Abstract
We have conducted the first study of sleep in the platypus Ornithorhynchus anatinus. Periods of quiet sleep, characterized by raised arousal thresholds, elevated electroencephalogram amplitude and motor and autonomic quiescence, occupied 6–8 h/day. The platypus also had rapid eye movement sleep as defined by atonia with rapid eye movements, twitching and the electrocardiogram pattern of rapid eye movement. However, this state occurred while the electroencephalogram was moderate or high in voltage, as in non-rapid eye movement sleep in adult and marsupial mammals. This suggests that the low-voltage electroencephalogram is a more recently evolved feature of mammalian rapid eye movement sleep. Rapid eye movement sleep occupied 5.8–8 h/day in the platypus, more than in any other animal.
Our findings indicate that rapid eye movement sleep may have been present in large amounts in the first mammals and suggest that it may have evolved in pre-mammalian reptiles.
Keywords: phylogeny, monotreme, duck-billed, evolution, development
Sleep in mammals consists of two stages, rapid eye movement (REM) and non-REM. REM sleep has been observed with behavioral or electrophysiological measures in virtually all placental and marsupial mammals studied.55 The monotremes comprise the third branch of the mammalian tree. There are just three extant monotreme species, the short-beaked and long-beaked echidna and the platypus. Fossil and genetic evidence indicates that the monotreme line diverged from the other mammalian lines about 150 million years ago and that both echidna species are derived from a platypus-like ancestor.10,16,53
The monotremes have shown a remarkably conservative evolutionary course since their divergence from the rest of the mammalian line. For example, fossil teeth from Steropodon galmani dated at 110 million years ago show many similarities to the vestigial teeth of the current-day platypus, Ornithorhyncus anatinus.3 Analyses of fossilized skull remains indicate remarkably little change in platypus morphology over at least 60 million years.2,37
The low level of speciation throughout the fossil record is another indicator of the uniquely conservative lineage of monotremes. Apart from the echidna line, the 150 million years of platypus evolution has produced no species radiation, although the fragmentary skull evidence available for the identification of Monotrematum sudamericanum has led to its tentative classification as a separate taxon.37 The echidna line has a similar history. The classification of the giant echidna, Zaglossus hacketti, is uncertain because of the lack of cranial material.19 However, apart from this specimen, there has only been the relatively recent divergence of the short- and long-beaked echidna over the echidna’s 60-million-year-long evolutionary course.20 In contrast, more than 4000 placental and marsupial species have evolved since the emergence of the monotremes. While monotremes are distinctly mammalian, they do display a number of reptilian features, making study of their physiology a unique opportunity to determine the commonalties and divergences in mammalian evolution.19,26,53
The first study of monotreme sleep was performed on the short-beaked echidna,1 and reported the presence of non-REM sleep and the complete absence of REM sleep. A more recent study of unit activity in the echidna46 found that, whereas there was no sleep state with rapid eye movements and twitching, during sleep with high-voltage electroencephalogram (EEG), brainstem reticular formation units fired in the irregular burst–pause pattern that characterizes REM sleep, not in the slow regular pattern of non-REM sleep.24,44 This finding suggests that, while the monotremes may not have the low-voltage EEG of placental and marsupial REM sleep, they may have aspects of the brainstem activation that underlies its principal features. Examination of the platypus was undertaken to elucidate the nature of sleep in this most plesiomorphic mammal.
EXPERIMENTAL PROCEDURES
We were allowed access to one adult female and three adult male platypus for the current study. They were captured in southeast Queensland, Australia, and weighed between 0.9 and 1.5 kg, with the smallest being a female. All studies were done at the University of Queensland in Brisbane. The research was carried out according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes under Queensland National Parks and Wildlife permit T00803 and K01782.
Because the platypus is a semi-aquatic mammal and cannot be confined without severe stress, it has been difficult to maintain and study in captivity.18,54 Eighty per cent of platypus die during the first year of captivity in zoos within Australia, most within the first month.54 One possible explanation for this high mortality rate is stress resulting from the electrical noise generated by the pumps and other electrical equipment used in conventional aquaria. Platypus are unique among mammals in possessing a highly developed electrosensory system.31,41 This system processes information derived from specialized electroreceptors arrayed over the bill.32 We have dealt with this problem by constructing a Faraday cage around the 2.0-m-diameter, 1-m-high (water depth 40 cm) fiber-glass tank where the platypus could swim and feed. This reduced the ambient electrical noise to 10–20 μV/cm. The Faraday cage also enclosed the 13 m × 30 cm × 30 cm burrow system. Platypus typically slept in one of two nesting boxes (250 mm high × 300 mm wide × 400 mm long) within the burrow system. Hay and leaf litter was available in the tunnels and used by the platypus to customize their sleep areas.31 Plexiglas ports placed over the nest areas allowed video monitoring and recording of sleep postures, twitching and eye movements. These ports were covered during polygraphic recording of normative sleep parameters.
Platypus were fed fresh water crustaceans and worms, as described previously.31 Platypus typically consumed 500 g (up to half their body weight) each day. An active gravel and charcoal filtration system maintained water cleanliness. The pool and burrow system was in an unheated room open at the ceiling to allow natural cycles of temperature and light into the room. Of the 30 platypus maintained for one to three months (mean of six weeks) in this enclosure over a six-year period for a variety of behavioral, anatomical and physiological studies, only one died prior to experimentation.
The present experiments were done in June and July (Winter). In Brisbane at this time of year, dawn is at 6.00 a.m. and sunset at 5.30 p.m. Temperatures within the pool ranged from 20.5 to 21.5°C, and in the burrow from 20 to 23°C.
To maintain good health in captivity, platypus must be free to swim and explore their burrow system to approximate conditions in the wild. It is likely that any prolonged confinement or direct connection to a recording cable would cause severe stress, the major cause of death in captive platypus.54 Therefore, we used an implanted telemetry device with one- or three-channel capability (DSI, St Paul, MN, U.S.A.) and an array of six telemetry receivers, two placed underwater in the tank and four placed under the burrow system. This system continuously transmitted EEG, electro-oculogram (EOG) and electromyogram (EMG) while the platypus was active and inactive, in the burrow and underwater. The frequency response of the system was 0.5–100 Hz (sampling rate of 500/Hz/channel). We recorded continuously with the three-channel system in one implanted animal (a 1.5-kg male) for two weeks, and derived the sleep duration and periodicity measurements from this recording. This animal was videotaped continuously for 48 h prior to implantation and for 72 h starting 11 days after implantation. We videotaped sleep behavior in a second animal (a male), then implanted it with EEG telemetry electrodes, but it died within 24 h of implantation, so its physiological data were of limited use. We recorded the electrocardiogram (ECG) with a one-channel telemetry system and videotaped behavior in a third animal, also for a two-week period. This animal was used for the Poincaré analysis. A fourth unimplanted animal was also observed visually and videotaped during sleep for 12 h. Arousal threshold testing was done during the long-duration EEG recording study and on the fourth unimplanted animal (a 0.9-kg female).
