Abstract
Exosomes are nano-sized lipid vesicles that are produced by all eukaryotic cells, and they typically range in size from 30-150 nm. Exosomes were discovered almost 40 years ago; however, the last two decades have attracted considerable attention due to exosomes’ inherent abilities to shuttle nucleic acids, lipids and proteins between cells, along with their natural affinity to exosome target cells. From a pharmaceutical perspective, exosomes are regarded as naturally produced nanoparticle drug delivery vehicles. The application of exosomes as a means of drug delivery offers critical advantages compared to other nanoparticulate drug delivery systems, such as liposomes and polymeric nanoparticles. These advantages are due to the exosomes’ intrinsic features, such as low immunogenicity, biocompatibility, stability, and their ability to overcome biological barriers. Herein, we outline the structure and origin of exosomes, as well as their biological functions. We also touch upon recent advances in exosome labeling, imaging and drug loading. Finally, we discuss exosomes in targeted drug delivery and clinical trial development.
Keywords: Ceramide, exosomes, cancer, neurodegeneration
Graphical Abstract
1. Introduction
Cellular communication is essential for the regulation of physiological functions [1]. While cells attached to one another transfer signals through physical channels, known as gap junctions, distant communication occurs via chemical messengers, such as hormones, secreted lipid vesicles or extracellular vesicles (EVs), which transport DNA, RNA, proteins and lipids from a donor to a recipient cell [2-7]. EVs are heterogeneously classified based on their cellular source of origin, size, content and function. There are three main groups of EVs: apoptotic bodies, microvesicles and exosomes [2].
The term “exosome” was coined by Rose Johnstone to refer to any extracellular body secreted by cells. In 1983, two independent studies, published within one week of each other, showed that small vesicles of ~50 nm are secreted by mature blood reticulocytes and associated with transferrin receptors [8, 9]. Initially, exosomes were thought to be a “membrane enclosed structure of an intracellular sac” that discard unwanted protein, lipids or nucleic acids from cells. Over the past few decades, extensive research has been carried out to define exosomes and identify their contribution to different aspects of physiological and pathological conditions. Exosomes have long been considered a marker of physiological function and pathological conditions, but now they are also used as a delivery platform for potential therapeutic agents.
Exosomes are secreted by all cell types and contained in almost all body fluids, such as milk, saliva and blood. Typically, the exosome size ranges from 30-150 nm. Using asymmetric flow field flow fractionation (AF4) technology, Zhang et al. characterized distinct sub-populations of exosomes as large exosomes (exo-L, 90-120 nm), small exosomes (exo-S, 60-80 nm) and exomeres (~35 nm) [10].
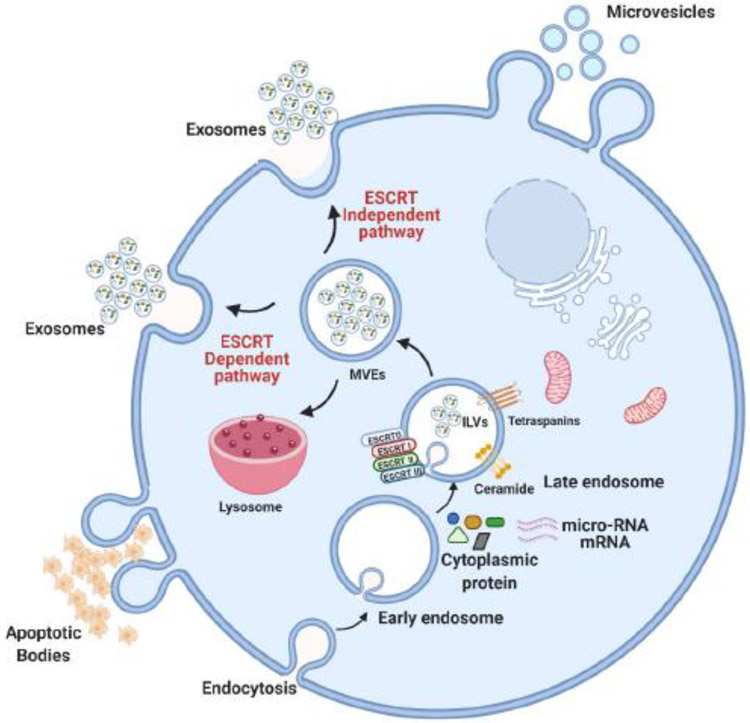
Exosomes are typically formed through a process that involves a two-step invagination of the plasma membrane, leading to the formation of endosomal multivesicular bodies (MVEs) that contain intraluminal vesicles (ILVs). While some MVEs fuse to the plasma membrane to release their ILVs (exosomes) into the extracellular milieu, others fuse with lysosomes or autophagosomes for degradation, or contribute to specialized organelle generation, such as secretory granules (in mast cells), Weibel-Palade bodies (endothelial cells) and melanosomes (in melanocytes). The biogenesis of exosomes within MVEs occurs by two pathways, the endosomal sorting complex required for transport machinery (ESCRT)-dependent and the ESCRT-independent pathway [11] (Figure 1).
Figure 1. Illustrated scheme for extracellular vesicles biogenesis.
Apoptotic bodies, microvesicles and exosomes are synthesized within cells and are secreted to the extracellular environment. Endocytosis is followed by generation of multivesicular endosomes (MVEs) which can fuse with lysosomes, or they can generate intraluminal vesicles (ILVs) that are secreted as exosomes. The exosome biogenesis pathway is either ESCRT-dependent or ESCRT independent, involving distinct types of protein and lipids. Microvesicles are heterogenous vesicles that are formed by outward budding of the cell membrane and are of different size than exosomes, which typically range from 50 nm to 1 μm.
The ESCRT machinery consists of a distinct group of cytosolic protein complexes: ESCRT-0, -I, -II and -III. ESCRT 0 has a ubiquitin binding domain, which recognizes and binds ubiquitin cargos, a process that is assisted by the Tsg101 protein of ESCRT I and facilitates the activation of ESCRT II for inward budding of the MVE membrane. ESCRT III uses VPS4 and ATPase to remove ubiquitin from the cargo complex, leading to the final step of ILV generation. The function of the ESCRT pathway in generating ILV and exosome biogenesis has been demonstrated by significantly decreased exosome secretion upon ESCRT 0 inhibition [12]. However, several studies also suggested that exosome generation involved an ESCRT-independent pathway regulated by tetraspanins, heat shock protein and lipids [13-15].
The tetraspanin superfamily (CD9, CD63 and CD81) is among the most abundant membrane proteins of exosomes, and it is often used as an exosomal biomarker. Tetraspanins are involved in many biological processes, including exosomal biogenesis [15], cell adhesion [16], antigen presentation to T-cells [17] and exosome fusion to recipient cells [18]. The exosome release in tetraspanin protein CD9 knock out mice is significantly reduced [19], indicating a critical function of CD9, and other tetraspanins, in the regulation of exosome biosynthesis. Heat shock protein 90α (Hsp90α), which was found on the external surface of exosomes, was shown to mediate tumor stromal cell communication [20].
In the ESCRT-independent pathway, the sphingolipid ceramide was identified as a critical factor in the biogenesis of exosomes. In a pioneering study, Trajkovic et al. showed that the biogenesis of exosomes is dependent on the sphingolipid ceramide, a cone-shaped sphingolipid that triggers spontaneous curvature of membranes, leading to invagination and budding of exosomes into the MVE [21]. One key enzyme that is involved in the generation of ceramide is neutral sphingomyelinase 2 (nSMase2) through the hydrolysis of the membrane lipid sphingomyelin [22-24]. The key function of nSMase2 in the ESCRT-independent pathway of exosome biogenesis is also supported by studies from several groups, including ours, using a noncompetitive inhibitor of nSMase2 (GW4869) to prevent exosome secretion [25-27]. Ceramide is a long chain fatty acid amide derivative of sphingosine that induces formation of ILVs [28]. Ceramide generation at the MVEs leads to its enrichment in ILVs, and eventually it results in exosomes with a specific lipid composition that is distinct from that of the donor cell.
In spite of these known characteristics, it is not clear with which proteins ceramide interacts and if ESCRT-independent exosome biogenesis relies on several distinct pathways involving ceramide. Recently, Rab31, a GTPase of the RAS oncogene family, was shown to participate in the ESCRT-independent pathway by preventing ILV generation. Rab31 was suggested to interact with ceramide and cholesterol in lipid microdomains (lipid rafts) important for ILV formation [29]. This observation aligns with our previously published proposed model describing that ceramide-enriched platforms (CRPs) form “mobile rafts”, which are important for EV biogenesis and function [30]. Since ceramide generation can be specifically modulated by drugs that inhibit or activate enzymes in ceramide metabolism, it constitutes a bona fide target for manipulating exosome biogenesis and function in pharmacology and therapy.
2. The biological function of exosomes and relevance to pharmacology
2.1. Exosomes in Immune Biology
Exosomes play a crucial role in mediating the immune response [31, 32]. The induction of the immune response by exosomes is likely due to carrying major histocompatibility complex (MHC)-peptide complexes, allowing for efficient activation of T lymphocytes, thus mediating the function of exosomes as promoters of the adaptive immune response. Additionally, one of the mechanisms to induce the immune response elicited by exosomes is the delivery of exosomal cargo to recipient cells, resulting in signaling and gene regulation. Exosomes derived from Antigen-presenting cells (APC) carry major histocompatibility complex II (MHC II) with the antigenic peptide, stimulating T-cell activation [33-35]. Exosomes shed from dendritic cells and pulsed with a tumor peptide were injected into a tumor mouse model and showed delayed tumor growth [33]. In addition, dendritic cells shed different sizes of EVs with equal efficacy to induce CD4 T-cell activation. However in-vitro studies suggest that smaller EVs favor secretion of Th2 cytokines, while larger EVs promote Th1 cytokine secretion [36].
The capacity of exosomes to induce antigen-specific CD8 T-cells has been characterized and compared to other EVs like microvesicles [37], which directly bud from the cell surface. Exosomes and microvesicles were obtained from ovalbumin-pulsed dendritic cells, and it was found that exosomes were more efficient in inducing antigen-specific CD8 T-cells and eliciting antigen specific IgG production when compared to microvesicles [37]. Moreover, compared to microvesicles, exosomes carry higher levels of ovalbumin and showed significant ex vivo Interferon-γ (IFN- γ) production in response to antigen stimulation, indicating that microvesicles and exosomes differ in their capacity to incorporate antigens and induce an immune response [37]. These studies suggest a heterogeneity in the immunogenic effect of exosomes and microvesicles.
The role of exosomes was also investigated in the field of bacterial infection, particularly with Mycobacterium tuberculosis (M. tuberculosis) and Listeria monocytogenes (L. monocytogenes). It has been shown that M. tuberculosis-infected macrophages secrete exosomes that contain M. tuberculosis proteins [38] and are able to induce antigen specific Interferon-α (IFN-α) and Interleukin-2 (IL-2) expressing CD4 (+) and CD8 (+) T-cells [39]. Exosomes isolated from M. tuberculosis-infected macrophages were injected into healthy mice and the Th1 immune response was measured, which was comparable to BCG-vaccinated mice [39]. Exosomes were shown to partake in the communication between gut microbiota and immune cells of the mucosa involving microbe recognition, due to secretion of exosomes from epithelial cells to immune cells [40]. Therefore, cross-talk was established between host and host gut microbiota through exosomes.
Exosomes containing nucleic acid cargoes (DNA, RNA, micro-RNA) also play a significant role in the immune response. DNA of L. monocytogenes is packed inside exosomes of a host’s infected cells, which stimulates the cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)–stimulator of the interferon genes (STING) pathway [41], suggesting that the antibacterial defense mechanism is activated by L. monocytogenes exosomal DNA [41]. M. tuberculosis-infected, macrophage-derived exosomes contain M. tuberculosis RNA, which stimulates the host RIG-I/MAVS/TBK1/IRF3 RNA sensing pathway, leading to type I interferon production in recipient cells [42]. Injecting a mixture of exosomes isolated from M. tuberculosis infected macrophages with moxifloxacin (a drug for tuberculosis) into M. tuberculosis-infected mice, significantly lowered the bacterial burden relative to treatment alone, demonstrating the potential for exosomes to combine with drugs for anti-bacterial therapy [42].
Melanoma-derived exosomes were found to modulate the immune response by suppressing the function of CD8-T cells and facilitating tumor growth [43]. PDL-1 (programmed death-ligand 1) from melanoma cells is incorporated into the exosome surface, which stimulates CD8-T cells by binding to the PD1 (Programmed death-1) receptor. IFN-γ stimulates exosomal PDL-1 expression, which is positively correlated with IFN-γ treatment, thereby providing a target for anti-PD1 therapy by IFN-γ stimulation [43]. Moreover, exosomal PDL-1 is reported to play a role in immune suppression through blocking the maturation of dendritic cells, decreasing the immune response of T-cells [44]. Also, pancreatic ductal adenocarcinoma (PDAC)-derived exosomes were shown to exhibit tumor antigens on their surface, which provokes an autoantibody response and induces serum-mediated complement cytotoxicity [45]. Exosomes play a crucial role in the anti-tumor immune response in the tumor microenvironment (TME) by triggering the myeloid-derived suppressor cell activation by STAT3 [46], or by inducing the STAT3-mediated immune suppressive switch of the macrophage phenotype [47].
Another area of interest is the regulation of the immune response by exosome-mediated pathogen infection by enabling the dissemination of viral components [48]. Several viruses, such as West Nile Virus (WNV), Zika virus (ZV), Hepatitis C Virus (HCV) and Dengue virus (DENV), have been reported to hijack the exosomal pathway for propagation and pathogenesis. By exploiting clathrin-mediated or receptor-mediated endocytosis and back-fusion to MVEs, these viral particles are delivered into the host cytoplasm via exosomes [49]. Some viral genomes, such as the HCV genome, can stay inside exosomes and are delivered to host cells via exosome fusion [50].
