Highlights
-
•
No presence of anti-SARS-CoV-2 antibodies in individuals before the pandemic
-
•
28% seroprevalence of anti-SARS-CoV-2 antibodies in asymptomatic adults
-
•
4% active SARS-CoV-2 infection in villagers
-
•
57% of IgG-positive individuals also had neutralizing antibodies
Keywords: SARS-CoV-2, IgG and IgM, antibody, Bomassa, Republic of Congo
Abstract
Objectives
With limited data available from Central Africa, the aim of our study was to evaluate the anti-SARS-CoV-2 Ab prevalence in indigenous residents of Bomassa, a village located in the Sangha region in the Republic of Congo.
Methods
Plasma and oropharyngeal swab samples were collected from 304 healthy adult individuals, randomly recruited in May 2021 before vaccine introduction in the area. In addition, 82 plasma samples from the same area in 2019 were included as controls for the investigation of cross-reactivity against other coronaviruses. The SARS-CoV-2 virus was detected by qRT-PCR and sequenced using next-generation sequencing. ELISA was used for detecting IgG, IgM, and neutralizing Ab against SARS-CoV-2 antigens.
Results
Around 4.9% (15/304) of the participants were SARS-CoV-2 positive, with B.1.631 being the only variant identified. Of 109 individuals harboring anti-SARS-CoV-2 IgG and/or IgM Ab, 45.9% (50/109) had anti-SARS-CoV-2 neutralizing Ab. Of the control samples collected before the pandemic, 3.7% (3/82) were positive for IgG, but negative for neutralizing Ab.
Conclusions
Seroprevalence against SARS-CoV-2 occurred in 25% of the indigenous population sample, with almost 50% of these seropositive participants possessing neutralizing antibodies. These findings suggest that the spread of SARS-CoV-2 has been underestimated in the Republic of Congo.
Introduction
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently the most important public health problem worldwide. Since its emergence in China in December 2019, this novel coronavirus had infected over 213 million people across the globe by August 23, 2021, leading to over 4 million deaths worldwide (WHO, 2021). The African continent, which has a population of more than one billion, had reported around 5 539 401 confirmed cases and 131 857 associated deaths for the same period, with 201 154 cases and 3133 deaths in Central Africa (WHO, 2021).
Diagnosis of SARS-CoV-2 infection is based on reliable testing, while determining the spread of the disease is highly dependent on the testing capacity (Kobia and Gitaka, 2020), which can be especially challenging in rural areas of sub-Saharan Africa (United Nations, 2020). Sero-epidemiological studies have reported the presence of specific IgM and IgG Ab against SARS-CoV-2 virus (Graham, 2020; Zhu et al., 2020), which seem to persist from some days (IgM) to many months (IgG) (WHO, 2020). Thus, several antibody-based serological rapid diagnostic tests (Graham, 2020; Zhu et al., 2020) and ELISA tests (CDC, 2020) have been developed for the detection of current or past SARS-CoV-2 infection.
The seroprevalence of anti-SARS-CoV-2 IgG has been reported as 42.3% among truck drivers and assistants in Kenya (Uyoga et al., 2021), 42% among blood donors in Nigeria, 45% among frontline health workers with no COVID-19 symptoms from Ibadan, Nigeria (Olayanju et al. 2021), 40% among public-sector patients in Cape Town, South Africa (Hsiao et al. 2020), and 12.3% among healthcare workers with no COVID-19 in Blantyre, Malawi (Chibwana et al., 2020). Similarly, anti-SARS-CoV-2 IgM prevalence has been estimated at 41% among blood donors from Nigeria (Nna et al., 2021). Together, these sero-epidemiological data reveal the high exposure of the African population to SARS-CoV-2 infection, even though pre-pandemic samples from sub-Saharan Africa had higher cross-reactivity against SARS-CoV-2 than those from the Americas and Europe (Nkuba Ndaye et al., 2021).
A recent cohort study from the UK reported the association of a past history of SARS-CoV-2 infection with an 84% lower risk of reinfection, with a median protective effect observed 7 months following the primary infection (Hall et al., 2021), showing that previous infections provide strong immunity to future infections. This evidence has been confirmed by another report showing the persistence SARS-CoV-2-specific IgG-secreting memory B cells and durable CD8 and CD4 T cells recognizing distinct viral epitope regions in many individuals for over 200 days post infection (Cohen et al., 2021). These findings suggest that reduced doses of vaccine, as compared with current recommended doses, might be sufficient for an Ab response boosting in individuals with naturally acquired anti-SARS-CoV-2 Ab. Thus, data on natural anti-SARS-CoV-2 Ab responses might help to improve vaccination strategies in the non-vaccinated population.
The Republic of Congo reported its first SARS-CoV-2 infection case on March 14, 2020, with a total of 13 493 cases identified and 179 deaths reported within the first 17 months of the pandemic in the country (SITREP-171, 2021). To date, only seroprevalence data from the capital Brazzaville have been reported, as these account for more than 60% of cases. Limited RT-PCR testing capacity restricts the evaluation of the pandemic in the country, particularly in remote regions like the Sangha region in the north, where since the beginning of the pandemic 154 cases have been reported so far, with 130 cases in August 2020 and 15 cases in April 2021 (SITREP-141 and SITREP-146, 2021). Therefore, this study investigated anti-SARS-CoV-2 seroprevalence in Bomassa village, situated in the rainforest in the Sangha region. The collected data will contribute to improving the implementation of preventive measures, including vaccination strategies, in the community.