For implantation, animals were anesthetized with 30 mg ketamine mixed with 1.3 mg xylazine/kg. Screw electrodes were placed over the motor cortex29,45 for EEG recording. The EOG was recorded from a pair of electrodes inserted through the conjunctiva of the right eye. The EMG was recorded from electrodes threaded through the dorsal neck muscles. The ECG was recorded from thoracic electrodes. All electrodes were connected to a three-channel telemetry device inserted subcutaneously in the back region or, in the case where just ECG was recorded, attached externally to the fur on the back. Signals were recorded polygraphically and on a Racal tape recorder along with a digital time code. Power spectra and Poincaré plots were calculated with a CED digitizing and analysis system (Cambridge, U.K.) sampling at 100 Hz on all channels. Poincaré plots give an indication of the amplitude of respiratory and other sources of beat-to-beat heart rate variability. The dispersion of points is maximal in non-REM sleep but is minimal in REM sleep owing to a loss of the respiratory-related heart rate rhythmicity.39
RESULTS
We identified five sleep–waking states on the basis of EEG, EOG and EMG recordings: waking (Wake), quiet sleep with moderate-voltage EEG (QS-M), quiet sleep with high-voltage EEG (QS-H), REM sleep with moderate-voltage EEG (REM-M) and REM sleep with high-voltage EEG (REM-H). Figure 1 shows the polygraphic patterns of the sleep–waking states of the platypus. In the Wake state, when the platypus was underwater and quiet, holding its breath and showing its typical diving response,12,31 EEG voltage was at its lowest level. A similar low-voltage EEG was also present when the animal was observed to be awake and immediately after movement periods in the burrow. No periods with comparable low-voltage EEG occurred when the animal had been in a sleep posture for more than a few seconds. When the animal was quiescent, there was little or no tonic muscle tone, even if the animal’s eyes were open and it was responsive to sensory stimuli, i.e. awake. Therefore, tonic muscle activity was not a useful indicator of sleep state.
Fig. 1.

EOG, EMG and EEG power spectra of samples shown of sleep–wake states in the platypus: Wake (in pool and burrow), QS-M and QS-H, REM-M and REM-H.
At sleep onset, EEG amplitude increased, particularly at frequencies below 4 Hz, producing QS-M. Eyes were closed throughout these periods. QS-M was scored if EEG voltages exceeded 40 μV for more than half of the 1-min epoch. The presence of phasic events observed on the EOG channel, beginning as soon as 30–90 s after the start of the QS period, were used to score REM and to discriminate REM from QS.
We first identified periods of REM sleep by video recording and direct visual observation of posture and behavior through the Plexiglas windows placed over the nesting regions in the burrow. We saw REM sleep, as defined by twitching of the bill, head and eyes, in large amounts in all four animals. The behavioral phenomena (eye, bill and head movements) did not differ in implanted vs unimplanted animals. We found that all of the REM episodes occurred while the animal was immobile in a curled or prone sleep posture. Figure 2 presents an episode in which direct visual observation was used to operate event markers to record bill and neck movements on the polygraph record. We found that phasic potentials from the electrodes placed adjacent to the eyes were correlated with rapid movements of the eyes, neck and bill. These included mastication-like movements of the bill, as well as side-to-side movements of the head, that resembled, but were much smaller and less complete, than the movements seen during feeding and swimming in the water tank.31 We saw sleep behavior of similar type in all of the three platypus that were visually observed in the burrow, prior to, as well as after, implantation. We never saw movements of the eyes, bill or head resembling those seen during sleep in the burrow, when the animals were in the water tank.
Fig. 2.
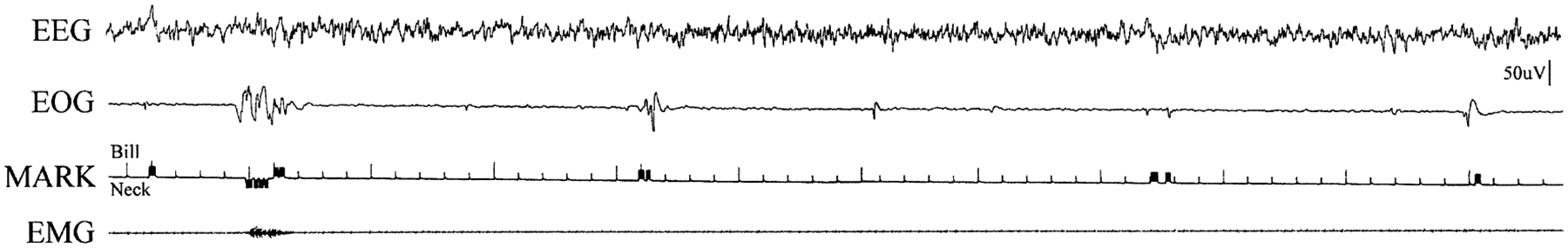
Visually observed REM sleep episode. Bill deflections are indicated with an upward movement of the pushbutton activated marker pen. Neck movement is indicated by a downward deflection of the pen. Ticks on the third channel are at 1-s intervals.
To determine a threshold for phasic event detection using the polygraphic recording of the EOG, we observed the sleeping platypus continuously for a period of 2 h. Using the marker channel, we labeled phasic bill movements on the polygraph record (Fig. 2). We then determined the median amplitude of the EOG signal accompanying each observed bill movement. We used this median amplitude as the criterion for phasic event scoring. We then examined each 1-min epoch for the presence or absence of phasic events.
We found that the median amplitude of the deflection recorded on the EOG channel during visually observed head and bill movements was 40 μV. Therefore, we scored as REM sleep any 60-s epoch with muscle atonia and one or more EOG potentials > 40 μV. This scoring criterion follows the conventions used in young animals, in which EEG is not a useful discriminator between REM and non-REM sleep.21,25 We note that, by our scoring rule, half of the visually observable visually phasic events (those below the median) would not be scored. In order to further assess the effect of scoring rules on sleep epoch duration, we also re-scored the data using three phasic events/1-min epoch as a criterion for REM sleep. One-minute sleep epochs with one or two phasic events were scored as QS-M or QS-H when using this scoring criterion.