Both enveloped and non-enveloped virus infections are regulated by exosomes, as they exist as pseudo-envelope forms inside MVEs [51, 52], turning them into a “Trojan Horse” favoring viral entry into cells, thereby enhancing infectivity [53]. Exosomes promote viral entry into host cells by stabilizing the interaction between exosomal tetraspanin proteins (CD81 and CD9) and the host cell plasma membrane [54]. On one side, viral infection is propagated by exosomes, on the other side, host cell immunity is also mediated by exosomes. For instance, it appears that exosomes may serve as a mechanism of transferring resistance to virus infection induced by IFN-α from nonpermissive liver nonparenchymal cells (LNPCs) to permissive hepatocytes [55]. The resistance mechanism is achieved through exosome shedding from IFN-α-stimulated macrophages, which leads to the delivery of enzymes to uninfected cells, particularly Apolipoprotein B mRNA editing enzymes and catalytic polypeptides like 3G (APOBEC3G) [55]. APOBEC3G mediates DNA hyper-mutation, resulting in viral resistance. Moreover, APOBEC3G also confers Human Immunodeficiency Virus (HIV) resistance to recipient cells [56], providing a mechanism of immune response regulation by exosomes carrying viral particles.
As discussed above, exosomes have the potential to modify the innate and adaptive immune response, including B-cells, T-cells and macrophages, which provides pharmacological relevance of exosomes in immune biology. As exosomes are sufficient to deliver specific cargos and stimulate the immune response, designing, packaging and delivery of key molecules to exosomes to elicit an immune response in a specific cell type is of pharmacological relevance to immune response-mediated therapy. For example, exosome-based vaccines against the spike S protein of SARS-associated coronavirus (SARS-CoV) have efficiently neutralized the antibody titer [57]. Exosomes derived from Multiple Myeloma (MM) taken up by bone marrow stromal cells (BMSC) were shown to regulate MM progression, cell growth, proliferation and drug resistance. Meanwhile, inhibiting the uptake of exosomes derived from MM suppresses the functional response in bone marrow stromal cells [58]. Exosomes isolated from antigen-presenting cells and pulsed with a tumor peptide, eradicate growth of established murine tumors in a T-cell dependent manner [33, 59]. Exosomal PDL-1 expression weakens the immune response by either blocking the maturation of dendritic cells or suppressing the function of T-cells. Therefore, blocking the exosomal PDL-1 ligand, using a pharmacological inhibitor, can be used as a tool for a therapeutics approach in cancer treatment.
2.2. Exosomes in Cancer Biology
Extracellular fluids surrounding tumors provide a tumor microenvironment (TME) to facilitate cellular communication involving stromal cells, immune cells and extracellular vesicles [60]. Exosomes modulate the TME as they carry multiple angiogenesis-related proteins, thereby contributing to the enhancement of angiogenesis and immunosuppression, resulting in tumor progression [61]. Cancer-associated fibroblasts (CAFs) are one of the major components of the TME, and they secrete exosomes that reprogram the tumor’s metabolism by various mechanisms, including inhibiting mitochondrial oxidation and increasing glycolysis and glutamine-dependent reductive carboxylation [62]. Exosomes, secreted by CAFs and tumors communicate with each other, as exosomes derived from CAFs modulate the tumor phenotype, whereas exosomes secreted by the tumor activate CAFs [63].
Cancer cell regulation and propagation has been observed by various micro-RNAs delivered via exosomes. Relative expression levels of micro-RNAs derived from breast CAFs (miR-21, miR-378e and miR-143) are found to be significantly higher when compared to normal fibroblasts [64]. When these micro-RNAs were transfected to normal fibroblasts, they promote stemness, mammosphere formation and Epithelia-Mesenchymal Transition (EMT), which are the typical features of breast cancer cell progression and transformation [64]. It is known that miR-21 regulates the tumor suppressor gene by binding to mRNA target of phosphatase and tension homolog (PTEN) [65] and programmed cell death protein 4 (PDCD4) [66, 67]. The miR-21 level isolated from CAF-derived exosomes was significantly higher, leading to the suppression of ovarian cancer apoptosis and induction of chemoresistance by binding miR-21 to the mRNA of Apoptotic Peptidase Activating Factor 1 (APAF1) [68], which has been shown to be associated with chemoresistance and apoptosis [68].
CAFs associated with PDAC are resistant to gemcitabine, a drug used in the chemotherapy of pancreatic ductal adenocarcinoma. Exposure of CAFs to gemcitabine significantly increases the release of exosomes, which contain the chemoresistance-inducing factor Snail that promotes tumor proliferation and drug resistance [69]. CAF-derived exosomes were found to be enriched with transforming growth factor-β1 (TGFβ1), a cytokine that induces malignant behavior, promotes EMT and contributes to the generation of more CAFs [70]. CAF-derived exosomes stimulate breast cancer cell protrusion and motility via the Wnt-planar cell polarity signaling pathway [71]. In addition, exosomes derived from tumor cells are reported to play a role in the resistance of cancer cells to antibodies, as exosomes secreted from B-cell lymphomas contain CD20, which acts as a decoy for the binding of anti-CD20 antibody to B-cells [72].
Various mechanisms have already been reviewed by other groups discussing the “gain of cancer resistance” mediated through exosomes, which is achieved either by specific cargo proteins or the nucleic acids (non-coding RNA and DNA) they contain [73]. Out of many, one crucial mechanism to drug resistance attributed by exosomes is the enrichment of a certain type of micro-RNAs that are transported to tumor cells they target. Among the micro-RNAs carried by exosomes, miR-100, miR-222 and miR-30a participate in breast cancer pathogenesis and therapy failure by altering target gene expression [74]. The exosomal miR-100-5p and miR-222-3p derived from non-small cell lung cancer are delivered to tumor cells and induce drug resistance by targeting either to mammalian target of rapamycin (mTOR) [75] or targeting the 3’UTR of the suppressor of cytokine signaling 3 (SOCS3) gene [76]. In addition to micro-RNA, other types of non-coding RNA, mRNA and DNA also have been reported to confer anti-tumor drug resistance. For example, exosomal long non-coding RNA PART1 promotes gefitinib resistance in esophageal squamous cell carcinoma [77], and exosomal circular RNA CirxN-FIX induces temozolomide (TMZ) resistance in glioma [78]. TMZ resistance is also observed by exosomal O-6-methylguanine-DNA methyltransferase (MGMT) mRNA in neurological tumors [79] and exosomal CAFs-derived mitochondrial DNA in hormonal therapy in breast cancer [80].
Cancer-related exosomes, secreted by cancer cells and CAFs, packaged with specific molecules such as mRNA, micro-RNA and proteins, can be used as biomarkers. As exosomes deliver micro-RNA to tumor cells and enhance tumorigenesis, identification of specific exosomal micro-RNAs and inhibition of exosome fusion to recipient cells are crucial steps towards utilizing exosomes in a pharmacological application. Exosomes engineered with a miR-21 sponge construct have the potential to suppress miR-21 and consequently upregulate miR-21 target genes PDCD4 and RECK, which are key regulators of apoptotic and metastatic pathways [81].
Additionally, miR-21 plays a key role in radiotherapy resistance of non-small cell lung cancers (NSCLC). Downregulating miR-21, using specific anti-miR to NSCLC A549 cells, induces apoptosis and inhibits the proliferation of cancer cells [81]. Interestingly, a therapeutic approach has been performed by engineering an exosomal peptide surface for the triple-negative breast cancer cell surface (TNBCCS) [82]. Modified exosomal peptides target MET factor, which is over-expressed in TNBCCS, improving the cellular uptake efficiency and antitumor efficacy of doxorubicin [83]. Further, as exosomes mediate pathology by cellular communication, strategies can be developed to inhibit exosome release. For example, exosomes secreted by melanoma cells contain miR-494, which promotes tumor growth and induces apoptosis. Silencing Rab27a reduced exosomal release, and hence the accumulation of miR-494 in exosomes, significantly suppressed the malignant phenotype of melanoma cells. Hence, inhibiting the exosome-mediated transfer of miR-494 can be used in melanoma therapy [84]. Likewise, ascites-derived exosomes have been used in combination with the granulocyte-macrophage colony-stimulating factor (GM-CSF) in the immunotherapy of colorectal cancer (CRC) [85].
2.3. Exosomes in Neurodegeneration
The role of exosomes in spreading disease is not only limited to cancers but also other pathological conditions, including neurodegenerative diseases. The ability of exosomes to promote the spread of disease is thought to play a role in diverse neurological diseases, including Alzheimer’s disease (AD), stroke, prion disease, amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD) [86].
Deposition of extracellular Amyloid beta (Aβ) and intracellular neurofibrillary tau tangles are the hallmark of AD [87]. Aβ is a peptide of 34-42 amino acids and processed from amyloidogenic cleavage of amyloid precursor protein (APP) via subsequent cleavage by β-secretase and γ-secretase enzymes [88, 89]. Rajendran et al. showed that in N2a and Hela cells expressing APP, β-cleavage of APP occurs in early endosomes, which is followed by the routing of Aβ to MVEs. The same study estimated that a minute fraction of Aβ (< 1%) is secreted by exosomes from N2a cells, overexpressing the Swedish mutant of APP [90]. Moreover, enrichment of amyloid plaques with exosomal proteins, such as Flotillin and Alix1, suggested the involvement of exosomes in plaque formation [90]. Specifically, higher expression levels of APP, C-terminal fragment (CTF) of APP and Aβ peptide have been found in exosomes isolated from AD transgenic mouse models, such as Tg2576 and 5XFAD [91].
Elsherbini et al. defined a new mechanistic pathway for AD pathogenesis mediated by exosomes derived from astrocytes, known as “astrosomes” [92]. Astrosomes are ceramide-enriched exosomes that can be associated with Aβ when isolated from serum and brain tissue of AD patients and 5XFAD mice. When taken up by neurons in culture, Aβ-associated astrosomes are transported to mitochondria, where they enhance mitochondrial clustering and bind to voltage-dependent anion channel 1 (VDAC1). Binding of Aβ leads to oligomerization of VDAC1, which results in the formation of a pro-apoptotic pore. Release of cytochrome c leads to activation of caspase 3, eventually resulting in neuronal death [92]. In addition to Aβ, secretases were also found in exosomes, suggesting that APP cleavage may occur inside the vesicles [93]. Interestingly, Aβ peptide stimulates exosome secretion from astrocytes in vitro [94], suggesting a positive feedback mechanism in which the production of Aβ and exosome secretion participate in AD pathogenesis.
A novel mechanism of apoptosis induction by exosomes, which are secreted by Aβ-exposed astrocytes has also been described [94]. In brief, up-regulation of prostate apoptosis response 4 (PAR-4) and ceramide sensitizes astrocytes to amyloid-induced cell death [94]. Inhibiting nSMase2 using GW4869 in 5XFAD mice remarkably reduces brain ceramide, brain and serum exosomes and Aβ plaque load [26], indicating that plaque formation is reduced by inhibiting nSMase2 and secretion of exosomes. Exosomes isolated from cerebral spinal fluid (CSF) derived from AD patients contain more Aβ than controls. Quantification of Aβ peptide, and other factors associated with AD pathogenesis, has been described by analyzing astrocyte-derived exosomes (ADEs) and neuron-derived exosomes (NDEs) [95], which were isolated from the plasma of patients with AD pathogenesis. The cargo content in the ADEs, such as β-site amyloid precursor protein 1 (BACE-1), γ-secretase, soluble Aβ42, soluble APP, glial-derived neurotrophic factors (GDNF), pThr-181 Tau and pSer-396 Tau, was significantly higher than the respective cargo content of NDEs when compared to controls [95], suggesting a critical role of ADE’s cargo proteins in AD pathogenesis.
The C-terminal truncated and oligomeric form of tau is another potentially neurotoxic protein delivered by exosomes [96]. Neurofibrillary tangles are intracellular depositions of tau, which are hyper-phosphorylated (phosphorylation at threonine 181 (Thr-181) and serine 396 (ser-396) residues of tau), and aggregated, which induces cellular senescence resulting in death [97]. The progression of neuropathological disease induced and promoted by tau is known as tauopathy. Tau is transmitted and communicated to other cells by either cell-to-cell transmission via extracellular fluid [98] or by exosomes.
Various factors affect the release of tau to the extracellular fluid such as the increase in neuronal activity by AMPA receptor activation, which induces tau release from healthy cortical neurons [99]. Intracellular tau accumulation [100], neuronal death [101] and mutations of the MAPT gene [102] also induce tau release to the extracellular fluid. Tau can be secreted via an exosome-mediated mechanism from M1C cells (a human neuroblastoma tauopathy cell model), which is enriched with hyperphosphorylated tau (Thr-181 tau) [103]. The elevated level of Thr-181 tau in exosomal CSF from early AD patients [103] indicates the significance of pathological tau in driving the pathology. Exosomes isolated from AD patients’ blood were quantified for phosphorylated tau (Thr-181 tau and Ser-396 tau) and Aβ peptide, and they were found to be different in expression compared to control. They also differ in expression at different stages of AD progression [104].
In addition to these factors, there is the crucial role of micro-RNA carried by exosomes in AD. TaqMan micro-RNA analysis from exosomes isolated from the plasma of AD patients (compared to control), indicates a significant dysregulation in the expression level of several AD signature micro-RNAs (miR-23a-3p, miR-223-3p, miR-100-3p and miR-190-5p) [105]. In addition, the expression level of exosomal miR-223-3p, isolated from the orbitofrontal cortex, is significantly elevated in schizophrenia and bipolar disorder patients when compared to controls [106]. miR-223-3p targets the 3’UTR of glutamate receptor mRNAs encoding Grin2b and Gria2, and hence they regulate protein expression and control synapse-mediated communications [106]. Profiling of exosomal micro-RNA, obtained from the CSF of early onset of AD patients, shows a differential expression of AD biomarkers when compared to controls, such as miR-16-5p, miR-125b-5p, miR-451a and miR-605-5p [107].
