ||||
| Pre-treatment | Mean | 3.14 | |||
| Range | 2.18–4.32 | ||||
| Post-treatment | Mean | 3.16 | |||
| Range | 2.13–4.42 |
2.2. Treatment regime and clinical evaluation
The denosumab treatment regimen was as follows: subcutaneous injection of denosumab 120 mg once every four weeks with the addition of a single dose on day 8 and day 15 after the initial treatment. The patients received both calcium and vitamin D supplements. Clinical evaluations and recordings were performed before and after denosumab treatment. The visual analogue scale (VAS) pain score, musculoskeletal tumor society (MSTS) limb function score, and adverse reactions related to the treatment were recorded.
2.3. Imaging (enhanced CT) evaluation
All patients underwent enhanced CT examination of the lesion site before the first denosumab treatment (Fig. 1). Enhanced CT examination of the lesion was again performed at the first, third, and sixth month after treatment. The examinations were performed on the same CT machine before and after treatment. The agent and dose of contrast as well as the scanning time were consistent. The enhanced CT value was measured and evaluated before and after treatment. Based on the size and scope of the tumor, 5–10 CT slices were selected for measurement of the lesion. The plain and enhanced CT values of the lesion area at the same level were measured before and after treatment; the plain and enhanced CT values of the main arteries at the same level were also measured. The CT enhancement rate was calculated as the ratio of the enhanced CT value to the plain CT value. The final calculated value was the average value of all slices.
Fig. 1.
Enhanced CT of a sacral GCTB. The tumor had a wide range of bone destruction with a large soft tissue mass and no obvious bone boundary (axial bone window of CT, A and B), so it was difficult to perform curettage. The tumor showed obvious enhancement and abundant blood supply in the CT enhancement window (C).
If an unresectable tumor transformed into a resectable tumor, a non-curettable tumor transformed into a curettable tumor, or the important structures were able to be preserved, the denosumab treatment was stopped and surgical treatment was performed. Otherwise, denosumab treatment continued if the above criteria were not met.
2.4. Biological activity (PET-CT) evaluation
Fourteen patients received PET-CT examination before and three months after denosumab treatment. The average SUV max values of the tumor before and after denosumab treatment were compared.
2.5. Statistical analysis
SPSS 22.0 was used for statistical analysis. The mean CT enhancement rate before treatment and at various time points after treatment were compared. The mean VAS and MSTS scores before and after treatment were compared. The continuous variables were compared by means tests and the categorical variables were compared by the chi-square test or Fisher’s exact test. Pearson correlations were used for continuous variables and Spearman correlations were used for categorical variables. P ≤ 0.05 was considered statistically significant.
3. Results
3.1. CT value before treatment
Before treatment, the plain CT and enhanced CT values of the main arteries were 45.2 (39–55) and 142.1 (94–182), respectively, and the average enhancement rate was 3.14 (2.18–4.32). The plain CT and enhanced CT values of the tumor were 43.2 (30–67) and 92.5 (47–153), respectively, and the average enhancement rate was 2.14 (1.22–4.05). The average CT enhancement rates of sacral or pelvic lesions and limb lesions before treatment were 2.40 (1.40–4.05) and 1.94 (1.22–2.49), respectively. The enhancement rate of sacral or pelvic lesions before treatment was significantly higher than that of limb lesions (P = 0.004, F = 9.270).
3.2. Imaging evaluation results after treatment
3.2.1. Comparison of the CT enhancement rate of blood arteries before and after treatment
After denosumab treatment, the plain CT value and enhanced CT value of the main arteries in the same layer of the lesion were 44.2 (37–55) and 139.5 (92–178), respectively. The average enhancement rate was 3.16 (2.13–4.42), which was not significantly different from that before treatment (P = 0.623, F = 0.305). This result suggests that the enhanced CT before and after treatment is consistent and comparable (Table 1).
