Abstract
In response to the widespread COVID-19 pandemic, cryopreservation of allogeneic donor apheresis products was implemented to mitigate the challenges of donor availability and product transport. Although logistically beneficial, the impact of cryopreservation on clinical outcomes and graft composition remains unclear. In this study, we compared outcomes and graft composition with cryopreserved versus fresh allografts in the setting of allogeneic hematopoietic cell transplantation (allo-HCT). We retrospectively analyzed the clinical outcomes of 30 consecutive patients who received cryopreserved allografts between March and August 2020 and 60 consecutive patients who received fresh allografts before the COVID-19 pandemic. Primary endpoints were hematopoietic engraftment and graft failure (GF), and secondary outcomes were overall survival (OS), relapse-free survival (RFS) and nonrelapse mortality (NRM). In addition, extended immunophenotype analysis was performed on cryopreserved and prospectively collected fresh apheresis samples. Compared with recipients of fresh allografts, both neutrophil and platelet recovery were delayed in recipients of cryopreserved reduced-intensity conditioning (RIC) allo-HCT, with a median time to engraftment of 24 days versus 18 days (P = .01) for neutrophils and 27 days versus 18 days (P = .069) for platelets. We observed primary GF in 4 of 30 patients in the cryopreserved cohort (13.3%) versus only 1 of 60 patients (1.7 %) in the fresh cohort (P = .03). Cryopreserved RIC allo-HCT was associated with significantly lower median total, myeloid, and T cell donor chimerism at 1 month. OS and RFS were inferior for cryopreserved graft recipients (hazard ratio [HR], 2.16; 95% confidence interval [CI], 1.00 to 4.67) and HR, 1.90; 95% CI, 0.95 to 3.79, respectively. Using an extended immunophenotype analysis, we compared 14 samples from the cryopreserved cohort to 6 prospectively collected fresh apheresis donor samples. These analyses showed both a decrease in total cell viability and a significantly reduced absolute number of natural killer cells (CD3−CD56+) in the cryopreserved apheresis samples. In this single-institution study, we found delayed engraftment and a trend toward clinical inferiority of cryopreserved allografts compared with fresh allografts. Further evaluation of the use of cryopreserved allografts and their impact on clinical and laboratory outcomes is warranted.
Key Words: Cryopreserved allografts, Engraftment failure, Reduced-intensity conditioning, Graft composition
INTRODUCTION
Cryopreservation of autologous hematopoietic grafts is a standard-of-care approach that allows adequate collection and storage of patients’ blood-forming stem cells in advance of high-dose myeloablative chemotherapy and hematopoietic cell rescue. In contrast, cryopreserved grafts are not routinely used for allogeneic hematopoietic cell transplantation (allo-HCT), because healthy donors are available at the time recipients undergo conditioning for their transplantation procedure. Despite some advantages in the logistics of cryopreserved allo-HCT, to date there are no studies supporting the use of cryopreserved allografts over fresh allografts as a gold standard for allo-HCT, and data on the impact of cryopreservation on graft content and clinical outcomes are limited [1].
In 2020, in response to the COVID-19 pandemic, widespread cryopreservation of allogeneic donor apheresis products was implemented to mitigate the challenges of donor availability and product transport. During the early phase of the pandemic from March to August 2020, the US National Marrow Donor Program (NMDP) required the cryopreservation of all unrelated donor (URD) grafts. Before the pandemic, data available from single-center or small multicenter reports showed conflicting results regarding post-HCT clinical outcomes of cryopreserved allografts 2, 3, 4. One of these studies comparing cryopreserved and fresh peripheral blood stem cell (PBSC) grafts from related donors showed similar clinical outcomes in terms of overall survival (OS), nonrelapse mortality (NRM), acute and chronic graft-versus-host disease (GVHD), and hematopoietic recovery [2]. In contrast, 2 other studies showed increased rates of graft failure (GF) with cryopreserved URD PBSC grafts [3] and delayed platelet recovery with cryopreserved related donor and URD PBSC grafts [4].
Since the institution of pandemic restrictions, the Center for International Blood and Marrow Transplant Research (CIBMTR) has reported on 3 retrospective studies comparing the clinical outcomes of cryopreserved and fresh allogeneic donor products 5, 6, 7. The study by Eapen et al. [5], which focused on patients with severe aplastic anemia, showed higher rates of GF and inferior OS with cryopreserved allografts, whereas the study by Hamadani et al. [6], which included only patients undergoing allo-HCT with post-transplantation cyclophosphamide (Cy) as GVHD prophylaxis, showed similar outcomes for cryopreserved and fresh allografts. The more recent and largest study by Hsu et al. [7] examined a total of 7379 patients from the CIBMTR database who underwent transplantation between 2013 and 2018 and separately assessed the impact of cryopreservation on clinical outcomes of transplantation with (1) URD PBSC grafts, (2) related PBSC grafts, and (3) bone marrow (BM) grafts. That study demonstrated a clear inferiority of cryopreserved URD PBSC grafts, with delayed hematopoietic recovery, increased NRM and relapse, and decreased progression-free survival (PFS) and OS. In addition, cryopreserved PBSC grafts from related donors showed delayed platelet recovery, inferior NRM and OS, and a higher rate of acute GVHD. Recipients of BM cryopreserved grafts from both related donors and URDs had similar outcomes as recipients of fresh BM grafts. These latter results from the CIBMTR are in contrast to the findings of a large retrospective single-center analysis of 958 patients at the Princess Margaret Cancer Centre who underwent allo-HCT between 2010 and 2018 that found no differences in hematopoietic recovery, GF, acute and chronic GVHD, and OS in recipients of cryopreserved grafts compared with recipients of fresh grafts [8]. The different outcomes reported in the latter study could be attributed mainly to the difference in graft sources used in the analysis, because the majority of patients (91%) who received cryopreserved grafts had a matched related donor graft and only 9% received a URD graft, compared with 83% of URD graft recipients in the fresh graft cohort. Importantly, this imbalance in graft source differs substantially from the real-time experience during the COVID-19 pandemic, because nearly all URD grafts were cryopreserved. A recent publication from the Dana-Farber Cancer Institute comparing cryopreserved and fresh URD PBSC grafts in the context of the COVID-19 pandemic reported lower T cell chimerism, impaired immune reconstitution the first months post-allo-HCT, as well as increased GF linked to longer transit time (>48 hours) independent of cryopreservation. No differences in 6-month OS, PFS, or NRM were noted [9].
