Abstract
Background
Dogs are one of the important asymptomatic carriers of antimicrobial resistant and potentially pathogenic strains of Salmonella. They can harbor large bacterial load in the intestines and mesenteric lymph nodes which can be shed in their feces with the possibility of transmission to humans. Therefore, a cross-sectional study was conducted with the objectives of estimating the prevalence of non-typhoidal Salmonella, assessing the risk factors for dog’s Salmonella carriage, and profiling the antimicrobial resistance pattern of Salmonella isolates among housed dogs in Harar town, Eastern Ethiopia. A total of 415 rectal swab samples were collected from randomly selected dogs. Samples were examined for non-typhoidal Salmonella using standard bacteriologic culture and biochemical tests. The disk diffusion method (Kirby-Bauer test) was employed to evaluate the isolates for their susceptibility against five antimicrobials.
Results
Non-typhoidal Salmonella were isolated from 26 (6.3%) of the rectal swab samples, with significantly higher occurrence in diarrheic (15.2%) than non-diarrheic (5.5%) dogs. The risk of Salmonella harboring was significantly higher in female dogs than in male dogs (OR = 2.5, p = 0.027). Dogs fecal shedding of Salmonella was relatively higher in households who used offal as a main feed type for their dogs (23.1%; 95% CI = 5–53.8) than those who used leftover food (10.1%; 95% CI = 5.7–16.1) and practiced mixed feeding system (17%; 95% CI = 7.6–30.8). Salmonella isolates showed higher resistance to ampicillin (41.7%), while all isolates were fully susceptible to gentamicin. Moreover, 58.3% of Salmonella isolates showed resistance to at least one of the tested antimicrobials. Majorities (72.7%) of the dog owners had no awareness on the risk of zoonotic salmonellosis from dog and all of the respondents use bare hand to clean dog kennel.
Conclusion
Our study reveals the importance of both diarrheic and apparently healthy housed dogs in the harboring and shedding of antimicrobial resistant non-typhoidal Salmonella. The risk of non-typhoidal Salmonella spread among pet owners is not negligible, especially in households who use offal as main feed type. Therefore, an integrated approach such as: proper dog handling practices; continuous evaluation of antimicrobial resistance; and rational use of antimicrobials in the field of veterinary sector are necessary to tackle the problem.
Keywords: Antimicrobial resistance, Dog, Non-typhoidal Salmonella, Prevalence, Risk factors
Introduction
Salmonella is the causative agent of both human and animal salmonellosis. The bacterium causes infections ranging from subclinical carrier state to acute fatal septicemia [1]. It is a potential cause of acute and chronic diarrhea and death in numerous animal species and in human beings [2]. Particularly, salmonellosis in animals is a major concern, because animals can shed Salmonella serotypes into the environment without any apparent clinical signs [3]. Salmonella is widespread in the environment and commonly found in farm effluents, human sewage and in any material subjected to fecal contamination [4]. Due to considerable geographical and temporal variation in the prevalence of Salmonella species in animals and humans, understanding the role of animals in zoonotic transmission is important to monitor salmonellosis [5].
Non-typhoidal Salmonella is an important zoonosis worldwide. It is reported that globally an estimated 65–380 million illnesses and 43–88 thousand deaths of human beings were associated with non-typhoidal S. enterica from the year 1990 to 2012 [6]. As of 2002, zoonotic Salmonella strains such as S. typhimurium, S. Heidelberg, and S. enteritidis accounts for 17, 11, and 9% of Salmonella sourced from non-human subjects [7]. One of the sources for human salmonellosis is feces of pet dogs [8] and there have been reports on transmission of Salmonella from dogs to humans [9, 10]. It was reported that dogs can harbor large bacterial load (102–106 per 100 g of feces) in their intestine, which can be shed in their feces for several months [11]. Thus, this carriage could be of significant importance to public health as dogs have close contact with family members in households [12].
Different scholars reported antimicrobial resistant Salmonella isolates from food samples [13], animals [14, 15] and human [16, 17] in Ethiopia. Due to the emergence and spread of antimicrobial-resistant strains, there is an increasing concern with this pathogen [18]. The concern of antimicrobial resistance is particularly important in developing countries, because of inadequate adherence to prudent use of antimicrobials; unhygienic living conditions; and close contact and sharing of houses between animals and humans [19].
Some reports have shown the occurrence of Salmonella in dogs from different parts of the globe. For instance, United States [20, 21], the United Kingdom [22], Thailand [23], Taiwan [24], Turkey [25, 26], and Trinidad [27]. It has been well known for several decades that dogs may carry Salmonella species in their intestinal tracts, mainly as an asymptomatic carrier state [9, 28]. Gastrointestinal disease manifested as enterocolitis and endotoxemia can occur and is often associated with fever, vomiting, anorexia, dehydration, and depression [29, 30]. Pet feed preparations play crucial role in the transmission of Salmonella among housed dogs, because raw meat-based dogs feed tends to contain significantly higher Salmonella spp. than commercial dry feed [31]. Furthermore, it was reported that Salmonella was found in 21% commercial raw food diets, representing combinations of raw meat, vegetables, grain, and eggs or fruit [32]. Contamination rates in dry or canned foods are thought to be considerably lower, and Salmonella has not been isolated from canned dog food [33].
Studies in Ethiopia showed that majority of livestock owners have the habit of using antimicrobials to treat animal diseases [34, 35]. However, the antimicrobial usage is characterized by shortcomings such as: inability to define the specific purposes of prescribed drug and lack of awareness on the risks of antimicrobial resistance [34]; the use of human preparation for veterinary purposes, inappropriate dosages, incomplete treatment regimens, lack awareness on the recommended withdrawal periods [35]; and limited access to antimicrobial varieties [34, 35]. In Ethiopia, pet dogs are integral part of the society, which is evidenced by household’s dog ownership ranging from 33 to 40.5% in towns [36, 37] and 75.5% in rural communities [37]. Despite the increasing urbanization in major towns of Ethiopia, only few studies have shown the status of Salmonella in housed dogs [15, 38, 39]. Moreover, these studies failed to provide detailed information on the risk factors for dog salmonellosis and there is limited information on the antimicrobial susceptibility profiles of clinical isolates. Therefore, the objectives of this study were to estimate the prevalence of non-typhoidal Salmonella isolates, to assess the risk factors associated with Salmonella occurrence, and to identify antimicrobial susceptibility profiles of the isolates from apparently healthy and diarrheic dogs in Harar town, Eastern Ethiopia.
Results
Overall prevalence of Salmonella in dogs
From 415 dogs examined, 26 (6.3%) were positive for Salmonella. The present study showed that the point estimates for prevalence of Salmonella in apparently healthy and diarrheic dogs was 5.5 and 15.2% respectively. Confidence intervals are given in Table 1.
Table 1.