3.2.2. Changes in the tumor CT enhancement rate after treatment
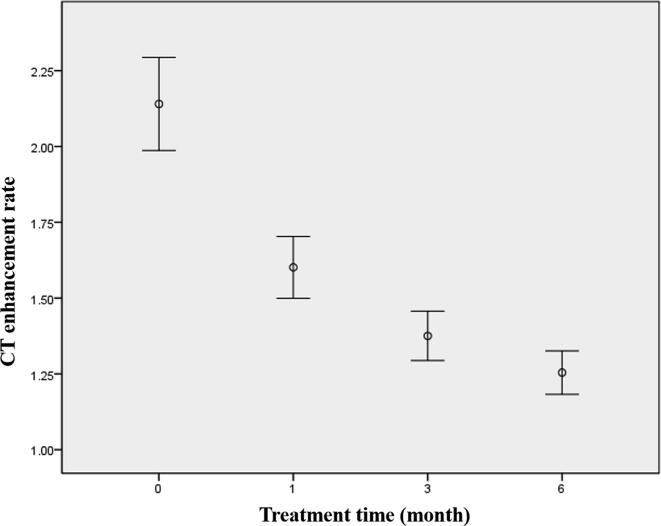
The average enhancement rates at one month, three months, and six months post-treatment were 1.60 (1.12–2.53), 1.38 (1.02–2.24), and 1.25 (1–2.11), respectively (Fig. 2). The tumor CT enhancement rate decreased over time as follows: the average reduction was 0.54 (25.2%) one month post-treatment; the cumulative average decrease was 0.76 (35.5%) three months post-treatment, with the an average reduction of 0.22 (10.3%) from the second to the third month; the cumulative average decrease was 0.89 (41.6%) six months post-treatment, with an average reduction of 0.12 (5.6%) from the fourth to the sixth month. After denosumab treatment, the average monthly reduction in the CT enhancement rate was as follows: 0.54 (25.2%) in the first month, 0.11 (5.15%) from the second to the third month, and 0.04 (1.87%) from the fourth to the sixth month. The above calculation results demonstrate that with the prolongation of denosumab treatment, the reduction in the absolute value and the degree of reduction in the tumor CT enhancement rate reduced month by month (Fig. 3). After three months of treatment, the tumor enhancement rate was no longer significantly reduced (Table 2).
Fig. 2.
The average values and distribution of the CT enhancement rate before treatment (0) and at different times (1 month, 3 months and 6 months) after treatment. The enhancement rates showed a significant difference between pre-treatment and 1 month post-treatment (P = 0.000, F = 33.353). There was no significant difference between 1 month and 3 months post-treatment (P = 0.062, F = 5.282), nor between 3 months and 6 months post-treatment (P = 0.290, F = 0.914).
Fig. 3.
Comparison of enhanced CT of sacral tumor before treatment (A: plain CT, B: enhanced CT), 1 month after treatment (C: plain CT, D: enhanced CT), and 3 months after treatment (E: plain CT, F: enhanced CT). The enhancement rates were 2.67, 1.65, and 1.38 before treatment and 1 month and 3 months after treatment, respectively.
Table 2.
Changes of CT enhancement rate pre- and post-denosumab treatment
| Time | CT enhancement rate | P value | ||||
|---|---|---|---|---|---|---|
| Value | Cumulative decrease value(proportion) | Monthly decrease value(proportion) | Compared with pre-treatment | Compared with 1 month post-treatment | Compared with 2–3 months post-treatment | |
| Pre-treatment | 2.14 | |||||
| 1 month post-treatment | 1.60 | 0.54(25.2%) | 0.54(25.2%) | P = 0.000 F = 33.353 |
||
| 2–3 months post-treatment | 1.38 | 0.76(35.5%) | 0.11(5.15%) | P = 0.000 F = 77.267 |
P = 0.062 F = 5.282 |
|
| 4–6 months post-treatment | 1.25 | 0.89(41.6%) | 0.04(1.87%) | P = 0.000 F = 98.524 |
P = 0.079 F = 6.471 |
P = 0.290 F = 0.914 |
The average plain CT values of the tumor pre-treatment and one month, three months, and six months post-treatment were 43.2 (30–67), 51.6 (32–74), 68.9 (46–118), and 93.8 (65–186), respectively. The plain CT values of the tumor increased significantly with the extension of treatment time (P = 0.000, F = 151.352) (Fig. 4).
Fig. 4.
The average plain CT values of the tumor before treatment (0) and at different times (1 month, 3 months, and 6 months) after treatment.
3.2.3. Correlation between the reduction in the CT enhancement rate and the initial CT enhancement rate
The reduction in the CT enhancement rate one month post-treatment was significantly correlated with the initial CT enhancement rate (P = 0.000, Pearson correlation coefficient = 0.846). The higher the baseline CT enhancement rate pre-treatment, the more the CT enhancement rate decreased post-treatment.