Another neurodegenerative disease involving exosomes is Parkinson Disease (PD). PD is caused by progressive loss of dopaminergic neurons and occurrence of Lewis Bodies, which predominantly contain misfolded alpha-synuclein (α-Syn). The packaging of α-Syn into exosomes and their direct release to the extracellular environment has been described [108]. α-Syn, with the assistance of other proteins and factors, transitions from the early endosome to the late endosome, which then fuses with the plasma membrane, resulting in the release of exosomes containing α–Syn delivered to recipient cells. Exosomes isolated from overexpressing α–Syn proteins in SH-SY5Y cells, when incubated with normal SH-SY5Y cells, causes cell death of recipient cells, which is reversed after α–Syn immunodepletion, suggesting a role of exosomes in propagating PD-related pathology [109].
Exosomes promote the progression of PD pathology by providing an ideal environment for α–Syn aggregation [110]. Exosomes containing oligomeric α-Syn are readily taken up by recipient cells compared to free α-Syn, suggesting that exosomes provide a better medium for delivery of α-Syn to spread PD [111]. Further, there is significant cell death after the delivery of exosomal α-Syn to recipient cells, providing support for the hypothesis that neuronal atrophy induced by α-Syn is mediated by exosomes containing α-Syn [109]. α-Syn induces and activates exosome secretion, which activates apoptosis, supporting the hypothesis that exosomes mediate neurodegeneration [112].
Exosomes can freely cross the blood brain barrier (BBB) in both directions[113-115]. This unique capability makes exosomes an attractive candidate for drug delivery to the brain. In addition, their presence in the peripheral circulation and biological fluids allows researchers to capture them away from the brain and study their content and cargo in search for unique exosomal biomarkers relevant to neurodegenerative diseases, making exosomes a potential diagnostic tool. The utilization of exosomes as valuable biomarkers, as well as vehicles for effective drug transfer to distinct tissues including the brain, heart and cancer tissues, is discussed in the following sections.
3. Pharmacokinetics and biodistribution of exosomes
As mentioned in the previous section, it is well established that exosomes are secreted from all cell types as a mean of information and function transfer. This concept has opened a new research venue, revolving around the application of exosomes as drug delivery vehicles, therapeutic agents and diagnostic tools. Therefore, it has become necessary to better understand the pharmacokinetics of exosomes: that is, the in vivo behavior of exosomes inside the body. In this section, we will briefly cover the recent advances in the methods to analyze the biodistribution of exosomes and factors affecting the biodistribution and potential uses of exosomes in therapeutics.
3.1. Advances in exosome labeling and in vivo imaging
The two main modalities of exosome in vivo imaging are optical and nuclear [116, 117]. By default, each method has its own specifics and sample preparation. Optical imaging encompasses fluorescence and bioluminescent imaging, both of which are powerful tools for exosome tracking in small animals without the need to sacrifice the subjects [117]. Fluorescent molecules could be endogenous or exogenous proteins or other particles that emit light upon activation by an external light source [118]. Bioluminescence involves the use of a natural light-emitting protein that is activated by a chemical reaction, such as luciferase, to trace the movement of certain proteins or to identify the location of specific chemical reactions within the body [119].
While fluorescence imaging, using fluorescent proteins, has the highest spatial resolution, the low penetration of the protein fluorescence does not allow noninvasive in vivo imaging. Bioluminescence is characterized by having the highest sensitivity and high signal-to-noise ratio; however, it requires the addition of molecules to activate the reaction [120]. On the other hand, nuclear imaging, using radionuclides, has been applied for tracking exosomes in mouse models utilizing specific cameras [116]. The advantage of nuclear imaging, such as magnetic resonance imaging (MRI), is that it provides excellent sensitivity and great tissue penetration, which has been adopted for the exosome biodistribution research [116].
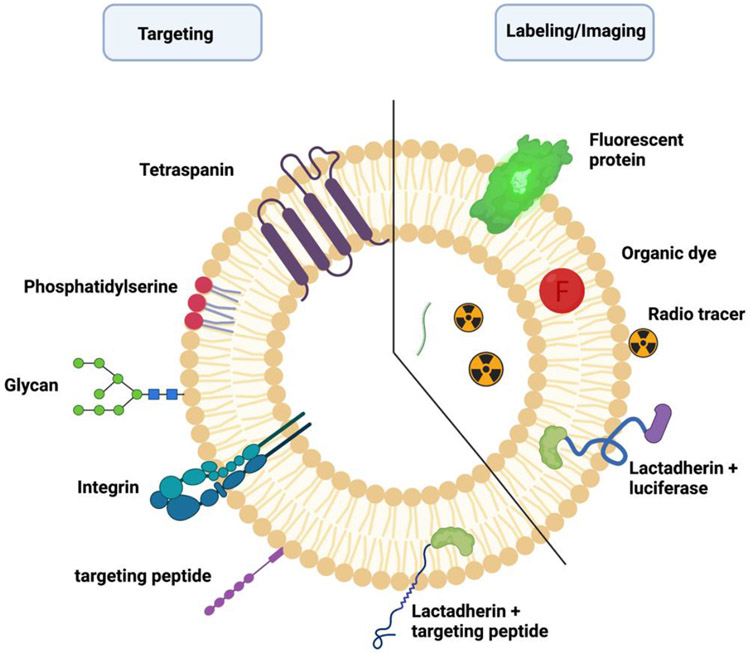
As with imaging, exosome labeling also falls under different categories, including fluorescence, bioluminescence and radiolabeling. Fluorescence labeling is the most used method since it is widely applied in simple in vitro and in vivo studies. Fluorescence labeling is either protein or organic dye-based; both provide excellent spatial resolution under optical microscopy. The first use of fluorescent proteins for labeling exosomes was done by Mittelbrunn et al. when the group fused exosomal CD63 with GFP to serve as a reporter for imaging, utilizing Raji B and J77 T cells [121]. The fluorescently-labeled exosomes were then used to treat wild type cells. After 16 hours of incubation, the signal was detected on the recipient cell surface. A similar strategy was used to track the uptake of cancer cell-derived exosomes into lung cells [122].
Many organic fluorescent dyes have been repurposed from labeling cells to exosome labeling. The organic dyes generally combine fluorophores with different functional groups to label the lipid bilayer or proteins of interest on EVs [123]. Despite having their own limitation, such as aggregation in exosome-like vesicles and leakage into surrounding cells, these dyes have been widely used to label and track exosomes. Carbocyanine dyes (such as DiD [(1,1′-dioctadecyl-3,3,3′,3′- tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt], DiR [(1,1’-dioctadecyltetramethyl indotricarbocyanine iodide], DiO [(3,3′-dioctadecyloxacarbocyanine, perchlorate], DiA [(4-(4-dihexadecylaminostyryl)-N-methylpyridinium iodide], and DiI [1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate]) are lipophilic dyes that exhibit a strong fluorescent signal when incorporated into exosome lipid membranes [124]. For example, Wiklander et al. used DiR to study the biodistribution of conditioned media-derived exosomes in vivo [125]. Using In Vivo Imaging System (IVIS) imaging, the group reported different biodistribution patterns based on the exosomes’ origin and route of administration [125]. Another lipid labeling dye that has been used to study exosomes is Octadecyl rhodamine B chloride (R18) as reported by Tian et al. [126]. PKH26 and PKH67 are lipophilic dyes that use aliphatic tails to anchor into the lipid bilayer of exosomes [116] Most recently, Shimomura et al. reported developing three types of exosomal membrane binding fluorescent probes, namely Mem Dye-Green, Mem Dye-Red and Mem Dye-Deep Red [127]. These dyes overcome the reported PKH dye issues by employing polyethylene glycol to improve hydrophilicity and introduce a negatively-charged moiety to a symmetric two-armed cyanine. The group proposed that the newly developed dyes are superior to PKH dyes in the sense that they do not aggregate or change the zeta potential or the particle size of exosomes [127].
As for bioluminescence exosome labeling, it is accomplished by expressing exosome-reporter luciferases via plasmid transfection or lentivirus transduction to the donor cells followed by imaging the released exosomes [128]. Takahashi et al. transfected B16BL6 murine melanoma cells with Gaussia luciferase(gLuc)-lactadherin plasmid prior to the collection of exosomes via ultracentrifugation [129]. Within five hours of intravenous injection into mice, labeled exosomes were shown to be quickly distributed to different organs [129].
Radiolabeling of exosomes can be accomplished via covalent binding, encapsulation of radiolabeled radionuclides and membrane radiolabeling. Covalent binding makes use of the presence of reactive amine/carboxylic terminated phospholipids or transmembrane proteins on the surface of exosomes that are available for binding of probes [130]. Another approach is to introduce non-native binding groups to the membrane of exosomes prior to targeting them with desired probes [131-133]. In order to avoid modifications on the surface of exosomes, many researchers have opted to encapsulate radiolabeled hydrophilic probes, which can passively diffuse across the exosomal membrane into the vesicle lumen, or they have used active loading strategies for hydrophobic probes [134].
3.2. Biodistribution of exosomes
Several groups have studied the in vivo biodistribution of exosomes using different isolation, labeling and imaging methods. While we discuss the recent advances of exosomal biodistribution and pharmacokinetics, the reader should be aware that the interpersonal and between-laboratory variations could influence the interpretation of each study. In addition, the reliability of in vivo analysis is presumably impaired by the free dye released from exosomes, among other technical hurdles. Nevertheless, this strategy has proven to be a useful approach to evaluate the localization of exosomes delivered to tissues and organs.
For instance, B16F10 murine melanoma cell-derived exosomes were labeled with PKH67 prior to their intravenous injection. The injected exosomes were found to accumulate in the spleen, liver, bone marrow and lung [135]. Particularly in the lung, exosomes were able to increase endothelial permeability, ultimately leading to tumor metastasis [135]. In another study, intravenously-injected lipophilic near infrared dyes, such as DiD-labeled mesenchymal stem cells (MSC), exosomes were distributed to the spleen and liver in wild-type mice [136]. Notably, when the exosomes were injected into an acute kidney mouse model, they demonstrated a different distribution pattern [137]. In addition to the liver and spleen, exosomes accumulated in the kidney, facilitating the recovery from acute kidney injury [137]. Moreover, Gangadaran et al. used DiR-labeled thyroid cancer (CAL62)-derived exosomes to study their biodistribution after intravenous injection [138]. The group reported that intravenously injected exosomes were predominantly distributed to the liver and the spleen and, to a lesser extent, to the lung and kidney. Lai et al. fused biotinylated gLuc to HEK239T derived exosomes prior to intravenous injection into mice [139]. The administered gLuc-labeled exosomes predominantly accumulated in the spleen, followed by the liver, lungs, and kidneys.
While the previous studies used fluorescent proteins for in vivo imaging, it has been reported that radiolabeled exosomes are more suitable for the quantitative evaluation of the pharmacokinetics and biodistribution of exosomes. One group modified B16BL6 exosomes with a streptavidin fusion protein prior to the addition of biotin derivatives labeled with 125I, resulting in the formulation of 125I labeled B16BL6 exosomes [132]. After 4 hours of intravenous injection of the radiolabeled exosomes, the group reported radioactivity/organ of 28%, 7%, and 1.6% in the liver, lung and spleen, respectively [132]. Exosomes isolated from human prostate adenocarcinoma PC3 cells labeled with 111In were also used for evaluating the pharmacokinetics of exosomes. In that case, 111In-labeled PC3 exosomes primarily distributed to the liver following intravenous injection [140].
There are, however, factors that could influence the biodistribution of exosomes. The influence of the route of administration was elucidated using HEK293T cell exosomes. While the intravenously injected exosomes mainly localized in the liver, HEK293T exosomes administered by intraperitoneal or subcutaneous injection accumulated in the liver, pancreas and gastrointestinal tract [141]. The specific hepatic and splenic uptake of intravenously-administered exosomes have been attributed to macrophage clearance, while the uptake of exosomes in the lung was an action of endothelial cells [141]. The clearance of intravenously injected B16BL6 exosomes from circulation in macrophage-depleted mice was considerably delayed compared to untreated mice, suggesting that macrophages play an integral role in the pharmacokinetics of intravenously-injected exosomes [142]. In another study, exosomes derived from bovine milk and labeled with DiR were administered orally or intravenously into mice [143]. While the orally administered exosomes distributed to the liver, lung, spleen, ovaries, colon, kidney, pancreas, and lastly brain at 4 days of administration, intravenously injected exosomes predominantly accumulated in the liver [143]. This is in line with previous findings regarding the tissue distribution of intravenously-administered exosomes.
Another factor that has been reported to influence the exosome biodistribution is the administered dose. Smyth et al. used two different doses of DiR-labeled 4T1 murine mammary carcinoma cell derived exosomes to investigate this issue. Intravenous injection of 60 μg of those exosomes led to accumulation in the liver and spleen. However, when the dose was raised to 400 μg, a significant accumulation of exosomes was observed in the lung, ultimately leading to asphyxiation of the mice [140].
The source of exosomes is of particular importance when it comes to pharmacokinetics, especially in regard to clearance kinetics. While we mentioned the sequestering of the intravenously injected exosomes by macrophages and their accumulation in the liver and spleen, this is not the case when exosomes are derived from certain origins that endow exosomes with receptors or other specific surface markers. For instance, immunocyte-derived exosomes express the CD47 receptor, which in turn interacts with the signal regulatory protein SIRP, resulting in blocking exosome uptake by phagocytes [144]. To support the same argument, it has been shown that primary fibroblast-like mesenchymal cells secrete exosomes that can bypass immune clearance by macrophages and monocytes, hence allowing extended blood circulation [145]. In general, the problem of rapid exosome clearance could be solved by the introduction of polyethylene glycol (PEG) molecules to coat the surface of the vesicles, as successfully applied in the liposome field. Kooijmans et al. have recently shown that the introduction of PEG to exosomes results in stealth properties, significantly increasing the circulation time of exosomes in mice [145].