Prevalence of Salmonella based on clinical status of sampled dogs in Harar town
| Clinical state | Number of dogs examined | Number positive for Salmonella | Prevalence in % (95% CI) |
|---|---|---|---|
| Apparently healthy | 382 | 21 | 5.5 (3.4–8.3) |
| Diarrheic | 33 | 5 | 15.2 (5.1–31.9) |
| Total | 415 | 26 | 6.3 (4.1–9.0) |
Prevalence of Salmonella in dogs and households among Kebeles
The prevalence varied among kebeles, in that it was higher in kebele 10 (10%) followed by kebeles 15 (9.9%), 16 (7.0%), 18 (3.4%), 13 (1.6%), and 17 (0%) (Table 2) but with no significant variation among the kebeles. Among the 209 households, Salmonella was detected in 12.4% with varied frequencies among the kebeles (Table 2). However, kebele had no significant association with the occurrence of Salmonella both at animal and household levels. Except in kebele 17, Salmonella positive dogs were found among households in all kebeles and the prevalence varied numerically with the highest being in kebele 10 (25%) (Table 2).
Table 2.
Prevalence of Salmonella across the studied kebeles of Harar town, eastern Ethiopia
| Kebeles | Total No. of dogs examined | Number of dogs positive for Salmonella (%) | Total household examined | Number of household positive For Salmonella (%) |
|---|---|---|---|---|
| 15 | 101 | 10 (9.9) | 72 | 10 (13.9) |
| 16 | 142 | 10 (7.0) | 66 | 10 (14.9) |
| 13 | 61 | 1 (1.6) | 23 | 1 (4.3) |
| 10 | 30 | 3 (10) | 12 | 3 (25) |
| 18 | 59 | 2 (3.4) | 29 | 2 (6.9) |
| 17 | 22 | 0 (0) | 7 | 0 (0) |
| Total | 415 | 26 (6.3) | 209 | 26 (12.4) |
Risk factors for Salmonella in dogs
As shown in Table 3, Salmonella prevalence was significantly higher in female (10.1%) than males (4.3%) and in diarrheic dogs (15.2%) than apparently healthy (5.5%). Female dogs had 2.5 times the odd of shedding Salmonella in their feces than male dogs (P < 0.05). The odds of Salmonella shedding in thin and fat body conditioned dogs were 2.8 and 1.5 times, respectively higher than medium body conditioned once. Meanwhile, dogs fed uncooked preparations had 2.0 times the odds of harboring Salmonella than those fed with cooked preparations. However, there was no significant difference with respect to breed, age, feeding, feed treatment, BCS, and educational status of dog owners (Table 3).
Table 3.
Results of analysis on potential risk factors for Salmonella shedding by dogs in Harar town, Eastern Ethiopia
| Variables | No. of Animals examined | No. of Animals with Salmonella (%) | χ2 value (p-value) | Univariable LG analysis | |
|---|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | ||||
| Sex | |||||
| Female | 139 | 14 (10.1) | 5.158 (0.023) | 2.5 (1.1–5.5) | 0.027 |
| Male | 276 | 12 (4.3) | * | ||
| Breed | |||||
| Local | 306 | 20 (6.5) | 0.146 (0.732) | 1.2 (0.5–3.1) | 0.703 |
| Cross | 109 | 6 (5.5) | * | ||
| Age | |||||
| Young | 189 | 11 (5.8) | 0.117 (0.732) | * | |
| Old | 226 | 15 (6.6) | 1.2 (0.5–2.6) | 0.732 | |
| BSC | |||||
| Medium | 284 | 14 (4.9) | 3.600 (0.135) | * | |
| Fat | 97 | 8 (8.2) | 1.5 (0.4–5.3) | 0.543 | |
| Thin | 34 | 4 (11.8) | 2.8 (0.8–8.3) | 0.115 | |
| Feeding | |||||
| Leftover | 288 | 15 (5.2) | 2.596 (0.262) | * | |
| Offal’s | 27 | 3 (11.1) | 1.6 (0.6–4) | 0.312 | |
| Both | 100 | 8 (8) | 1.4 (0.4–5.8) | 0.612 | |
| Feed Rx | |||||
| Uncooked | 31 | 1 (3.2) | 0.527 (0.404) | 2.0 (0.3–1.6) | 0.478 |
| Mixed | 384 | 25 (6.5) | * | ||
| Diarrheic | |||||
| No | 382 | 21 (5.5) | 4.821 (0.045) | – | – |
| Yes | 33 | 5 (15.2) | – | – | |
| Educational status: | |||||
| Below high school | 251 | 17 (6.8) | 0.279 (0.597) | 1.3 (0.5–2.9) | 0.598 |
| High school and above | 164 | 9 (5.5) | * | ||
No. Number, LG Logistic regression, CI Confidence Interval, BCS Body condition score, Rx Treatment
*Explanatory variables
In this study, a relatively higher prevalence of Salmonella shedding was observed in households who used offal as main feed type for their dogs (23.1%) than those who used leftover food (10.1%) and practiced mixed feeding system (17%) (Table 4).
Table 4.
Owners’ awareness on the risk of zoonotic transmission of dog Salmonella among households of Harar town, Eastern Ethiopia (n = 209)
| Variable items | Category | No. of HH respondents | No. positive | Prevalence in % (95% CI) | Chi-square (p value) |
|---|---|---|---|---|---|
| Feed type | Leftover food | 149 | 15 | 10.1 (5.7–16.1) | 3.026 (0.220) |
| Offal | 13 | 3 | 23.1 (5–53.8) | ||
| Mixed | 47 | 8 | 17 (7.6–30.8) | ||
| Feed treatment | Uncooked | 19 | 1 | 5.3 (0.1–26.0) | 0.988 (0.320) |
| Mixed | 190 | 25 | 13.2 (8.7–18.8) | ||
| Educational status of dog owners | Below high school | 119 | 17 | 14.3 (8.5–21.9) | 0.864 (0.238) |
| High school and above | 90 | 9 | 10 (4.7–18.1) | ||
| Knowledge on transmission of Salmonella to human | Yes | 57 | 10 | 17.5 (6.1–16.5) | 1.874 (0.171) |
| No | 152 | 16 | 10.5 (8.7–29.9) |
n Number of households examined, No. Number, HH Households
Antimicrobial susceptibility profiles of Salmonella isolates
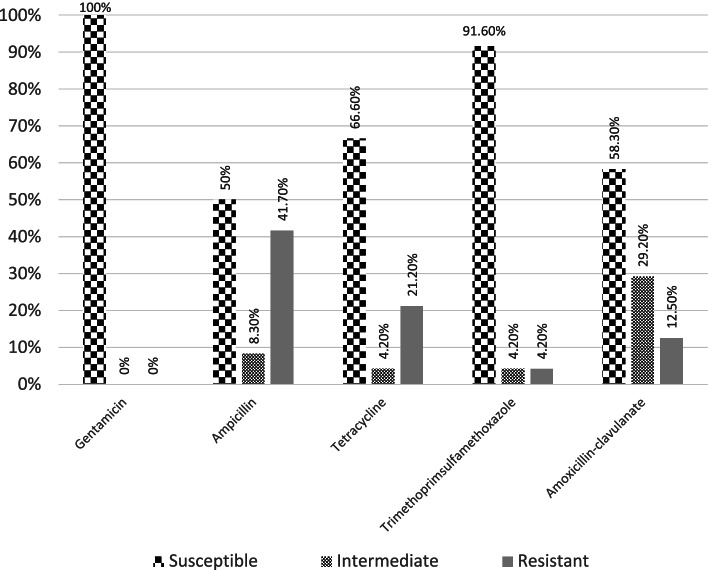
All (n = 24) the tested isolates were susceptible to gentamicin, while varied proportions of resistance were observed against the other tested antimicrobials. Thus, relatively high resistance was observed against ampicillin (41.7%) followed by tetracycline (21.2%), amoxicillin-clavulanate (12.5%), and trimethoprim-sulfamethoxazole (4.2%) (Fig. 1). The control organism was susceptible to all tested antimicrobials.