At our center, we observed a highly unusual cluster of GF in patients undergoing reduced-intensity conditioning (RIC) allo-HCT which correlated with the institution of URD cryopreservation beginning in March 2020. Therefore, we compared clinical outcomes of 30 consecutive patients who underwent allo-HCT with cryopreserved PBSC grafts during the COVID-19 pandemic with 60 consecutive patients who received fresh PBSC grafts prior to the pandemic cryopreservation requirement. No previous study has reported on the variable of conditioning intensity; here we separately assessed the effect of graft cryopreservation on the outcomes of patients receiving a RIC regimen versus those receiving a myeloablative conditioning (MAC) regimen. Furthermore, to study whether differences in cell composition might be present between cryopreserved and fresh grafts, we carried out extended phenotype analyses of hematopoietic stem cell (HSC) and lymphocyte subsets on available samples from the cryopreserved PBSC products and prospectively collected and analyzed fresh allogeneic PBSC samples. To our knowledge, our study is the first to include extended phenotyping of the graft composition, which we believe will further elucidate the biological relevance of the different cellular subpopulations for clinical outcomes.
METHODS
Patients
Data were collected retrospectively from 30 consecutive patients who underwent allo-HCT with cryopreserved PBSCs between March and August 2020 (cryopreserved cohort) and a control cohort of 60 consecutive patients who underwent allo-HSCT with fresh PBSCs between June 2019 and March 2020 (fresh cohort). All patients included in the analysis received either MAC or RIC regimen. Patients receiving total lymphoid irradiation (TLI), antithymocyte globulin (ATG), and low-dose total body irradiation (TBI) RIC were excluded from this analysis, because (1) this TLI/ATG/TBI reduced-intensity regimen is center-specific and (2) this regimen leads to higher rates of mixed donor chimerism compared with other RIC regimens [10]. Donors included HLA-matched siblings, HLA-matched unrelated, haploidentical relatives, and HLA-mismatched URDs. All patients had provided written informed consent, and the study was approved by the Institutional Review Board of Stanford University.
Definitions and Study Endpoints
Primary endpoints were the time to hematopoietic engraftment of neutrophils and platelets and GF. Neutrophil recovery was defined as the date of the first of 3 consecutive days with an absolute neutrophil count (ANC) ≥0.5 × 109 /L, and platelet recovery was defined as the date of the first of 3 consecutive days with a platelet count ≥20 × 109 /L without transfusion within the previous 7 days. Primary GF was defined as initial donor chimerism <5% or failure to achieve an ANC ≥0.5 × 109 /L without morphologic evidence of disease relapse. Secondary GF was defined as decrease in donor chimerism to <5% after initial successful engraftment or decrease in ANC to ≤0.5 × 109 /L without morphologic evidence of disease relapse. Poor graft function was defined as 2 or 3 cytopenic lines (hemoglobin <10 g/dL, ANC <1.0 × 109 /L, platelet count <30 × 109 /L) for >2 consecutive weeks beyond day +28, without transfusion support and in the presence of complete donor chimerism [11]. Secondary endpoints were chimerism level post allo-HCT, OS, relapse-free survival (RFS), and NRM. Donor cell chimerism of whole blood and lineage subsets was assessed by short tandem repeat analysis as described previously [12]. To assess T cell and myeloid chimerism, immunomagnetic beads coated with monoclonal antibodies against CD3 and CD15 were used to separate these fractions in peripheral blood [10]. For OS, death from any cause was considered an event, and surviving patients were censored at last contact. For RFS, relapse, or death, whichever occurred earlier, was considered an event, and surviving patients without relapse were censored.
Disease state was reported as myeloid, lymphoid, or mixed. The Disease Risk Index (DRI) was calculated for each patient according to disease and stage risk using standard criteria [13]. Cytomegalovirus (CMV) risk category was reported as high (recipient (R)+/ donor (D)- or +], intermediate (R-/D+) and low (R-/D-) based on pre-HCT CMV serostatus. The cell doses of CD34+ x 106/kg and CD3+ x 108/kg that patients received as part of their grafts were available only at the time of collection and not obtained at the time of product thaw.
Apheresis Sample Collection and Processing
In the cryopreserved cohort 28 apheresis products were cryopreserved at our center, whereas only 2 apheresis products underwent cryopreservation at the donor center. Cryopreservation at our center was performed according to standard operating procedure, using 10% dimethylsulfoxide (DMSO)/Normosol media, followed by cryopreservation in an automated controlled-rate freezer. On the day of infusion cryopreserved products were transported to the patient unit in liquid nitrogen transporter, thawed using 37 °C bath, and infused.
Analysis of cryopreserved grafts was performed on available quality control (QC) samples collected on patients in the cryopreserved cohort. In brief, QC vials were obtained from the actual products after adding 10% DMSO/Normosol medium and were frozen in the same controlled-rate freezer as the apheresis products. On the day of analysis, the QC vials were thawed in warm bath for 1 to 2 minutes, resuspended in warm RPMI medium (containing 10% fetal bovine serum) and DNAse to avoid clumping, and centrifuged at 500× g for 10 minutes at room temperature. Cells were counted on an Invitrogen Countess automated cell counter (Thermo Fisher Scientific, Waltham, MA), and mononuclear cells (MNCs) were isolated using Ficoll density gradient separation; in cases with a low cell count at baseline, the Ficoll step was not performed. Fresh apheresis samples were obtained prospectively from related donors at the day of collection and processed immediately. After cell counting, MNCs were isolated using Ficoll density gradient separation as described above.
Immunophenotype Analysis
MNCs obtained as described above were stained for surface markers for 30 minutes at room temperature in the dark. An extended table describing the monoclonal antibodies used for immunophenotype analysis of hematopoietic stem and progenitor cell (HSPC) and lymphocyte subsets is provided in the Supplementary Data. Propidium iodide or Zombie Aqua viability dye (catalog no. 423101; BioLegend, San Diego, CA) were used to exclude dead cells. Data were collected on a BD FACSAria II (BD Biosciences, San Jose, CA) and analyzed with FlowJo version 9.9.4 (FlowJo Software, Ashland, OR). Precision count beads (catalog no. 424902; BioLegend) were used to obtain absolute cell counts for CD34+ cells, HSCs, and lymphocyte subpopulations according to the manufacturer's instructions. In brief, 100 µL of precision count beads was added to 200 µL of sample, and absolute cell counts/µL were calculated using the following equation: (cell count × bead volume)/(bead count × cell volume) × bead concentration/µL.
Statistical Analysis
Patient, disease, and transplantation characteristics were summarized, and differences between the 2 graft type cohorts were compared. P values based on the t test for continuous variables, the chi-square test for categorical variables, and Fisher's exact test for binary variables were provided for descriptive purposes.