Importantly, for development of exosome-based therapeutics, it is crucial to control the pharmacokinetics of exosomes, that is, the selective delivery of exosomes to target organs and cells. Controlling the pharmacokinetics of exosomes by modification of the exosomal surface with targeting proteins or peptides has been widely used in different fields as discussed in the following section.
3.3. Exosomes in targeted therapeutics
The feasibility of loading drugs into exosomes, penetrating diverse biological barriers, including BBB, and the easiness of altering their membrane composition, has brought exosomes to the forefront as an ideal drug-delivery model. Specifically, advancement in modifying the exosomal surface to carry different molecules provided scientists with the ability to engineer exosomes to target selective cell types or tissues. These capabilities have been utilized to deliver drug-loaded exosomes to different tissues, including the brain, heart and specific cancer tissues. The most commonly modified transmembrane proteins include tetraspanins (CD63, CD9, CD81), lysosome-associated membrane glycoprotein 2b (Lamp-2b), glycosyl-phosphatidyl-inositol (GPI), platelet-derived growth-factor receptors (PDGFRs) and lactadherin (C1C2 domain) [146]
3.3.1. Brain delivery
Alvarez-Erviti et al. were the first to introduce the concept of engineering exosomes for targeted delivery to the brain [147]. This was accomplished by loading dendritic cell-derived exosomes with small interference (si) RNA prior to successfully fusing rabies viral glycoprotein (RVG) with Lamp-2b. After intravenous injection, the targeted exosomes were able to cross the BBB and deliver the BACE1-targeted siRNA into neurons, leading to BACE1 knockdown. Liu et al. also engineered the membrane surface of exosomes to express the RVG peptide effectively delivering opioid receptor mu siRNA into the brain [148].
More recently, Cui et al. modified MSC-derived exosomes with RVG peptide to target them to the cortex and hippocampus in an AD mouse model [149]. The intravenously injected exosomes were shown to reduce the plaque burden and astrocyte activation, as well as decrease the expression levels of pro-inflammatory mediators, such as IL-β, IL-6 and TNF-α. BM-MSC-derived exosomes containing miR-17-92 were then injected into a brain stroke model and revealed improvement of neuroplasticity, neurogenesis, and oligodendrogenesis with functional recovery [149].
Another approach to make use of the fact that exosomes can cross the BBB is to administer them intranasally for brain delivery. In BTBR T+tf/J (BTBR) mice (a mouse model of autistic-like behavior), Perets et al. investigated the effects of intranasal administration of MSC-exosomes and reported reduced repetitive behavior, increased male-male social interaction, and improvement in maternal behavior [150]. In a similar experiment using a pilocarpine-induced status epilepticus mice model, MSC-derived exosomes were administrated intranasally and were reported to reach the hippocampus within 6 h, where they had neuroprotective and anti-inflammatory effects [151].
Tian et al. used bioorthogonal copper-free azide-alkyne cycloaddition to conjugate functional ligands onto exosomal surfaces - a technique called click-chemistry [152]. The cyclo (Arg-Gly-Asp-D-Tyr-Lys) peptide, which exhibits high affinity to integrin αvβ3 in reactive cerebral vascular endothelial cells after ischemia specifically, was conjugated onto the mesenchymal stromal cell (MSC)-derived exosome surface. This strategy resulted in a significant enrichment of EVs carrying curcumin in the brain compared to non-decorated vesicles. Guo et al. reported the detection of MSC exosomes in spinal cord lesions of a spinal cord injury model following intranasal delivery [153]. Precisely, exosomes loaded with phosphatase and tensin-homolog, small-interfering RNA (ExoPTEN) were able to reduce the expression of PTEN, ultimately improving structural as well as electrophysiological functions in spinal cord injury [153].
3.3.2. Tumor delivery
It is not surprising that there is substantial interest in researching the drug delivery potential of exosomes in various diseases, especially cancer. Searching PubMed using “exosomes AND drug delivery AND cancer” resulted in over 730 articles, the majority of those articles, over 450, were published within the last three years. Indeed, researchers used various exosome sources coupled with different targeting strategies to deliver compounds to specific cancer types.
One characteristic of exosomes that is being studied in exosome cancer drug delivery is tumor-homing [154]. Tissue tropism is governed by the surface composition of exosomes, with different integrin compositions regulating the organotropism of exosomes derived from deferent tumors. For instance, the therapeutic effect of hypoxic cancer-homing exosomes loaded with a poly ADP ribose polymerase (PARP) inhibitor, Olaparib was shown to increase apoptosis and decrease tumor growth in xenograft mice [155]. Moreover, MSC-exosomes have been reported to exhibit tumor-homing properties similar to those of MSCs. Exosomes generated by human umbilical cord MSC (UC-MSC) were reported to accumulate in tumors of mouse osteosarcoma K7M2 cells in nude mice. The treated mice exhibited reduced proliferation of human osteosarcoma 143B and mouse osteosarcoma K7M2 cells in vitro in a dose-dependent manner by inducing apoptosis [156].
Apart from natural tissue-targeting abilities, exosomes have been extensively engineered to deliver drugs for cancer therapy. Diverse therapeutic molecules have been delivered by exosomes, including anti-cancer drugs, oncolytic viruses, and small molecules. For instance, Zhou et al. used BM-MSC exosomes loaded with siRNA and oxaliplatin as an experimental treatment for pancreatic cancer in a mouse model [157]. The exosomes enhanced the uptake of oxaliplatin compared to free drug treatment. Another group used dental-pulp MSC exosomes loaded with miR-34a as a treatment for breast cancer, and they reported downregulated cancerous phenotype [158]. In addition, the effect of miRNA-126 packaged into MDA-MB-231 exosomes on non-small lung cell cancer was studied both in vitro and in vivo [159]. A reduction of proliferation and migration in vitro, as well as reduced metastatic nodules in vivo with minimal toxicity, was reported. Kamerkar et al. demonstrated the use of kRAS siRNA-loaded exosomes in pancreatic-cancer mouse models, which exhibited a greater reduction in tumor growth as well as a reduced clearance from the body [145].
The chemotherapeutic doxorubicin has been the target of several studies for cancerous tissue delivery via exosomes. For example, Li et al. demonstrated that milk exosomes coated with hyaluronan for CD44-targeting and loaded with doxorubicin significantly increased the uptake and therapeutic effect of the drug against cancer cells in vitro. Notably, the application of bovine-milk-derived exosomes as a drug delivery vehicle has been gathering considerable interest. For example, milk-derived exosomes have been used to deliver paclitaxel in vivo as reported by Agrawal et al. [160]. Paclitaxel-loaded exosomes led to a greater inhibition of tumor growth than free paclitaxel, with reduced systemic side effects. In addition, several other molecules have been packaged into exosomes for cancer treatment [161-164].
3.3.3. Cardiac delivery
Therapeutic application of exosomes in the context of cardiovascular disease (CVD) is of utmost interest. For the past few decades, a wide spectrum of stem cells has been studied for CVD treatment due to their ability to differentiate themselves into several cell types [165, 166]. In addition, stem cells have been differentiated into specific cells that aid in the repair and regeneration of damaged tissues, including cardiac tissues [166]. However, due to clinical, ethical and safety issues, researchers have identified stem-cell exosomes as a focal point to offer cardiac protection and restore function, ultimately treating CVD [167]. This novel research area has become known as “stem cell therapy without stem cells” or “exosome therapy”.
Indeed, exosomes obtained from stem cells are found to be cardioprotective and offer great hope in the treatment of CVD. Hematopoietic stem cell (HSC)-derived exosomes provide support to repair cardiac tissues by differentiating into cardiomyocytes [168]. Moreover, in a rat model of ischemia/reperfusion injury, intravenous injection of cardiac progenitor stem cell-derived exosomes that overexpress the C-X-C chemokine receptor type 4 (CXCR4) were shown to improve cardiac function [169]. As demonstrated by Jung et al., therapeutic efficacies of induced Pluripotent Stem Cell (iPSC)-derived exosomes have also been shown to contribute to neovascularization and survival of cardiomyocytes in experimental animal models of CVDs [170]. Research of the application of exosomes in cardiac ischemia has also gathered much interest. Lai et al. observed that human embryonic stem cells (hESC) are able to reduce the infarct size in an ex vivo Langendorff model of ischemia/reperfusion (I/R) mouse injury [171]. In addition, it has been demonstrated that intravenous injection of stem cell-derived exosomes prior to reperfusion reduced the size of infarcts by about 45% in mice models [172].
As in the case of cancer tissue, homing peptides have also been utilized to guide exosomes to cardiac tissues. In cardiac stem cell-derived exosomes, conjugating a cardiac-homing peptide using a dioleoyl phosphatidylethanolamine N-hydroxy succinimide (DOPE-NHS) linker resulted in enhanced exosome uptake by cardiomyocytes [173]. In a similar fashion, Lamp2b has been used as a modified anchor on exosomes to attach cardiac-homing peptides for better tissue selectivity. Several other reports showcased the potential advantages of using exosomes to treat CVD, making use of the inherent abilities of stem cell-derived exosomes to replicate and repair diseased tissues via targeted delivery of exosomes to cardiac tissues [174-176]. Collectively, these studies conclude that exosomes could be a promising foundation for CVD treatment.
4. Exosomes in disease diagnosis
The presence of exosomes in all forms of biological fluids, in addition to their specific donor-cell inspired cargo, gives them a significant superiority as an ideal diagnostic tool in “liquid biopsy”. In addition, exosomes are biologically stable, which makes them easy to store and handle. Over the past decade, mounting evidence has shown the feasibility of using exosomes as biomarkers in different diseases and pathophysiological conditions, including but not limited to neurodegenerative diseases, cardiovascular diseases, cancer and pregnancy disorders.
4.1. Exosomes in neurodegenerative diseases diagnosis
Particularly for neurodegenerative disease, the recent development of using exosomes as a diagnostic tool is of importance. First, the brain is in an enclosed environment with no means of access except for CSF collection via lumbar puncture. Even though lumbar puncture is currently considered safe and reliable, many diseases develop much earlier than clinical diagnosis can be inferred, thus there is an urgent need for easily detectable and reliable biomarkers in neurodegenerative diseases. The presence of a biological entity with a distinct signature secreted from brain cells and captured in the blood has opened a “window to the brain” for researchers. Utilizing this concept, efforts have been made to enrich exosomes from distinct brain cells, especially neurons and astrocytes.
In the context of AD, it does not come as a surprise that the first sought-after targets were the major culprits in AD pathophysiology, namely Aβ, tau and p-tau. Specifically, the Aβ42/40 ratio, total tau (t-tau) and phosphorylated tau (p-tau) are already being used as biomarkers in CSF-based neurochemical diagnosis, and the same molecules have been identified in serum exosomes [177]. Fiandaca and colleagues reported that neuronal exosomes enriched from the blood of AD patients showed significantly higher levels of Aβ42, total tau, p-tau181 and p-tau 396 compared to controls [177]. The levels of these candidates were higher in preclinical individuals up to ten years prior to the clinical onset of AD. Similar results were reported by another group, claiming that plasma exosomes from AD patients significantly differed from healthy controls based on their morphology, content and count, which might provide a basis for early diagnosis of AD [178].
In a recent study, a new technology termed amplified plasmonic exosome (APEX) analysis was developed to subtype blood exosome-bound Aβ in relation to the cells of origin. The authors found that plasma neuronal exosomes favor binding to prefibrillar Aβ, especially Aβ42. Analyzing these exosomes with APEX presented a precise correlation to the amyloid plaque load in AD patients with higher sensitivity than positron emission topography (PET), especially in early stages of AD [179]. Although astrocyte-derived exosomes (ADE) are reported to be present at lower levels in plasma compared to neuronal ones (NDE), they contain a higher amount of amyloid precursor protein (APP)-derived metabolites (including Aβ42) and APP-processing enzymes, such as BACE-1, in addition to p-tau [95]. This observation allowed the use of ADE analyses to distinguish AD patients from study controls. More recently, a proteomic study demonstrated elevated levels of Thr(P)-181 tau in AD CSF and brain tissues exosomes. Notably, Thr(P)-181 tau is a well-established marker for early-onset AD diagnosis. Moreover, another comprehensive study utilizing label-free quantitative proteomics, coupled with a machine learning method, identified a distinct panel of exosome proteins that could distinguish AD patients from age-matched controls with 88% accuracy [180].
Similar to AD, peripheral neuronal exosomes have been proposed as a diagnostic tool in mild TBI (mTBI). The recent work of Gill et al. examined the mechanisms underlying mTBI and its chronic symptoms using neuronal-derived exosomes and high-sensitivity detection of neurodegenerative and inflammatory biomarkers [181]. Interestingly, the levels of Aβ42, tau and IL-10 were significantly higher in military personnel with mTBI when compared to normal individuals. The authors argue that elevated levels of tau were related to chronic post-concussive symptoms. On the other hand, higher IL-10 levels were related to post-traumatic stress disorder (PTSD) symptoms. These exciting findings suggest that exosomes may serve as prognostic and diagnostic biomarkers to identify patients with mTBI at risk for developing chronic symptoms [181].
The use of peripheral exosomes as biomarkers for neurodegenerative diseases has also expanded to Parkinson’s Disease (PD), Huntington’s Disease (HD), and amyotrophic lateral sclerosis (ALS). A recent study on exosomes derived from astrocytes and oligodendrocytes from PD patients’ plasma revealed an exponential increase in exosome concentration in relation to disease severity [182]. A few other groups showed that exosomal α-Syn oligomer, α-Syn oligomer/α-Syn total and apolipoprotein A1 can be used as potential biomarkers to monitor disease progression of PD [183-185]. Proteomic analysis of sporadic ALS patients’ CSF-derived exosomes showed a significant upregulation of the INHAT repressor (INR) protein compared to the control group [186]. In addition, the pathological hallmark of ALS TDP-43 is identified in the exosomes isolated from brain-tissues from ALS patients [187].
