Abstract
The intracellular sensing protein termed NLRP3 (for NACHT, LRR, and PYD domains-containing protein 3) forms a macromolecular structure called the NLRP3 inflammasome. The NLRP3 inflammasome plays a major role in inflammation, particular in the production of interleukin-1β (IL-1β). IL-1β is the most studied of the the IL-1 family of cytokines, including 11 members among which IL-1α and IL-18. Here, we summarize pre-clinical and clinical findings supporting the key pathogenetic role of the NLRP3 inflammasome and IL-1 cytokines in the formation, progression and complications of atherosclerosis, in ischemic (acute myocardial infarction, AMI), and non-ischemic injury to the myocardium (myocarditis) and the progression to heart failure (HF). We also review the clinically available IL-1 inhibitors, although not currently approved for a cardiovascular indications, and discuss other IL-1 inhibitors, not currently approved, as well as oral NLRP3 inflammasome inhibitors currently in clinical development. Canakinumab, IL-1β antibody, prevented the recurrence of ischemic events in patients with prior AMI in a large phase III clinical trial including 10,061 patients world-wide. Phase II clinical trials show promising data with anakinra, recombinant IL-1 receptor antagonist, in patients with ST segment elevation AMI or HF with reduced ejection fraction. Anakinra also improved outcomes in patients with pericarditis and it is now considered standard of care as second line treatment for patients with recurrent/refractory pericarditis. Rilonacept, a soluble IL-1 receptor chimeric fusion protein neutralizing IL-1α and IL-1β, has also shown promising results in a phase II study in recurrent/ refractory pericarditis. In conclusion, there is overwhelming evidence linking the NLRP3 inflammasome and the IL-1 cytokines with the pathogenesis of cardiovascular diseases. The future will likely include targeted inhibitors to block the IL-1 isoforms, and possibly oral NLRP3 inflammasome inhibitors, across a wide spectrum of cardiovascular diseases.
Keywords: atherosclerosis, atherothrombosis, inflammation, interleukin, inflammasome
Subject Terms: Vascular Disease
Introduction
Inflammation is broadly defined as a cellular and humoral response to infectious or non-infectious injury. While inflammatory responses are necessary for survival against infection and vigilance against cancer, an exuberant or dysregulated inflammation contributes to the pathogenesis of numerous acute and chronic conditions. Cytokines, often termed interleukins, are signaling proteins regulating the inflammatory response by communicating pro- and anti-inflammatory signals. Interleukin-1 (IL-1) is the prototypical pro-inflammatory cytokine, occupying an apical role in the innate immune response.1, 2 Heightened IL-1 activity contributes to the pathogenesis of several pro-inflammatory conditions, and, more recently, it has been linked to an increased risk and greater severity of cardiovascular diseases (CVD).3–5
We herein review the mechanisms by which active IL-1 is produced and contributes to CVD. Treatments aimed at inhibiting IL-1 activity in acute myocardial infarction, heart failure, refractory/recurrent pericarditis and in other chronic inflammatory heart diseases are being developed for the treatment of CVD.
The Biology of the IL-1 Family in Cardiovascular Diseases
The IL-1 Family has 11 cytokine members and 10 receptors; IL-1β and IL-18 are the most studied members of the Family.1, 6 A depiction of the 11 members, their receptors and co-receptors as well as their prominent function is available in Supplemental Table I. There are 4 members that function as anti-inflammatory cytokines and of these, IL-1 Receptor antagonist (IL-1Ra) and IL-36 Receptor antagonist (IL-36Ra) are specific, whereas IL-37 and IL-38 are non-specific and broadly inhibit innate immunity. As described in this review, a recombinant form of the naturally occurring IL-1Ra is anakinra. Anakinra is used to treat a wide spectrum of inflammatory conditions, including CVD.3, 4 IL-36Ra, IL-37 and IL-38 are not presently approved for humans but preclinical studies reveal several indications for treating human diseases.1 In addition to the anti-inflammatory members of the IL-1 Family, the extracellular domains of receptors termed soluble receptors also suppress inflammation. For example, soluble IL-1R2 neutralizes IL-1β and the IL-18 Binding Protein (IL-18BP) neutralizes IL-18.
Inflammation and IL-1
IL-1β is an inducible cytokine primarily produced by monocytes and macrophages but also neutrophils. IL-1α is quite different; although IL-1α is also inducible in myeloid cells, the IL-1α precursor in present constitutively in all mesenchymal cells in health, including the myocardium (Figure 1). First synthesized as precursors but lacking a signal peptide, both IL-1α and IL-1β initially remain intracellularly. The inactive IL-1β precursor requires processing into a mature, active cytokine and released into the intracellular compartment as described in Figure 2. The IL-1α precursor is released upon necrotic cell death7, is active in its precursor form8 and can induce IL-1β (Figure 2). IL-1α is also present anchored to the membrane and functions upon cell-cell contact.9, 10 Both IL-1α and IL-1β are highly inflammatory cytokines and when administered in humans induces systemic inflammation in subnanomolar concentrations.2 In healthy subjects, IL-1β gene expression is low or absent in blood monocytes but is markedly increased in disease states. Circulating IL-1β is at unusually low concentrations; for example, in 500 healthy subjects, the mean level of IL-1β was 0.33 pg/mL 11 but in diseases states increases to 5-fold to barely over 1 pg/mL.12 In health, the IL-1α precursor is present in most mesenchymal tissues such as the myocardium and the endothelium. Measuring circulating IL-1 in patients rarely correlates with disease severity. For example, specific neutralization of IL-1β significantly reduces cardiovascular events in high risk patients13 but circulating levels of IL-1β are below detection even in high risk patients. Similarly, specific neutralization of IL-1α in patients with metastatic colorectal cancer results in improved outcomes yet circulating IL-1α is not detected.14
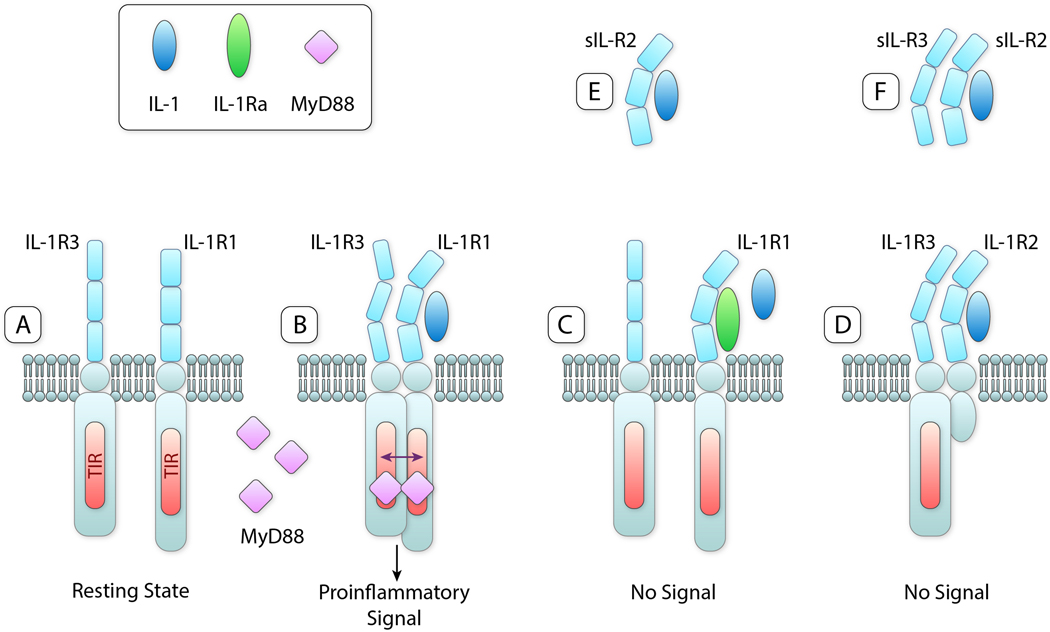
Figure 1. IL-1 and IL-1 Receptors.
A. Resting state of ligand binding chain of IL-1R1 and the co-receptor IL-1R3 on cell surfaces. B. Binding of IL-1β (or IL-1α not shown) to IL-1R1 results in a conformational change allowing IL-1R3 to bind resulting in a heterotrimeric complex. The TIR domains in the intracellular compartment approximate, MyD88 binds and triggers a pro-inflammatory signal via NFκB (not shown). C. IL-1Ra binds to IL-1R1 but does not cause a conformational change. IL-1R3 does not bind, a trimeric complex is not formed and there is no signal despite the presence of IL-1β. The affinity for IL-1Ra for IL-1R1 is greater than that for IL-1α and least for IL-1β. D. IL-1β binds to IL-1R2 and a conformational change occurs, IL-1R3 binds and there is a trimeric complex. Since IL-1R2 lacks an intracellular domain, there is no TIR domain to dimerize with the TIR domain on IL-1R3. There is no signal. E. soluble IL-1R2 in the extracellular space binds IL-1β and sequesters IL-1β away from IL-1R1 on cells. F. Soluble IL-1R2 binds IL-1β and forms a complex with soluble IL-1R3 resulting in a higher affinity for IL-1β.
Abbreviations: IL-1, interleukin-1; IL-1R1, ligand binding IL-1 Receptor type 1; IL-1R3, co-receptor IL-1 Receptor type 3; IL-1Ra, IL-1 Receptor antagonist; MyD88, Myeloid Differentiation Factor 88 IL-1R2, IL-1 receptor type 2; sIL-1R2, soluble (extracellular domain) IL-1R2sIL-1R3, soluble IL-1R3; TIR, Toll-IL-1-Receptor domain. IIllustration credit: Ben Smith
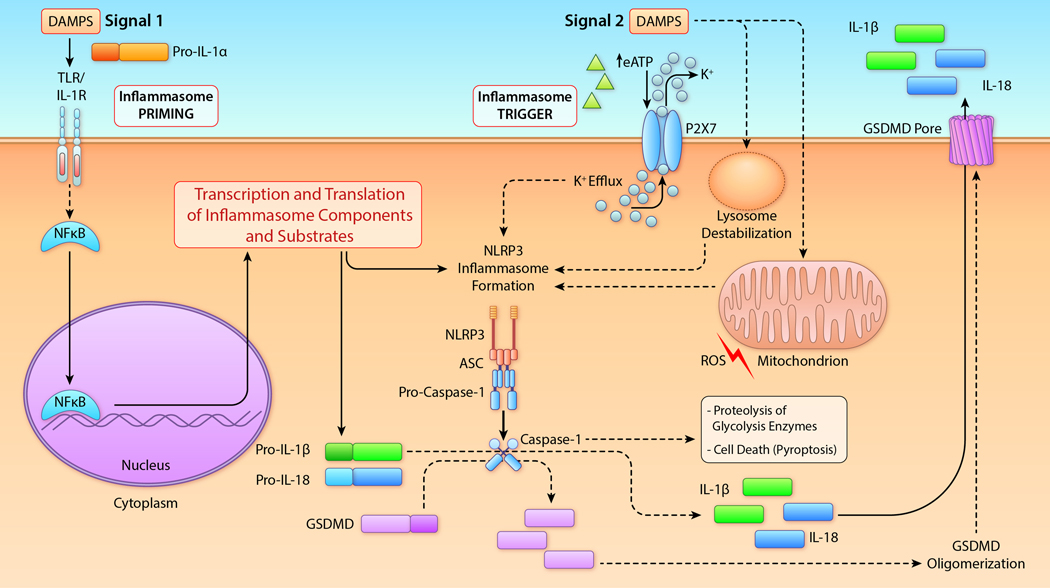
Figure 2. Schematic of the NLRP3 inflammasome formation.
In most cells, including macrophages resident cells, activation of the NLRP3 inflammasome activation requires two signals. Signal 1 (left) is a priming step. Tissue damage, such as ischemia reperfusion injury, promotes the release of DAMPs, including the IL-1α precursor (pro-IL-1α) and eATP. The Signal 1 priming is initiated when DAMPs activate membrane receptors, including the TLRs or the IL-1R1, leading to the translocation of NF-κB into the nucleus. This event promotes the transcription and translation of several of pro-inflammatory genes, particularly the precursors of IL-1β and IL-18, as well as components of the NLRP3 inflammasome. IL-1β and IL-18 precursors accumulate in the cytosol and signal 1 does not directly result in NLRP3 activation. Signal 2 provides the trigger for activation of the inflammasome. This signal is promoted by eATP or intracellular DAMPs (e.g. mitochondrial molecules, like ROS, or lysosomal content) which in most cases involve the efflux of K+. In the heart, eATP activates the purinergic receptor P2X7, resulting in K+ efflux with subsequent activation of NLRP3. NLRP3 oligomerizes and binds ASC and pro-caspase-1 resulting in the auto-catalytic cleavage of pro-caspase-1 to active caspase-1. Caspase-1 cleaves pro-IL-1β and pro-IL-18 into their mature and active forms. Caspase-1 also cleaves gasdermind D (GSDMD) producing an N-terminal fragment that oligomerizes and forms plasma membrane pores. These pores facilitate the release of mature IL-1β and IL-18 into the extracellular space. Active Caspase-1 induces degradation of glycolysis enzymes thus reducing the energy production in the cell. Caspase-1 and GSDMD pores also mediate a form of regulated cell death termed pyroptosis. IIllustration credit: Ben Smith
Abbreviations: ASC, apoptosis-associated spec-like protein containing a carboxy-terminal containing a caspase recruiting domain; DAMPs, damage associated molecular patterns; eATP, extracellular adenosine triphosphate; GSDMD, gasdermin D; IL-1α, interleukin-1α; IL-1β, interleukin-1β; IL-18, interleukin-18; IL-1R, Interleukin-1 receptor type 1; NF-κB, Nuclear factor-κB; NLRP3, NACHT LRR and PYD domains-containing protein 3; P2X7, purinergic receptor 2X7; ROS, reactive oxygen species; TLR, Toll-Like Receptors.
IL-1 Receptors and IL-1 signaling.