3.2.4. Comparison of the influence of denosumab on the CT enhancement rate of limb lesions and sacral or pelvic lesions
The average enhancement rates of sacral or pelvic tumors and limb bone tumors were 1.72 (1.25–2.81) and 1.48 (1.12–1.97) (P = 0.016, F = 6.294), respectively, one month post-treatment, 1.45 (1.11–2.24) and 1.31 (1.04–1.77) (P = 0.086, F = 3.061) three months post-treatment, and 1.25 (1.04–2.11) and 1.25 (1.02–1.49) (P = 0.985, F = 0.000) six months post-treatment. The above statistical results suggest that the enhancement rate of sacral or pelvic lesions in the initial stage was significantly higher than that of limb lesions, and the enhancement rate of sacral or pelvic lesions gradually approached that of limb lesions after three months of treatment.
3.3. Clinical evaluation
All patients exhibited clinical benefits, including pain relief and improvement of limb function (Table 1). The average VAS scores before and after treatment were 4.0 (1–8) and 0.4 (0–2), respectively, with a significant decrease after treatment (P = 0.000, F = 283.521). The mean MSTS scores before and after treatment were 23.8 (17–27) and 26.4 (22–28), respectively, with a significant increase after treatment (P = 0.000, F = 42.387). There were no serious adverse reactions after treatment.
3.4. PET-CT evaluation
The average SUV max values of the tumor before and three months after denosumab treatment were 11.1 (7.8–15.6) and 6.5 (3.3–12.5), respectively. The average SUV max values of the tumor were significantly decreased after treatment (P = 0.000, F = 18.922).
4. Discussion
Denosumab is a new targeted therapeutic drug that can specifically combine with RANKL to block the RANK-RANKL pathway and inhibit the function of osteoclasts. Denosumab has been gradually applied in the treatment of GCTB, especially for recurrent, unresectable, and metastatic GCTB [16]. As such, denosumab has become the first targeted drug for adjuvant therapy in GCTB. In clinical practice, we observed that denosumab can not only relieve the clinical symptoms of patients and inhibit bone destruction behavior, but can also reduce the blood supply to the tumor. Thus, the blood loss during the curettage of sacral tumors may be reduced. The enhancement rate of enhanced CT can reflect the arterial blood supply to the tumor [17], [18], [19]. We carried out several studies and observed that the CT enhancement rate of the tumor decreased after treatment with denosumab, and the density of the micro arteries in the postoperative specimen was also lower than that of the pre-treatment biopsy specimen [12], [20].
In the current study, the enhancement rate was measured and the blood flow characteristics of GCTB after denosumab treatment were evaluated. There was no significant difference in the average enhancement rate of the main arteries before and after treatment. This result suggests that the contrast CT examination results before and after treatment were comparable with the CT enhancement rate we calculated. The CT enhancement rate reflects the blood flow of the tissue examined. Therefore, if the blood flow in a tumor is decreased or increased, the enhancement degree of an enhanced CT scan will also be weakened or strengthened accordingly [17], [18], [19].
Before analyzing the effect of treatment, the baseline CT enhancement rate of the tumor was analyzed before and after treatment. The enhancement rate of sacrum or pelvis lesions was significantly higher than that of limb lesions. This result is similar to our preliminary clinical results [20]. Sacral or pelvic lesions presented with a higher CT enhancement rate and more abundant blood supply. This is consistent with our clinical experience; that is, significant blood loss commonly occurs during surgery on sacrum or pelvic tumors. This finding also provides useful data support for the clinical practice of bone oncologists Thus, it can be considered that preoperative treatment with denosumab is more valuable in the treatment of sacrum or pelvic tumor.
The results of this study indicated that the enhanced CT rate of the tumor was significantly reduced after denosumab treatment, suggesting that denosumab can reduce the tumor blood supply in GCTB. The PET-CT results demonstrated that the average SUV max values of the tumor were significantly decreased after treatment, suggesting a decrease in the biological activity of the tumor. With the prolongation of denosumab treatment, the absolute value and the degree of reduction in the CT enhancement rate of the tumor decreased gradually. The difference in the average CT enhancement rate between three months and six months after treatment was not as significant as that in the early stage. In most of the cases in this study, the CT enhancement rate and the blood supply to the tumor had significantly reduced after one to three months of treatment. However, the tumor plain CT value increased significantly with the prolongation of treatment time, even after three months of treatment. The continuous aggravation of sclerosis may increase the difficulty of tumor curettage. These results suggest that it is necessary for clinicians to consider the rational treatment time before surgery. In order to reduce the blood supply to the tumor and the intraoperative blood loss, the denosumab treatment time should be controlled be no more than three months.