Materials and methods
Ethical considerations
The study protocol was reviewed and approved by the Institutional Ethics Committee of the Congolese Foundation for Medical Research (Ethical Clearance No. 030/CIE/FCRM/2020). Participation in the study was voluntary, with written informed consent obtained from each individual. SARS-CoV-2 diagnosis was performed for each participant at the time of enrollment, using reverse transcription PCR (qRT-PCR), and all positive results were reported to the physician for care planning according to national policy.
Study area
This descriptive cross-sectional study was conducted in Bomassa village in northern Republic of Congo (2°12′N, 16°11′E), located on the Sangha River and the border with Central African Republic (Figure 1). The village lies 800 m from the Nouabale-Ndoki National Park, which is known for its local rainforests and home to wide variety of large mammals. The inhabitants of the village are mostly indigenous (Pygmies and Bantous) and predominantly illiterate (61%), with hunting, fishing and fruit picking as their main activities.
Figure 1.
Map showing the geographical location of Bomassa village
Study population and sample collection
The study was carried out between May 20 and June 2, 2021. In total, 304 Congolese residents aged from 18 to 60 years old, and with no known COVID-19 symptoms, were randomly recruited across all of Bomassa village. In addition, 82 plasma samples collected from individuals living in the same area between June and July 2019 (before the COVID-19 pandemic) were included in the analysis as controls for the investigation of cross-reactivity between SARS-CoV-2 antigens and Ab induced by other coronavirus antigens. Data on the participants’ health, age, address, and sex were collected using a standard questionnaire. Oropharyngeal swabs collected in viral transport media were transported at −80°C to the laboratory for the diagnosis of SARS-CoV-2 infection by qRT-PCR and sequencing of the virus. Peripheral blood samples collected in EDTA tubes from each participant, were centrifuged, and the obtained plasma stored at −80°C for antibody assay.
Diagnosis of SARS-CoV-2 infection
SARS-CoV-2 was detected using a multiplex qRT-PCR kit, according to the manufacturer's protocol. In brief, total nucleic acid extraction was performed using a QIAamp RNA mini kit (Qiagen GmbH, Hilden, Germany), with 140 µL of the supernatant used to yield a nucleic acid elution volume of 50 µL. The detection of SARS-CoV-2 was performed by qRT-PCR using a Light-Cycler 480 Instrument II (Roche Diagnostics, Mannheim, Germany) and a RealStar SARS-CoV-2 RT-PCR kit 1 (Altona Diagnostics, Germany), according to the manufacturer's protocol. This kit uses three probes: the first targets an E gene specific for B-βCoV; the second probe targets an S gene specific for SARS-CoV-2; and the last targets the internal control (IC). All specimens with an S gene probe cycle threshold (Ct) less than or equal to 30 were considered positive.
Viral genome sequencing
For all SARS-CoV-2-positive samples, cDNA synthesis was performed with NEB LunaScript RT SuperMix Kit (E3010) using 8 µl of extracted viral RNA and 2 µl of LunaScript RT SuperMix (5x) according to manufacturer's instructions. Direct amplification of the viral genome cDNA was then performed. After synthesis, cDNA was amplified using 29 pairs of multiplex primers subdivided into two pools (15 and 14 pairs of primers in pool 1 and pool 2, respectively). Each primer pair amplified a 1200 bp DNA fragment. PCR amplification was carried out using Q5 Hot Start DNA Taq Polymerase with 2.5 µl of cDNA in a 22.5 µl reaction volume of the mix. The PCR program used was as follows: an initial step of 98°C for 30 s, then 35 cycles of 98°C for 15 s, followed by 5 minutes at 65°C. After PCR, amplicons from both pools were mixed and validated using agarose gel electrophoresis.
A volume of 45 µl of PCR amplicons was purified using AMPure XP beads. In brief, for each sample, 25 µl of beads were added, followed by washing steps and elution with 20 µl of 10 mM Tris, HCl, and 50 mM NaCl, at pH 7.5–8. Next, 3 µl of the purified DNA was used to quantify the nucleic acid, in order to normalize the concentration for each sample to 100 ng in a volume of 7.5 µl. Then, 2.5 µl of corresponding barcode was added to each sample and incubation performed at 30°C for 1 minute and then at 80°C for 1 minute. Samples with barcode were pooled in a single tube, and purification as well as elution of 10 µl of DNA was performed. Adapter ligation and cleanup were performed using the Oxford Nanopore kit, according to manufacturer's specifications. Libraries were sequenced on the Nanopore MinION device using FLO-MIN106D flow cells. For read-quality filtering, sequence reads were aligned to a SARS-CoV-2 reference genome sequence, and consensus sequence generation was performed using ARTIC Network software. Isolate lineages were determined with PANGOLIN, and consensus genome sequences deposited in the GISAID database.
Measurement of plasma IgG and IgM Ab
IgG and IgM Ab against SARS-CoV-2 antigens were measured using the Virotech SARS-CoV-2 IgG, IgM ELISA kit (Virotech Diagnostics GmbH, Germany), according to the manufacturer's protocol. The Virotech unit (VU) or the cut-off value for seropositivity of IgG and IgM Ab was calculated for each plasma sample as follows:
VU = [optical density (OD) (individual plasma)/OD (calibrator control × correction factor)] × 10
According to the kit manufacturer, any sample with VU > 11 was positive for the assayed Ab. Negative control, positive control, and calibrator control (mix of positive control and negative control) were included in each assay for quality control. As recommended by the kit manufacturer, the control results for a valid assay were as follows: OD450 value < 0.09 for negative control, OD450 value > 0.10 for calibrator control, and OD450 value > 0.30 for positive control.
