Abstract
The functions of mammalian sleep remain unclear. Most theories suggest a role for non-rapid eye movement (NREM) sleep in energy conservation and in nervous system recuperation. Theories of REM sleep have suggested a role for this state in periodic brain activation during sleep, in localized recuperative processes and in emotional regulation. Across mammals, the amount and nature of sleep are correlated with age, body size and ecological variables, such as whether the animals live in a terrestrial or an aquatic environment, their diet and the safety of their sleeping site. Sleep may be an efficient time for the completion of a number of functions, but variations in sleep expression indicate that these functions may differ across species.
Saying that it is desirable to be well rested and that the body seeks lost sleep with a vigour comparable to or greater than that displayed for food or sex does not answer the question of the functional role of sleep. Why do we spend one-third of our lives asleep? Why has our body evolved to press us relentlessly to make up for lost sleep? Can we separate the drive for sleep, manifested in sleepiness, from the function of sleep, as we can separate hunger from the benefits of food consumption? Why do so many species habitually sleep much more than humans, and others much less, and how do species that sleep for only short periods accomplish the functions of sleep in less time? Why does the daily sleep amount decrease from birth to maturity in all species of terrestrial mammals? And why do we have two kinds of sleep, rapid eye movement (REM) and non-REM (NREM) sleep?
Sleep can be defined as a state of immobility with greatly reduced responsiveness, which can be distinguished from coma or anaesthesia by its rapid reversibility. An additional defining characteristic of sleep is that when it is prevented, the body tries to recover the lost amount. The existence of sleep ‘rebound’ after deprivation1 demonstrates that sleep is not simply a period of reduced activity or alertness regulated by circadian or ultradian rhythms, a phenomenon that can be seen even in non-sleeping organisms2–4.
The amplitude of the changes in brain metabolism and neuronal activity that occurs during sleep exceeds those which occur during most waking periods5–7. The argument that sleep serves a vital function is compelling. Sleep deprivation in rodents and flies can cause death more quickly than food deprivation8. Nevertheless, we must not assume that the effects of sleep loss are independent of the deprivation technique used or that sleep loss has equally dire effects in all animals9,10.
In this review we will consider the vast knowledge that has been gained about the physiological nature of sleep and sleep-control mechanisms, evidence from sleep-deprivation studies and the distribution of sleep across species in the context of theories of sleep function. These data support theories that suggest that sleep saves energy, keeps species from being active at inopportune times and reverses waking-induced changes in brain function. The evidence suggests distinct roles for REM and NREM sleep. It is also clear that sleep expression is adapted to ecological factors and may differ qualitatively across species.
Sleep-controlling brain regions in mammals
Neurophysiological studies have provided considerable information about the mechanisms controlling sleep states. These data can guide theories of sleep functions. Detailed reviews of the physiological control of sleep are available elsewhere11, but for the purposes of the current review, several aspects will be highlighted.
NREM sleep phenomena can be generated by the isolated forebrain12–14. Groups of sleep-active neurons have been discovered in the preoptic and basal forebrain regions (Fig. 1). These cells are maximally active during NREM sleep, and when stimulated will induce this state. Conversely, damage to these regions greatly reduces sleep. These neurons act through direct and indirect inhibitory projections to aminergic, cholinergic and hypocretinergic (also called orexinergic) neurons in the forebrain and brainstem. These and other neuronal groups maintain waking. The preoptic and anterior hypothalamic regions, within which most of these sleep-active neurons are embedded, have central roles in controlling the body and brain’s temperature15. Many sleep-active neurons are thermosensitive; when studied in tissue slices and in the intact brain they increase their activity at higher temperatures12. Heating of the preoptic regions increases NREM sleep.
Figure 1 |. Distribution of some key sleep-regulating neuronal populations plotted on a sagittal section of a rat brain97.

Circles indicate ‘REM sleep off’ neurons; purple represents serotonergic neurons (located on the midline), orange represents adrenergic or noradrenergic neurons, blue represents histaminergic neurons, red represents hypocretinergic (orexinergic) neurons. Squares indicate ‘sleep on’ neurons. The green star indicates ‘REM sleep on’ neurons. The area shaded in grey is both necessary and sufficient for REM sleep generation. The area shaded in yellow is both necessary and sufficient for NREM sleep generation. In the intact animal both REM sleep and NREM sleep involve interactions between brainstem and forebrain structures. Vlpo, ventrolateral preoptic area; Mpo, median preoptic.
REM sleep phenomena can be generated by the isolated brainstem, specifically by the pons and adjacent midbrain14. This region contains a subgroup of neurons that are maximally active during REM sleep. These ‘REM sleep on’ cells cause a complete loss of muscle tone in the postural muscles during REM sleep by triggering simultaneous inhibition of and withdrawal of excitation to motoneurons. They also have a crucial role in the regulation of REM sleep itself. When these neurons are activated, which can be accomplished by microinjecting acetylcholine agonists into specific regions of the pons, prolonged REM sleep periods are elicited16. Damage to these neurons greatly diminishes or prevents REM sleep for long periods14. Although the thermosensitivity of the REM sleep on cell populations has not been characterized, it has been shown that cooling of the isolated brainstem produces a marked increase in REM sleep amount17, just as heating of the forebrain produces a marked increase in NREM sleep12. This suggests that, in the intact animal, brainstem mechanisms triggering REM sleep may be facilitated by brainstem cooling or correlated metabolic changes.
The effects of localized temperature changes in brainstem and forebrain regions on sleep should not be confused with the effects of environmental temperature. Sleep is reduced at temperatures outside the thermoneutral zone, and REM sleep amounts are maximal at the upper levels within this zone15.
Neuronal activity across the sleep cycle
With the important exception of the comparatively small populations of sleep-active and REM sleep-active neurons described above, and other small groups of ‘REM sleep off’ neurons to be considered below, most brainstem neurons are maximally active during waking with movement and during REM sleep and are minimally active during NREM sleep14. In neocortical neurons, the decrease in activity during NREM sleep compared with active waking is smaller than that seen in the brainstem, but the neuronal activity pattern differs greatly from the REM sleep and waking pattern. The asynchronous activity of adjacent neocortical neurons in both REM sleep and waking changes to a rhythmic discharge, synchronized across large regions of the neocortex. This causes a summation of excitatory and inhibitory postsynaptic potentials, resulting in the high-voltage 2–12-Hz neocortical waves that are seen by electroencephalogram (EEG)18. These activity patterns are linked to maximal metabolic activity during waking and REM sleep, and minimal metabolic activity during NREM sleep in both the neocortex and the brainstem6,7.
If cortical and subcortical neuronal activity is similar during waking and REM sleep, why are these states so different? The differences between these two states, including the difference in muscle tone19 and the different levels of awareness of the environment, can presumably be attributed to the small number of neurons whose activity differs between these two states. Two groups of cells whose activity differs between waking and REM sleep are the REM on cells, discussed above, and the REM sleep off cells. The latter cells, which contain noradrenaline, epinephrine, serotonin, histamine or hypocretin, are continuously active during waking. They all have decreased activity during NREM sleep (this decrease is particularly marked for hypocretin cells, which may also be silent during quiet waking) and cease discharge during REM sleep14,20–23. Recent work suggests that the cessation of histamine neuron activity is linked to the loss of consciousness in sleep, whereas the cessation of noradrenaline neuron activity is linked to muscle tone suppression during sleep24.