IL-1-mediated inflammation is initiated when IL-1 bind to its receptors. There are two receptors for IL-1; IL-1 Receptor type 1 (IL-1R1) is the ligand binding chain and IL-1R3 is the co-receptor. Both receptors are present on all nucleated cells in the resting state (Figure 1A). When either IL-1β or IL-1α binds to IL-1R1, a structural change takes place allowing IL-1R3 to bind and to form a heterotrimeric complex (Figure 1B). The pro-inflammatory functions of IL-1β are initiated with the approximation of the intracellular domains of IL-1R1 and IL-1R3. The intracellular domain of IL-1R1 and IL-1R3 contain the Toll-IL-1-Receptor (TIR) domain. Found in all members of the IL-1 family of receptors, the TIR domain is nearly identical to the TIR domain of Toll-like receptors (TLRs) associated with microbial pathogens. Thus, the IL-1 family and the TLR family share the same pro-inflammatory mechanisms for inducing inflammation. As shown in Figure 1B, the TIR domains of IL-1R1 and IL-1R3 approximate and the adapter protein myeloid differentiation factor 88 (MyD88) binds to the TIR domain. MyD88 binding leads to a rapid cascade of phosphorylations resulting in activation of NFκB.
The IL-1 family of cytokines and receptors is balanced in that anti-inflammatory members function to limit and or even prevent inflammation from pro-inflammatory members. IL-1Ra acts as a classic receptor antagonist. When IL-1Ra binds to IL-1R1 (Figure 1C), the conformational change (shown in Figure 1B) does not result IL-1R3 binding. Therefore, there is no signal when IL-1Ra occupies of IL-1R1. IL-1R2 preferentially binds IL-1β (Figure 1D) and in doing so sequesters IL-1β from binding to IL-1R1. Following binding, IL-1R2 undergoes a similar conformational change, IL-1R3 binds to form a complex and this complex increases the affinity of IL-1R2 for IL-1β. However, lacking an intracellular domain, IL-1R2 has no TIR domain and there is no signal (Figure 1D). Thus, IL-1R2 is considered a “decoy” receptor as it functions to sequester IL-1β.15 The soluble form of IL-1R2 is found in the circulation in health where it is thought to provide a backup mechanism to limit inflammation by IL-1Ra. Although binding of soluble IL-1R2 binding to IL-1β (Figure 1E) takes place, the affinity increases significantly when soluble IL-1R3 forms joins the complex (Figure 1F).
NLRP3 inflammasome
The processing of pro-IL-1β into its active form is largely regulated by the enzymatic activity of caspase-1 within the cell (Figure 2). Caspase-1 is activated by the inflammasome, a macromolecular structure of several components with different functions.16, 17 The sensor protein is an intracellular receptor termed NLRP3 (for NACHT, LRR, and PYD domains-containing protein 3 ), which responds to intracellular or extracellular danger-associated signals. The discovery of the role of NLRP3 is attributed to Hal Hoffman’s group,18 whereas the identification of NLRP3 as costitutive part of the inflammasome in the processing of IL-1β was first shown by Jurg Tschopp’s group.19 In macrophages resident cells, activation of the NLRP3 inflammasome requires two signals. Signal 1 (Figure 2, left) is a priming step. Tissue damage, such as ischemia reperfusion injury, promotes the release of danger-associate molecular patterns (DAMPs), including the IL-1α precursor and extracellular adenosine triphosphate (eATP). This event promotes the transcription and translation of several of pro-inflammatory genes, particularly the precursors of IL-1β and IL-18, as well as components of the NLRP3 inflammasome. Signal 2 provides the trigger for activation of the inflammasome. This signal is promoted by eATP or intracellular DAMPs (e.g. mitochondrial molecules, like reactive oxygen species or lysosomal content) which in most cases involve the efflux of K+. NLRP3 oligomerizes and binds the adaptor protein ASC (apoptosis-associated spec-like protein containing a carboxy-terminal containing a caspase recruiting domain) and pro-caspase-1 resulting in the auto-catalytic cleavage of pro-caspase-1 to active caspase-1. Caspase-1 cleaves pro-IL-1β and pro-IL-18 into their mature and active forms. Caspase-1 also provides for the formation of the gasdermin channel for the release of IL-1β and IL-18 from the cell (Figure 2, right). Circulating monocytes, however, release active IL-1β after a one-time stimulation of the Toll-like Receptors (TLR), resulting from constitutively activated caspase-1 and release of endogenous adenosine triphosphate.20 Moreover, when the IL-1β precursor is released at inflammatory sites such as in ischemic tissues, proteolytic enzymes from infiltrating neutrophils can process the IL-1β precursor extracellularly into an active cytokine independent of NLRP3 and caspase-11, 2, 21.
IL-18
The IL-1 family member IL-18 is similar to IL-1β in that both cytokines precursors are inactive and both require caspase-1 for processing into active cytokines (Figure 2). The activity of IL-18 is regulated by the IL-18BP. IL-18BP has an unusually high affinity kD of 0.5 nM for mature IL-18.22 This high affinity binding allows for the calculation of the level of circulating “free” from IL-18 bound to IL-18BP23 and the role of IL-18 in cardiovascular diseases is best understood by free IL-18. Circulating free IL-18 is markedly elevated in patients with Macrophage Activation Syndrome (MAS), a syndrome observed in cancer and autoinflammatory conditions. In septic patients with MAS, anakinra significantly reduced 28-day mortality compared to placebo treated patients 24. Since IL-18 is a risk factor for cardiovascular events, the therapeutic efficacy of anakinra or canakinumab in patients with heart failure likely includes suppression of IL-18. The reduction in IL-18 activity by blocking IL-1 is in part due to a reduction in the activation of the NLRP3 inflammasome (Figure 2).
IL-33
Interleukin-33 (IL-33) is another member of the IL-1 Family.1, 2 Unlike pro-IL-1β and pro-IL-18, caspase-1 inactivates the IL-33 precursor. The primarly function of IL-33 is to shift the inflammatory reaction toward a T helper response type 2, thus reducing the T helper type 1 response, and favoring the resolution of inflammation.1, 2 IL-33 triggers IL-1R4 (also known as ST2), however it also translocates to the nucleus and suppresses the inflammatory response, independent of IL-1R4.
IL-37 and IL-38
IL-1Ra, IL-1R2 and soluble IL-1R2 are specific in solely reducing the activities of IL-1β as well as IL-1α. In contrast, IL-37 and IL-38 reduce inflammation broadly; for example, these cytokines reduce levels of IL-1, TNFα, IL-6 and several chemokines. IL-37 is particularly broad in its anti-inflammatory properties25, including inhibition the NLRP3 inflammasome.26 The first studies identified that recombinant human IL-38 binds to the IL-36 receptor (IL-1R6) and inhibits the production of IL-1727 but increasing evidence reveals that IL-38 broadly reduces inflammation.28, 29
Atherosclerosis and the NLRP3 inflammasome
IL-1 and the pathogenesis of atherosclerosis.
Once considered a mere accumulation of lipids in the wall of blood vessels, seminal work in the past decades have defined atherosclerosis as an inflammatory disease.30–32 Circulating pro-inflammatory lipoproteins increase endothelial cell permeability, leading to accumulation of lipids and inflammatory macrophages termed “foam cells” in the intima of the vessels. In the vessel wall, a central necrotic core develops with a fibrous cap, and outward remodeling of the vessel wall: the atherosclerotic plaque.30–32 IL-1 contributes to the initiation, formation, growth and rupture of the plaques (Figure 3).33–36 Both IL-1α and IL-1β are produced locally and directly affect the function of endothelial cells. For example, both cytokines impair vasodilation, increases oxidative stress, the production of procoagulant mediators and are released from platelets, each property predisposing to atherothrombosis, a dreaded complication of the atherosclerotic plaque.37–39
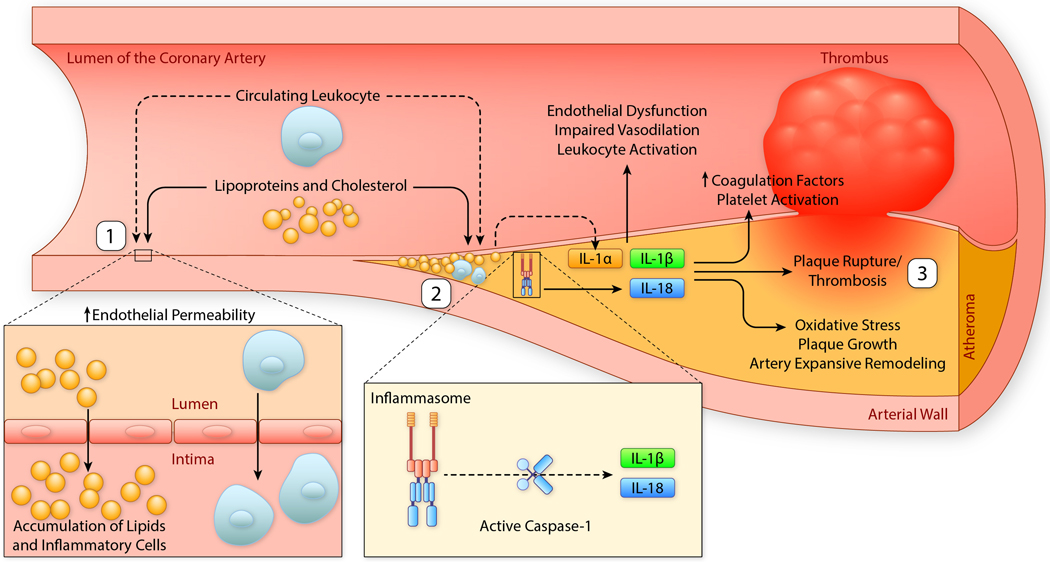
Figure 3. IL-1, inflammasome and atherothrombosis.
Atherosclerosis is a chronic process that can culminate with plaque rupture and atherothrombosis. (1) Pro-inflammatory lipoproteins in the lumen of the arteries promote endothelial dysfunction and permeability, leading to accumulations of lipids and migration of pro-inflammatory cells (leukocytes) in the intima of the vessel. (2) Over time, the accumulation of lipids, including cholesterol, and inflammatory cells leads to production of cytokines, including IL-1α, NLRP3 inflammasome activation, and production of inflammasome dependent cytokines IL-1β and IL-18. This process perpetuates endothelial dysfunction, impairs vasodilation, activates leukocytes and promotes oxidative stress, plaque growth and arterial expansive (outward) remodeling. (3) IL-1 activity increases coagulation factors, contributes to platelet activation and promotes plaque rupture and thrombosis. IIllustration credit: Ben Smith
Abbreviations: IL-1α, interleukin-1α; IL-1β, interleukin-1β; IL-18, interleukin-18; NLRP3, NACHT, LRR, and PYD domains-containing protein 3.
Systemically, IL-1 induces the production of interleukin-6 (IL-6) as well as increases pro-inflammatory and procoagulant events, thus creating a pro-thrombotic environment further promoting adverse CV events. Histological studies show the presence of IL-1β in human atherosclerotic plaques.40 Indeed, pro-inflammatory stimuli such as oxidized lipoproteins stimulate IL-1 gene expression and further enhance disease progression in a autostimulatory cycle.41 IL-1β also has a significant role in the initiation and progression of abdominal aorta aneurysms. In mouse models of elastase infusion, deficiency of IL-1β or blockade of the IL-1R1 with anakinra attenuates aneurysm formation.42
The balance of IL-1 and IL-1Ra affects the wellbeing of the endothelium and the vessel wall. This is best elucidated in the mouse with homozygous deletion of the gene coding for IL-1Ra resulting in unopposed IL-1 activity in the resting state.43 This mouse develops a severe vasculitis with transmural infiltration of inflammatory cells in the wall of medium size arteries and subsequent collapse of vessel wall, stenosis and organ infarction.43 Supplemental Table II summarizes the key preclinical findings in atherosclerosis.
The atherogenic properties of IL-1β but also of IL-1α have been studied in hyperlipidemic mice in which genetic mutations that decrease lipoprotein clearance from the blood and leads to hypercholesterolemia. There are two strains of mice for these studies: mice deficient in Apo E and mice deficient in low-density lipoprotein receptor (LDL-R). Mice deficient in both Apo E as well as deficient in IL-1Ra develop atheromatous plaques when fed a high fat diet.34 Administration of recombinant IL-1Ra or neutralizing antibody against IL-1β to Apo E deficient mice have significantly reduced atheroma formation.44, 45 Similarly, mice deficient in LDL-R but overexpressing IL-1Ra showed significant reduction in atherosclerotic lesions.35 The Apo E deficient mouse also deficient in IL-1R1 exhibited a reduction in the burden of atherosclerotic plaques in the aortic root, but not in the brachiocephalic artery.46
Other biologic differences were noted in mice deficient in IL-1R1, such as differences in outward remodeling of the vessel wall46 and maintenance of the collagen in the fibrous cap of the atheromatous lesions.47 Using mice deficient in IL-1R1, one study proposed IL-1 signaling to be ‘protective’ against rupture of the plaque in the mouse 46, nevertheless, whether this is the case remains debatable. In regards to outward remodeling, a feature of progressive atherosclerosis, one study suggested that IL-1β is responsible for the outward remodeling47, yet a separate study comparing IL-1α and IL-1β suggests a pivotal role for IL-1α during early experimental atherogenesis, whereas IL-1β drives inflammation during atherogenesis in the evolution of advanced atheroma in mice.48
The NLRP3 inflammasome, active IL-1β and atherosclerosis.