Fig. 1.
In-vitro antimicrobial susceptibility of the isolated Salmonella (n = 24)
The study showed that 58.3% of Salmonella isolates were resistant to at least one of the tested antimicrobials (Table 5). The dominant isolates were those showed resistance against ampicillin only at a proportion of 20.8%. Meanwhile, from the total Salmonella isolates examined, 2 (8.3%) had shown resistance to two antimicrobial classes, with a resistance pattern to ampicillin, tetracycline, and amoxicillin-clavulanate. Moreover, the study revealed that resistant isolates showed similar distribution across the candidate risk factors for dog salmonellosis (Table 6).
Table 5.
Drug resistance patterns of Salmonella isolates (n = 24)
| Resistant to: | Name of the antimicrobial | Resistant isolates | |
|---|---|---|---|
| Number | % | ||
| None | – | 10 | 41.7 |
| One drug | AMP | 5 | 20.8 |
| TTC | 4 | 16.6 | |
| Two drugs | AMP, TMS | 1 | 4.2 |
| AMP, AMC | 1 | 4.2 | |
| AMP, TTC | 1 | 4.2 | |
| Three drugs | AMP, TTC, AMC | 2 | 8.3 |
| Overall | – | 24 | 100 |
Key: n Number, AMP Ampicillin, TTC Tetracycline, TMS Trimethoprim-sulfamethoxazole, AMC Amoxicillin-clavulanate
Table 6.
Antimicrobial susceptibility profiles of Salmonella isolates based on risk categories
| Categories | Number (%) of isolates: | ||
|---|---|---|---|
| Resistant to TTC | Resistant to AMP | Susceptible to all | |
| Age | |||
| Young (n = 10) | 3 (30) | 4 (40) | 5 (50) |
| Old (n = 14) | 4 (28.6) | 6 (42.9) | 5 (35.7) |
| Feed | |||
| Leftover food (n = 13) | 2 (15.9) | 4 (30.8) | 6 (46.2) |
| Offal based (n = 11) | 5 (45.5) | 6 (54.5) | 4 (36.4) |
| Sex | |||
| Male (n = 11) | 3 (27.3) | 6 (54.5) | 3 (27.3) |
| Female (n = 13) | 4 (30.8) | 4 (30.8) | 7 (53.8) |
| Breed | |||
| Cross (n = 5) | 2 (40) | 2 (40) | 3 (60) |
| Local (n = 19) | 5 (26.3) | 8 (42.1) | 7 (36.8) |
n Number of Salmonella isolates tested from each variable category, AMP Ampicillin, TTC Tetracycline
Dog handling practices in relation to Salmonella control
Practices related to dog handling, feeding, and hygiene had varied among households (Table 7). Thus, it was observed that majority (71.3%) of dog owners used leftover food as dog feed and none of them used commercial diet for the feeding of dog. Regarding feed treatment, majority (90.9%) of the households used occasional cooking of feed. Moreover, it was recorded that majority (72.7%) of the dog owners had no awareness on the risk of zoonotic dog salmonellosis. In addition, all of the owners responded that they used to clean dog’s kennel with bare hands.
Table 7.
Summary of dog management practices and dog owner’s awareness on the risk of zoonotic transmission of Salmonella (n = 209)
| Variable items | Response | Number of respondents | % |
|---|---|---|---|
| Feed types | Commercial diet | 0 | 0 |
| Leftover food | 149 | 71.3 | |
| Offal | 11 | 5.3 | |
| Mixed | 49 | 23.4 | |
| Feed treatment | Uncooked | 19 | 9.1 |
| Always cooked | 0 | 0 | |
| Sometimes cooked | 190 | 90.9 | |
| House cleaning | Use glove | 0 | 0 |
| Bare hand | 209 | 100 | |
| No clean | 0 | 0 | |
| Water source | Tap water | 209 | 100 |
| Ground water | 0 | 0 | |
| Addition of drug to feed | Yes | 0 | 0 |
| No | 209 | 100 | |
| Knowledge on transmission of Salmonella to human | Yes | 57 | 27.3 |
| No | 152 | 72.7 |
n Number of households examined
Discussion
Our study has focused on prevalence study for Salmonella carriage in apparently healthy and diarrheic dogs based on bacteriologic culture and biochemical identification. In addition, an invitro antimicrobial test was conducted using disc diffusion method to observe the resistance profiles of Salmonella isolates against five antimicrobials used in the veterinary as well as human medicine. The study also attempts to elucidate the potential risks for the transmission of salmonellosis in dogs as well as humans using a prepared questionnaire format.
Our study showed that the fecal shedding of Salmonella among pet dogs located in Harar town of eastern Ethiopia was 6.3%, in which significantly higher prevalence was recorded in diarrheic dogs (15.2%) as compared to the apparently healthy once (5.5%). This finding is within the range of 0 to 44% subclinical carriage of Salmonella in dogs [40]. This higher prevalence of Salmonella in diarrheic dogs is supported by previous findings in different parts of the globe [8, 41–43]. However, authors like Sultan et al. [38] and Zewdu et al. [39] reported that the prevalence did not vary significantly between clinically healthy and diarrheic dogs in Ethiopia.
The sub-clinical shedding of Salmonella by housed dogs has been reported from different countries, but the prevalence varies. For instance, overall sub-clinical Salmonella shedding in our study (5.5%) is in line with the report of Amadi et al. [44] and Leahy et al. [20] from Grenada (5.6%) and USA (4.9%), respectively. In contrary to our finding, studies showed lower sub-clinical carriage, such as: 0% [45]; 2.3% [46]; 1% [26]; 1.2% [47]; and 0.2% [48] from New Zealand, USA, Turkey, Canada, and United Kingdom, respectively. This indicates the fact that owners in developed countries may be more focused on the importance of hygiene and make use of the available veterinary care for their animals [49]. On the other hand, different authors have reported higher prevalence such as: 20.8% [50]; 10.5% [3]; 43.7% [51]; 13.2% [43]; 11.7% [15]; and 17.1% [38] from USA, Iran, Northeastern Nigeria, Thailand, Addis Ababa, and Holeta town of Ethiopia, respectively. Generally, prevalence is influenced by factors such as pet sanitary practices, feeding habit, difference in public awareness about dog zoonosis, and socioeconomic status of the owners. Despite the above facts, season of study, geographical areas, and diagnostic methods employed might have also accounted for the observed difference as described by Seepersadsingh et al. [27].