Sleep studies in terrestrial mammals
To evaluate theories of REM and NREM sleep function, one must consider how sleep amounts differ across species. Daily sleep amounts vary substantially from mammal to mammal. Some animals, such as bats and opossums, sleep for 18–20 hours a day (Fig. 2). Others, such as the elephant and giraffe, sleep for as little as 3–4 hours a day. One might expect species in each mammalian order to have a similar sleep pattern because of their genetic, behavioural and anatomical similarities. This is not the case. Differences in order do not simply explain differences in sleep amounts25. Primates as a group do not have sleep characteristics that distinguish them from Rodentia, Insectivora or other orders. Humans, in particular, do not seem to have amounts or aspects of REM sleep or NREM sleep that distinguish them from other species, although they do have less sleep (more waking) than most omnivores (Fig. 2).
Figure 2 |. Sleep time in mammals.
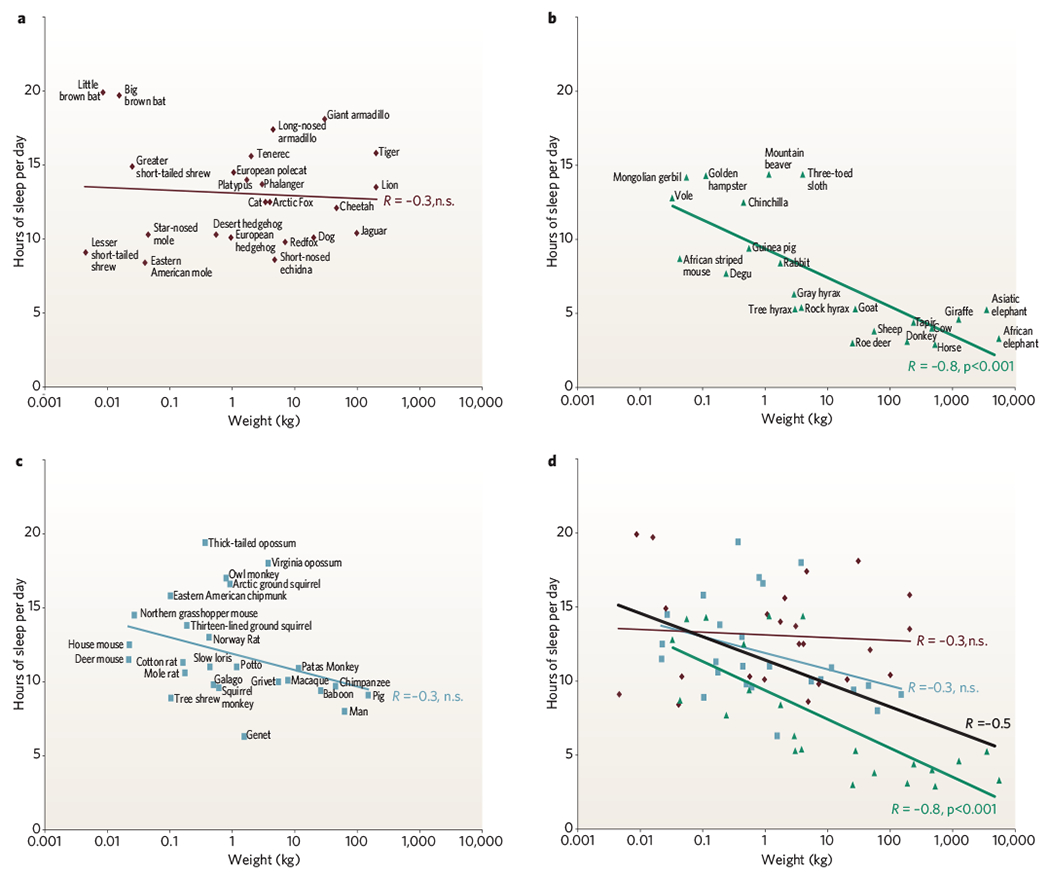
a, Carnivores are shown in dark red; b, herbivores are in green and c, omnivores in grey. Sleep times in carnivores, omnivores and herbivores differ significantly (P < 0.0002, F test, d.f. 2,68), with carnivore sleep amounts significantly greater than those of herbivores (P < 0.001, t-test, d.f. 24, 22). Sleep amount is an inverse function of body mass over all terrestrial mammals (black line). This function accounts for approximately 25% of the interspecies variance (d) in reported sleep amounts (regression of log weight against sleep amount, R = −0.5, P < 0.0001, n = 71). Herbivores are responsible for this relation because body mass and sleep time were significantly and inversely correlated in herbivores (R = −0.77, P < 0.001, d.f. 24), but were not in carnivores (R = −0.28, d.f. 24) or omnivores (R = −0.25, d.f. 25).
It is not easy to quantify sleep parameters throughout the animal kingdom and as a result all desired parameters are rarely measured. For example, arousal thresholds are rarely systematically measured as part of a phylogenetic sleep study. Evidence for homeostatic regulation of sleep (sleep rebound) is seldom sought. Most species have not been implanted with electrodes for monitoring muscle tone and other variables. Instead, estimates are often based on visual observations, with the observer forced to intuit the differences between quiet waking and sleep. Other factors such as temperature, light cycle, food and noise conditions, which all affect sleep, have often not been controlled for. The age and health of the animals observed can vary, particularly depending on whether observations are made on animals in the wild or in the zoo. Often, observations of only one or two individuals are the source of the reported sleep amount of a given species. In animals observed in the wild, the weight of the subject is often not known, and in many cases the typical adult body weight, brain weight and other anatomical and physiological parameters cannot be or have not been precisely determined. Despite these sources of noise, significant relationships between weight, sleep time and diet are apparent. Source data for this figure were mainly from ref. 25.
A species’ customary diet is correlated with sleep time. Daily sleep amounts are highest in carnivores, lower in omnivores and lowest in herbivores. Sleep time is inversely correlated with body mass in herbivores. This correlation is responsible for a significant overall correlation between body mass and sleep time in all mammals studied so far25; however, sleep time and body mass are not significantly correlated in carnivores or omnivores when they are evaluated separately (Fig. 2).
Another aspect of sleep strongly linked to body mass and brain size is the duration of the sleep cycle, that is, the average time taken to cycle from NREM sleep onset, through REM sleep, to waking. Small animals have shorter sleep cycles, and cycle times range from about 8 minutes in the short-tailed shrew (Blarina brevicauda) to 1.8 hours in the Asiatic elephant (Elephas maximus)25. The reason for this robust correlation is not known. Possible explanations include the inverse correlation of metabolic rate with body mass and brain mass, the thermal inertia of the brain and body, the time required for diffusion of substances through the brain parenchyma or the time required to complete a particular anabolic or catabolic biochemical task.