Measurement of anti-SARS-CoV-2 neutralizing Ab
The presence of anti-SARS-CoV-2 Ab in the study participants’ plasma was investigated using the cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit (Nanjing GenScript Biotech, China) according to the manufacturer's protocol. Plasma samples and controls were diluted with sample dilution buffer (1:9), and the peroxidase conjugated spike protein receptor binding domain (HRP-RBD) diluted with HRP dilution buffer (1:1000). Diluted positive controls and negative controls, as well as each of diluted plasma samples, were mixed with diluted HRP-RBD in tubes at a volume ratio of 1:1, and incubated at 37°C for 30 minutes to allow the binding of the circulating neutralization antibodies to HRP-RBD. Next, 100 µL volumes of the positive control mixture, the negative control mixture, and each plasma sample mixture were added to the corresponding wells of the capture microplate, which was pre-coated with the human receptor for angiotensin-converting enzyme 2 (hACE2) protein, and incubated at 37°C for 15 minutes. After this incubation step, the microplate was washed four times with 260 µL of wash solution per well to remove the circulating neutralization Ab-HRP-RBD complexes remaining in the supernatant. Following a wash cycle, 100 µL of enzyme substrate, tetramethyl benzidine (TMB), was added to each well and the microplate was incubated in the dark at 25°C for 15 minutes. Finally, 50 µL of stop solution was added to each well to stop the reaction, and the absorbance of the final solution in each well immediately measured at 450 nm using an ELISA microplate reader. Both negative and positive controls were included in each assay for quality control. Control results for a valid assay were as follows: OD450 value > 1.0 for negative control and OD450 value < 0.3 for positive control. In addition, the optical density (OD450) value of the negative control was used to calculate the percentage inhibition as follows:
inhibition = [1 − (OD value of sample/OD value of negative control)] × 100%
A sample was declared positive for neutralizing Ab if its inhibition was at least 30%.
Serologigal and virological (RT-PCR and sequencing) tests were performed at the same time at the Centre de Recherches sur les Maladies Infectieuses – Christophe Méreiux in Brazzaville.
Statistical analysis
Graph Pad Prism 6.0.1 was used for the statistical analyses. Continuous variables were reported as medians with interquartile ranges (IQR), while categorical variables were reported as percentages and compared using Fisher's exact test or the chi-square test. Values of p < 0.05 were considered statistically significant.
Results
Study population
The characteristics of individuals enrolled in this study are summarized in Table 1. Overall, 304 healthy adult individuals were recruited in Bomassa from May to June, 2021. The study population was stratified into four age groups – 18–24, 25–34, 35–44, and 45–60 years. In total, 63 individuals (20.8%) were unable to give their date of birth (age unknown). Males represented 63.8% (194/304) of the study participants. All participants were subjected to oropharyngeal swab for investigation of SARS-CoV-2 infection, with 15 individuals testing qRT-PCR SARS-CoV-2 positive, giving a prevalence of asymptomatic SARS-CoV-2 infection of 4.9% (15/304).
Table 1.
Characteristics of the study population.
| Variables | Study participants (n = 304) |
|---|---|
| Age in years (median with ranges) | 30 (18–60) |
| 18–24 years, n (%) | 71 (23.3) |
| 25–34 years, n (%) | 88 (28.9) |
| 35–44 years, n (%) | 41 (13.5) |
| 45–60 years, n (%) | 41 (13.5) |
| Unknown, n (%) | 63 (20.8) |
| Sex: | |
| Male, n (%) | 194 (63.8) |
| Female, n (%) | 110 (36.2) |
| RT-PCR positive, n (%) | 15 (4.9) |
Table 2.
Seroprevalence of anti-SARS-CoV-2 antibodies in qRT-PCR-positive and qRT-PCR-negative individuals.
| All individuals | qRT-PCR+ | qRT-PCR− | p-value | |
|---|---|---|---|---|
| IgG | 87/304 (28.7%) | 8/15 (53.3%) | 79/289 (27.3%) | 0.040 |
| IgM | 38/304 (12.5%) | 2/15 (13.3%) | 36/289 (12.5%) | 0.99 |
| Neutralizing Ab | 50/109 (45.8%) | 3/8 (37.5%) | 47/101 (46.5%) | 0.72 |
The seroprevalence of anti-SARS-CoV-2 Ab was compared between qRT-PCR-positive and qRT-PCR-negative groups using the Fisher exact test. Ab: antibody
SARS-CoV-2 variants detected in Congolese participants
Of the 15 positive samples detected in this study, those with low Ct were subjected to sequencing. All sequences corresponded to the B.1.631 lineage, belonging to clade G. It was found that all sequences carried the E484K mutation and many additional mutations of interest, including like spike P681H, spike A243del, spike D614G, spike H69del, spike H245Y, spike L242del, spike L244del, and spike P26S.