The endpoints considered were neutrophil engraftment, platelet engraftment, donor chimerism, primary GF, OS, RFS, and NRM. Cumulative incidence of neutrophil and platelet engraftment and NRM were calculated based on competing-risks models. Probabilities of OS and RFS were estimated using the Kaplan-Meier method. A Cox proportional hazards model was used to assess the effect of graft type (cryopreserved versus fresh) on OS while adjusting for other covariates of interest. A logistic regression model was built similarly for the primary GF endpoint. These statistical analyses were performed using R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Analyses of chimerism and immunophenotype data were performed with the Mann-Whitney U test using Prism version 8.4.2 (GraphPad Software, San Diego, CA). All P values were 2-sided, and statistical significance was assessed at the nominal level of .05 without multiplicity adjustment.
RESULTS
Patient, Disease, and Transplantation Characteristics
A total of 90 patients were included in our analysis, including 30 in the cryopreserved cohort and 60 in the fresh cohort. Patient baseline and transplantation characteristics are shown in Table 1 . Both cohorts were similar in terms of patient age and sex, type of malignancy (myeloid versus lymphoid), CMV risk group, and DRI. Conditioning intensity was equally distributed (cryopreserved cohort: 70% RIC and 30% MAC; fresh cohort: 65% RIC and 35% MAC). The median donor age was 34 years in the cryopreserved cohort and 41 years in the fresh cohort. The median postcollection (prefreezing) dose of CD34+ cells was lower in the cryopreserved cohort (7.4 × 106/kg versus 9.3 × 106/kg in the fresh cohort), but there was no between-group difference in CD3+ cell dose. The cryopreserved cohort included more HLA-matched URDs (73% versus 42%), whereas the fresh cohort included more HLA-matched sibling donors (33% versus 6.7%) and slightly more haplo/HLA-mismatched donors (25% versus 20%). We noted that the imbalance between the cryopreserved and fresh cohorts was seen mainly in the haploidentical and identical sibling donors; however, the cohorts were balanced in the URDs (n=22 in the cryopreserved cohort versus n=25 in the fresh cohort).
Table 1.
Baseline Characteristics of the Cryopreserved and Fresh Cohorts
| Characteristic | N* | Cryopreserved (N = 30) | Fresh (N = 60) | P Value† |
|---|---|---|---|---|
| Patient age, yr, mean | 90 | 56 | 57 | .74 |
| Donor age, yr, mean | 87 | 34 | 41 | .017 |
| Sex, n/N (%) | 90 | .11 | ||
| Female | 17/30 (57) | 22/60 (37) | ||
| Male | 13/30 (43) | 38/60 (63) | ||
| Karnofsky Performance Scale, n/N | 88 | .006 | ||
| ≥90 | 13/29 | 16/59 | ||
| <90 | 16/29 | 43/59 | ||
| Graft type, n/N (%) | 90 | .28 | ||
| Allogeneic | 27/30 (90) | 47/60 (78) | ||
| Haploidentical | 3/30 (10) | 13/60 (22) | ||
| Donor type, n/N (%) | 90 | .003 | ||
| HLA-identical sibling | 2/30 (6.7) | 20/60 (33) | ||
| HLA-matched unrelated | 22/30 (73) | 25/60 (42) | ||
| HLA-haploidentical | 3/30 (10) | 13/60 (22) | ||
| HLA-mismatched unrelated | 3/30 (10) | 2/60 (3.3) | ||
| Conditioning intensity, n/N (%) | 90 | .81 | ||
| MAC | 9/30 (30) | 21/60 (35) | ||
| RIC | 21/30 (70) | 39/60 (65) | ||
| CMV risk group, n/N (%) | 90 | .17 | ||
| High (R+) | 18/30 (60) | 47/60 (78) | ||
| Intermediate (R-, D+) | 4/30 (13) | 3/60 (5) | ||
| Low (R-, D-) | 8/30 (27) | 10/60 (17) | ||
| ABO match, n/N (%) | 90 | .033 | ||
| Matched | 16/30 (53) | 42/60 (70) | ||
| Minor mismatch | 11/30 (37) | 7/60 (12) | ||
| Major mismatch | 3/30 (10) | 10/60 (17) | ||
| Bidirectional | 0/30 (0) | 1/60 (1.7) | ||
| Disease type, n/N (%) | 90 | .34 | ||
| Myeloid | 23/30 (77) | 52/60 (87) | ||
| Lymphoid | 6/30 (20) | 6/60 (10) | ||
| Mixed | 1/30 (3.3) | 2/60 (3.3) | ||
| DRI, n/N (%) | 90 | .18 | ||
| High | 12/30 (40) | 18/60 (30) | ||
| Intermediate | 17/30 (57) | 42/60 (70) | ||
| Low | 1/30 (3.3) | 0/60 (0) | ||
| CD34 dose, × 106/kg, mean | 90 | 7.4 | 9.3 | .034 |
| CD3 dose, × 108/kg, mean | 89 | 3.1 | 2.56 | .061 |
R indicates recipient; D, donor.
Number of patients with available data.
Statistical test performed: t test, chi-square test of independence, Fisher's exact test.
ABO major mismatch was documented in 17% of the fresh cohort and 10% of the cryopreserved cohort. More patients in the fresh cohort had a Karnofsky Performance Status <90 prior to allo-HCT (P = .006). The most common MAC regimen was busulfan/Cy (Bu/Cy; n = 22), followed by fludarabine/thiotepa/melphalan (Flu/TT/Mel; n = 5), TBI/Cy (n = 1), Flu/Cy/TBI (n = 1), and Flu/TBI (n = 1). The most common RIC regimen was Flu/Mel (140 mg/m2; n = 43), followed by Flu/Cy/TBI (200 to 300 cGy; n = 9), Flu/TBI (300 cGy; n = 7), and Flu/Mel/post-transplantation Cy (n = 1).
Hematologic Recovery
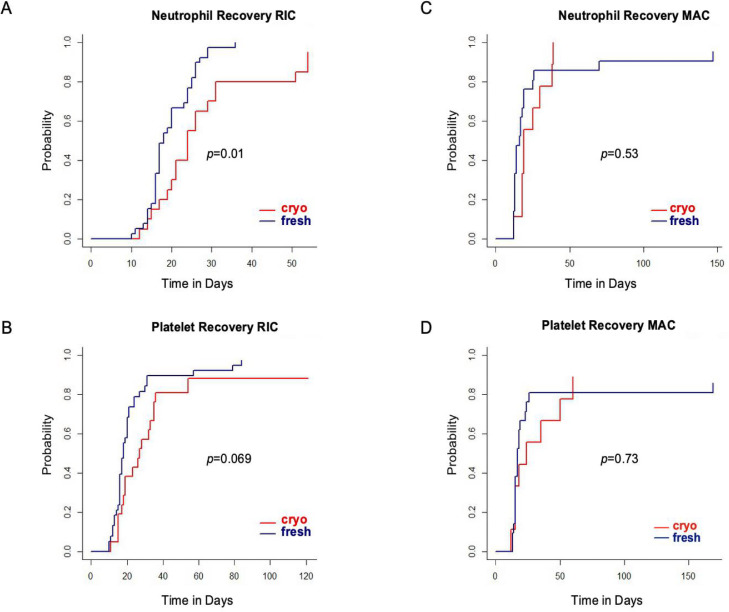
Neutrophil recovery was significantly delayed in the RIC cryopreserved cohort, with a median time to neutrophil engraftment of 24 days compared with 18 days in the RIC fresh cohort (P = .01; Figure 1 A). Platelet engraftment was also delayed in the RIC cryopreserved cohort, with a median time to engraftment of 27 days compared with 18 days in the RIC fresh cohort (P = .069; Figure 1B). The median time to neutrophil engraftment in MAC allo-HCT recipients was 19 days in the cryopreserved cohort versus 14 days in the fresh cohort (P = .53), and the median time to platelet engraftment was 24 days in the cryopreserved cohort versus 17 days in the fresh cohort (P = .73; Figure 1C and D). In a subgroup analysis examining only URDs, we observed similar results with significant delays in both neutrophil and platelet recovery (Supplementary Figure S1).