Prior to mouse studies, the link between high fat diets, IL-1 and atherosclerosis had been established in rabbits. For example, typical plaques developed in New Zealand rabbits fed high cholesterol diets. Plaques also develop in the Watanabe genetically hyperlipidemic rabbit and in rabbits deficient in the LDL receptor.49 In the New Zealand rabbit, plaques develop in the iliac artery with macrophage-derived foam cells expressing IL-1β. The atherosclerotic lesions however regress with the cessation of the high saturated fat diets.50 In other studies, plaques develop with high mRNA levels of IL-1R1 and IL-1β.51
The mechanism by which high cholesterol triggers the processing and release of mature IL-1β has been further elucidated by in vitro experiments linking intraplaque cholesterol crystals and the activation of the NLRP3 inflammasome.52, 53 Cholesterol crystals directly activate the NLRP3 inflammasome by inducing leakage of the lysosomal protease cathepsin B into the cytoplasm.53 Using chimeric mice with bone marrow restricted deficiency of NLRP3, ASC, IL-1β or IL-1α in LDL-R deficient mice fed a high-fat diet, development of lesions was significantly decreased compared to mice transplanted with wild-type bone marrow.52 The link between the NLRP3 inflammasome and atherosclerosis was however not supported by a single study in the Apo E deficient mouse.54
Mice deficient in caspase-1 are protected because they have a dysfunctional inflammasome machinery. Hypercholesterolemic mice deficient in caspase-1 but also deficient in either ApoE or LDL-R are protected compared with the mice with Apo E or LDL-R deficiency and functional caspase-1.55–57 Expression of the NLRP3 inflammasome in atherosclerotic plaques from patients has been documented and correlated with the severity of coronary artery disease.58 NLRP3 expression in peripheral blood monocytes in patients with acute coronary syndrome also predicts adverse cardiac events.59 A small molecule inhibitor of the NLRP3 inflammasome, administered for 4 weeks in the Apo E deficient mouse resulted in a reduction in the atherosclerotic plaque burden.60 Several NLRP3 inhibitors are currently in drug development.61–63
IL-18, IL-33 and IL-37 atherosclerosis
The advantage of targeting the NLRP3 inflammasome includes the reduction in mature IL-18. The IL-18 precursor is also processed by the NLRP3 inflammasome. Circulating IL-18 is a strong and independent predictor of adverse events in patients with coronary artery disease.64 Genetic deletion of IL-18 or transgenic expression of human IL-18BP in the Apo E knock out mouse reduced the development of atherosclerotic plaque in the aorta.65, 66
IL-33 regulates the inflammatory response shifting toward a T helper 2 response by binding the IL-1R4 receptor. When recombinant IL-33 was administered to Apo E deficient mice fed high-fat diets, it reduced atherosclerotic plaque burden.67, 68
IL-37 is an anti-inflammatory member of the IL-1 family. IL-37 is present in human atherosclerotic plaques and also in coronary artery smooth muscle cells69 and circulating levels of IL-37 are elevated in patients with acute coronary syndrome.70 Transgenic expression of human IL-37 reduces plaque burden and increases plaque stability.69 Treatment of the Apo E deficient mouse with recombinant IL-37 reduces vascular calcification and atherosclerosis.71, 72 In human calcific aortic valves, IL-37 expression is significantly reduced compared to healthy aortic valves and treatment with recombinant human IL-37 reduces the osteogenic responses in cultured aortic valves in vitro.73
Translational Studies
Studies of atherosclerosis and atherothrombosis in experimental models are based on several assumptions and have limitations. For example, whereas most of the clinical trials are focused on events surrounding an AMI, in mice, the coronary arteries are generally spared from atherosclerosis. In contrast, atheromatous changes are assessed in other vascular districts such as the aortic arch, the thoracic and abdominal aorta, and the major branches of the aorta. Moreover, mice are either subjected to extreme hypercholesterolemia or the vessel wall is injured in order to enhance the inflammatory response. In both instances, the phenotype is exaggerated in mice compared to what occurs in patients. Clinical assessment is quantification of atherosclerotic plaque burden, that does not always correlate with clinical disease severity.
An additional impetus for exploring IL-1 as a therapeutic target is derived from biomarkers in patients with or at risk for CVD.74 Elevated circulating levels of IL-1Ra serve as surrogate for IL-1 activity and predict atherosclerotic outcomes.75, 76 IL-1 induces IL-6 and serum levels of IL-6 are strong independent predictors of outcomes.64, 77 Likewise, IL-18 levels predict outcomes64, 78 and similar to IL-6, IL-18 is also downstream from IL-1.79, 80 Since IL-6 is downstream from IL-1, IL-6 induces C-reactive protein (CRP), CRP is also a surrogate for IL-1 activity; at present, CRP is the preferred inflammatory biomarker for cardiovascular risk stratification.81, 82
The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) trial.
The CANTOS trial13 randomized 10,061 patients with prior acute myocardial infarction (>30 days prior to screening) and evidence of systemic inflammation, defined as a CRP level of at least 2 mg/L, to either placebo or canakinumab 50, 150 or 300 mg every 3 months. In a previous study, canakinumab dose dependently reduced CRP in 556 patients with type 2 diabetes.83 The primary endpoint of the CANTOS trial was a composite of nonfatal myocardial infarction, nonfatal stroke or cardiovascular death. Over a median follow-up of 3.7 years, there was a reduction by 15% in patients treated with 150 mg of canakinumab compared to placebo (HR, 0.85; 95% CI, 0.74–0.98; P=0.021) (Table 1). The dose of 150 mg also reduced the need for coronary revascularization (HR, 0.83; 95% CI, 0.73–0.95; P=0.005).13 These definitive data establish that IL-1β blockade with canakinumab in patients with stable atherosclerotic CVD prevents recurrent cardiovascular events and thus provide compelling proof for the IL-1 atherothrombosis concept.84 The benefit of canakinumab on clinical events was independent of any effect on lipoproteins or blood pressure and it was closely related with the inflammatory response, as patients showing the greatest reduction in CRP had improved survival with canakinumab.85
Table 1.
Overview of clinical trials with IL-1 targeted strategies in patients with AMI.
| Study | Population | Sample Size | Study Design | Intervention | Duration | Outcomes | PMID or NCT number | |||
|---|---|---|---|---|---|---|---|---|---|---|
| R | DB | PC | MC | |||||||
| CANTOS | Prior AMI (>30 days) with CRP >2 mg/l | 10,061 | X | X | X | X | Canakinumab 50, 150, 300 mg every 3 months | 3.7 years (median) | ↓CRP, ↓ischemic events, ↓HF hospitalization ↑infection-related deaths ↓cancer-related deaths ↓rheumatologic diseases | 28845751 30586730 |
| CIRT | Prior AMI (>30 days) or diabetes and multivessel CAD | 4,786 | X | X | X | X | Low dose methotrexate (15–20 mg weekly) | 2.3 years (median) | no effect on clinical events (no reduction in CRP levels) | 30415610 |
| COLCOT | Recent AMI (<30 days) | 4,745 | X | X | X | X | Colchicine 0.5 mg daily | 1.9 years (median) | ↓ischemic events, ↑pneumonia (no effect on CRP beyond reduction seen in placebo; no effect on HF events) | 31733140 |
| LODOCO | Stable CAD (125 with prior AMI/UA) | 532 | X | X | Colchicine 0.5 mg daily | 2.4 years (median) | ↓acute coronary syndromes (CRP not reported) | 23265346 | ||
| LODOCO-MI | Recent MI (Prior 7 days) | 237 | X | X | X | Colchicine 0.5 mg daily | 30 days | no effect on CRP beyond reduction seen in placebo (↓total re-hospitalization) | 31284074 | |
| MRC-ILA | Acute NSTEMI (<48 hours) | 182 | X | X | X | Anakinra 100 mg daily | 2 weeks treatment (1 year follow up) |
↓CRP at 7 and 14 days (no effect on ischemic events at 30 days and 3 months, but ↑at 1 year) | 25079365 | |
| VCUART / VCUART2 | Acute STEMI (<12 hours) | 40 | X | X | X | Anakinra 100 mg daily | 2 weeks treatment (3 months follow up) | ↓CRP, ↓ incidence of HF (no effect on ischemic events) | 23453459 | |
| VCUART3 | Acute STEMI (<12 hours) | 99 | X | X | X | X | Anakinra 100 mg once or twice daily | 2 weeks treatment (1 year follow up) |
↓CRP, ↓ incidence of HF, ↓ hospitalization for HF (no effect on ischemic events) | NCT01950299 |
Abbreviations: CRP = C-reactive protein; DB = Double blind; HF = Heart failure; hsCRP= high sensitivity CRP; PC = Placebo-controlled; R = Randomized.
The Cardiovascular Inflammation Reduction Trial (CIRT)
Two other clinical trials have tested the inflammatory hypothesis of atherosclerosis/atherothrombosis. The Cardiovascular Inflammation Reduction Trial (CIRT) explored low-dose methotrexate for the prevention of atherothrombotic events.86 At difference with the CANTOS trial employing a targeted anti-IL-1β therapy, methotrexate is a non-targeted anti-metabolite drug mainly reducing cell proliferation. Methotrexate is commonly used in rheumatologic diseases, particularly rheumatoid arthritis for its anti-proliferative and reduced tissue remodeling properties. Since IL-1 blockade is also used to treat rheumatoid arthritis, and since IL-1 blockade reduces IL-6, there was a rationale for using methotrexate to reduce treat CVD, as IL-6 drives smooth muscle proliferation including smooth muscle cells.87 In the CIRT trial, methotrexate failed to improve the primary or secondary endpoints, and it also did not significantly lower CRP levels, nor IL-1β or IL-6 levels (Table 1).86 It is worth noting, however, that baseline CRP levels in CIRT were only 1.6 mg/l. This likely reflects the lack of CRP elevation as entry criteria, at difference with the CANTOS trial. Whether the lower CRP levels may have influenced the lack of benefit of methotrexate in the CIRT trial is unknown.
Colchicine Cardiovascular Outcome Trial (COLCOT)
An additional trial is the COLCOT study.88 Colchicine inhibits myeloid cell migration, for example neutrophils infiltration in gout; more recently, colchicine appears to also non-specifically inhibit the NLRP3 inflammasome.89, 90 A clinical trial in patients with stable coronary artery disease randomly assigned to Low Dose Colchicine (LoDoCo trial)91 or no treatment showed a significant reduction in the incidence of acute atherothrombotic events. In the COLCOT trial, 4745 patients with recent AMI were randomly assigned to colchicine or placebo within 30 days (median time of 13 days) of the index event. Colchicine continued for a median of 1.88 years significantly reduced the composite endpoint of recurrent ischemic events (Table 1), largely drive by a reduction in unstable angina and revascularization.88 Additional large scale colchicine outcome trials are ongoing. It remains undetermined whether the protective effects of colchicine in atherothrombosis are due to a reduction in IL-1 activity as treatment in familial Mediterranean fever, gout, pericarditis, and other inflammatory conditions or due to a different mechanism.
In summary, preclinical data show that the IL-1 family of cytokines and the NLRP3 inflammasome are involved in the formation and progression of the atherosclerotic plaques, and IL-1 blockade with canakinumab or treatment with colchicine reduces cardiovascular events in patients with previous coronary atherothrombotic events.
Formation of the inflammasome, IL-1 activity and acute myocardial infarction
Acute myocardial infarction (AMI) refers to the myocardial necrosis following acute ischemia as a result of a severe reduction in coronary artery blood supply. AMI is the most dreaded consequence of coronary atherosclerosis by the formation of a thrombus occluding coronary blood flow.92 The severe and sudden reduction in supply of oxygen and nutrients to the myocardium leads to a critical energy failure and loss of essential functions, leading to cell swelling and rupture, with ensuing release of intracellular components.92 Reperfusion strategies, central in the treatment of AMI, have revolutionized the treatment of AMI and significantly improved the outcomes by reducing the overall amount of myocardium loss.92 Although reperfusion limits the inflammatory response in AMI, necrotic cell death from the initial ischemic event releases cell contents referred to as DAMPs. DAMPS include intracellular cytokines such as IL-1α and IL-33, contributing to local and systemic inflammatory responses.16, 17, 93
The NLRP3 inflammasome in sterile injury
In the setting of AMI, the injury is sterile and not a result of a microbial invasion. NLRP3 is the sensing component of the inflammasome most commonly involved in the response to sterile injury. The formation of the inflammasome in most cells, including cardiomyocytes, is a 2-step process, requiring a priming process followed by an the activation or triggering step.94 The cell debris released during AMI functions as DAMPs, which induce the expression of key components of the inflammasome via cell surface receptors such as Toll-like Receptors (TLR) and IL-1R1 through activation of the nuclear factor kB (NF-kB)(Figure 2).94 NLRP3 activation requires a trigger and during the early phases of AMI, when ischemia dominates, the reduction of intracellular K+ due to failure of the Na+/K+ ATPase is likely the major trigger. Later in the course of the injury, NLRP3 may become active from lysosomal destabilization or from activation of the P2X7 purinergic receptor recognizing eATP, released from leukocytes or dying cells.16, 17 Activation of the NLRP3 inflammasome is a formidable step in cardiomyocytes after the ischemic injury with the fate of the cell towards an inflammatory type of cell death termed pyroptosis. Activation of the NLRP3 inflammasome also amplifies the inflammatory response by processing and secretion of IL-1β and IL-18.16, 17, 95 As shown in Figure 2, mature IL-1β and IL-18 exit the cell via the gasdermin D channel. Gasdermin D, a substrate for caspase-1, forms N-terminal fragment oligomers within the cell membrane after its cleavage by caspase-1 and is followed by the formation of gasdermin pores. Gasdermin D pores are permeable to macromolecules and mediate the unconventional extracellular release of mature IL-1β, IL-18, and active caspase-1.16, 17 In addition, caspase-1 cleaves several proteins involved in the glycolysis, resulting in a dramatic decrease in cell energy production that eventually leads to cell swelling and rupture.96 Several intermediate steps dependent on additional cytoplasmic adaptor proteins or kinases are also involved in the activation process.16, 17
Detrimental effect of NLRP3 inflammasome activation in AMI
The role of the NLRP3 inflammasome in AMI has been explored in preclinical mouse models using mice lacking the Nlrp3 gene (Nlrp3 deficient), mice treated with siRNA silencing to suppress Nlrp3 expression, and the use of NLRP3 inflammasome inhibitors. Nlrp3 deficient mice subjected to myocardial ischemia-reperfusion injury have a smaller infarct size and preserved cardiac function as compared with wild-type mice (Figure 4, Supplemental Table III).97, 98 These findings are consistent with those seen with ASC and Casp1 deficient mice.99 The role of NLRP3 in AMI is also time-dependent, consistent with the 2-step process required for activation, and accordingly the Nlrp3 deficient mouse shows no protection when the clinical assessment is limited to the first few hours of AMI.95, 100, 101 Six different NLRP3 inflammasome inhibitors given at time of reperfusion significantly reduce infarct size in experimental AMI (Supplemental Table III).97, 102–106
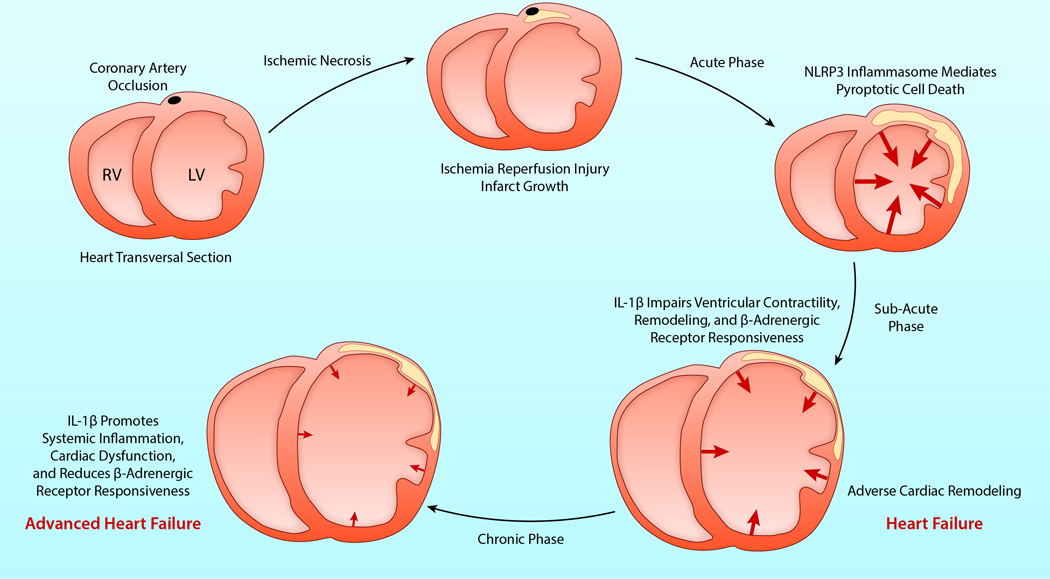
Figure 4. IL-1 and the inflammasome in AMI and heart failure.