In our findings, there was no significance difference between feeding of leftover, offal and both (leftover and offal). But the prevalence is higher in dogs fed on offal (11.1%) as compared to dogs fed on household leftover food (5.2%) and mixed diet (8%). In agreement with the present finding, Finley et al. [52] reported higher fecal shedding of Salmonella in dogs fed on raw meat and offal diets. Schotte et al. [53] stated that feeding raw meat and other uncooked diets were risk factors for carriage of Salmonella in dogs. PHAC (Public Health Agency of Canada) [54] reported that raw meat and meat products were frequently contaminated with Salmonella, and consequently, homemade raw diets were considered as a potential source of Salmonella. Freeman et al. [55] observed that the known infection risk to owners is highly relevant when pets are consuming Salmonella contaminated feed. Reports from Ethiopia showed that 8.5–13.5% of examined chicken, pork, mutton, and beef harbor different serotypes of Salmonella [13, 56]. Other reports indicate that Salmonella is prevalent in animals, humans, and food items in different parts of Ethiopia, suggesting that Salmonella can be prevalent in dogs [57, 58].
Our study shows that Salmonella shedding was significantly higher in female than male dogs. However, Jajere et al. [59] from Nigerian reported that male dogs had significantly higher Salmonella infection than females. In contrary, previous studies from Taiwan [24], Ontario [42], and Mexico [60] showed insignificant difference among male and female dogs. Similarly, from Ethiopia various authors indicated that the prevalence didn’t vary significantly among sex categories of studied dogs [15, 38, 39]. These disparities might not in fact reflect a real phenomenon, but just statistical variation resulting from confounding factors/variables.
In our findings, there was no significance difference between medium, fat and thin body condition score of the dogs, which is in accordance with the reports of Kiflu et al. [15], Sultan et al. [38], and Zewdu et al. [39], in that insignificant difference in the prevalence of Salmonella was recorded between body condition categories of studied dogs. Similarly, our study didn’t show significance difference between age categories of dogs. Furthermore, Sultan et al. [38] and Kiflu et al. [15] reported insignificant difference in the prevalence of Salmonella between age groups examined. In contrary to our finding, Núñez-Castro et al. [61] from Mexico reported that dogs under 1 year are more likely to acquire Salmonella than dogs older than 1 year, while Zewdu et al. [39] from Ethiopia reported that older dogs harbor more Salmonella than younger dogs. Often it is difficult to compare different findings, because different age profiles are seen in different studies and it is confounded by differences in sampled population lifestyles and owners dog caring practices. Literatures generally mentioned that younger animals are more susceptible to most of bacterial infections, mainly due to the immature immune system. However, both young and adult animal can be asymptomatic carriers of Salmonella [1, 11, 62].
Our finding shows that all Salmonella isolates were susceptible to gentamicin. However, previous antimicrobial resistance studies on dog isolates of Salmonella species reported resistance to gentamicin in Taiwan (5%) [24] and Nigeria (35.3%) [8]. This may reflect the fact that gentamicin is not commonly used in veterinary sector in Ethiopia, particularly Harar town (Source: researcher’s personal observations and clinical experiences). Meanwhile, some isolates have shown resistance against ampicillin (41.7%), tetracycline (21.2%), amoxicillin-clavulanate (12.5%), and trimethoprim-sulfamethoxazole (4.2%). This high proportion of resistance against ampicillin and tetracycline might reflect their frequent use in veterinary medications. From Ethiopia, Beyene et al. [63] suggest that high-rate of resistance to oxytetracycline is due to the fact that this drug is the most commonly used antimicrobial agent in animal medications. Similarly, a previous study in Taiwan showed resistant isolates to tetracycline (77.5%) and sulfamethoxazole/trimethoprim (37.5%) [24]. From Nigeria, it was reported that Salmonella isolates showed resistance to tetracycline (70.6%), ampicillin (47.1%), and amoxicillin-clavulanic acid (87.6%) [8, 51]. A previous report from Ethiopia indicates that 30.9 and 59.5% of Salmonella isolates from dogs showed resistance to ampicillin and tetracycline, respectively [15]. These findings indicate that Salmonella drug resistance can vary from country to country and even from one area to another area in the same country. The feeding habits of dogs play an important role in contracting drug resistant strains. For instance, Kiflu et al. [15] from Addis Ababa reported that majority of dog owners in the city used raw animal products to feed their dogs. In relation to this, Bedada and Molla [64] showed that 71.3% of beef obtained from cattle slaughtered in central Ethiopia contained oxytetracycline residues.
Majority of the Salmonella isolates (58.3%) were resistant to at least one of the tested antimicrobials. Moreover, 8.3% of the isolates showed resistance against three drug types (i.e., ampicillin, tetracycline, and amoxicillin-clavulanate). This shows that apparently healthy dogs could harbor drug resistant Salmonella thereby serving as a source of human infection. A better understanding of the interplay of factors that contribute to the dissemination and establishment of multidrug resistant isolates is necessary.
Conclusion
Our study revealed that non-typhoidal Salmonella occurred at higher frequency in diarrheic than apparently healthy dogs with an occurrence in almost all studied small administration units (kebeles). Salmonella occurrence was relatively higher in dogs managed at households who used offal as main feed type for their dogs than those who used leftover food and practiced mixed feeding system. Thus, dogs might play a significant role in spreading of the organism to humans as well as other animals. Moreover, the high carriage rate of Salmonella isolates resistant to varied antimicrobials used in the medications of humans and animals signals an important threat in both the veterinary and public health sectors as it limits antimicrobial drugs available for the effective control of Salmonella infections. Regular investigations on the circulating serotype as well as assessing the multi-drug resistance profiles may assist in controlling the occurrence of zoonotic salmonellosis in areas where large proportion of households use dogs as a pet animal.
Materials and methods
Study area
The study was conducted in Harar town, which is located around 9oN latitude and 42°E longitude and at a distance of about 526 km East of Addis Ababa, the capital of Ethiopia. Harar town has mean annual temperature of 28 °C [65]. The altitude of the town is 1850 m above sea level and its mean annual rainfall and humidity measures 596 mm and 60.3%, respectively [66]. The total human population of the town was estimated at 125,000 with annual growth rate of 2.6% as of the year 2014 [67].
Study population and sampling units
The study population was dogs owned by residents of Harar town. Among the 19 kebeles (i.e. the smallest administrative units of the town) in Harar town, six were randomly selected. Apparently healthy and diarrheic dogs regardless of age, sex, breed, and dog care practices were included in the study. All dogs included were those who didn’t took any medication with antimicrobial activity for the past 4 weeks prior to sampling.
Study design
A cross-sectional study was conducted from January 2020 to August 2020 to estimate the prevalence of Salmonella from rectal swab sample of dogs in selected kebeles of Harar towns, Harari Regional State, eastern Ethiopia. Dogs were sampled through door-to-door visit from households. Invitro-experimental study was employed to identify the antimicrobial susceptibility patterns of Salmonella isolates.