Most studies of mammalian sleep have been performed on placental (eutherian) or marsupial mammals. The third subclass of mammals is the monotremes, found in Australia and New Guinea. These egg-laying mammals have more genetic and physiological similarities to reptiles and birds than do other mammals and are thought to possess characteristics of the common mammalian ancestor26. Although an initial study of the echidna suggested that monotremes do not have REM sleep27, further observations indicated that they have an unusual form of this state. Both the echidna and platypus show evidence of brainstem activation during sleep, with the platypus displaying intense rapid eye, limb and bill movements periodically during sleep. However, the low-voltage neocortical EEG typically seen in eutherian and marsupial mammals during REM sleep is not consistently present during sleep in either the echidna or platypus during these activities28,29. Thus, these ‘primitive’ mammals seem to have a form of REM sleep that is mainly localized to the brainstem.
Sleep in marine mammals
All terrestrial mammals show relatively high-voltage neocortical EEG activity bilaterally during NREM sleep. By contrast, cetaceans (whales and dolphins) almost never have high-voltage slow waves in both hemispheres at the same time (Fig. 3; refs 30, 31). Manatees (Trichechus inunguis, a member of the order Sirenia) also have unihemispheric slow waves32. In all marine mammals studied to date, the eye contralateral to the brain hemisphere with slow waves is almost always closed while the other eye is almost always open. There have been no published reports documenting REM sleep in cetaceans, making them the only studied mammals in which this state has not been observed.
Figure 3 |. Unihemispheric slow waves in cetaceans.

Top, photos of immature beluga, adult dolphin and section of adult dolphin brain. Electroencephalogram (EEG) of adult cetaceans, represented here by the beluga, during sleep are shown. All species of cetacean so far recorded have unihemispheric slow waves30,31,98–100. Top traces show left and right EEG activity. The spectral plots show 1–3-Hz power in the two hemispheres over a 12-hour period. The pattern in the cetaceans contrasts with the bilateral pattern of slow waves seen under normal conditions in all terrestrial mammals, represented here by the rat (bottom traces). The brain photograph is from the University of Wisconsin, Michigan State, and the National Museum of Health Comparative Mammalian Brain Collections.
The bottlenose dolphin (Tursiops truncatus), when not floating or resting on the bottom, generally swims in a single direction (usually counterclockwise) even as the hemisphere with slow waves alternates. Some smaller cetacean species are rarely, if ever, immobile. They move and avoid obstacles 24 hours a day from birth until death, even during unihemispheric slow-wave activity. These animals may never exhibit the immobility that we use in terrestrial mammals to define the state of sleep33.
Slow (high-voltage 1–4-Hz) waves and spindle (high-voltage 8–12 Hz) waves are not by themselves conclusive evidence for sleep as defined at the beginning of this review. They have been linked to sleep because they generally accompany the behavioural signs of sleep in terrestrial mammals. However, even in terrestrial mammals, certain drugs can induce bilateral high-voltage EEG activity in individuals that are clearly awake34. Moreover, high-voltage neocortical activity is not a required indicator of sleep; a NREM sleep state with low-voltage neocortical activity has been documented in rodents35. REM sleep is characterized by a low-voltage EEG, indistinguishable from that of waking in many animals.
Studies of arousal threshold have not been performed on cetaceans across putative sleep–wake cycles. Only one study has looked for sleep rebound after EEG slow waves were prevented by disturbing cetaceans. Although some evidence for rebound was seen, the response was highly variable, with some animals showing little or no recovery of lost slow waves. The amount of slow waves after deprivation bore no consistent relation to the amount of slow-wave activity that had been lost36. Neuronal recording studies in terrestrial mammals suggest that the axial movements occurring in cetaceans during both normal swimming and swimming with unihemispheric slow waves are accompanied by activation of large regions of the brainstem reticular formation. This is quite different from the great reduction in brainstem reticular activity that characterizes NREM sleep in terrestrial mammals14,37. Cetaceans deftly avoid obstacles during this constant motion, suggesting accurate bilateral processing of sensory information.
If sensory and motor systems do not show typical sleep inactivation, if behavioural and sleep rebound evidence for sleep debt is weak, do cetaceans sleep as conventionally defined? Certainly further work is necessary to determine which, if any, neurochemical and neurophysiological aspects of sleep, other than unilateral neocortical slow waves and eye closure, are preserved in cetaceans and might constitute the ‘essence’ of sleep. Alternatively, we may need to revise our assumption that sleep is fundamentally similar in cetaceans and terrestrial mammals and focus on how cetaceans can dispense with some of the most readily detectable aspects of sleep.
Postpartum sleep behaviour in cetaceans
Further evidence for the unique properties of ‘sleep’ in cetaceans is the near absence of sleep behaviour in neonates and a postpartum reduction in sleep behaviour in their mothers38. All terrestrial mammals have minimal activity and maximal total sleep and REM sleep amounts at birth, with sleep gradually decreasing and activity gradually increasing to adult levels as the animals grow to maturity39. This is not the pattern in cetaceans. We have found that killer whales (Orcinus orca) and dolphins have minimal amounts of sleep behaviour (that is, immobility or eye closure) at birth, with sleep behaviour slowly increasing to adult levels over a period of months. This minimal amount of sleep behaviour occurs during the period of most rapid growth of the body and brain for the newborn, during a period of bonding to the mother and learning how to nurse, find food, avoid predators and swim efficiently.
We have hypothesized that the continuous activity of cetaceans has adaptive value in allowing the neonate, which is much less insulated by body fat than the adult, to thermoregulate in cold ocean water. The suppression of sleep behaviour also allows the neonate to swim with and be protected by its mother during development. Unlike the terrestrial environment, there are few warm, safe places to sleep in the ocean. As the animal gains mass and blubber and approaches adult size, adult-like ‘sleep’ or rest behaviour, including periods of immobility, emerges.
Equally striking is the near absence of typical sleep behaviour in the mother during the postpartum period. Both mother and calf go without substantial amounts of immobility and the eye closure linked to unihemispheric slow waves, for periods that greatly exceed those of sleep deprivation that are reported to kill rats8. Moreover, neither the mother nor the calf shows any rebound increase in the amount of sleep behaviour after this period.
The suppression of sleep behaviour seen in cetaceans after birth is analogous to a reduction in sleep that has been seen during the migration season in one bird species studied, the white crowned sparrow. This sleep reduction was accompanied by high levels of alertness and was not followed by sleep rebound. It has been argued that this phenomenon may be similar to mania seen in humans, which is also accompanied by a major reduction in sleep40.
Fur seals
Fur seals and other otariids (seals with external ear flaps) exhibit another difference from the sleep of terrestrial mammals. When in the water, fur seals show a pattern of unihemispheric slow waves similar to that of cetaceans. However, fur seal sleep, unlike that of cetaceans, is accompanied by striking motor asymmetries. The flipper contralateral to the hemisphere with slow waves is immobile, the flipper contralateral to the hemisphere with low-voltage activity paddles to maintain the animal’s position, and the whisker contralateral to the hemisphere with low-voltage activity is used to monitor the seal’s position in the water. When slow-wave activity shifts to the opposite side, so do the motor asymmetries. When in the water, the fur seal has an extremely small amount of REM sleep, and may go without any REM sleep for a week or two. Surprisingly, when the fur seal moves onto land after spending weeks in the water, a change in its sleep structure occurs immediately. Unihemispheric slow waves largely disappear, and bilateral NREM and REM sleep are present, similar in amount and form to those of comparably sized land mammals. A remarkable feature of the transition from water to land is that fur seals immediately adopt their land sleep amounts without any evidence of REM sleep rebound, despite the absence or near absence of REM sleep while in the water41.
