Abstract
Background
Vaccination with ChAdOx1 n‐CoV‐19 is associated with a rare syndrome called vaccine‐induced immune thrombotic thrombocytopenia (VITT). VITT has been reported mainly in Western countries, whereas the report of VITT in Asians is sparse.
Objectives
To report a case series of VITT following ChAdOx1 n‐CoV‐19 in Thailand and to estimate the incidence of VITT among Asian countries.
Methods
We retrieved the number of VITT patients and the total inoculation doses from the database of the Thai Ministry of Public Health. We performed a literature search including published articles and gray literature to estimate the incidence of VITT. The incidences of VITT by countries and respective confidence intervals were calculated.
Results
By the end of August 2021, five VITT cases occurred after 15 million doses of ChAdOx1 n‐CoV‐19 in Thailand. The median age was 31 years, and 60% were women. The incidence of VITT is estimated at 1 in 3 million. In other Asian countries, only a few cases of VITT have been reported. The incidence of VITT is much lower than in those of Western countries, which is estimated at 1 in 100, 000. The fatality rate was 44% in this study.
Conclusions
Although the incidence of VITT in Asians is low, the mortality rate is substantially higher. We urge that public awareness of this syndrome be raised, as early recognition and appropriate treatment of this syndrome following ChAdOx1 n‐CoV‐19 are crucial to improve the outcome.
Keywords: Asian, COVID‐19 vaccines, incidence, Thailand, vaccine‐induced thrombotic thrombocytopenia
Essentials.
Vaccine‐induced immune thrombotic thrombocytopenia (VITT) is a rare syndrome.
The incidence of VITT after ChAdOx1 nCoV‐19 vaccination is lower in Asians than in White persons.
Despite the lower incidence of VITT in Asians, the mortality rate is higher.
Early diagnosis and appropriate treatment are crucial to improve the outcome.
1. INTRODUCTION
By the end of April 2021, there were several case reports of thrombosis with thrombocytopenia after receiving the first dose of the ChAdOx1 nCoV‐19 vaccine. Most cases presented with thrombosis at unusual sites. 1 , 2 , 3 In addition, anti–platelet factor 4 (PF4)/polyanionic antibodies were positive in almost all patients. This resulted in many countries halting coronavirus disease 2019 (COVID‐19) vaccination with ChAdOx1 nCoV‐19. While many countries resumed the vaccination after their regulators suggested that the benefits of the vaccine outweighed the risk of thrombosis, several countries have restricted the vaccine to older populations, such as those aged >50 years. In Asia, Thailand, Taiwan, Malaysia, and South Korea have resumed inoculation. To date, the occurrences of VITT have been mostly reported in White persons, 4 while data in Asia have been sparsely reported. In Thailand, where about 15 million doses of ChAdOx1 nCoV‐19 have been administered, only five cases of vaccine‐induced thrombotic thrombocytopenia (VITT) have been reported. Herein, we report our case series of VITT and review the incidence of VITT in Asian population.
2. METHODS
We retrieved the number of VITT cases and the total number of inoculations from the database of the Thai Ministry of Public Health. We performed a literature search including published articles and gray literature to estimate the incidence of VITT. Baseline characteristics of patients with VITT in Thailand and South Korea were summarized using descriptive statistics. The incidences of VITT by countries and respective confidence intervals were estimated from the reported incidences and the standard errors of proportion. All statistical analyses were performed using Stata software version 16 (StataCorp, College Station, TX, USA).
3. RESULTS
At the end of August 2021, there were five cases of VITT reported in Thailand (Table 1). We classified the definition of VITT according to the proposed criteria. 5 All five criteria, including timing, thrombosis, thrombocytopenia, D‐dimer level >4,000 ng/mL fibrinogen equivalent units (FEU), and positive anti–platelet factor 4 (PF4)/polyanionic antibodies, are required for the diagnosis of definite VITT. If D‐dimer level was >4,000 ng/mL FEU but one of the other four criteria was absent, the diagnosis of probable VITT was made. If D‐dimer level was unknown or in the range of 2,000 to 4,000 ng/mL FEU with one other criterion absent, or two other criteria (timing, thrombosis, thrombocytopenia, or anti‐PF4/polyanionic antibodies) were not met, the diagnosis of possible VITT was made. One of our cases was thus classified as definite VITT, two cases were labeled probable, and two were designated possible VITT.
TABLE 1.