Sample size determination
The sample size was determined using the formula given by Thrusfield [68] by assuming simple random sampling. As there was no previous study on dog salmonellosis, the sample size was determined by assuming 50% expected prevalence; 5% desired absolute precision at 95% confidence interval; and based on the assumption of large dog population existing in the town. Thus, with two missed samples, 382 dogs were sampled. In addition, 33 dogs with signs of salmonellosis (diarrhea and septicemia) encountered during the study period were purposively included in the study. Diarrheic dog was defined as an animal presented by owner with a current problem of diarrhea [68].
Where n = sample size.
Pexp = expected prevalence.
d = desired absolute precision.
Sample and data collection
Prior to sample collection, individual animal’s history of medication with antimicrobial agents was noted. Then rectal swab sample was collected from each dog after proper restraining with the help of the owner. The samples were placed into a sterile buffered peptone water (HiMedia, India) and transported to Haramaya University Veterinary Microbiology Laboratory in box containing ice packs. Samples were processed for bacterial culture within 12 h of arrival. In addition, questionnaire and observational survey were used to gather data on feeding practices (cooked animal products and mixed [raw meat, cooked animal products and household leftover]) and sampled animal attributes such as sex, breed, body condition, and age.
Isolation and identification of Salmonella
Isolation and identification of Salmonella from rectal swab samples were performed according to the procedure recommended by the international standard organization (ISO) for isolation of Salmonella [69]. Rectal swab samples were transferred into a tube with 9 ml of buffered peptone water (HiMedia, India), shaken for approximately 2 min and incubated at 37 ± 1 °C for 18 ± 2 h. A portion of the culture (0.1 ml) was transferred into a tube containing 10 ml of selective enrichment liquid media (Rappaport-Vassiliadis, HiMedia, India) and incubated at 42 °C for 24 ± 3 h. Similarly, 1 ml of the culture was transferred to a tube containing 10 ml of tetrathionate broth (Conda S.A., Spain) and incubated at 37 °C for 24 ± 3 h. A loopful of inoculum from each of enrichment cultures was then inoculated on the surface of two different plates, xylose lysine deoxycholate (XLD) agar (Sisco research lab, India) and brilliant green agar (BGA) (HiMedia, India) and then incubated at 37 °C for 24 ± 3 h. For confirmation, presumptive Salmonella colonies from both XLD and BGA agar were selected and streaked onto the surface of pre-dried nutrient agar (Oxoid, England) plates and incubated at 37 °C for 24 ± 3 h. Colonies from nutrient agar were tested for catalase, oxidase, and Gram’s reaction. Presumptive isolates were inoculated into the following biochemical test tubes for identification: triple sugar iron (TSI) agar (HiMedia, India), Simmon‟s citrate agar (HiMedia, India), Sulphide Indole Motility (SIM) medium (Sisco research lab, India) and incubated for 24 or 48 h at 37 °C. Colonies producing an alkaline (red) slant with acid (yellow) butt with hydrogen sulphide production (blackening) on TSI, positive for citrate utilization (blue color), and negative for tryptophan utilization (Indole test) (yellow-brown ring), and negative for urea utilization were considered as Salmonella [70]. In addition, all of the tested isolates were motile. Positive control isolate/strain was obtained from Ethiopian Public Health Institute (EPHI), Addis Ababa, Ethiopia.
Antimicrobial susceptibility test of Salmonella isolates
Susceptibility of the isolates to five antimicrobials was determined using the disk diffusion method according to the guidelines of Clinical and Laboratory Standards Institute [71]. Briefly, frozen isolates were sub-cultured on tryptic soy agar (Becton, Dickinson and Company, USA) from which 3 to 4 pure colonies were further inoculated in to a tube containing 5 ml of tryptic soy broth (TSB) (Becton, Dickinson and Company, USA). The tubes were then incubated at 37 °C for 4–5 h. The turbidity of each suspension was then adjusted to 0.5 McFarland turbidity standard using sterile saline solution. Sterile cotton swab was dipped and rotated several times and pressed firmly on the inside wall of the tube above the fluid level to remove excess inoculum. It was then spreading on to the entire surface of Mueller-Hinton agar plate (Oxoid, Ltd). The inoculated plates were left at room temperature to for 5–10 min until excess moisture is removed and antimicrobial discs were placed by pressing on the plate with sterile forceps. The plates were then inverted and incubated overnight at 35 °C. Diameters of the zone of inhibition were measured to the nearest millimeter using a plastic transparent ruler. The interpretation of the categories of susceptible, intermediate or resistant was based on the CLSI guidelines [71]. For the purpose of analysis, all readings classified as intermediate were considered as resistant unless indicated. Reference strain of Salmonella Typhi ATCC 27853 was used as a quality control. The antimicrobial discs (Sensi-Discs, Becton, Dickinson and Company, Loveton, USA) were amoxicillin + clavulanic acid (20/10 μg), gentamicin (10 μg), tetracycline (30 μg), sulfamethoxazole and trimethoprim (23.75 and 1.25 μg), and ampicillin (10 μg).
Data management and analysis
All collected data were entered and coded using Microsoft Excel Spreadsheet. Statistical analysis was made using STATA software version 11.0 (STATACORP, 2009). Before analysis, the age of dog was classified in to two group young (less than 2 years) and old (> 2 years). in addition, body condition score was done based on 5 scale (emaciated, thin, ideal (medium), fat, and obese) according to AAHA (American Animal Hospital Association). Descriptive statistics such as frequency and percentage were used to describe the practices, knowledge and awareness in the community regarding the disease. Chi-square, Fisher exact test and logistic regression analyses were used to assess the association of risk factors with the prevalence of Salmonella. In all the cases, P < 0.05 was considered as significant association.
Acknowledgements
The authors are thankful to Haramaya University for facilitating the work and availing the laboratory reagents, consumables, and facilities. Particularly, we are thankful to staff members in veterinary microbiology laboratory of Haramaya University for their support during the laboratory work.
Abbreviations
- BGA
Brilliant Green Agar
- TSI
Triple Sugar Iron
- XLD
Xylose Lysine Deoxycholate
Authors’ contributions
B.U., B.A., and S.A. wrote the main manuscript. All authors contributed starting from the inception, data collection, analysis, and interpretation. All authors reviewed the manuscript. The authors read and approved the final manuscript.
Authors’ information
Belisa Usmael is MSc graduate in Veterinary Microbiology and working as researcher in Haramaya University owned research projects. Bruk Abraha (DVM) is an Associate Professor in Veterinary Microbiology in College of Veterinary Medicine, Haramaya University, Ethiopia. Sisay Alemu is an Assistant Professor in College of Veterinary Medicine, Haramaya University, Ethiopia. Bahar Mummed is a Lecturer in College of Veterinary Medicine, Haramaya University, Ethiopia. Adem Hiko is a Professor in College of Veterinary Medicine, Haramaya University, Ethiopia. Abdallahi Abdurehman is a Lecturer in College of Veterinary Medicine, Haramaya University, Ethiopia.