Most interestingly, a new branch of exosomal diagnostics, focused on salivary exosomes, has attracted considerable attention. Using salivary exosomes instead of the whole saliva overcomes the limitations innate to the saliva, like the presence of contaminating elements and the richness of amylase enzyme that to mask target biomolecules. Several studies elucidated the use of salivary exosomes in neurodegenerative diseases diagnosis. For instance, the level of the α-syn oligomer, α-syn oligomer/α-syn total in salivary exosomes were found to be higher in PD patients compared to healthy controls [188].
4.2. Exosomes in cardiovascular diseases (CVD) diagnosis
Currently, there are a few circulating biomarkers of CVD, including total cholesterol levels, high-sensitivity C-reactive protein, low-density lipoproteins (LDL), and high-sensitivity cardiac troponin and creatine kinase MB [189]. However, these biomarkers can barely estimate the risk of disease occurrence and progression, and they cannot provide reliable information regarding the onset and development of the disease process. Therefore, novel blood-based exosome biopsy can offer a promising platform for more accurate clinical diagnosis and prediction. In general, micro-RNAs are the most prevalent molecules in CVD-associated exosomes, revealing superiority as a diagnostic biomarker [190]. For instance, Kuwabara et al. showed that serum exosomes of patients with an injured myocardium contain significantly higher levels of circulating miR-133a, proposing it as a biomarker for cardiomyocyte death [191]. Additionally, it has been reported that some micro-RNAs (miR-22, miR-320a, miR-423-5p and miR-92b) are overexpressed in serum exosomes from heart failure patients, indicating that they can be used as prognostic and diagnostic biomarkers. Also, in heart failure, many serum exosomes containing p53- responsive micro-RNAs, including miR-34a, miR-194 and miR-192, were upregulated in exosomes from heart failure patients within one year of acute myocardial infarction onset [192].
Apart from micro-RNA, exosomal proteins have also been identified as potential biomarkers for CVD. Several groups correlated the presence of many exosomal proteins to a high risk for CVD incident and mortality, including Cystatin C, CD14 and Serpin F2 [193-195]. Lastly, Cheow et al. utilized liquid chromatography coupled with tandem mass spectrometry to identify 252 upregulated exosomal proteins after myocardial infarction (MI), which created a potential panel for the early MI diagnosis [196].
4.3. Exosomes in pregnancy disorders
Even though the use of exosomes in urine, amniotic fluid and in maternal peripheral blood as a biomarker during pregnancy is still in its infancy, there is sufficient evidence that suggests a great potential for exosomes in diagnostics during pregnancy. It has been shown that placental cells can release exosomes to communicate with the maternal body [197]. In addition, the biogenesis and secretion of placenta-derived exosomes (PDEs) are described as regulated by the microenvironment, such as glucose concentration and oxygen tension. This promotes exosomes as a noninvasive and promising tool for the early diagnosis and prognosis of pregnancy disorders [198-200].
Preeclampsia (PE), which is a hypertensive disorder of pregnancy, has been shown to stimulate the secretion of exosomes from placental cells. One placental-specific marker that has been differentially altered in placental exosomes is alkaline phosphatase (PLAP) [201]. Based on the PLAP expression, Pillay et al. demonstrated that the ratio of PDEs to the total number of exosomes was significantly reduced in early and late onset PE [202]. The relative concentration of PDEs was significantly increased compared to that in normotensive patients. Biro et al. also showed that plasma-derived exosomes from pregnant women with PE, gestational hypertension or chronic hypertension have significantly higher levels of total miRNA and hypoxia-sensitive miR-210 compared to healthy controls [203]. Another study addressing the placental exosome changes found that miR-486-1-5p and miR-486-2-5p were strikingly higher in women with PE than in healthy controls [204]. This observation suggests that diagnosis using exosomes associated with pregnancy disorders may greatly improve the management of pregnancy hypertension.
4.4. Exosomes as a prognostic and diagnostic tool in cancer
Even though solid biopsy is still considered the gold standard for pathological diagnosis and is essentially the basis for cancer treatment, it is invasive, sometimes difficult to execute, and tumor heterogeneity is inevitable. Hence, the use of tumor-derived exosomes (TDEs) as a non-invasive liquid biopsy shows great advantages for individualized and precise diagnosis, as well as for treatment via personalized medicine. This is owed to the relation between TDEs and metastatic niche formation, immune evasion, and tumor progression [205-207].
Several groups have investigated exosomes for early screening and accurate diagnosis of cancer. For instance, exosomal DNA in early stages of pancreatic cancer were shown to have a high probability of KRAS mutation [208]. Circulating exosomes from patients with pancreatic ductal carcinoma and colorectal cancer contained elevated levels of Glypican 1 (GPC1), an observation that might serve as an early detection tool for tumors in the digestive system [209]. This observation was followed up with a clinical trial to detect the presence of CTCs and GPC1-containing exosomes for diagnosis accuracy assessment and comparison (NCT03032913). Other exosomal RNAs have also been proposed as candidates for early diagnosis of colorectal cancer, including lncRNA (BCAR4) and the two mRNAs, KRTAP5-4 and MAGEA3 [210].
Aside from exosomal RNAs, various exosomal proteins have shown powerful efficacy in differentiating cancerous from noncancerous patients. Sandfeld-Paulsen et al. used exosome arrays containing 49 antibodies and showed that CD171 and CD151 stood out as the most significant molecules to distinguish lung cancer patients from cancer-free individuals [211]. Similarly, Chen et al. identified 144 distinctly elevated phosphorylated proteins in exosomes from breast cancer patients compared to noncancer patients using MS [211]. Numerous other studies investigated the use of exosomes in the prognosis and diagnosis of cancer. Table 1 summarizes the list of those studies that developed into clinical trials evaluating the use of exosomes in cancer diagnostics.
Table.1.
Summary of clinical trials using exosomes for cancer prognosis and diagnosis.
| Cancer type |
Exosome source |
No. of patients |
Target | Extraction method | Detection method |
Reference |
|---|---|---|---|---|---|---|
| Colorectal Cancer | Serum | 40 | miRNA | ExoQuick | qRT-PCR | [212] |
| Serum | 140 | lncRNA | Ultracentrifugation | qPCR | [210] | |
| Serum | 116 | CEA | ExoQuick ™ | ELISA | [213] | |
| Tissue homogenate | 102 | GPC1 | ExoCap ™ | Flow cytometry | [214] | |
| Plasma | 124 | Copine 111 | Ultracentrifugation | ELISA | [215] | |
| Serum | 87 | miR-25-3p | Ultracentrifugation | qPCR | [216] | |
| Plasma | 93 | miR-357 | Exiqion ™ miRCURY Exosome kit. | qPCR | [217] | |
| Serum | 108 | miR-548c-5p | Invitrogen Total exosomes isolation kit | qPCR | [218] | |
| Serum | 28 | miR-17-5p | qEV size exclusion Columns | qRT-PCR | [219] | |
| Serum | 225 | miR-19a | Ultracentrifugation/Invitrogen Total exosomes isolation kit | qRT-PCR | [220] | |
| Breast cancer | Plasma | 32 | miR-21, miR1246 | ExoQuick | qRT-PCR | [221] |
| Serum | 38 | miR-105 | Ultracentrifugation | qRT-PCR | [222] | |
| Serum | 240 | CD82 | ExoQuick | ELISA/WB | [223] | |
| Plasma | 44 | Phosphoproteins | Ultracentrifugation. | LC-MS/MS | [224] | |
| Serum | 53 | miR-222 | Density gradient ultracentrifugation. | qPCR | [225] | |
| Lung cancer | Plasma | 20 | Amphiregulin | Ultracentrifugation | ELISA | [226] |
| Serum | 85 | PD-L1 | Invitrogen Total exosomes isolation kit | ELISA | [227] | |
| Plasma | 276 | NY-ESO-1 | Exosome array | Exosome Array | [211] | |
| Serum | 106 | FLI1 exonic circular RNA | Exoeasy Maxi kit | qRT-PCR | [228] | |
| Plasma | 210 | EGFR T790M | Ultracentrifugation | Allelle specific qPCR | [229] | |
| Plasma | 105 | miRNAs | Ultracentrifugation | qRT-PCR | [230] | |
| Plasma | 581 | Proteins | Exosome array | Exosome array | [231] | |
| Serum | 171 | Proteins | Ultracentrifugation | Western blot. | [232] | |
| Pancreatic cancer | Plasma | 263 | KRAS | Ultracentrifugation | ddPCR | [233] |
| Serum | 221 | GPC1 | Sucrose gradient ultracentrifugation | Flow cytometry | [234] | |
| Serum | 85 | CKAP4 | PS capture ELISA kit | PS capture exosomes ELISA | [235] | |
| Plasma | 194 | KRAS | Ultracentrifugation | ddPCR | [236] | |
| Serum | 91 | PD-L1, c-MET | Invitrogen Total exosomes isolation kit | Flow cytometry | [237] | |
| Melanoma | Serum | 96 | S100B, MIA | ExoQuick | ELISA | [238] |
| Serum | 56 | miRNA-125b | ExoQuick | qRT-PCR | [239] | |
| Bladder cancer | Urine | 69 | miRNA | Differential centrifugation | miRNA microarray | [240] |
| Esophageal carcinoma | Saliva | 602 | Chimeric RNAs | Exoquick | qRT-PCR | |
| Prostate cancer | Urine | 13 | ITGA3, ITGB1 | Ultracentrifugation | Western blot | [241] |
Interestingly, several commercial organizations have already started developing exosome-based cancer diagnostics, such as Caris Life Sciences, Exosomes Diagnostics and Humsa Bio Med.
For instance, Exosome Diagnostics sponsored an observational clinical trial investigating the efficacy of the diagnostic test “ExoDx Prostate Intelliscore (EPI)” [242]. The 532 participants enrolled in the study had early clinical presentation of prostate cancer based in part on high prostate-specific antigen levels (limit range: 2.0–10 ng/mL). The enrolled participants were divided into two cohorts: the first cohort, consisting of men already scheduled for an initial prostate biopsy; and the second cohort comprised of men without a scheduled prostate biopsy. The aims of the study were to evaluate the performance of the urine test in men already belonging to the first cohort, and to assess how the results of the urine test could influence the decision process for determining whether to perform biopsy. Currently, ExoDx is the only exosome-based prostate cancer test delivering a unique data point to help guide the prostate biopsy decision. The ExoDx prostate test is included in the NCCN Guidelines, and it is independent of PSA and other standard of care (SOC) information.
5. Exosomes in clinical trials
Accessed on August 21st, 2021, the database www.ClinicalTrials.gov shows 231 trials registered under the term “exosomes” and 84 under the more comprehensive term “extracellular vesicles”. Further analysis shows that the majority of these trials use exosomes as a biomarker, and only a few have exosome treatment results as a primary outcome. Cancer applications dominated most of the exosome trials, with over 100 registered studies. Again, as summarized in Section 4.4, the majority of these trials utilized exosomes as a diagnostic tool rather than a therapeutic agent. However, some early studies have attempted to study the potential of exosomes as “cancer vaccine” [243, 244].
Dendritic cell-derived exosomes (DEX) were the basis of two phase I clinical trials testing exosomes for cancer vaccination. In the first trial, DEXs loaded with HLA-restricted melanoma-associated antigen (MAGE) peptides were infused into patients with HLA A2+ non-small cell lung cancer [245]. The vaccine was reported to be tolerated by all subjects after four weekly doses. However, only one-third of the patients presented with MAGE-specific T-cell responses, and half of the analyzed patients showed an increase in NK cell activity. The second trial described the usage of DEX generated by dendritic cells after being transfected with MAGE to conduct immunization of melanoma patients [246]. Similar to the first study using DEX, no major toxicity event was reported; however, no MAGE-specific response of CD4+ and CD8+ cells were detected in peripheral blood. Apart from the DEX vaccine, one phase I clinical trial reported the use of ascites-derived exosomes (AEXs) in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF) as an immunotherapy for colorectal cancer [85]. While the vaccine was well tolerated by patients, the injected patients demonstrated a strong anti-tumor cytotoxic T-lymphocyte response against the carcinoembryonic antigen, which is a colorectal cancer biomarker. The concept of exosome-based vaccines has also been explored in diseases other than cancer. One phase II/III clinical trial was conducted using umbilical cord MSCs derived exosomes in patients with chronic kidney diseases, including Type 1 diabetes and interstitial nephritis. The participants in the study reported no significant adverse effects during or after the treatment.
For neurodegenerative and behavioral diseases, there have been only 13 trials including the research topic “exosomes”. While most of these trials have exosome treatment results as a secondary outcome, a few have included exosome treatment results as a primary outcome. For instance, the Jordan group from Neurological Associates of West Los Angeles has initiated two separate trials targeting neurodegenerative, disease-driven depression, anxiety and dementia (NCT04202770), and craniofacial neuralgia (NCT04202783). In addition, the Wang group from Ruijin Hospital in China is conducting a phase I/II clinical trial to investigate the safety and efficacy of allogeneic adipose tissue derived-MSCs exosomes for the treatment of mild to moderate dementia due to Alzheimer’s disease (NCT04388982).
The Michael J. Fox Foundation for Parkinson’s Research has also considered exosomes in the diagnosis of Parkinson Disease (PD) as a part of the “Fox BioNet Project”. The Foundation has sponsored an observational study aiming to optimize an isolation protocol for cerebrospinal fluid (CSF)-derived exosomes. The aim of the study is to enrich and increase the detection of specific PD-associated mutations in Leucine-rich repeat kinase 2 (LRRK2). Most recently, an observation study in the Tang-Du Hospital is currently enrolling subjects to study changes in circulating exosomes and search for early serum markers and potential intervention targets for disease monitoring in patients with intracerebral hemorrhage (NCT05035134).