Figure 1.
Hematopoietic recovery in recipients of cryopreserved grafts versus fresh grafts. (A) Neutrophil engraftment in RIC allo-HCT. (B) Platelet engraftment in RIC allo-HCT. (C) Neutrophil engraftment in MAC allo-HCT. (D) Platelet engraftment in MAC allo-HCT.
Engraftment Failure
Primary GF was documented in 5 patients among the 90 patients analyzed, including 4 of 30 patients in the cryopreserved cohort (13.3%) and 1 of 60 patients (1.7 %) in the fresh cohort (P = .03). In a subgroup analysis including only URDs, we observed similar results with a significantly higher rate of GF (P = .03).
Disease and transplantation characteristics for the 5 patients with GF are presented in Table 2 . All patients in the cryopreserved cohort received a graft from a matched URD following RIC (3 with Flu/Mel 140 mg/m2 and 1 with Flu/TBI 300 cGy), whereas the 1 patient in the fresh cohort received a haploidentical graft following MAC with Bu (14.4 mg/kg)/Cy. Three of the 5 patients had DRI high before allo-HCT. One patient in the cryopreserved cohort had undergone prior allo-HCT with a fresh graft from a different donor, followed by GF. One patient in the cryopreserved cohort died at day 114 post-HCT from disease relapse. This patient, who had refractory high-risk myelodysplastic syndrome and progression to acute myelogenous leukemia before allo-HCT, showed initial morphologic remission with blast counts <5%, followed by disease recurrence in the absence of donor engraftment. Three of 4 patients in the cryopreserved cohort were alive at the time of this report, including 2 who underwent a rescue second allo-HCT with fresh allografts and 1 who developed autologous hematopoietic recovery followed by disease progression. The single patient with GF in the fresh cohort underwent a rescue second allo-HCT from a different haploidentical donor following RIC (with Flu/Cy/ATG) and demonstrated >95% total donor and myeloid chimerism after the second allo-HCT. However, despite prompt hematopoietic engraftment, this patient died shortly after the second allo-HCT from multiorgan dysfunction.
Table 2.
Characteristics and Outcome of Patients Who Developed Primary GF
| Disease | Graft Type | Donor | Prior HCT | DRI | Conditioning | Outcome |
|---|---|---|---|---|---|---|
| Mycosis fungoides | Cryopreserved | HLA-matched unrelated | Yes | High | RIC (Flu/TBI) | Autologous reconstitution; alive |
| High-risk MDS > AML | Cryopreserved | HLA-matched unrelated | No | High | RIC (Flu/Mel) | Died |
| AML | Cryopreserved | HLA-matched unrelated | No | Int | RIC (Flu/Mel) | Underwent second HCT with fresh allografts; alive |
| CML | Cryopreserved | HLA-matched unrelated | No | Low | RIC (Flu/Mel) | Underwent second HCT with fresh allografts; alive |
| MDS/CMML | Fresh | Haploidentical | No | High | MAC (Bu/Cy) | Underwent second HCT; died |
MDS indicates myelodysplastic syndrome; AML, acute myelogenous leukemia; Int, intermediate; CML, chronic myelogenous leukemia; CMML, chronic myelomonocytic leukemia.
Poor graft function was observed in 8 of the 90 patients analyzed, including 5 of 30 (16.7%) in the cryopreserved group and 3 of 60 (5%) in the fresh group, suggesting a trend toward an increased rate of poor graft function in the cryopreserved group. However, this trend did not reach statistical significance (P = .09). The transit time of the cryopreserved grafts did not appear to be associated with GF or poor graft function (Supplementary Figure S2A and B).
Donor Chimerism
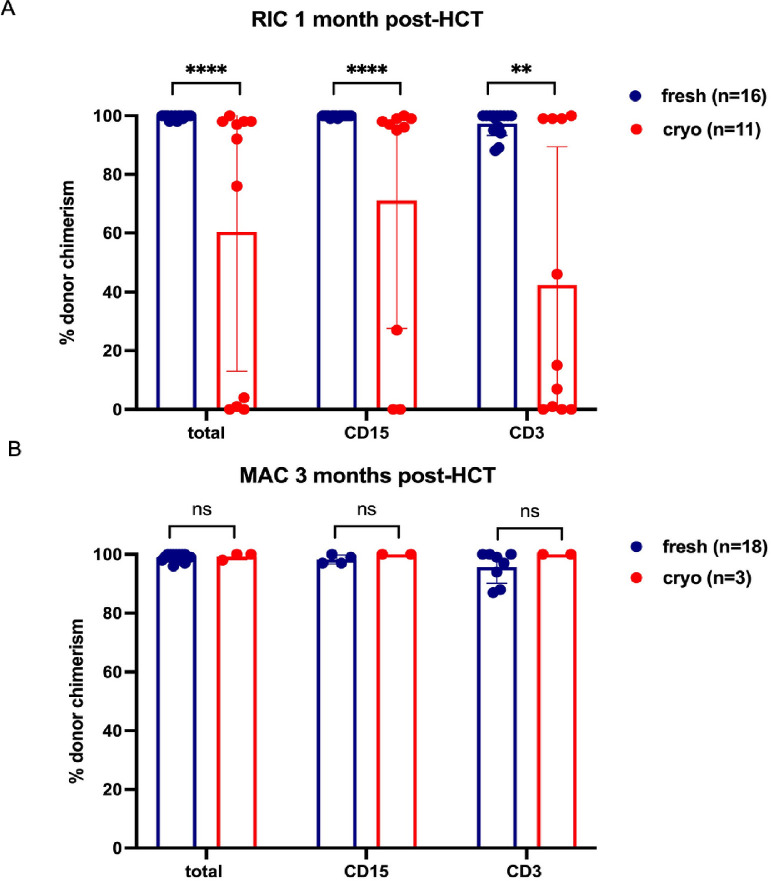
Total cell, myeloid cell, and T cell donor chimerism levels measured in blood at 1 month post-transplantation for RIC allo-HCT recipients and 3 months post-transplantation for MAC allo-HCT recipients are shown in Figure 2 . Cryopreserved RIC allo-HCT was associated with significantly lower median total, myeloid, and T cell donor chimerism at 1 month (Figure 2A), whereas no differences in donor chimerism were observed when MAC conditioning was used (Figure 2B). Of note, the patient with GF in the fresh cohort who had undergone MAC allo-HCT was not included in the 3-month chimerism assessment, because this patient died very early in the post-transplantation course and chimerism levels were available only at 1 month. In the RIC cryopreserved cohort, besides the 4 patients who developed primary GF with levels of donor chimerism <5% at 1 month post-HCT, 3 more patients were observed to be mixed donor T cell chimeras. Their donor T cell levels increased over time without further interventions (Supplementary Figure S3).