Theories of NREM sleep function
There is no shortage of theories to explain the functions of REM and NREM sleep. Recent reviews have critically evaluated many of these theories8,15,42,43. There are several themes that can be seen in some of these theories and for which the data reviewed above provide support, and other theories that are inconsistent with these data. In general, most theories have assumed that sleep serves the same function in all animals, although most data supporting such theories have been derived from observations limited to just a few species of terrestrial mammal.
Neocortical maintenance
A recurrent theme in theories of sleep function is that sleep time is determined by neuronal activity in the neocortex. Although neocortical EEG changes are the most easily observed electrical correlate of sleep because they are recordable from scalp electrodes in humans and from electrodes placed on the surface of the cortex in other animals, sleep produces large changes in the rates and patterns of neuronal activity in nearly all brain regions, not just in the neocortex. Cortical EEG phenomena are controlled by and reflect activity in thalamic, hypothalamic and brainstem reticular regions. The cellular activity changes underlying the changes in neocortical EEG include calcium fluxes into and hyperpolarization of neocortical and thalamic neurons that are synchronized in large populations, producing high-voltage brain waves18,44. However, neocortical size does not correlate positively with sleep amount. Both total brain weight and encephalization correlate poorly and negatively with total NREM and REM sleep amounts25. The elephant, which has the largest neocortex of any terrestrial mammal, has one of the smallest amounts of sleep. Conversely, the rat and the platypus, which have smooth cortices with small total neocortical volumes, have extremely large amounts of sleep, with the platypus having more REM sleep than any other animal studied so far (Fig. 4; ref. 29).
Figure 4 |. Size of the neocortex does not correlate positively with daily sleep amount.

Sleep amount is not proportional to the relative size of the cerebral cortex or to the degree of encephalization, as illustrated by these two examples. Brain photographs are from the University of Wisconsin, Michigan State, and the National Museum of Health Comparative Mammalian Brain Collections.
Although neocortical size does not seem to be a major determinant of either NREM or REM sleep amounts, recent work has indicated that neocortical activity during sleep may be altered by prior waking activity. Some such changes dissipate with continued waking44–46, suggesting that localized recuperative processes may take place during either waking or sleep in systems projecting to, or within, the neocortex.
Effects of sleep deprivation
Sleep restriction leads to a feeling of sleepiness and, depending on the nature of the sleep lost, to increases in the amplitude of the brain-wave signals that characterize NREM sleep and the amplitudes and frequencies of the eye movements and twitches that characterize REM sleep, when sleep is allowed47,48. Sleep loss causes intrusions of sleep into waking that can displace behaviours that have obvious survival value. The fact that sleep debt can be accumulated suggests that sleep serves important functions that require some portion of the missed sleep amount to be made up. Although motivated humans can overcome sleepiness for short periods, they cannot perform at high levels for sustained periods49,50.
The signs of long-term sleep deprivation, including skin lesions, hyperthermia followed by hypothermia, increased food intake and death, that have been noted in the rat, the subject whose response has been most thoroughly investigated, do not occur in the rat or cat even with total, long-term decortication51, consistent with the lack of a positive correlation between cortical size and sleep time noted above. Many of these signs of sleep loss can be seen after hypothalamic damage and concomitant abnormal functioning of the endocrine and immune systems52.
Energy conservation
Sleep may be adaptive because it conserves energy and suppresses behaviour across portions of the 24-hour day, just as hibernation does across certain seasons53. Large herbivores may have evolved reduced sleep amounts because they are more vulnerable to predators than small herbivores54. A second hypothesis is that these grazing animals may need to spend more time awake in order to eat because of the low calorific density of their food. A complementary hypothesis is that small herbivores and other mammals may need to maximize sleep amounts to conserve energy because their relatively high ratio of surface area to body mass makes it costly to maintain their body temperature. Retreating to a warm, protected nest may minimize this cost. A striking feature of sleep in animals with small daily sleep amounts, such as many herbivores, is that sleep depth, as judged by EEG and sensory response threshold, seems to be less than that in animals requiring more sleep. In other words, animals with low sleep amounts do not seem to ‘make up’ for low sleep quantity by sleeping more ‘deeply’. This contrasts with the raised arousal thresholds and increased amplitudes of low-frequency brain-wave activity shown in individual animals after sleep deprivation55,56.
Energy conservation may be particularly important in newborns. Their high ratio of surface area to body mass makes the energy conservation achieved by sleep highly adaptive. Furthermore, animals that are immature at birth benefit from the sleep-induced reduction in exposure to danger. When body size increases and sensory-motor systems mature, young animals derive greater benefits from waking activities, consistent with the developmental decrease in sleep time.
Body mass, metabolism and sleep control
One of the best-established relationships in mammalian biology is the inverse link between body mass and mass-specific metabolic rate. Small animals have high metabolic rates; large animals have low metabolic rates. Brain metabolic rate is correlated with body metabolic rate57. Elevated metabolism is linked to a number of biochemical changes, several of which have been linked to sleep control.
Sleep time may be related to defence against oxidative stress, particularly in, but not limited to, herbivores. A high metabolic rate results in the generation of high levels of reactive oxygen species (ROS) by mitochondria. This ROS generation has been linked to ageing, producing a wrinkled, arthritic, demented mouse by two years of age, whereas larger animals such as humans do not experience such effects of ageing until they are 70–80 years old. Previous studies challenging the phylogenetic relationship between sleep time and metabolic rate have used the partial correlation statistical approach. This statistical procedure is confounded when used with highly correlated variables such as metabolic rate and body mass.
We and others have shown that sleep deprivation in the rat is accompanied by increased oxidative stress and evidence of membrane disruption in the hippocampus, subcortical brain regions and peripheral tissues58–60. There were no such changes in the neocortex59,61. An interesting aspect of our findings was that the most marked changes occurred in a brain region with the highest rate of protein synthesis, and presumably with one of the highest rates of ROS generation, the supraoptic nucleus of the hypothalamus58. We also saw changes in the brainstem, hippocampus and hypothalamus as a whole59. We hypothesize that, all other things being equal, higher metabolic rates in the brain require longer periods of sleep to interrupt ROS-induced damage to brain cells, facilitate the synthesis and activities of molecules that protect brain cells from oxidative stress, allow sufficient time for the repair or replacement of essential cellular components in neurons and glia62 and deal with other biochemical consequences of waking metabolic activity. This would account for an inverse relationship between body mass and sleep time.