Prevalence of anti-SARS-CoV-2 antibodies in plasma samples from villagers in the Republic of Congo before the COVID-19 pandemic
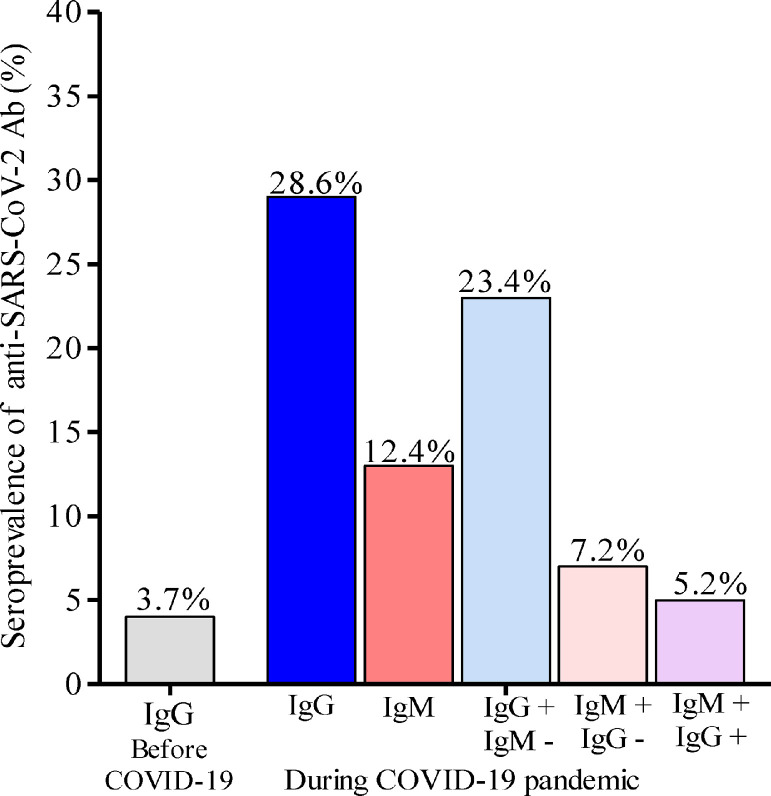
During an investigation conducted in June and July 2019, healthy adults residing in Bomassa were enrolled and venous blood samples collected for planned immunological investigations. In total, 82 plasma samples from residents who provided consent for future use of their biological materials were tested in 2021 for the presence of total IgG against SARS-CoV-2. Of the 82 plasma samples, three were positive for anti-SARS-CoV-2 IgG Ab, giving a prevalence of 3.7% (Figure 2). These three plasmas subsequently tested negative using the neutralizing assay.
Figure 2.
Seroprevalence of anti-SARS-CoV-2 in individuals from Bomassa village. IgG+: anti-SARS-CoV-2 IgG positive; IgG−: anti-SARS-CoV-2 IgG negative; IgM+: anti-SARS-CoV-2 IgM positive; IgM−: anti-SARS-CoV-2 IgM negative.
Prevalences of anti-SARS-CoV-2 IgG and IgM Ab during the pandemic
Overall seroprevalences of anti-SARS-CoV-2 Ab were 28.6% (87/304) for IgG and 12.5% (38/304) for IgM in the enrolled individuals (Figure 2; Table 3). However, the seroprevalence of IgG Ab was significantly higher in SARS-CoV-2 qRT-PCR-positive individuals (53.3%, 8/15) compared with SARS-CoV-2 qRT-PCR-negative individuals (27.3%, 79/289) (p = 0.04), while the seroprevalence of IgM Ab did not differ between SARS-CoV-2 qRT-PCR-positive individuals (13.3%, 2/15) and SARS-CoV-2 qRT-PCR-negative individuals (12.3%, 36/289) (p = 0.99).
Table 3.
Seroprevalence of anti-SARS-CoV-2 antibodies, stratified by age group.
| Age group | IgG | IgM |
|---|---|---|
| 18–24 years | 20/71 (28.2%) | 7/71 (9.9%) |
| 25–34 years | 22/87 (25.3%) | 8/87 (9.2%) |
| 35–44 years | 15/41 (36.6%) | 3/41 (7.3%) |
| 45–60 years | 16/42 (38.1%) | 8/42 (19.0%) |
| p-value | 0.37 | 0.29 |
Seroprevalences of anti-SARS-CoV-2 IgG and IgM Ab in non-infected individuals in relation with age and sex
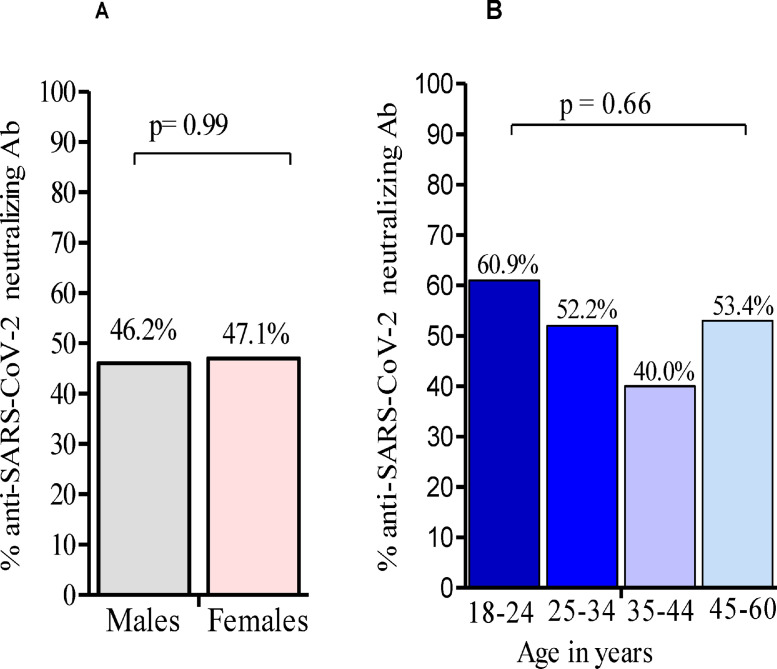
Anti-SARS-CoV-2 IgG and IgM Ab prevalences did not differ significantly according to age groups (Table 3). In addition, the seroprevalences of IgG and IgM Ab did not differ significantly between males (28.8% and 12.9% respectively), and females (28.2% and 11.8%, respectively).