Figure 2.
Donor chimerism in PBSCs or BM in recipients of cryopreserved versus fresh grafts as assessed by short tandem repeat analysis and immunomagnetic beads coated with monoclonal antibodies against CD3 and CD15. (A) Total, myeloid (CD15), and T cell (CD3) donor chimerism (%) at 1 month post-RIC allo-HCT. (B) Total, myeloid (CD15), and T cell (CD3) donor chimerism (%) at 3 months post MAC allo-HCT. ****P < .0001; **P < .001; ns, nonsignificant.
Univariable Analysis for OS, RFS, and NRM
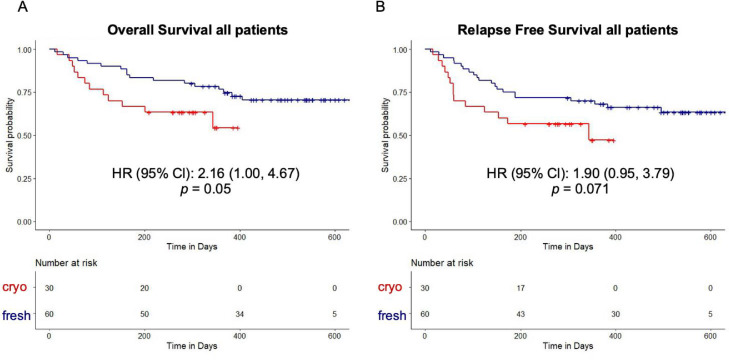
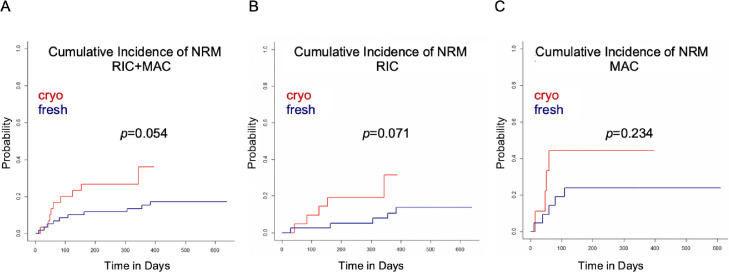
The median duration of follow-up was 15.4 months in the fresh cohort compared with 9.9 months in the cryopreserved cohort. In a univariate analysis, OS was inferior in the cryopreserved cohort (hazard ratio [HR], 2.16; 95% confidence interval [CI], 1.00 to 4.67; P = .050, Figure 3 A). In the cryopreserved graft recipients who received either a RIC or MAC regimen, there was a trend toward inferior OS (Supplementary Figure S4A and B). The magnitude of the difference was similar in the RIC and MAC recipients, with an HR of 2.19 (95% CI, 0.80 to 6.01) and 2.38 (95% CI, 0.72 to 7.83), respectively. We also performed a subgroup analysis including only URDs and observed a similar trend toward inferior OS in the cryopreserved cohort (Supplementary Figure S4C). The cryopreserved graft recipients showed a trend toward lower RFS (HR, 1.90; 95% CI, 0.95 to 3.79; Figure 3B). This trend was more pronounced in the MAC subgroup (HR, 2.63; 95% CI, 0.79 to 8.69) compared with the RIC subgroup (HR, 1.63; 95% CI, 0.69 to 3.83; Supplementary Figure S5A and B); however, the difference was of borderline statistical significance (P = .071) for the entire patient cohort and did not reach statistical significance in the conditioning subgroups. The cumulative incidence of NRM was higher in the cryopreserved cohort (Figure 4 A), and a trend toward a higher cumulative incidence of NRM was also observed in the conditioning subgroups (Figure 4B and C).
Figure 3.
Kaplan-Meier curves for OS (A) and RFS (B) in recipients of allo-HCT with cryopreserved grafts and fresh grafts.
Figure 4.
Cumulative incidence of NRM in recipients of allo-HCT with cryopreserved grafts and fresh grafts for all patients (A), patients receiving RIC regimens (B), and patients receiving MAC regimens.
Multivariable Analysis for OS and GF
We performed a multivariable Cox model for OS by graft type (cryopreserved versus fresh) while adjusting for donor age, patient sex, donor type, CD34+ cell dose, conditioning intensity, and DRI. There was no statistically significant difference in OS by graft type (HR, 1.48; 95% CI, 0.62 to 3.52; P = .4; Table 3 ). Similarly, in a multivariable logistic regression model for GF by graft type (cryopreserved versus fresh), adjusted for donor age, patient sex, CD34+ cell dose, conditioning intensity, and DRI, no statistically significant increase in GF was observed in the cryopreserved cohort (OR, 6.99; 95% CI, 0.80 to 168; P = .12; Table 4 ).
Table 3.
Multivariable Cox Model for OS
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Graft type (cryopreserved vs fresh) | 1.48 (0.62-3.52) | .4 |
| Donor age | 0.99 (0.96-1.04) | .8 |
| Patient sex | .077 | |
| Female | — | |
| Male | 0.48 (0.21-1.08) | |
| Donor type | .3 | |
| HLA-identical sibling | — | .3 |
| Haploidentical | 2.44 (0.50-11.9) | |
| HLA unrelated (matched + mismatched) | 2.56 (0.49-13.5) | |
| CD34+ cells/kg | 1.05 (0.96-1.14) | .3 |
| Conditioning intensity | .037 | |
| MAC | — | |
| RIC | 0.38 (0.15-0.94) | |
| DRI high | .043 | |
| No | — | |
| Yes | 2.55 (1.03-6.31) |
Table 4.