Prolonged coronary artery occlusion leads to necrosis of the cardiomyocytes and acute myocardial infarction (AMI). In the acute phase, reperfusion limits necrosis but it does not interrupt the inflammatory response. Activation of the inflammasome following ischemia and reperfusion contributes to the acute loss of cardiomyocytes through pyroptotic cells death. In the subacute phase, locally produced IL-1β reduces myocardial contractility and the myocardial response to β-adrenergic receptor agonists, favoring adverse ventricular remodeling and heart failure. In the chronic phase, locally or systemically produced IL-1β perpetuates the contractile dysfunction, impaired β-adrenergic receptor responsiveness, progression of adverse ventricular remodeling and worsening heart failure. Cross-sections of the ventricles are depicted. The arrows represent the movement and force of the ventricular walls, with progressive weakening of contractility over time. IIllustration credit: Ben Smith
Abbreviations: AMI, acute myocardial infarction; IL-1β, interleukin-1β; LV, left ventricle; RV, right ventricle.
Colchicine, a nonspecific inhibitor of the NLRP3 inflammasome, was used in the AMI mouse model without reperfusion, and when administered at high doses (0.1 mg/kg/day) for 7 days, significantly reduced inflammasome activation, decreased infarct size, ventricular remodeling and prolonged 7-day survival.107 In a Phase II clinical trial, 151 patients were randomly assigned to colchicine (0.5 mg once or twice daily according to body weight) or placebo for 5 days and infarct size was measured as area-under-the-curve for biomarkers of myocardial necrosis, and with cardiac magnetic resonance in a subgroup of cases.108 Colchicine was well tolerated and significantly reduced infarct size and inflammatory biomarkers, thus supporting the beneficial effect of this strategy (Table 1).108 A second trial, low dose colchicine (LoDoCoMI trial)91, colchicine added during the course of AMI failed to reduce CRP levels beyond the reductions seen with placebo treated patients (Table 1). The trial was underpowered for clinical events, however a reduction in the number of all-cause re-hospitalizations was observed.91
These findings indicate that the NLRP3 inflammasome preferentially functions in the response to ischemia (and reperfusion) and targeting the NLRP3 inflammasome leads to smaller infarct size and improved outcomes in mice. Activation of the NLRP3 inflammasome within cardiomyocytes has an obvious detrimental effect for loss of functional myocardium.95, 101 Moreover, other cell types in the heart activate the NLRP3 inflammasome during AMI, with cell-type specific consequences. The activation of the NLRP3 inflammasome in cells other than cardiomyocytes does not directly lead to loss of contractile structures but indirectly contributes to cardiac dysfunction via IL-1β and IL-18, which amplify the inflammatory response. In neutrophils, IL-1β and IL-18 promote the release of radical oxygen species, enzymes, and cytokines that further injure the cardiomyocyte. In endothelial cells, the activation of the inflammasome is associated with loss of function, further impairing coronary flow, whereas IL-1β and IL-18 can determine inappropriate vasodilation (vasoplegia). In fibroblasts, inflammasome activation leads to release of IL-1β which in turn induces pro-fibrotic changes contributing to enlargement of infarct scar. On the other hand, cardiomyocytes fail to secrete IL-1β, despite having an active caspase-1 and pyroptosis.16, 17, 95, 98, 99
Unopposed IL-1 activity in AMI
The mechanisms by which NLRP3 inflammasome activation contributes to infarct size is largely independent of the processing and release of active IL-1β and IL-18 and dependent on cardiomyocyte loss through pyroptosis.95, 101 The activation of the NLRP3 inflammasome during AMI, however, leads to an intense inflammatory response and IL-1 activity. Unopposed IL-1 activity during AMI mobilizes myeloid cells from bone marrow to the infarction site inducing a pathologic myocardial healing and favoring cardiac rupture in experimental models.109, 110 The role of IL-1 in AMI has been studied in mice with genetic deletion of the Il1r1 gene and not responsive to IL-1, in mice with deletion of Il1ra gene 109 and in mice treated with blockers of IL-1α, IL-1β, or IL-1R1.110–116 The early phase of IL-1 activity during AMI is the release of the IL-1α precursor from dying cells, rather than the processing and secretion of IL-1β.113, 115 A reduction in infarct size in experimental AMI was seen when the activity of IL-1α was blocked at the receptor level in the IL-1R1 deficient mouse, by using recombinant IL-1Ra or neutralization of IL-1α by a monoclonal antibody.109, 113, 117 In contrast, IL-1β neutralization using a monoclonal antibody had no effects on infarct size.110, 115 Regardless on how IL-1 activity is blocked, adverse cardiac remodeling and cardiac dysfunction after an AMI is consistently reduced (Supplemental Table III).
Similar to the administration of IL-1Ra targeting the IL-1R1, administration of recombinant IL-37 or IL-38, broad inflammatory inhibitors of the IL-1 family, also ameliorated myocardial ischemia reperfusion injury in the mouse.118–120 IL-33 is a cytokine of the IL-1 family that modulates the inflammatory response toward a T response helper 2. When IL-33 is administered to mice during AMI, IL-33 reduced infarct size and prevented adverse remodeling, through the activity on the IL-1R4 (ST2) receptor.121, 122 IL-33 is also released by endothelial cells during pressure overload, in which case IL-33 favorably regulates the cardiac remodeling process.123, 124 The IL-33 pathway is of particular interest in HF because, the soluble form of the ST2 (sST2) is being developed as a biomarker for acute decompensated HF.125, 126 IL-1Ra may also exert an endogenous intracellular protective signaling, independent of IL-1R1.127
The Virginia Commonwealth University Anakinra Remodeling Trials (VCUART)
The effects of IL-1 blockade in patients with AMI has been studied in patients with large infarcts, such as those with ST-segment elevation (STEMI) who show an intense inflammatory response. The levels of the inflammatory marker CRP predicts outcomes including cardiac rupture and incidence of HF in patients with STEMI.128 The VCUART Phase II clinical trial program included 3 sequential studies (N=139).129–133 In each study, patients with STEMI received 14 days of anakinra or placebo. Treatment with anakinra was well tolerated and led to a significant reduction in the acute inflammatory response measured as area-under-the-curve for CRP.130, 131, 133 Anakinra for 14 days in patients with STEMI also showed a significantly lower incidence of new onset heart failure and of heart failure hospitalization versus placebo (Table 1).131, 133. In a sub-analysis of the CANTOS trial, canakinumab resulted in a reduction in the hospitalizations for HF (Table 1).134 In the MRC-ILA-Heart study of 182 patients with AMI of smaller size, without ST-segment elevation (non-STEMI), IL-1 blockade with anakinra also reduced C-reactive protein levels at 7 days, but failed to improve clinical outcomes.135 We conclude that NLRP3 inflammasome activation with enhanced IL-1 activity during AMI contribute to the risk of heart failure and that IL-1 targeting therapy may prevent recurrent atherothrombotic events but also the incidence of HF. Table 1 summarizes clinical trials with IL-1 targeted strategies in patients with AMI.
In summary, preclinical data show that the IL-1 family of cytokines and the NLRP3 inflammasome are involved in the myocardial response to ischemia-reperfusion injury, and IL-1 blockade with anakinra in patients with AMI may reduces progression to heart failure.
IL-1, inflammasome, and heart failure
Once HF is established, the condition tends to become chronic and patients experience symptoms due to impaired cardiac function at rest or with exertion, often on a daily basis.136 Patients with HF experience a progressive decline over time and are predisposed to periods of exacerbations, which accelerate the decline and shorten survival.136 Given that HF represents a final common pathway for many CVD disorders, the incidence and prevalence of HF continue to increase137 despite significant progress in the diagnosis and management. Across the spectrum of CVD in the past, many patients would not have survived yet today they are alive but face multiple disabilities due to impaired cardiac function. There is a certain urgency for more and better treatments to prevent and treat HF.
IL-1 and heart failure
Inflammation promotes and aggravates HF.93, 138 As discussed above, cytokines of the IL-1 family contribute to atherothrombosis and AMI, common events in for development of HF in addition the metabolic risk factors in HF.139, 140 Moreover, IL-1 is also known to directly modulate cardiac contractility, being recognized as one of the ‘soluble cardiodepressant factors’ in sepsis.80 Indeed, plasma from patients with severe sepsis and shock suppressed contractility and β-adrenergic receptor signaling in cardiomyocytes in vitro via a IL-1 dependent mechanism.80 Similar cardiodepressant properties were observed using serum of patients with acute decompensated systolic heart failure through an IL-1- and IL-18-mediated mechanism.141, 142 Endogenous IL-1β and IL-18 are also contribute to contractile dysfunction following myocardial ischemia.143 The evolutionary advantage of the vascular effects of the IL-1 family of cytokines may be related to the promotion of local vasodilatation in order to redirect blood flow to bring leukocytes to an area of infection. The cardiodepressant effects of IL-1β and IL-18 counterbalance the increase in oxygen consumption due to reflex tachycardia. These protective mechanisms for combatting infection become counter-productive in humans with HF. Once released, IL-1β and IL-18 impair contractility by multiple mechanisms, including those that disrupt cytoplasmic calcium handling through modulation of protein synthesis (Figure 4, Supplemental Table III).4, 5 These cumulative effects reduce the intrinsic property of cardiomyocytes to contract (inotropy) and to relax (lusitropy), observed in reductions of systolic and diastolic function.4, 5 IL-1β also appears to induce pro-arrythmic changes in vitro and in vivo.4, 5
In mice, the effects of IL-1β and IL-18 on contractility are transient and reversible,144 supporting the hypothesis that blocking these cytokine could improve or restore cardiac function in humans. In a study of 23 patients with rheumatoid arthritis - a condition with elevated IL-1β levels - a single dose of anakinra of 150 mg resulted in a significant improvement in cardiac and vascular function.145 The link between IL-1 and HF is also supported by elevated circulating levels of IL-1β as well as surrogate biomarkers such as IL-1Ra, IL-6 or CRP, each of which correlate with worsening HF symptoms and outcomes.134, 146–149 Data on plasma IL-18 are limited; in one study of 38 patients with HF, those with acute decompensation had higher IL-18 levels compared to those without decompensation.150 In preclinical models of HF due to ischemia/infarction, pressure overload, anthracycline toxicity, radiation injury or septic cardiomyopathy, reducing IL-1 or IL-18 activities directly or by blocking downstream signaling of IL-1 resulted in a more favorable remodeling pattern and an amelioration of the HF phenotype (Figure 4, Supplemental Table III).
IL-1 blockade in heart failure: early clinical trial experience
Anakinra adminstered to patients with stable chronic systolic HF resulted in a significant reduction in circulating levels of CRP and IL-6 levels; these patients experienced a significant improvement in cardiorespiratory function (measured as peak oxygen consumption) and quality of life related to cardiac fitness (Table 2).142 In patients with acute decompensated systolic heart failure, anakinra initated within 24 hours of admission had significantly reduced acute inflammatory responses compared to placebo and after 14 days of treatment, improved left ventricular ejection fraction.151 In another study in patients hospitalized for acute decompensated systolic HF, anakinra was initiated at discharge and continued for 12 weeks. Improved cardiorespiratory fitness (peak oxygen consumption), reduced levels of N-terminal pro-brain natriuretic peptide (NT-proBNP), and increased quality of life (REDHART study) was observed (Table 2).152 A larger clinical trial of anakinra for 24 weeks after hospital discharge is currently ongoing (REDHART2 study).153
Table 2.