REM sleep was always accompanied by an EEG that was of moderate (REM-M) or high amplitude (REM-H), with consistently more power in all of the frequency bands assessed than that during waking states. EEG amplitude in REM-M was always equal to or greater than that in QS-M. REM periods in the 48-h sample that we quantified had an average of 13 EOG events/min exceeding 25 μV. REM-M occupied 50.6% of sleep time using the one phasic event criterion (Table 1) and 40.8% of sleep time using the three phasic events criterion, and typically followed QS-M or Wake. REM-H was scored if the criteria for REM-M were met and there were three or more EEG slow waves exceeding 80 μV in each 1-min epoch. REM-H periods typically followed REM-M periods and occupied 5.7% of sleep time using the one phasic event criterion and 4.0% of sleep time using the three phasic event criterion. Figure 3 shows the state transition probabilities in the 48-h recording block. The most common sleep–wake progression was Wake → QS-M → REM-M → Wake. However, in contrast to adult placental and marsupial mammals, REM sleep could begin from waking at sleep onset. Figure 4 is a hypnogram showing the sequence and duration of states over a continuous 24-h period. In Fig. 5, the length of the REM sleep cycle is plotted. Periodicity was calculated by measuring the time from REM sleep onset to the onset of the subsequent REM sleep period, excluding intervening waking periods.55 A new REM sleep period was scored if more than 3 min had elapsed since the prior REM sleep epoch.
Table 1.
Sleep state durations in minutes and percentage sleep time, based on a continuous 48-h period of electroencephalogram, electro-oculogram and electromyogram telemetry recording, scored in 1-min epochs
| State | Hours/day | Sleep time (%) | Episode duration | Episode range |
|---|---|---|---|---|
| Wake | 9.7 | — | 25.1 ± 73.5 | 0.3–390 |
| QS-M | 4.8 | 33.8 | 13.1 ± 10.7 | 0.3–42.7 |
| QS-H | 1.4 | 9.9 | 7.7 ± 9.7 | 1.0–31 |
| REM-M | 7.2 | 50.6 | 12.1 ± 13.5 | 1.0–74 |
| REM-H | 0.8 | 5.7 | 7.81 ± 6.5 | 1.0–24.5 |
The criterion for REM-M and REM-H was more than one phasic event exceeding the mean phasic event amplitude per epoch.
Fig. 3.
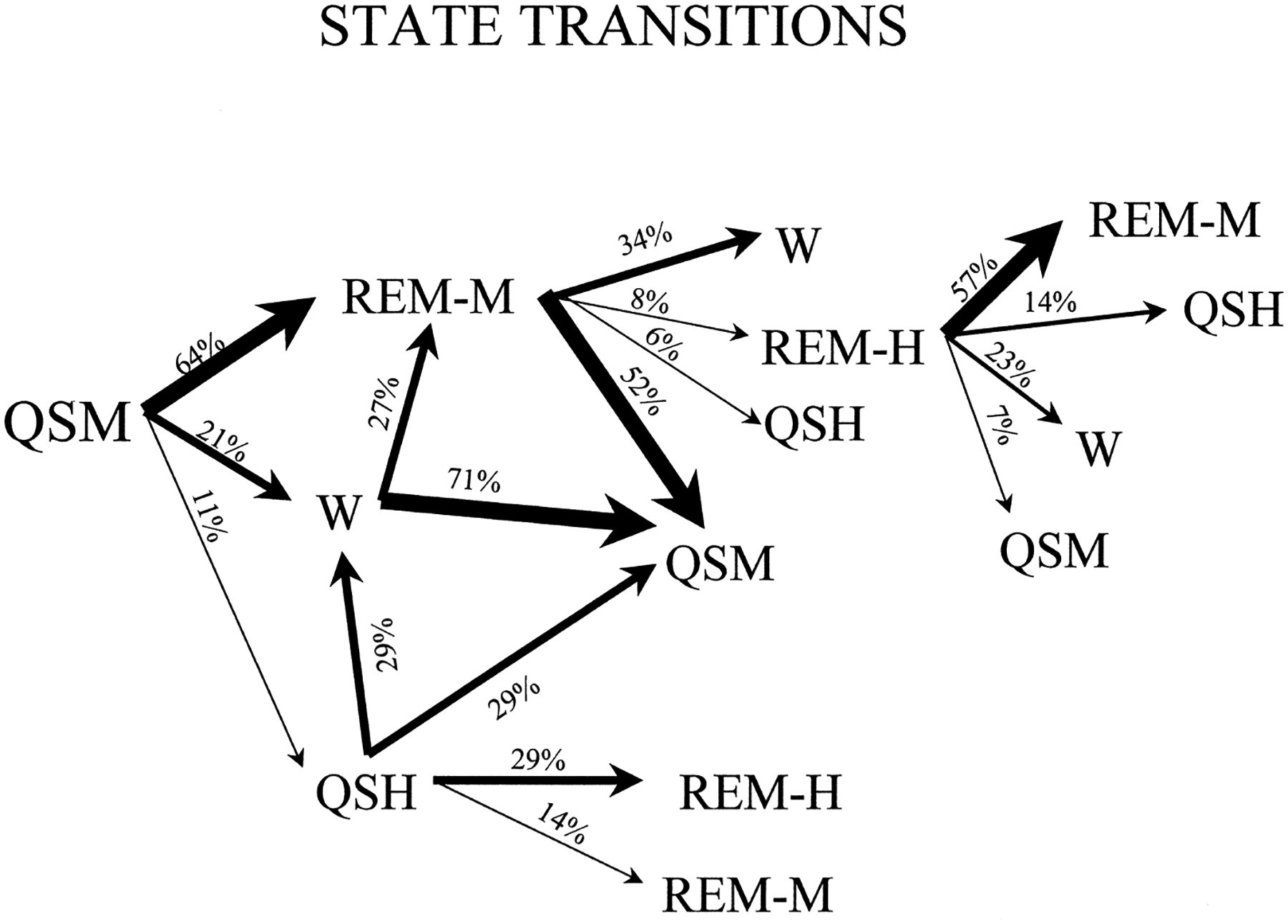
State transition probabilities during sleep in the platypus, based on a 48-h recording and using the one phasic event criterion for scoring REM sleep (Table 1). Only transition types that occurred two or more times within the period of observation are shown. The most common sleep cycle course in the platypus was QS-M to REM-M to Wake.