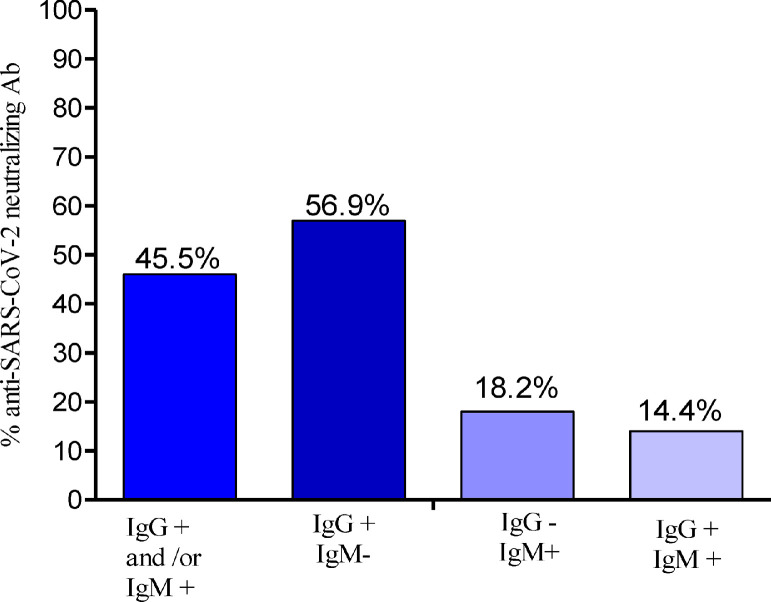
Seroprevalences of anti-SARS-CoV-2 neutralizing antibodies in IgG- and/or IgM-positive individuals
The seroprevalence of anti-SARS-CoV-2 neutralizing Ab was 45.9% (50/125) in IgG-and/or IgM-positive individuals (Figure 3). Individuals harboring only IgG Ab were more frequently positive for neutralizing Ab (56.9%, 37/65) compared with individuals harboring IgM Ab exclusively (18.2%, 4/22), and those harboring both IgM and IgG Ab (14.4%, 2/14) (data not shown). The seroprevalence of neutralizing Ab was not significantly higher in SARS-CoV-2 qRT-PCR-negative individuals (46.5%,) compared with SARS-CoV-2 qRT-PCR-positive individuals (37.5%) (p = 0.72) (Table 3). Sex and age had no impact on the seroprevalence of neutralizing Ab (Figures 4A and 4B).
Figure 3.
Seroprevalence of anti-SARS-CoV-2 neutralizing Ab in IgG/IgM-positive individuals. Ab: antibody; IgG+: anti-SARS-CoV-2 IgG positive; IgG−: anti-SARS-CoV-2 IgG negative; IgM+: anti-SARS-CoV-2 IgM positive; IgM−: anti-SARS-CoV-2 IgM negative.
Figure 4.
Seroprevalence of anti-SARS-CoV-2 neutralizing Ab in IgG/IgM-positive individuals by age group, and sex. Fisher's exact test was used for the comparison of Ab seroprevalence between males and females, while the comparison of Ab seroprevalence across age groups was performed using the chi-square test. Ab: antibody
Discussion
Our study aimed to evaluate the anti-SARS-CoV-2 Ab prevalence in Congolese individuals with no known related COVID-19 symptoms, living in Bomassa village. The results indicated that 29%, and 12.5% of individuals harbored anti-SARS-CoV-2 IgG and IgM, respectively. Therefore, it could be stated that more than 25% of the indigenous population living in Bomassa village might have been in contact with SARS-CoV-2, to which they have produced specific IgG and/or IgM antibodies, with about 12.5% experiencing recent exposure.
The prevalence of anti-SARS-CoV-2 Ab found in this study was either higher or lower than estimates presented in previous studies carried out across the African continent. Studies carried out in Nigeria have shown anti-SARS-CoV-2 seroprevalences ranging from 25% to 45%, depending on the population sampled (Olayanju et al., 2021; Nna et al., 2021; Majiya et al., 2021). Similarly, the seroprevalence of anti-SARS-CoV-2 Ab was found to be 40% in public-sector patients in Cape Town, South Africa (Hsiao et al., 2020), 12% (8.2–16.5) among asymptomatic healthcare workers in Blantyre, Malawi (Chibwana et al., 2020), and 25% among gold mine workers in Côte d'Ivoire (Milleliri et al., 2021). In Addis Ababa, Ethiopia, the Ab seroprevalence among those reporting no close contact with SARS-CoV-2-infected individuals was 9% in April 2020 (Alemu et al., 2020). Anti-SARS-CoV-2 Ab seroprevalence was lower, at 4.3% (2.9–5.8), in blood donors in Kenya in June (Uyoga et al., 2021), increasing to 9% (7.6–10.8) by September 2020 (Adetifa et al., 2021).
The higher or lower anti-SARS-CoV-2 Ab prevalence observed in this study compared with previous reports may be due to variation in SARS-CoV-2 epidemiology in different locations, time periods, or sub-populations, as well as difficulty in accessing vaccination. Moreover, our study was carried out from May to June 2021, in rural area, whereas the majority of other studies were undertaken during the first 5 months of the pandemic in 2020, and mostly in cities, which are densely populated. Furthermore, the use of non-internationally standardized serological tests may have contributed to these differences; a study in Kinshasa, Democratic Republic of the Congo showed that seropositivity in health-facility staff ranged from 8% to 36% depending on the serological test used (Iyer et al., 2020).
These studies together indicate that SARS-CoV-2 has spread widely in sub-Saharan Africa, including rural areas. Antibody titers have been shown to be important for the evaluation of protective anti-SARS-CoV-2 Ab responses. The lack of standard anti-SARS-CoV-2 IgG and IgM Ab did not allow the determination of these parameters in our study. Nevertheless, the ELISA test used is more sensitive compared with antibody-based serological immunochromatographic tests, which were used in over 50% of previous sero-epidemiological studies carried out in Africa (Olayanju et al., 2021; Majiya et al., 2021; Nna et al., 2021).