Overview of IL-1 targeted studies in heart failure patients.
| Study | Population | Sample Size | Study Design | Intervention | Main Outcomes | PMID or NCT number | |||
|---|---|---|---|---|---|---|---|---|---|
| R | DB | PC | MC | ||||||
| Anakinra ADHF | Acute decompensated HFrEF with CRP >3 mg/l | 30 | X | X | X | Anakinra 100 mg twice daily for 3 days and then once daily for 11 | ↓hsCRP, ↓IL-6, ↑LVEF | 26906034 | |
| AIR-HF | Stable HFrEF with hsCRP >2 mg/l | 7 | Anakinra 100 mg daily for 14 days | ↓hsCRP, ↓IL-6, ↑Peak VO2, ↑exercise time, ↑QOL | 22438931 | ||||
| D-HART | Stable HFpEF with hsCRP >2 mg/l | 12 | X* | X | X | Anakinra 100 mg daily for 14 days | ↓hsCRP, ↑ Peak VO2, ↑exercise time, ↑QOL | 24262762 | |
| D-HART2 | Stable HFpEF with hsCRP >2 mg/l | 31 | X | X | X | Anakinra 100 mg daily for 12 weeks | ↓hsCRP, ↑exercise time, ↑QOL, ↓NTproBNP (no effect on peak VO2) |
30354558 | |
| Colchicine in HF | Stable HFrEF | 279 | X | X | X | Colchicine 0.5 mg twice daily for 6 months | ↓hsCRP, (no effect on exercise time, or peak VO2) | 24720919 | |
| REDHART | Post-discharge HFrEF with hsCRP >2 mg/l | 60 | X | X | X | Anakinra 100 mg daily for 12 weeks | ↓hsCRP, ↑peak VO2, ↑exercise time, ↑QOL, ↓NTproBNP | 9141858 | |
| OLATEC-HF | Stable HFrEF with hsCRP >2 mg/l | 30 | X | X | X | OLT1177 500 mg 1–4 times daily for 14 days | Completed but results not yet available | NCT03534297 | |
| REDHART2 | Post-discharge HFrEF with hsCRP >2 mg/l | 102 | X | X | X | Anakinra 100 mg daily for 24 weeks | Ongoing | NCT03797001 | |
Abbreviations: CRP = C-reactive protein; DB = Double blind; HF = Heart failure; hsCRP= high sensitivity CRP; PC = Placebo-controlled; R = Randomized.
cross-over trial.
Approximately half of HF patients have preserved ejection fraction (EF) and are therefore classified as HF with preserved EF (HFpEF). Impaired cardiac diastolic function is commonly present, in addition to other cardiac and non-cardiac abnormalities.154 Daily anakinra was administred to 12 patients with HFpEF and resulted in a modest but significant improvement in cardiorespiratory fitness (peak oxygen consumption) in a pilot cross-over trial with placebo treatment (D-HART).155 A follow-up study in 31 patients with HFpEF (D-HART2) showed significant increases in treadmill exercise time, lower NT-proBNP levels, and improved quality of life measures without significant change in peak oxygen consumption.156 In the CANTOS trial, as discussed above, patients having experienced an AMI at least 30 days before enrollment were largely free of HF. In this population, those randomized to canakinumab treatment had significantly lower hospitalizations for HF.134 Moreover, baseline IL-6 and CRP levels were independent predictors of HF hospitalizations.134 In 279 patients with stable systolic HF treated for 6 months with colchicine, a non-specific of the NLRP3 inflammasome inhibitor and of IL-1-mediated migration of myeloid cells, did not reduce HF or any significant improvement in clinical outcomes.157 A phase IB trial with dapansutrile, a specific NLRP3 inhibitor, is ongoing.158 Table 2 summarize the results IL-1-targeted clinical trials in patients with HF.
In summary, preclinical data show that the IL-1 family of cytokines contribute to cardiac dysfunction, and IL-1 blockade with anakinra or canakinumab in patients with HF improves cardiorespiratory fitness and prevents HF hospitalizations.
IL-1, the NLRP3 inflammasome and inflammatory heart disease
Inflammatory heart disease is a condition in which inflammation is considered the primary mechanism of disease.136 In such conditions, inflammation may have been initiated following an injury, often a viral infection, but the inflammation persists and can lead to organ dysfunction, pathological remodeling and heart failure.159 The various syndromes are defined by how the heart is affected. For example, myocarditis refers to inflammation affecting the myocardium159, whereas pericarditis is inflammation of pericardial covering of the heart.160, 161 Inflammatory heart syndromes are highly heterogeneous conditions with varied pathogenesis. Only recently, preclinical and clinical studies have explored the role of IL-1 and the inflammasome in these syndromes. The injury during a viral infections or from chemotherapy often activates NLRP3 inflammasome, similar to that during AMI.
Myocarditis
Patients with acute, idiopathic myocarditis following a viral infection show intense NLRP3 inflammasome formation in the heart.162 This finding was also observed in a mouse model of viral myocarditis due to Coxsackie B virus; treatment with a caspase-1 inhibitor downstream from NLRP3 rescued the animals.163 Rescue has been observed in mice with acute myocarditis but deficient in MyD88 signaling of IL-1, in transgenic mice overexpressing IL-1Ra and in mice treated with a neutralizing anti-IL-1β monoclonal antibody.163–165 In humans, anakinra has improved heart function in selected cases of acute myocarditis refractory to standard treatment.166, 167 IL-1 blockers have also been studied in non-infectious autoimmune mouse models of myocarditis, in which the disease is induced by microbial antigens triggering the inflammatory response.168 Rejection after heart transplantation represent a severe example of deregulated inflammation and myocarditis. Activation of the NLRP3 inflammasomes in the heart of patients with rejection parallels the clinical severity of the condition.169
Sarcoidosis
Cardiac sarcoidosis is a particular form of myocarditis that affects at least 25% of patients with sarcoidosis based on autopsy and imaging data.170 The clinical manifestations of cardiac sarcoidosis depend on the location of involvement and include cardiac conduction abnormalities, ventricular arrhythmias, and heart failure. The etiology sarcoidosis is not known but is likely an immunologic response to an unidentified antigenic trigger in genetically susceptible hosts. Although primary treatment is glucocosteroids and/or immunosuppressive agents, there is little convincing data of improved outcomes. Recent data reveal the formation of the NLRP3 inflammasome in myocardial granulomata of patients with cardiac sarcoidosis, opening the pathway for novel treatment.171 In a small randomized controlled trial of patients with symptomatic cardiac sarcoidosis, IL-1 blockade with anakinra is being used for evaluation of safety and efficacy.172
Pericarditis
A similar pattern of NLRP3 inflammasome activation was demonstrated within the pericardial layers of patients with chronic pericarditis.173 The benefit of colchicine, a non-specific NLRP3 inflammasome inhibitor, in treating pericarditis is well established, and is first line therapy for a first and recurrent pericarditis (Table 3).174–177 The benefits of colchicine are particularly evident in patients with recurrent pericarditis who are at greater risk of recurrences and complications.161, 174, 176
Table 3.
Evidence Supporting the Role for NLRP3 Inflammasome in Inflammatory Heart Disease
| Condition | Etiology | Evidence |
|---|---|---|
|
| ||
| Acute Myocarditis | Injury induced by virus or other irritant | • NLRP3 inflammasome is present in humans with acute idiopathic myocarditis [PMID 24439778] • Mouse model of viral myocarditis due to Coxsackie B showed inhibition of inflammasome activation led to symptom alleviation [PMID 25260607] • Anakinra has been successful in treating selective cases of acute myocarditis refractory to standard treatment [PMID 27031379, 27428134] • IL-1 blockade with anakinra prevented myocardial dysfunction in non-infectious autoimmune mouse model of myocarditis [PMID 31099056] |
|
| ||
| Cardiac Sarcoidosis | Immunologic response to unknown antigenic trigger | • NLRP3 inflammasome is present in granulomas in hearts of patients with cardiac sarcoidosis [ PMID 31522533] |
|
| ||
| Post-cardiac transplantation rejection | Acute rejection is caused by injury and death of the cardiomyocytes due to an immune (cell or antibody) response | • NLRP3 inflammasome is present in hearts of post-transplant patients with rejection and parallels the clinical severity of the disease [PMID 26301674] |
|
| ||
| Acute (or recurrent) Pericarditis | 80–90% presumed to be post-viral; May also be related to myocardial infarction, percutaneous coronary intervention, cardiac procedures, autoimmune disease, chest irradiation and cancer |
• Colchicine, a non-specific NLRP3 inflammasome inhibitor, is effective in reducing the risk recurrence after a first or recurrent episode of pericarditis [PMID 16186437; 16186468; 23992557; 24694983] • AIRTRIP trial: randomized, placebo-controlled trial of 21 patients with recurrent pericarditis refractory to colchicine and dependent on corticocosteroids who were treated with anakinra 100 mg daily for 60 days. [PMID 27825009] ➢ All patients responded and were able to come off corticosteroids. ➢ Patients were then randomized to continuation of anakinra vs. placebo, with 90% survival free of recurrence with anakinra vs. 18% in placebo. • 16 patients treated with Rilonacept (recombinant chimeric fusion protein that acts as a trap for IL-1α and IL-1β) in a 24 week phase II study showed significant decrease in pericardial pain and CRP levels with 100% response rate [Abstract - J Am Coll Cardiol 2019;73 Suppl 1:1261] |
Abbreviations: IL-1, interleukin-1; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3
A causal role for IL-1-mediated inflammation in pericarditis is also found in the AnakInRa for Treatment of Recurrent Idiopathic Pericarditis (AIRTRIP trial).160, 178 The AIRTRIP trial included patients with recurrent pericarditis, who were resistant to colchicine and dependent on corticosteroid therapy, receiving anakinra 100 mg daily for 60 days.178 All patients responded to anakinra and stopped glucorticoid therapy. After remission of symptoms, patients were assigned continuation of anakinra or switching to placebo. Those randomized to anakinra experienced a 90% survival free of recurrence as compared with 18% in placebo.178 Other studies report the efficacy of anakinra in acute and chronic pericarditis.160, 179 A 24-week follow-up Phase II study with rilonacept, a soluble IL-1 receptor chimeric fusion protein neutralizing IL-1α and IL-1β, was performed in 16 patients with recurrent pericarditis and showed a significant decrease in both pericardial pain and CRP within 24 hours following the first dose with a 100% response rate.180 A Phase III clinical trial with rilonacept is currently ongoing.181 A small proof-of-concept clinical trial of anakinra in acute pericarditis is currently ongoing.182
Together, these data validate the role of the NLRP3 inflammasome and IL-1 in myocarditis and pericarditis. Although colchicine remains the first-line treatment for patients with pericarditis, IL-1 blockade with anakinra has become standard of care in patients refractory to initial treatment.161 Table 3 summarizes pre-clinical and clinical evidence supporting a role for IL-1-targeted therapy in inflammatory heart disease.
Clinically Available Pharmacologic Inhibitors
There are three approved biologics for blocking IL-1: anakinra, a recombinant form of the naturally occurring IL-1Ra; rilonacept, a soluble chimeric Fc fusion protein of IL-1R1 and IL-1R3 and canakinumab, a humanized monoclonal antibody specific for neutralizing IL-1β (Table 4). Although none of these IL-1 blockers have an indication for a CVD at the present time, each has been explored in patients with heart diseases. Other IL-1 blocking biologics in clinical trial but not approved for any indication are bermekimab, a naturally occuring monoclonal antibody specific for neutralizing IL-1α; gevokizumab, a humanized monoclonal antibody specific for neutralizing IL-1β; lutikizumab, a humanized monoclonal antibody with dual affinity for IL-1α and IL-1β; and AMG108, a humanized monoclonal antibody targeting IL-1R1 that blocks IL-1α and IL-1β. Tadekinig alfa is a recombinant form of naturally occurring IL-18BP with a high affinity for neutralizing IL-18. In addition, dapansutrile, an orally active specific inhibitor of NLRP3, is used for reducing the processing and release of IL-1β. Both approved and those in clinical studies are well-tolerated and have broad safety margins when used in efficacious doses.
Table 4.
Interleukin-1 inhibitors in clinical practice.
| Drug | Class | Target | Dose, route and frequency | Approved indications | Adverse reactions, precautions and warnings (apply to the entire class) |
|---|---|---|---|---|---|
| Anakinra (Kineret ®) | Receptor antagonist | IL-1R1 | 100 mg (or 1–8 mg/kg in children) SC once daily | • Rheumatoid arthritis • Cryopyrin-associated periodic syndromes |
• These drugs should not be initiated, and should be discontinued, in patients with active infections. • Monitoring of neutrophil count is recommended during their use • These drugs should not be used, or used cautiously, together with other immune modulating drug. • Increased awareness and monitoring for infection are indicated during treatment - blunting of symptoms and signs of infections may occur delaying diagnosis and treatment possibly increasing the risk of death due to infection due to common pathogens (the rates of opportunistic infections are not increased). • Injection site reactions are common (1–15%), yet generally self-limiting. • Hypersensitivity reactions may occur, but anaphylaxis and angioedema are extremely rare. |
| Rilonacept (Arcalyst ®) | Soluble decoy receptor | IL-1α, IL-1β (IL-1Ra) | 320 mg (or 4.4 mg/kg in children) SC as loading dose then 160 mg (or 2.2 mg/kg) SC every week | • Cryopyrin-associated periodic syndromes | |
| Canakinumab (Ilaris®) | Monoclonal antibody | IL-1β | 150–300 mg (or 2–4 mg/kg in children) SC every 4–8 weeks | • Cryopyrin-associated periodic syndromes • Tumor Necrosis Factor Receptor Associated Periodic Syndrome • Hyperimmunoglobulin D Syndrome /Mevalonate Kinase Deficiency • Familial Mediterranean Fever • Systemic juvenile idiopathic arthritis |
Abbreviations: IL-1 = Isnterleukin-1; IL-1Ra= IL-1 receptor antagonist; IL-1R1 = Intereukin-1 receptor 1; SC = subcutaneous
Anakinra, recombinant IL-1 receptor antagonist
Anakinra (Kineret®, Swedish Orphan Biovitrum, Stockholm, Sweden) blocks the IL-1R1 inhibiting the activities of IL-1α and IL-1β. Anakinra is approved for rheumatoid arthritis and Cryopyrin Associated Periodic Syndrome, a multisystemic IL-1β-mediated disease due to a gain of function in NLRP3. A single 100 mg dose of anakinra is administered subcutaneously once daily and in exploratory cardiovascular diseases, increasing the daily does does not significantly improve outcomes.133 The most common side effect is injection site reactions such as pain and erythema in approximately 20% of patients that often abate with continued use. A large number of patients, both adults and children, have been treated with daily anakinra for more than 15 years and the safety of anakinra is well-established compared to other biologics.183 Although rare allergic reactions and anaphylaxis to anakinra have been reported, anakinra is not associated with untoward effects on blood pressure, renal function, metabolism or hepatic toxicity. Anakinra can lead to decreases in neutrophil counts, which is reversible; nevertheless, counts should monitor periodically with chronic use. Anakinra is contraindicated in patients with active or recurrent infections. While anakinra does not increase the risk of reactivation of M. tuberculosis or opportunistic infections, an increase in the severity of common infections can be expected. An intrinsic risk for infection as with the use of all anti-inflammatory biologics, the blunting of signs and symptoms of inflammation, for example fever, can result in delayed recognition of an indulant infection. A distinct advantage of anakinra is the rapid half-life such that withdrawal of treatment is recommended for patients with minor symptoms of infection. Relevant to the safety of anakinra over compared to other biologics, three randomized controlled trials of anakinra in over 2,000 patients with severe sepsis and septic shock revealed that 28-day mortality was not worse for those treated with anakinra. In fact, there was a potential signal for benefit in patients with more severe sepsis.24, 184 Since approval in 2001, off-label use of anakinra use in thousands or patients supports its safety.