Fig. 4.
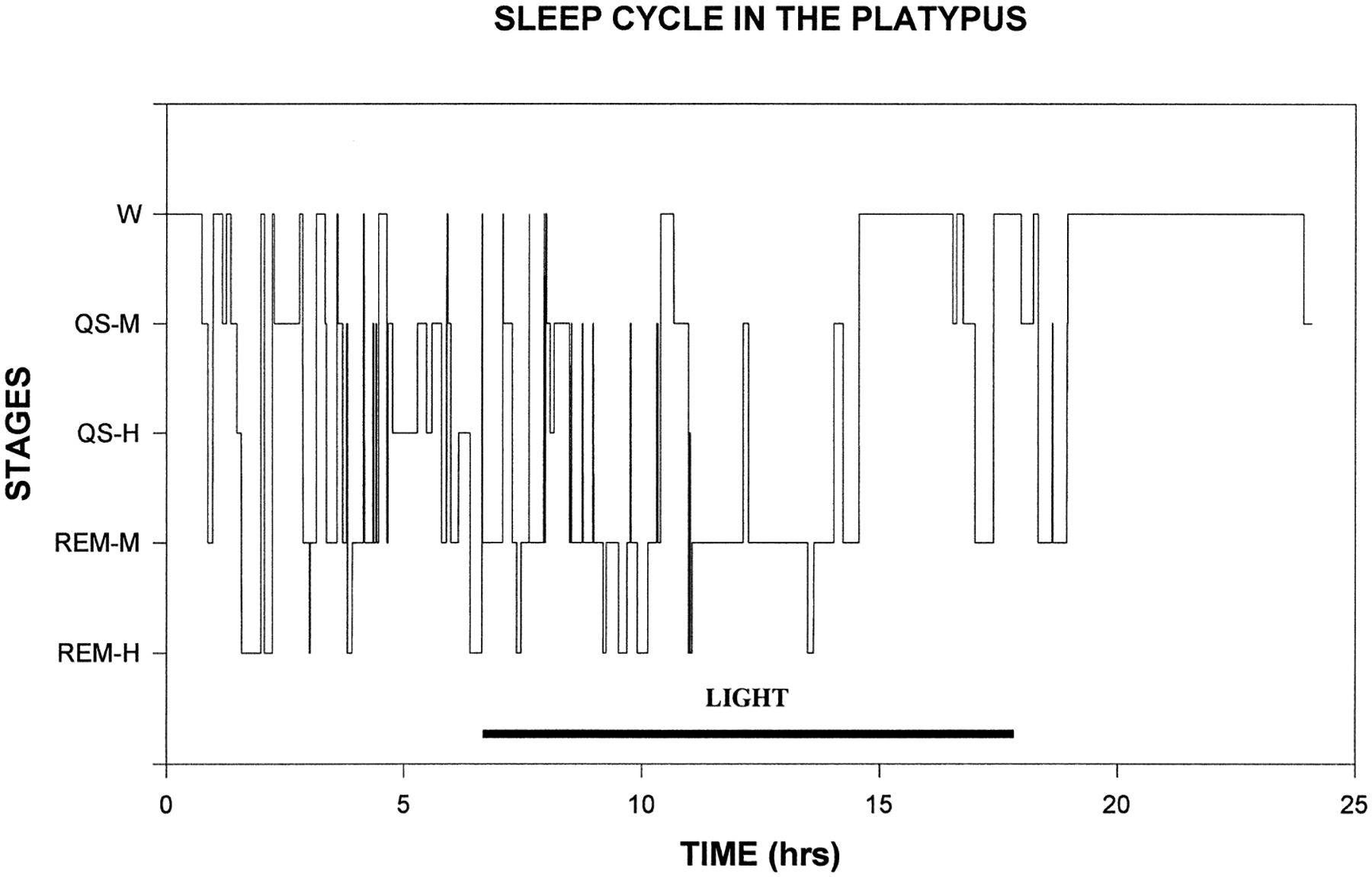
Hypnogram showing the state transitions occurring over a 24-h period beginning at 11.00 a.m. Sleep states had a wide range of durations.
Fig. 5.

Histogram showing distribution of intervals from onset of REM sleep period to onset of subsequent REM sleep based on 48-h recording period. The modal sleep cycle interval in the platypus is 15 min.
QS-M occupied 33.8–47% of sleep time. Epochs with no phasic events and high-voltage EEG, defined as three or more slow waves exceeding 80 μV in each 1-min epoch, were designated QS-H. QS-H periods followed some periods of QS-M, and occupied 9.9–12.2% of sleep time (Tables 1, 2).
Table 2.
Sleep state durations in minutes and percentage sleep time, based on a continuous 48-h period of electroencephalogram, electro-oculogram and electromyogram telemetry recording, scored in 1-min epochs
| State | Hours/day | Sleep time (%) | Episode duration | Episode range |
|---|---|---|---|---|
| Wake | 9.7 | — | 25.4 ± 73.4 | 0.3–390 |
| QS-M | 6.7 | 47.0 | 5.5 ± 7.8 | 0.3–42.7 |
| QS-H | 1.7 | 12.2 | 7.2 ± 7.7 | 4.6–31 |
| REM-M | 5.2 | 36.8 | 5.3 ± 6.3 | 1.7–32.8 |
| REM-H | 0.6 | 4.0 | 3.1 ± 2.7 | 1.0–10.8 |
The criterion for REM-M and REM-H was more than three phasic events exceeding the mean phasic event amplitude per epoch. Using these criteria instead of the one phasic event criterion used in Table 1 caused a large drop in the mean duration of REM periods, but a relatively small change in total REM time.
In placental mammals, REM sleep periods are characterized by a distinctive ECG pattern. During REM sleep, there is a decreased beat-to-beat interval dispersion due to decreased respiratory-related ECG rhythmicity, relative to non-REM sleep. These patterns are best identified with Poincaré plots.39 Figure 6 presents Poincaré plots of QS and REM ECG. During REM, there is relatively little dispersion, reflecting the lack of ECG rhythmicity and elevated ECG rate. During QS, the dispersion around the mean interbeat interval is maximal, reflecting respiratory-related cardiac rhythmicity. The ECG pattern of platypus REM as identified by Poincaré plots resembles that of REM sleep, whereas the QS pattern resembles that of non-REM sleep.39 Thus, from the standpoint of this fundamental indicator of autonomic control, REM in the platypus resembles REM in placental mammals.