The estimated level of SARS-CoV-2 spread in Bomassa village reported by this study should be a cause for concern for the health authorities, who should reinforce COVID-19 preventive measures in this vulnerable population. COVID-19 represents a grave health threat to this indigenous community, which already experiences poor access to healthcare, significantly higher rates of communicable diseases, and a lack of access to essential services, sanitation, and other key preventive measures, such as clean water, soap, and disinfectant (United Nations, 2020).
In this study, 45.8% (50/109) of individuals harboring IgG and/or IgM Ab had anti-SARS-CoV-2 neutralizing Ab. This was consistent with a recent cohort study that reported the association of a previous history of SARS-CoV-2 infection with the persistence of SARS-CoV-2-specific IgG-secreting memory B cells and durable, specific CD8 and CD4 T cells in many individuals for over 200 days post infection (Cohen et al., 2021). It also suggests a potential contribution of natural SARS-CoV-2 infection to acquired Ab-mediated immunity against COVID-19. On other hand, the detection of anti-SARS-CoV-2 IgG Ab in 3.7% of plasma samples collected in Bomassa village before the COVID-19 pandemic was in agreement with previous studies showing the existence of cross-reactivity of the tests with other circulating coronaviruses in African countries, which could lower their specificity. In fact, the presence of pre-existing antibodies recognizing SARS-CoV-2 in uninfected individuals due to seasonal coronaviruses was also identified by Ng et al. (Ng et al., 2020), while Tso et al. showed that pre-pandemic samples from sub-Saharan Africa had higher cross-reactivity against SARS-CoV-2 than those from the USA (Tso et al., 2021). Among the three pre-pandemic plasma samples that tested positive for anti-SARS-CoV-2 IgG in our study, none was positive to anti-SARS-CoV-2 neutralizing Ab, suggesting high specificity of the Ab neutralization test used in the study.
Although males and females are known to differ in their immunological responses to foreign pathogens, conflicting data have been reported concerning sex and anti-SARS-CoV-2 Ab responses, with some reports showing a higher prevalence of IgM Ab in females than in males (Borges et al., 2020), with others showing no association between IgM Ab and sex (Uyoga et al., 2021; Adetifa et al., 2021). In our study, no significant impact of sex or age was found on the assayed anti-SARS-CoV-2 Ab prevalence, suggesting that the Ab response against SARS-CoV-2 in Bomassa village, as well as the viral spread, was similar in males and females.
Among the 15 SARS-CoV-2 qRT-PCR-positive individuals found in this study, seven (46.6%) individuals were negative for anti-SARS-CoV-2 Ab. Since these individuals had active SARS-CoV-2 infection, the question arises as to why they did not produce detectable Ab against the virus. Several explanations seem feasible. First, some of the individuals may have become infected too close to the date of sample collection to have produced Ab. Second, the SARS-CoV-2 viral load could have been below the level required to induce an immune response.
Finally, not all allelic forms of SARS-CoV-2 antigen were used in this study. Since some of these antigens have been shown to be very polymorphic (Ntoumi et al., 2021; Hou et al., 2020), it is possible that some individuals had Ab against SARS-CoV-2 antigens that were not detected. In support of this possibility are data from our study and others conducted in Africa. In fact, although B.1.631 was the only SARS-CoV-2 variant identified, all the sequences found in our study carried the E484K mutation, which has been associated with decreased therapeutic/vaccine efficacy. Many other mutations of interest, including P681H, spike A243del, spike D614G, spike H69del, spike H245Y, spike L242del, spike L244del, and spike P26S, were also identified in these sequences. These data were in line with the findings of a previous study by Ntoumi et al. carried out in Brazzaville, where all SARS-CoV-2 genomes carried the spike mutation D614G (Ntoumi et al. 2021), which has been associated with efficient replication ex vivo and transmission in vivo (Ntoumi et al., 2021).
Conclusion
The results from our study suggest that by March 2021, around 25% of the indigenous population living in Bomassa village, with no known COVID-19 symptoms, might have been in contact with SARS-CoV-2, with 4% presenting asymptomatic infection. The detection of neutralizing Ab in almost 50% of IgG- and/or IgM-positive individuals in this study population suggest that these individuals had developed active protection against SARS-COV2 infection. With regard to vaccination, these individuals would be eligible for one dose.
Acknowledgments
Acknowledgements
The authors are grateful to all participants in this study, and to the Wildlife Conservation Society (WCS). FN is member of the Central Africa Clinical Network (CANTAM) and PANDORA-ID-Net. JCD is supported by the Fondation Mérieux.
Funding
This work was funded by PANDORA-ID-Net (EDCTP grant agreement RIA2016E-1609) and the ITAIL project (EDCTP grant agreement RIA2020EF-2947).
Author contributions
FN and EK were involved in the study design. LLI, JCD, FM, SDK, and CCMM collected and processed the samples. JCD, CV, and LLI were involved in data analysis. All the authors contributed to writing the manuscript.
Competing interests
The authors declare no competing interests.
Contributor Information
Line Lobaloba Ingoba, Email: linelobaloba@gmail.com.
Jean Claude Djontu, Email: cdjontu@yahoo.fr.
Claujens Chastel Mfoutou Mapanguy, Email: chastelmapanguy@gmail.com.
Freisnel Mouzinga, Email: freisnelm@gmail.com.
Steve Diafouka Kietela, Email: diafkietelas@fcrm-congo.com.
Christevy Vouvoungui, Email: vjchristevy@gmail.com.