Rilonacept, chimeric soluble decoy receptor for IL-1
Rilonacept (Arcalyst®, Regeneron Pharmaceuticals, Tarrytown, NY, USA) is a chimeric recombinant fusion protein consisting of the IL-1 receptor 1 and the IL-1 receptor accessory protein, functioning as a soluble decoy protein that binds IL-1α, IL-1β, and IL-1Ra. Rilonacept is approved for the treatment of CAPS and is administered as a loading dose of 320 mg subcutaneous injection once followed by 160 mg every 2 weeks. Rilonacept is also associated with injection site reactions. Similar to all IL-1 blockers, as discussed above, awareness for the risk of infections also apply for rilonacept. The clinical trial and real life experiences with rilonacept is rather limited, when compared with anakinra, but the safety profile appears to be comparable.
Canakinumab, monoclonal IL-1β antibody
Canakinumab (Ilaris®, Novartis, Basel, Switzerland) is a humanized monoclonal antibody neutralizing IL-1β and not IL-1α, and as such canakinumab differs from anakinra and rilonacept. Canakinumb has a long half-life and is dosed once monthly at a dose of 150 to 300 mg (or 2–4 mg/kg in pediatric patients) for the CAPS, Tumor Necrosis Factor Receptor Associated Periodic Syndrome, Hyperimmunoglobulin D Syndrome/Mevalonate Kinase Deficiency, Familial Mediterranean Fever, and Systemic Juvenile Idiopathic Arthritis. The Phase II pilot study prelude to the CANTOS trial185 showed that a single dose of canakinumab at 1.5 mg/kg was sufficient to reduce CRP by >40% for up to 12 weeks. Therefore, in the CANTOS trial canakinumab was administered every 12 weeks.13 Nevertheless, the long duration of action, however advantageous for many reasons, has a down-side when managing side effects or complications. The CANTOS trial represents the largest cytokine inhibition study ever completed in any indication and provides several thousands of patient-years of exposure.13 Although canakinumab did increase infections, including infection-related deaths, there was a highly significant reduction in cancer-related deaths.13 Infection-related deaths was approximately 0.1% of all infections and related to common bacterial infections, not to opportunistic infections. Infections appeared to be present across all dose groups and was statistically significant versus placebo only when all 3 group canakinumab treatment groups (50, 150 and 300 mg) were pooled together.13 A 77% reduction in mortality in patients with lung cancer was observed with the 300 mg dose. 186. During the CANTOS trial, patients who developed a cancer did not receive further canakinumab but rather were treated with standard of care, suggesting that only a short duration neutralizing IL-1β improves anti-tumor standard of care.
Other inhibitors
Gevocizumab, a humanized monoclonal antibody against IL-1β with residual agonistic activity, is in clinical development but not approved.187 Recent interest in the NLRP3 inflammasome has led to preclinical studies of several inhibitors. Colchicine has been proposed as a non-specific NLRP3 inflammasome inhibitor and has explored in cardiovascular clinical trials. Whether the anti-inflammatory properties of colchicine are due to inhibition of the inflammasome or an inhibitor of IL-1 induced migration of leukocytes, requires further research. To date, colchicine is not approved for any cardiovascular indication. Dapansutrile (OLT1177) is a specific NLRP3 inflammasome inhibitor being tested in humans for cardiovascular and other indications.105, 158 No NLRP3 inflammasome inhibitors are currently approved for any indication. There are several recent in-depth reviews on NLRP3 inflammasome inhibitors.16, 62, 188
Conclusions
The NLRP3 inflammasome and IL-1 family of cytokines are central to the pathologic response to injury and represent a key pathogenetic mechanism in the formation, progression, and complication of atherosclerosis and the myocardial response to ischemic and non-ischemic injury. IL-1 targeted therapies have shown alredy to improve cardiovascular outcomes in clinical trials in patients with or at risk for AMI, HF, and recurrent pericarditis. Canakinumab, IL-1β antibody, prevented the recurrence of ischemic events in patients with prior AMI in a large phase III clinical trial including 10,061 patients world-wide. Phase II clinical trials show promising data with anakinra, recombinant IL-1 receptor antagonist, in patients with ST segment elevation AMI or HF with reduced ejection fraction. Anakinra also improved outcomes in patients with pericarditis and it is now considered standard of care as second line treatment for patients with recurrent/refractory pericarditis. Rilonacept, a soluble IL-1 receptor chimeric fusion protein neutralizing IL-1α and IL-1β, has also shown promising results in a phase II study in recurrent/ refractory pericarditis. Colchicine, a non-specific NLRP3 inflammasome inhibitor and inhibitor of IL-1 mediated leukocyte migration, is also an effective treatment for pericarditis, and it has been recently shown to reduce the risk of recurrent ischemic events in patients with AMI. Whether the effects of colchicine in cardiovascular disease are mediated by an effect on the NLRP3 inflammasome and/or IL-1 signaling remains to be determined. Specific oral NLRP3 inflammasome inhibitors are under clinical development. There is therefore overwhelming evidence linking the NLRP3 inflammasome and the IL-1 cytokines with the pathogenesis of cardiovascular diseases. The future will likely include targeted inhibitors to block the IL-1 isoforms, and possibly oral NLRP3 inflammasome inhibitors, across a wide spectrum of cardiovascular diseases.
Supplementary Material
Acknowledgments
Sources of funding: none.
Disclosures: A.A. has received research grant funding and has served as a paid scientific advisor to GSK, Kiniksa, Merck, Novartis, Olatec, Serpin Pharma, and Swedish Orphan Biovitrum; S.T. has received research grant funding from Kiniksa, Olatec and Serpin Pharma. C.M. serves as Director for Olatec’s Innovative Science Program and has equity in Olatec; B.V.T. has received research grant funding from Swedish Orphan Biovitrum and he has served as a paid scientific advisor to Serpin Pharma; C.A.D. serves as Chairman of Olatec’s Scientific Advisory Board, is co-Chief Scientific Officer, receives compensation and has equity in Olatec. J.K. has no disclosures related to this manuscript.
Non-standard Abbreviations and Acronyms
- AIRTRIP
AnakInRa for Treatment of Recurrent Idiopathic Pericarditis
- AMI
acute myocardial infarction
- ASC
apoptosis-associated spec-like protein containing a carboxy-terminal containing a caspase recruiting domain
- ATPase
adenosine triphosphatase
- BP
binding protein
- CIRT
Cardiovascular Inflammation Reduction Trial
- CANTOS
Canakinumab Anti-inflammatory Thrombosis Outcomes Study
- COLCOT
Colchicine Cardiovascular Outcome Trial
- CRP
C-reactive protein
- CV
cardiovascular
- CVD
CV disease
- DAMPs
damage associated molecular patterns
- D-HART
Diastolic Heart Failure Anakinra Response Trial(s)
- eATP
extracellular adenosine triphosphate
- GSDMD
gasdermin D
- HF
heart failure
- HFpEF
HF with preserved EF
- IL
interleukin
- MAS
Macrophage Activation Syndrome
- LDL-R
low-density lipoprotein receptor
- LoDoCo
Low Dose Colchicine
- MRC-ILA-Heart study
title of a UK anakinra in non-STEMI trial
- MyD88
myeloid differentiation factor 88
- NF-κB
Nuclear factor-κB
- NLRP3
NACHT LRR and PYD domains-containing protein 3
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- P2X7
purinergic receptor 2X7
- R
receptor
- Ra
receptor antagonist
- REDHART
Recently Decompensated Heart Failure Anakinra Response Trial(s)
- STEMI
ST-segment elevation myocardial infarction
- ST2
suppressor of tumoregenicity 2
- sST2
soluble ST2
- TIR
Toll-IL-1-Receptor
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
- VCUART
Virginia Commonwealth University Anakinra Remodeling/Response Trial(s)
References
- 1.Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat Rev Rheumatol. 2019;15:612–632. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Tassell BW, Toldo S, Mezzaroma E and Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128:1910–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley LF and Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J. 2018;39:2063–2069. [DOI] [PubMed] [Google Scholar]
- 5.Buckley LF and Abbate A. Interleukin-1 Blockade in Cardiovascular Diseases: From Bench to Bedside. BioDrugs. 2018;32:111–118. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CJ, Kono H, Golenbock D, Reed G, Akira S and Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–6. [DOI] [PubMed] [Google Scholar]
- 8.Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S and Dinarello CA. The Interleukin-1alpha Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front Immunol. 2013;4:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurt-Jones EA, Beller DI, Mizel SB and Unanue ER. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985;82:1204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brody DT and Durum SK. Membrane IL-1: IL-1 alpha precursor binds to the plasma membrane via a lectin-like interaction. J Immunol. 1989;143:1183–7. [PubMed] [Google Scholar]
- 11.Ter Horst R, Jaeger M, Smeekens SP, et al. Host and Environmental Factors Influencing Individual Human Cytokine Responses. Cell. 2016;167:1111–1124 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, Wittkowski H, Bek S, Hartmann N, Bosset S, Hawkins PN and Jung T. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 14.Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J, Nemecek R, Rogowski W, Lesniewski-Kmak K, Petruzelka L, Apte RN, Mohanty P, Stecher M, Simard J and de Gramont A. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18:192–201. [DOI] [PubMed] [Google Scholar]
- 15.Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE and Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–5. [DOI] [PubMed] [Google Scholar]
- 16.Toldo S and Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–214. [DOI] [PubMed] [Google Scholar]
- 17.Toldo S, Mauro AG, Cutter Z and Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315:H1553–H1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman HM, Gregory SG, Mueller JL, Tresierras M, Broide DH, Wanderer AA and Kolodner RD. Fine structure mapping of CIAS1: identification of an ancestral haplotype and a common FCAS mutation, L353P. Hum Genet. 2003;112:209–16. [DOI] [PubMed] [Google Scholar]
- 19.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN and Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25. [DOI] [PubMed] [Google Scholar]
- 20.Netea MG, Nold-Petry CA, Nold MF, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fantuzzi G, Ku G, Harding MW, Livingston DJ, Sipe JD, Kuida K, Flavell RA and Dinarello CA. Response to local inflammation of IL-1 beta-converting enzyme- deficient mice. J Immunol. 1997;158:1818–24. [PubMed] [Google Scholar]
- 22.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA and Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–36. [DOI] [PubMed] [Google Scholar]
- 23.Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, Sthoeger Z, Keane WF, Chvatchko Y, Kim SH, Fantuzzi G, Dinarello CA and Rubinstein M. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine. 2001;14:334–42. [DOI] [PubMed] [Google Scholar]
- 24.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ and Opal SM. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016;44:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinarello CA, Nold-Petry C, Nold M, Fujita M, Li S, Kim S and Bufler P. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol. 2016;46:1067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG, Puccetti M, Garlanda C, Kim S, Li S, van de Veerdonk FL, Dinarello CA and Romani L. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Pathog. 2014;10:e1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, Joosten LA, van der Meer JW, Hao R, Kalabokis V and Dinarello CA. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci U S A. 2012;109:3001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Veerdonk FL, de Graaf DM, Joosten LA and Dinarello CA. Biology of IL-38 and its role in disease. Immunol Rev. 2018;281:191–196. [DOI] [PubMed] [Google Scholar]
- 29.Xu WD and Huang AF. Role of Interleukin-38 in Chronic Inflammatory Diseases: A Comprehensive Review. Front Immunol. 2018;9:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 31.Libby P and Hansson GK. From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:1594–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgozoglu L and Lewis EF. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. [DOI] [PubMed] [Google Scholar]
- 33.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, Takayanagi T, Egashira K and Takeshita A. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest. 1996;97:769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, Iwakura Y and Ohsuzu F. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–73. [DOI] [PubMed] [Google Scholar]
- 35.Devlin CM, Kuriakose G, Hirsch E and Tabas I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc Natl Acad Sci U S A. 2002;99:6280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamberlain J, Evans D, King A, Dewberry R, Dower S, Crossman D and Francis S. Interleukin-1beta and signaling of interleukin-1 in vascular wall and circulating cells modulates the extent of neointima formation in mice. Am J Pathol. 2006;168:1396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bevilacqua MP, Pober JS, Majeau GR, Cotran RS and Gimbrone MA Jr. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS and Gimbrone MA Jr. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76:2003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamberlain J, Francis S, Brookes Z, Shaw G, Graham D, Alp NJ, Dower S and Crossman DC. Interleukin-1 regulates multiple atherogenic mechanisms in response to fat feeding. PLoS One. 2009;4:e5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dewberry R, Holden H, Crossman D and Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2394–400. [DOI] [PubMed] [Google Scholar]