Fig. 6.
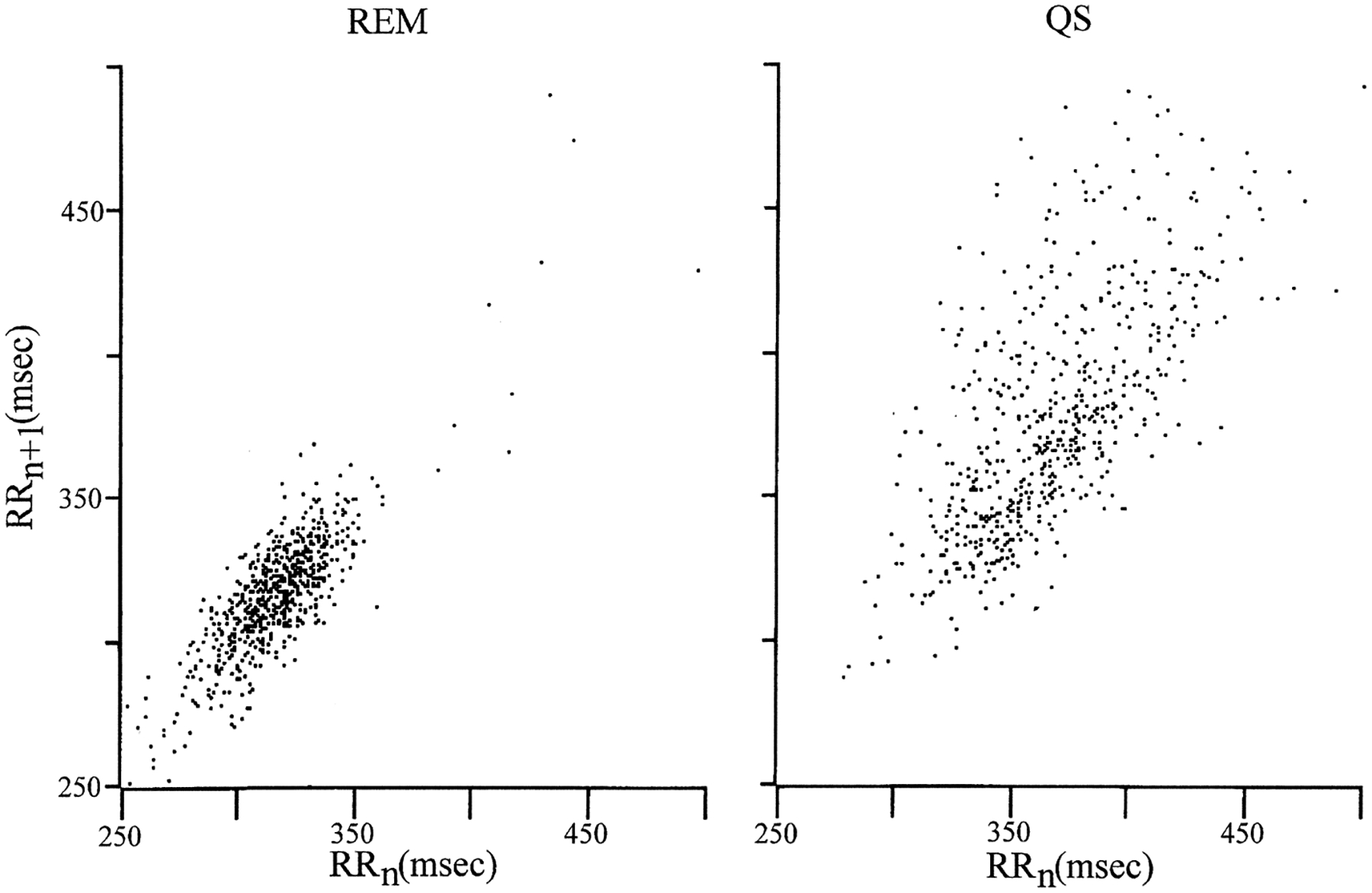
Poincaré plots of ECG (R wave to R wave interval plotted against subsequent interval) of REM sleep (REM) and quiet sleep (QS). REM and QS had differing patterns of interbeat intervals. The decreased beat-to-beat dispersion of the REM period relative to QS resembles that of REM sleep in eutherians.
A series of Von Frey hairs, applied to the dorsal midline of the neck, was used to measure arousal threshold in an unimplanted male, an implanted male and an implanted female.35,50 Immediately after the onset of a quiet sleep period, stimuli of 2 g (n = 10 in each animal, range 1–3 g) were sufficient to arouse the platypus. However, during the periods of behaviorally identified REM sleep, the platypus were difficult to arouse. Pressures required for arousal always exceeded 20 g and were often as high as 50 g (n = 10, mean = 35 g, range 20–50 g). When aroused in this way, the platypus would run from the stimulus, often leaving the burrow and entering the pool.
DISCUSSION
Sleep in the platypus
We found that the platypus has a state with the EOG, EMG, ECG and arousal threshold changes typical of non-REM sleep, and a state meeting generally employed criteria for REM sleep. The REM sleep state was characterized by phasic head, bill and eye movements. The ECG lost the regular sinus arrhythmia of quiet sleep during the REM sleep state, as is the case in placental mammals.
Platypus are present only along the eastern coast of Queensland, Victoria, New South Wales and Tasmania. Despite this restricted range, their population is stable and they are not considered endangered.17 However, the use of monotremes in research is subject to very rigorous regulation by the State governments of Australia. In order to meet the requirements of the Queensland Government and minimize use of these unique animals, we restricted our work to four platypus.
We observed abundant REM sleep, by behavioral criteria, in all four of the animals we monitored. The quantitative analysis of sleep time described in Tables 1 and 2 was derived from observation and continuous recording of polygraphic variables in a 1.5-kg male platypus occurring on days 9 and 10 of a two-week observation period. We did not feel that we could justify, or obtain approval for, further studies aimed at repeating our recordings for the purpose of assessing individual differences in sleep time in a larger group of animals. In cats,51 rats9 and humans,8 the most thoroughly studied mammals, there is relatively little individual to individual variation in sleep stage times, with 1–3% standard deviations in the proportion of the 24-h period devoted to REM sleep. Therefore, it is likely that the sleep state durations that we found are close to the mean values for all platypus studied in a laboratory environment. However, further studies would be needed to determine the distribution of sleep states in the general population of adult platypus and to evaluate the extent of age and sex differences.