One may hypothesize that the ‘ratio’ of the energy conservation benefit of sleep to the waking metabolic-activity-derived need for sleep for brain recuperation varies across species. Carnivores and omnivores, which tend to have more sleep than predicted on the basis of body mass alone, may make more use of the energy-conservation aspects of sleep because their generally safe sleep places63 and ability to eat meals with high calorific density may make continuous activity unnecessary. In such a situation, reproductive fitness might best be served by energy conservation, which would reduce the need for hunting, aid nurturing of the young and speed development. This additional sleep time would obscure any underlying sleep requirement that is linked to metabolic rate.
Protein synthesis in the brain is increased during slow-wave sleep64. Recent work suggests another role for sleep. New neurons are generated in adult animals in the olfactory bulb, the subventricular zone lining the lateral ventricles, and in the subgranular cell layer of the dentate gyrus of the hippocampus, in a process that produces functional neurons in 3–4 weeks. This neurogenesis is facilitated by exercise and blocked by stress. Short-term (2–3-day) total sleep deprivation, even when other forms of stress are controlled for, also blocks subsequent proliferation of cells in the dentate gyrus65. Thus, sleep may have a general role in allowing or facilitating neurogenesis.
Several substances whose activities or levels may vary with overall metabolic activity in the brain have been proposed to have roles in sleep control or function. One hypothesis was that brain glycogen is depleted during waking and restored during sleep66,67; however, subsequent work showed that this effect did not occur in all strains of mice, suggesting that this could not be a general regulatory mechanism or function of sleep68. Cytokines such as interleukin-1 have been implicated in sleep control69. Other substances, including adenosine, prostaglandin D2, a muramyl dipeptide, delta sleep inducing peptide, corticostatin, growth-hormone-releasing hormone, oxidized glutathione, uridine, tumour necrosis factor-alpha, oleamide, cortistatin, cholecystokinin, insulin, nitric oxide69 and neuropeptide S70, have significant effects on sleep. The relative importance of each of these ‘sleep factors’, the pathways by which they interact with each other and the way in which they might act on sleep-triggering mechanisms remain to be more fully elucidated.
Theories of REM sleep function
REM sleep, the state in which our most vivid dreams occur, has inspired a multitude of functional theories. The identification of the ‘dream state’ as a periodic physiological process during sleep71 has encouraged the addition of physiological and psychological theories to the more mystical theories of the ancients.
REM sleep is ‘paradoxical’ in the sense that although an animal in REM sleep is behaviourally asleep, brain metabolic and neuronal activity are high, respiration and heart rate are variable, rapid eye movements and twitches of the extremities occur and males frequently develop erections72. These phenomena and the vivid dreams that humans report upon awakening from REM sleep have made the function of this state particularly mysterious and intriguing. Although behavioural immobility and reduced overall body metabolic rate relative to active waking are maintained, why has this state evolved when continued NREM sleep, with its reduction in brain metabolic activity, would seem to be more efficient at achieving the recuperative and energy-saving effects of sleep?
Memory consolidation
The idea that either REM or NREM sleep is ‘absolutely required’ for memory consolidation has received much attention recently. I and others have reviewed this issue elsewhere and concluded that this is unlikely to be the case. Certainly disturbed sleep is not conducive to concentration and learning, but an essential role for sleep in memory consolidation remains unproven43,73,74.
REM sleep and development
REM sleep amount is positively correlated with total sleep amount and negatively correlated with body weight. However, if one statistically controls for body weight or brain weight, REM sleep amount is most strongly correlated with immaturity at birth25. Altricial mammals, those that are immature at birth, tend to have more REM sleep than mammals that are mature at birth, or precocial. This tendency is marked in the neonatal period. But perhaps more remarkable is that altricial animals continue to have more REM sleep as adults. The platypus has 8 hours of REM sleep per day as an adult and the neonate cannot thermoregulate, locomote, acquire food or defend itself at birth and lives attached to its mother. The ferret, likewise, is immature at birth, and the adult has over 6 hours of REM sleep per day. By contrast, the guinea pig has only 1 hour of REM sleep per day as an adult75. The guinea pig is born with teeth, claws, fur and open eyes; it thermoregulates at birth, locomotes within an hour of birth and eats solid food within a day of birth. Similarly, the sheep and giraffe are relatively mature at birth and have little REM sleep (less than 1 hour per day) at maturity25.
Although the nature of sleep states early in the development of the rat differs from adult patterns, the brainstem mechanisms generating REM sleep are present at birth76,77. The extremely high levels of REM sleep seen at birth, followed by a slow decrease to adult levels seen in many terrestrial mammals, must be an important clue to its function. This time course, combined with the observation that neuronal activity levels are high during REM sleep, led to the hypothesis that this sleep state is involved in the development of the brain. Wiesel and Hubel discovered that reducing light input into one eye for a period of several days in neonates led to a reduction in the number of cells in the visual system receiving input from that eye. This reduction persisted into adulthood, long after normal visual input had been restored. Monocular light deprivation during the ‘critical’ neonatal period also caused a reduction in the thickness of the lateral geniculate layers innervated by the closed eye. Others subsequently found that when animals were also REM sleep deprived during the critical period of susceptibility, this shrinkage was accelerated78. This finding indicates that the activity of the visual system known to occur in REM sleep normally compensates for any asymmetrical, abnormal or absent input, preventing processes that prune away unused connections during development. One can imagine that REM sleep serves this function for other sensory systems and perhaps for motor systems as well, given the intense central motor activation that occurs during this state (whose peripheral expression is blocked by the inhibition of motoneurons)14,19. As long as the extended NREM sleep of neonates is interrupted by the increased neuronal activity of REM sleep, neuronal development proceeds according to genetic programmes79.
REM sleep in adults
If we accept that the large amount of REM sleep early in life serves to maintain or establish brain connections during crucial periods of development, what is the function of this state later in life? One idea that has been proposed repeatedly is that REM sleep stimulates the adult brain during sleep to reverse the effects of NREM sleep on immediately subsequent waking behaviour. Animals typically awaken spontaneously from REM sleep80. Animals that are awakened from NREM sleep have poor sensory-motor function compared with those awakened from REM sleep81. Awakening in a more alert state would convey a substantial selective advantage.
In humans, the duration of REM sleep episodes progressively increases throughout the sleep period and is maximal at the expected time of awakening. The initial REM sleep period of the night may last only 5–10 minutes, whereas the last REM sleep period may last more than 25 minutes. The intensity of REM sleep, as measured by the density of eye movements and twitches, the prevalence of erections in males and the vividness of dream reports also increases as the night progresses. REM sleep amounts are maximal near the nadir of the brain and core body temperature cycles. And temperature in several brain regions increases during REM sleep82,83, even though the regulation of body temperature is largely suppressed during REM sleep15. One would not expect an increase in duration and intensity of REM sleep across the sleep period if REM sleep intensity were linked to one or more aspects of the previous waking episode. If a strong relationship with previous waking existed, one would expect to see maximal REM sleep intensity and duration in the early part of the night, alternating with NREM sleep, which is most intense at this time. When ambient temperature is outside the thermoneutral range, REM sleep is suppressed more strongly than is NREM sleep15, suggesting that the preservation of NREM sleep is of higher priority than that of REM sleep.