- 41.Libby P, Ordovas JM, Auger KR, Robbins AH, Birinyi LK and Dinarello CA. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986;124:179–85. [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, Owens GK, Upchurch GR Jr. and Ailawadi G. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2013;33:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicklin MJ, Hughes DE, Barton JL, Ure JM and Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhaskar V, Yin J, Mirza AM, Phan D, Vanegas S, Issafras H, Michelson K, Hunter JJ and Kantak SS. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011;216:313–20. [DOI] [PubMed] [Google Scholar]
- 45.Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF and Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–4. [DOI] [PubMed] [Google Scholar]
- 46.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL and Owens GK. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St Hilaire C, Muller W, Waisman A, Francis SE, Pinteaux E, Randolph GJ, Gram H and Owens GK. Interleukin-1beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med. 2018;24:1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vromman A, Ruvkun V, Shvartz E, Wojtkiewicz G, Santos Masson G, Tesmenitsky Y, Folco E, Gram H, Nahrendorf M, Swirski FK, Sukhova GK and Libby P. Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur Heart J. 2019;40:2482–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E and Chen YE. Rabbit models for the study of human atherosclerosis: from pathophysiological mechanisms to translational medicine. Pharmacol Ther. 2015;146:104–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khanna V, Jain M, Singh V, Kanshana JS, Prakash P, Barthwal MK, Murthy PS and Dikshit M. Cholesterol diet withdrawal leads to an initial plaque instability and subsequent regression of accelerated iliac artery atherosclerosis in rabbits. PLoS One. 2013;8:e77037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clinton SK, Fleet JC, Loppnow H, Salomon RN, Clark BD, Cannon JG, Shaw AR, Dinarello CA and Libby P. Interleukin-1 gene expression in rabbit vascular tissue in vivo. Am J Pathol. 1991;138:1005–14. [PMC free article] [PubMed] [Google Scholar]
- 52.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT and Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menu P, Pellegrin M, Aubert JF, Bouzourene K, Tardivel A, Mazzolai L and Tschopp J. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell Death Dis. 2011;2:e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gage J, Hasu M, Thabet M and Whitman SC. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Can J Cardiol. 2012;28:222–9. [DOI] [PubMed] [Google Scholar]
- 56.Hendrikx T, Jeurissen ML, van Gorp PJ, Gijbels MJ, Walenbergh SM, Houben T, van Gorp R, Pottgens CC, Stienstra R, Netea MG, Hofker MH, Donners MM and Shiri-Sverdlov R. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in Ldlr(−/−) mice. FEBS J. 2015;282:2327–38. [DOI] [PubMed] [Google Scholar]
- 57.Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Hida S, Sagara J, Taniguchi S and Takahashi M. Critical role of caspase-1 in vascular inflammation and development of atherosclerosis in Western diet-fed apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2012;425:162–8. [DOI] [PubMed] [Google Scholar]
- 58.Zheng F, Xing S, Gong Z and Xing Q. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ. 2013;22:746–50. [DOI] [PubMed] [Google Scholar]
- 59.Afrasyab A, Qu P, Zhao Y, Peng K, Wang H, Lou D, Niu N and Yuan D. Correlation of NLRP3 with severity and prognosis of coronary atherosclerosis in acute coronary syndrome patients. Heart Vessels. 2016;31:1218–29. [DOI] [PubMed] [Google Scholar]
- 60.van der Heijden T, Kritikou E, Venema W, van Duijn J, van Santbrink PJ, Slutter B, Foks AC, Bot I and Kuiper J. NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report. Arterioscler Thromb Vasc Biol. 2017;37:1457–1461. [DOI] [PubMed] [Google Scholar]
- 61.Bertinaria M, Gastaldi S, Marini E and Giorgis M. Development of covalent NLRP3 inflammasome inhibitors: Chemistry and biological activity. Arch Biochem Biophys. 2019;670:116–139. [DOI] [PubMed] [Google Scholar]
- 62.Marchetti C. The NLRP3 Inflammasome as a Pharmacological Target. J Cardiovasc Pharmacol. 2019;74:285–296. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y, Wang H, Kouadir M, Song H and Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ridker PM, MacFadyen JG, Thuren T and Libby P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1beta inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur Heart J. 2019. [DOI] [PubMed] [Google Scholar]
- 65.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, Bayard F and Hansson GK. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–40. [DOI] [PubMed] [Google Scholar]
- 66.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y and Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89:E41–5. [DOI] [PubMed] [Google Scholar]
- 67.McLaren JE, Michael DR, Salter RC, Ashlin TG, Calder CJ, Miller AM, Liew FY and Ramji DP. IL-33 reduces macrophage foam cell formation. J Immunol. 2010;185:1222–9. [DOI] [PubMed] [Google Scholar]
- 68.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB and Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J, Lin J, He S, et al. Transgenic Overexpression of IL-37 Protects Against Atherosclerosis and Strengthens Plaque Stability. Cell Physiol Biochem. 2018;45:1034–1050. [DOI] [PubMed] [Google Scholar]
- 70.Ji Q, Zeng Q, Huang Y, Shi Y, Lin Y, Lu Z, Meng K, Wu B, Yu K, Chai M, Liu Y and Zhou Y. Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm. 2014;2014:165742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chai M, Ji Q, Zhang H, et al. The Protective Effect of Interleukin-37 on Vascular Calcification and Atherosclerosis in Apolipoprotein E-Deficient Mice with Diabetes. J Interferon Cytokine Res. 2015;35:530–9. [DOI] [PubMed] [Google Scholar]
- 72.Ji Q, Meng K, Yu K, Huang S, Huang Y, Min X, Zhong Y, Wu B, Liu Y, Nie S, Zhang J, Zhou Y and Zeng Q. Exogenous interleukin 37 ameliorates atherosclerosis via inducing the Treg response in ApoE-deficient mice. Sci Rep. 2017;7:3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng Q, Song R, Fullerton DA, Ao L, Zhai Y, Li S, Ballak DB, Cleveland JC Jr., Reece TB, McKinsey TA, Xu D, Dinarello CA and Meng X. Interleukin-37 suppresses the osteogenic responses of human aortic valve interstitial cells in vitro and alleviates valve lesions in mice. Proc Natl Acad Sci U S A. 2017;114:1631–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res. 2016;118:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herder C, de Las Heras Gala T, Carstensen-Kirberg M, et al. Circulating Levels of Interleukin 1-Receptor Antagonist and Risk of Cardiovascular Disease: Meta-Analysis of Six Population-Based Cohorts. Arterioscler Thromb Vasc Biol. 2017;37:1222–1227. [DOI] [PubMed] [Google Scholar]
- 76.Schofer N, Ludwig S, Rubsamen N, Schnabel R, Lackner KJ, Ruprecht HJ, Bickel C, Landmesser U, Blankenberg S and Zeller T. Prognostic impact of Interleukin-1 receptor antagonist in patients with documented coronary artery disease. Int J Cardiol. 2018;257:24–29. [DOI] [PubMed] [Google Scholar]
- 77.Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM and Glynn RJ. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39:3499–3507. [DOI] [PubMed] [Google Scholar]
- 78.Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, Rupprecht HJ and AtheroGene I. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. [DOI] [PubMed] [Google Scholar]
- 79.Fitzgerald AA, Leclercq SA, Yan A, Homik JE and Dinarello CA. Rapid responses to anakinra in patients with refractory adult-onset Still’s disease. Arthritis Rheum. 2005;52:1794–803. [DOI] [PubMed] [Google Scholar]
- 80.Kumar A, Thota V, Dee L, Olson J, Uretz E and Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collaboration CRPCHDG, Wensley F, Gao P, et al. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R and Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T and Group CPI. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48. [DOI] [PubMed] [Google Scholar]
- 84.Abbate A. Why the CANTOS Is a Game Changer in Cardiovascular Medicine. J Cardiovasc Pharmacol. 2017;70:353–355. [DOI] [PubMed] [Google Scholar]
- 85.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ and Group CT. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 86.Ridker PM, Everett BM, Pradhan A, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loppnow H and Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest. 1990;85:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tardif JC, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 89.Leung YY, Yao Hui LL and Kraus VB. Colchicine--Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taskiran EZ, Cetinkaya A, Balci-Peynircioglu B, Akkaya YZ and Yilmaz E. The effect of colchicine on pyrin and pyrin interacting proteins. J Cell Biochem. 2012;113:3536–46. [DOI] [PubMed] [Google Scholar]
- 91.Nidorf SM, Eikelboom JW, Budgeon CA and Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. [DOI] [PubMed] [Google Scholar]
- 92.Anderson JL and Morrow DA. Acute Myocardial Infarction. N Engl J Med. 2017;376:2053–2064. [DOI] [PubMed] [Google Scholar]
- 93.Zuurbier CJ, Abbate A, Cabrera-Fuentes HA, Cohen MV, Collino M, De Kleijn DPV, Downey JM, Pagliaro P, Preissner KT, Takahashi M and Davidson SM. Innate immunity as a target for acute cardioprotection. Cardiovasc Res. 2019;115:1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toldo S, Mezzaroma E, McGeough MD, Pena CA, Marchetti C, Sonnino C, Van Tassell BW, Salloum FN, Voelkel NF, Hoffman HM and Abbate A. Independent roles of the priming and the triggering of the NLRP3 inflammasome in the heart. Cardiovasc Res. 2015;105:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF and Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shao W, Yeretssian G, Doiron K, Hussain SN and Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–9. [DOI] [PubMed] [Google Scholar]
- 97.Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao C, Xin C, Zhu D, Li Y, Yan W, Xiong L, Gao E, Wang H and Tao L. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2014;109:415. [DOI] [PubMed] [Google Scholar]
- 98.Sandanger O, Ranheim T, Vinge LE, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99:164–74. [DOI] [PubMed] [Google Scholar]
- 99.Kawaguchi M, Takahashi M, Hata T, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. [DOI] [PubMed] [Google Scholar]
- 100.Jong WM, Leemans JC, Weber NC, Juffermans NP, Schultz MJ, Hollmann MW and Zuurbier CJ. Nlrp3 plays no role in acute cardiac infarction due to low cardiac expression. Int J Cardiol. 2014;177:41–3. [DOI] [PubMed] [Google Scholar]
- 101.Toldo S, Marchetti C, Mauro AG, Chojnacki J, Mezzaroma E, Carbone S, Zhang S, Van Tassell B, Salloum FN and Abbate A. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int J Cardiol. 2016;209:215–20. [DOI] [PubMed] [Google Scholar]
- 102.Fulp J, He L, Toldo S, Jiang Y, Boice A, Guo C, Li X, Rolfe A, Sun D, Abbate A, Wang XY and Zhang S. Structural Insights of Benzenesulfonamide Analogues as NLRP3 Inflammasome Inhibitors: Design, Synthesis, and Biological Characterization. J Med Chem. 2018;61:5412–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marchetti C, Chojnacki J, Toldo S, Mezzaroma E, Tranchida N, Rose SW, Federici M, Van Tassell BW, Zhang S and Abbate A. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol. 2014;63:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mastrocola R, Penna C, Tullio F, Femmino S, Nigro D, Chiazza F, Serpe L, Collotta D, Alloatti G, Cocco M, Bertinaria M, Pagliaro P, Aragno M and Collino M. Pharmacological Inhibition of NLRP3 Inflammasome Attenuates Myocardial Ischemia/Reperfusion Injury by Activation of RISK and Mitochondrial Pathways. Oxid Med Cell Longev. 2016;2016:5271251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toldo S, Mauro AG, Cutter Z, Van Tassell BW, Mezzaroma E, Del Buono MG, Prestamburgo A, Potere N and Abbate A. The NLRP3 Inflammasome Inhibitor, OLT1177 (Dapansutrile), Reduces Infarct Size and Preserves Contractile Function After Ischemia Reperfusion Injury in the Mouse. J Cardiovasc Pharmacol. 2019;73:215–222. [DOI] [PubMed] [Google Scholar]
- 106.van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA, Arslan F, de Jager SC, Robertson AA, Pasterkamp G and Hoefer IE. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J. 2017;38:828–836. [DOI] [PubMed] [Google Scholar]
- 107.Fujisue K, Sugamura K, Kurokawa H, Matsubara J, Ishii M, Izumiya Y, Kaikita K and Sugiyama S. Colchicine Improves Survival, Left Ventricular Remodeling, and Chronic Cardiac Function After Acute Myocardial Infarction. Circ J. 2017;81:1174–1182. [DOI] [PubMed] [Google Scholar]
- 108.Deftereos S, Giannopoulos G, Angelidis C, Alexopoulos N, Filippatos G, Papoutsidakis N, Sianos G, Goudevenos J, Alexopoulos D, Pyrgakis V, Cleman MW, Manolis AS, Tousoulis D and Lekakis J. Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation. 2015;132:1395–403. [DOI] [PubMed] [Google Scholar]
- 109.Abbate A, Salloum FN, Van Tassell BW, Vecile E, Toldo S, Seropian I, Mezzaroma E and Dobrina A. Alterations in the interleukin-1/interleukin-1 receptor antagonist balance modulate cardiac remodeling following myocardial infarction in the mouse. PLoS One. 2011;6:e27923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK and Nahrendorf M. Targeting Interleukin-1beta Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation. 2015;132:1880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abbate A, Salloum FN, Vecile E, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–83. [DOI] [PubMed] [Google Scholar]
- 112.Abbate A, Van Tassell BW, Seropian IM, Toldo S, Robati R, Varma A, Salloum FN, Smithson L and Dinarello CA. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12:319–22. [DOI] [PubMed] [Google Scholar]
- 113.Mauro AG, Mezzaroma E, Torrado J, Kundur P, Joshi P, Stroud K, Quaini F, Lagrasta CA, Abbate A and Toldo S. Reduction of Myocardial Ischemia-Reperfusion Injury by Inhibiting Interleukin-1 Alpha. J Cardiovasc Pharmacol. 2017;69:156–160. [DOI] [PubMed] [Google Scholar]
- 114.Toldo S, Mezzaroma E, Bressi E, Marchetti C, Carbone S, Sonnino C, Van Tassell BW and Abbate A. Interleukin-1beta blockade improves left ventricular systolic/diastolic function and restores contractility reserve in severe ischemic cardiomyopathy in the mouse. J Cardiovasc Pharmacol. 2014;64:1–6. [DOI] [PubMed] [Google Scholar]