Obvious differences in the reduction of the CT enhancement rate were observed among different lesions. The correlation between the reduction in the CT enhancement rate and the initial CT enhancement rate was analyzed and the results showed that the reduction in the CT enhancement rate after one month of treatment was significantly correlated with the initial CT enhancement rate. Therefore, the higher the baseline CT enhancement rate of the lesion, the more significant the reduction in the value after treatment.
The characteristics of blood supply changes of tumors in different sites (sacral or pelvic tumors versus limb tumors) after denosumab treatment were also compared. After the first month of treatment, the average CT enhancement rate of sacral or pelvic lesions was still significantly higher than that of limb bone lesions However, the enhancement rate of sacrum or pelvis tumors gradually became closer to that of limb bone tumors with the prolongation of treatment time, which also suggests that the treatment time should not be extended excessively in the treatment of sacrum or pelvis tumors. Our previous study [20] showed that the enhancement rate of sacral or pelvis tumors decreased more obviously and rapidly than that of limb tumors. The above analysis demonstrates that the reduction in sacrum or pelvis lesions was more obvious, which is consistent with the result indicating that the reduction value was related to the baseline enhancement rate. Based on the above results, it can be concluded that most cases of sacral or pelvis GCTB will exhibit a decrease in the tumor blood supply one month after treatment. If surgery is performed after one month of treatment, not only will reduced blood loss and improved safety of the surgery be observed, but curettage difficulty due to excessive sclerosis of tumor will be avoided.
All patients in this study experienced clinical benefits after treatment with denosumab, regardless of the duration of treatment. The patients presented with pain relief, increased limb mobility, and improved function. No serious adverse reactions were observed in the clinical follow-up. Several previous reports also suggest that patients experience clinical benefits [7], [8], [13], [21]; however, there has been no analysis of timeliness. Bukata et al. [22] analyzed the clinical efficacy of denosumab treatment in 131 patients and a clinical benefit was reported in 83% of patients. Different from our study, most of the patients had surgically unsalvageable GCTB and received long-term treatment with denosumab. The probabilities of disease progression were 3% at year one and 7.4% at year three in unsalvageable GCTB. However, the results of the current study suggested that the time should be carefully controlled when denosumab is administered as a preoperative adjuvant treatment in surgically salvageable GCTB. Boriani et al. [23] suggested that denosumab is an excellent solution for spine GCT where surgical treatment is not appropriate or is associated with unacceptable morbidity or loss of function. The role of post-operative treatment is valued, but it is unclear when to safely stop the treatment.
A recent clinical study [24] suggested that denosumab treatment may increase the risk of recurrence. However, there were only three cases of preoperative treatment in the above study. The small number of cases and the existence of selection bias may affect the conclusions of the research. Several systematic reviews [25], [26], [27] suggest that denosumab treatment may be associated with increased postoperative recurrence. However, most of the existing research reports and clinical cases are of Campanacci stage 3 cases, and in most of these cases, resection is very difficult or will cause serious morbidity. Therefore, treatment of such cases may lead to selection bias. At present, there is still no convincing randomized controlled study.
An in vitro experiment [28] showed that denosumab inhibited osteoclast differentiation and osteoclastic activity but did not kill osteoclasts and tumor stromal cells. Another clinical study [29] showed that tumor progression occurred in 40% of cases at a median time of eight months after discontinuing denosumab. This suggests that denosumab cannot completely eliminate the tumor cells in GCTB, and incomplete surgery after treatment will lead to tumor recurrence. In addition, some cases of malignant transformation of GCTB have been reported in recent years [30,31]. Due to the small number of cases, the mechanism is not clear. Therefore, whether long-term treatment will lead to malignant transformation requires careful consideration and exploration.
The current study has several limitations that should be considered. First, there may be bias in the selection of patients. Most patients with GCTB received surgery without preoperative treatment. The cases that received denosumab treatment were those where surgery was relatively difficult or where a large amount of intraoperative blood loss was expected. They may not represent the general population of GCTB patients. In addition, this clinical study did not have a control group of cases who received different treatment times. Instead, this was a self-control study comparing the same case at different times.
In conclusion, preoperative application of denosumab can reduce tumor blood supply. The decrease in the blood supply is most significant in the initial stage of treatment. The decrease in the tumor blood supply gradually reduces with the prolongation of treatment time. Therefore, for the purpose of reducing intra-operative bleedingand facilitating surgery, the administration of denosumab treatment more than three monthsbefore surgery is not recommended. Clinicians should perform individualized analysis and appropriate adjustment of each patient’s treatment plan based on the individual’s biological characteristics and tumor development.