The current global pandemic, caused by the widespread infection with the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARSCoV-2), has become a severe burden on global health and economy. SARS-CoV-2 mostly targets the human respiratory system, which is similar to other coronaviruses including Middle East respiratory syndrome (MERS-CoV) and severe acute respiratory syndrome (SARSCoV). Unlike other corona viruses, SARS-CoV-2 affects the lower respiratory tract, leading to infiltration of the upper lobes of the lungs and ultimately to severe acute respiratory syndrome (SARSCoV) [247]. In addition, reduction of the T cells, as well as dysregulation of immune effector cells, have been reported in patients with COVID-19, collectively leading to acute respiratory distress syndrome (ARDS) [248-250]. Of note, MSC exosomes have already been employed to treat airway diseases such as bronchopulmonary dysplasia, asthma, and more importantly ARDS [251, 252]. Currently, there are a total of 13 active clinical trials using exosome interventions for COVID-19 as per ClinicalTrials.gov, four of which investigate the usage of MSC exosomes as a supportive treatment for COVID-19-infected patients.
The first phase I trial was conducted to investigate the therapeutic potential of aerosol inhalation of allogenic bone marrow MSC derived exosomes in patients hospitalized with severe conditions, sponsored by the Ruijin Hospital (NCT04276987). The results associated with the study have been submitted but are not yet available to the public. Another study led by Russian clinicians is testing whether inhalation of MSC-derived exosomes can suppress the hyper-response of the immune system to the virus and stimulate regenerative processes (NCT04602442). Lastly, intravenously-injected BM-MSCs are being evaluated as a potential treatment for moderate-to-severe ARDS in COVTD-19 patients (NCT04493242). The efficacy and safety of intravenous infection of MSC-derived exosomes every other day on an escalating dose of 2:4:8 is also being investigated in a phase I/II trial (NCT04798716).
So far, one clinical trial has been completed with results submitted to ClinicalTrials.gov (NCT04491240), which reported no adverse effects in patients who received MSC-derived exosomes aerosol twice a day for 10 days. However, details about the aerosol formulation and the source of the donor MSCs used in the clinical trial are not yet available. The costimulatory molecule CD24 is also an attractive target for COVID-19 clinical trials. CD24 is expressed on several hematopoietic cells, such as B cell progenitors, and it is also associated with autoimmune diseases. Currently, two independent phase I and phase II (NCT04747574 and NCT04902183, respectively) clinical trials are recruiting subjects with moderate to severe COVID-19 infection to assess the safety and efficacy of exosomes overexpressing CD24 purified from T-REx™-293 cells engineered to express CD24.
When completed, the current clinical trials can deliver the foundation for future studies using exosomes, especially MSC-derived exosomes since they are the main donor of exosomes in clinical trials to this point. Recently, the International Society for Extracellular Vesicles (ISEV), together with the International Society for Cell and Gene Therapy (ISCT), has issued a statement to encourage the conduction of further research and clinical trials using exosomes as a therapeutic strategy against COVID-19 [253]. This statement also underscores the need for good clinical practice and rational clinical trial design.
Of note, while considering exosomes for mass production beyond the clinical trial stage, a standardized manufacturing process, such as a process in compliance with good manufacturing practice (GMP) for exosomes is of utmost importance [254, 255]. There are three areas where GMP is imperative when it comes to exosomes: (1) The process preceding cell cultivation; (2) the steps downstream of exosome purification; and (3) standardized quality control. Indeed, in the past few years, a few groups ought to standardize a GMP-compliant process for large-scale production of exosomes for therapeutic use with promising results. However, a more universal comprehensive protocol needs to be agreed upon before moving exosomes into the focus of a valid therapeutic agent.
Conclusions
Nearly two decades ago, exosomes were relatively unknown. Today, numerous clinical trials are investigating the therapeutic potential of exosome in diverse diseases. The continuous surge of interest in exosome research will continue and more exciting data will be generated at laboratories worldwide.
As for the development of exosomes into valid therapies, Clara Biotech internal analysis claims that the worldwide exosome market is projected to be more than $50 billion by 2026. Nevertheless, the use of exosomes is not without challenges, and more efforts are needed to understand their behaviors inside the body, standardize the manufacturing process and control for quality. The isolation and purification of exosomes, utilizing consistent and reproducible methods, remain challenges. The scientific community will inevitably continue to evolve, such that our understanding of exosomes nature, behavior and purification methods will bring about new knowledge, diagnostics and novel therapeutics that will improve global health outcomes.
Figure 2. Labeling and surface modification strategies for biodistribution analysis and targeted drug delivery.
Targeting of exosomes to specific organs or cells can be achieved through engineering the exosomal membrane to express certain molecules such as targeting peptides conjugated with exosomal membrane-associated domains like lysosome-associated membrane glycoprotein 2b (Lamp2b) or the C1C2 domain of lactadherin (LA). Labeling of exosomes for tracking and imaging purposes is achieved via fluorescence, bioluminescence, and radiolabeling approaches.
Acknowledgements
The authors acknowledge funding by NIH grants R01AG034389 and R01AG064234, and VA grant I01BX003643. The authors thank the Department of Physiology (Chair Dr. Alan Daugherty), University of Kentucky College of Medicine, for institutional support. The authors also thank Ms. Krista E. Callahan-Caudill for editing the manuscript.
Footnotes
Declaration of Competing Interest
None
CRediT authorship contribution statement
Zainuddin Quadri: Completed Section 1 and Section 2. Ahmed Elsherbini: Completed Section 3 and Section 4. Erhard Bieberich: Supervised, reviewed and edited manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Perbal B, Communication is the key. Cell Communication and Signaling, 2003. 1(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle LM and Wang MZ, Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells, 2019. 8(7): p. 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gruner HN and McManus MT, Examining the evidence for extracellular RNA function in mammals. Nature Reviews Genetics, 2021. 22(7): p. 448–458. [DOI] [PubMed] [Google Scholar]
- 4.Kanada M, et al. , Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A, 2015. 112(12): p. E1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mighty J, et al. , Analysis of Adult Neural Retina Extracellular Vesicle Release, RNA Transport and Proteomic Cargo. Invest Ophthalmol Vis Sci, 2020. 61(2): p. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roefs MT, Sluijter JPG, and Vader P, Extracellular Vesicle-Associated Proteins in Tissue Repair. Trends Cell Biol, 2020. 30(12): p. 990–1013. [DOI] [PubMed] [Google Scholar]
- 7.Garcia NA, et al. , Circulating exosomes deliver free fatty acids from the bloodstream to cardiac cells: Possible role of CD36. PLoS One, 2019. 14(5): p. e0217546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding C, Heuser J, and Stahl P, Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol, 1983. 97(2): p. 329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan BT and Johnstone RM, Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell, 1983. 33(3): p. 967–78. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H and Lyden D, Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc, 2019. 14(4): p. 1027–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, et al. , Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci, 2019. 9: p. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamai K, et al. , Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun, 2010. 399(3): p. 384–90. [DOI] [PubMed] [Google Scholar]
- 13.Kowal J, Tkach M, and Thery C, Biogenesis and secretion of exosomes. Curr Opin Cell Biol, 2014. 29: p. 116–25. [DOI] [PubMed] [Google Scholar]
- 14.Reddy VS, et al. , Extracellular small heat shock proteins: exosomal biogenesis and function. Cell Stress and Chaperones, 2018. 23(3): p. 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Niel G, et al. , The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell, 2011. 21(4): p. 708–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiuchi R, et al. , Potentiation of the ligand-binding activity of integrin alpha3beta1 via association with tetraspanin CD151. Proc Natl Acad Sci U S A, 2005. 102(6): p. 1939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolte-'t Hoen EN, et al. , Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood, 2009. 113(9): p. 1977–81. [DOI] [PubMed] [Google Scholar]
- 18.Rana S, et al. , Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol, 2012. 44(9): p. 1574–84. [DOI] [PubMed] [Google Scholar]
- 19.Chairoungdua A, et al. , Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol, 2010. 190(6): p. 1079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang X, et al. , Tumour-Secreted Hsp90alpha on External Surface of Exosomes Mediates Tumour - Stromal Cell Communication via Autocrine and Paracrine Mechanisms. Sci Rep, 2019. 9(1): p. 15108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trajkovic K, et al. , Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science, 2008. 319(5867): p. 1244–7. [DOI] [PubMed] [Google Scholar]
- 22.Shamseddine AA, Airola MV, and Hannun YA, Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes. Advances in biological regulation, 2015. 57: p. 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannun YA and Obeid LM, Many ceramides. The Journal of biological chemistry, 2011. 286(32): p. 27855–27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miura Y, et al. , Hydrolysis of sphingosylphosphocholine by neutral sphingomyelinases. FEBS Letters, 2004. 557(1-3): p. 288–292. [DOI] [PubMed] [Google Scholar]
- 25.Guo BB, Bellingham SA, and Hill AF, The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem, 2015. 290(6): p. 3455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinkins MB, et al. , Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer's disease. Neurobiol Aging, 2014. 35(8): p. 1792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Essandoh K, et al. , Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 2015. 1852(11): p. 2362–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinrichs A, Ceramide buds in. Nature Reviews Molecular Cell Biology, 2008. 9(4): p. 265–265. [Google Scholar]
- 29.Wei D, et al. , RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Research, 2021. 31(2): p. 157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elsherbini A and Bieberich E, Ceramide and Exosomes: A Novel Target in Cancer Biology and Therapy. Adv Cancer Res, 2018. 140: p. 121–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shenoda BB and Ajit SK, Modulation of Immune Responses by Exosomes Derived from Antigen-Presenting Cells. Clinical medicine insights. Pathology, 2016. 9(Suppl 1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu P, et al. , Exosome: The Regulator of the Immune System in Sepsis. 2021. 12(880). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zitvogel L, et al. , Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med, 1998. 4(5): p. 594–600. [DOI] [PubMed] [Google Scholar]
- 34.Vincent-Schneider H, et al. , Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol, 2002. 14(7): p. 713–22. [DOI] [PubMed] [Google Scholar]
- 35.Raposo G, et al. , B lymphocytes secrete antigen-presenting vesicles. J Exp Med, 1996. 183(3): p. 1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tkach M, et al. , Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J, 2017. 36(20): p. 3012–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wahlund CJE, et al. , Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep, 2017. 7(1): p. 17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatnagar S, et al. , Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood, 2007. 110(9): p. 3234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Y and Schorey JS, Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol, 2013. 43(12): p. 3279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smythies LE and Smythies JR, Exosomes in the gut. Front Immunol, 2014. 5: p. 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nandakumar R, et al. , Intracellular bacteria engage a STING-TBK1-MVB12b pathway to enable paracrine cGAS-STING signalling. Nat Microbiol, 2019. 4(4): p. 701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Y and Schorey JS, Extracellular vesicles deliver Mycobacterium RNA to promote host immunity and bacterial killing. EMBO Rep, 2019. 20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G, et al. , Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature, 2018. 560(7718): p. 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ning Y, et al. , Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett, 2018. 199: p. 36–43. [DOI] [PubMed] [Google Scholar]
- 45.Capello M, et al. , Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat Commun, 2019. 10(1): p. 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalmin F, et al. , Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest, 2010. 120(2): p. 457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrusiewicz K, et al. , Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology, 2018. 7(4): p. e1412909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crenshaw BJ, et al. , Exosome Biogenesis and Biological Function in Response to Viral Infections. Open Virol J, 2018. 12: p. 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nour AM and Modis Y, Endosomal vesicles as vehicles for viral genomes. Trends Cell Biol, 2014. 24(8): p. 449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramakrishnaiah V, et al. , Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A, 2013. 110(32): p. 13109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng Z, et al. , A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature, 2013. 496(7445): p. 367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagashima S, et al. , Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J Gen Virol, 2014. 95(Pt 10): p. 2166–2175. [DOI] [PubMed] [Google Scholar]
- 53.Gould SJ, Booth AM, and Hildreth JE, The Trojan exosome hypothesis. Proc Natl Acad Sci U S A, 2003. 100(19): p. 10592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sims B, et al. , Tetraspanin blockage reduces exosome-mediated HIV-1 entry. Arch Virol, 2018. 163(6): p. 1683–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, et al. , Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol, 2013. 14(8): p. 793–803. [DOI] [PubMed] [Google Scholar]
- 56.Khatua AK, et al. , Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol, 2009. 83(2): p. 512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuate S, et al. , Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology, 2007. 362(1): p. 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Y, et al. , Inhibition of multiple myelomaderived exosomes uptake suppresses the functional response in bone marrow stromal cell. Int J Oncol, 2019. 54(3): p. 1061–1070. [DOI] [PubMed] [Google Scholar]
- 59.Amigorena S, Anti-tumour immunotherapy using dendritic-cell-derived exosomes. Res Immunol, 1998. 149(7-8): p. 661–2. [DOI] [PubMed] [Google Scholar]
- 60.Roma-Rodrigues C, et al. , Targeting Tumor Microenvironment for Cancer Therapy. Int J Mol Sci, 2019. 20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, et al. , Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol, 2016. 239(2): p. 162–73. [DOI] [PubMed] [Google Scholar]
- 62.Zhao H, et al. , Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife, 2016. 5: p. e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang X, et al. , Role of Exosomes in Crosstalk Between Cancer-Associated Fibroblasts and Cancer Cells. Front Oncol, 2019. 9: p. 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donnarumma E, et al. , Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget, 2017. 8(12): p. 19592–19608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng F, et al. , MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology, 2007. 133(2): p. 647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang HS, et al. , Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene, 2003. 22(24): p. 3712–20. [DOI] [PubMed] [Google Scholar]