Characteristics of VITT cases reported in Asia
| Country | Diagnosis classification | Age | Sex | Days after vaccine | Clinical presentation | Initial platelet count (×109/L) | Platelet count nadir (×109/L) | D‐dimer (ng/mL FEU) | Anti‐PF4 antibodies (OD) | Thrombosis | ICH | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thailand | Probable | 20s | Female | 8 | Headache, petechiae | 22 | 15 | 9,452 | Positive (2.1) | Not seen on imaging | No | Alive |
| Definite | 30s | Male | 15 | Headache, back pain, petechiae | 19 | 18 | 46, 383 | Positive (1.8) | CVT, PVT | No | Alive | |
| Probable | 20s | Female | 6 | Headache | 192 | 100 | 87, 536 | Negative (0.2) | CVT | Yes | Died | |
| Possible | 50s | Male | 15 | Headache, hemiparesis | 72 | 32 | ND | ND | CVT | Yes | Died | |
| Possible | 70s | Female | 3 | Headache, drowsiness | 218 | 142 | 8,400 | Negative (0.1) | CVT | Yes | Died | |
| Korea | Definite | 30s | Male | 11 | Headache, seizure | 77 | 65 | >20, 000 | Positive (3.1) | CVT | Yes | Alive |
| Definite | 30s | Male | 8 | Headache, hemiparesis, drowsiness | 14 | 10 | >35, 200 | Positive (0.7) | CVT | Yes | Died | |
| Taiwan | Definite | 30s | Male | 10 | Headache, abdominal pain | 34 | 23 | >10, 000 | Positive (1.2) | Lacunar infarction | No | Alive |
| Unknown | 30s | Female | 8 | Bleeding per gums | Low | NR | High | NR | NR | NR | Alive |
Abbreviations: CVT, cerebral vein thrombosis; ICH, intracerebral hemorrhage; ND, not done; NR, not reported; PVT, portal vein thrombosis.
D‐dimer levels were determined by Innovance D‐Dimer microparticle‐enhanced immunoassay (Dade Behring, Deerfield, IL, USA). Results for D‐dimer levels were reported in nanograms per milliliter FEU. The normal level was <500 ng/mL FEU. Anti‐PF4/polyanionic antibodies were detected by a heparin‐induced thrombocytopenia (HIT) ELISA method with the use of Zymutest HIA (Hyphen BioMed, Neuville sur Oise, France) monospecific (IgG) assay. The positive threshold was based on the manufacturer’s optical density (OD) threshold (>0.3).
3.1. Case 1
A woman in her 20s presented with severe headache 9 days after vaccination with the first dose of ChAdOx1 nCoV‐19. The patient also noted petechiae on both lower extremities. Her initial platelet count was 22 × 109/L. However, no thrombosis was demonstrated on the magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) of the brain. Computed tomography (CT) angiography of the pulmonary artery and CT scan of the abdomen were also negative. D‐dimer level was 9,452 ng/mL FEU. Anti‐PF4/polyanionic antibodies were positive, with an OD of 2.1. Given a highly elevated D‐dimer level and strongly positive anti‐PF4/polyanionic antibodies, the diagnosis of probable VITT was made. She was promptly treated with intravenous immunoglobulin (IVIG), corticosteroids, and apixaban on day 1. She completely recovered after treatment.
3.2. Case 2
A man in his 30s presented with progressive headache and back pain 8 days after the first dose of the ChAdOx1 nCoV‐19 vaccine. His initial platelet count was 19 × 109/L. CT of the brain revealed cerebral venous thrombosis. CT scan of the whole abdomen also demonstrated acute portal vein thrombosis. D‐dimer level was 46, 383 ng/mL FEU. Anti‐PF4/polyanionic antibodies were positive, with an OD of 1.8. Given the presence of all five criteria, the diagnosis of definite VITT was made. He was treated with IVIG, corticosteroids, and apixaban started on day 1, before the result of anti‐PF4/polyanionic antibodies. His symptoms and thrombocytopenia subsequently resolved.
3.3. Case 3
A woman in her 20s presented with progressive headache and vomiting 6 days after the first dose of ChAdOx1 nCoV‐19. CT of the brain revealed cerebral sinus thrombosis with intracranial hemorrhage. Her initial platelet count was 192 × 109/L, with a subsequent nadir of 100 × 109/L. D‐dimer level was 87, 536 ng/mL FEU. Anti‐PF4/polyanionic antibodies were negative, with an OD of 0.2. Given the elevated D‐dimer and the fact that one criterion (anti‐PF4/polyanionic antibodies) was absent, the patient was classified as probable VITT. She was treated with enoxaparin but later died from brain herniation.