In intact, adult placental and marsupial mammals, EEG voltage reduction occurs at REM sleep onset and is maintained throughout this state. In some cases, muscle atonia begins at REM sleep onset, although it is often present in non-REM sleep. These continuous indicators of state make it relatively easy to score onset and offset. In the platypus, these indicators are not present, as is the case in neonatal humans and other altricial mammals. To make the most meaningful estimate of REM sleep time, we observed the platypus for extended periods of time and, using an event marker channel, indicated observed head and bill movements on the polygraph record. We then calculated the average amplitudes of the correlated deflections seen on the EMG and EOG channels. We required that each 1-min epoch contain one deflection exceeding the mean amplitude of these deflections to score an epoch as REM sleep. To determine the effect of scoring rules, we also scored the same data using three deflections exceeding the threshold set by visual observation as a requirement for REM sleep. The stricter three-event criterion resulted in shorter average durations for REM sleep epochs, but not a great change in total REM sleep time (Tables 1, 2).
We worked exclusively with adult animals. If the general mammalian pattern is followed in the platypus, one would expect that younger animals would have more REM sleep. Mammals typically have maximal amounts of REM sleep at thermo-neutrality.27,42 Whereas the ambient temperatures at which the recording were conducted were within the normal range (20–23°C), it certainly is possible that REM sleep amounts would differ at other ambient temperatures. Light cycles can have a small effect on REM sleep durations.11,13 Our animals were exposed to a natural light cycle and slept in their dark burrows. However, it is possible that exposure to different light cycles during waking could alter their sleep times in the burrow. All of these considerations indicate that the parameters of sleep that we have recorded may not be precisely reflected in the wild. To the extent that conditions in the laboratory were less hospitable than those in the wild, one would expect even more REM sleep in the natural population than we have seen here. Perhaps other conditions in the laboratory that we are unaware of increased REM sleep. However, we know of no manipulations in other species that would cause an elevation in REM sleep time to the extraordinary high levels seen in the platypus.
Our studies, under laboratory conditions comparable to those used for measuring REM sleep amounts in other animals, show that the platypus not only has REM sleep, but also has very large amounts of REM sleep. Using standard scoring criteria,25,51 we find that the platypus spends more time in REM sleep than any other animal.33,55
Electroencephalograms of the platypus and the echidna
The moderate- and high-voltage EEGs of platypus REM sleep are unlike the low-voltage REM sleep EEG seen in adult placental and marsupial mammals. In our previous work, we have reported that, in the echidna, activation of brainstem reticulo-motor systems also occurs while the EEG is high in amplitude. These findings suggest that the low-voltage EEG activation of REM sleep seen in placentals and marsupials is derived and evolved after the divergence of the monotreme line. Cortical desynchronization may be a relatively recent development in the history of mammalian REM sleep and is unlikely to have been linked to its original function(s). Rather, these functions are more likely to have been linked to brainstem processes, in keeping with the fact that brainstem mechanisms are both necessary and sufficient to generate this state.43
The REM sleep periods without low-voltage EEG seen in the platypus are similar to the “active sleep” periods seen early in development in altricial mammals.22 In altricial mammals, with development, these periods gradually transition to the REM sleep state with low-voltage EEG. The occurrence of sleep-onset REM periods is also a well-known property of sleep early in development.36 These properties of monotreme sleep suggest that, in these instances, ontogeny is “recapitulating phylogeny”.
Similarities and differences in platypus and echidna sleep
Our previous study showed that, during sleep in the echidna, medial reticular formation neurons have an irregular burst–pause discharge pattern that resembles the discharge pattern during placental REM sleep. However, unlike other mammals, their sleep-related discharge is usually asynchronous in simultaneously recorded neurons and we saw no twitching of the eye or neck.46 This suggests that this terrestrial monotreme has lost the high degree of synchrony between bursting brainstem neurons that is responsible for REM sleep twitching.
In contrast to the high somatosensory pressures required to arouse the platypus, the echidna always aroused with Von Frey pressures less than 2 g (Siegel J. M., Manger P. R. and Pettigrew J. D., unpublished observations). The arousal threshold differences illustrate the evolutionary divergence between these species in sleep characteristics and are consistent with the idea that the echidna has had to adapt to a much less secure sleeping state. In contrast to the platypus, which is not vulnerable to predation when sleeping in its burrow,7 the echidna is exposed during sleep.19 Although its spines provide some protection, twitching movements of the spines during sleep would attract predators. The echidna’s sleep may represent an evolutionary adaptation of the extraordinarily deep sleep of its platypus-like ancestor to the requirements of the echidna’s more vulnerable ecological niche.
CONCLUSIONS
Our findings do not support the concept that REM sleep evolved after the appearance of the first mammals. Rather, they are consistent with the hypothesis that REM sleep was present in very large amounts in the earliest mammals. They suggest that the immediate reptilian ancestors of the early mammals either had REM sleep or had a state with many of the neural correlates of REM sleep, or that REM sleep evolved very rapidly in the mammalian line. Since REM sleep is present in birds, the most parsimonious hypothesis is that REM sleep evolved only once and was present in the common ancestors of birds and mammals. If this is the case, the dinosaurs ancestral to the birds6 may also have had REM sleep.
If the stem reptiles, the common ancestors of birds and mammals, had REM sleep or some neuronal aspects of REM sleep in an REM sleep precursor state, one would expect that extant reptiles would also have some of these same sleep state characteristics. Investigation of reptilian sleep has not produced conclusive evidence on this question. Whereas several papers have claimed that reptiles have REM sleep,4,5,23,38,40,48,49,52 other studies performed after the study by Allison et al.1 of the echidna concluded that reptiles do not have REM sleep.14,15,34 All of these studies used polygraphic recordings of “EEG” and EMG to categorize state. It is particularly difficult to interpret EEG signals as a state indicator in reptiles, since the current work shows that monotremes, the most “reptilian” mammals, have REM sleep without the low-voltage pattern seen in adult placental and marsupial mammals. Further insight into the nature of sleep and its probable evolutionary history could be achieved by examining, in reptiles, the activity of the brainstem neuronal groups that are involved in mammalian REM sleep control,43 particularly the aminergic and cholinergic cell groups that exist in the same regions of reptilian and mammalian brains.28,30,47
Acknowledgements—
This work was supported by the Medical Research Service of the Veterans Administration, USPHS grants NS32819 and NS14610, and the Australian Research Council Special Research Centres Budget.