The absence or very small amounts of REM sleep in marine mammals displaying unihemispheric sleep with brainstem-regulated motor activity supports the hypothesis that the stimulation of brainstem-activating systems is an important function of REM sleep. In these animals, the presence of continuous motor activity would be expected to maintain a high level of metabolic activity in brainstem structures 24 hours a day, eliminating any need for REM sleep activation of these systems. Similarly, the manifestation of REM sleep in monotremes as a largely brainstem state, without marked neocortical activation, suggests that REM sleep may have evolved as a state of brainstem activation, with cortical stimulation functions added later in evolution. The cold-induced increase in REM sleep amount in the isolated brain-stem17, the increased REM sleep amount at the minimum of the circadian brain and body temperature cycles and the increase in the temperatures of brain regions during REM sleep82,83 are consistent with this brainstem-activation hypothesis.
Surprisingly, in humans, one can increase the amount of REM sleep and percentage of total sleep time devoted to this state simply by extending sleep time. Furthermore, this increased REM sleep time does not seem to produce a homeostatic reduction in REM sleep time during the subsequent night42,84. Although anxiety can decrease the amount of REM sleep85, acute stress can greatly increase REM sleep time in the rat, despite the absence of sleep loss during the previous stress86, suggesting that under some conditions REM sleep ‘rebound’ may be related to the emotional or autonomic concomitants of the REM sleep-deprivation procedure rather than the loss of REM sleep itself.
The proposed functions of REM sleep in adults are consistent with the lack of any easily detectable cognitive or physiological symptoms in humans in whom REM sleep has been suppressed for months or years by monoamine oxidase inhibitors or brain lesions43. One might hypothesize that these individuals would be less alert upon waking, a change that would have minimal survival implications in most contemporary humans but might have significant effects upon reproductive success in other animals.
Regulation of monoaminergic systems
The linked cessation of activity in noradrenaline, serotonin, histamine and hypocretin cells that occurs during normal REM sleep, and to a lesser extent during NREM sleep, suggests another possible function for sleep. Sleep may resensitize these REM off or sleep off transmitter systems, which are tonically active during waking, by increasing the quantities and activities of their synthetic enzymes, transporter mechanisms and receptors87,88. Three studies favour a role for REM sleep in the maintenance of central noradrenergic receptors, whereas one study questions this role. If this hypothesis were valid, it seems clear that the nature of such resensitization would be specific for particular receptor types and brain regions89–92.
REM sleep deprivation has antidepressive effects. It may be that a normal function of REM sleep is to dampen activity and emotional expression42,93 by causing the changes in monoaminergic systems proposed above. These systems are well known to be involved in emotional regulation, and most antidepressive medications act through effects on monoaminergic systems and suppress REM sleep.
REM sleep deprivation causes an acceleration of body heat loss during subsequent waking in the rat94,95. REM sleep itself is a state in which thermoregulation is attenuated96, suggesting that recuperative changes in thermoregulatory control mechanisms may occur during REM sleep periods, perhaps through the maintenance, repair or sensitization of peripheral monoaminergic control mechanisms, in much the same way that an improvement in the central functioning of these systems has been proposed to take place during REM sleep. The unique thermoregulatory challenges faced by cetaceans may also be a factor in the apparent absence of significant amounts of REM sleep in this order. Although a high level of heat generation is vital to maintaining their body temperature, sea temperature varies little over the 24-hour day, making the ‘recalibration’ proposed for thermoregulatory systems during their relative suspension in REM sleep less necessary8.
Future directions
Sleep probably has multiple functions for the brain and body. An important task will be the identification of which of the hypothesized functions may only be achieved during sleep, and which may be executed during both waking and sleep, with sleep being a more efficient time for their accomplishment. It also remains to be determined which, if any, of the proposed functions are universal across mammalian species and across the lifespan, and which may be limited to particular species or phases of development.
Acknowledgements
Supported by NIH and NSF and DARPA. I thank O. Lyamin for the beluga and dolphin photo and the graph of beluga sleep, and A. Siegel, L. Boehmer, R. Nienhuis, A. Rechtschaffen, I. Tobler, C. Heller, S. Ridgway, J. Horne, D. McGinty, C. Amlaner and J. Lesku for very helpful comments.
Footnotes
The author declares no competing interests.
References
- 1.Dinges DF, Rogers NL & Baynard MD in Principles and Practice of Sleep Medicine Vol. 4 (eds Kryger MH, Roth T & Dement WC) 67–76 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 2.Huber R et al. Sleep homeostasis in Drosophila melanogaster. Sleep 27, 628–639 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Czeisler CA, Buxton O & Khalsa SBS in Principles and Practice of Sleep Medicine Vol. 4 (eds Kryger MH, Roth T & Dement WC) 375–394 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 4.Bell-Pedersen D et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nature Rev. Genet 6, 554–556 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everson CA, Smith CB & Sokoloff L Effects of prolonged sleep deprivation on local rates of cerebral energy metabolism in freely moving rats. J. Neurosci 14, 6769–6778 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nofzinger EA et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am. J. Psychiatry 161, 2126–2128 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Maquet P et al. Regional organisation of brain activity during paradoxical sleep (PS). Arch. Ital. Biol 142, 413–419 (2004). [PubMed] [Google Scholar]
- 8.Rechtschaffen A Current perspectives on the function of sleep. Perspect. Biol. Med 41, 359–390 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Cirelli C et al. Reduced sleep in Drosophila Shaker mutants. Nature 434, 1087–1092 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Kume K, Kume S, Park SK, Hirsh J & Jackson FR Dopamine is a regulator of arousal in the fruit fly. J. Neurosci 25, 7377–7384 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kryger MH, Roth T & Dement WC Principles and Practice of Sleep Medicine (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 12.McGinty DJ & Szymusiak RS in Principles and Practice of Sleep Medicine Vol. 4 (eds Kryger MH, Roth T & Dement WC) 169–184 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 13.Saper CB, Chou TC & Scammell TE The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 24, 726–731 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Siegel JM in Principles and Practice of Sleep Medicine Vol. 4 (eds Kryger MH, Roth T & Dement WC) 120–135 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 15.Heller HC in Principles and Practice of Sleep Medicine Vol. 4 (eds Kryger MH, Roth T & Dement WC) 292–304 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 16.Coleman CG, Lydic R & Baghdoyan HA M2 muscarinic receptors in pontine reticular formation of C57BL/6J mouse contribute to rapid eye movement sleep generation. Neuroscience 126, 821–830 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Jouvet M, Buda C, Debilly G, Dittmar A & Sastre JP Glycine immunoreactive neurons in the medulla oblongata in cat. C. R. Acad. Sci. III 306, 69–73 (1988). [PubMed] [Google Scholar]