Eeva Kuisma, Email: ekuisma@wcs.org.
Etienne Nguimbi, Email: etienne.ng1612@gmail.com.
Francine Ntoumi, Email: fntoumi@fcrm-congo.com.
References
- Adetifa I.M.O., Uyoga S., Gitonga J.N., Mugo D., Otiende M., Nyagwange J., Karanja H.K., Tuju J., Wanjiku P., Aman R., Mwangangi M., Amoth P., Kasera K., Ng'ang'a W., Rombo C., Yegon C., Kithi K., Odhiambo E., Rotich T., Orgut I., Kihara S., Bottomley C., Kagucia E.W., Gallagher K.E., Etyang A., Voller S., Lambe T., Wright D., Barasa E., Tsofa B., Bejon P., Ochola-Oyier L.I., Agweyu A., Scott J.A.G., Warimwe G.M. Temporal trends of SARS-CoV-2 seroprevalence during the first wave of the COVID-19 epidemic in Kenya. Nat Commun. 2021;12:3966. doi: 10.1038/s41467-021-24062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemu, B. N., A. Adamu, M. Gemechis, D. Negussie, A. Tamrat, A. Abdulnasir, A. Wondimu, A. Workeabeba, H. Tewodros, A. Rahel, A. Wondwossen, B. Ayele, D. Zelalem, T. Brhanu, K. Eva, W. Mesfin, A. Saro, T. Getachew, and T. Lia. 2020. Sero-prevalence of anti-SARS-CoV-2 antibodies in Addis Ababa, Ethiopia. In 10.1101/2020.10.13.337287. [DOI]
- Borges L.P., Martins A.F., de Melo M.S., de Oliveira M.G.B., Neto J.M.R., Dosea M.B., Cabral B.C.M., Menezes R.F., Santos A.A., Matos I.L.S., Borges P.C., Dos Santos K.A., Ribeiro A.A., Menendez A.I.M., Serafini M.R., Walker C.B., Quintans Junior L.J., Araujo A.A.S., de Souza D.R.V. Seroprevalence of SARS-CoV-2 IgM and IgG antibodies in an asymptomatic population in Sergipe, Brazil. Rev Panam Salud Publica. 2020;44:e108. doi: 10.26633/RPSP.2020.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2020. Centers for Disease Control and Prevention Vaccines COVID-19 phase 3. In https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different [PubMed]
- Chibwana M.G., Jere K.C., Kamn'gona R., Mandolo J., Katunga-Phiri V., Tembo D., Mitole N., Musasa S., Sichone S., Lakudzala A., Sibale L., Matambo P., Kadwala I., Byrne R.L., Mbewe A., Henrion M.Y.R., Morton B., Phiri C., Mallewa J., Mwandumba H.C., Adams E.R., Gordon S.B., Jambo K.C. High SARS-CoV-2 seroprevalence in health care workers but relatively low numbers of deaths in urban Malawi. medRxiv. 2020 [Google Scholar]
- Cohen K.W., Linderman S.L., Moodie Z., Czartoski J., Lai L., Mantus G., Norwood C., Nyhoff L.E., Edara V.V., Floyd K., De Rosa S.C., Ahmed H., Whaley R., Patel S.N., Prigmore B., Lemos M.P., Davis C.W., Furth S., O'Keefe J.B., Gharpure M.P., Gunisetty S., Stephens K., Antia R., Zarnitsyna V.I., Stephens D.S., Edupuganti S., Rouphael N., Anderson E.J., Mehta A.K., Wrammert J., Suthar M.S., Ahmed R., McElrath M.J. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J.M., Simmons R., Wellington E., Cole M.J., Saei A., Oguti B., Munro K., Wallace S., Kirwan P.D., Shrotri M., Vusirikala A., Rokadiya S., Kall M., Zambon M., Ramsay M., Brooks T., Brown C.S., Chand M.A., Hopkins S., Group Siren Study. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.J., Chiba S., Halfmann P., Ehre C., Kuroda M., Dinnon K.H., 3rd, Leist S.R., Schafer A., Nakajima N., Takahashi K., Lee R.E., Mascenik T.M., Graham R., Edwards C.E., Tse L.V., Okuda K., Markmann A.J., Bartelt L., de Silva A., Margolis D.M., Boucher R.C., Randell S.H., Suzuki T., Gralinski L.E., Kawaoka Y., Baric R.S. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370:1464–1468. doi: 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao., M, M.-A. Davies., E. Kalk., D. Hardie., and M. Naidoo. 2020. SARS-CoV-2 seroprevalence in the Cape Town metropolitan sub-districts after the peak of infections. In http//www.nicd.ac.za/wp-content/uploads/2020/06/COVID-19-Special-PublicHealth-Bulletin. (Accessed: 08/02/2021).
- Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., Astudillo M., Yang D., Miller T.E., Oliver E., Fischinger S., Atyeo C., Iafrate A.J., Calderwood S.B., Lauer S.A., Yu J., Li Z., Feldman J., Hauser B.M., Caradonna T.M., Branda J.A., Turbett S.E., LaRocque R.C., Mellon G., Barouch D.H., Schmidt A.G., Azman A.S., Alter G., Ryan E.T., Harris J.B., Charles R.C. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020:5. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobia F., Gitaka J. COVID-19: are Africa's diagnostic challenges blunting response effectiveness? AAS Open Res. 2020 doi: 10.12688/aasopenres.13061.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majiya, H., M. Aliyu-Paiko, V.T. Balogu, D.A. Musa, I.M. Salihu, A.A. Kawu, Y.I. Bashir, R.A. Sani, J. Baba, A.T. Muhammad, F.L. Jibril, E. Bala, N.G. Obaje, B.Y. Aliyu, R.G. Muhammad, H. Mohammed, N.U. Gimba, A. Uthman, H.M. Liman, A.A. Sule, K.J. Joseph, M.M. Makusidi, M.D. Isah, I. Abdullahi, U. Ndagi, B. Waziri, C.I. Bisallah, N.J. Dadi-Mamud, and A.K. Adamu K. Ibrahim. 2021. Seroprevalence of COVID-19 in Niger State. In 10.1101/2020.08.04.20168112. [DOI]
- Milleliri J.M., Coulibaly D., Nyobe B., Rey J.L., Lamontagne F., Hocqueloux L., Giache S., Valery A., Prazuck T. SARS-CoV-2 infection in Ivory Coast: a serosurveillance survey among gold mine workers. Am J Trop Med Hyg. 2021 doi: 10.4269/ajtmh.21-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K.W., Faulkner N., Cornish G.H., Rosa A., Harvey R., Hussain S., Ulferts R., Earl C., Wrobel A.G., Benton D.J., Roustan C., Bolland W., Thompson R., Agua-Doce A., Hobson P., Heaney J., Rickman H., Paraskevopoulou S., Houlihan C.F., Thomson K., Sanchez E., Shin G.Y., Spyer M.J., Joshi D., O'Reilly N., Walker P.A., Kjaer S., Riddell A., Moore C., Jebson B.R., Wilkinson M., Marshall L.R., Rosser E.C., Radziszewska A., Peckham H., Ciurtin C., Wedderburn L.R., Beale R., Swanton C., Gandhi S., Stockinger B., McCauley J., Gamblin S.J., McCoy L.E., Cherepanov P., Nastouli E., Kassiotis G. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370:1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkuba Ndaye A., Hoxha A., Madinga J., Marien J., Peeters M., Leendertz F.H., Ahuka Mundeke S., Arien K.K., Muyembe Tanfumu J.J., Mbala Kingebeni P., Vanlerberghe V. Challenges in interpreting SARS-CoV-2 serological results in African countries. Lancet Glob Health. 2021;9:e588–ee89. doi: 10.1016/S2214-109X(21)00060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nna, Emmanuel, Uchenna Okeke, Emo Ivo, Ojor Ayemoba, Thairu Yunusa, Ogbeche Ochagu, Ngozi Ugwu, Henrietta Okafor, Amaka Nnamani, Nneka Iloanusi, Chika Onu, and Ifeoma Okoye. 2021. Sero-prevalence of SARS-CoV-2 IgM and IgG antibodies amongst blood donors in Nigeria. In 10.21203/rs-151037/v1: Research Square.
- Ntoumi F., Mfoutou Mapanguy C.C., Tomazatos A., Pallerla S.R., Linh L.T.K., Casadei N., Angelov A., Sonnabend M., Peter S., Kremsner P.G., Velavan T.P. Genomic surveillance of SARS-CoV-2 in the Republic of Congo. Int J Infect Dis. 2021;105:735–738. doi: 10.1016/j.ijid.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayanju O., Bamidele O., Edem F., Eseile B., Amoo A., Nwaokenye J., Udeh C., Oluwole G., Odok G., Awah N. SARS-CoV-2 seropositivity in asymptomatic frontline health workers in Ibadan, Nigeria. Am J Trop Med Hyg. 2021;104:91–94. doi: 10.4269/ajtmh.20-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SITREP-146 Rapport de situation de la maladie à Corona virus, République du Congo. SITREP N°. 2021;146 https://SITREP-N-146-COVID-19-CONGO-DU-25-MAI2021-VF.pdf [Google Scholar]
- SITREP-171 Rapport de situation de la maladie à Corona virus, République du Congo. SITREP N°. 2021;171 https://SITREP-N-171-COVID-19-CONGO-DU-20-AOUT2021-VF.pdf [Google Scholar]
- Tso F.Y., Lidenge S.J., Pena P.B., Clegg A.A., Ngowi J.R., Mwaiselage J., Ngalamika O., Julius P., West J.T., Wood C. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int J Infect Dis. 2021;102:577–583. doi: 10.1016/j.ijid.2020.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations. 2020. COVID-19 and indigenous peoples. In un.org/development/desa/indigenous peoples/covid-19.html.
- Uyoga S., Adetifa I.M.O., Karanja H.K., Nyagwange J., Tuju J., Wanjiku P., Aman R., Mwangangi M., Amoth P., Kasera K., Ng'ang'a W., Rombo C., Yegon C., Kithi K., Odhiambo E., Rotich T., Orgut I., Kihara S., Otiende M., Bottomley C., Mupe Z.N., Kagucia E.W., Gallagher K.E., Etyang A., Voller S., Gitonga J.N., Mugo D., Agoti C.N., Otieno E., Ndwiga L., Lambe T., Wright D., Barasa E., Tsofa B., Bejon P., Ochola-Oyier L.I., Agweyu A., Scott J.A.G., Warimwe G.M. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science. 2021;371:79–82. doi: 10.1126/science.abe1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2020. The latest on the COVID-19 immunity and the current global situation.https://www.who.int/publications/m/item/ [Google Scholar]
- WHO. 2021. World Health Organization coronavirus disease (COVID-19) situation dashboard. In https://covid19.who.int.
- Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., Wu S.P., Wang Z., Wu X.H., Xu J.J., Zhang Z., Jia S.Y., Wang B.S., Hu Y., Liu J.J., Zhang J., Qian X.A., Li Q., Pan H.X., Jiang H.D., Deng P., Gou J.B., Wang X.W., Wang X.H., Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]