3.4. Case 4
A man in his 50s with diabetes, hypertension, and hyperlipidemia presented with progressive headache and left hemiparesis 15 days after the first dose of ChAdOx1 nCoV‐19. CT of the brain demonstrated cerebral venous sinus thrombosis with intracerebral hemorrhage. His platelet count was 72 × 109/L. Anti‐PF4/polyanionic antibodies and D‐dimer were not performed. Given the unknown D‐dimer level and the fact that one criterion was not met (anti‐PF4/polyanionic antibodies), the patient was classified as possible VITT. He was treated with decompressive craniectomy but, unfortunately, died following brain herniation.
3.5. Case 5
A woman in her 70s presented with headache and drowsiness 3 days after the first dose of ChAdOx1 nCoV‐19. CT of the brain revealed cerebral venous sinus thrombosis and intracranial hemorrhage. Her initial platelet count was 218 × 109/L, which dropped to 142 × 109/L during hospitalization. D‐dimer level was 8,400 ng/mL FEU. Anti‐PF4/polyanionic antibodies were negative, with an OD of 0.1. Given the high D‐dimer level and the absence of two criteria (timing, anti‐PF4/polyanionic antibodies), the diagnosis of possible VITT was made. She was treated with IVIG, corticosteroids, and apixaban. Her symptoms and thrombocytopenia improved but the patient died later from urinary tract infection and sepsis.
4. DISCUSSION
To date, though vaccination using ChAdOx1 nCoV‐19 has been used in many Asian countries since March 2021, VITT cases have rarely been reported. Our four of five VITT cases met the criteria of thrombotic thrombocytopenia syndrome as defined by the Brighton Collaboration, which is used by the World Health Organization. 6 In Thailand, after 15 million doses of ChAdOx1 nCoV‐19 vaccines (10 million first doses and 5 million second doses), five cases of VITT have been reported. All occurred after the first ChAdOx1 nCoV‐19 vaccination. Therefore, the rate for first‐dose vaccination is 5 in 10 million and for second‐dose vaccination is 0 in 5 million. To our knowledge, only four additional cases of VITT were reported in other Asian countries by the end of August 2021. Two cases of definite VITT were reported from South Korea 7 , 8 and Taiwan. 9 , 10 , 11 Most affected individuals were in their 30s, and most presented with clinical thrombosis and bleeding. Of the nine mentioned cases, four were fatal, yielding a 44% fatality rate. Despite the low incidence of VITT in Asians, the mortality rate is higher than that reported in the United Kingdom (Table 2). 5 This indicates that early recognition and treatment of this syndrome is important. All vaccinees should be informed of abnormal symptoms and signs suggestive of thrombosis, and physicians should have a high index of suspicion of VITT in those who received ChAdOx1 nCoV‐19. Two of our cases received early treatment with IVIG, and the outcome was favorable. One patient received early IVIG treatment but died later from sepsis. Two cases did not receive treatment with IVIG and died from progressive cerebral sinus thrombosis with intracranial hemorrhage.
TABLE 2.
Comparison of clinical features of VITT cases in Asia and the United Kingdom
| Features | Thai cases | Korean cases | UK cases |
|---|---|---|---|
| Total number of cases | 5 | 2 | 220 |
| Number after first‐dose vaccine (%) | 5 (100) | 2 (100) | 220 (100) |
| Days since vaccination (median) | 3‐15 (8) | 8‐11 | 5‐48 (14) |
| Female (%) | 3 (60) | 0 | 119 (55) |
| Age range, y (median) | 26‐76 (31) | 33 | 18‐79 (48) |
| Number of patients <50 years old (%) | 3 (60) | 2 (100) | 123 (56) |
| Platelet count range, ×109/L (median) | 19‐142 (72) | 10‐65 | 6‐344 (47) |
| Cerebral vein thrombosis (%) | 4 (80) | 2 (100) | 110 (50) |
| Intracerebral hemorrhage (%) | 3 (60) | 2 (100) | 42 (19) |
| Death (%) | 3 (60) | 1 (50) | 44 (22) |
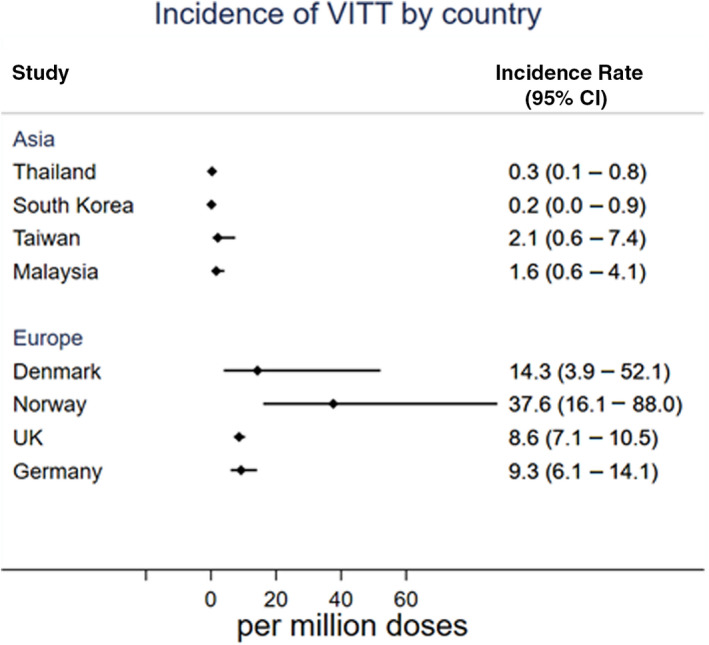