Multivariable Logistic Regression Model for GF
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Graft type (cryopreserved vs fresh) | 6.99 (0.80-168) | .12 |
| Donor age | 1.00 (0.92-1.07) | .8 |
| Patient sex | .7 | |
| Female | — | |
| Male | 0.67 (0.07-5.06) | |
| CD34+ cells/kg | 0.80 (0.50-1.12) | .3 |
| Conditioning intensity | .8 | |
| MAC | — | |
| RIC | 1.46 (0.15-32.9) | |
| DRI high | .4 | |
| No | — | |
| Yes | 2.42 (0.33-21.8) |
Analysis of Cellular Components in Cryopreserved Grafts versus Fresh Grafts
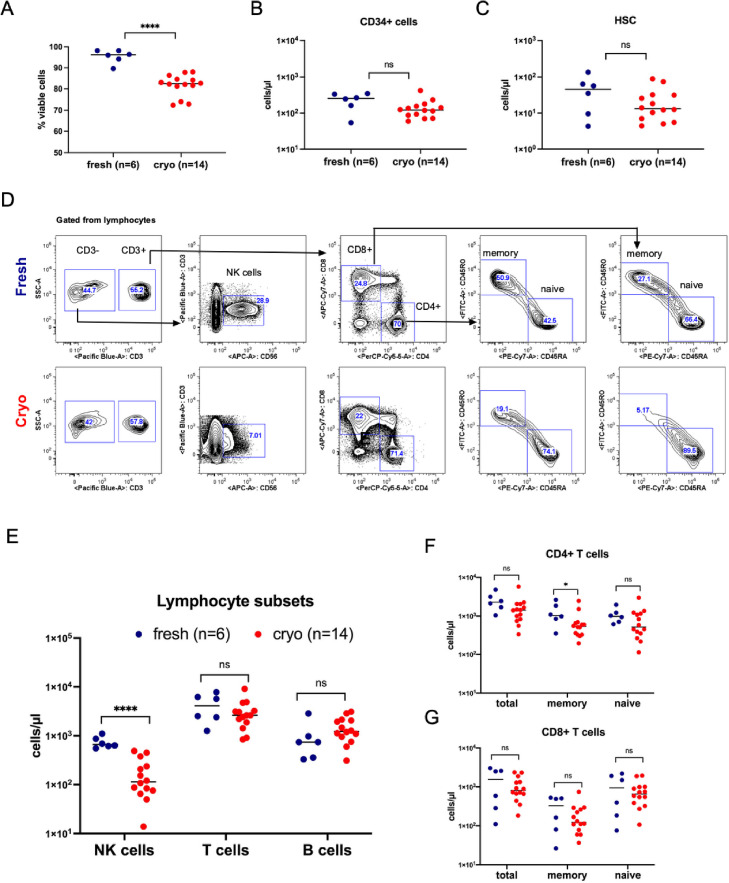
We performed extended immunophenotype analysis by flow cytometry on 14 available donor apheresis samples from the cryopreserved cohort and 6 prospectively collected fresh donor apheresis samples. The analyses included cell viability, HSPC content, and immune cell subsets. Cell viability as assessed by propidium iodide was significantly reduced in the cryopreserved grafts compared with fresh grafts (P < .0001; Figure 5 A). However, the absolute counts of CD34+ cells and of more stringent HSCs (CD34+CD38−CD90+CD45RA−) were similar in the 2 types of grafts (Figure 5B and C). The flow cytometry gating strategy used to identify the CD34+ cells and HSCs is shown in Supplementary Figure S6.
Figure 5.
Characteristics of the apheresis products in cryopreserved versus fresh allografts. (A) Percentage of viable cells as assessed by FACS using propidium iodide dead cell exclusion in fresh and cryopreserved grafts. (B) Absolute counts (cells/µL) of CD34+ cells as assessed by FACS in fresh and cryopreserved apheresis samples. (C) Absolute counts (cells/µL) of HSCs (CD34+CD38-CD90+CD45RA-) as assessed by FACS in fresh and cryopreserved apheresis samples. (D) Representative FACS plots in 1 fresh and 1 cryopreserved apheresis sample from allogeneic donors. Gated from lymphocytes. T cells (CD56−CD3+), NK cells (CD56+CD3−), memory T cells (CD45RO+CD45RA−), naïve T cells (CD45RO−CD45RA+). (E) Absolute counts (cells/µl) of NK, T and B (CD19+CD20+) cells as assessed by FACS in fresh as compared to cryopreserved apheresis samples. (F) Absolute counts (cells/µl) of total, memory and naïve CD4+ T cells as assessed by FACS in fresh as compared to cryopreserved apheresis samples. (G) Absolute counts (cells/µl) of total, memory and naïve CD8+ T cells as assessed by FACS in fresh as compared to cryopreserved apheresis samples.
In contrast to the stable quantity of HSPC populations present in grafts under the conditions of cryopreservation and thaw, analysis of the lymphocyte subsets revealed a significant decrease in the absolute numbers of natural killer (NK) cells, phenotypically defined as CD3−CD56+ cells (Figure 5D and E), in the cryopreserved apheresis samples compared with the fresh apheresis samples. Absolute counts of total T cells (CD3+CD56−) and B cells (CD19+CD20+) did not differ between the cryopreserved and fresh samples (Figure 5E). However, there was a trend toward decreased absolute numbers of CD4 memory T cells (CD45RO+) in cryopreserved samples, but no differences in the absolute numbers of total and naïve (CD45RA+) CD4+ T cells (Figure 5F) were observed. Total, naïve, and memory CD8 T-cell counts were similar in the fresh and cryopreserved samples (Figure 5G). In addition, no statistically significant between-group differences were observed in regulatory T cells (CD3+CD4+CD25+CD127−) (Supplementary Figure S7A and B).
DISCUSSION
This single-center retrospective analysis reports our real-world experience with cryopreserved allogeneic PBSC grafts during the COVID-19 pandemic, examining both clinical impact and graft characteristics. On univariate analysis, we observed that cryopreserved allo-HCT was associated with delayed hematopoietic recovery, increased primary GF, decreased OS, and trends toward decreased RFS and increased NRM. These data are consistent with the recently reported results from the largest retrospective study of the CIBMTR database [7]. However, in a multivariable analysis, cryopreservation was not associated with a statistically significant inferior OS or an increased rate of GF. Given the small study size and the observed trend toward inferiority for the cryopreserved cohort, future studies with larger numbers of patients are warranted.
Assessing the subgroups of RIC and MAC allo-HCT recipients revealed that the differences in neutrophil and platelet engraftment between the cryopreserved and fresh cohorts were associated primarily with RIC allo-HCT. In addition, all patients with GF in the cryopreserved cohort had undergone RIC allo-HCT. At our institution, standard-of-care RIC regimens include either Flu/Mel or Flu/TBI, and for these regimens, in vivo lymphodepletive serotherapy, such as ATG and alemtuzumab, is not standard of care. Interestingly, we did not observe an increase in GF in patients who received our institution-specific RIC with TLI/ATG/TBI and underwent cryopreserved allo-HCT (data not shown).