Abbreviations:
- ECG
electrocardiogram
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electro-oculogram
- QS
quiet sleep
- QS-H
quiet sleep with high-voltage EEG
- QS-M
quiet sleep with moderate-voltage EEG
- REM
rapid eye movement
- REM-H
REM sleep with high-voltage EEG
- REM-M
REM sleep with moderate-voltage EEG
REFERENCES
- 1.Allison T, Van Twyver H and Goff WR (1972) Electrophysiological studies of the echidna, Tachyglossus aculeatus. I. Waking and sleep. Archs ital. Biol 110, 145–184. [PubMed] [Google Scholar]
- 2.Archer M, Jenkins F, Hand S, Murray P and Godthelp H (1992) Description of the skull and non-vestigial dentition of a Miocene platypus (Obdurodon dicksoni n. sp.) from Riversleigh, Australia, and the problem of monotreme origins. In Platypus and Echidnas (ed. Augee M), pp. 15–27. Royal Zoological Society of NSW, Mosman. [Google Scholar]
- 3.Archer M, Flannery TF, Ritchie A and Molnar RE (1985) First Mesozoic mammal from Australia—an early cretaceous monotreme. Nature 318, 363–365. [Google Scholar]
- 4.Ayala-Guerrero F and Huitron-Resendiz S (1991) Behavioral and electrophysiological patterns of wakefulness–sleep states in a lizard. Bol. Estud. Med. Biol 39, 9–14. [PubMed] [Google Scholar]
- 5.Ayala-Guerrero F and Huitron-Resendiz S (1991) Sleep patterns in the lizard Ctenosaura pectinata. Physiol. Behav 49, 1305–1307. [DOI] [PubMed] [Google Scholar]
- 6.Bakker RT (1993) The Dinosaur Heresies. Zebra Books, New York. [Google Scholar]
- 7.Burrell CMZS (1927) The Platypus. Angus & Robinson, Sydney. [Google Scholar]
- 8.Carskadon MA and Dement WC (1994) Normal human sleep: an overview. In Principles and Practice of Sleep Medicine (eds Kryger MH, Roth T and Dement WC), pp. 16–25. W. B. Saunders, Philadelphia. [Google Scholar]
- 9.Clancy JJ, Cladwell DF, Villeneuve MJ and Sangiah S (1978) Daytime sleep–wake cycle in the rat. Physiol. Behav 21, 457–459. [DOI] [PubMed] [Google Scholar]
- 10.Clemens WA (1989) Diagnosis of the class mammalia. In Fauna of Australia (eds Walton DW and Richardson BJ), pp. 401–406. Australian Government Publishing, Canberra. [Google Scholar]
- 11.Deboer T and Tobler I (1996) Shortening of the photoperiod affects sleep distribution, EEG and cortical temperature in the Djungarian hamster. J. comp. Physiol.: Sensory, Neural, Behav. Physiol 179, 483–492. [DOI] [PubMed] [Google Scholar]
- 12.Evans BK, Jones DR, Baldwin J and Gabbot GRJ (1994) Diving ability of the platypus. Aust. J. Zool 42, 17–27. [Google Scholar]
- 13.Faradji H, Cespuglio R, Rondot G, Paut L and Jouvet M (1980) Absence of light–dark entrainment on the sleep–waking cycle in mice with intact visual perception. Brain Res. 202, 41–49. [PubMed] [Google Scholar]
- 14.Flanigan WF Jr, Knight CP, Hartse KM and Rechtschaffen A (1974) Sleep and wakefulness in chelonian reptiles. I. The box turtle, Terrapene carolina. Archs ital. Biol 112, 227–252. [PubMed] [Google Scholar]
- 15.Flanigan WF Jr, Wilcox RH and Rechtschaffen A (1973) The EEG and behavioral continuum of the crocodilian Caiman sclerops. Electroenceph. clin. Neurophysiol 34, 521–538. [DOI] [PubMed] [Google Scholar]
- 16.Flannery TF (1989) Origins of the Australo-Pacific mammal fauna. Aust. zool. Rev 1, 15–24. [Google Scholar]
- 17.Grant T (1992) Historical and current distribution of the platypus, Ornithorhynchus anatinus, in Australia. In Platypus and Echidnas (ed. Augee M), pp. 232–254. Royal Zoological Society of NSW, Mosman. [Google Scholar]
- 18.Grant TR, Williams R and Carrick FN (1977) Maintenance of the platypus (Ornithorhynchus anatinus) in captivity under laboratory conditions. Aust. Zool 19, 117–124. [Google Scholar]
- 19.Griffiths M (1978) The Biology of the Monotremes. Academic, New York. [Google Scholar]
- 20.Griffiths M, Wells RT and Barrie DJ (1991) Observations on the skulls of fossil and extant echidnas (Monotremata tachyglossidae). Aust. Mammal 14, 87–101. [Google Scholar]
- 21.Harper RM, Leake B, Miyahara L, Mason J, Hoppenbrouwers T, Sterman MB and Hodgman J (1981) Temporal sequencing in sleep and waking states during the first 6 months of life. Expl Neurol. 72, 294–307. [DOI] [PubMed] [Google Scholar]
- 22.Harper RM, Leake B, Miyahara L, Hoppenbrouwers T, Sterman MB and Hodgman J (1981) Development of ultradian periodicity and coalescence at 1 cycle per hour in electroencephalographic activity. Expl Neurol. 73, 127–143. [DOI] [PubMed] [Google Scholar]
- 23.Huntley AC (1987) Electrophysiological and behavioral correlates of sleep in the desert iguana, Dipsosaurus dorsalis Hallowell. Comp. Biochem. Physiol 86A, 325–330. [DOI] [PubMed] [Google Scholar]
- 24.Huttenlocher PR (1961) Evoked and spontaneous activity in single units of medial brain stem during natural sleep and waking. J. Neurophysiol 24, 451–468. [Google Scholar]
- 25.Jouvet-Mounier D, Astic L and Lacote D (1970) Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Devl Psychobiol. 2, 216–239. [DOI] [PubMed] [Google Scholar]
- 26.Kemp T (1982) Mammal-like Reptile and the Origin of Mammals. Academic, London. [Google Scholar]
- 27.Kent S and Satinoff E (1990) Influence of ambient temperature on sleep and body temperature after phentolamine in rats. Brain Res. 511, 227–233. [DOI] [PubMed] [Google Scholar]
- 28.Kiehn O, Rostrup E and Moller M (1992) Monoaminergic systems in the brainstem and spinal cord of the turtle Pseudemys scripta elegans as revealed by antibodies against serotonin and tyrosine hydroxylase. J. comp. Neurol 325, 527–547. [DOI] [PubMed] [Google Scholar]