The incidence of VITT is estimated at 1 in 3 million in Thailand. The incidence of VITT in South Korea is estimated at 1 in 6.4 million. 8 The incidence of VITT is estimated at 1 in 0.48 million in Taiwan according to the report from Taiwan’s Central Epidemic Command Center. 9 , 10 VITT occurred in 4 of 2.5 million doses given in Malaysia (Selvaratnam V., personal communication, September 8, 2021). The incidence of VITT reported in Asians is much lower than those in White persons (Figure 1). The incidence of VITT is estimated at 1 in 20, 000 to 1 in 50, 000 in the younger population (aged <50 years), while the incidence is ≈1 in 100, 000 in the older population (aged >50 years). 5 , 12 Given the rarity of VITT in Asia, we hypothesize that the Asian population is less susceptible to the development of VITT than those in the White population. Of relevance, a lower incidence of HIT in Asian patients compared to White patients has also been reported. 13
FIGURE 1.
The exact mechanism of VITT is still under investigation. However, given the clinical picture of thrombosis and thrombocytopenia with positive anti‐PF4/polyanionic antibodies, the clinical picture bears some resemblance to HIT. Heparin can form a complex with PF4 released from platelets. This complex induces autoantibodies that cause intense platelet activation, resulting in thrombocytopenia and thrombosis. While HIT requires heparin as a trigger, VITT does not. This has been described as being analogous to the syndrome of “spontaneous HIT.” 14 In VITT, the vaccine itself and/or its effect upon the host is assumed to be the trigger. It has been variously hypothesized that vaccine constituents form antigenic complexes with PF4, that EDTA in the vaccine induces microvascular permeability, and that vaccine components cause acute inflammatory reactions. Any or all of these mechanisms may promote strong platelet activation and cause the VITT prothrombotic response. 15
Cerebral venous sinus thrombosis is the most common clinical presentation in patients with VITT. An increase in the incidence of cerebral sinus thrombosis after the vaccination compared to the general population has also been observed. 16 , 17 The predilection for the cerebral sinus is unclear. One hypothesis is that it may be due to the non‐unidirectional blood flow in the cerebral venous sinus that is missing the usual venous valves causing stasis of blood flow. 18
The first case in our cohort presented with severe headache, marked thrombocytopenia, and a markedly elevated D‐dimer level but no evidence of cerebral vein thrombosis on MRI and MRV. This could represent a “pre‐VITT” syndrome. In a cohort of 11 patients from Germany, 4 patients presented with severe headache that preceded any evidence of thrombosis. All 4 patients developed thrombosis in the 6 to 9 days following the onset of headache. 19 The other 7 cases received early treatment with either IVIG, anticoagulants, or corticosteroids and did not develop subsequent thrombosis. These findings emphasize that early detection and treatment of VITT, especially in the pre‐VITT phase, is important. In addition, 3 cases (60%) in our cohort had concurrent cerebral sinus thrombosis and intracranial hemorrhage. All patients with intracranial hemorrhage died. This aligned with the finding from the cohort from United Kingdom that intracranial hemorrhage is associated with increased mortality.
Our study has a limitation. Since the data were rarely reported, we performed a narrative review of the literature rather than a systematic review. The reporting systems of adverse events are different among Asian countries; thus, the underreporting might be an issue. However, in Thailand, we have three surveillance reporting systems including reports from medical personnel and vaccinees themselves. All serious adverse events after vaccination are reviewed weekly by the National Immunization Center of the Thai Ministry of Public Health.
In summary, we report a case series of VITT in Thailand. Current experience suggests a low incidence of VITT in Asians compared to people in Western countries. However, the mortality rate was high. Early diagnosis and appropriate treatment are crucial.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
KB reviewed literature, analyzed the data, and drafted the manuscript. PA conceived of the study idea and critically revised the manuscript.