- 18.Steriade M in Principles and Practice of Sleep Medicine Vol. 4 (eds Kryger MH, Roth T & Dement WC) 101–119 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 19.Chase MH & Morales FR in Principles of Sleep Medicine Vol. 4 (eds Kryger MH, Roth, & Dement WC) 154–168 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 20.Aston-Jones G & Bloom FE Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J.Neurosci 1, 876–886 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs BL & Azmitia EC Structure and function of the brain serotonin system. Physiol. Rev 72, 165–229 (1992). [DOI] [PubMed] [Google Scholar]
- 22.McGinty DJ & Harper RM Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 101, 569–575 (1976). [DOI] [PubMed] [Google Scholar]
- 23.Mileykovskiy BY, Kiyashchenko LI & Siegel JM Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46, 787–798 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John J, Wu M-F, Boehmer LN & Siegel JM Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron 42, 619–634 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zepelin H, Siegel JM & Tobler I in Principles and Practice of Sleep Medicine Vol. 4(eds Kryger MH, Roth T & Dement WC) 91–100 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 26.Grutzner F et al. In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes. Nature 432, 913–917 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Allison T & Van Twyver H Electrophysiological studies of the echidna, Tachyglossus aculeatus. II. Dormancy and hibernation. Arch. Ital. Biol 110, 145–184 (1972). [PubMed] [Google Scholar]
- 28.Siegel JM, Manger P, Nienhuis R, Fahringer HM & Pettigrew J The echidna Tachyglossus aculeatus combines REM and non-REM aspects in a single sleep state: implications for the evolution of sleep. J. Neurosci 16, 3500–3506 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel JM et al. Sleep in the platypus. Neuroscience 91, 391–400 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukhametov LM, Supin AY & Polyakova IG Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res. 134, 581–584 (1977). [DOI] [PubMed] [Google Scholar]
- 31.Lyamin OI, Mukhametov LM & Siegel JM Relationship between sleep and eye state in Cetaceans and Pinnipeds. Arch. Ital. Biol 142, 557–568 (2004). [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhametov LM, Lyamin OI, Chetyrbok IS, Vassilyev AA & Diaz RP Sleep in an Amazonian manatee, Trichechus inunguis. Experientia 48, 417–419 (1992). [DOI] [PubMed] [Google Scholar]
- 33.Mukhametov LM, Lyamin OI, Shpak OV, Manger P & Siegel JM Swimming styles and their relationship to rest and activity states in captive Commerson’s dolphins. Proc. 14th Biennial Conference on the Biology of Marine Mammals 152 (2002). [Google Scholar]
- 34.Vanderwolf CH & Baker GB Evidence that serotonin mediates non-cholinergic neocortical low voltage fast activity, non-cholinergic hippocampal rhythmical slow activity and contributes to intelligent behavior. Brain Res. 374, 342–356 (1986). [DOI] [PubMed] [Google Scholar]
- 35.Bergmann BM, Winter JB, Rosenberg RS & Rechtschaffen A NREM sleep with low-voltage EEG in the rat. Sleep 10, 1–11 (1987). [DOI] [PubMed] [Google Scholar]
- 36.Oleksenko AI, Mukhametov LM, Polykova IG, Supin AY & Kovalzon VM Unihemispheric sleep deprivation in bottlenose dolphins. J. Sleep Res 1, 40–44 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Siegel JM & Tomaszewski KS Behavioral organization of reticular formation: studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements. J. Neurophysiol 50, 696–716 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyamin O, Pryaslova J, Lance V & Siegel J Animal behaviour: continuous activity in cetaceans after birth. Nature 435, 1177 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carskadon MA & Dement WC in Principles and Practice of Sleep Medicine Vol. 4 (eds Kryger MH, Roth T & Dement WC) 13–23 (Elsevier Saunders, Philadelphia, 2005). [Google Scholar]
- 40.Rattenborg NC et al. Migratory sleeplessness in the white-crowned sparrow (Zonotrichia leucophrys gambelii). PLoS Biol. 2, E212 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyamin OI, Oleksenko AI, Polyakova IG & Mukhametov LM Paradoxical sleep in northern fur seals in water and on land. J. Sleep. Res 5 (suppl.), 130–130 (1996). [Google Scholar]
- 42.Horne JA REM sleep — by default? Neurosci. Biobehav. Rev 24, 777–797 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Siegel JM The REM sleep-memory consolidation hypothesis. Science 294, 1058–1063 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber R, Ghilardi MF, Massimini M & Tononi G Local sleep and learning. Nature 430, 78–81 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Krueger JM, Obal FJ & Fang J Why we sleep: a theoretical view of sleep function. Sleep Med. Rev 3, 119–129 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Vyazovskiy VV, Welker E, Fritschy JM & Tobler I Regional pattern of metabolic activation is reflected in the sleep EEG after sleep deprivation combined with unilateral whisker stimulation in mice. Eur. J. Neurosci 20, 1363–1370 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Achermann P & Borbely AA Mathematical models of sleep regulation. Front. Biosci 8, s683–s693(2003). [DOI] [PubMed] [Google Scholar]
- 48.Verret L, Leger L, Fort P & Luppi PH Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur. J. Neurosci 21, 2488–2504 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Binks PG, Waters WF & Hurry M Short-term total sleep deprivation does not selectively impair higher cortical functioning. Sleep 22, 328–334 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Balkin TJ et al. On the importance of countermeasures in sleep and performance models. Aviat. Space Environ. Med 75, A155–A157(2004). [PubMed] [Google Scholar]
- 51.Villablanca JR Counterpointing the functional role of the forebrain and of the brainstem in the control of the sleep-waking system. J. Sleep Res 13, 179–208 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Everson CA & Crowley WR Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am. J. Physiol. Endocrinol. Metab 286, E1060–E1070(2004). [DOI] [PubMed] [Google Scholar]
- 53.O’Hara BF et al. Gene expression in the brain across the hibernation cycle. J. Neurosci 19, 3781–3790 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lima SL, Rattenborg NC, Lesku JA & Amlaner CJ Sleep under the risk of predation. Anim. Behav 70, 723–736 (2005). [Google Scholar]
- 55.Merrick AW & Scharp DW Electroencephalography of resting behavior in cattle, with observations on the question of sleep. Am. J. Vet. Res 32, 1893–1897 (1971). [PubMed] [Google Scholar]
- 56.Klemm WR Sleep and paradoxical sleep in ruminants. Proc. Soc. Exp. Biol. Med 121, 635–638 (1966). [DOI] [PubMed] [Google Scholar]