Funding
Beijing Hospitals Authority Youth Programme (QML20200403).
Beijing Jishuitan Hospital Elite Young Scholar Programme (XKGG202108); Beijing JST Research Funding (ZR-202104).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None
References
- 1.Errani C., Ruggieri P., Asenzio M.A.N., Toscano A., Colangeli S., Rimondi E., Rossi G., Longhi A., Mercuri M. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1–7. doi: 10.1016/j.ctrv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Arbeitsgemeinschaft K., Becker W.T., Dohle J., et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvanttherapy. J Bone Joint Surg Am. 2008;90(5):1060–1067. doi: 10.2106/JBJS.D.02771. [DOI] [PubMed] [Google Scholar]
- 3.X. Niu Q. Zhang L. Hao Y.i. Ding Y. Li H. Xu W. Liu Giant cell tumor of the extremity:retrospective analysis of 621 Chinese patients from one institution 94 5 2012 461 467 [DOI] [PubMed]
- 4.Leggon R.E., Zlotecki R., Reith J., Scarborough M.T. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004;423:196–207. doi: 10.1097/01.blo.0000128643.38390.07. [DOI] [PubMed] [Google Scholar]
- 5.Zheng K., Wang Z., Wu S.-J., Ye Z.-M., Xu S.-F., Xu M., Hu Y.-C., Yu X.-C. Giant cell tumor of the pelvis: a systematic review. Orthop Surg. 2015;7(2):102–107. doi: 10.1111/os.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung H.W., Kuo D.P., Shu W.P., Chai Y.B., Liu C.C., Li S.M. Giant-cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am. 1982;64(5):755–761. [PubMed] [Google Scholar]
- 7.Martin-Broto J., Cleeland C.S., Glare P.A., Engellau J., Skubitz K.M., Blum R.H., Ganjoo K.N., Staddon A., Dominkus M., Feng A., Qian Y.i., Braun A., Jacobs I., Chung K., Atchison C. Effects of denosumab on pain and analgesic use in giant cell tumor of bone:interim results from a phase II study. Acta Oncol. 2014;53(9):1173–1179. doi: 10.3109/0284186X.2014.910313. [DOI] [PubMed] [Google Scholar]
- 8.Rutkowski P., Ferrari S., Grimer R.J., Stalley P.D., Dijkstra S.P.D., Pienkowski A., Vaz G., Wunder J.S., Seeger L.L., Feng A., Roberts Z.J., Bach B.A. Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22(9):2860–2868. doi: 10.1245/s10434-015-4634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutkowski P., Gaston L., Borkowska A., Stacchiotti S., Gelderblom H., Baldi G.G., Palmerini E., Casali P., Gronchi A., Parry M., Campanacci D.A., Scoccianti G., Wagrodzki M., Ferrari S., Dijkstra S., Pieńkowski A., Grimer R. Denosumab treatment of inoperable or locally advanced giant cell tumor of bone-multicenter analysis outside clinical trial. Eur J Surg Oncol. 2018;44(9):1384–1390. doi: 10.1016/j.ejso.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Chinder P.S., Hindiskere S., Doddarangappa S., Pal U. Evaluation of local recurrence in giant-cell tumor of bone treated by neoadjuvant denosumab. Clin Orthop Surg. 2019;11(3):352. doi: 10.4055/cios.2019.11.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri A., Gulia A., Hegde P., Verma V., Rekhi B. Neoadjuvant denosumab: its role and results in operable cases of giant cell tumour of bone. Bone Joint J. 2019;101-B(2):170–177. doi: 10.1302/0301-620X.101B2.BJJ-2018-0907.R2. [DOI] [PubMed] [Google Scholar]
- 12.Niu X., Yang Y., Wong K.C., Huang Z., Ding Y.i., Zhang W. Giant cell tumour of the bone treated with denosumab: How has the blood supply and oncological prognosis of the tumour changed? J Orthop Transl. 2019;18:100–108. doi: 10.1016/j.jot.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costantino E, Shinji T, Andreas FM. How safe and effective is denosumab for bone giant cell tumour? International Orthopaedics (SICOT). 2017; 41(11): 2397-400 [DOI] [PubMed]
- 14.Müller D.A., Beltrami G., Scoccianti G., Campanacci D.A., Franchi A., Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone—a case series. World J Surg Oncol. 2016;14(1) doi: 10.1186/s12957-016-1034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Y. Yang Y. Li W. Liu H. Xu X. Niu A nonrandomized controlled study of sacral giant cell tumors with preoperative treatment of denosumab 97 46 2018 e13139 10.1097/MD.0000000000013139 [DOI] [PMC free article] [PubMed]