The negative experience with frozen allogeneic products contrasts with the decades-long experience in autologous HCT that relies exclusively on frozen grafts. Allografting contrasts with autografting in that the former requires overcoming the immunologic barrier, and both HSC and non-HSC cellular components in the allografts are important in overcoming engraftment resistance. Hence, variables in the processing that negatively impact allograft content are more likely to be observable in allograft recipients. Based on our data and review of the studies reported to date, we conclude that multiple factors are responsible for the observed differences between cryopreserved and fresh products including alterations in the graft composition, which may be impacted by product age, including transit time between donor collection centers and transplantation centers; the cryopreservation manipulation itself; and inadequacy of RIC regimens to sufficiently deplete endogenous HSC and reduce the immunologic resistance to engraftment, thus requiring graft cellular elements to aid in overcoming these barriers. Purtill et al. [14] have reported on the variable quality of post-thaw apheresis products. Specifically, they found that longer transit time, higher initial white cell counts, and graft manipulation (ie, CD34 selection and washing) before cryopreservation can negatively impact CD34+ cell post-thaw recovery and viability [14]. Similarly, a recent study by Maurer et al. [9] that included allo-HCTs performed before and during the COVID-19 pandemic showed that older PBSC product age (>48 hours), independent of cryopreservation, was associated with increased GF, and impaired immune reconstitution was observed in recipients of cryopreserved grafts. Wiercinska et al. [15] reported a median post-thaw recovery of viable CD34+ cells of 42% compared with a viability of >90% before cryopreservation of HSC products [15]. Although at our center, cell viability and CD34+ cell content are not routinely measured after thawing of cryopreserved grafts, we were able to evaluate these parameters by flow cytometry in available samples from the cryopreserved grafts and compare them with prospectively freshly collected allogeneic graft samples. Importantly, we saw no decrease in the absolute counts of CD34+ cells and HSCs and only a slight decrease in viability that was consistent among the cryopreserved samples.
Given that (1) prefreeze CD34+ cell count was not notably different in the cryopreserved and fresh cohorts and (2) post-thaw numbers of CD34+ cells and HSCs were similar in the apheresis samples of the 2 cohorts, our experience suggests that cryopreservation and storage might have negatively impacted the amount or activity of non-CD34 graft “facilitating cells.” Replete allogeneic hematopoietic grafts are known to contain non-HSCs that facilitate donor HSC engraftment 16, 17, 18, 19. In the absence of sufficient myeloablation and lymphoablation, these non-HSC facilitating cells play a more prominent role in promoting HSC engraftment. T cells are known to facilitate engraftment by virtue of marrow-directed graft-versus-host activity [16,19, 20, 21], but other populations and mechanisms also have been implicated in enhancing donor HSC engraftment [18,22, 23, 24]. We performed immunophenotype profiling to examine the different lymphocyte subsets present in fresh versus cryopreserved products. These studies revealed a significant loss of NK cells (CD3−CD56+), with no changes in T cell and B cell compartments. Although little is known about NK cell stability or cell surface molecule changes in cryopreserved apheresis products, our results are consistent with a prior study demonstrating a significant quantitative decrease in the percentage of NK cells in donor lymphocyte products upon cryopreservation/thaw [25]. Along with induction of the graft-versus-leukemia effect and impairment of GVHD, NK cells have been shown to have potential in facilitating HSC engraftment [24,26,27]. In preclinical mouse models, NK cells were shown to increase the in vitro clonogenicity and in vivo repopulation potential of cord blood hematopoietic cells [22], and alloreactive NK cells have been shown to enhance HSC engraftment in MHC-mismatched HCT in the setting of RIC [26]. Importantly, several correlative clinical studies have reported beneficial effects of higher NK cell transplant dose on hematopoietic recovery, infections, OS, and NRM post allo-HCT [24,27].
Our data are consistent with previous reports that raise concerns about a universal practice of cryopreservation for allo-HCT [5,7,9]. A strength of our study is that patients in both the cryopreserved and fresh cohorts were collected consecutively, thereby minimizing selection bias. Moreover, patients who underwent cryopreserved allo-HCT underwent transplantation between March and August 2020 and represent our real-time experience during the COVID-19 pandemic. In addition, our results provide important observations regarding the consequences of cryopreserved allo-HCT in the context of conditioning intensity. Finally, we have identified changes in the allograft composition elicited by the process of cryopreservation/thaw that had not been reported previously.
We acknowledge that our study has some limitations, including the imbalances between the 2 groups in terms of donor type at baseline and the relatively shorter observation period for the cryopreserved cohort compared with the fresh cohort. Furthermore, because immunophenotype analysis of the cryopreserved products was not performed at the time of infusion, it is possible that the duration of storage in liquid nitrogen might have negatively impacted the NK cell population.
In summary, our analysis revealed detrimental clinical outcomes associated with the cryopreservation of allogeneic grafts. Alterations in graft composition caused by prolonged product storage before freezing and the process of cryopreservation itself should be considered as contributing factors to this effect. Given the need to optimize strategies for HCT care during the COVID-19 pandemic, which continues to impose challenges in transportation and donor availability worldwide, further evaluation of the use of cryopreserved grafts and its impact on both clinical and laboratory outcomes is warranted.
ACKNOWLEDGMENTS
The authors thank Jessica Poyser for excellent laboratory management and technical support of this study.
Financial disclosure statement: This work was supported by the California Institute for Regenerative Medicine (Grant DR2A-05365, to J.A.S.), the National Institutes of Health (Grants NCI P01CA049605 and NHLBI OT2HL152830, to J.A.S.), the Gunn/Oliver Research Fund (to J.A.S.), the D.K. Ludwig Fund for Cancer Research (to J.A.S.), and the H.L. Snyder Medical Foundation (to J.A.S.).
Conflict of interest statement: L.M. reports research funding from Adaptive Biotechnologies, Servier, Astellas, and Jasper Therapeutics and consulting for Amgen and Pfizer. E.H.M. reports research funding from Orca Bio. R.S.N. reports research funding from Amgen, Kuur, and Magenta; equity in the public company Magenta and private companies BioEclipse and Biosource; and honoraria from Uptodate. A.R.R. reports research funding from Pharmcyclics. S.S. reports consultancy for Janssen. P.S. reports research funding from Orca Bio. J.A.S. reports research funding from Magenta and equity in the public company Jasper Therapeutics.