- 57.Turner N, Else PL & Hulbert AJ An allometric comparison of microsomal membrane lipid composition and sodium pump molecular activity in the brain of mammals and birds. J. Exp. Biol 208, 371–381 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Eiland MM et al. Increases in amino-cupric-silver staining of the supraoptic nucleus after sleep deprivation. Brain Res. 945, 1–8 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramanathan L, Gulyani S, Nienhuis R & Siegel JM Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport 13, 1387–1390 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Everson CA, Laatsch CD & Hogg N Antioxidant defense responses to sleep loss and sleep recovery. Am. J. Physiol. Regul. Integr. Comp. Physiol 288, R374–R383(2005). [DOI] [PubMed] [Google Scholar]
- 61.Gopalakrishnan A, Ji LL & Cirelli C Sleep deprivation and cellular responses to oxidative stress. Sleep 27, 27–35 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Zimmerman JE, Mackiewicz M, Galante RJ et al. Glycogen in the brain of Drosophila melanogaster: diurnal rhythm and the effect of rest deprivation. J. Neurochem 88, 32–40 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Allison T & Cicchetti DV Sleep in mammals: ecological and constitutional correlates. Science 194, 732–734 (1976). [DOI] [PubMed] [Google Scholar]
- 64.Nakanishi H et al. Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. Eur. J. Neurosci 9, 271–279 (1997). [DOI] [PubMed] [Google Scholar]
- 65.Guzman-Marin R et al. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J. Physiol 549, 563–571 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benington JH & Heller HC Restoration of brain energy metabolism as the function of sleep. Prog. Neurobiol 45, 347–360 (1995). [DOI] [PubMed] [Google Scholar]
- 67.Kong J et al. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J. Neurosci 22, 5581–5587 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franken P, Gip P, Hagiwara G, Ruby NF & Heller HC Changes in brain glycogen after sleep deprivation vary with genotype. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R413–R419 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Opp MR & Krueger JM Interleukin-1 is involved in responses to sleep deprivation in the rabbit. Brain. Res 639, 57–65 (1994). [DOI] [PubMed] [Google Scholar]
- 70.Xu YL et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron 43, 487–497 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Aserinsky E & Kleitman N Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118, 273–274 (1953). [DOI] [PubMed] [Google Scholar]
- 72.Schmidt MH, Valatx JL, Sakai K, Fort P & Jouvet M Role of the lateral preoptic area in sleep-related erectile mechanisms and sleep generation in the rat. J. Neurosci 20, 6640–6647 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siegel JM & Vertes RP Sleep and memory: The ongoing debate, Rebuttal. Sleep 28, 1232–1233 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vertes RP Memory consolidation in sleep; dream or reality. Neuron 44, 135–148 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Jouvet-Mounier D, Astic L & Lacote D Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev. Psychobiol 2, 216–239 (1970). [DOI] [PubMed] [Google Scholar]
- 76.Frank MG & Heller HC The ontogeny of mammalian sleep: a reappraisal of alternative hypotheses. J. Sleep Res 12, 25–34 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Karlsson KAE, Gall AJ, Mohns EJ, Seelke AMH & Blumberg MS The neural substrates of infant sleep in rats. PLoS Biol. 3, e143 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaffery JP, Roffwarg HP, Speciale SG & Marks GA Ponto-geniculo-occipital-wave suppression amplifies lateral geniculate nucleus cell-size changes in monocularly deprived kittens. Brain Res. Dev. Brain Res 114, 109–119 (1999). [DOI] [PubMed] [Google Scholar]
- 79.Jouvet M in Cerebral Correlates of Conscious Experience. INSERM Symposium Vol. 6 (eds Buser P. & Rougeul-Buser A.) 245–261 ( Elsevier/North-Holland Biomed., Amsterdam, 1978). [Google Scholar]
- 80.Snyder F Toward an evolutionary theory of dreaming. Am. J. Psychiatry 123, 121–136 (1966). [DOI] [PubMed] [Google Scholar]
- 81.Horner RL, Sanford LD, Pack AI & Morrison AR Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Res. 778, 127–134 (1997). [DOI] [PubMed] [Google Scholar]
- 82.Baker FC, Angara C, Szymusiak R & McGinty D. Persistence of sleep-temperature coupling after suprachiasmatic nuclei lesions in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol (2005). [DOI] [PubMed] [Google Scholar]
- 83.Wehr TA A brain-warming function for REM sleep. Neurosci. Biobehav. Rev 16, 379–397 (1992). [DOI] [PubMed] [Google Scholar]
- 84.Darchia N, Campbell IG, Palagini L & Feinberg I Rapid eye movement density shows trends across REM periods but is uncorrelated with NREM delta in young and elderly human subjects. Brain Res. Bull 63, 433–438 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Sanford LD, Tang X, Ross RJ & Morrison AR Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav. Genet 33, 43–58 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Gonzalez MM, Debilly G, Valatx JL & Jouvet M Sleep increase after immobilization stress: role of the noradrenergic locus coeruleus system in the rat. Neurosci. Lett 202, 5–8 (1995). [DOI] [PubMed] [Google Scholar]
- 87.Shaw PJ, Cirelli C, Greenspan RJ & Tononi G Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Siegel JM & Rogawski MA A function for REM sleep: regulation of noradrenergic receptor sensitivity. Brain Res. Rev 13, 213–233 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pedrazzoli M & Benedito MA Rapid eye movement sleep deprivation-induced down-regulation of beta-adrenergic receptors in the rat brainstem and hippocampus. Pharmacol. Biochem. Behav 79, 31–36 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Tsai LL, Bergmann B, Perry B & Rechtschaffen A Effects of chronic total sleep deprivation on central noradrenergic receptors in rat brain. Brain Res. 602, 221–227 (1993). [DOI] [PubMed] [Google Scholar]
- 91.Hipolide DC, Tufik S, Raymond R & Nobrega JN Heterogeneous effects of rapid eye movement sleep deprivation on binding to alpha- and beta-adrenergic receptor subtypes in rat brain. Neuroscience 86, 977–987 (1998). [DOI] [PubMed] [Google Scholar]
- 92.Hipolide DC et al. Distinct effects of sleep deprivation on binding to norepinephrine and serotonin transporters in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 297–303 (2005). [DOI] [PubMed] [Google Scholar]
- 93.Vogel GW An alternative view of the neurobiology of dreaming. Am. J. Psychiatry 135, 1531–1535 (1978). [DOI] [PubMed] [Google Scholar]
- 94.Rechtschaffen A & Bergmann BM Sleep deprivation in the rat: an update of the 1989 paper. Sleep 25, 18–24 (2002). [DOI] [PubMed] [Google Scholar]
- 95.Zenko CE, Bergmann BM & Rechtschaffen A Vascular resistance in the rat during baseline, chronic total sleep deprivation, and recovery from total sleep deprivation. Sleep 23, 341–346 (2000). [PubMed] [Google Scholar]
- 96.Parmeggiani PL, Azzaroni A & Calasso M Systemic hemodynamic changes raising brain temperature in REM sleep. Brain Res. 940, 55–60 (2002). [DOI] [PubMed] [Google Scholar]
- 97.Paxinos G & Watson C The Rat Brain in Stereotaxic Coordinates (Elsevier Academic Press, London, 2005). [Google Scholar]
- 98.Lyamin OI et al. Unihemispheric slow wave sleep and the state of the eyes in a white whale. Behav. Brain Res 129, 125–129 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mukhametov LM & Poliakova IG [Electroencephalographic study of sleep in Sea of Azov porpoises.] Zh. Vyssh. Nerv. Deiat. Im. I. P. Pavlova 31, 333–339 (1981). [PubMed] [Google Scholar]
- 100.Mukhametov LM Unihemispheric slow-wave sleep in the Amazonian dolphin, Inia geoffrensis. Neurosci. Lett 79, 128–132 (1987). [DOI] [PubMed] [Google Scholar]