- 16.Singh A.S., Chawla N.S., Chawla S.E. Giant-cell tumor of bone: treatment options and role of denosumab. Biologics. 2015;9:69–74. doi: 10.2147/BTT.S57359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miles K.A. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: a review. European journal of radiology. 1999;30(3):198–205. doi: 10.1016/s0720-048x(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 18.Michael G. X-ray computed tomography. Physics Education. 2001;36(6):442–451. [Google Scholar]
- 19.Bushberg J.T., Seibert J.A., Leidholdt E.M., Boone J.M., Goldschmidt E.J. The essential physics of medical imaging. Eur J Nucl Med Mol Imaging. 2003;30(7):1936. [Google Scholar]
- 20.Yongkun Y., Zhen H., Yi D., et al. Effects of preoperative denosumab therapy on blood supply of bone giant cell tumors. Chin J Bone Joint. 2019;8(9):667–671. [Google Scholar]
- 21.S. Nishimura K. Hashimoto A. Tan Y. Yagyu M. Akagi Successful treatment with denosumab in a patient with sacral giant cell tumor of bone refractory to combination therapy with arterial embolization and zoledronic acid: A case report 6 3 2017 307 310 [DOI] [PMC free article] [PubMed]
- 22.Bukata SV, Blay JY, Rutkowski P, et al. Denosumab Treatment for Giant Cell Tumor of the Spine Including the Sacrum. Spine. 2021; 46 (5): 277-84.23. Boriani S, Cecchinato R, Cuzzocrea F, et al. Denosumab in the treatment of giant cell tumor of the spine. Preliminary report, review of the literature and protocol proposal. European Spine Journal. 2020; 29: 257-71
- 23.Kei S., Yoshiyuki S., Taketo O., et al. Preoperative denosumab treatment with curettage may be a risk factor for recurrence of giant cell tumor of bone. J Orthop Surg (Hong Kong). 2020;28(2):1–8. doi: 10.1177/2309499020929786. [DOI] [PubMed] [Google Scholar]
- 24.Tsukamoto S., Tanaka Y., Mavrogenis A.F., et al. Is Treatment with Denosumab Associated with Local Recurrence in Patients with Giant Cell Tumor of Bone Treated with Curettage? A Systematic Review. Clin Orthop Relat Res. 2019;478(5):1–10. doi: 10.1097/CORR.0000000000001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X.i., Li H., Zhu S., Wang Y., Qian W. Pre-operative denosumab is associated with higher risk of local recurrence in giant cell tumor of bone: a systematic review and meta-analysis. BMC Musculoskeletal Disorders. 2020;21(1) doi: 10.1186/s12891-020-03294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Errani C., Tsukamoto S., Leone G., Righi A., Akahane M., Tanaka Y., Donati D.M. Denosumab May Increase the Risk of Local Recurrence in Patients with Giant-Cell Tumor of Bone Treated with Curettage. J Bone Joint Surg Am. 2018;100(6):496–504. doi: 10.2106/JBJS.17.00057. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya I., Takami M., Miyamoto A., Karakawa A., Dezawa A., Nakamura S., Kamijo R. Invitro study of the effects of denosumab on giant cell tumor of bone: comparison with zoledronicacid. Pathol Oncol Res. 2019;25(1):409–419. doi: 10.1007/s12253-017-0362-8. [DOI] [PubMed] [Google Scholar]
- 28.Palmerini E., Chawla N.S., Ferrari S., Sudan M., Picci P., Marchesi E., Leopardi M.P., Syed I., Sankhala K.K., Parthasarathy P., Mendanha W.E., Pierini M., Paioli A., Chawla S.P. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Cancer. 2017;76:118–124. doi: 10.1016/j.ejca.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Broehm C.J., Garbrecht E.L., Wood J., Bocklage T. Two cases of sarcoma arising in giant cell tumor of bone treated with denosumab. Case Rep Med. 2015;2015:1–6. doi: 10.1155/2015/767198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aponte-Tinao L.A., Piuzzi N.S., Roitman P., Farfalli G.L. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop. 2015;473:3050–3055. doi: 10.1007/s11999-015-4249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]