- 67.Frankel LB, et al. , Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem, 2008. 283(2): p. 1026–33. [DOI] [PubMed] [Google Scholar]
- 68.Au Yeung CL, et al. , Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun, 2016. 7: p. 11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richards KE, et al. , Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene, 2017. 36(13): p. 1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoon H, et al. , TGF-beta1-mediated transition of resident fibroblasts to cancer-associated fibroblasts promotes cancer metastasis in gastrointestinal stromal tumor. Oncogenesis, 2021. 10(2): p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luga V, et al. , Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell, 2012. 151(7): p. 1542–56. [DOI] [PubMed] [Google Scholar]
- 72.Aung T, et al. , Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A, 2011. 108(37): p. 15336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li S, et al. , The roles of exosomes in cancer drug resistance and its therapeutic application. Clin Transl Med, 2020. 10(8): p. e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen WX, et al. , Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One, 2014. 9(4): p. e95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qin X, et al. , Cisplatin-resistant lung cancer cell-derived exosomes increase cisplatin resistance of recipient cells in exosomal miR-100-5p-dependent manner. Int J Nanomedicine, 2017. 12: p. 3721–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei F, et al. , Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer, 2017. 16(1): p. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang M, et al. , Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J Exp Clin Cancer Res, 2018. 37(1): p. 171. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Ding C, et al. , Exosome-mediated transfer of circRNA CircNFIX enhances temozolomide resistance in glioma. Cancer Lett, 2020. 479: p. 1–12. [DOI] [PubMed] [Google Scholar]
- 79.Yu T, et al. , Delivery of MGMT mRNA to glioma cells by reactive astrocyte-derived exosomes confers a temozolomide resistance phenotype. Cancer Lett, 2018. 433: p. 210–220. [DOI] [PubMed] [Google Scholar]
- 80.Sansone P, et al. , Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A, 2017. 114(43): p. E9066–E9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monfared H, et al. , Potential Therapeutic Effects of Exosomes Packed With a miR-21-Sponge Construct in a Rat Model of Glioblastoma. Front Oncol, 2019. 9: p. 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raghav KP, et al. , cMET and phospho-cMET protein levels in breast cancers and survival outcomes. Clin Cancer Res, 2012. 18(8): p. 2269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li S, et al. , Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer. Nanoscale, 2020. 12(19): p. 10854–10862. [DOI] [PubMed] [Google Scholar]
- 84.Li J, et al. , Blockage of transferred exosome-shuttled miR-494 inhibits melanoma growth and metastasis. J Cell Physiol, 2019. [DOI] [PubMed] [Google Scholar]
- 85.Dai S, et al. , Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther, 2008. 16(4): p. 782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu W, et al. , Role of Exosomes in Central Nervous System Diseases. Frontiers in Molecular Neuroscience, 2019. 12(240). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Serrano-Pozo A, et al. , Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine, 2011. 1(1): p. a006189–a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun X, Chen W-D, and Wang Y-D, β-Amyloid: The Key Peptide in the Pathogenesis of Alzheimer’s Disease. Frontiers in Pharmacology, 2015. 6(221). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chow VW, et al. , An overview of APP processing enzymes and products. Neuromolecular medicine, 2010. 12(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rajendran L, et al. , Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A, 2006. 103(30): p. 11172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perez-Gonzalez R, et al. , The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem, 2012. 287(51): p. 43108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elsherbini A, et al. , Association of Abeta with ceramide-enriched astrosomes mediates Abeta neurotoxicity. Acta Neuropathol Commun, 2020. 8(1): p. 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharpies RA, et al. , Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J, 2008. 22(5): p. 1469–78. [DOI] [PubMed] [Google Scholar]
- 94.Wang G, et al. , Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J Biol Chem, 2012. 287(25): p. 21384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goetzl EJ, et al. , Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer's disease. FASEB J, 2016. 30(11): p. 3853–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miyoshi E, et al. , Exosomal tau with seeding activity is released from Alzheimer’s disease synapses, and seeding potential is associated with amyloid beta. Laboratory Investigation, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.d’Errico P and Meyer-Luehmann M, Mechanisms of Pathogenic Tau and Aβ Protein Spreading in Alzheimer’s Disease. Frontiers in Aging Neuroscience, 2020. 12(265). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mohamed NV, et al. , Spreading of tau pathology in Alzheimer's disease by cell-to-cell transmission. Eur J Neurosci, 2013. 37(12): p. 1939–48. [DOI] [PubMed] [Google Scholar]
- 99.Pooler AM, et al. , Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep, 2013. 14(4): p. 389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simon D, et al. , Proteostasis of tau. Tau overexpression results in its secretion via membrane vesicles. FEBS Lett, 2012. 586(1): p. 47–54. [DOI] [PubMed] [Google Scholar]
- 101.Gomez-Ramos A, et al. , Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol Cell Neurosci, 2008. 37(4): p. 673–81. [DOI] [PubMed] [Google Scholar]
- 102.Karch CM, Jeng AT, and Goate AM, Extracellular Tau levels are influenced by variability in Tau that is associated with tauopathies. J Biol Chem, 2012. 287(51): p. 42751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saman S, et al. , Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem, 2012. 287(6): p. 3842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fiandaca MS, et al. , Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement, 2015. 11(6): p. 600–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serpente M, et al. , MiRNA Profiling in Plasma Neural-Derived Small Extracellular Vesicles from Patients with Alzheimer's Disease. Cells, 2020. 9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amoah SK, et al. , Exosomal secretion of a psychosis-altered miRNA that regulates glutamate receptor expression is affected by antipsychotics. Neuropsychopharmacology, 2020. 45(4): p. 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McKeever PM, et al. , MicroRNA Expression Levels Are Altered in the Cerebrospinal Fluid of Patients with Young-Onset Alzheimer's Disease. Mol Neurobiol, 2018. 55(12): p. 8826–8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee HJ, Patel S, and Lee SJ, Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci, 2005. 25(25): p. 6016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Emmanouilidou E, et al. , Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci, 2010. 30(20): p. 6838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grey M, et al. , Acceleration of alpha-synuclein aggregation by exosomes. J Biol Chem, 2015. 290(5): p. 2969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Danzer KM, et al. , Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener, 2012. 7: p. 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang C, et al. , Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett, 2013. 548: p. 190–5. [DOI] [PubMed] [Google Scholar]
- 113.Banks WA, et al. , Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int J Mol Sci, 2020. 21(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hornung S, Dutta S, and Bitan G, CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front Mol Neurosci, 2020. 13: p. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saint-Pol J, et al. , Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells, 2020. 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gangadaran P, Hong CM, and Ahn B-C, Current Perspectives on In Vivo Noninvasive Tracking of Extracellular Vesicles with Molecular Imaging. BioMed Research International, 2017. 2017: p. 9158319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Almeida S, et al. , In Vivo Tracking of Extracellular Vesicles by Nuclear Imaging: Advances in Radiolabeling Strategies. International journal of molecular sciences, 2020. 21(24): p. 9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berezin MY and Achilefu S, Fluorescence lifetime measurements and biological imaging. Chemical reviews, 2010. 110(5): p. 2641–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fleiss A and Sarkisyan KS, A brief review of bioluminescent systems (2019). Current genetics, 2019. 65(4): p. 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Syed AJ and Anderson JC, Applications of bioluminescence in biotechnology and beyond. Chemical Society Reviews, 2021. 50(9): p. 5668–5705. [DOI] [PubMed] [Google Scholar]
- 121.Mittelbrunn M, et al. , Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature Communications, 2011. 2(1): p. 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suetsugu A, et al. , Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev, 2013. 65(3): p. 383–90. [DOI] [PubMed] [Google Scholar]
- 123.Gangadaran P, Hong CM, and Ahn B-C, An Update on in Vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Frontiers in pharmacology, 2018. 9: p. 169–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chuo ST-Y, Chien JC-Y, and Lai CP-K, Imaging extracellular vesicles: current and emerging methods. Journal of biomedical science, 2018. 25(1): p. 91–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wiklander OPB, et al. , Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. Journal of extracellular vesicles, 2015. 4: p. 26316–26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tian T, et al. , Dynamics of exosome internalization and trafficking. 2013. 228(7): p. 1487–1495. [DOI] [PubMed] [Google Scholar]
- 127.Shimomura T, et al. , New Lipophilic Fluorescent Dyes for Exosome Labeling: Monitoring of Cellular Uptake of Exosomes. 2020: p. 2020.02.02.931295. [Google Scholar]
- 128.Gupta D, et al. , Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. Journal of Extracellular Vesicles, 2020. 9(1): p. 1800222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takahashi Y, et al. , Visualization and in vivo tracking of the exosomes of murine melanoma B16BL6 cells in mice after intravenous injection. J Biotechnol, 2013. 165(2): p. 77–84. [DOI] [PubMed] [Google Scholar]
- 130.Kumar S and Mansson A, Covalent and non-covalent chemical engineering of actin for biotechnological applications. Biotechnology Advances, 2017. 35(7): p. 867–888. [DOI] [PubMed] [Google Scholar]
- 131.Morishita M, et al. , Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials, 2016. 111: p. 55–65. [DOI] [PubMed] [Google Scholar]
- 132.Morishita M, et al. , Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J Pharm Sci, 2015. 104(2): p. 705–13. [DOI] [PubMed] [Google Scholar]
- 133.Morishita M, et al. , Pharmacokinetics of Exosomes-An Important Factor for Elucidating the Biological Roles of Exosomes and for the Development of Exosome-Based Therapeutics. J Pharm Sci, 2017. 106(9): p. 2265–2269. [DOI] [PubMed] [Google Scholar]
- 134.Faruqu FN, et al. , Membrane Radiolabelling of Exosomes for Comparative Biodistribution Analysis in Immunocompetent and Immunodeficient Mice - A Novel and Universal Approach. Theranostics, 2019. 9(6): p. 1666–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peinado H, et al. , Melanoma exosomes educate bone marrow progenitor cells toward a prometastatic phenotype through MET. Nat Med, 2012. 18(6): p. 883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grange C, et al. , Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med, 2014. 33(5): p. 1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bruno S, et al. , Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One, 2012. 7(3): p. e33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gangadaran P, et al. , A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice. Oncotarget, 2017. 8(66): p. 109894–109914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lai CP, et al. , Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano, 2014. 8(1): p. 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smyth T, et al. , Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release, 2015. 199: p. 145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wiklander OP, et al. , Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles, 2015. 4: p. 26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Imai T, et al. , Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles, 2015. 4: p. 26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Munagala R, et al. , Bovine milk-derived exosomes for drug delivery. Cancer Lett, 2016. 371(1): p. 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Koh E, et al. , Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials, 2017. 121: p. 121–129. [DOI] [PubMed] [Google Scholar]
- 145.Kamerkar S, et al. , Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature, 2017. 546(7659): p. 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mentkowski KI, et al. , Therapeutic Potential of Engineered Extracellular Vesicles. Aaps j, 2018. 20(3): p. 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alvarez-Erviti L, et al. , Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol, 2011. 29(4): p. 341–5. [DOI] [PubMed] [Google Scholar]
- 148.Liu Y, et al. , Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep, 2015. 5: p. 17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cui G.-h., et al. , RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immunity & Ageing, 2019. 16(1): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Perets N, et al. , Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Molecular autism, 2018. 9: p. 57–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Long Q, et al. , Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proceedings of the National Academy of Sciences of the United States of America, 2017. 114(17): p. E3536–E3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tian T, et al. , Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials, 2018. 150: p. 137–149. [DOI] [PubMed] [Google Scholar]
- 153.Guo S, et al. , Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano, 2019. 13(9): p. 10015–10028. [DOI] [PubMed] [Google Scholar]
- 154.Shao J, Zaro J, and Shen Y, Advances in Exosome-Based Drug Delivery and Tumor Targeting: From Tissue Distribution to Intracellular Fate. International journal of nanomedicine, 2020. 15: p. 9355–9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jung KO, et al. , Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials, 2018. 177: p. 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abello J, et al. , Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics, 2019. 9(8): p. 2325–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou W, et al. , Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials, 2021. 268: p. 120546. [DOI] [PubMed] [Google Scholar]
- 158.Vakhshiteh F, et al. , Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: A new approach for drug delivery. Life Sciences, 2021. 266: p. 118871. [DOI] [PubMed] [Google Scholar]
- 159.Nie H, et al. , Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale, 2020. 12(2): p. 877–887. [DOI] [PubMed] [Google Scholar]
- 160.Agrawal AK, et al. , Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine, 2017. 13(5): p. 1627–1636. [DOI] [PubMed] [Google Scholar]
- 161.Massey AE, et al. , Clinical Implications of Exosomes: Targeted Drug Delivery for Cancer Treatment. Int J Mol Sci, 2021. 22(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao X, et al. , Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomedicine & Pharmacotherapy, 2020. 128: p. 110237. [DOI] [PubMed] [Google Scholar]
- 163.Pullan JE, et al. , Exosomes as Drug Carriers for Cancer Therapy. Mol Pharm, 2019. 16(5): p. 1789–1798. [DOI] [PubMed] [Google Scholar]