Authorship statement: A.K.B., J.C., J.A.S. and S.A. conceived the project and designed the studies; A.K.B., J.C., M.L.J. and S.A. collected clinical data. A.K.B. processed apheresis samples and analyzed the data; B.Y. conducted the statistical analysis. A.K.B., J.C., J.A.S. and S.A. wrote the manuscript; B.Y., T.L.R., J.B., K.H., N.D., M.L.J., R.L., J.B., L.J., A.R.R., M.J.F., L.M., W.K.W., S.S., R.S.N., D.B.M., P.S., and E.H.M. edited the manuscript; and J.A.S. and S.A. supervised the project. J.A.S. and S.A. contributed equally to this work.
Footnotes
Financial disclosure: See Acknowledgments on page 215.e9.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2022.01.010.
Appendix. Supplementary materials
REFERENCES
- 1.Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the “cold shoulder”? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38:399–405. doi: 10.1038/sj.bmt.1705462. [DOI] [PubMed] [Google Scholar]
- 2.Kim DH, Jamal N, Saragosa R, et al. Similar outcomes of cryopreserved allogeneic peripheral stem cell transplants (PBSCT) compared to fresh allografts. Biol Blood Marrow Transplant. 2007;13:1233–1243. doi: 10.1016/j.bbmt.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Lioznov M, Dellbrugger C, Sputtek A, Fehse B, Kröger N, Zander AR. Transportation and cryopreservation may impair haematopoietic stem cell function and engraftment of allogeneic PBSCs, but not BM. Bone Marrow Transplant. 2008;42:121–128. doi: 10.1038/bmt.2008.93. [DOI] [PubMed] [Google Scholar]
- 4.Medd P, Nagra S, Hollyman D, Craddock C, Malladi R. Cryopreservation of allogeneic PBSC from related and unrelated donors is associated with delayed platelet engraftment but has no impact on survival. Bone Marrow Transplant. 2013;48:243–248. doi: 10.1038/bmt.2012.118. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Zhang MJ, Tang XY, et al. Hematopoietic cell transplantation with cryopreserved grafts for severe aplastic anemia. Biol Blood Marrow Transplant. 2020;26:e161–e166. doi: 10.1016/j.bbmt.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamadani M, Zhang MJ, Tang XY, et al. Graft cryopreservation does not impact overall survival after allogeneic hematopoietic cell transplantation using post-transplantation cyclophosphamide for graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2020;26:1312–1317. doi: 10.1016/j.bbmt.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu JW, Farhadfar N, Murthy H, et al. The effect of donor graft cryopreservation on allogeneic hematopoietic cell transplantation outcomes: a Center for International Blood and Marrow Transplant Research analysis. Implications during the COVID-19 pandemic. Transplant Cell Ther. 2021;27:507–516. doi: 10.1016/j.jtct.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alotaibi AS, Prem S, Chen S, et al. Fresh vs frozen allogeneic peripheral blood stem cell grafts: a successful timely option. Am J Hematol. 2021;96:179–187. doi: 10.1002/ajh.26033. [DOI] [PubMed] [Google Scholar]
- 9.Maurer K, Kim HT, Kuczmarski TM, et al. Impact of cryopreservation and transit times of allogeneic grafts on hematopoietic and immune reconstitution. Blood Adv. 2021;5:5140–5149. doi: 10.1182/bloodadvances.2021005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohrt HE, Turnbull BB, Heydari K, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114:1099–1109. doi: 10.1182/blood-2009-03-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowsky R, Messner HA. Mechanisms and treatment of graft failure. Forman SJ, Negrin RS, Antin JH, Appelbaum FR, editors. Mechanisms and treatment of graft failureThomas’ Hematopoietic Cell Transplantation. 2016:944–954. [Google Scholar]
- 12.Millan MT, Shizuru JA, Hoffmann P, et al. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73:1386–1391. doi: 10.1097/00007890-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purtill D, Antonenas V, Chiappini P, et al. Variable CD34+ recovery of cryopreserved allogeneic HPC products: transplant implications during the COVID-19 pandemic. Blood Adv. 2020;4:4147–4150. doi: 10.1182/bloodadvances.2020002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiercinska E, Schlipfenbacher V, Bug G, et al. Allogeneic transplant procurement in the times of COVID-19: quality report from the central European cryopreservation site. J Transl Med. 2021;19:145. doi: 10.1186/s12967-021-02810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin PJ. Donor CD8 cells prevent allogeneic marrow graft rejection in mice: potential implications for marrow transplantation in humans. J Exp Med. 1993;178:703–712. doi: 10.1084/jem.178.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shizuru JA, Jerabek L, Edwards CT, Weissman IL. Transplantation of purified hematopoietic stem cells: requirements for overcoming the barriers of allogeneic engraftment. Biol Blood Marrow Transplant. 1996;2:3–14. [PubMed] [Google Scholar]
- 18.Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994;84:2436–2446. [PubMed] [Google Scholar]
- 19.Gandy KL, Domen J, Aguila H, Weissman IL. CD8+TCR+ and CD8+TCR- cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity. 1999;11:579–590. doi: 10.1016/s1074-7613(00)80133-8. [DOI] [PubMed] [Google Scholar]
- 20.Hexner E. The role of T cells in hematopoietic stem cell engraftment. ScientificWorldJournal. 2006;6:246–253. doi: 10.1100/tsw.2006.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panse JP, Bastianelli C, Santos EB, et al. Dog leukocyte antigen nonidentical unrelated canine marrow grafts: enhancement of engraftment by CD4 and CD8 T cells. Transplantation. 2003;76:474–480. doi: 10.1097/01.TP.0000076625.18877.02. [DOI] [PubMed] [Google Scholar]
- 22.Escobedo-Cousin M, Jackson N, Laza-Briviesca R, et al. Natural killer cells improve hematopoietic stem cell engraftment by increasing stem cell clonogenicity in vitro and in a humanized mouse model. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu B, Bao G, Zhang Y, et al. Donor NK Cells and IL-15 promoted engraftment in nonmyeloablative allogeneic bone marrow transplantation. J Immunol. 2012;189:1661–1670. doi: 10.4049/jimmunol.1103199. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Won DI, Lee NY, Sohn SK, Suh JS, Lee KB. Non-CD34+ cells, especially CD8+ cytotoxic T cells and CD56+ natural killer cells, rather than CD34 cells, predict early engraftment and better transplantation outcomes in patients with hematologic malignancies after allogeneic peripheral stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:719–728. doi: 10.1016/j.bbmt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Schäfer AK, Waterhouse M, Follo M, et al. Phenotypical and functional analysis of donor lymphocyte infusion products after long-term cryopreservation. Transfus Apher Sci. 2020;59 doi: 10.1016/j.transci.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 27.Kim DH, Sohn SK, Lee NY, et al. Transplantation with higher dose of natural killer cells associated with better outcomes in terms of non-relapse mortality and infectious events after allogeneic peripheral blood stem cell transplantation from HLA-matched sibling donors. Eur J Haematol. 2005;75:299–308. doi: 10.1111/j.1600-0609.2005.00514.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.