ACKNOWLEDGMENTS
We sincerely thank Professor Nigel Key (University of North Carolina at Chapel Hill, USA) for reviewing the manuscript and offering comments.
Boonyawat K, Angchaisuksiri P. Vaccine‐induced immune thrombotic thrombocytopenia with ChAdOx1 nCoV‐19 is rare in Asia. Res Pract Thromb Haemost.2022;6:e12644. doi: 10.1002/rth2.12644
Handling Editor: Dr Cihan Ay
REFERENCES
- 1. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChadOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2092‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2124‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2202‐2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan BTB, Bobos P, Odutayo A, Pai M. Meta‐analysis of risk of vaccine‐induced immune thrombotic thrombocytopenia following ChAdox1‐A recombinant vaccine. medRxiv. 2021. doi: 10.1101/2021.05.04.21256613 [DOI] [Google Scholar]
- 5. Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine‐induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680‐1689. doi: 10.1056/NEJMoa2109908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steve Black M & Brighton Collaboration Updated Proposed Brighton Collaboration process for developing a standard case definition for study of new clinical syndrome X, as applied to thrombosis with thrombocytopenia syndrome (TTS). https://brightoncollaboration.us/thrombosis‐with‐thrombocytopenia‐syndrome‐interim‐case‐definition/. [Accessed 2021/10/21].
- 7. Arin K. Korea confirms first rare blood clot case linked to AstraZeneca COVID‐19 vaccine The Korea Herald2021. http://www.koreaherald.com/view.php?ud=20210531000968. [Accessed 2021/08/27].
- 8. Bang SM, Na SH, Kim JH, Kim SR, Kim SR, Jang S. Platelet count as an important prognostic factor for vaccine‐induced immune thrombotic thrombocytopenia. Blood Res. 2021;56:129‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Everington K. Taiwanese man in his 30s develops blood clots after AZ shot Taiwan News. https://www.taiwannews.com.tw/en/news/4214977. [Accessed 2021/08/21].
- 10. Chieh‐ling H & Kao E. Taiwan reports another vaccine‐induced blood clot case Focus Taiwan, CNA English News. https://focustaiwan.tw/society/202106070025. [Accessed 2021/08/27].
- 11. Huang CT, Hsu SY, Wang CH, et al. Double high‐dose immunoglobulin for ChAdOx1 nCov‐19 vaccine‐induced immune thrombotic thrombocytopenia. Thromb Res. 2021;206:14‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiedmann M, Skattor T, Stray‐Pedersen A, et al. Vaccine induced immune thrombotic thrombocytopenia causing a severe form of cerebral venous thrombosis with high fatality rate: a case series. Front Neurol. 2021;12:721146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boonyawat K, Angchaisuksiri P, Aryurachai K, Chaiyaroj S, Ahmadi Z, Chong BH. Low prevalence of heparin‐induced thrombocytopenia after cardiac surgery in Thai patients. Thromb Res. 2014;134:957‐962. [DOI] [PubMed] [Google Scholar]
- 14. Warkentin TE, Greinacher A. Spontaneous HIT syndrome: knee replacement, infection, and parallels with vaccine‐induced immune thrombotic thrombocytopenia. Thromb Res. 2021;204:40‐51. [DOI] [PubMed] [Google Scholar]
- 15. Greinacher A, Selleng K, Palankar R, et al. Insights in ChAdOx1 nCov‐19 vaccine‐induced immune thrombotic thrombocytopenia (VITT). Blood. 2021;138:2256‐2268. doi: 10.1182/blood.2021013231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schulz JB, Berlit P, Diener H‐C, et al. COVID‐19 vaccine‐associated cerebral venous thrombosis in Germany. Ann Neurol. 2021;90:627‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pottegård A, Lund LC, Karlstad Ø, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford‐AstraZeneca ChAdOx1‐S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kowarz E, Krutzke L, Reis J, Bracharz S, Kochanek S, Marschalek R. “Vaccine‐induced covid‐19 mimicry” syndrome: splice reactions within the SARS‐CoV‐2 spike open reading frame result in spike protein variants that may cause thromboembolic events in patients immunized with vector‐based vaccines. Res Square. 2021. doi: 10.21203/rs.3.rs-440461/v1 [DOI] [Google Scholar]
- 19. Salih F, Schonborn L, Kohler S, et al. Vaccine‐induced thrombocytopenia with severe headache. N Engl J Med. 2021;385:2103–2105. doi: 10.1056/NEJMc2112974 [DOI] [PMC free article] [PubMed] [Google Scholar]