- 164.Kibria G, et al. , Exosomes as a Drug Delivery System in Cancer Therapy: Potential and Challenges. Molecular Pharmaceutics, 2018. 15(9): p. 3625–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jadczyk T, Faulkner A, and Madeddu P, Stem cell therapy for cardiovascular disease: the demise of alchemy and rise of pharmacology. British journal of pharmacology, 2013. 169(2): p. 247–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lippi M, et al. , Human Cell Modeling for Cardiovascular Diseases. 2020. 21(17): p. 6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jayaraman S, et al. , Stem Cell-Derived Exosomes Potential Therapeutic Roles in Cardiovascular Diseases. Frontiers in cardiovascular medicine, 2021. 8: p. 723236–723236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Burt R, et al. , Hematopoietic stem cell transplantation for cardiac and peripheral vascular disease. Bone Marrow Transplant, 2003. 32 Suppl 1: p. S29–31. [DOI] [PubMed] [Google Scholar]
- 169.Ciullo A, et al. , Exosomal Expression of CXCR4 Targets Cardioprotective Vesicles to Myocardial Infarction and Improves Outcome after Systemic Administration. International journal of molecular sciences, 2019. 20(3): p. 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jung J-H, Fu X, and Yang PC, Exosomes Generated From iPSC-Derivatives: New Direction for Stem Cell Therapy in Human Heart Diseases. Circulation research, 2017. 120(2): p. 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lai RC, et al. , Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research, 2010. 4(3): p. 214–222. [DOI] [PubMed] [Google Scholar]
- 172.Arslan F, et al. , Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research, 2013. 10(3): p. 301–312. [DOI] [PubMed] [Google Scholar]
- 173.Vandergriff A, et al. , Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics, 2018. 8(7): p. 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yuan Y, et al. , Stem Cell-Derived Exosome in Cardiovascular Diseases: Macro Roles of Micro Particles. 2018. 9(547). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yuan Y, et al. , Stem Cell-Derived Exosome in Cardiovascular Diseases: Macro Roles of Micro Particles. Frontiers in pharmacology, 2018. 9: p. 547–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Guo D, et al. , Roles and Clinical Applications of Exosomes in Cardiovascular Disease. BioMed Research International, 2020. 2020: p. 5424281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fiandaca MS, et al. , Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer's & dementia : the journal of the Alzheimer's Association, 2015. 11(6): p. 600–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun R, et al. , Changes in the Morphology, Number, and Pathological Protein Levels of Plasma Exosomes May Help Diagnose Alzheimer's Disease. J Alzheimers Dis, 2020. 73(3): p. 909–917. [DOI] [PubMed] [Google Scholar]
- 179.Lim CZJ, et al. , Subtyping of circulating exosome-bound amyloid β reflects brain plaque deposition. Nature Communications, 2019. 10(1): p. 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Muraoka S, et al. , Proteomic and biological profiling of extracellular vesicles from Alzheimer's disease human brain tissues. Alzheimer's & Dementia, 2020. 16(6): p. 896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gill J, et al. , Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Injury, 2018. 32(11): p. 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cerri S, et al. , The Exosomal/Total α-Synuclein Ratio in Plasma Is Associated With Glucocerebrosidase Activity and Correlates With Measures of Disease Severity in PD Patients. Frontiers in cellular neuroscience, 2018. 12: p. 125–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu H, et al. , Potential Roles of Exosomes in Parkinson's Disease: From Pathogenesis, Diagnosis, and Treatment to Prognosis. Frontiers in cell and developmental biology, 2020. 8: p. 86–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jiang C, et al. , Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. Journal of Neurology, Neurosurgery & Psychiatry, 2020. 91(7): p. 720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lemprière S, Exosomal α-synuclein as a biomarker for Parkinson disease. Nature Reviews Neurology, 2020. 16(5): p. 242–243. [DOI] [PubMed] [Google Scholar]
- 186.Hayashi N, et al. , Proteomic analysis of exosome-enriched fractions derived from cerebrospinal fluid of amyotrophic lateral sclerosis patients. Neuroscience Research, 2020. 160: p. 43–49. [DOI] [PubMed] [Google Scholar]
- 187.Jo M, et al. , The role of TDP-43 propagation in neurodegenerative diseases: integrating insights from clinical and experimental studies. Experimental & Molecular Medicine, 2020. 52(10): p. 1652–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cao Z, et al. , α-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson's disease. Neurosci Lett, 2019. 696: p. 114–120. [DOI] [PubMed] [Google Scholar]
- 189.van Holten TC, et al. , Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One, 2013. 8(4): p. e62080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zheng D, et al. , The Role of Exosomes and Exosomal MicroRNA in Cardiovascular Disease. Frontiers in cell and developmental biology, 2021. 8: p. 616161–616161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kuwabara Y, et al. , Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet, 2011. 4(4): p. 446–54. [DOI] [PubMed] [Google Scholar]
- 192.Matsumoto S, et al. , Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res, 2013. 113(3): p. 322–6. [DOI] [PubMed] [Google Scholar]
- 193.Zhang Y-N, et al. , Extracellular Vesicle Proteins Associated with Systemic Vascular Events Correlate with Heart Failure: An Observational Study in a Dyspnoea Cohort. PloS one, 2016. 11(1): p. e0148073–e0148073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dekker M, et al. , Extracellular Vesicle cystatin c is associated with unstable angina in troponin negative patients with acute chest pain. PLoS One, 2020. 15(8): p. e0237036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Timmerman N, et al. , Pre-Operative Plasma Extracellular Vesicle Proteins are Associated with a High Risk of Long Term Secondary Major Cardiovascular Events in Patients Undergoing Carotid Endarterectomy. European Journal of Vascular and Endovascular Surgery, 2021. [DOI] [PubMed] [Google Scholar]
- 196.Cheow ES, et al. , Plasma-derived Extracellular Vesicles Contain Predictive Biomarkers and Potential Therapeutic Targets for Myocardial Ischemic (MI) Injury. Mol Cell Proteomics, 2016. 15(8): p. 2628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jin J and Menon R, Placental exosomes: A proxy to understand pregnancy complications. American journal of reproductive immunology (New York, N.Y. : 1989), 2018. 79(5): p. e12788–e12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Salomon C, et al. , Oxygen tension regulates glucose-induced biogenesis and release of different subpopulations of exosome vesicles from trophoblast cells: A gestational age profile of placental exosomes in maternal plasma with gestational diabetes mellitus. Placenta, 2015. 36(4): p. 488. [Google Scholar]
- 199.Truong G, et al. , Oxygen tension regulates the miRNA profile and bioactivity of exosomes released from extravillous trophoblast cells - Liquid biopsies for monitoring complications of pregnancy. PloS one, 2017. 12(3): p. e0174514–e0174514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mitchell MD, et al. , Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol, 2015. 213(4 Suppl): p. S173–81. [DOI] [PubMed] [Google Scholar]
- 201.Sarker S, et al. , Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. Journal of translational medicine, 2014. 12: p. 204–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pillay P, et al. , Placental exosomes and pre-eclampsia: Maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta, 2016. 46: p. 18–25. [DOI] [PubMed] [Google Scholar]
- 203.Biró O, et al. , Various levels of circulating exosomal total-miRNA and miR-210 hypoxamiR in different forms of pregnancy hypertension. Pregnancy Hypertens, 2017. 10: p. 207–212. [DOI] [PubMed] [Google Scholar]
- 204.Salomon C, et al. , Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation. The Journal of Clinical Endocrinology & Metabolism, 2017. 102(9): p. 3182–3194. [DOI] [PubMed] [Google Scholar]
- 205.Hosseini R, et al. , The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Molecular cancer, 2021. 20(1): p. 83–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Doglioni G, Parik S, and Fendt S-M, Interactions in the (Pre)metastatic Niche Support Metastasis Formation. Frontiers in Oncology, 2019. 9(219). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wan M, et al. , Tumor-derived exosomes (TDEs): How to avoid the sting in the tail. Medicinal Research Reviews, 2020. 40(1): p. 385–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Allenson K, et al. , High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Annals of oncology : official journal of the European Society for Medical Oncology, 2017. 28(4): p. 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Buscail E, et al. , CD63-GPC1-Positive Exosomes Coupled with CA19-9 Offer Good Diagnostic Potential for Resectable Pancreatic Ductal Adenocarcinoma. Translational Oncology, 2019. 12(11): p. 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dong L, et al. , Circulating Long RNAs in Serum Extracellular Vesicles: Their Characterization and Potential Application as Biomarkers for Diagnosis of Colorectal Cancer. Cancer Epidemiol Biomarkers Prev, 2016. 25(7): p. 1158–66. [DOI] [PubMed] [Google Scholar]
- 211.Sandfeld-Paulsen B, et al. , Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol, 2016. 10(10): p. 1595–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhao L, et al. , Isolation and Identification of miRNAs in exosomes derived from serum of colon cancer patients. Journal of Cancer, 2017. 8(7): p. 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yokoyama S, et al. , Clinical implications of carcinoembryonic antigen distribution in serum exosomal fraction-Measurement by ELISA. PLoS One, 2017. 12(8): p. e0183337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li J, et al. , GPC1 exosome and its regulatory miRNAs are specific markers for the detection and target therapy of colorectal cancer. J Cell Mol Med, 2017. 21(5): p. 838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sun B, et al. , Circulating exosomal CPNE3 as a diagnostic and prognostic biomarker for colorectal cancer. J Cell Physiol, 2019. 234(2): p. 1416–1425. [DOI] [PubMed] [Google Scholar]
- 216.Zeng Z, et al. , Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nature Communications, 2018. 9(1): p. 5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Meltzer S, et al. , Circulating Exosomal miR-141-3p and miR-375 in Metastatic Progression of Rectal Cancer. Translational oncology, 2019. 12(8): p. 1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Peng ZY, Gu RH, and Yan B, Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer. J Cell Biochem, 2018. [DOI] [PubMed] [Google Scholar]
- 219.Fu F, et al. , Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl Oncol, 2018. 11(2): p. 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Matsumura T, et al. , Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer, 2015. 113(2): p. 275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hannafon BN, et al. , Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res, 2016. 18(1): p. 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhou W, et al. , Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell, 2014. 25(4): p. 501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang X, et al. , Exosomal protein CD82 as a diagnostic biomarker for precision medicine for breast cancer. Mol Carcinog, 2019. 58(5): p. 674–685. [DOI] [PubMed] [Google Scholar]
- 224.Chen IH, et al. , Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc Natl Acad Sci U S A, 2017. 114(12): p. 3175–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rodríguez-Martínez A, et al. , Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy. Breast Cancer Res, 2019. 21(1): p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Taverna S, et al. , Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci Rep, 2017. 7(1): p. 3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li C, et al. , Clinical significance of PD-L1 expression in serum-derived exosomes in NSCLC patients. J Transl Med, 2019. 17(1): p. 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li L, et al. , FLI1 Exonic Circular RNAs as a Novel Oncogenic Driver to Promote Tumor Metastasis in Small Cell Lung Cancer. Clin Cancer Res, 2019. 25(4): p. 1302–1317. [DOI] [PubMed] [Google Scholar]
- 229.Castellanos-Rizaldos E, et al. , Exosome-Based Detection of EGFR T790M in Plasma from Non-Small Cell Lung Cancer Patients. Clin Cancer Res, 2018. 24(12): p. 2944–2950. [DOI] [PubMed] [Google Scholar]
- 230.Rodríguez M, et al. , Different exosome cargo from plasma/bronchoalveolar lavage in non-small-cell lung cancer. Genes Chromosomes Cancer, 2014. 53(9): p. 713–24. [DOI] [PubMed] [Google Scholar]
- 231.Sandfeld-Paulsen B, et al. , Exosomal Proteins as Diagnostic Biomarkers in Lung Cancer. J Thorac Oncol, 2016. 11(10): p. 1701–10. [DOI] [PubMed] [Google Scholar]
- 232.Niu L, et al. , Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci, 2019. 110(1): p. 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Allenson K, et al. , High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann Oncol, 2017. 28(4): p. 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Melo SA, et al. , Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature, 2015. 523(7559): p. 177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kimura H, et al. , CKAP4, a DKK1 Receptor, Is a Biomarker in Exosomes Derived from Pancreatic Cancer and a Molecular Target for Therapy. 2019. 25(6): p. 1936–1947. [DOI] [PubMed] [Google Scholar]
- 236.Bernard V, et al. , Circulating Nucleic Acids Are Associated With Outcomes of Patients With Pancreatic Cancer. Gastroenterology, 2019. 156(1): p. 108–118.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lux A, et al. , c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. Int J Mol Sci, 2019. 20(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Alegre E, et al. , Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clin Chim Acta, 2016. 454: p. 28–32. [DOI] [PubMed] [Google Scholar]
- 239.Alegre E, et al. , Study of circulating microRNA-125b levels in serum exosomes in advanced melanoma. Arch Pathol Lab Med, 2014. 138(6): p. 828–32. [DOI] [PubMed] [Google Scholar]
- 240.Matsuzaki K, et al. , MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget, 2017. 8(15): p. 24668–24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bijnsdorp IV, et al. , Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J Extracell Vesicles, 2013. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.McKiernan J, et al. , A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA oncology, 2016. 2. [DOI] [PubMed] [Google Scholar]
- 243.Naseri M, et al. , Tumor-derived exosomes: the next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology, 2020. 9(1): p. 1779991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hsu DH, et al. , Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J Immunother, 2003. 26(5): p. 440–50. [DOI] [PubMed] [Google Scholar]
- 245.Morse MA, et al. , A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. Journal of Translational Medicine, 2005. 3(1): p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Escudier B, et al. , Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. Journal of translational medicine, 2005. 3(1): p. 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Shan C, et al. , Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques. Cell Research, 2020. 30(8): p. 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bartleson JM, et al. , SARS-CoV-2, COVID-19 and the aging immune system. Nature Aging, 2021. 1(9): p. 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.de Candia P, et al. , T Cells: Warriors of SARS-CoV-2 Infection. Trends in immunology, 2021. 42(1): p. 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shrotri M, et al. , T cell response to SARS-CoV-2 infection in humans: A systematic review. PLoS One, 2021. 16(1): p. e0245532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Worthington EN and Hagood JS, Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. International journal of molecular sciences, 2020. 21(7): p. 2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Guo H, Su Y, and Deng F, Effects of Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Lung Diseases: Current Status and Future Perspectives. Stem Cell Reviews and Reports, 2021. 17(2): p. 440–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Börger V, et al. , International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy, 2020. 22(9): p. 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chen Y-S, et al. , Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci ji yi xue za zhi = Tzu-chi medical journal, 2019. 32(2): p. 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Andriolo G, et al. , Exosomes From Human Cardiac Progenitor Cells for Therapeutic Applications: Development of a GMP-Grade Manufacturing Method. Front Physiol, 2018. 9: p. 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]