Funding
Not applicable.
Availability of data and materials
The data used to validate the results of this analysis are available from the first and correspondent authors upon reasonable request.
Declarations
Ethics approval and consent to participate
Even thou the study subjects (dogs) are not exposed to damages due to the nature of sample (swab sample), the best practice guidelines for veterinary care were applied during sampling. Because our study didn’t include animal experimentation, our study was not subjected to ethical review by “Animal Research Ethical Review Committee of Haramaya University, College of Veterinary Medicine”. Rectal swab sample collection was carried out under aseptic conditions without affecting an animal’s life. The dog owners were informed and aware about the purpose of the study and verbal informed consent was obtained to conduct the study. In doing so, we don’t include any personal data, images, videos, etc. that can violate individual participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Belisa Usmael, Email: ubelisa58@gmail.com.
Bruk Abraha, Email: abraha.ashebr@yahoo.com, Email: Bruk.Abraha@haramaya.edu.et.
Sisay Alemu, Email: ssayalemu@gmail.com.
Bahar Mummed, Email: mummedbahar@yahoo.com.
Adem Hiko, Email: adex.2010ph@gmail.com.
Abdallahi Abdurehman, Email: amboabdallahi@gmail.com.
References
- 1.Quinn P, Markey B, Leonard F, Fitzpatrick E, Fanning S, Hartigan P. Veterinary microbiology and microbial disease. 2. Syndey: Hometra; 2011. [Google Scholar]
- 2.McGavin D, Carlton W, Zachary J. Thompson’s special veterinary pathology. Philadephia: Mosby; 2001. [Google Scholar]
- 3.Zahraei-Salehi T, Askari-Badouei M, Madadgar O, Ghiasi SR, Ashrafi-Tamai I. Shepherd dogs as a common source for Salmonella enterica serovar reading in Garmsar, Iran. Turk J Vet Anim Sci. 2013;37:102–105. doi: 10.3906/vet-1107-1. [DOI] [Google Scholar]
- 4.OIE (World Organization for Animal Health) Report of the meeting of the OIE ad hoc group on Salmonellosis. May 2010 ed. Paris: World Organisation for Animal Health; 2010. [Google Scholar]
- 5.Leonard F. Salmonella infection and carriage: the importance of dogs and their owners. Vet Rec. 2014;174(4):92–93. doi: 10.1136/vr.g367. [DOI] [PubMed] [Google Scholar]
- 6.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, Protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12(12):e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galanis E, Lo Fo Wong DMA, Patrick ME, Binsztein N, Cieslik A, Chalermchikit T, Aidara-Kane A, Ellis A, Angulo FJ, Wegener HC. Web-based surveillance and global Salmonella distribution, 2000-2002. Emerg Infect Dis. 2006;12(3):381–388. doi: 10.3201/eid1205.050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojo O, Adetosoye A. Salmonella Typhimurium infection in diarrhoeic and none-diarrhoeic infection dogs in Ibadan, Nigeria dogs in Ibadan, Nigeria. Veterinarski Arhiv. 2009;79:371–377. [Google Scholar]
- 9.Morse E, Duncan M, Estep D, Riggs W, Blackburn B. Canine salmonellosis: a review and report of dog to child transmission of Salmonella Enteritidis. Am J Public Health. 1976;66:82–84. doi: 10.2105/AJPH.66.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato Y, Mori T, Koyama T, Nagase H. Salmonella Virchow infection in an infant transmitted by household dogs. J Vet Med Sci. 2000;62(7):767–769. doi: 10.1292/jvms.62.767. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Katsube Y, Imaizumi K. Distribution of salmonellae in the digestive tract and lymph node of carrier-dogs. Japan J Vet Sci. 1976;38:215–224. doi: 10.1292/jvms1939.38.215. [DOI] [PubMed] [Google Scholar]
- 12.Hoelzer K, Moreno-Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res. 2011;42:34. doi: 10.1186/1297-9716-42-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ejo M, Garedew L, Alebachew Z, Worku W. Prevalence and antimicrobial resistance of Salmonella isolated from animal-origin food items in Gondar, Ethiopia. Biomed Res Int. 2016;4290506. 10.1155/2016/4290506. [DOI] [PMC free article] [PubMed]
- 14.Abdi RD, Mengstie F, Beyi AF, Beyene T, Waktole H, Mammo B, Ayana D, Abunna F. Determination of the sources and antimicrobial resistance patterns of Salmonella isolated from the poultry industry in Southern Ethiopia. BMC Infect Dis. 2017;17(1):352. doi: 10.1186/s12879-017-2437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiflu B, Alemayehu H, Abdurahaman M, Negash Y, Eguale T. Salmonella serotypes and their antimicrobial susceptibility in apparently healthy dogs in Addis Ababa Ethiopia. BMC Vet Res. 2017;13:1–9. doi: 10.1186/s12917-017-1055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eguale T, Gebreyes W, Asrat D, Alemayehu H, Gunn JS, Engidawork E. Non-typhoidal Salmonella serotypes, antimicrobial resistance and coinfection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect Dis. 2015;15:497. doi: 10.1186/s12879-015-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marami D, Hailu K, Tolera M. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella species among asymptomatic food handlers working in Haramaya University cafeterias, Eastern Ethiopia. BMC Res Notes. 2018;11(1):74. doi: 10.1186/s13104-018-3189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. The evolving threat of antimicrobial resistance: options for action. Geneva: World Health Organization; 2012. https://apps.who.int/iris/handle/10665/44812
- 19.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379(9835):2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leahy AM, Cummings KJ, Rodriguez-Rivera LD, Rankin SC, Hamer SA. Evaluation of faecal Salmonella shedding among dogs at seven animal shelters across Texas. Zoonoses Public Health. 2016;63(7):515–521. doi: 10.1111/zph.12257. [DOI] [PubMed] [Google Scholar]
- 21.Reimschuessel R, Grabenstein M, Guag J, Nemser SM, Song K, Qiu J, et al. Multilaboratory Survey to Evaluate Salmonella Prevalence in Diarrheic and Nondiarrheic Dogs and Cats in the United States between 2012 and 2014. J Clin Microbiol. 2017;55(5). 10.1128/JCM.02137-16. [DOI] [PMC free article] [PubMed]
- 22.Philbey AW, Mather HA, Gibbons JF, Thompson H, Taylor DJ, Coia JE. Serovars, bacteriophage types and antimicrobial sensitivities associated with salmonellosis in dogs in the UK (1954-2012) Vet Rec. 2014;174:94. doi: 10.1136/vr.101864. [DOI] [PubMed] [Google Scholar]