- 29.Krubitzer L, Manger P, Pettigrew J and Calford M (1995) Organization of somatosensory cortex in monotremes: in search of the prototypical plan. J. comp. Neurol 351, 261–306. [DOI] [PubMed] [Google Scholar]
- 30.Luebke J, Weider J, McCarley R and Greene R (1992) Distribution of NADPH diaphorase positive somata in the brainstem of the monitor lizard Varanus exanthematicus. Neurosci. Lett 148, 129–132. [DOI] [PubMed] [Google Scholar]
- 31.Manger PR and Pettigrew JD (1995) Electroreception and the feeding behavior of platypus Ornithorhynchus anatinus: Monotremata: Mammalia. Phil. Trans. R. Soc. Lond B347, 359–381. [Google Scholar]
- 32.Manger PR and Pettigrew JD (1996) Ultrastructure, number, distribution and innervation of electroreceptors and mechanoreceptors in the bill of the platypus, Ornithorhynchus anatinus. Brain Behav. Evol 48, 27–54. [DOI] [PubMed] [Google Scholar]
- 33.Marks GA and Shaffery JP (1996) A preliminary study of sleep in the ferret, Mustela putorius furo: a carnivore with an extremely high proportion of REM sleep. Sleep 19, 83–93. [DOI] [PubMed] [Google Scholar]
- 34.Meglasson MD and Huggins SE (1979) Sleep in a crocodilian, Caiman sclerops. Comp. Biochem. Physiol 63A, 561–567. [Google Scholar]
- 35.Murray RA, Essick GK and Kelly DG (1994) Effect of stimulus force on perioral direction discrimination: clinical implications. J. oral maxillofac. Surg 52, 688–697. [DOI] [PubMed] [Google Scholar]
- 36.Parmelee AH and Stern E (1972) Development of states in infants. In Sleep and the Maturing Nervous System (eds Clemente C, Purpura D and Mayer F). Academic, New York. [Google Scholar]
- 37.Pascual R, Archer M, Ortiz Jaureguizar E, Prado J, Godthelp H and Hand S (1992) The first non-Australian monotreme an early Paleocene South American platypus (Monotremata, Ornithorhynchidae). In Platypus and Echidnas (ed. Augee M), pp. 1–14. Royal Zoological Society of NSW, Mosman. [Google Scholar]
- 38.Peyrethon J and Dusan-Peyrethon D (1968) Etude polygraphique de cycle veille-sommeil chez trois genres de reptiles. C. r. Séanc. Soc. Biol 162, 181–186. [PubMed] [Google Scholar]
- 39.Raetz SL, Richard CA, Garfinkel A and Harper RM (1991) Dynamic characteristics of cardiac R–R intervals during sleep and waking states. Sleep 14, 526–533. [DOI] [PubMed] [Google Scholar]
- 40.Rial RV (1997) The evolution of waking states. In Sleep and Sleep Disorders: From Molecule to Behavior (eds Hayaishi O and Inoue S), pp. 189–193. Academic, Tokyo. [Google Scholar]
- 41.Scheich H, Langner G, Tidemann C, Coles R and Guppy A (1986) Electroreception and electrolocation in platypus. Nature 319, 401–412. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt-Kessen W and Kendel K (1973) Influence of room temperature on night sleep in man. Res. exp. Med., Berlin 160, 220–233. [DOI] [PubMed] [Google Scholar]
- 43.Siegel JM (1994) Brainstem mechanisms generating REM sleep. In Principles and Practices of Sleep Medicine (eds Kryger MH, Roth T and Dement WC), pp. 125–144. W. B. Saunders, Philadelphia. [Google Scholar]
- 44.Siegel JM and Tomaszewski KS (1983) Behavioral organization of reticular formation: studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements. J. Neurophysiol 50, 696–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel JM, McGinty DJ and Breedlove SM (1977) Sleep and waking activity of pontine gigantocellular field neurons. Expl Neurol. 56, 553–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegel JM, Manger P, Nienhuis R, Fahringer HM and Pettigrew J (1996) The echidna Tachyglossus aculeatus combines REM and nonREM aspects in a single sleep state: implications for the evolution of sleep. J. Neurosci 16, 3500–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smeets WJAJ, Alonso JR and Gonzalez A (1997) Distribution of NADPH-diaphorase and nitric oxide synthase in relation to catecholaminergic neuronal structures in the brain of the lizard Gecko gecko. J. comp. Neurol 377, 121–141. [PubMed] [Google Scholar]
- 48.Tauber ES, Roffwarg HP and Weitzman ED (1966) Eye movements and electroencephalogram activity during sleep in diurnal lizards. Nature, Lond 213, 1612–1613. [DOI] [PubMed] [Google Scholar]
- 49.Tauber ES, Rojas-Ramirez J and Hernandez-Peon R (1968) Electrophysiological and behavioral correlates of wakefulness and sleep in the lizard (Ctenosaura pectinata). Electroenceph. clin. Neurophysiol 24, 424–443. [DOI] [PubMed] [Google Scholar]
- 50.Terashima SI and Lian YF (1994) Touch and vibrotactile neurons in a crotaline snake’s trigeminal ganglia. Somatosensory Motor Res. 11, 169–181. [DOI] [PubMed] [Google Scholar]
- 51.Ursin R and Sterman MB (1981) A Manual for Standardized Scoring of Sleep and Waking States in the Adult Cat. Brain Information Service/Brain Research Institute, University of California, Los Angeles. [Google Scholar]
- 52.Vasilescu E (1970) Sleep and wakefulness in the tortoise (Emys orbicularis). Rev. Roum. Biol.-Zool 15, 177–179. [Google Scholar]
- 53.Westerman M and Edwards D (1992) DNA hybridization and the phylogeny of monotremes. In Platypus and Echidnas (ed. Augee M), pp. 28–34. Royal Zoological Society of NSW, Mosman. [Google Scholar]
- 54.Whittington R (1991) The survival of platypuses in captivity. Aust. vet. J 68, 32–35. [DOI] [PubMed] [Google Scholar]
- 55.Zepelin H (1994) Mammalian sleep. In Principles and Practice of Sleep Medicine (eds Kryger MH, Roth T and Dement WC), pp. 69–80. W. B. Saunders, Philadelphia. [Google Scholar]