- 115.Toldo S, Mezzaroma E, Van Tassell BW, Farkas D, Marchetti C, Voelkel NF and Abbate A. Interleukin-1beta blockade improves cardiac remodelling after myocardial infarction without interrupting the inflammasome in the mouse. Exp Physiol. 2013;98:734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Tassell BW, Varma A, Salloum FN, Das A, Seropian IM, Toldo S, Smithson L, Hoke NN, Chau VQ, Robati R and Abbate A. Interleukin-1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;55:117–22. [DOI] [PubMed] [Google Scholar]
- 117.Toldo S, Schatz AM, Mezzaroma E, Chawla R, Stallard TW, Stallard WC, Jahangiri A, Van Tassell BW and Abbate A. Recombinant human interleukin-1 receptor antagonist provides cardioprotection during myocardial ischemia reperfusion in the mouse. Cardiovasc Drugs Ther. 2012;26:273–6. [DOI] [PubMed] [Google Scholar]
- 118.Wei Y, Lan Y, Zhong Y, Yu K, Xu W, Zhu R, Sun H, Ding Y, Wang Y and Zeng Q. Interleukin-38 alleviates cardiac remodelling after myocardial infarction. J Cell Mol Med. 2020;24:371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu B, Meng K, Ji Q, et al. Interleukin-37 ameliorates myocardial ischaemia/reperfusion injury in mice. Clin Exp Immunol. 2014;176:438–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu D, Wang A, Jiang F, Hu J and Zhang X. Effects of interleukin-37 on cardiac function after myocardial infarction in mice. Int J Clin Exp Pathol. 2015;8:5247–51. [PMC free article] [PubMed] [Google Scholar]
- 121.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J and Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2:684–91. [DOI] [PubMed] [Google Scholar]
- 122.Yin H, Li P, Hu F, Wang Y, Chai X and Zhang Y. IL-33 attenuates cardiac remodeling following myocardial infarction via inhibition of the p38 MAPK and NF-kappaB pathways. Mol Med Rep. 2014;9:1834–8. [DOI] [PubMed] [Google Scholar]
- 123.Chen WY, Hong J, Gannon J, Kakkar R and Lee RT. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc Natl Acad Sci U S A. 2015;112:7249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN and Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Januzzi JL, Pascual-Figal D and Daniels LB. ST2 testing for chronic heart failure therapy monitoring: the International ST2 Consensus Panel. Am J Cardiol. 2015;115:70B–5B. [DOI] [PubMed] [Google Scholar]
- 126.Pascual-Figal DA and Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. 2015;115:3B–7B. [DOI] [PubMed] [Google Scholar]
- 127.Vecile E, Dobrina A, Salloum FN, Van Tassell BW, Falcione A, Gustini E, Secchiero S, Crovella S, Sinagra G, Finato N, Nicklin MJ and Abbate A. Intracellular function of interleukin-1 receptor antagonist in ischemic cardiomyocytes. PLoS One. 2013;8:e53265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seropian IM, Toldo S, Van Tassell BW and Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–603. [DOI] [PubMed] [Google Scholar]
- 129.Abbate A, Kontos MC, Abouzaki NA, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol. 2015;115:288–92. [DOI] [PubMed] [Google Scholar]
- 130.Abbate A, Kontos MC, Grizzard JD, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol. 2010;105:1371–1377 e1. [DOI] [PubMed] [Google Scholar]
- 131.Abbate A, Van Tassell BW, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111:1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Van Tassell BW, Lipinski MJ, Appleton D, et al. Rationale and design of the Virginia Commonwealth University-Anakinra Remodeling Trial-3 (VCU-ART3): A randomized, placebo-controlled, double-blinded, multicenter study. Clin Cardiol. 2018;41:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abbate A, Trankle CR, Buckley L, et al. Interleukin-1 blockade Inhibits the Acute Inflammatory Response in Patients with ST-segment Elevation Myocardial Infarction. J Am Heart Association 2020. – in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ and Ridker PM. Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation. 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 135.Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD and Crossman DC. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Van Tassell BW, Raleigh JM and Abbate A. Targeting interleukin-1 in heart failure and inflammatory heart disease. Curr Heart Fail Rep. 2015;12:33–41. [DOI] [PubMed] [Google Scholar]
- 137.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 138.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Herder C, Dalmas E, Boni-Schnetzler M and Donath MY. The IL-1 Pathway in Type 2 Diabetes and Cardiovascular Complications. Trends Endocrinol Metab. 2015;26:551–563. [DOI] [PubMed] [Google Scholar]
- 140.Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD and Crowley SD. Interleukin-1 Receptor Activation Potentiates Salt Reabsorption in Angiotensin II-Induced Hypertension via the NKCC2 Co-transporter in the Nephron. Cell Metab. 2016;23:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Toldo S, Mezzaroma E, O’Brien L, Marchetti C, Seropian IM, Voelkel NF, Van Tassell BW, Dinarello CA and Abbate A. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2014;306:H1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Van Tassell BW, Arena RA, Toldo S, Mezzaroma E, Azam T, Seropian IM, Shah K, Canada J, Voelkel NF, Dinarello CA and Abbate A. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7:e33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pomerantz BJ, Reznikov LL, Harken AH and Dinarello CA. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci U S A. 2001;98:2871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Van Tassell BW, Seropian IM, Toldo S, Mezzaroma E and Abbate A. Interleukin-1beta induces a reversible cardiomyopathy in the mouse. Inflamm Res. 2013;62:637–40. [DOI] [PubMed] [Google Scholar]
- 145.Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos PN and Kremastinos DT. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–9. [DOI] [PubMed] [Google Scholar]
- 146.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG and Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055–9. [DOI] [PubMed] [Google Scholar]
- 147.Testa M, Yeh M, Lee P, Fanelli R, Loperfido F, Berman JW and LeJemtel TH. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol. 1996;28:964–71. [DOI] [PubMed] [Google Scholar]
- 148.van Wezenbeek J, Canada JM, Ravindra K, et al. Determinants of Cardiorespiratory Fitness in Patients with Heart Failure Across a Wide Range of Ejection Fractions. Am J Cardiol. 2020;125:76–81. [DOI] [PubMed] [Google Scholar]
- 149.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D’Agostino RB and Framingham Heart S. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–91. [DOI] [PubMed] [Google Scholar]
- 150.Di Somma S, Pittoni V, Raffa S, Magrini L, Gagliano G, Marino R, Nobili V and Torrisi MR. IL-18 stimulates B-type natriuretic peptide synthesis by cardiomyocytes in vitro and its plasma levels correlate with B-type natriuretic peptide in non-overloaded acute heart failure patients. Eur Heart J Acute Cardiovasc Care. 2017;6:450–461. [DOI] [PubMed] [Google Scholar]
- 151.Van Tassell BW, Abouzaki NA, Oddi Erdle C, et al. Interleukin-1 Blockade in Acute Decompensated Heart Failure: A Randomized, Double-Blinded, Placebo-Controlled Pilot Study. J Cardiovasc Pharmacol. 2016;67:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Van Tassell BW, Canada J, Carbone S, et al. Interleukin-1 Blockade in Recently Decompensated Systolic Heart Failure: Results From REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. https://www.clinicaltrials.gov/ct2/show/NCT03797001.
- 154.Del Buono MG, Buckley L and Abbate A. Primary and Secondary Diastolic Dysfunction in Heart Failure With Preserved Ejection Fraction. Am J Cardiol. 2018;122:1578–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Van Tassell BW, Arena R, Biondi-Zoccai G, Canada JM, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, Voelkel NF, Dinarello CA and Abbate A. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol. 2014;113:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Tassell BW, Trankle CR, Canada JM, et al. IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2018;11:e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Deftereos S, Giannopoulos G, Panagopoulou V, Bouras G, Raisakis K, Kossyvakis C, Karageorgiou S, Papadimitriou C, Vastaki M, Kaoukis A, Angelidis C, Pagoni S, Pyrgakis V, Alexopoulos D, Manolis AS, Stefanadis C and Cleman MW. Anti-inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. JACC Heart Fail. 2014;2:131–7. [DOI] [PubMed] [Google Scholar]
- 158. https://www.clinicaltrials.gov/ct2/show/NCT03534297.
- 159.Heymans S, Eriksson U, Lehtonen J and Cooper LT Jr. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. [DOI] [PubMed] [Google Scholar]
- 160.Buckley LF, Viscusi MM, Van Tassell BW and Abbate A. Interleukin-1 blockade for the treatment of pericarditis. Eur Heart J Cardiovasc Pharmacother. 2018;4:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chiabrando JG, Bonaventura A, Vecchie A, Wohlford GF, Mauro AG, Jordan JH, Grizzard JD, Montecucco F, Berrocal DH, Brucato A, Imazio M and Abbate A. Management of Acute and Recurrent Pericarditis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:76–92. [DOI] [PubMed] [Google Scholar]
- 162.Toldo S, Kannan H, Bussani R, Anzini M, Sonnino C, Sinagra G, Merlo M, Mezzaroma E, De-Giorgio F, Silvestri F, Van Tassell BW, Baldi A and Abbate A. Formation of the inflammasome in acute myocarditis. Int J Cardiol. 2014;171:e119–21. [DOI] [PubMed] [Google Scholar]
- 163.Wang Y, Gao B and Xiong S. Involvement of NLRP3 inflammasome in CVB3-induced viral myocarditis. Am J Physiol Heart Circ Physiol. 2014;307:H1438–47. [DOI] [PubMed] [Google Scholar]
- 164.Fuse K, Chan G, Liu Y, Gudgeon P, Husain M, Chen M, Yeh WC, Akira S and Liu PP. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. 2005;112:2276–85. [DOI] [PubMed] [Google Scholar]
- 165.Lim BK, Choe SC, Shin JO, Ho SH, Kim JM, Yu SS, Kim S and Jeon ES. Local expression of interleukin-1 receptor antagonist by plasmid DNA improves mortality and decreases myocardial inflammation in experimental coxsackieviral myocarditis. Circulation. 2002;105:1278–81. [PubMed] [Google Scholar]
- 166.Cavalli G, Pappalardo F, Mangieri A, Dinarello CA, Dagna L and Tresoldi M. Treating Life-Threatening Myocarditis by Blocking Interleukin-1. Crit Care Med. 2016;44:e751–4. [DOI] [PubMed] [Google Scholar]
- 167.Noji Y. Anakinra in Fulminant Myocarditis: Targeting Interleukin-1 and the Inflammasome Formation. Crit Care Med. 2016;44:1630–1. [DOI] [PubMed] [Google Scholar]
- 168.Gorelik M, Lee Y, Abe M, Andrews T, Davis L, Patterson J, Chen S, Crother TR, Aune GJ, Noval Rivas M and Arditi M. IL-1 receptor antagonist, anakinra, prevents myocardial dysfunction in a mouse model of Kawasaki disease vasculitis and myocarditis. Clin Exp Immunol. 2019;198:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shah KB, Mauro AG, Flattery M, Toldo S and Abbate A. Formation of the inflammasome during cardiac allograft rejection. Int J Cardiol. 2015;201:328–30. [DOI] [PubMed] [Google Scholar]
- 170.Birnie DH, Nery PB, Ha AC and Beanlands RS. Cardiac Sarcoidosis. J Am Coll Cardiol. 2016;68:411–21. [DOI] [PubMed] [Google Scholar]
- 171.Kron J, Mauro AG, Bonaventura A, Toldo S, Salloum FN, Ellenbogen KA and Abbate A. Inflammasome Formation in Granulomas in Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol. 2019;12:e007582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. https://clinicaltrials.gov/ct2/show/NCT04017936.
- 173.Bonaventura A, Cannatà A, Mezzaroma E, Sinagra G, Bussani R, Toldo S, Gandhi R, Kim M, Paolini J, Montecucco F, Abbate A, Mauro AG. Intensification of the Inflammasome Formation in the Pericardium of Patients With Chronic Severe Pericarditis. Circulation 2019; 140:A13363. [Google Scholar]
- 174.Imazio M, Belli R, Brucato A, Cemin R, et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet. 2014;383:2232–7. [DOI] [PubMed] [Google Scholar]
- 175.Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F, Moratti M, Gaschino G, Giammaria M, Ghisio A, Belli R and Trinchero R. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation. 2005;112:2012–6. [DOI] [PubMed] [Google Scholar]
- 176.Imazio M, Bobbio M, Cecchi E, Demarie D, Pomari F, Moratti M, Ghisio A, Belli R and Trinchero R. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med. 2005;165:1987–91. [DOI] [PubMed] [Google Scholar]
- 177.Imazio M, Brucato A, Cemin R, et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med. 2013;369:1522–8. [DOI] [PubMed] [Google Scholar]
- 178.Brucato A, Imazio M, Gattorno M, et al. Effect of Anakinra on Recurrent Pericarditis Among Patients With Colchicine Resistance and Corticosteroid Dependence: The AIRTRIP Randomized Clinical Trial. JAMA. 2016;316:1906–1912. [DOI] [PubMed] [Google Scholar]
- 179.Imazio M, Andreis A, De Ferrari GM, et al. Anakinra for corticosteroid-dependent and colchicine-resistant pericarditis: The IRAP (International Registry of Anakinra for Pericarditis) study. Eur J Prev Cardiol. 2019:2047487319879534. [DOI] [PubMed] [Google Scholar]
- 180.Klein Allan, Lin David, Cremer Paul, Nasir Saifullah, Crugnale Sharon, Collins Larisa, Fang Fang, Beutler Anna and Paolini John F. Rilonacept in recurrent pericarditis: first efficacy and safety data from an ongoing Phase 2 pilot clinical trial. J Am Coll Cardiol. 2019; 1204–052. [Google Scholar]
- 181. https://www.clinicaltrials.gov/ct2/show/NCT03737110.
- 182. https://www.clinicaltrials.gov/ct2/show/NCT03224585.
- 183.Dinarello CA, Simon A and van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Opal SM, Fisher CJ Jr., et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25:1115–24. [DOI] [PubMed] [Google Scholar]
- 185.Noe A, Howard C, Thuren T, Taylor A and Skerjanec A. Pharmacokinetic and pharmacodynamic characteristics of single-dose Canakinumab in patients with type 2 diabetes mellitus. Clin Ther. 2014;36:1625–37. [DOI] [PubMed] [Google Scholar]
- 186.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ and Group CT. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 187.Cavelti-Weder C, Babians-Brunner A, Keller C, Stahel MA, Kurz-Levin M, Zayed H, Solinger AM, Mandrup-Poulsen T, Dinarello CA and Donath MY. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35:1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Buckley LF and Libby P. Inhibiting NLRP3 Inflammasome Activity in Acute Myocardial Infarction: A Review of Pharmacologic Agents and Clinical Outcomes. J Cardiovasc Pharmacol. 2019;74:297–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.