- 23.Srisanga S, Angkititrakul S, Sringam P, Ho PT, Vo A, Chuanchuen R. Phenotypic and genotypic antimicrobial resistance and virulence genes of Salmonella enterica isolated from pet dogs and cats. J Vet Sci. 2016;18:273–281. doi: 10.4142/jvs.2017.18.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai H, Huang H, Lin C, Lien Y, Chou H. Salmonellae and campylobacters in household and stray dogs in northern Taiwan. Vet Res Commun. 2007;31(8):931–939. doi: 10.1007/s11259-007-0009-4. [DOI] [PubMed] [Google Scholar]
- 25.Kocabiyik AL, Cetin C, Dedicova D. Detection of Salmonella spp. in stray dogs in Bursa Province, Turkey: first isolation of Salmonella Corvallis from dogs. J Vet Med B Infect Dis Vet Public Health. 2006;53(4):194–196. doi: 10.1111/j.1439-0450.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 26.Bagcigil AF, Ikiz S, Dokuzeylu B, Basaran B, Or E, Ozgur NK. Fecal shedding of Salmonella species in dogs. J Vet Med Sci. 2007;69(7):775–777. doi: 10.1292/jvms.69.775. [DOI] [PubMed] [Google Scholar]
- 27.Seepersadsingh N, Adesiyun A, Seebaransingh R. Prevalence and antimicrobial resistance of Salmonella spp. in non- diarrhoeic dogs in Trinidad. Journal of veterinary medicine. B. Infect Dis Vet Public Health. 2004;51:337–342. doi: 10.1111/j.1439-0450.2004.00785.x. [DOI] [PubMed] [Google Scholar]
- 28.Morse E, Duncan M. Canine salmonellosis: prevalence, epizootiology, signs, and public health significance. J Am Vet Med Assoc. 1975;167(9):817–820. [PubMed] [Google Scholar]
- 29.Carter M, Quinn J. Salmonella infections in dogs and cats. In: Wray C, Wray A, editors. Salmonella in Domestic Animals. Wallingford: CAB International; 2000. pp. 231–244. [Google Scholar]
- 30.Philbey AW, Brown FM, Mather HA, Coia JE, Taylor DJ. Salmonellosis in cats in the United Kingdom. 1955 to 2007. Vet Rec. 2009;164(4):120–122. doi: 10.1136/vr.164.4.120. [DOI] [PubMed] [Google Scholar]
- 31.Viegas FM, Ramos CP, Xavier RGC, Lopes EO, Júnior CAO, Bagno RM, et al. Fecal shedding of Salmonella spp., Clostridium perfringens, and Clostridioides difficile in dogs fed raw meat-based diets in Brazil and their owners’ motivation. PLoS One. 2020;15(4):e0231275. doi: 10.1371/journal.pone.0231275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finley R, Reid-Smith R, Ribble C, Popa M, Vandermeer M, Aramini J. The occurrence and antimicrobial susceptibility of salmonellae isolated from commercially available canine raw food diets in three Canadian cities. Zoonoses Public Health. 2008;55(8–10):462–469. doi: 10.1111/j.1863-2378.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- 33.CDC (Centers for Disease Control and Prevention) Update: recall of dry dog and cat food products associated with human Salmonella Schwarzengrund infections–United States, Morbidity and Mortality Weekly Report. 2008. [PubMed] [Google Scholar]
- 34.Tufa TB, Gurmu F, Beyi AF, Hogeveen H, Beyene TJ, Ayana D, Woldemariyam FT, et al. Veterinary medicinal product usage among food animal producers and its health implications in Central Ethiopia. BMC Vet Res. 2018;14:409. doi: 10.1186/s12917-018-1737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gemeda BA, Amenu K, Magnusson U, Dohoo I, Hallenberg GS, Alemayehu G, Desta H, Wieland B. Antimicrobial use in extensive smallholder livestock farming Systems in Ethiopia: knowledge, attitudes, and practices of livestock keepers. Front Vet Sci. 2020;7:55. doi: 10.3389/fvets.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yimer E, Mesfin A, Beyene M, Bekele A, Taye G, Zewdie B, Alemayehu T. Study on knowledge, attitude and dog ownership patterns related to rabies prevention and control in Addis Ababa, Ethiopia. Ethiop Vet J. 2012;16(2):27–39. doi: 10.4314/evj.v16i2.3. [DOI] [Google Scholar]
- 37.Tschopp R, Bekele S, Aseffa A. Dog demography, animal bite management and rabies knowledge-attitude and practices in the Awash Basin, Eastern Ethiopia. PLoS Negl Trop Dis. 2016;10(2):e0004471. doi: 10.1371/journal.pntd.0004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sultan A, Eyob H, Olifan Z, Yohannes E. Isolation and Identification of Salmonella from Apparently Healthy Pet Dogs in Holeta Town, Western Shoa, Ethiopia. Curr Trends Biomed Eng Biosci. 2018;15(2):555907. doi: 10.19080/CTBEB.2018.15.555907. [DOI] [Google Scholar]
- 39.Zewdu E, Miheretu S, Megersa L, Jorga E, Kebebew G, Shiferaw S. Prevalence, risk factors and antimicrobial susceptibility profile of Salmonella isolated from dogs of ambo, Bako and Gojo towns of west Shoa, Ethiopia. Ethiop Vet J. 2019;23:59–77. doi: 10.4314/evj.v23i1.5. [DOI] [Google Scholar]
- 40.Sanchez S, Hofarce CL, Lee MD, Maurer JJ, Doyle MP. Animal sources of Salmonellosis in humans. J Am Vet Med Assoc. 2002;221(4):492–497. doi: 10.2460/javma.2002.221.492. [DOI] [PubMed] [Google Scholar]
- 41.Fukata T, Naito F, Yoshida N, Yamaguchi T, Mizumura Y, Hirai K. Incidence of Salmonella infection in healthy dogs in Gifu prefecture, Japan. J Vet Med Sci. 2002;64(11):1079–1080. doi: 10.1292/jvms.64.1079. [DOI] [PubMed] [Google Scholar]
- 42.Leonard EK, Pearl DL, Finley RL, Janecko N, Reid-Smith RJ, Peregrine AS, Weese JS. Comparison of antimicrobial resistance patterns of Salmonella spp. and Escherichia coli recovered from pet dogs from volunteer households in Ontario (2005-06) J Antimicrob Chemother. 2012;67(1):174–181. doi: 10.1093/jac/dkr430. [DOI] [PubMed] [Google Scholar]
- 43.Polpakdee A, Angkititrakul S, Suksawat F, Sparagano O, Kanistanon K. Epidemiology and antimicrobial resistance of Salmonella sp. isolated from dogs and cats in northeastern Thailand. J Anim Vet Adv. 2012;11(5):618–621. doi: 10.3923/javaa.2012.618.621. [DOI] [Google Scholar]
- 44.Amadi VA, Hariharan H, Arya G, Matthew-Belmar V, Nicholas-Thomas R, Pinckney R, Sharma R, Johnson R. Serovars and antimicrobial resistance of non-typhoidal Salmonella isolated from non-diarrhoeic dogs in Grenada, West Indies. Vet Med Sci. 2018;4(1):26–34. doi: 10.1002/vms3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timbs D, Davis G, Carter M, Carman M. The Salmonella excretory incidence of dogs in Hawke’s bay. N Z Vet J. 1975;23:54–56. doi: 10.1080/00480169.1975.34193. [DOI] [PubMed] [Google Scholar]
- 46.Hackett T, Lappin MR. Prevalence of enteric pathogens in dogs of north-Central Colorado. J Am Anim Hosp Assoc. 2003;39(1):52–56. doi: 10.5326/0390052. [DOI] [PubMed] [Google Scholar]
- 47.Procter TD, Pearl DL, Finley RL, Leonard EK, Janecko N, Reid-Smith RJ, et al. A cross-sectional study examining Campylobacter and other zoonotic enteric pathogens in dogs that frequent dog parks in three cities in South-Western Ontario and risk factors for shedding of Campylobacter spp. Zoonoses Public Health. 2014;61(3):208–218. doi: 10.1111/zph.12062. [DOI] [PubMed] [Google Scholar]
- 48.Lowden P, Wallis C, Gee N, Hilton A. Investigating the prevalence of Salmonella in dogs within the midlands region of the United Kingdom. BMC Vet Res. 2015;11:239. doi: 10.1186/s12917-015-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stull JW, Peregrine AS, Sargeant JM, Weese JS. Pet husbandry and infection control practices related to zoonotic disease risks in Ontario, Canada. BMC Public Health. 2013;13:520. doi: 10.1186/1471-2458-13-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frye JG, Fedorka-Cray PJ. Prevalence, distribution and characterization of ceftiofur resistance in Salmonella enterica isolated from animals in the USA from 1999 to 2003. Int J Antimicrob Agents. 2007;30(2):134–142. doi: 10.1016/j.ijantimicag.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 51.Saleh M, Samson A, Nuhu B, Naphtali N, Adamu S, Shuaibu G, Fatima B. Prevalence of Salmonella infection in dogs in Maiduguri, northeastern Nigeria. Int J Microbiol. 2014;1:5. doi: 10.1155/2014/392548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finley R, Ribble C, Aramini J, Vandermeer M, Popa M, Litman M, Reid-Smith R. The risk of salmonellae shedding by dogs fed Salmonella-contaminated commercial raw food diets. Can Vet J. 2007;48(1):69–75. [PMC free article] [PubMed] [Google Scholar]
- 53.Schotte U, Borchers D, Wulff C, Geue L. Salmonella Montevideo outbreak in military kennel dogs caused by contaminated commercial feed, which was only recognized through monitoring. Vet Microbiol. 2007;119(2–4):316–323. doi: 10.1016/j.vetmic.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 54.PHAC . C-EnterNet 2007 Annual Report: National Integrated Enteric Pathogen Surveillance Program. 2009. [Google Scholar]
- 55.Freeman LM, Chandler ML, Hamper BA, Weeth LP. Current knowledge about the risks and benefits of raw meat-based diets for dogs and cats. JAVMA. 2013;243(11):1549–1558. doi: 10.2460/javma.243.11.1549. [DOI] [PubMed] [Google Scholar]
- 56.Zewdu E, Cornelius P. Antimicrobial resistance pattern of Salmonella serotypes isolated from food items and personnel in Addis Ababa, Ethiopia. Trop Anim Health Prod. 2009;41(2):241–249. doi: 10.1007/s11250-008-9181-y. [DOI] [PubMed] [Google Scholar]
- 57.Addis Z, Kebede N, Worku Z, Gezahegn H, Yirsaw A, Kassa T. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross-sectional study. BMC Infect Dis. 2011;11:222. doi: 10.1186/1471-2334-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wabeto W, Abraham Y, Anjulo AA. Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. J Health Popul Nutr. 2017;36:52. doi: 10.1186/s41043-017-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jajere SM, Onyilokwu SA, Adamu NB, Atsanda NN, Saidu AS, Adamu SG, et al. Prevalence of Salmonella infection in dogs in Maiduguri, Northeastern Nigeria. Int J Microbiol. 2014:392548. 10.1155/2014/392548. [DOI] [PMC free article] [PubMed]
- 60.Tupler T, Levy JK, Sabshin SJ, Tucker SJ, Greiner EC, Leutenegger CM. Enteropathogens identified in dogs entering a Florida animal shelter with normal feces or diarrhea. J Am Vet Med Assoc. 2012;241(3):338–343. doi: 10.2460/javma.241.3.338. [DOI] [PubMed] [Google Scholar]
- 61.Núñez-Castro KM, Muñoz ET, García GF, Herrera-Ramírez JC, Valencia GL, Medina-Basulto GE, Pujol-Manríquez LC, Rentería-Evangelista TB. Prevalence, risk factors, and identification of Salmonella spp. in stray dogs of northwest Mexico. Austral J Vet Sci. 2019;51(1):37–40. doi: 10.4067/S0719-81322019000100107. [DOI] [Google Scholar]
- 62.Wray C, Wray A. Salmonella in Domestic Animals. Wallingford: CAB International; 2000. [Google Scholar]
- 63.Beyene T, Endalamaw D, Tolossa Y, Feyisa A. Evaluation of rational use of veterinary drugs especially antimicrobials and anthelmintics in Bishoftu, Central Ethiopia. BMC Res Notes. 2015;8:482. doi: 10.1186/s13104-015-1466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bedada AH, Zewde BM. Tetracycline residue levels in slaughtered beef cattle from three slaughterhouses in Central Ethiopia. Global Veterinaria. 2012;8(6):546–554. [Google Scholar]
- 65.Abdulwasi S. An assessment of the effectiveness of the upgrading and revitalization strategies for housing development in case of Harar city. Addis Ababa: MSc Thesis, Ethiopian Civil Service University; 2009. [Google Scholar]
- 66.Adimasu DA, Kebede A, Menkir S. Prevalence of antibiotic resistance Salmonella isolates, Entermoeba histolytica and Giardia lomblia in Harar, Eastern Ethiopia. Afr J Microbiol Res. 2014;8(20):2044–2053. doi: 10.5897/AJMR2014.6714. [DOI] [Google Scholar]
- 67.CSA . Federal Democratic Republic of Ethiopia, central statistical agency. Addis Ababa: Population Projection of Ethiopia for the Year 2014; 2013. pp. 4–38. [Google Scholar]
- 68.Thrusfield M. Veterinary epidemiology fourth edition. Edinburgh: Veterinary Clinical Sciences Royal (Dick) School of Veterinary Studies, University of Edinburgh; 2018. [Google Scholar]
- 69.ISO (International Organization for Standardization) 6579:2002/amd 1:2007 . Detection of Salmonella species in animal faeces and in environmental samples from the primary production stage, amendment 1, Annex D. 2007. [Google Scholar]
- 70.ISO (International Organization for Standardization) 6579. Microbiology of food and animal feeding stuff- horizontal method for the detection of Salmonella species. Geneva: International Organization for Standardization; 2002. p. 511–25.
- 71.CLSI . Performance Standards for Antimicrobial Susceptibility Testing; document M100. 28. Wayne: Second informational supplement, Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to validate the results of this analysis are available from the first and correspondent authors upon reasonable request.